Abstract
Synthetic compounds that are used in the clinic to regulate skin hyperpigmentation, such as arbutin, hydroquinone, and kojic acid, are only moderately effective. But, their use is limited by side effects. As part of an effort to overcome the limitations, we developed resveratrol-enriched rice (RR) using genetic engineering technique. Each of resveratrol and rice has been reported to produce anti-melanogenic effects. Therefore, we hypothesized that RR would show more anti-melanogenic effects than those of resveratrol or rice alone. Anti-melanogenic effect of RR was done by using melan-a mouse melanocytes. The depigmenting efficacy was then observed following topical application of the RR to UVB-stimulated hyperpigmented dorsal skin of guinea pigs. Treatment with RR extract resulted a 21.4 ± 0.7% decrease in tyrosinase expression at melan-a cells. Colorimetric analysis showed a significantly lower depigmenting value by day 9 following treatment with RR in UVB-irradiated guinea pigs the dorsal skin (p<0.01), indicating that RR produced a depigmentation effect. By staining with Fontana-Masson stain, we found that the RR-treated group had more effect histopathologically in epidermal melanin production than resveratrol or rice alone-treated group. RR was associated with reduction in the levels of microphthalmia-associated transcription factor (MITF), and downregulation of tyrosinase and tyrosinase-related protein (TRP-2) expression, leading to inhibit epidermal melanin production by western blot analysis. This study suggests that the resveratrol-enriched rice may be a promising candidate in regulating skin pigmentation with UVB exposure.
Keywords: Resveratrol, Rice, Melanogenesis, Resveratrol-enriched rice, Ultraviolet B, Guinea pig
INTRODUCTION
Botanically derived products are used frequently in the cosmetics industry because of the increased preference of the consumer for environmentally friendly cosmetics. Resveratrol, a natural product-derived polyphenolic compound, has been widely used in the cosmetics and pharmaceutical industries because of its pharmacological activities, which include anti-tumor, anti-inflammation, and anti-aging effects (Chen et al., 2013). Recent reports have shown that resveratrol produced therapeutic effects against hyperplastic skin disorders by inhibiting keratinocyte proliferation (Wu et al., 2014), inhibited skin photoaging by acting as a Sirt-1 agonist (Lee et al., 2010), and showed anti-cancer effects in human melanoma cell lines (Niles et al., 2003). The anti-melanogenic effects of resveratrol and resveratrol analogs have already been reported by many other groups (Ohguchi et al., 2003; Song et al., 2012; Bae et al., 2013; Park and Boo, 2013; Park et al., 2014). We previously demonstrated the depigmentation effects of resveratrol in UVB-treated guinea pigs (Lee et al., 2014), too. In this previous report, resveratrol strongly down-regulated tyrosinase, tyrosinase-related protein (TPR)-1, TPR-2, and microphthalmia-associated transcription factor (MITF) in animal study (Lee et al., 2014). Tyrosinase plays an important role in determining melanin production (Peng et al., 2013) by initiating melanin synthesis through the conversion of tyrosine to 3,4-dihydroxyphenylalanine (L-DOPA). TRP-1 and TPR-2 stabilize tyrosinase and regulate its function in melanin formation; these three types of enzymes are key mediators of melanogenesis that is promoted by the binding of MITF (Newton et al., 2007).
Being widening the scope of its usage, our collaborative group produced the resveratrol enriched-rice (RR) using genetic engineering methods. In previous report, long-term supplement of RR showed anti-obesity effects, as manifested by reduced body weights and abdominal fat volumes, without causing serious side effects (Baek et al., 2014). This previous study suggests that RR may be a natural source of biologically active resveratrol, showing a synergistic interaction between resveratrol and rice in the control of metabolic syndrome and related diseases (Baek et al., 2013).
Rice (Oryza sativa var. japonica) is one of the most consumed cereal grains and has been used in cosmetics industry in Korea, China, and Japan (Jun et al., 2012). Several studies have reported that rice has beneficial effects on skin aging, including skin lightening, decreased roughness, and increased thickness and elasticity. (Seo et al., 2009; Manosroi et al., 2012a). Moreover, it has been reported that rice-derived glucosylceramides improved epidermal water loss and barrier function by regulating ceramides and glucosylceramides in mouse skin and human epidermal equivalents. (Shimoda et al., 2012). Given these studies on the biological effects of resveratrol and rice, we expected that RR might have synergistic anti-melanogenic effects stronger than that of resveratrol or rice alone.
Previous in vitro studies have shown that resveratrol produced beneficial effects on skin aging; however, the high polarity of resveratrol severely restricts its penetration into the skin and limits its topical use. We expect that the topical use of RR will overcome this limitation of resveratrol, because rice has shown good biocompatibility and increases penetration into the skin (Manosroi et al., 2012b). Therefore, the aim of this study is to determine whether the RR is more effective on anti-melanogenesis than resveratrol alone as a skin whitening agent. To evaluate the depigmentation effect of RR, we used UVB-induced epidermal hyperpigmentation model in guinea pig, and focused on evaluating for the regulation of levels of melanogenic-relating enzymes by treatment of RR.
MATERIALS AND METHODS
Chemicals
Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), and penicillin streptomycin were purchased from Gibco BRL (Grand Island, NY, USA). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and dimethyl sulfoxide (DMSO) were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Standard and sample preparation for HPLC analysis
Resveratrol was purchased from Sigma-Aldrich (St Louis, Missouri, USA). Rice (Oryza sativa var. japonica) and resveratrol enriched rice (RR) were supplied by the Rural Development Administration of South Korea. The standard stock solutions of the experimental compounds were prepared by dissolving 1 mg of each compound in 1 mL MeOH, and the resulting solutions were stored at −20°C. Rice and RR samples (10 g) were extracted in 100 mL MeOH and then placed in an ultrasonic bath for 60 min. After the extracts were filtered and evaporated, they were dissolved in MeOH at a final concentration of 10 mg/mL.
HPLC analysis
Analysis was carried out on a Waters system (Waters Corp., Milford, MA, USA), consisting of a separation module (e2695) with a photodiode array detector (2998). UV absorbance was monitored from 200 to 700 nm. Quantification was carried out by integration of the peak areas at 660 nm. The injection volume was 10 μL. Separation was carried out using a YMC-Triart C18 column (250×4.6 mm; particle size, 5 μm; YMC Co. Ltd., Kyoto, Japan). The mobile phase was composed of 1% acetic acid-water (v/v solvent A) and acetonitrile (solvent B). The flow rate was 1 mL/min, and the gradient was as followings; 0.0–3.0 min, 95% A; 20.0 min, 85% A; 44.0 min, 75% A.
Experimental animals
Five-week-old male brown guinea pigs (KIWA:A1) (weighing 272 ± 3 g, n=4) were obtained from Japan Kiwa Laboratory Animals Co., Ltd. (Wakayama, Japan). The guinea pigs were kept in a temperature- and humidity-controlled room (22 ± 1°C, 50 ± 5% humidity) with 12 h light/dark cycles. The animals were acclimated to the laboratory environment for 7 days. During the experimental period, the mice were allowed free access to food and water. Animal experiment was approved by the Institutional Animal Care and Use Committee of Korea Conformity Laboratories (IA13-00229).
UVB irradiation for hyperpigmentation
The UV source was supplied by a closely spaced array of 5 Sankyo Denki sun lamps with peak irradiance at 310 nm (Kanagawa, Japan). The bulbs were positioned 15 cm above the guinea pigs. Irradiation (0.1 mW/cm2) was measured with an IL1700 Research Radiometer (International Light, Inc., Newburyport, MA, USA) equipped with a UVB sensor. After hair removal, the dorsal skin of the guinea pigs was exposed to 390 mJ/cm2 UVB radiation 3 times per week for 2 weeks.
Sample administration in guinea pigs
RR extract was dissolved in a mixture of ethanol and propylene glycol (3:7, v/v). The sample solution was applied topically to the dorsal skin once per day for 9 days after the final UVB treatment. Solutions of 1% resveratrol, 1% arbutin, 1% rice, or 1% RR (200 μL; 10 mg/mL) were applied to separate 2 cm2 areas of the dorsal skin. We applied 4 different sample solutions (1% resveratrol, 1% rice, 1% RR, or 1% arbutin) to separate skin areas for 9 days and measured dorsal skin color using the Dermalab® Combo system (Cortex Technology ApS, Hudsund, Denmark).
Western blot
Melan-a immortal mouse melanocytes and treated guinea pig skin were homogenized separately and lysed in lysis buffer (50 mM Tris-Cl, pH 8.0, 0.1% SDS, 150 mM NaCl, 1% NP-40, 0.02% sodium azide, 0.5% sodium deoxycholate, 100 μg/mL PMSF, 1 μg/mL aprotinin) on ice for 2 h, after which the supernatant was collected by centrifuging at 12000 g. Protein amounts were estimated via the Bradford assay using bovine serum albumin (BSA). Protein (40 μg) was separated by 10% SDS-PAGE and transferred to a nitrocellulose membrane, which was blocked for 1 h using 5% skim milk and incubated overnight at 4°C with a primary antibody: α-tubulin (1:3000 dilution, Sigma-Aldrich, St. Louis, MO, USA), MITF (1:500 dilution, Cell Signaling Technology, Inc., Danvers, MA, USA), tyrosinase (1:500 dilution, Abcam, Cambridge, MA, USA), TRP-1 (1:500 dilution, Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA), or TRP-2 (1:500 dilution, Santa Cruz Biotechnology Inc.). After incubation, the membranes were washed using TBST and incubated with a horseradish peroxidase conjugated secondary antibody for 1 h. Finally, immune complexes were detected with a ChemiDocXRS+imaging system (Bio-Rad, Hercules, CA, USA).
Histological study and densitometry analysis
Fontana-Masson stain was performed using a Fontana-Masson staining kit (IHC WORLD, GA, USA) according to the manufacture’s protocol. Briefly, the dorsal skin of guinea pigs fixed in 4% paraformaldehyde for 24 h and embedded in paraffin. Sections, approximately 4 μm thick, were stained with Fontana-Masson staining solution. Melanin production was measured in a 435×325 μm area of stained section. Densitometry analysis for the skin areas was conducted using the imaging software NIS elements version 4.0 (Nikon, Japan).
Statistical analysis
All experiments were carried out in triplicate. The data are expressed as means ± standard deviation (SD). Statistical comparisons between different groups were performed using one-way analysis of variance (ANOVA) with Tukey’ multiple comparisons test. The level of statistical significance was set at *p<0.05, **p<0.01, and ***p<0.001.
RESULTS
Analysis of resveratrol in genetically modified rice
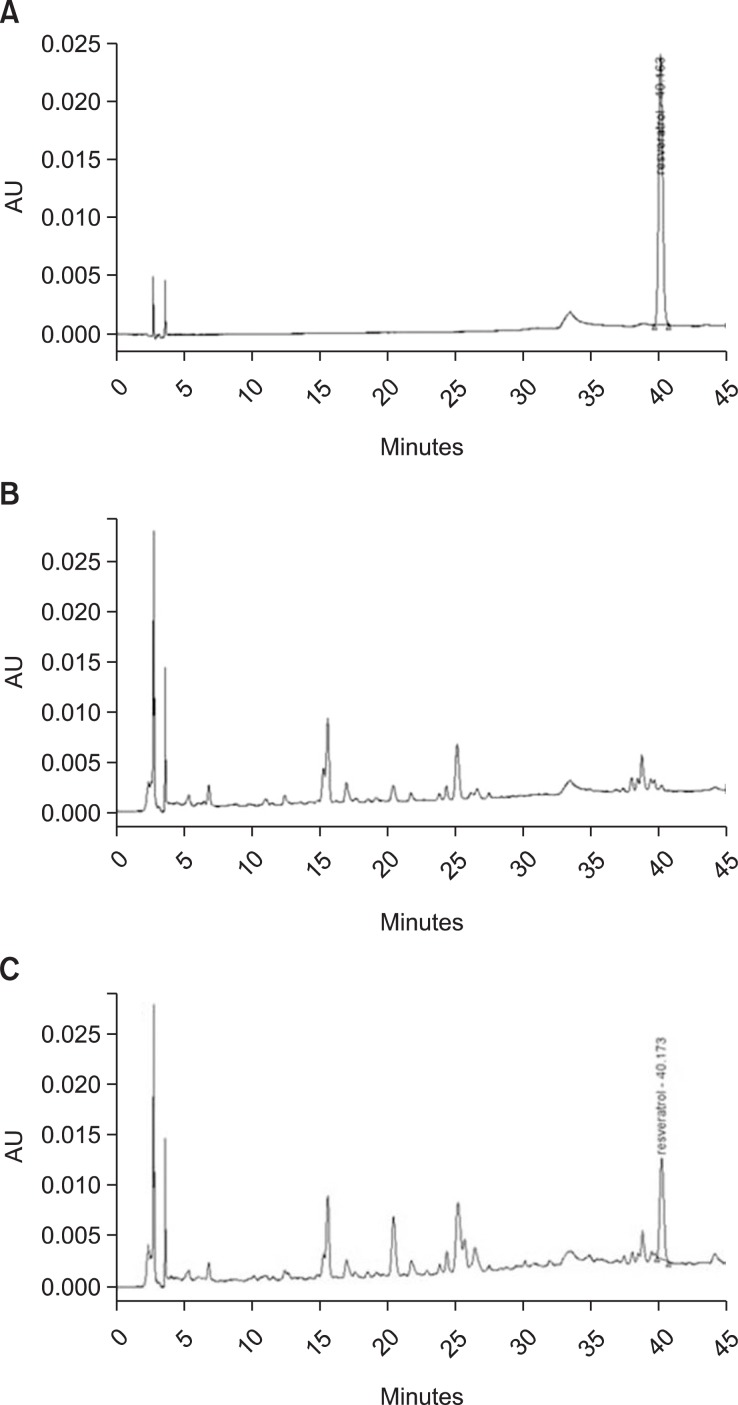
We measured content of resveratrol in RR using a HPLC. The retention time of resveratrol is shown in Fig. 1A. Resveratrol content was calculated by constructing a standard calibration curve. The resveratrol content in RR was 0.246 μg/mg. But, resveratrol in rice which used a as control sample was not measured under the same analytical conditions (Fig. 1B).
Fig. 1.
HPLC chromatogram. Resveratrol standard (A); control rice (B); resveratrol-enriched rice (C).
Inhibitory effect of RR on tyrosinase expression in melan-a cells
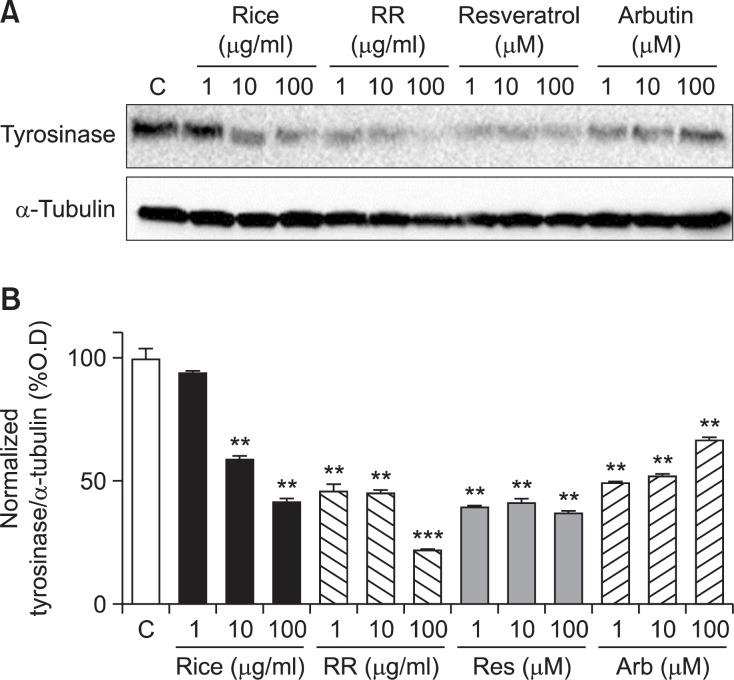
We found the inhibitory effect of RR on tyrosinase expression in melan-a cells by western blot analysis. Melan-a cells were treated with RR at concentrations of 1, 10, or 100 μg/mL for 3 days. Arbutin, a potential whitening agent, was used as the positive compound in this study. As shown Fig. 2, resveratrol decreased tyrosinase expression in melan-a cells. Interestingly, RR at a concentration of 100 μg/mL showed a more highly inhibitory effect than that of resveratrol.
Fig. 2.
Tyrosinase expression in melan-a cells. Melan-a cells were treated with each sample for 72 h. Forty micrograms of total protein from the cell lysates were used for western blot analysis (A). Tyrosinase expression was normalized to that of α-tubulin. Significance was determined using one-way ANOVA followed by Tukey’s multiple comparison test. **p<0.01 and ***p<0.001 indicate statistically significant differences compared to the control.
Depigmentation effects of RR on UVB-induced hyperpigmentation in guinea pigs
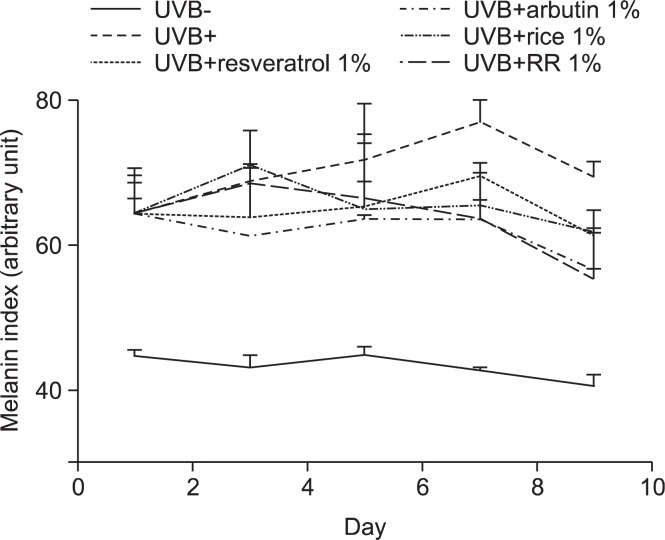
Hyperpigmentation in the dorsal skin of brown guinea pigs was induced by UVB irradiation. Changes in dorsal skin pigmentation were observed after 1 week of UVB treatment, and the maximum pigmentation of dorsal skin was induced after 2 weeks. We evaluated the anti-melanogenic efficacy of RR by comparing it with that of each material applied alone. Each of resveratrol and rice decreased dorsal skin pigmentation. Interestingly, RR produced a stronger depigmentation effect than that of resveratrol or rice (Fig. 3), suggesting that RR treated group has a synergistic effect.
Fig. 3.
Measurements of skin color. Hyperpigmentation of the brown guinea pigs skin was induced by UVB irradiation. The guinea pigs were exposed to 390 mJ/cm2 UVB radiation 3 times per week for 2 weeks. After UVB irradiation, each material (1% resveratrol with UVB, 1% arbutin with UVB, 1% rice extract with UVB, and 1% RR extract with UVB) was applied for 9 days. We daily measured change of skin color using a Dermalab® Combo system.
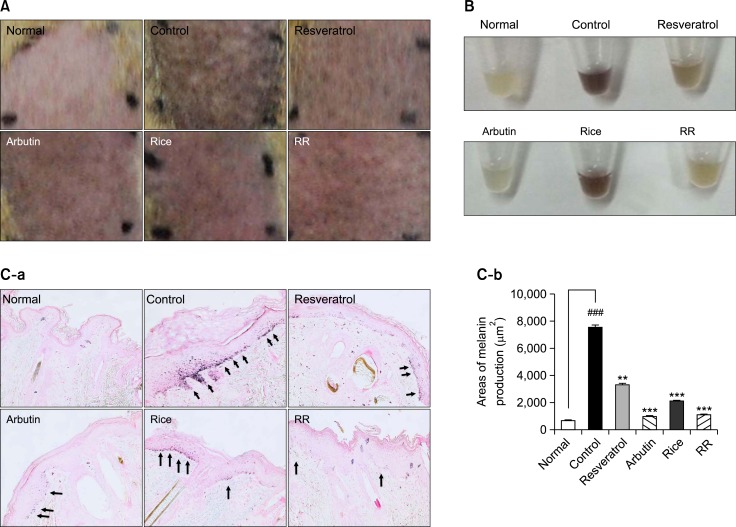
Contrary to the results obtained with the Dermalab® Combo system, visual differences in skin color between the dorsal skin from the rice- and RR-treated groups were not significant (Fig. 4A). Therefore, we extracted melanin from the epidermis using a sodium hydroxide solution to confirm the significant reduction of melanin in the RR-treated group (Fig. 4B).
Fig. 4.
A visible decrease and histological change in hyperpigmentation. The treated region was divided into 6 areas; Normal (Nor) (UVB-), Control (Con) (UVB+), Resveratrol (Res) (1% resveratrol with UVB), Arbutin (Arb) (1% arbutin with UVB), Rice (1% rice extract with UVB) and RR (1% RR extract with UVB). Representative features of the dorsal skin of brown guinea pigs were obtained after treatment of the animals for 9 days (A). Melanin was extracted from the epidermis using a sodium hydroxide solution (B). Epidermal melanin production was detected by Fontana-Masson stain. Arrows indicate melanin (C-a). Densitometry analysis of the areas was conducted using the NIS elements version 4.0 imaging software (C-b). One-way analysis of variance (ANOVA) with Tukey’ multiple comparisons test was used for statiscal analyses. ###p<0.001 indicate statistically significant differences compared to the normal. **p<0.01 and ***p<0.001. indicate statistically significant differences compared to the control.
Histological change of epidermal skin by treatment of RR
Epidermal melanin content was measured by Fontana-Masson staining after treatment with resveratrol, rice, RR, or arbutin. Epidermal melanin levels in skin tissue specimens treated with resveratrol, rice, RR, and arbutin were lower than those of the control group. RR produced a depigmentation effect which is similar to that of arbutin (Fig. 4C). RR significantly decreased melanin production (p<0.001) and its potency was similar to that of arbutin.
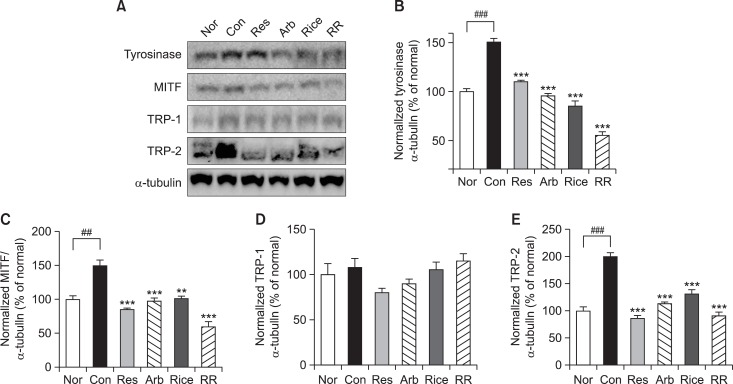
Effect on RR on down-regulation of melanogenic enzymes by western blot analysis
Melanin synthesis is controlled by an enzyme cascade including tyrosinase, MITF, TPR-1, and TRP-2 by western blot analysis. The expression of these enzymes was measured in the dorsal skin tissue after treatment with resveratrol, rice, RR, or arbutin. Epidermal protein levels of tyrosinase, MITF, TPR-1, and TRP-2 were significantly increased to 150.6%, 134.5%, 116.1%, and 270.1% of control levels, respectively, as a result of exposure to UVB irradiation. However, with the exception of TRP-1, protein expression was decreased by treatment with resveratrol, rice, RR, and arbutin. RR showed a particularly strong inhibitory effect on the expression of tyrosinase, MITF, and TRP-2, which were expressed at 54.6%, 49.4%, and 120.2% of control levels, respectively (Fig. 5).
Fig. 5.
Effects of resveratrol, arbutin, rice extract, and RR on melanogenesis-related proteins in UVB irradiated guinea pig dorsal skin. Forty micrograms of total protein from the skin tissue lysates [Nor (UVB-), Con (UVB+), Res (1% resveratrol with UVB), Arb (1% arbutin with UVB), Rice (1% rice extract with UVB) and RR (1% RR extract with UVB)] were used for western blot analysis (A). For western blot analysis, the data are represented as the relative density of tyrosinase (B), MITF (C), TRP-1 (D), and TRP-2 (E) bands normalized to α-tubulin. One-way analysis of variance (ANOVA) with Tukey’ multiple comparisons test was used for statiscal analyses. ##p<0.01 and ###p<0.001 indicate statistically significant differences compared to the normal. **p<0.01 and ***p<0.001 indicate statistically significant differences compared to the control.
DISCUSSION
RR is a resveratrol-enriched transgenic rice that overexpresses the stilbene synthase gene isolated from the peanut (Arachis hypogaea var. Palkwang) and contains high levels of the resveratrol (Sobolev et al., 2011). We hypothesized that RR might possess biological activities similar to those of resveratrol or more active than that of resveratrol. Baek et al. reported that resveratrol-enriched rice might treat obesity in high-fat diet fed mice (Baek et al., 2014). Our collaborative group reported that RR might act against metabolic syndrome and related diseases (Baek et al., 2013; Baek et al., 2014). Interestingly, RR shows synergistic effects in comparison that of each resveratrol or rice alone (Baek et al., 2013; Baek et al., 2014). Several reports show that resveratrol decreases the expression of tyrosinase (Franco et al., 2012; Zheng et al., 2012; Bae et al., 2013; Park and Boo, 2013; Park et al., 2014), and Jun et al. reported that gamma-oryzanol from rice extract dually inhibited cellular melanogenesis, too (Jun et al., 2012). Because each of resveratrol and rice had anti-melanogenic effects, we expected that RR would produce synergistic anti-melanogenic effects and thus have some advantages over treatment with rice or resveratrol alone. To demonstrate positive and/or synergistic effects, we used an animal model to conduct multiple comparative studies of the anti-melanogenic effects of resveratrol, arbutin, rice extract, and RR extract. Before the in vivo experiments, we conducted in vitro experiments using the melan-a cells to investigate the decreasing effects of RR on the expression of tyrosinase, which is a key protein in the process of melanin synthesis. Melan-a cell is an immortal line of pigmented, non-tumorigenic mouse melanocytes that are syngeneic with B16 melanoma cells (Bennett et al., 1987). In a previous report, we showed that resveratrol decreased tyrosinase expression in B16F10 mouse melanoma cells (Lee et al., 2014). Therefore, the cells were also treated with resveratrol, with comparing its effects to those of RR. We confirmed that it showed similar effects in melan-a cells. We found that treatment with resveratrol or rice extract inhibited tyrosinase expression. Interestingly, RR inhibited tyrosinase expression more strongly than did resveratrol or rice alone (Fig. 2). This result supported that RR might produce synergistic anti-melanogenic effects.
UVB-irradiated guinea pigs, a commonly used animal model for hyperpigmentation research, were used for the in vivo experiments reported herein (Kim et al., 2008; Fujii et al., 2011; Peng et al., 2014). All were treated with a dose of 1% (w/v) sample. Hyperpigmentation was induced by 390 mJ/cm2 UVB irradiation for 2 weeks. From that time, samples were applied to the dorsal skin of the guinea pigs. After 2 weeks of UVB irradiation, the skin color of the UVB-irradiated control group had uniformly changed into black. In our colorimetric analysis, although RR did not suppress pigmentation as rapidly as did arbutin (positive control), the skin lightening of the RR-treated group was equivalent to that of the arbutin-treated group at the end (Fig. 3).
In addition, histological change due to depigmenting effect of RR was observed using Fontana-Masson staining. Melanin is produced by melanocytes that are located in the epidermis and are stimulated by cytokines and growth factors released from UV-irradiated keratinocytes (Yamada et al., 2013). We observed the increase of melanin in the epidermal skin of the UV-irradiated control group. However, UV-induced melanin production was decreased in the each groups treated with rice and resveratrol. The inhibitory effect of RR on melanin production was more potent than that of each rice extract or resveratrol (Fig. 4). To strengthen our understanding of the mechanisms underlying the depigmenting effects of RR, we measured the expression of tyrosinase, TRP-1, TRP-2, and MITF, which are involved in melanin synthesis by western blotting analysis. Initial melanin synthesis is controlled primarily through regulating tyrosinase. Tyrosinase converts tyrosine, the initial substrate for melanin synthesis, into L-DOPA. In addition, tyrosinase is involved in the next step of melanin synthesis, which is the conversion of DOPA into DOPAquinone (Moon et al., 2012). Therefore, regulation of tyrosinase is a powerful strategy with which to decrease melanin synthesis. In our result, RR significantly inhibited tyrosinase expression in the dorsal skin of guinea pigs. After DOPAquinone is formed, it is converted into DOPAchrome. TRP-2 participates in the conversion of DOPAchrome to 5,6-dihydroxyindole-2-carboxylic acid, which is an intermediate in the synthesis of eumelanin. We found that treatment with RR inhibited TRP-2 expression in the dorsal skin; however, RR did not inhibit the expression of TRP-1, which is involved in the final step of eumelanin synthesis. Taken together, these results suggest that the depigmentation effect of RR may be a result of its suppression of melanin synthesis via regulation of tyrosinase. And that the anti-melanogenic effect of RR is more active than that of rice or resveratrol alone. Based on HPLC analysis, however, the amount of resveratrol in the skin tissue of RR-treated group (5.6 μg) was much less than that was measured in the resveratrol-treated group (18 mg). Interestingly, the depigmentation effect of RR was stronger than that of resveratrol, indicating that this effect of RR is not caused by resveratrol alone. It was supposed that there may be, therefore, a further factor which acting on hypopigmentation. Namely, rice may be an important candidate for regulation of melanogenesis. Rice (Oryza sativa) contains several types of lipids, including triacylglycerols, monoacylglycerols, diacylglycerols, sterols, and free fatty acids (Hemavathy and Prabhakar, 1987), which might enhance the penetration of resveratrol into the skin tissue. Moreover, our study indicated that RR had no toxic effects (data not shown), and therefore it can be considered to be a safe natural source of whitening agents. Although synthetic chemicals such as arbutin, hydroquinone, and kojic acid have strong whitening effects, long term treatment with these compounds can produce negative side effects (Tse, 2010). In conclusion, resveratrol-enriched rice may be a novel, safe and effective natural source for skin whitening agent.
Acknowledgments
This work was supported by a grant from the Next-Generation BioGreen 21 Program (No.PJ009511), Rural Development Administration, Republic of Korea.
REFERENCES
- Bae SJ, Ha YM, Kim JA, Park JY, Ha TK, Park D, Chun P, Park NH, Moon HR, Chung HY. A novel synthesized tyrosinase inhibitor: (E)-2-((2,4-dihydroxyphenyl)diazenyl) phenyl 4-methylbenzenesulfonate as an azo-resveratrol analog. Biosci Biotechnol Biochem. 2013;77:65–72. doi: 10.1271/bbb.120547. [DOI] [PubMed] [Google Scholar]
- Baek SH, Chung HJ, Lee HK, D’Souza R, Jeon Y, Kim HJ, Kweon SJ, Hong ST. Treatment of obesity with the resveratrol-enriched rice DJ-526. Sci Rep. 2014;4:3879. doi: 10.1038/srep03879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek SH, Shin WC, Ryu HS, Lee DW, Moon E, Seo CS, Hwang E, Lee HS, Ahn MH, Jeon Y, Kang HJ, Lee SW, Kim SY, D’Souza R, Kim HJ, Hong ST, Jeon JS. Creation of resveratrol-enriched rice for the treatment of metabolic syndrome and related diseases. PloS one. 2013;8:e57930. doi: 10.1371/journal.pone.0057930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DC, Cooper PJ, Hart IR. A line of non-tumorigenic mouse melanocytes, syngeneic with the B16 melanoma and requiring a tumour promoter for growth. Int J Cancer. 1987;39:414–418. doi: 10.1002/ijc.2910390324. [DOI] [PubMed] [Google Scholar]
- Chen YJ, Chen YY, Lin YF, Hu HY, Liao HF. Resveratrol inhibits alpha-melanocyte-stimulating hormone signaling, viability, and invasiveness in melanoma cells. Evid Based Complement Alternat Med. 2013;2013 doi: 10.1155/2013/632121. 632121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco DC, de Carvalho GS, Rocha PR, da Silva Teixeira R, da Silva AD, Raposo NR. Inhibitory effects of resveratrol analogs on mushroom tyrosinase activity. Molecules. 2012;17:11816–11825. doi: 10.3390/molecules171011816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii T, Ikeda K, Saito M. Inhibitory effect of rose hip (Rosa canina L.) on melanogenesis in mouse melanoma cells and on pigmentation in brown guinea pigs. Biosci Biotechnol Biochem. 2011;75:489–495. doi: 10.1271/bbb.100702. [DOI] [PubMed] [Google Scholar]
- Hemavathy J, Prabhakar JV. Lipid composition of rice (Oryza sativa L.) bran. J Am Oil Chem Soc. 1987;64:1016–1019. [Google Scholar]
- Jun HJ, Lee JH, Cho BR, Seo WD, Kang HW, Kim DW, Cho KJ, Lee SJ. Dual inhibition of gamma-oryzanol on cellular melanogenesis: inhibition of tyrosinase activity and reduction of melanogenic gene expression by a protein kinase A-dependent mechanism. J Nat Prod. 2012;75:1706–1711. doi: 10.1021/np300250m. [DOI] [PubMed] [Google Scholar]
- Kim JH, Baek SH, Kim DH, Choi TY, Yoon TJ, Hwang JS, Kim MR, Kwon HJ, Lee CH. Downregulation of melanin synthesis by haginin A and its application to in vivo lightening model. J Invest Dermatol. 2008;128:1227–1235. doi: 10.1038/sj.jid.5701177. [DOI] [PubMed] [Google Scholar]
- Lee JS, Park KY, Min HG, Lee SJ, Kim JJ, Choi JS, Kim WS, Cha HJ. Negative regulation of stress-induced matrix metalloproteinase-9 by Sirt1 in skin tissue. Exp Dermatol. 2010;19:1060–1066. doi: 10.1111/j.1600-0625.2010.01129.x. [DOI] [PubMed] [Google Scholar]
- Lee TH, Seo JO, Baek SH, Kim SY. Inhibitory effects of resveratrol on melanin synthesis in ultraviolet B-induced pigmentation in Guinea pig skin. Biomol Ther. 2014;22:35–40. doi: 10.4062/biomolther.2013.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manosroi A, Chutoprapat R, Abe M, Manosroi W, Manosroi J. Anti-aging efficacy of topical formulations containing niosomes entrapped with rice bran bioactive compounds. Pharm Biol. 2012a;50:208–224. doi: 10.3109/13880209.2011.596206. [DOI] [PubMed] [Google Scholar]
- Manosroi A, Ruksiriwanich W, Abe M, Manosroi W, Manosroi J. Transfollicular enhancement of gel containing cationic niosomes loaded with unsaturated fatty acids in rice (Oryza sativa) bran semi-purified fraction. Eur J Pharm Biopharm. 2012b;81:303–313. doi: 10.1016/j.ejpb.2012.03.014. [DOI] [PubMed] [Google Scholar]
- Moon E, Kim AJ, Kim SY. Sarsasapogenin Increases Melanin Synthesis via Induction of Tyrosinase and Microphthalmia-Associated Transcription Factor Expression in Melan-a Cells. Biomol Ther. 2012;20:340–345. doi: 10.4062/biomolther.2012.20.3.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton RA, Cook AL, Roberts DW, Leonard JH, Sturm RA. Post-transcriptional regulation of melanin biosynthetic enzymes by cAMP and resveratrol in human melanocytes. J Invest Dermatol. 2007;127:2216–2227. doi: 10.1038/sj.jid.5700840. [DOI] [PubMed] [Google Scholar]
- Niles RM, McFarland M, Weimer MB, Redkar A, Fu YM, Meadows GG. Resveratrol is a potent inducer of apoptosis in human melanoma cells. Cancer Lett. 2003;190:157–163. doi: 10.1016/s0304-3835(02)00676-6. [DOI] [PubMed] [Google Scholar]
- Ohguchi K, Tanaka T, Ito T, Iinuma M, Matsumoto K, Akao Y, Nozawa Y. Inhibitory effects of resveratrol derivatives from dipterocarpaceae plants on tyrosinase activity. Biosci Biotechnol Biochem. 2003;67:1587–1589. doi: 10.1271/bbb.67.1587. [DOI] [PubMed] [Google Scholar]
- Park J, Boo YC. Isolation of resveratrol from vitis viniferae caulis and its potent inhibition of human tyrosinase. Evid Based Complement Alternat Med. 2013;2013 doi: 10.1155/2013/645257. 645257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Park JH, Suh HJ, Lee IC, Koh J, Boo YC. Effects of resveratrol, oxyresveratrol, and their acetylated derivatives on cellular melanogenesis. Arch Dermatol Res. 2014;306:475–487. doi: 10.1007/s00403-014-1440-3. [DOI] [PubMed] [Google Scholar]
- Peng LH, Liu S, Xu SY, Chen L, Shan YH, Wei W, Liang WQ, Gao JQ. Inhibitory effects of salidroside and paeonol on tyrosinase activity and melanin synthesis in mouse B16F10 melanoma cells and ultraviolet B-induced pigmentation in guinea pig skin. Phytomedicine. 2013;20:1082–1087. doi: 10.1016/j.phymed.2013.04.015. [DOI] [PubMed] [Google Scholar]
- Peng LH, Xu SY, Shan YH, Wei W, Liu S, Zhang CZ, Wu JH, Liang WQ, Gao JQ. Sequential release of salidroside and paeonol from a nanosphere-hydrogel system inhibits ultraviolet B-induced melanogenesis in guinea pig skin. Int J Nanomedicine. 2014;9:1897–1908. doi: 10.2147/IJN.S59290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo MY, Chung SY, Choi WK, Seo YK, Jung SH, Park JM, Seo MJ, Park JK, Kim JW, Park CS. Anti-aging effect of rice wine in cultured human fibroblasts and keratinocytes. J Biosci Bioeng. 2009;107:266–271. doi: 10.1016/j.jbiosc.2008.11.016. [DOI] [PubMed] [Google Scholar]
- Shimoda H, Terazawa S, Hitoe S, Tanaka J, Nakamura S, Matsuda H, Yoshikawa M. Changes in ceramides and glucosylceramides in mouse skin and human epidermal equivalents by rice-derived glucosylceramide. J Med Food. 2012;15:1064–1072. doi: 10.1089/jmf.2011.2137. [DOI] [PubMed] [Google Scholar]
- Sobolev VS, Khan SI, Tabanca N, Wedge DE, Manly SP, Cutler SJ, Coy MR, Becnel JJ, Neff SA, Gloer JB. Biological activity of peanut (Arachis hypogaea) phytoalexins and selected natural and synthetic Stilbenoids. J Agric Food Chem. 2011;59:1673–1682. doi: 10.1021/jf104742n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song YM, Ha YM, Kim JA, Chung KW, Uehara Y, Lee KJ, Chun P, Byun Y, Chung HY, Moon HR. Synthesis of novel azo-resveratrol, azo-oxyresveratrol and their derivatives as potent tyrosinase inhibitors. Bioorg Med Chem Lett. 2012;22:7451–7455. doi: 10.1016/j.bmcl.2012.10.050. [DOI] [PubMed] [Google Scholar]
- Tse TW. Hydroquinone for skin lightening: safety profile, duration of use and when should we stop? J Dermatol Treat. 2010;21:272–275. doi: 10.3109/09546630903341945. [DOI] [PubMed] [Google Scholar]
- Wu Z, Uchi H, Morino-Koga S, Shi W, Furue M. Resveratrol inhibition of human keratinocyte proliferation via SIRT1/ARNT/ERK dependent downregulation of aquaporin 3. J Dermatol Sci. 2014;75:16–23. doi: 10.1016/j.jdermsci.2014.03.004. [DOI] [PubMed] [Google Scholar]
- Yamada T, Hasegawa S, Inoue Y, Date Y, Yamamoto N, Mizutani H, Nakata S, Matsunaga K, Akamatsu H. Wnt/beta-catenin and kit signaling sequentially regulate melanocyte stem cell differentiation in UVB-induced epidermal pigmentation. J Invest Dermatol. 2013;133:2753–2762. doi: 10.1038/jid.2013.235. [DOI] [PubMed] [Google Scholar]
- Zheng ZP, Tan HY, Wang M. Tyrosinase inhibition constituents from the roots of Morus australis. Fitoterapia. 2012;83:1008–1013. doi: 10.1016/j.fitote.2012.06.001. [DOI] [PubMed] [Google Scholar]