Abstract
In the three decades since the discovery of the Wnt1 proto-oncogene in virus-induced mouse mammary tumours, our understanding of the signalling pathways that are regulated by the Wnt proteins has progressively expanded. Wnts are involved in an complex signalling network that governs multiple biological processes and cross-talk with multiple additional signalling cascades, including the Notch, FGF (fibroblast growth factor), SHH (Sonic hedgehog), EGF (epidermal growth factor) and Hippo pathways. The Wnt signalling pathway also illustrates the link between abnormal regulation of the developmental processes and disease manifestation. Here we provide an overview of Wnt-regulated signalling cascades and highlight recent advances. We focus on new findings regarding the dedicated Wnt production and secretion pathway with potential therapeutic targets that might be beneficial for patients with Wnt-related diseases.
Keywords: adenomatous polyposis coli, planar cell polarity (PCP), Wnt
Abbreviations: APC, adenomatous polyposis coli; BAR, Bin-Amphiphysin-Rvs; CBP, CREB (cAMP response element-binding)-binding protein; COP, coat protein complex; CRD, cysteine-rich domain; CTD, C-terminal domain; CK1α, casein kinase 1 α; ER, endoplasmic reticulum FAP, familial adenomatous polyposis; FDH, focal dermal hypoplasia; GSK3β, glycogen synthase kinase 3β; LEF, lymphoid enhancer-binding factor; LRP, lipoprotein receptor-related protein; NTD, N-terminal domain; PCP, planar cell polarity; PORCN, protein Porcupine; Ror2, receptor tyrosine kinase-like orphan receptor 2; RSPO, R-Spondin; sFRP, secreted Frizzled-related protein; SNX-1, sorting nexin-1; swim, Wingless-interacting molecule; TCF, T cell-specific factor
INTRODUCTION
In multicellular organisms, cell–cell communication is critical for development, homoeostasis, and response to injury. Long and short-range signalling molecules (ligands) bind to cellular receptors and convey messages by initiating a cascade of cytoplasmic signalling events. These signals can lead to dramatic changes in cellular protein abundance, localization and activity, as well as the changes in the transcriptome, and ultimately, in the epigenome. All steps in these pathway can potentially be regulated and the intricate interactions among the ligands, receptors, and intracellular signalling molecules add both complexity and robustness into the system.
The Wnt family of signalling ligands and associated downstream mediators play key roles in short range cell–cell signalling within specific tissues that is essential for both developmental and homoeostatic processes. Adding to their importance, dysregulation of Wnt pathways can result in diverse pathologic disorders (recently reviewed by Clevers and Nusse [1]). The classification of Wnt signalling pathways is complex and historical. One of the most intensively studied consequence of Wnt signalling is the stabilization of the multifunctional protein β-catenin, a process that can activate gene transcription and is often referred to as canonical signalling. This Wnt/β-catenin pathway is readily studied by the use of artificial reporter constructs containing synthetic TCF (T cell-specific factor)/LEF (lymphoid enhancer-binding factor) bindings sites, such as TOPFLASH and SuperTOPFLASH [2,3]. The ease of use of these tools has facilitated intense study of this specific subset of the Wnt signalling, perhaps at the expense of other equally important but less easily studied responses. There are diverse additional consequences of Wnt–receptor interactions, such as β-catenin independent regulation of PCP (planar cell polarity) pathway, and these harder-to-study pathways are sometimes lumped into a category referred to non-canonical signalling. However, since there are 19 mammalian Wnt genes, ten Frizzled receptors and additional Wnt receptors and co-activators, the adverb ‘non-canonical’ is not particularly informative. Given the complexity of these pathways, it is preferable to specify as much as possible which downstream pathways are regulated by specific ligands. For example, referring to Wnt/β-catenin signalling, or Wnt/PCP signalling is more informative than a less specific statement about non-canonical Wnt signalling.
A BRIEF OVERVIEW OF THE Wnt/β-CATENIN SIGNALLING CASCADE
A simplified view of the transcriptional activation pathway
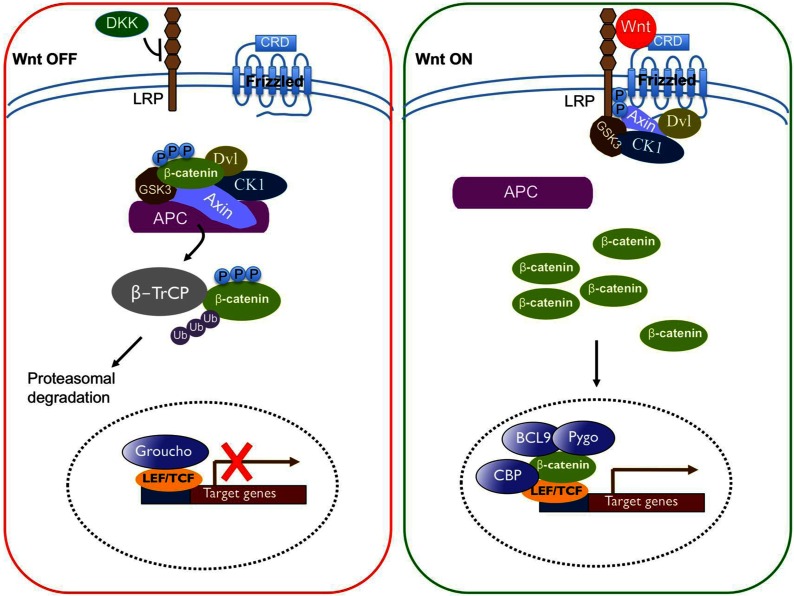
Free cytoplasmic β-catenin is a key player in a major subset of Wnt signalling cascades. When specific Wnt ligands are absent, cytoplasmic β-catenin levels are kept at low via constant targeting by a multiprotein destruction complex, recently comprehensively reviewed by Niehrs [4]. The destruction complex is composed of two scaffold proteins, Axin and APC (adenomatous polyposis coli), which facilitate the phosphorylation of β-catenin by CK1α (casein kinase 1 α) and GSK3β (glycogen synthase kinase 3β). Phosphorylation of β-catenin results in its recognition and ubiquitination by the E3 ubiquitin ligase β-TrCP (β-transducin repeats containing protein), which earmarks β-catenin for proteasomal degradation. Under such conditions, the nuclear transcription factor lymphoid enhancer-binding factor/T cell-specific (LEF/TCF) is associated with Groucho and represses target gene expression [5,6]. (Figure 1, Wnt OFF state).
Figure 1. A classical view of the canonical Wnt pathway.
Refer to the text for detailed explanation
Conversely, when Wnt ligands bind to Frizzled (Fzd) and its co-receptor, the low-density LRP (lipoprotein receptor-related protein)5/6, the receptors multimerize and form larger multiprotein complexes, which are visible as distinct punctate structures in the cytoplasm [7]. These aggregates, termed signalosomes, are the locations where a cascade of phosphorylation events take place that prevents β-catenin proteasomal degradation [8]. Stabilized β-catenin then accumulates in the cytoplasm. A subset of β-catenin can be translocated into the nucleus via a process that in some cases requires activated Ras signalling [9–11]. Nuclear β-catenin displaces Groucho and forms a complex with the BCL9 (B-cell lymphoma 9 protein), Pygopus, histone modifier CBP [CREB (cAMP response element-binding)-binding protein] as well as tissue-specific transcriptional activators, and converts LEF/TCF from a transcriptional repressor to an activator that turns on gene expression in a very cell-type-specific manner [12,13] (Figure 1, Wnt ON state).
Alternative models for how Wnt/GSK3 signalling regulates cell fate have recently been proposed. The Wnt/LRP6-mediated down-regulation of GSK3 activity can occur through internalization of GSK3 into multivesicular bodies [14–16]. This causes a global decrease in cytosolic GSK3 activity, and hence decreased phosphorylation-mediated degradation of a large number of cellular proteins. This transcription-independent mode of Wnt regulation may be especially important in G2/M phase of the cell cycle when CDK14/Cyclin Y, which phosphorylates LRP6 to drive GSK3 inhibition, is most active [14,17]. This emerging protein stabilization paradigm may also explain why more specific Wnt/β-catenin transcriptional signatures have been elusive and unreliable.
Updating the destruction complex
Detailed kinetic studies have been carried out in cell culture to investigate how β-catenin levels are regulated after Wnt stimulation [18]. By carefully comparing the changes in differentially phosphorylated β-catenin (i.e. either by GSK3 or CK1α), unphosphorylated, and total β-catenin, the authors conclude that β-catenin degradation is still occurring after long-term Wnt stimulation when a new steady state has been reached. More importantly, Wnt only partially inhibits the function of the destruction complex for both phosphorylation events [18], which is in contrast to the general belief that Wnt ligand binding blocks β-catenin phosphorylation as well as ubiquitination-mediated degradation. Using endogenous Axin immunoprecipitation, it has also been reported that the Axin destruction complex remains intact after Wnt stimulation [19]. Instead of inhibiting phosphorylation, β-catenin ubiquitination is hindered, resulting in the saturation of the destruction complex with phosphorylated β-catenin. In this model, the newly synthesized and accumulating free β-catenin is the key player for the initiation of transcription [19].
The exact mechanisms underlying how the signals perceived by the receptors are transduced to the destruction complex remain controversial. Upon Wnt stimulation, the PPPSP motif at the cytoplasmic tail of LRP6 is phosphorylated, leading to the recruitment of Axin to LRP6 [20]. Multiple kinases can phosphorylate and regulate LRP6 activity, including membrane associated CK1γ (casein kinase 1γ) [20], GSK3 [21], other casein kinase 1 family members [22], PKA (protein kinase A) [23], GRK (G protein-coupled receptor kinase) 5/6 [24], and in mitosis, the cell-cycle-dependent kinase CDK14 with its cyclin, cyclin Y [17,25]. After LRP6 activation, GSK3 and PP1 (protein phosphatase 1) together modulate Axin phosphorylation and hence conformation. This in turn regulates Axin's scaffolding function [26–28]. When Wnt is absent, Axin is phosphorylated by GSK3, resulting an open state of Axin ready for activation. Upon Wnt stimulation, the phosphorylated LRP6 cytoplasmic tail can inhibit GSK3-mediated phosphorylation [29,30]. Thus the balance of Axin phosphorylation shifts to dephosphorylation via the activity of PP1. As a result, Axin is in a closed state via intramolecular interactions, unable to associate with β-catenin and LRP6. The freed phospho-LRP6 is thus able to inactivate other open state and activated phospho-Axin molecules [26]. It should be noted that there are several alternative models that explain the regulation of β-catenin abundance following phosphorylation of LRP6, including axin degradation or sequestration, or displacement of YAP and TAZ [15,19,31–33]. Several other studies suggest that regulation of axin abundance is a critical control point for Wnt/β-catenin signalling [31,34,35]. Different modes of pathway regulation may co-exist within the same cells or tissues, with alternative pathways varying in importance depending on cellular context.
WNT RECEPTOR COMPLEXITY AND DYNAMICS
Context-dependent response with other ligands and receptors
The interaction of Wnts with Frizzled and LRP5/6 at the membrane is regulated by a dense network of additional signalling agonists and antagonists. Diverse non-Wnt proteins also bind to the Wnts and to the Frizzled receptors to either activate or inhibit Wnt/β–catenin pathway. For example, Norrie disease protein Norrin binds to Fzd4 receptor with high affinity to activate the Wnt pathway and mutations of either Norrin or Fzd4 lead to incomplete retinal vascularization [36]. Norrin has also recently been proposed to be a ligand for the LGR4 receptor [37]. Several sFRPs (secreted Frizzled-related proteins) are expressed that can competitively bind to Wnt ligands and prevent the interaction between Wnt and its receptors [38]. sFRPs may also bind to Fzd receptor through the dimerization of their homologous CRDs (cysteine-rich domains) [38], and sFRPs can activates Wnt signalling at lower, perhaps more physiological concentrations in certain cellular contexts [39]. Another class of Wnt inhibitors, the DKK (Dickkopf) family of proteins, potently inhibits Wnt/β-catenin signalling by binding to the Wnt co-receptors LRP5/6 [40]. The WISE and Sclerostin (SOST) family of proteins similarly antagonize Wnt signalling by binding to LRP4, 5 and 6 [41–43]. Inherited mutations in several of these components underlie human skeletal disorders including osteoporosis and increased bone mass syndromes (reviewed recently in [44]). Reconstitution and monoclonal antibody studies have demonstrated that different classes of Wnts interacts with distinct propeller regions of LRP6 [45–47]. These findings provide structural and mechanistic insights as to how different Wnts can interact differently with the same sets of receptors.
The Wnt-binding CRD of the frizzled family of Wnt receptors is also found in the single pass Ror2 (receptor tyrosine kinase-like orphan receptor 2). Wnt5a may interact with Ror2 through this domain [48]. Furthermore, another well-characterized Wnt-binding motif, WIF, is present in the atypical receptor tyrosine kinase Ryk [49]. It appears that the specific signalling cascades induced by Wnts are determined by the combination of ligands and specific receptors in a cellular context. This is exemplified by the activation of JNK (c-Jun N-terminal kinase) and Src kinases after binding of Wnt ligands to Ror2 and Ryk receptors, respectively [49].
Surface Frizzled levels are regulated by post-translational modifications
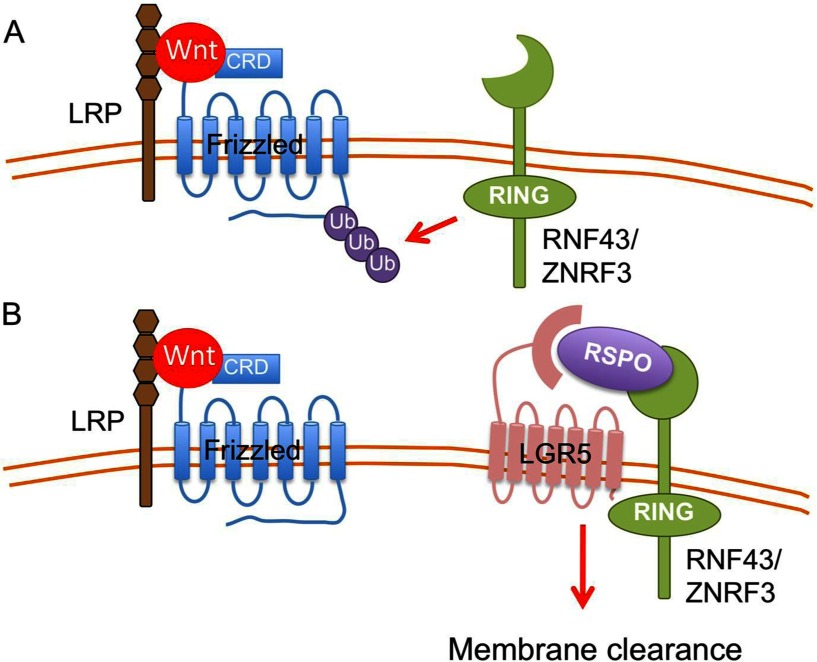
A complex new mechanism that controls the abundance of Wnt receptors on the cell surface has recently emerged. Ubiquitination of Frizzled receptors induces their endocytosis, thus reducing their surface receptor abundance and decreasing sensitivity to Wnts [50,51]. RNF43 and ZNRF3 are related transmembrane RING domain-containing E3 ubiquitin ligases whose expression is up-regulated by Wnt signalling. They are expressed in diverse tissues, and are co-expressed with Lgr5 in intestinal stem cells [51]. By ubiquitination of a cytosolic domain of Frizzled, they induce their internalization and degradation. Importantly, these ubiquitin ligases are tightly regulated. RSPOs (R-Spondins) are secreted proteins that sensitize cells to Wnts. They do this by complexing with the extracellular domains of both LGR4/5 and RNF43/ZNRF3, leading to decreased activity of the ubiquitin ligase and hence increased Frizzled abundance [50,52–54]. This mechanism is consistent with the observation that RSPOs cooperate with Wnts but on their own do not activate Wnt/β-catenin signalling.
The RSPO/LGR5/RNF43/Frizzled pathway is critical in both normal stem cell maintenance and in cancer. Inactivating mutations in RNF43 and ZNRF3 occur in ~5% of pancreatic, ovarian, gastric and biliary cancers [55–58]. Pancreatic cancer cells with RNF43 mutations have increased Wnt/β-catenin activity and are especially sensitive to Wnt inhibition [59]. RSPOs were known to be important in the ex vivo survival and proliferation of intestinal stem cells [60,61]. The RSPO in the intestinal stem cell niche appears to be RSPO3, where it is supplied by the stroma [62]. Notably, RSPO2 and RSPO3 are overexpressed by chromosome translocations in a subset of colorectal cancers [63]; this translocation product markedly stimulates Wnt/β-catenin signalling. RSPO2 and RSPO3 translocations appear to be mutually exclusive with APC mutations, consistent with the idea that the Wnt/β–catenin pathway needs to be activated one way or another in colorectal cancers.
The crystal structure of R-Spondin binding to the ectodomains of LGR5, RNF43 and ZNRF3 has been solved recently [64,65]. Together with other structural studies, this supports the model that R-Spondin is bridging LGR5 and RNF43/ZNRF3 through its Furin domains to form a ternary complex [66–68] (Figure 2). It has also been reported that binding of R-Spondin stabilizes ZNRF3 dimerization [64]. In Drosophila, it has been suggested that UBPY could be the deubiquitinating enzyme for Frizzled [69] and might reverse the effects of RNF43/ZNRF3. Although the counterpart has not been studied comprehensively in the mammalian system, the mutations of RNF43, ZNRF3 and RSPO2/3 in cancer supports the importance of this mechanism to fine-tune cell responsiveness to Wnt.
Figure 2. Surface level of Frizzled receptor is regulated by R-Spondin and RNF43/ZNRF3.
(A) In the absence of RSPO (R-Spondin), negative regulator E3 ubiquitin ligase RNF43 and ZNRF3 ubiquitinate Frizzled and lead to receptor degradation. (B) When RSPO is present, it binds to the ectodomain of RNF43/ZNRF3 and forms a complex through association with LGR5 ectodomain. This results the membrane clearance of RNF43/ZNRF3 and increases the receptor stability.
Dedicated Wnt production and secretion pathway
The downstream Wnt signalling cascades have been extensively studied in the past three decades. More recently, the study of the production and secretion of Wnt proteins has garnered increased attention as well.
Wnt ligand post-translational modification
Protein maturation and proper folding require several cellular processes after its translation. Wnt proteins contain 22 or 24 conserved cysteine residues that are important for proper folding or oligomerization of the proteins through the formation of 11 or 12 intra–molecular disulphide bonds [70,71]. Post-translation modifications of the murine WNT3A protein are the most extensively characterized [72,73]. Although the primary sequence of WNT3A is hydrophilic, the endogenous protein is hydrophobic and partitions in the detergent-phase in the Triton X-114 phase separation assay [74]. While initial studies suggested that cys-77 (Cys55 in XWnt8) was lipid modified, subsequent investigation supports the conclusion that Wnts contains only O-linked palmitoleate, on a conserved serine corresponding to Ser209 of WNT3A [70,72,75]. Desaturation of the palmitate to palmitoleate occurs via SCD (stearoyl CoA desaturase) prior to its attachment to the Wnts [75].
Wnt and receptor complex crystal structures
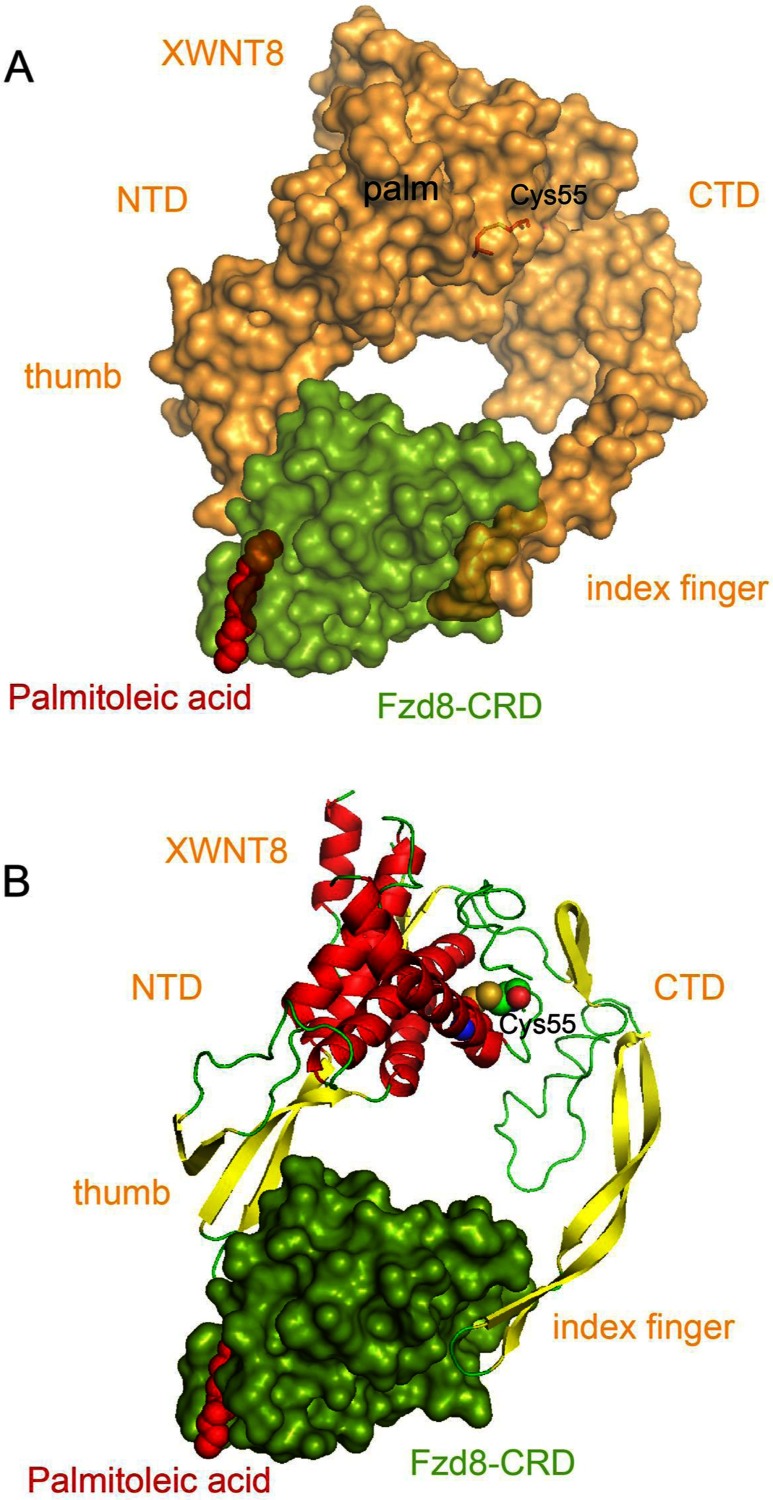
The hydrophobic nature of Wnt made its purification difficult, and its crystallization near impossible, until Xenopus WNT8 (XWNT8) was co-expressed, co-purified and co-crystallized with the Wnt-binding CRD of Fzd8 [70]. The structure of XWNT8 comprises two subdomains, an NTD (N-terminal domain) and a CTD (C-terminal domain), connected by a flexible linker region. Overall, the structure resembles a striking ‘thumb and index finger’ grasping the Fzd8–CRD at two sites, with the palmitoleate extending from the thumb to increase the interaction with Fzd8. Variation in the sequence of various Wnts and Frizzled in the interaction domains is likely to determine Wnt–Fzd-binding specificity (Figure 3). The mono-unsaturation of the palmitoleate causes a kink in the fatty acid chain and this specific structural feature may also play a role in the interaction of Wnts with both Frizzled and the carrier protein WLS (Wntless) [76].
Figure 3. Structure of XWNT8 complexed with Fzd8-CRD.
(A) Surface representation of XWNT8 (yellow) and Fzd8-CRD (green). (B) Ribbon model of XWNT8, with red α-helix and yellow β-sheets secondary structures. The palmitoleic acid at Ser187 (red) is positioned at the tip of the ‘thumb’ of XWNT8. The ‘index finger’ of XWNT8 forms the second interaction site with Fzd8–CRD. The Cys55 originally proposed to be acylated instead forms an intramolecular disulphide bond with Cys66 [shown in sticks in the palm region in (A), and spheres in (B)]. The structure was obtained from Protein Data Bank, ID: 4F0A. The images were generated using MacPyMOL. NTD, N-terminal domain. CTD, C-terminal domain.
Notably, the palmitoleated serine residue is conserved in all Wnt members across different species except in WntD, a Drosophila Wnt family member that does not undergo lipid modification [77]. Comparison of the crystal structure of a WntD fragment with the XWNT8 structure suggest that the positively-charged linker is responsible for interaction with the negative-charged LRP extracellular repeat 3 propeller domain [78,79].
Porcupine/PORCN (protein Porcupine) is the acyltransferase for Wnt
The ER resident PORCN is both necessary and sufficient to catalyse the lipid modification of Wnts [80–84]. Initially identified as a segment polarity gene in Drosophila, PORCN belongs to the superfamily of MBOATs (membrane-bound O-acyltransferases) [85]. Since Wnt signalling is critical for embryonic development, it is not surprising that mutations in the pathway genes have been reported in many human genetic diseases [1]. Inherited and post-zygotic mutations in PORCN, an X-linked gene, cause FDH (focal dermal hypoplasia, also known as Goltz syndrome), a disease that predominantly affects females [86,87]. Global ablation of Porcn in mice is embryonic lethal, as the embryo fails to complete gastrulation [88]. This explains the female-specific inheritance of FDH., Mutant males suffer embryonic lethality, whereas mutant females survive and exhibit variable clinical manifestations as a result of random X-chromosome inactivation. To study PORCN developmental functions after gastrulation, conditional knockout mice have been generated using the epiblast-active Sox2-Cre driver [89]. Female heterozygous Porcn mutant (PORCNΔ/+) mice showed a range of abnormalities in limbs and dermis that resemble the human FDH disease [89]. These findings provide strong evidence of the aetiology of human FDH, suggesting the tissue-specific failure of Wnt ligand secretion as the cause of the disease.
PORCN appears to be the only acyl-transferase capable of modifying Wnts. Using specific zinc-finger nuclease technology, the single PORCN allele in the X-chromosome was inactivated in male HT1080 fibrosarcoma cells [83,84]. In these PORCN-null HT1080 cells, all 19 human Wnts lose activity, and re-expression of PORCN was required for either Wnt/β-catenin signalling, Wnt-induced Dvl-2 phosphorylation, or Wnt–WLS binding. Although WNT5B did not have any known signalling activity in these assays, it still required PORCN for binding to WLS. This underscores the importance of PORCN as the single acyltransferase in the regulation of Wnt ligand secretion and Wnt signalling. Using this cell line, our group studied the activity of the PORCN mutants found in human FDH patients. Consistent with the notion that PORCN is exquisitely required for Wnt signalling, mutants with only subtle reduction in the enzyme activity are associated with the severe form of the disease [84]. Interestingly, knockdown of PORCN in some cell lines produces a slow growth phenotype that is rescued by catalytically inactive PORCN, suggesting PORCN has additional functions in addition to acylation of Wnts in some cancer cells [90].
Studies with PORCN knockouts are consistent with prior studies of Wnt, β-catenin and LRP5/6 germline mouse knockouts that show that cell-autonomous Wnt signalling is not essential for the proliferation of embryonic stem cells. While several Wnt mRNAs are detectable in blastocysts, knockout of PORCN, WLS and the double knockout of LRP5 and LRP6 all demonstrate that the embryonic requirement for Wnt protein function only appears at gastrulation, and then later in a subset of tissues in the adult mouse. [62,88,89,91–95]. Studies with potent small molecule PORCN inhibitors further indicate that Wnt secretion is not required for the proliferation of most cultured cell lines [96].
Wntless/WLS is the carrier protein for Wnt
A second essential Wnt secretion protein was identified through screening in the Drosophila system. The multipass transmembrane protein Wntless (WLS) is a Wnt-sorting receptor that carries Wnt out to the cell surface for release [97–99]. It is also highly conserved from Cnidarians to human [100].
In Xenopus, WLS is required for XWNT4 secretion and morpholino-mediated knockdown of WLS resulted in eye development defects [101]. In mice, Wls knockout is embryonic lethal similar to the Porcn knockout with failure of mesoderm induction and gastrulation [95]. It has been reported that in the Drosophila larval neuromuscular junction, Wingless (Wg, the homologue of Wnt1 in the fly) is secreted in the WLS-containing exosome-like vesicles from the presynaptic neurons [102]. Jin et al. further identified the mu-opioid receptor as a WLS-binding partner, which sequesters WLS on the cell surface in the presence of an opioid agonist, thus inhibiting Wnt protein secretion [103]. These data suggest that the regulation of Wnt secretion by WLS is complex.
Several conditional Wls knockout mouse models have been developed that highlight the essential role of WLS in development. When the floxed Wls allele is excised by Wnt1-Cre, the resulting mice exhibit a phenotype similar to the Wnt1 null mice, with developmental abnormalities in the midbrain and hindbrain [104]. Recently, genome-wide association studies identified intronic SNPs (single-nucleotide polymorphisms) in the WLS gene that are strongly associated with reduced bone mineral density [105]. Consistent with the association study, when Wls was specifically deleted in mature osteoblast and osteocytes, the mice developed severe low bone mass due to reduced bone formation and increased matrix resorption [106]. These mice were prone to fractures and could not survive long after birth. Wnt/β-catenin signalling is also critical in skin and hair. Accordingly, conditional deletion of Wls with Keratin 14-Cre driver results in the loss of hair follicles and infiltration of immune cells into the skin together with up-regulation of inflammation-related genes [107,108].
Unlike PORCN, WLS-coding region mutations are not reported to date in any human diseases. WLS expression levels vary widely, however, and are elevated in some cancers, including astrocytic gliomas and glioblastoma cell lines [109]. On the other hand, WLS expression is reduced in melanoma tumours as compared to the normal skin and benign lesions [110]. Knockdown of WLS in melanoma cell lines increased cell proliferation in vitro and promoted lung metastasis in a xenograft model [110], consistent with a tumour suppressive role of Wnt/β-catenin signalling in this disease [111]. These findings further highlight the importance of cellular context in determining the role of Wnt signalling in a specific disease.
Factors that regulate WLS trafficking-retromer complex and SNX3
At the molecular level, WLS is a cargo receptor that transports Wnt from the Golgi apparatus to the plasma membrane. The interaction of Wnt and WLS is dependent on the palmitoleation of Wnt at the serine residue mediated by PORCN [76,84,112]. Upon dissociation from Wnts at the cell surface, WLS is recycled via clathrin-mediated endocytosis. The tyrosine-containing motif YXXΦ (where Φ is any hydrophobic amino acid) in the third intracellular loop of WLS is required for its endocytosis from the cell surface, as WLS accumulates on the plasma membrane when the corresponding Y425 is mutated [113,114]. Following internalization, the retromer complex drives retrieval of WLS from the endosomes to the trans-Golgi network [115–117]. The involvement of the retromer complex in Wnt signalling was first discovered in Caenorhabditis elegans, as the vps-35 mutant produced an EGL-20/Wnt-defect phenotype [118]. In the budding yeast Saccharomyces cerevisiae, the retromer complex is composed of two subcomplexes, cargo-selection subunits consisting of Vps35p, Vps29p and Vps26p; and structure subunits, consisting of Vps5p and Vps17p dimer [119]. WLS interacts with Vps35 in immunoprecipitation experiments [115]. In the absence of Vps35, WLS accumulates instead in multi-vesicular bodies, as shown by electron microscopy [116]. Therefore the improper recycling of WLS results in the reduced levels of the protein in Vps35-depleted cells [115]. To date, the sorting signal on WLS that enables cargo-selection by Vps35 has not been identified.
The retromer complex is associated with the endosomal membrane, probably through interaction with lipid-enriched elements found in the endocytic network [119]. SNX-1 (sorting nexin-1), the homologue of yeast Vps5p, has a Phox-homology (PX) domain that binds to phosphatidylinositol 3,5-bisphosphate (Ptd(3,5)P2) commonly found on the early endosomes [120]. Interestingly, MTM-6 and MTM-9, members of the myotubularin family, have also been implicated in the MIG-14/WLS trafficking in C. elegans [121]. Myotubularin is a lipid phosphatase that dephosphorylates phosphatidylinositol 3-phosphate (Ptd3P) and Ptd(3,5)P2, which could potentially affect endosomal trafficking. However, in contrast to the conventional SNX1-/SNX-2 and SNX-5/SNX-6 (homologous to yeast Vps17p) dependent retromer pathway, an unrelated SNX-3 appears to participate in the retrograde transport of WLS [122,123]. SNX-3 co-localizes with WLS and can co-immunoprecipitate with the cargo selective subunits, Vps35 and Vps26, of the retromer complex [122]. Conventional SNXs possess a BAR (Bin-Amphiphysin-Rvs) domain, which drives the membrane curvature and cargo segregation into the tubular structure [124]. However, it is still unknown how SNX-3, which lacks the BAR domain, regulates the recycling of WLS.
When retromer complex function is partially disrupted in C. elegans by a vps29 mutant, the retrieval of WLS/MIG-14 can be restored by blocking endosomal maturation. Moreover, WLS/MIG-14 that accumulates in the late endosomes can be partially retrieved through the classical SNX–BAR-dependent pathway in vps29 mutant, but not in snx-3 mutant [125]. The authors therefore proposed that both the spatial distribution of SNX3 (in early endosomes) and SNX–BAR (in early-to-late and late endosomes), as well as cargo-specific mechanisms contributes to the retromer-dependent retrieval of WLS [125].
p24 protein family members in Wnt secretion
The transport of Wnt from the ER (endoplasmic reticulum) to the Golgi complex relies on a sequence of protein-sorting events in the early secretory pathway. Specific COP (coat protein) complexes are involved in this bidirectional trafficking of proteins. Generally, COPII mediates the anterograde transport from the ER to the Golgi, whereas COPI generates the retrograde trafficking from the Golgi back to the ER. However, there is also evidence showing that anterograde-directed cargos are present in a distinct population of COPI vesicles [126].
Genome-wide RNAi screening in Drosophila revealed the a role for the p24 family of proteins in Wnt secretion [127,128]. p24 proteins are components of the COP vesicles, and have previously been implicated in the regulation of cargo sorting and trafficking [129]. In mammals, there are at least eleven p24 family members. It has been suggested that p24 proteins are involved in the ER exit of GPI (glycosylphosphatidylinositol)-anchored proteins in yeast [130,131]. Similar roles of p24 proteins have also been proposed by Port et al. [128] as to facilitate Wg export from the ER in Drosophila. However, there is only limited evidence supporting the role of p24 in mammalian Wnt secretion and the redundancy of p24 family members complicates their study.
Retrograde trafficking to the ER
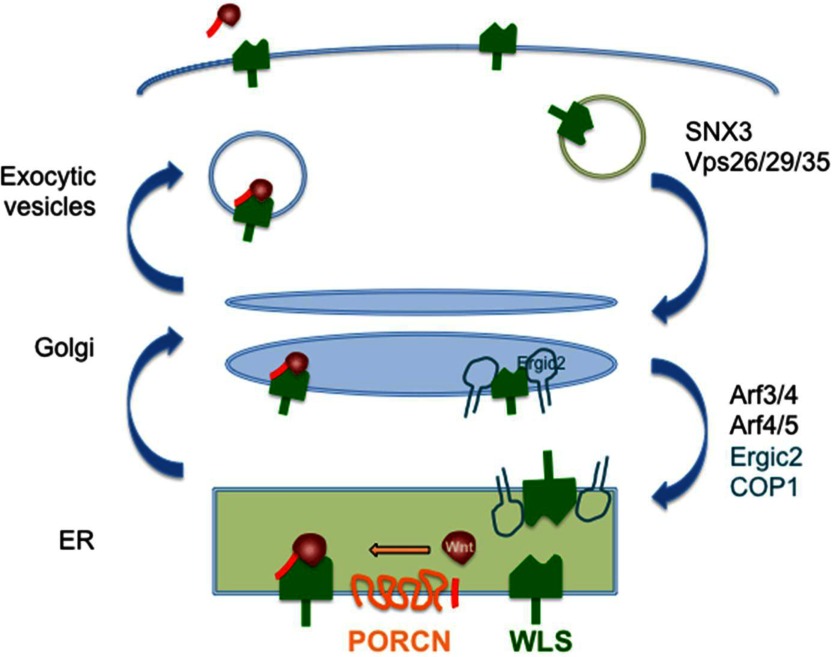
WLS cycles from the Golgi to the plasma membrane and then back to the Golgi via the retromer complex after endocytosis, but it is not known how the palmitoleated Wnts in the ER meet up with WLS in the Golgi. A high-affinity monoclonal antibody against human WLS was developed that specifically recognizes endogenous WLS [76]. Using a diverse set of approaches, we found that after endogenous WLS cycles to the cell surface, it then returns to the ER through the Golgi [114]. WLS contains a unique ER targeting sequence at its C-terminus that is critical for both its ER recycling and biological function. C-terminal deletion, point mutation or epitope tagging inhibits WLS ER localization and activity in Wnt/β-catenin signalling. In addition, Golgi to ER retrograde recycling of WLS requires the COPI transport machinery involving the function of the small GTPase ARF as well as ERGIC2 [114]. These findings solve the problem of how to get lipid-modified Wnts out of the ER. Whether release of WLS from the ER is itself regulated remains to be determined, it is notable that WLS is the first example of an integral membrane secretory protein that cycle from ER to the PM and back to the ER. The Wnt secretion pathway is shown in Figure 4.
Figure 4. The Wnt secretion pathway.
Newly synthesized Wnts are palmitoleated (red line) by PORCN in the ER, facilitating binding to the specialized transporter WLS (green). WLS carries Wnts to the plasma membrane for release after acidification in exocytic vesicles [76]. WLS is recycled via clathrin-coated vesicles and the retromer complex to the Golgi, and then to the ER using COP1 vesicles and ERGIC2 [114]. Alternative routes for Wnt exit also exist (see text).
Wnt extracellular trafficking
As noted above, Wnt secretion is a complex process involving specialized cellular machinery that controls the export of Wnt. In addition, the presence of a fatty acid chain on Wnts also increases their hydrophobicity, posing challenges to the spreading of Wnt in the extracellular matrix that is required to exert signalling as a morphogen. Although this process is still poorly understood, a few models have been proposed to better account for Wnt extracellular trafficking. Some examples of these models are described below.
In Drosophila wing imaginal disc, filopodia-like cellular extensions called cytonemes have been observed in outlying (signal receiving) cells oriented towards the disc centre (signal secreting) cells [132]. This type of cell–cell contact might mediate the intermediate-range transport of Wnt, although no direct evidence has been reported so far.
HSPGs (heparan sulphate proteoglycans) are another group of molecules that mediate the lateral diffusion of Wnt proteins. Glypican Dally and Dlp (Dally-like proteins) are involved in gradient formation by transferring Wg to neighbouring cells, thus inhibiting the binding of Wg to its local receptor for signalling [133].
Alternatively, a complex Wnt-containing structure might be formed, which shields the lipids attached to the protein from the extracellular environment. Self-aggregation into a micelle might reduce the hydrophobicity of the Wnt molecule. However, monounsaturated palmitoleate, unlike palmitate, might not favour micelle formation. This mechanism has been reported for the Hedgehog protein, another palmitoylated signalling protein that requires long-range signalling during development [134]. It has been suggested that Wnts can bind to lipoprotein particles such as lipophorin in Drosophila to facilitate their movement [135,136]. Secreted Swim (Wingless-interacting molecule), a member of the lipocalin family proteins that bind lipids in a tertiary barrel-like structure, has been identified recently to bind Drosophila Wg with high affinity [137]. More importantly, Swim is both necessary and sufficient to solubilize purified Wg proteins in vitro [137].
Recent studies from the Budnik laboratory provide strong evidence that Wg is transported across synapses in WLS-containing exosome-like vesicles at the larval NMJ (neuromuscular junction) in Drosophila [102]. The release of WLS-containing exosomes at the synaptic boutons seems to require the small GTPase Rab11 and Syntaxin 1A [138]. In cultured Drosophila S2 cells, Wg is also secreted on exosome-like vesicles, which depends on the Rab11 function as well [139]. However, the Wg gradient formation in the wing imaginal disc is not affected when Rab11 is depleted by RNAi in the tissue [139]. In mammalian cells, Wnt proteins can be secreted on exosomes derived from endosomal compartments in a process that requires the R-SNARE (SNAP receptor) protein Ykt6 [140]. Exosome-mediated Wnt signalling may play a role in cell migration and wound healing [141–143].
DRUG DISCOVERY IN THE WNT FIELD
Wnt signalling and cancers
Since Wnt signalling controls cell growth, differentiation and migration, the aberrant activation of Wnt signalling can lead to uncontrolled cell growth and contribute to tumourigenesis. The molecular evidence that Wnt is involved in both embryogenesis and cancer development demonstrates the convergence for these two fields [144].
A well-known example that links the Wnt pathway with cancer development is a hereditary disease condition known as FAP (familial adenomatous polyposis). FAP patients inherit a mutated copy of the APC gene, and the second APC allele is often lost later in life [145–147]. These mutations found on APC are usually nonsense or frame shift mutations, which generate truncated forms of APC. The loss-of-heterozygosity phenomenon observed in the disease suggests that APC may exert a potential tumour suppressive function. Affected individuals usually develop multiple adenomatous polyps in the colon at a relatively early age. These polyps can transform into carcinomas upon acquisition of additional mutations. Besides mutations in APC, inactivating mutations in Axin2 as well as gain-of-function mutations in β-catenin have also been found in colon cancer patients, albeit with relatively low frequency [148–150]. The Cancer Genome Atlas Network in 2012 reported Wnt/β-catenin pathway mutations in up to 93% of colorectal cancers, and that was prior to the identification of RPSO2/3 translocations and RNF43/ZNRF3 mutations in the Wnt pathway [151]. Stabilizing mutations in β-catenin are commonly found in gastric, liver and kidney cancers as well.
Current advances in Wnt pathway inhibitors
Given the therapeutic potential in the treatment of Wnt-related cancers, there are diverse attempts to target the Wnt pathway with small molecule inhibitors. The identification of valid drug targets is challenging, although an increasing number of proof-of-concepts studies in animal models have been reported and recently reviewed [152]. The effective therapies can be divided into downstream inhibitors, those that target β-catenin abundance and/or activity [153,154], and upstream inhibitors, those that target Wnt production or Wnt interaction with extracellular receptors.
Downstream inhibitors include tankyrase inhibitors. This class of compounds stabilizes Axin protein and thus promotes the function of the destruction complex. The earliest examples of these include IWR-1 [155] and XAV939 [156]. Both XAV939 and IWR are inhibitors of the poly-ADP-ribosylating enzyme tankryase, which facilitates Axin turnover by PARsylation-mediated ubiquitination and proteosomal degradation [156].
ICG-001 inhibits the interaction of β-catenin with the histone acetyltransferase and transcriptional co-activator CBP, hence blocking transcriptional activation of a subset of β-catenin regulated genes. A related compound, PRI-724, is now in clinical trials for acute myeloid leukaemia (clinicaltrials.gov NCT01606579) and advanced solid tumours (clinicaltrials.gov NCT01302405) [154].
Inhibition of upstream Wnt activity can be achieved by small molecule inhibition of PORCN activity [76]. Novartis has developed a PORCN inhibitor LGK974, which entered Phase I clinical trial in 2012 (clinicaltrials.gov NCT01351103). This compound, as well as another related compound C59, showed efficacy in Wnt-driven tumours in mouse models at well-tolerated doses [96,157]. Importantly, pancreatic ductal cancer cell lines with inactivating mutations in RNF43 are sensitive to LGK974, providing a genetic biomarker for responsive patients [59]. A second promising approach to upstream inhibition of Wnt signalling is the use of monoclonal antibodies such as vantictumab that bind to the extracellular domain of Frizzled receptors and prevent the interactions with Wnts [158] (clinicaltrials.gov NCT02005315 and others). One concern in all these studies is whether effective Wnt pathway inhibition will have significant side effects due to inhibition of essential Wnt-driven processes such as bone formation.
Remaining questions in the field
As we learn more about Wnt biology, new questions continue to arise. There continues to be controversy about how Wnts are transported outside the cell. Lipoproteins and argosomes were identified first, while the exosome model is gaining traction. More work is needed to define the molecular origin of the exosomes and how Wnts are incorporated into these vesicles. Even the concept of Wg as a secreted morphogen in the Drosophila wing imaginal disc has been challenged [159]. When a membrane-tethered Wg was expressed in Drosophila in the absence of the endogenous alleles, the resulting flies were viable with almost normal wing size [160]. This implies that Wg spreading across distance is not necessary for patterning and growth in Drosophila. The authors reasoned that the Wg transcription is active during the initial stage of wing patterning, and only restricted to the dorsoventral boundary later on. Nevertheless, the expression of target genes such as vestigial and Distal-less persists at a lower level. This partially explains the slow growth phenotype of flies with membrane-bound Wg [160]. This study prompts us to question some long-held assumptions of the Wg morphogen model.
Limited studies have been done to investigate the Wnt secretory pathway in a polarized cell system, which is more relevant to normal physiology. At least for WNT3A and WNT11, glycosylation seems to regulate whether Wnt is secreted from the apical or basolateral surface of polarized epithelial cells [161]. However, the role of WLS in this polarized trafficking is less well defined.
In the context of the tissue or organ, it will be important to identify the source of the Wnt. For example, Paneth cells at the bottom of the small intestine crypts were reported to be the critical Wnt-producing cells that support the LGR5-positive CBC (crypt base columnar) cells [162], based on ex vivo data. However, several recent in vivo studies call this model into question. In the absence of Paneth cells, or if epithelial Wnt secretion is blocked by knockout of PORCN or WLS, intestinal stem cells are still functional in vivo [62,163,164]. In fact, our group found that underlying stromal intestinal myofibroblasts robustly support Wnt-deficient Lgr5+ intestinal stem cells in organoid formation and in this setting, organoids no longer require RSPO supplementation. Importantly, these myofibroblasts express multiple Wnts, and high levels of RSPO3. Hence, as in many other stem cell niches, critical trophic factors derive from the stroma rather than the proliferating compartment.
SUMMARY
Because of their central role in both development and disease, only a few signalling pathways have received as much scrutiny over the years as Wnt signalling. This intensive study continues to yield surprising insights and unexpected mechanisms. Our ability to target the pathway therapeutically is gaining in sophistication, and the number of clinical trials ongoing suggests we will learn in the coming years if therapeutic intervention in the Wnt pathway is safe and effective.
References
- 1.Clevers H., Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 2.Korinek V., Barker N., Morin P. J., van Wichen D., de Weger R., Kinzler K. W., Vogelstein B., Clevers H. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC−/− colon carcinoma. Science. 1997;275:1784–1787. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- 3.Veeman M. T., Slusarski D. C., Kaykas A., Louie S. H., Moon R. T. Zebrafish prickle, a modulator of noncanonical Wnt/Fz signaling, regulates gastrulation movements. Curr. Biol. 2003;13:680–685. doi: 10.1016/S0960-9822(03)00240-9. [DOI] [PubMed] [Google Scholar]
- 4.Niehrs C. The complex world of WNT receptor signalling. Nat. Rev. Mol. Cell. Biol. 2012;13:767–779. doi: 10.1038/nrm3470. [DOI] [PubMed] [Google Scholar]
- 5.Cavallo R. A., Cox R. T., Moline M. M., Roose J., Polevoy G. A., Clevers H., Peifer M., Bejsovec A. Drosophila Tcf and Groucho interact to repress Wingless signalling activity. Nature. 1998;395:604–608. doi: 10.1038/26982. [DOI] [PubMed] [Google Scholar]
- 6.Roose J., Molenaar M., Peterson J., Hurenkamp J., Brantjes H., Moerer P., van de Wetering M., Destrée O., Clevers H. The Xenopus Wnt effector XTcf-3 interacts with Groucho-related transcriptional repressors. Nature. 1998;395:608–612. doi: 10.1038/26989. [DOI] [PubMed] [Google Scholar]
- 7.Bilic J., Huang Y.-L., Davidson G., Zimmermann T., Cruciat C.-M., Bienz M., Niehrs C. Wnt induces LRP6 signalosomes and promotes dishevelled-dependent LRP6 phosphorylation. Science. 2007;316:1619–1622. doi: 10.1126/science.1137065. [DOI] [PubMed] [Google Scholar]
- 8.Zeng X., Huang H., Tamai K., Zhang X., Yokota C., Almeida K., Wang J., Doble B., Woodgett J., Wynshaw-Boris A., et al. Initiation of Wnt signaling: control of Wnt coreceptor Lrp6 phosphorylation/activation via frizzled, dishevelled and axin functions. Development (Cambridge, England) 2008;135:367–375. doi: 10.1242/dev.013540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Janssen K.-P., Alberici P., Fsihi H., Gaspar C., Breukel C., Franken P., Rosty C., Abal M., Marjou, El F., Smits R., et al. APC and oncogenic KRAS are synergistic in enhancing Wnt signaling in intestinal tumor formation and progression. Gastroenterology. 2006;131:1096–1109. doi: 10.1053/j.gastro.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 10.Obrador-Hevia A., Chin S.-F., González S., Rees J., Vilardell F., Greenson J. K., Cordero D., Moreno V., Caldas C., Capellá G. Oncogenic KRAS is not necessary for Wnt signalling activation in APC-associated FAP adenomas. J. Pathol. 2010;221:57–67. doi: 10.1002/path.2685. [DOI] [PubMed] [Google Scholar]
- 11.Phelps R. A., Chidester S., Dehghanizadeh S., Phelps J., Sandoval I. T., Rai K., Broadbent T., Sarkar S., Burt R. W., Jones D. A. A two-step model for colon adenoma initiation and progression caused by APC loss. Cell. 2009;137:623–634. doi: 10.1016/j.cell.2009.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cadigan K. M., Waterman M. L. TCF/LEFs and Wnt signaling in the nucleus. Cold Spring Harb. Perspect. Biol. 2012;4:a007906. doi: 10.1101/cshperspect.a007906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mosimann C., Hausmann G., Basler K. Beta-catenin hits chromatin: regulation of Wnt target gene activation. Nat. Rev. Mol. Cell Biol. 2009;10:276–286. doi: 10.1038/nrm2654. [DOI] [PubMed] [Google Scholar]
- 14.Acebron S. P., Karaulanov E., Berger B. S., Huang Y.-L., Niehrs C. Mitotic wnt signaling promotes protein stabilization and regulates cell size. Mol. Cell. 2014;54:663–674. doi: 10.1016/j.molcel.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 15.Taelman V. F., Dobrowolski R., Plouhinec J.-L., Fuentealba L. C., Vorwald P. P., Gumper I., Sabatini D. D., De Robertis E. M. Wnt signaling requires sequestration of glycogen synthase kinase 3 inside multivesicular endosomes. Cell. 2010;143:1136–1148. doi: 10.1016/j.cell.2010.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vinyoles M., Del Valle-Perez B., Curto J., Viñas-Castells R., Alba-Castellón L., García de Herreros A., Dunach M. Multivesicular GSK3 sequestration upon Wnt signaling is controlled by p120–catenin/cadherin interaction with LRP5/6. Mol. Cell. 2014;53:444–457. doi: 10.1016/j.molcel.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 17.Davidson G., Shen J., Huang Y.-L., Su Y., Karaulanov E., Bartscherer K., Hassler C., Stannek P., Boutros M., Niehrs C. Cell cycle control of wnt receptor activation. Dev. Cell. 2009;17:788–799. doi: 10.1016/j.devcel.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 18.Hernandez A. R., Klein A. M., Kirschner M. W. Kinetic responses of β-catenin specify the sites of Wnt control. Science. 2012;338:1337–1340. doi: 10.1126/science.1228734. [DOI] [PubMed] [Google Scholar]
- 19.Li V. S. W., Ng S. S., Boersema P. J., Low T. Y., Karthaus W. R., Gerlach J. P., Mohammed S., Heck A. J. R., Maurice M. M., Mahmoudi T., et al. Wnt signaling through inhibition of β-catenin degradation in an intact Axin1 complex. Cell. 2012;149:1245–1256. doi: 10.1016/j.cell.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 20.Davidson G., Wu W., Shen J., Bilic J., Fenger U., Stannek P., Glinka A., Niehrs C. Casein kinase 1 γ couples Wnt receptor activation to cytoplasmic signal transduction. Nat. Cell Biol. 2005;438:867–872. doi: 10.1038/nature04170. [DOI] [PubMed] [Google Scholar]
- 21.Zeng X., Tamai K., Doble B., Li S., Huang H., Habas R., Okamura H., Woodgett J., He X. A dual-kinase mechanism for Wnt co-receptor phosphorylation and activation. Nature. 2005;438:873–877. doi: 10.1038/nature04185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Swiatek W., Kang H., Garcia B. A., Shabanowitz J., Coombs G. S., Hunt D. F., Virshup D. M. Negative regulation of LRP6 function by casein kinase I epsilon phosphorylation. J. Biol. Chem. 2006;281:12233–12241. doi: 10.1074/jbc.M510580200. [DOI] [PubMed] [Google Scholar]
- 23.Wan M., Yang C., Li J., Wu X., Yuan H., Ma H., He X., Nie S., Chang C., Cao X. Parathyroid hormone signaling through low-density lipoprotein-related protein 6. Genes Dev. 2008;22:2968–2979. doi: 10.1101/gad.1702708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen M., Philipp M., Wang J., Premont R. T., Garrison T. R., Caron M. G., Lefkowitz R. J., Chen W. G Protein-coupled receptor kinases phosphorylate LRP6 in the Wnt pathway. J. Biol. Chem. 2009;284:35040–35048. doi: 10.1074/jbc.M109.047456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Niehrs C., Shen J. Regulation of Lrp6 phosphorylation. Cell. Mol. Life Sci. 2010;67:2551–2562. doi: 10.1007/s00018-010-0329-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim S.-E., Huang H., Zhao M., Zhang X., Zhang A., Semonov M. V., Macdonald B. T., Zhang X., Abreu J. G., Peng L., et al. Wnt stabilization of β-catenin reveals principles for morphogen receptor-scaffold assemblies. Science. 2013;340:867–870. doi: 10.1126/science.1232389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luo W., Peterson A., Garcia B. A., Coombs G., Kofahl B., Heinrich R., Shabanowitz J., Hunt D. F., Yost H. J., Virshup D. M. Protein phosphatase 1 regulates assembly and function of the beta-catenin degradation complex. EMBO J. 2007;26:1511–1521. doi: 10.1038/sj.emboj.7601607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Willert K., Shibamoto S., Nusse R. Wnt-induced dephosphorylation of axin releases beta-catenin from the axin complex. Genes Dev. 1999;13:1768–1773. doi: 10.1101/gad.13.14.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu G., Huang H., Garcia Abreu J., He X. Inhibition of GSK3 phosphorylation of beta-catenin via phosphorylated PPPSPXS motifs of Wnt coreceptor LRP6. PLoS ONE. 2009;4:e4926. doi: 10.1371/journal.pone.0004926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cselenyi C. S., Jernigan K. K., Tahinci E., Thorne C. A., Lee L. A., Lee E. LRP6 transduces a canonical Wnt signal independently of Axin degradation by inhibiting GSK3′s phosphorylation of beta-catenin. Proc. Natl. Acad. Sci. U. S. A. 2008;105:8032–8037. doi: 10.1073/pnas.0803025105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salic A., Lee E., Mayer L., Kirschner M. W. Control of beta-catenin stability: reconstitution of the cytoplasmic steps of the wnt pathway in Xenopus egg extracts. Mol. Cell. 2000;5:523–532. doi: 10.1016/S1097-2765(00)80446-3. [DOI] [PubMed] [Google Scholar]
- 32.Azzolin L., Panciera T., Soligo S., Enzo E., Bicciato S., Dupont S., Bresolin S., Frasson C., Basso G., Guzzardo V., et al. YAP/TAZ incorporation in the β-catenin destruction complex orchestrates the Wnt response. Cell. 2014;158:157–170. doi: 10.1016/j.cell.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 33.Yamamoto H., Kishida S., Kishida M., Ikeda S., Takada S., Kikuchi A. Phosphorylation of axin, a Wnt signal negative regulator, by glycogen synthase kinase-3beta regulates its stability. J. Biol. Chem. 1999;274:10681–10684. doi: 10.1074/jbc.274.16.10681. [DOI] [PubMed] [Google Scholar]
- 34.Lee E., Salic A., Krüger R., Heinrich R., Kirschner M. W. The roles of APC and Axin derived from experimental and theoretical analysis of the Wnt pathway. PLoS Biol. 2003;1:E10. doi: 10.1371/journal.pbio.0000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee E., Salic A., Kirschner M. W. Physiological regulation of [beta]-catenin stability by Tcf3 and CK1epsilon. J. Cell Biol. 2001;154:983–993. doi: 10.1083/jcb.200102074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu Q., Wang Y., Dabdoub A., Smallwood P. M., Williams J., Woods C., Kelley M. W., Jiang L., Tasman W., Zhang K., et al. Vascular development in the retina and inner ear: control by Norrin and Frizzled-4, a high-affinity ligand-receptor pair. Cell. 2004;116:883–895. doi: 10.1016/S0092-8674(04)00216-8. [DOI] [PubMed] [Google Scholar]
- 37.Deng C., Reddy P., Cheng Y., Luo C.-W., Hsiao C.-L., Hsueh A. J. W. Multi-functional norrin is a ligand for the LGR4 receptor. J. Cell. Sci. 2013;126:2060–2068. doi: 10.1242/jcs.123471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kawano Y., Kypta R. Secreted antagonists of the Wnt signalling pathway. J. Cell Sci. 2003;116:2627–2634. doi: 10.1242/jcs.00623. [DOI] [PubMed] [Google Scholar]
- 39.Xavier C. P., Melikova M., Chuman Y., Uren A., Baljinnyam B., Rubin J. S. Secreted Frizzled-related protein potentiation versus inhibition of Wnt3a/β-catenin signaling. Cell Signal. 2014;26:94–101. doi: 10.1016/j.cellsig.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Glinka A., Wu W., Delius H., Monaghan A. P., Blumenstock C., Niehrs C. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature. 1998;391:357–362. doi: 10.1038/34848. [DOI] [PubMed] [Google Scholar]
- 41.Itasaki N., Jones C. M., Mercurio S., Rowe A., Domingos P. M., Smith J. C., Krumlauf R. Wise, a context-dependent activator and inhibitor of Wnt signalling. Development (Cambridge, England) 2003;130:4295–4305. doi: 10.1242/dev.00674. [DOI] [PubMed] [Google Scholar]
- 42.Semenov M., Tamai K., He X. SOST is a ligand for LRP5/LRP6 and a Wnt signaling inhibitor. J. Biol. Chem. 2005;280:26770–26775. doi: 10.1074/jbc.M504308200. [DOI] [PubMed] [Google Scholar]
- 43.Leupin O., Piters E., Halleux C., Hu S., Kramer I., Morvan F., Bouwmeester T., Schirle M., Bueno-Lozano M., Ramos Fuentes F. J., et al. Bone overgrowth-associated mutations in the LRP4 gene impair sclerostin facilitator function. J. Biol. Chem. 2011;286:19489–19500. doi: 10.1074/jbc.M110.190330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baron R., Kneissel M. WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat. Med. 2013;19:179–192. doi: 10.1038/nm.3074. [DOI] [PubMed] [Google Scholar]
- 45.Ettenberg S. A., Charlat O., Daley M. P., Liu S., Vincent K. J., Stuart D. D., Schuller A. G., Yuan J., Ospina B., Green J., et al. Inhibition of tumorigenesis driven by different Wnt proteins requires blockade of distinct ligand-binding regions by LRP6 antibodies. Proc. Natl. Acad. Sci. U. S. A. 2010;107:15473–15478. doi: 10.1073/pnas.1007428107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gong Y., Bourhis E., Chiu C., Stawicki S., DeAlmeida V. I., Liu B. Y., Phamluong K., Cao T. C., Carano R. A. D., Ernst J. A., et al. Wnt isoform-specific interactions with coreceptor specify inhibition or potentiation of signaling by LRP6 antibodies. PLoS ONE. 2010;5:e12682. doi: 10.1371/journal.pone.0012682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bourhis E., Tam C., Franke Y., Bazan J. F., Ernst J., Hwang J., Costa M., Cochran A. G., Hannoush R. N. Reconstitution of a frizzled8.Wnt3a.LRP6 signaling complex reveals multiple Wnt and Dkk1 binding sites on LRP6. J. Biol. Chem. 2010;285:9172–9179. doi: 10.1074/jbc.M109.092130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oishi I., Suzuki H., Onishi N., Takada R., Kani S., Ohkawara B., Koshida I., Suzuki K., Yamada G., Schwabe G. C., et al. The receptor tyrosine kinase Ror2 is involved in non-canonical Wnt5a/JNK signalling pathway. Genes Cells. 2003;8:645–654. doi: 10.1046/j.1365-2443.2003.00662.x. [DOI] [PubMed] [Google Scholar]
- 49.van Amerongen R., Mikels A., Nusse R. Alternative wnt signaling is initiated by distinct receptors. Sci. Signal. 2008;1:re9. doi: 10.1126/scisignal.135re9. [DOI] [PubMed] [Google Scholar]
- 50.Hao H.-X., Xie Y., Zhang Y., Charlat O., Oster E., Avello M., Lei H., Mickanin C., Liu D., Ruffner H., et al. ZNRF3 promotes Wnt receptor turnover in an R-spondin-sensitive manner. Nature. 2012;485:195–200. doi: 10.1038/nature11019. [DOI] [PubMed] [Google Scholar]
- 51.Koo B.-K., Spit M., Jordens I., Low T. Y., Stange D. E., van de Wetering M., van Es J. H., Mohammed S., Heck A. J. R., Maurice M. M., et al. Tumour suppressor RNF43 is a stem-cell E3 ligase that induces endocytosis of Wnt receptors. Nature. 2012;488:665–669. doi: 10.1038/nature11308. [DOI] [PubMed] [Google Scholar]
- 52.Carmon K. S., Gong X., Lin Q., Thomas A., Liu Q. R-spondins function as ligands of the orphan receptors LGR4 and LGR5 to regulate Wnt/beta-catenin signaling. Proc. Natl. Acad. Sci. U. S. A. 2011;108:11452–11457. doi: 10.1073/pnas.1106083108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Glinka A., Dolde C., Kirsch N., Huang Y.-L., Kazanskaya O., Ingelfinger D., Boutros M., Cruciat C.-M., Niehrs C. LGR4 and LGR5 are R-spondin receptors mediating Wnt/β-catenin and Wnt/PCP signalling. EMBO Rep. 2011;12:1055–1061. doi: 10.1038/embor.2011.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xie Y., Zamponi R., Charlat O., Ramones M., Swalley S., Jiang X., Rivera D., Tschantz W., Lu B., Quinn L., et al. Interaction with both ZNRF3 and LGR4 is required for the signalling activity of R-spondin. EMBO Rep. 2013;14:1120–1126. doi: 10.1038/embor.2013.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ryland G. L., Hunter S. M., Doyle M. A., Rowley S. M., Christie M., Allan P. E., Bowtell D. D. L., Australian Ovarian Cancer Study Group, Gorringe K. L., Campbell I. G. RNF43 is a tumour suppressor gene mutated in mucinous tumours of the ovary. J. Pathol. 2013;229:469–476. doi: 10.1002/path.4134. [DOI] [PubMed] [Google Scholar]
- 56.Ong C. K., Subimerb C., Pairojkul C., Wongkham S., Cutcutache I., Yu W., McPherson J. R., Allen G. E., Ng C. C. Y., Wong B. H., et al. Exome sequencing of liver fluke-associated cholangiocarcinoma. Nat. Genet. 2012;44:690–693. doi: 10.1038/ng.2273. [DOI] [PubMed] [Google Scholar]
- 57.Wu J., Jiao Y., Dal Molin M., Maitra A., de Wilde R. F., Wood L. D., Eshleman J. R., Goggins M. G., Wolfgang C. L., Canto M. I., et al. Whole-exome sequencing of neoplastic cysts of the pancreas reveals recurrent mutations in components of ubiquitin-dependent pathways. Proc. Natl. Acad. Sci. U. S. A. 2011;108:21188–21193. doi: 10.1073/pnas.1118046108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang K., Yuen S. T., Xu J., Lee S. P., Yan H. H. N., Shi S. T., Siu H. C., Deng S., Chu K.-M., Law S., et al. Whole-genome sequencing and comprehensive molecular profiling identify new driver mutations in gastric cancer. Nat. Genet. 2014;46:573–582. doi: 10.1038/ng.2983. [DOI] [PubMed] [Google Scholar]
- 59.Jiang X., Hao H.-X., Growney J. D., Woolfenden S., Bottiglio C., Ng N., Lu B., Hsieh M. H., Bagdasarian L., Meyer R., et al. Inactivating mutations of RNF43 confer Wnt dependency in pancreatic ductal adenocarcinoma. Proc. Natl. Acad. Sci. U. S. A. 2013;110:12649–12654. doi: 10.1073/pnas.1307218110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sato T., Vries R. G., Snippert H. J., van de Wetering M., Barker N., Stange D. E., van Es J. H., Abo A., Kujala P., Peters P. J., et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 61.Ootani A., Li X., Sangiorgi E., Ho Q. T., Ueno H., Toda S., Sugihara H., Fujimoto K., Weissman I. L., Capecchi M. R., et al. Sustained in vitro intestinal epithelial culture within a Wnt-dependent stem cell niche. Nat. Med. 2009;15:701–706. doi: 10.1038/nm.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kabiri Z., Greicius G., Madan B., Biechele S., Zhong Z., Zaribafzadeh H., Edison, Aliyev J., Wu Y., Bunte R., et al. Stroma provides an intestinal stem cell niche in the absence of epithelial Wnts. Development (Cambridge, England) 2014;141:2206–2215. doi: 10.1242/dev.104976. [DOI] [PubMed] [Google Scholar]
- 63.Seshagiri S., Stawiski E. W., Durinck S., Modrusan Z., Storm E. E., Conboy C. B., Chaudhuri S., Guan Y., Janakiraman V., Jaiswal B. S., et al. Recurrent R-spondin fusions in colon cancer. Nature. 2012;488:660–664. doi: 10.1038/nature11282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zebisch M., Xu Y., Krastev C., Macdonald B. T., Chen M., Gilbert R. J. C., He X., Jones E. Y. Structural and molecular basis of ZNRF3/RNF43 transmembrane ubiquitin ligase inhibition by the Wnt agonist R-spondin. Nat. Commun. 2013;4:2787. doi: 10.1038/ncomms3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen P.-H., Chen X., Lin Z., Fang D., He X. The structural basis of R-spondin recognition by LGR5 and RNF43. Genes Dev. 2013;27:1345–1350. doi: 10.1101/gad.219915.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang D., Huang B., Zhang S., Yu X., Wu W., Wang X. Structural basis for R-spondin recognition by LGR4/5/6 receptors. Genes Dev. 2013;27:1339–1344. doi: 10.1101/gad.219360.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xu K., Xu Y., Rajashankar K. R., Robev D., Nikolov D. B. Crystal structures of Lgr4 and its complex with R-spondin1. Structure. 2013;21:1683–1689. doi: 10.1016/j.str.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Peng W. C., de Lau W., Forneris F., Granneman J. C. M., Huch M., Clevers H., Gros P. Structure of stem cell growth factor R-spondin 1 in complex with the ectodomain of its receptor LGR5. Cell Rep. 2013;3:1885–1892. doi: 10.1016/j.celrep.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 69.Mukai A., Yamamoto-Hino M., Awano W., Watanabe W., Komada M., Goto S. Balanced ubiquitylation and deubiquitylation of Frizzled regulate cellular responsiveness to Wg/Wnt. EMBO J. 2010;29:2114–2125. doi: 10.1038/emboj.2010.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Janda C. Y., Waghray D., Levin A. M., Thomas C., Garcia K. C. Structural Basis of Wnt Recognition by Frizzled. Science. 2012;337:59–64. doi: 10.1126/science.1222879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Macdonald B. T., Hien A., Zhang X., Iranloye O., Virshup D. M., Waterman M. L., He X. Disulfide Bond Requirements for Active Wnt Ligands. J. Biol. Chem. 2014;289:18122–18136. doi: 10.1074/jbc.M114.575027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Takada R., Satomi Y., Kurata T., Ueno N., Norioka S., Kondoh H., Takao T., Takada S. Monounsaturated fatty acid modification of Wnt protein: its role in Wnt secretion. Dev. Cell. 2006;11:791–801. doi: 10.1016/j.devcel.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 73.Komekado H., Yamamoto H., Chiba T., Kikuchi A. Glycosylation and palmitoylation of Wnt-3a are coupled to produce an active form of Wnt-3a. Genes Cells. 2007;12:521–534. doi: 10.1111/j.1365-2443.2007.01068.x. [DOI] [PubMed] [Google Scholar]
- 74.Willert K., Brown J., Danenberg E., Duncan A., Weissman I., Reya T., Yates J., Nusse R. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448–452. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- 75.Rios-Esteves J., Resh M. D. Stearoyl CoA desaturase is required to produce active, lipid-modified Wnt proteins. Cell Rep. 2013;4:1072–1081. doi: 10.1016/j.celrep.2013.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Coombs G. S., Yu J., Veltri C. A., Covey T. M., Cheong J. K., Banerjee N., Zhang Z. H., Jadulco R. C., Concepcion G. P., Bugni T. S., et al. WLS-dependent secretion of WNT3A requires Ser209 acylation and vacuolar acidification. J. Cell Sci. 2010;123:3357–3367. doi: 10.1242/jcs.072132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ching W., Hang H. C., Nusse R. Lipid-independent secretion of a Drosophila Wnt protein. J. Biol. Chem. 2008;283:17092–17098. doi: 10.1074/jbc.M802059200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ahn V. E., Chu M. L.-H., Choi H.-J., Tran D., Abo A., Weis W. I. Structural basis of Wnt signaling inhibition by Dickkopf binding to LRP5/6. Dev. Cell. 2011;21:862–873. doi: 10.1016/j.devcel.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chu M. L.-H., Ahn V. E., Choi H.-J., Daniels D. L., Nusse R., Weis W. I. Structural studies of Wnts and identification of an LRP6 binding site. Structure. 2013;21:1235–1242. doi: 10.1016/j.str.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tanaka K., Okabayashi K., Asashima M., Perrimon N., Kadowaki T. The evolutionarily conserved porcupine gene family is involved in the processing of the Wnt family. Eur. J. Biochem. 2000;267:4300–4311. doi: 10.1046/j.1432-1033.2000.01478.x. [DOI] [PubMed] [Google Scholar]
- 81.van den Heuvel M., Harryman-Samos C., Klingensmith J., Perrimon N., Nusse R. Mutations in the segment polarity genes wingless and porcupine impair secretion of the wingless protein. EMBO J. 1993;12:5293–5302. doi: 10.1002/j.1460-2075.1993.tb06225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhai L., Chaturvedi D., Cumberledge S. Drosophila wnt-1 undergoes a hydrophobic modification and is targeted to lipid rafts, a process that requires porcupine. J. Biol. Chem. 2004;279:33220–33227. doi: 10.1074/jbc.M403407200. [DOI] [PubMed] [Google Scholar]
- 83.Najdi R., Proffitt K., Sprowl S., Kaur S., Yu J., Covey T. M., Virshup D. M., Waterman M. L. A uniform human Wnt expression library reveals a shared secretory pathway and unique signaling activities. Differentiation. 2012;84:203–213. doi: 10.1016/j.diff.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Proffitt K. D., Virshup D. M. Precise regulation of porcupine activity is required for physiological Wnt signaling. J. Biol. Chem. 2012;287:34167–34178. doi: 10.1074/jbc.M112.381970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hofmann K. A superfamily of membrane-bound O-acyltransferases with implications for wnt signaling. Trends Biochem. Sci. 2000;25:111–112. doi: 10.1016/S0968-0004(99)01539-X. [DOI] [PubMed] [Google Scholar]
- 86.Grzeschik K., Bornholdt D., Oeffner F., König A., del Carmen Boente M., Enders H., Fritz B., Hertl M., Grasshoff U., Höfling K., et al. Deficiency of PORCN, a regulator of Wnt signaling, is associated with focal dermal hypoplasia. Nat. Genet. 2007;39:833–835. doi: 10.1038/ng2052. [DOI] [PubMed] [Google Scholar]
- 87.Wang X., Reid Sutton V., Omar Peraza-Llanes J., Yu Z., Rosetta R., Kou Y., Eble T., Patel A., Thaller C., Fang P., et al. Mutations in X-linked PORCN, a putative regulator of Wnt signaling, cause focal dermal hypoplasia. Nat. Genet. 2007;39:836–838. doi: 10.1038/ng2057. [DOI] [PubMed] [Google Scholar]
- 88.Biechele S., Cox B. J., Rossant J. Porcupine homolog is required for canonical Wnt signaling and gastrulation in mouse embryos. Dev. Biol. 2011;355:275–285. doi: 10.1016/j.ydbio.2011.04.029. [DOI] [PubMed] [Google Scholar]
- 89.Barrott J. J., Cash G. M., Smith A. P., Barrow J. R., Murtaugh L. C. Deletion of mouse Porcn blocks Wnt ligand secretion and reveals an ectodermal etiology of human focal dermal hypoplasia/Goltz syndrome. Proc. Natl. Acad. Sci. U. S. A. 2011;108:12752–12757. doi: 10.1073/pnas.1006437108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Covey T. M., Kaur S., Tan Ong T., Proffitt K. D., Wu Y., Tan P., Virshup D. M. PORCN moonlights in a Wnt-independent pathway that regulates cancer cell proliferation. PLoS ONE. 2012;7:e34532. doi: 10.1371/journal.pone.0034532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Biechele S., Cockburn K., Lanner F., Cox B. J., Rossant J. Porcn-dependent Wnt signaling is not required prior to mouse gastrulation. Development (Cambridge, England) 2013;140:2961–2971. doi: 10.1242/dev.094458. [DOI] [PubMed] [Google Scholar]
- 92.Lyashenko N., Winter M., Migliorini D., Biechele T., Moon R. T., Hartmann C. Differential requirement for the dual functions of β-catenin in embryonic stem cell self-renewal and germ layer formation. Nat. Cell Biol. 2011;13:753–761. doi: 10.1038/ncb2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kemp C., Willems E., Abdo S., Lambiv L., Leyns L. Expression of all Wnt genes and their secreted antagonists during mouse blastocyst and postimplantation development. Dev. Dyn. 2005;233:1064–1075. doi: 10.1002/dvdy.20408. [DOI] [PubMed] [Google Scholar]
- 94.Kelly O. G., Pinson K. I., Skarnes W. C. The Wnt co-receptors Lrp5 and Lrp6 are essential for gastrulation in mice. Development (Cambridge, England) 2004;131:2803–2815. doi: 10.1242/dev.01137. [DOI] [PubMed] [Google Scholar]
- 95.Fu J., Jiang M., Mirando A. J., Yu H.-M. I., Hsu W. Reciprocal regulation of Wnt and Gpr177/mouse Wntless is required for embryonic axis formation. Proc. Natl. Acad. Sci. U. S. A. 2009;106:18598–18603. doi: 10.1073/pnas.0904894106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Proffitt K. D., Madan B., Ke Z., Pendharkar V., Ding L., Lee M. A., Hannoush R. N., Virshup D. M. Pharmacological inhibition of the Wnt acyltransferase PORCN prevents growth of WNT-driven mammary cancer. Cancer Res. 2013;73:502–507. doi: 10.1158/0008-5472.CAN-12-2258. [DOI] [PubMed] [Google Scholar]
- 97.Bänziger C., Soldini D., Schütt C., Zipperlen P., Hausmann G., Basler K. Wntless, a conserved membrane protein dedicated to the secretion of Wnt proteins from signaling cells. Cell. 2006;125:509–522. doi: 10.1016/j.cell.2006.02.049. [DOI] [PubMed] [Google Scholar]
- 98.Bartscherer K., Pelte N., Ingelfinger D., Boutros M. Secretion of Wnt ligands requires Evi, a conserved transmembrane protein. Cell. 2006;125:523–533. doi: 10.1016/j.cell.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 99.Goodman R. M., Thombre S., Firtina Z., Gray D., Betts D., Roebuck J., Spana E. P., Selva E. M. Sprinter: a novel transmembrane protein required for Wg secretion and signaling. Development (Cambridge, England) 2006;133:4901–4911. doi: 10.1242/dev.02674. [DOI] [PubMed] [Google Scholar]
- 100.Guder C., Philipp I., Lengfeld T., Watanabe H., Hobmayer B., Holstein T. W. The Wnt code: cnidarians signal the way. Oncogene. 2006;25:7450–7460. doi: 10.1038/sj.onc.1210052. [DOI] [PubMed] [Google Scholar]
- 101.Kim H., Cheong S.-M., Ryu J., Jung H.-J., Jho E.-H., Han J.-K. Xenopus Wntless and the retromer complex cooperate to regulate XWnt4 secretion. Mol. Cell Biol. 2009;29:2118–2128. doi: 10.1128/MCB.01503-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Korkut C., Ataman B., Ramachandran P., Ashley J., Barria R., Gherbesi N., Budnik V. Trans-synaptic transmission of vesicular Wnt signals through Evi/Wntless. Cell. 2009;139:393–404. doi: 10.1016/j.cell.2009.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jin J., Kittanakom S., Wong V., Reyes B. A. S., Van Bockstaele E. J., Stagljar I., Berrettini W., Levenson R. Interaction of the mu-opioid receptor with GPR177 (Wntless) inhibits Wnt secretion: potential implications for opioid dependence. BMC Neurosci. 2010;11:33–49. doi: 10.1186/1471-2202-11-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Carpenter A. C., Rao S., Wells J. M., Campbell K., Lang R. A. Generation of mice with a conditional null allele for Wntless. Genesis. 2010;48:554–558. doi: 10.1002/dvg.20651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rivadeneira F., Styrkársdottir U., Estrada K., Halldórsson B. V., Hsu Y.-H., Richards J. B., Zillikens M. C., Kavvoura F. K., Amin N., Aulchenko Y. S., et al. Twenty bone-mineral-density loci identified by large-scale meta-analysis of genome-wide association studies: Article: Nature Genetics. Nat. Genet. 2009;41:1199–1206. doi: 10.1038/ng.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhong Z., Zylstra-Diegel C. R., Schumacher C. A., Baker J. J., Carpenter A. C., Rao S., Helms J. A., Lang R. A., Williams B. O. Wntless functions in mature osteoblasts to regulate bone mass. Proc. Natl. Acad. Sci. U. S. A. 2012;109:E2197–204. doi: 10.1073/pnas.1120407109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Huang S., Zhu X., Liu Y., Tao Y., Feng G., He L., Guo X., Ma G. Wls is expressed in the epidermis and regulates embryonic hair follicle induction in mice. PLoS ONE. 2012;7:e45904. doi: 10.1371/journal.pone.0045904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Augustin I., Gross J., Baumann D., Korn C., Kerr G., Grigoryan T., Mauch C., Birchmeier W., Boutros M. Loss of epidermal Evi/Wls results in a phenotype resembling psoriasiform dermatitis. J. Exp. Med. 2013;210:1761–1777. doi: 10.1084/jem.20121871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Augustin I., Goidts V., Bongers A., Kerr G., Vollert G., Radlwimmer B., Hartmann C., Herold-Mende C., Reifenberger G., Deimling, von A., et al. The Wnt secretion protein Evi/Gpr177 promotes glioma tumourigenesis. EMBO Mol. Med. 2012;4:38–51. doi: 10.1002/emmm.201100186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yang P.-T., Anastas J. N., Toroni R. A., Shinohara M. M., Goodson J. M., Bosserhoff A. K., Chien A. J., Moon R. T. WLS inhibits melanoma cell proliferation through the β-catenin signalling pathway and induces spontaneous metastasis. EMBO Mol. Med. 2012;4:1294–1307. doi: 10.1002/emmm.201201486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chien A. J., Moore E. C., Lonsdorf A. S., Kulikauskas R. M., Rothberg B. G., Berger A. J., Major M. B., Hwang S. T., Rimm D. L., Moon R. T. Activated Wnt/beta-catenin signaling in melanoma is associated with decreased proliferation in patient tumors and a murine melanoma model. Proc. Natl. Acad. Sci. U. S. A. 2009;106:1193–1198. doi: 10.1073/pnas.0811902106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Herr P., Basler K. Porcupine-mediated lipidation is required for Wnt recognition by Wls. Dev. Biol. 2012;361:392–402. doi: 10.1016/j.ydbio.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 113.Gasnereau I., Herr P., Chia P. Z. C., Basler K., Gleeson P. A. Identification of an endocytosis motif in an intracellular loop of wntless protein, essential for its recycling and the control of wnt protein signaling. J. Biol. Chem. 2011;286:43324–43333. doi: 10.1074/jbc.M111.307231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yu J., Chia J., Canning C. A., Jones C. M., Bard F. A., Virshup D. M. WLS Retrograde Transport to the Endoplasmic Reticulum during Wnt Secretion. Dev. Cell. 2014;29:277–291. doi: 10.1016/j.devcel.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 115.Belenkaya T. Y., Wu Y., Tang X., Zhou B., Cheng L., Sharma Y. V., Yan D., Selva E. M., Lin X. The retromer complex influences Wnt secretion by recycling wntless from endosomes to the trans-Golgi network. Dev. Cell. 2008;14:120–131. doi: 10.1016/j.devcel.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 116.Franch-Marro X., Wendler F., Guidato S., Griffith J., Baena-Lopez A., Itasaki N., Maurice M. M., Vincent J.-P. Wingless secretion requires endosome-to-Golgi retrieval of Wntless/Evi/Sprinter by the retromer complex. Nat. Cell Biol. 2008;10:170–177. doi: 10.1038/ncb1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Port F., Kuster M., Herr P., Furger E., Bänziger C., Hausmann G., Basler K. Wingless secretion promotes and requires retromer-dependent cycling of Wntless. Nat. Cell Biol. 2008;10:178–185. doi: 10.1038/ncb1687. [DOI] [PubMed] [Google Scholar]
- 118.Coudreuse D. Y. M., Roël G., Betist M. C., Destrée O., Korswagen H. C. Wnt gradient formation requires retromer function in Wnt-producing cells. Science. 2006;312:921–924. doi: 10.1126/science.1124856. [DOI] [PubMed] [Google Scholar]
- 119.Seaman M. N. J. Recycle your receptors with retromer. Trends Cell Biol. 2005;15:68–75. doi: 10.1016/j.tcb.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 120.Cullen P. J. Endosomal sorting and signalling: an emerging role for sorting nexins. Nat. Rev. Mol. Cell Biol. 2008;9:574–582. doi: 10.1038/nrm2427. [DOI] [PubMed] [Google Scholar]
- 121.Silhankova M., Port F., Harterink M., Basler K., Korswagen H. C. Wnt signalling requires MTM-6 and MTM-9 myotubularin lipid-phosphatase function in Wnt-producing cells. EMBO J. 2010;29:4094–4105. doi: 10.1038/emboj.2010.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Harterink M., Port F., Lorenowicz M. J., McGough I. J., Silhankova M., Betist M. C., van Weering J. R. T., van Heesbeen R. G. H. P., Middelkoop T. C., Basler K., et al. A SNX3-dependent retromer pathway mediates retrograde transport of the Wnt sorting receptor Wntless and is required for Wnt secretion. Nat. Cell Biol. 2011;13:914–923. doi: 10.1038/ncb2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhang P., Wu Y., Belenkaya T. Y., Lin X. SNX3 controls Wingless/Wnt secretion through regulating retromer-dependent recycling of Wntless. Cell Res. 2011;21:1677–1690. doi: 10.1038/cr.2011.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rao Y., Haucke V. Membrane shaping by the Bin/amphiphysin/Rvs (BAR) domain protein superfamily. Cell. Mol. Life Sci. 2011;68:3983–3993. doi: 10.1007/s00018-011-0768-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lorenowicz M. J., Macurkova M., Harterink M., Middelkoop T. C., de Groot R., Betist M. C., Korswagen H. C. Inhibition of late endosomal maturation restores Wnt secretion in Caenorhabditis elegans vps-29 retromer mutants. Cell Signal. 2014;26:19–31. doi: 10.1016/j.cellsig.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 126.Orci L., Stamnes M., Ravazzola M., Amherdt M., Perrelet A., Söllner T. H., Rothman J. E. Bidirectional transport by distinct populations of COPI-coated vesicles. Cell. 1997;90:335–349. doi: 10.1016/S0092-8674(00)80341-4. [DOI] [PubMed] [Google Scholar]
- 127.Buechling T., Chaudhary V., Spirohn K., Weiss M., Boutros M. p24 proteins are required for secretion of Wnt ligands. EMBO Rep. 2011;12:1265–1272. doi: 10.1038/embor.2011.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Port F., Hausmann G., Basler K. A genome-wide RNA interference screen uncovers two p24 proteins as regulators of Wingless secretion. EMBO Rep. 2011;12:1144–1152. doi: 10.1038/embor.2011.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Springer S., Chen E., Duden R., Marzioch M., Rowley A., Hamamoto S., Merchant S., Schekman R. The p24 proteins are not essential for vesicular transport in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. U. S. A. 2000;97:4034–4039. doi: 10.1073/pnas.070044097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Castillon G. A., Watanabe R., Taylor M., Schwabe T. M. E., Riezman H. Concentration of GPI-anchored proteins upon ER exit in yeast. Traffic. 2009;10:186–200. doi: 10.1111/j.1600-0854.2008.00857.x. [DOI] [PubMed] [Google Scholar]
- 131.Castillon G. A., Aguilera-Romero A., Manzano-Lopez J., Epstein S., Kajiwara K., Funato K., Watanabe R., Riezman H., Muñiz M. The yeast p24 complex regulates GPI-anchored protein transport and quality control by monitoring anchor remodeling. Mol. Biol. Cell. 2011;22:2924–2936. doi: 10.1091/mbc.E11-04-0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ramírez-Weber F. A., Kornberg T. B. Cytonemes: cellular processes that project to the principal signaling center in Drosophila imaginal discs. Cell. 1999;97:599–607. doi: 10.1016/S0092-8674(00)80771-0. [DOI] [PubMed] [Google Scholar]
- 133.Franch-Marro X., Marchand O., Piddini E., Ricardo S., Alexandre C., Vincent J.-P. Glypicans shunt the Wingless signal between local signalling and further transport. Development (Cambridge, England) 2005;132:659–666. doi: 10.1242/dev.01639. [DOI] [PubMed] [Google Scholar]
- 134.Chen M.-H., Li Y.-J., Kawakami T., Xu S.-M., Chuang P.-T. Palmitoylation is required for the production of a soluble multimeric Hedgehog protein complex and long-range signaling in vertebrates. Genes Dev. 2004;18:641–659. doi: 10.1101/gad.1185804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Neumann S., Coudreuse D. Y. M., Van Der Westhuyzen D. R., Eckhardt E. R. M., Korswagen H. C., Schmitz G., Sprong H. Mammalian Wnt3a is released on lipoprotein particles. Traffic. 2009;10:334–343. doi: 10.1111/j.1600-0854.2008.00872.x. [DOI] [PubMed] [Google Scholar]
- 136.Panáková D., Sprong H., Marois E., Thiele C., Eaton S. Lipoprotein particles are required for Hedgehog and Wingless signalling. Nature. 2005;435:58–65. doi: 10.1038/nature03504. [DOI] [PubMed] [Google Scholar]
- 137.Mulligan K. A., Fuerer C., Ching W., Fish M., Willert K., Nusse R. Secreted Wingless-interacting molecule (Swim) promotes long-range signaling by maintaining Wingless solubility. Proc. Natl. Acad. Sci. U. S. A. 2012;109:370–377. doi: 10.1073/pnas.1119197109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Koles K., Nunnari J., Korkut C., Barria R., Brewer C., Li Y., Leszyk J., Zhang B., Budnik V. Mechanism of evenness interrupted (Evi)-exosome release at synaptic boutons. J. Biol. Chem. 2012;287:16820–16834. doi: 10.1074/jbc.M112.342667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Beckett K., Monier S., Palmer L., Alexandre C., Green H., Bonneil E., Raposo G., Thibault P., Le Borgne R., Vincent J.-P. Drosophila S2 cells secrete wingless on exosome-like vesicles but the wingless gradient forms independently of exosomes. Traffic. 2013;14:82–96. doi: 10.1111/tra.12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gross J. C., Chaudhary V., Bartscherer K., Boutros M. Active Wnt proteins are secreted on exosomes. Nat. Cell Biol. 2012;14:1036–1045. doi: 10.1038/ncb2574. [DOI] [PubMed] [Google Scholar]
- 141.Luga V., Zhang L., Viloria-Petit A. M., Ogunjimi A. A., Inanlou M. R., Chiu E., Buchanan M., Hosein A. N., Basik M., Wrana J. L. Exosomes mediate stromal mobilization of autocrine Wnt-PCP signaling in breast cancer cell migration. Cell (Elsevier) 2012;151:1542–1556. doi: 10.1016/j.cell.2012.11.024. [DOI] [PubMed] [Google Scholar]
- 142.Luga V., Wrana J. L. Tumor-stroma interaction: Revealing fibroblast-secreted exosomes as potent regulators of Wnt–planar cell polarity signaling in cancer metastasis. Cancer Res. 2013;73:6843–6847. doi: 10.1158/0008-5472.CAN-13-1791. [DOI] [PubMed] [Google Scholar]
- 143.Zhang B., Wang M., Gong A., Zhang X., Wu X., Zhu Y., Shi H., Wu L., Zhu W., Qian H., et al. HucMSC-exosome mediated -Wnt4 signaling is required for cutaneous wound healing. Stem Cells. 2014 doi: 10.1002/stem.1771. doi 10.1002-stem.1771. [DOI] [PubMed] [Google Scholar]
- 144.Nusse R., Varmus H. Three decades of Wnts: a personal perspective on how a scientific field developed. EMBO J. 2012;31:2670–2684. doi: 10.1038/emboj.2012.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Nishisho I., Nakamura Y., Miyoshi Y., Miki Y., Ando H., Horii A., Koyama K., Utsunomiya J., Baba S., Hedge P. Mutations of chromosome 5q21 genes in FAP and colorectal cancer patients. Science. 1991;253:665–669. doi: 10.1126/science.1651563. [DOI] [PubMed] [Google Scholar]
- 146.Kinzler K. W., Nilbert M. C., Su L. K., Vogelstein B., Bryan T. M., Levy D. B., Smith K. J., Preisinger A. C., Hedge P., McKechnie D. Identification of FAP locus genes from chromosome 5q21. Science. 1991;253:661–665. doi: 10.1126/science.1651562. [DOI] [PubMed] [Google Scholar]
- 147.Groden J., Thliveris A., Samowitz W., Carlson M., Gelbert L., Albertsen H., Joslyn G., Stevens J., Spirio L., Robertson M. Identification and characterization of the familial adenomatous polyposis coli gene. Cell. 1991;66:589–600. doi: 10.1016/0092-8674(81)90021-0. [DOI] [PubMed] [Google Scholar]
- 148.Morin P. J., Sparks A. B., Korinek V., Barker N., Clevers H., Vogelstein B., Kinzler K. W. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275:1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- 149.Samowitz W. S., Powers M. D., Spirio L. N., Nollet F., van Roy F., Slattery M. L. Beta-catenin mutations are more frequent in small colorectal adenomas than in larger adenomas and invasive carcinomas. Cancer Res. 1999;59:1442–1444. [PubMed] [Google Scholar]
- 150.Liu W., Dong X., Mai M., Seelan R. S., Taniguchi K., Krishnadath K. K., Halling K. C., Cunningham J. M., Boardman L. A., Qian C., et al. Mutations in AXIN2 cause colorectal cancer with defective mismatch repair by activating beta-catenin/TCF signalling. Nat. Genet. 2000;26:146–147. doi: 10.1038/79859. [DOI] [PubMed] [Google Scholar]
- 151.Muzny D. M., Bainbridge M. N., Chang K., Dinh H. H., Drummond J. A., Fowler G., Kovar C. L., Lewis L. R., Morgan M. B., Newsham I. F., et al. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487:330–337. doi: 10.1038/nature11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kahn M. Can we safely target the WNT pathway? Nat. Rev. Drug Discov. 2014;13:513–532. doi: 10.1038/nrd4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Teo J.-L., Ma H., Nguyen C., Lam C., Kahn M. Specific inhibition of CBP/beta-catenin interaction rescues defects in neuronal differentiation caused by a presenilin-1 mutation. Proc. Natl. Acad. Sci. U. S. A. 2005;102:12171–12176. doi: 10.1073/pnas.0504600102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lenz H.-J., Kahn M. Safely targeting cancer stem cells via selective catenin coactivator antagonism. Cancer Sci. 2014 doi: 10.1111/cas.12471. doi 10.1111-cas.12471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chen B., Dodge M. E., Tang W., Ma Z., Fan C.-W., Wei S., Williams N. S., Roth M. G., Amatruda J. F., Chen C., et al. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat. Chem. Biol. 2009;5:100–107. doi: 10.1038/nchembio.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Huang S.-M. A., Mishina Y. M., Liu S., Cheung A., Stegmeier F., Michaud G. A., Charlat O., Wiellette E., Zhang Y., Wiessner S., et al. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature. 2009;461:614–620. doi: 10.1038/nature08356. [DOI] [PubMed] [Google Scholar]
- 157.Liu J., Pan S., Hsieh M. H., Ng N., Sun F., Wang T., Kasibhatla S., Schuller A. G., Li A. G., Cheng D., et al. Targeting Wnt-driven cancer through the inhibition of Porcupine by LGK974. Proc. Natl. Acad. Sci. U. S. A. 2013;110:20224–20229. doi: 10.1073/pnas.1314239110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gurney A., Axelrod F., Bond C. J., Cain J., Chartier C., Donigan L., Fischer M., Chaudhari A., Ji M., Kapoun A. M., et al. Wnt pathway inhibition via the targeting of Frizzled receptors results in decreased growth and tumorigenicity of human tumors. Proc. Natl. Acad. Sci. U. S. A. 2012;109:11717–11722. doi: 10.1073/pnas.1120068109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Martinez Arias A. Wnts as morphogens? The view from the wing of Drosophila. Nat. Rev. Mol. Cell. Biol. 2003;4:321–325. doi: 10.1038/nrm1078. [DOI] [PubMed] [Google Scholar]
- 160.Alexandre C., Baena-Lopez A., Vincent J.-P. Patterning and growth control by membrane-tethered Wingless. Nature. 2014;505:180–185. doi: 10.1038/nature12879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Yamamoto H., Awada C., Hanaki H., Sakane H., Tsujimoto I., Takahashi Y., Takao T., Kikuchi A. The apical and basolateral secretion of Wnt11 and Wnt3a in polarized epithelial cells is regulated by different mechanisms. J. Cell Sci. 2013;126:2931–2943. doi: 10.1242/jcs.126052. [DOI] [PubMed] [Google Scholar]
- 162.Sato T., van Es J. H., Snippert H. J., Stange D. E., Vries R. G., van den Born M., Barker N., Shroyer N. F., van de Wetering M., Clevers H. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2011;469:415–418. doi: 10.1038/nature09637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Durand A., Donahue B., Peignon G., Letourneur F., Cagnard N., Slomianny C., Perret C., Shroyer N. F., Romagnolo B. Functional intestinal stem cells after Paneth cell ablation induced by the loss of transcription factor Math1 (Atoh1) Proc. Natl. Acad. Sci. U. S. A. 2012;109:8965–8970. doi: 10.1073/pnas.1201652109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Farin H. F., van Es J. H., Clevers H. Redundant sources of Wnt regulate intestinal stem cells and promote formation of Paneth cells. Gastroenterology. 2012;143:1518–1529.e7. doi: 10.1053/j.gastro.2012.08.031. [DOI] [PubMed] [Google Scholar]