Abstract
Chalcones (1,3-diaryl-2-propen-1-ones), a flavonoid subfamily, are widely known for their anti-inflammatory properties. Propenone moiety in chalcones is known to play an important role in generating biological responses by chalcones. In the present study, we synthesized chalcone derivatives structurally modified in propenone moiety and examined inhibitory effect on nitric oxide (NO) production and its potential mechanisms. Among the chalcone derivatives used for this study, TI-I-174 (3-(2-Hydroxyphenyl)-1-(thiophen-3-yl)prop-2-en-1-one) most potently inhibited lipopolysaccharide (LPS)-stimulated nitrite production in RAW 264.7 macrophages. TI-I-174 treatment also markedly inhibited inducible nitric oxide synthase (iNOS) expression. However, TI-I-174 did not significantly affect production of IL-6, cyclooxygenase-2 (COX-2) and tumor necrosis factor-α (TNF-α), implying that TI-I-174 inhibits production of inflammatory mediators in a selective manner. Treatment of macrophages with TI-I-174 significantly inhibited transcriptional activity of activator protein-1 (AP-1) as determined by luciferase reporter gene assay, whereas nuclear factor-κB (NF-κB) activity was not affected by TI-I-1744. In addition, TI-I-174 significantly inhibited activation of c-Jun-N-Terminal kinase (JNK) without affecting ERK1/2 and p38MAPK, indicating that down-regulation of iNOS gene expression by TI-I-174 is mainly attributed by blockade of JNK/AP-1 activation. We also demonstrated that TI-I-174 treatment led to an increase in heme oxygenase-1 (HO-1) expression both at mRNA and protein level. Transfection of siRNA targeting HO-1 reversed TI-I-174-mediated inhibition of nitrite production. Taken together, these results indicate that TI-I-174 suppresses NO production in LPS-stimulated RAW 264.7 macrophages via induction of HO-1 and blockade of AP-1 activation.
Keywords: Chalcone, Heme oxygenase-1, Inflammation, Lipopolysaccharide, Nitric oxide
INTRODUCTION
Inflammation, a complex biological response to harmful stimuli, is associated with various pathophysiological conditions. In response to inflammatory stimuli, macrophages release various pro-inflammatory molecules. Nitric oxide (NO) is a cellular signaling molecule that plays a key role in the pathogenesis of inflammation, while it is also required for normal physiological conditions (Gibaldi, 1993). NO is produced during oxidation of L-arginine to L-citrulline catalyzed by nitric oxide synthase (NOS) (Moncada and Higgs, 1993). Among three isoforms of NOSs, including endothelial NOS (eNOS), neuronal NOS (nNOS) and inducible NOS (iNOS), inducible form of NOS is responsible for the excessive production of NO by macrophages during inflammatory processes (Nussler and Billiar, 1993). It is widely accepted that NO production is a critical event leading to inflammatory process and therefore, suppression of NO production and iNOS expression is considered as a useful strategy for the treatment of diseases associated with inflammatory conditions.
Heme oxygenase (HO), a member of phase II detoxifying enzymes, is the rate-limiting enzyme in catabolism of heme into free iron, biliverdin and carbon monoxide (CO). Among the three isoforms, HO-1 is a highly inducible protein sensitive to cellular stress and catalyzes a series of reactions required for cellular defense. Previous studies have shown that HO-1 induction plays an important role in a number of cytoprotection responses, including anti-oxidative and anti-apoptotic effects (Motterlini et al., 2000) and a growing body of evidence has indicated that HO-1 induction acts an adaptive defense mechanism to protect cells from various pathophysiological conditions (Maines, 1997). In addition, recent studies have also revealed that HO-1 induction modulates inflammatory process via suppressing the production of pro-inflammatory mediators, such as COX-2, TNF-α and iNOS (Wang et al., 2001). Furthermore, deficiency of HO-1 in mice exhibited a severe inflammation (True et al., 2007), while HO-1 over-expression prevented inflammatory responses in various experimental conditions (Otterbein et al., 2003; Onyiah et al., 2013). Therefore, based on previous reports, modulation of HO-1 activity may be a promising strategy for the treatment of the diseases associated with inflammatory conditions.
Chalcones (1,3-diaryl-2-propen-1-ones), a group of polyphenolic and flavonoid family, are widely biosynthesized in plants. A number of previous studies have demonstrated that chalcones and its various derivatives regulate diverse biological functions and become the attractive molecule for the development of pharmacological agents in many diseases. In addition to the original observation showing the potent inhibitory effect on topoisomerase and subsequent on anti-cancer effects (Kim et al., 2013), chalcone and its derivatives also possess anti-bacterial (Avila et al., 2008), anti-fungal (Batovska et al., 2007), anti-oxidant (Dinkova-Kostova et al., 2001) properties. In particular, recent studies have shown the potent anti-inflammatory activities of the chalcones in various experimental conditions (Kontogiorgis et al., 2008). For examples, treatment of immune cells with chalcones significantly suppressed production of many inflammatory mediators, including COX-2, TNF-α and NO (Wu et al., 2011). We have previously observed that introduction of hydroxyl group on phenyl moiety of chalcone skeletone increased biological activities (Karki et al., 2010; Karki et al., 2012). In the present study, as part of effort to develop optimal anti-inflammatory agents, we prepared synthetic chalcone derivatives containing hydroxyl group at different positions of the phenyl ring, and investigated their inhibitory effects on NO production in macrophages and further its potential mechanisms.
MATERIALS AND METHODS
Materials
All the cell culture reagents were purchased from Hyclone Laboratories (South Logan, UT, USA). Lipopolysaccharide (LPS) was purchased from Sigma-Aldrich (St. Louis, MO, USA).Griess reagent system and Luciferase assay kit and all related products were purchased from Promega (Madison, WI, USA). HO-1 antibody was obtained from Enzo Life Sciences (Farmingdale, NY, USA). Antibodies against phosphorylated and total forms of ERK1/2, JNK, p38 MAPK and Nrf2 were obtained from Cell Signaling Technology Inc. (Beverly, MA, USA). HRP-conjugated anti-mouse and anti-rabbit were purchased from Pierce (Rockford, IL, USA).
Cell culture
The RAW 264.7 macrophage cell line was purchased from the Korean cell line bank (Seoul, Korea) and routinely cultured in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% (v/v) fetal bovine serum (FBS) and 1% (v/v) penicillin-streptomycin at 37°C in an incubator with a humidified atmosphere of 5% CO2.
Synthesis of chalcone derivatives
Compounds used as starting materials and reagents were obtained from Aldrich Chemical Co., Junsei or other chemical companies, and utilized without further purification. HPLC grade acetonitrile (ACN) and methanol were purchased from Burdick and Jackson, USA. Thin-layer chromatography (TLC) and column chromatography (CC) were performed with Kieselgel 60 F254 (Merck) and silica gel (Kieselgel 60, 230-400 mesh, Merck) respectively. Since all the compounds prepared contain aromatic ring, they were visualized and detected on TLC plates with UV light (short wave, long wave or both). NMR spectra were recorded on a Bruker AMX 250 (250 MHz, FT) for 1H NMR and 62.5 MHz for 13C NMR, and chemical shifts were calibrated according to TMS. Chemical shifts (δ) were recorded in ppm and coupling constants (J) in hertz (Hz). Melting points were determined in open capillary tubes on electrothermal 1A 9100 digital melting point apparatus and were uncorrected.
ESI LC/MS analyses were performed with a Finnigan LCQ Advantage® LC/MS/MS spectrometry utilizing Xcalibur® program. For ESI LC/MS, LC was performed with 10 μL injection volume on a Waters X Terra® 3.5 μm reverse-phase C18 column (2.1×100 mm) with a gradient elution: from 65% to 95% of B in A for 5 min followed by 95% to 65% of B in A for 5 min and 65% of B in A for 5 min, where mobile phase A was 100% distilled water with 20 mM AF and mobile phase B was 100% ACN. MS ionization conditions were: Sheath gas flow rate: 70 arb, aux gas flow rate: 20 arb, I spray voltage: 4.5 KV, capillary temp.: 215°C, capillary voltage: 21 V, tube lens offset: 10 V. Retention time is given in minutes.
Compound 3
To an ice cold solution of 85% KOH (1.2 eq.) in methanol (10 mL) and H2O (2 mL) methyl-3-thienylketone (1) (1.0 eq.) was added. After dissolution, aryl aldehyde 2 (1.0 eq.) was added dropwise. The reaction mixture was then stirred for 10 min to 3 h at 20°C. The mixture was neutralized with 4M aqueous HCl solution (pH adjusted to 2). The mixture was extracted with ethyl acetate (100 mL), washed with water (75 mL ×2) and saturated NaCl solution (50 mL). The organic layer was dried with magnesium sulfate and filtered. The filtrate was evaporated at reduced pressure, which was purified by silica gel column chromatography with a gradient elution of ethyl acetate/n-hexane to afford a solid compound 3 in 31.8 to 61.7% yield. Following the same procedure, four compounds were synthesized.
3-Phenyl-1-(thiophen-3-yl)prop-2-en-1-one (RK-I-105)
The same procedure described at general method was employed with 1 and 2 (R=a) to yield white solid 58.4%.
Rf(EtOAc: n-Hexane=1:5, v/v): 0.37
Retention time: 16.98 min, [MH]+: 215.1
1H NMR (250 MHz, CDCl3) δ 8.16 (dd, J=2.7, 1.1 Hz, 1 H, 1-thiophene H-2), 7.83 (d, J=15.7 Hz, 1 H, -CO-C=CH-), 7.67 (dd, J=5.2, 1.0 Hz, 1 H, 1-thiophene H-5), 7.63-7.60 (m, 2 H, 3-phenyl H-2, H-6), 7.42-7.39 (m, 3 H, 3-phenyl H-4, H-3, H-5), 7.41 (d, J=15.7 Hz, 1 H, -CO-CH=C-), 7.37 (dd, J=4.3, 3.0 Hz, 1 H, 1-thiophene H-4). 13C NMR (62.5 MHz, CDCl3) δ 183.96, 144.12, 143.04, 134.76, 132.08, 130.51, 128.94, 128.41, 127.43, 126.51, 122.65.
3-(2-Hydroxyphenyl)-1-(thiophen-3-yl)prop-2-en-1-one (TI-I-174)
The same procedure described at general method was employed with 1 and 2 (R=b) to yield dark green solid 34.9%.
Rf(EtOAc : n-Hexane=1:2 v/v): 0.34
Retention time: 16.78 min, [MH]+: 231.1
1H NMR (250 MHz, DMSO-d6) δ 10.29 (s, 1 H, 3-phenyl 2-OH), 8.73 (br, 1 H, 1-thiophene H-2), 7.99 (d, J=15.7 Hz, 1 H, -CO-C=CH-), 7.85 (dd, J=6.5, 1.2 Hz, 1 H, 3-phenyl H-6), 7.75 (d, J=15.7 Hz, 1 H, -CO-CH=C-), 7.68-7.61 (m, 2 H, 1-thiophene H-5, 3-phenyl H-4), 7.26 (td, J=8.37, 1.4 Hz, 1 H, 1-thiophene H-4), 6.93-6.83 (m, 2 H, 3-phenyl H-3, H-5). 13C NMR (62.5 MHz, DMSO-d6) δ 183.52, 157.40, 143.32, 138.53, 134.09, 132.30, 128.53, 127.90, 127.38, 128.97, 121.57, 119.64, 116.48.
3-(3-Hydroxyphenyl)-1-(thiophen-3-yl)prop-2-en-1-one (RK-III-277)
The same procedure described at general method was employed with 1 and 2 (R=c) to yield light yellow solid 61.7%.
Rf (EtOAc : n-Hexane=1:2 v / v): 0.47
Retention time: 13.68 min, [MH]+: 231.1
1H NMR (250 MHz, CDCl3) δ 8.17 (br, 1 H, 1-thiophene H-2), 7.78 (d, J=15.7 Hz, 1 H, -CO-C=CH-), 7.67 (d, J=5.1, Hz, 1 H, 1-thiophene H-5), 7.41-7.20 (m, 4 H, 1-thiophene H-4, -COCH=C-, 3-phenyl H-5, H-6), 7.13 (s, 1 H, 3-phenyl H-2), 6.91 (d, J=7.6 Hz, 1 H, 3-phenyl H-4). 13C NMR (62.5 MHz, CDCl3) δ 183.22, 157.93, 143.48, 143.07, 137.13, 134.51, 130.06, 127.87, 127.30, 123.13, 120.04, 117.93, 115.44.
3-(4-Hydroxyphenyl)-1-(thiophen-3-yl)prop-2-en-1-one (TI-I-176)
The same procedure described at general method was employed with 1 and 2 (R=d) to yield light yellow solid 31.8%.
Rf(EtOAc : n-Hexane=1:2 v / v): 0.54
Retention time: 14.58 min, [MH]+: 231.1
1H NMR (250 MHz, DMSO-d6) δ 10.3 (s, 1 H, 3-phenyl 4-OH), 8.71 (br, 1 H, 1-thiophene H-2), 7.69 (d, J=8.5 Hz, 2 H, 3-phenyl H-2, H-6), 7.64-7-57 (m, 4 H, 1-thiophene H-4, H-5, -CO-C=CH-, -CO-CH=C-), 6.84 (d, J=8.56 Hz, 2 H, 3-phenyl H-3, H-5). 13C NMR (62.5 MHz, DMSO-d6) δ 182.92, 159.98, 143.35, 143.13, 133.37, 130.79, 127.74, 127.06, 125.71, 190.63, 115.71.
Measurement of cell viability
Cell viability was assessed using the CellTiter 96 Aqueous One kit (Promega, Madison, WI, USA). Briefly, RAW 264.7 cells were seeded in 96-well plates at 5×104 cells/well. After overnight incubation, cells were treated with different concentrations of compounds. Then, 20 μl of 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfopheny)-2H-tetrazolium (MTS) solution was added and the cells were incubated for 2 h at 37°C. Cell viability was monitored via a SPECTROstar Nano microplate reader (BMG Labtech Inc., Ortenberg, Germany) by measuring absorbance at 490 nm.
Measurement of total Nitric Oxide production
Nitric oxide production was assessed spectrophotometrically as a formed nitrite (NO2). In brief, RAW 264.7 macrophages were seeded at a density of 5×104 cells/well in 96-well plates. Cells were pretreated with indicated concentration of compounds for 1 h followed by treatment with 100 ng/ml LPS for additional 24 h. The 50 μl of culture medium was reacted with 50 μl of sulfanilamide and 50 μl of N-1-napthylethylenediamine dihydrochloride (NED). Then, the absorbance was measured at 540 nm using a SPECTROstar Nano microplate reader (BMG Labtech Inc., Ortenberg, Germany).
ELISA for TNF-α detection
RAW 264.7 macrophages were seeded at a density of 5× 104 cells/well in 96-well plates. Cells were pretreated with indicated concentration of TI-I-174 (10 μM) for 2 h followed by treatment with 100 ng/ml LPS for additional 4 h. The culture media were collected and measured using TNF-α ELISA kits (Biolegend, San Diego, CA, USA), according to the manufacturer’s instructions.
Total RNA isolation, reverse transcription and quantitative PCR (qPCR)
To measure the mRNA levels of the target genes, total RNAs were isolated using Qiagen lysis solution (Qiagen, Hilden, Germany) according to the manufacturer’s instructions and cDNAs were synthesized from 1 μg of total RNA of each sample using the GoScript Reverse Transcription system (Promega). Quantitative Real-time PCR was then performed with LightCycler 1.5 (Roche Diagnostics, Mannheim, Germany) using QPCR SYBR Green Capillary Mix (ABgene, Surrey, UK) at 95°C for 15 min, followed by 40 cycles of 95°C for 15 sec, 60°C for 30 sec and 72°C for 30 sec. The primer sequences used for amplification of the target genes are listed in Table 1. The amount of target mRNA was measured via the comparative threshold (Ct) method after normalizing target mRNA Ct values to those for glyceraldehyde-3-phosphate dehydrogenase (GAPDH, ΔCt) as a housekeeping gene. The sequences of the siRNA used are listed in (Table 1).
Table 1.
Sequences of the primers used for quantitative RT-PCR
| Target gene | Primer | Sequence |
|---|---|---|
| IL-1β | Forward | 5′-GCCTCGTGCTGTCGGACCCATAT-3′ |
| Reverse | 5′-TCCTTTGAGGCCCAAGGCCACA-3′ | |
| COX-2 | Forward | 5′-GGGCTCAGCCAGGCAGCAAT-3′ |
| Reverse | 5′-GCACTGTGTTTGGGGTGGGCT-3′ | |
| IL-6 | Forward | 5′-ACAACCACGGCCTTCCCTACTT-3′ |
| Reverse | 5′-CACGATTTCCCAGAGAACATGTG-3′ | |
| iNOS | Forward | 5′-GCTCGCTTTGCCACGGACGA-3′ |
| Reverse | 5′-AAGGCAGCGGGCACATGCAA-3′ | |
| HO-1 | Forward | 5′-CACGCATATACCCGCTACCT-3′ |
| Reverse | 5′-CCAGAGTGTTCATTCGAGCA-3′ | |
| GAPDH | Forward | 5′-ACCACAGTCCATGCCATCAC-3′ |
| Reverse | 5′-TCCACCACCCTGTTGCTGTA-3′ |
Transient transfection and luciferase assay
Transcriptional activity was determined using luciferase reporter assay kit (Promega) according to the manufacturer’s instructions. Briefly, cells were initially seeded in 24-well plates at a density of 5×105 cells/well. After overnight culture, the cells were co-transfected with control (pRL-TK) and expression vectors (pGL4/NF-κB or pTL/AP-1) using Fugene HD (Promega). After 6 h incubation, media were replaced with DMEM containing 10% FBS and cells were cultured for additional 18 h. Thereafter, the cells were pretreated with indicated concentration of TI-I-174 for 1 h followed by 100 ng/ml LPS for additional 8 h and extracted with 0.1 ml of passive lysis buffer (Promega). Firefly and Renilla luciferase activities were measured by the Dual Luciferase Reporter Assay System (Promega). Statistical analyses for luciferase expression were carried out on the ratios of relative luciferase activity to Renilla luciferase.
Transient transfection with small interfering RNA (siRNA)
Cells were initially seeded on 35-mm dishes at a density of 7×105 cells/well. After overnight incubation, cells were transfected with scrambled control siRNA or siRNA targeting HO-1 (25 nM) or Nrf2 (50 nM) using Hiperfect transfection reagent (Qiagen) according to the manufacturer’s instructions. Gene silencing efficiency was assessed by qRT-PCR after 30 h of transfection. Murine specific siRNA targeting HO-1 and control siRNA were purchased from Bioneer (Daejeon, South Korea). The sequences of the siRNA used are listed in (Table 2).
Table 2.
Sequences of small interfering RNA used in transfection
| Target gene | Primer | Nucleotide sequence |
|---|---|---|
| HO-1 | Forward | 5′-CAGAUCAGCACUAGCUCAU-3′ |
| Reverse | 5′-AUGAGCUAGUGCUGAUCUG-3′ | |
| Nrf2 | Forward | 5′-GACUUUAGUCAGCGACAGA-3′ |
| Reverse | 5′-UCUGUCGCUGACUAAAGUC-3′ | |
| Scrambled | Forward | 5′-UGGUUUACAUGUCGACUAA-3′ |
| 5′-UGGUUUACAUGUUGUGUGA-3′ | ||
| 5′-UGGUUUACAUGUUUUCUGA-3′ | ||
| 5′-UGGUUUACAUGUUGUCUUA-3′ |
Preparation of cellular extracts and Western blot analysis
RAW 264.7 macrophages were seeded in 35-mm dishes at a density of 1×106 cells per well. After overnight incubation, cells were treated with TI-I-174 and/or LPS as indicated in figure legends. Total proteins were then isolated using RIPA lysis buffer containing Halt Protease and Phosphatase Inhibitor Single-Use Cocktail (Thermo scientific, Rockford) as described previously. For immunoblot analysis, 20 μg of solubilized proteins were loaded by 10% SDS-PAGE. The proteins were then transferred to PVDF membranes, blocked with 5% skim milk in PBS/ Tween20 for 1 h and incubated with the designated primary antibodies overnight at 4°C. Subsequently, the membrane was incubated with secondary HRP-conjugated antibody. The images of the blots were captured using Fujifilm LAS-4000 mini (Fujifilm, Tokyo, Japan). The membranes were then stripped and re-probed with total form of MAPKs or β-actin antibody as the loading control.
Statistical analysis
Values were presented as mean ± SEM derived from at least three separate experiments. Data were assessed by one-way analysis of variance (ANOVA) and Tukey’s multiple comparison tests using GraphPad prism software version 5.01 (California, USA). Differences between groups were considered to be significant at p<0.05.
RESULTS
Effects of chalcone derivatives on cell viability and LPS-induced nitrite production in RAW 264.7 macrophages
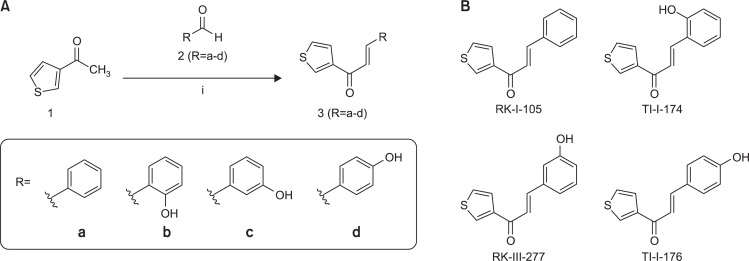
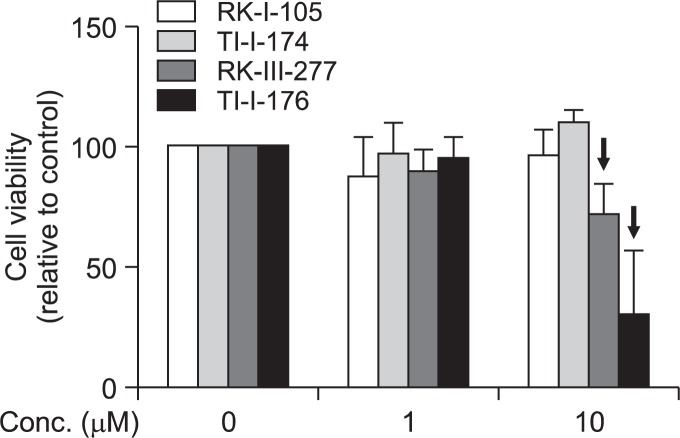
Previous studies have shown that chalcone derivatives potently suppress production of inflammatory mediators in macrophages (Kontogiorgis et al., 2008). In an effort to develop and optimize agent for treatment of inflammation-associated diseases, we prepared series of chalcone derivatives, RKI-105, TI-I-174, RK-III-277 and TI-I-176, containing hydroxyl groups at different positions (none, ortho, meta, or para) of the phenyl ring, respectively (Fig. 1). Before investigating the inhibitory effects of these compounds on the production of inflammatory mediators, we first examined if these compounds affect cell viability. As shown in Fig. 2, RK-I-105 and TI-I-174 did not generate any toxic effects up to 10 μM, while treatment with RK-III-277 and TL-I-176 caused significant cytotoxicity at 10 μM. These compounds were not further included for continuing study. For the investigation of the anti-inflammatory effects, we first examined the effects on LPS-stimulated nitric oxide (NO) production in RAW 264.7 macrophages. Pretreatment with TI-I-174 exhibited more potent inhibitory effect with the IC50 value of 5.75 μM roughly almost two-fold more potent than that of RK-I-105 (IC50 value of 9.63 μM) (Fig. 3). Based on these preliminary findings we focused subsequent efforts on TI-I-174.
Fig. 1.
Scheme for the preparation and chemical structures of the chalcone derivatives. (A) General synthetic scheme of phenyl/hydroxyphenyl-3-thienylpropenones. Reagents and conditions: (i) aryl aldehyde 2a-d (1.0 eq.), KOH (1.2 eq.), MeOH/H2O (5:1), 10 min to 3 h, 0°C, 31.8–61.7%. (B) Chemical structures of the chalcone derivatives.
Fig. 2.
Effects of chalcone derivatives on viability of RAW 264.7 macrophages. Cells were treated with the indicated concentrations of compounds for 24 h. Cell viability was measured by MTS assay as described in material and methods. Data represent the mean ± SEM (n=3).
Fig. 3.
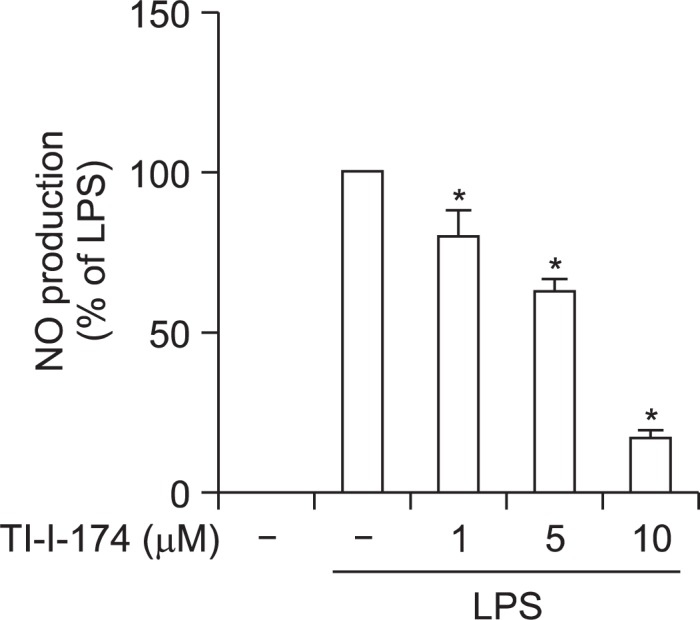
Effect of TI-I-174 on LPS-induced nitrite production in RAW 264.7 macrophages. Cells were pretreated with the indicated concentrations of TI-I-174 for 1 h followed by stimulation of LPS (100 ng/ml) for additional 24 h. Values are presented as percentage (%) compared to the cells treated with LPS and are expressed as mean ± SEM (n=3). *p<0.05 compared with the corresponding control values (2nd column group).
Effects of TI-I-174 on production of other inflammatory mediators in RAW 264.7 macrophages
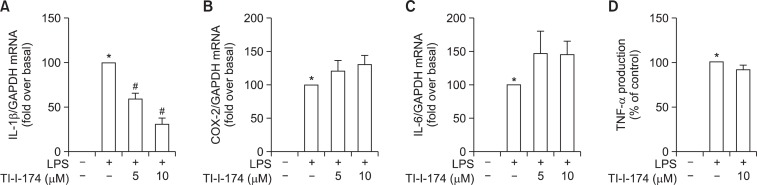
To further examine the anti-inflammatory properties of TI-I-174, we examined the effects of TI-I-174 on the expressions of other inflammatory mediators. As indicated in Fig. 4, treatment with TI-I-174 caused significant decrease in IL-1β expression (Fig. 4A). However, it did not generate significant effect on LPS-stimulated TNF-α secretion, IL-6 and COX-2 expression as determined by ELISA and quantitative RT-PCR, respectively (Fig. 4B–4D), implying that TI-I-174 regulates production of inflammatory mediators in a selective manner.
Fig. 4.
Effects of TI-I-174 on production of pro-inflammatory mediators in RAW 264.7 macrophages. (A) and (B) Cells were pretreated with TI-I-174 for 2 h followed by treatment of 100 ng/ml LPS additional for 6 h. IL-1β (A) and COX-2 (B) mRNA level was measured by qRT-PCR and the expression of target mRNA was normalized to GAPDH mRNA as described previously. Values are presented as mean ± SEM (n=3–4). *p<0.05 compared with control cells. (C) Cells were treated with TI-I-174 for 2 h followed by stimulation of 100 ng/ml LPS additional for 6 h. IL-6 mRNA level was normalized to GAPDH mRNA. Values are presented as mean ± SEM (n=3). *p<0.05 compared with control; #p<0.05 compared with cells treated with LPS. (D) Cells were pretreated with TI-I-174 for 2 h followed by LPS (100 ng/ml) additional for 4 h, and the production of TNF-α protein accumulated in the media was measured by ELISA. Values are presented as mean ± SEM (n=3). *p<0.05 compared with control.
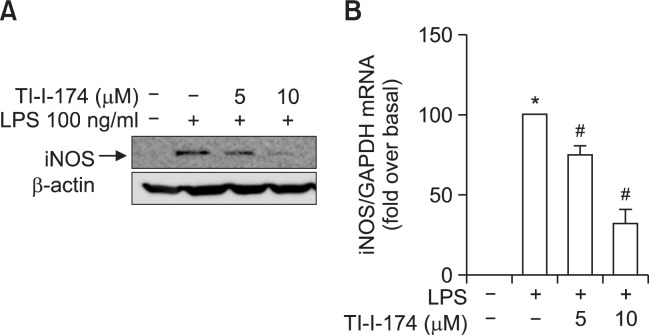
Effect of TI-I-174 on LPS-induced iNOS expression in RAW 264.7 macrophages
In continuing studies, we examined the molecular mechanisms underlying suppression of NO production. Nitric oxide is produced during the conversion of arginine into citrulline by nitric oxide synthase (NOS). Among the three isoforms of NOS, inducible form of NOS (iNOS) is responsible for NO production in response to LPS treatment. We therefore further examined the effect of TI-I-174 on iNOS expression. To elucidate the mechanisms underlying suppression of LPS-induced NO production, we investigate the effect of TI-I-174 on LPS-induced iNOS expression. As shown in Fig. 5A, TI-I-174 treatment significantly attenuated LPS-induced iNOS protein expression in a dose-dependent manner. Quantitative RT-PCR (qRT-PCR) analysis further indicated that LPS-induced increase in iNOS mRNA level was significantly inhibited by treatment with TI-I-174 (Fig. 5B) as expected, indicating that TI-I-174 suppresses NO production through inhibition of iNOS expression following LPS stimulation.
Fig. 5.
Effect of TI-I-174 on LPS-induced iNOS expression in RAW 264.7 macrophages. (A) Cells were pretreated with TI-I-174 for 2 h followed by incubation with 100 ng/ml LPS additional for 24 h. Expression of iNOS protein level was measured by Western blot analysis as described previously. Representative image from three independent experiments that showed similar results is shown along with β-actin for internal loading control. (B) Cells were treated with TI-I-174 for 2 h followed by stimulation of 100 ng/ml LPS additional for 6 h. iNOS mRNA level was normalized to GAPDH mRNA. Values are presented as mean ± SEM (n=4). *p<0.05 compared with control; #p<0.05 compared with cells treated with LPS.
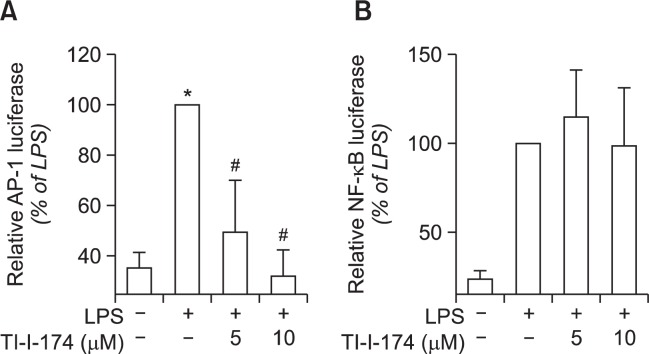
Effect of TI-I-174 on transcriptional activity of AP-1 and NF-κB in LPS-treated RAW 264.7 macrophages
Gene expression of iNOS is regulated by various transcription factors. In particular, AP-1 and NF-κB have shown to play a critical role in LPS-induced iNOS expression in macrophages. To further elucidate the mechanisms underlying down-regulation of iNOS expression, we therefore examined the effect of TI-I-174 on transcriptional activity of AP-1 and NF-κB using reporter gene assay. RAW 264.7 macrophages were transfected with the pAP-1-Luc plasmid (containing direct repeats of the AP-1 recognition sequences) or with the pNF-κB-Luc plasmid (containing repeats of NF-κB recognition sequences), and then stimulated with LPS in the absence or presence of TI-I-174. Reporter gene assay revealed that TI-I-174 treatment significantly inhibited transcriptional activation of AP-1 following LPS stimulation (Fig. 6A). However, TI-I-174 had no significant effect on the transcriptional activation of NF-κB by LPS stimulation (Fig. 6B), indicating that TI-I-174 regulates iNOS gene expression probably by inhibiting the activation of AP-1, but not NF-κB.
Fig. 6.
Effects of TI-I-174 on transcriptional activation of AP-1 and NF-κB in RAW 264.7 macrophages. (A) Cells were transiently cotransfected with pAP1-Luc plasmid and Renilla reporter gene as described previously. After 24 h of culture, cells were pretreated with indicated concentration of TI-I-174 for 1 h followed by incubation with 100 ng/ml LPS for additional 8 h. Transcriptional activity of AP-1 was assessed by luciferase reporter assay as described previously. Values are expressed as fold increase relative to control cells, mean ± SEM (n=3). *p<0.05 compared with the cells not treated with LPS; #p<0.05 compared with cells treated with LPS. (B) Cells were co-transfected with pGL4/NF-κB plasmid and Renilla reporter gene. After 24 h incubation, cells were pretreated with TI-I-174 for 1 h followed by stimulation with 100 ng/ml LPS for additional 8 h. NF-κB activity was measured by luciferase assay and values are expressed as fold increase relative to control cells, mean ± SEM (n=3).
Effects of TI-I-174 on LPS-induced activation of MAPKs in RAW 264.7 macrophages
Mitogen-activated protein kinases (MAPKs) signaling plays a key role in the activation of AP-1. To further identify the upstream signaling molecule involved in the modulation of transcriptional activity of AP-1 by TI-I-174, we further examined the effect of TI-I-174 on LPS-induced activation of MAPKs. As shown in Fig. 7, TI-I-174 significantly reduced phosphorylated level of JNK, while it did not significantly affect activation of p38MAPK and ERK1/2, suggesting that JNK would be an upstream signaling molecule to modulate activity of AP-1.
Fig. 7.
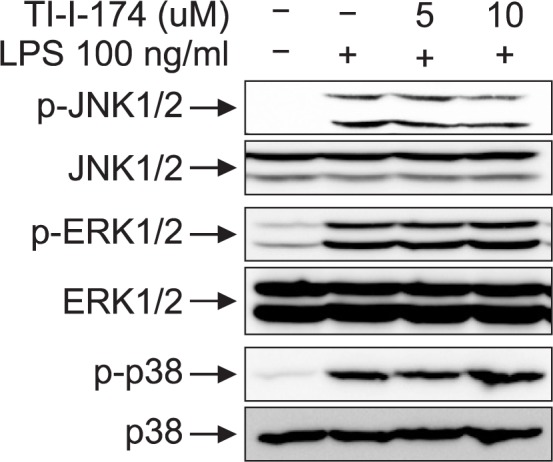
Effects of TI-I-174 on MAPKs activation in RAW 264.7 macrophages. Cells were treated with the indicated concentrations of TI-I-174 for 2 h followed by stimulation with LPS (100 ng/ml) for 20 min. Activation of each MAPK was investigated by Western blot analysis using corresponding phosphorylated forms of MAPKs. Representative image from three independent experiments is shown along with total form of MAPKs.
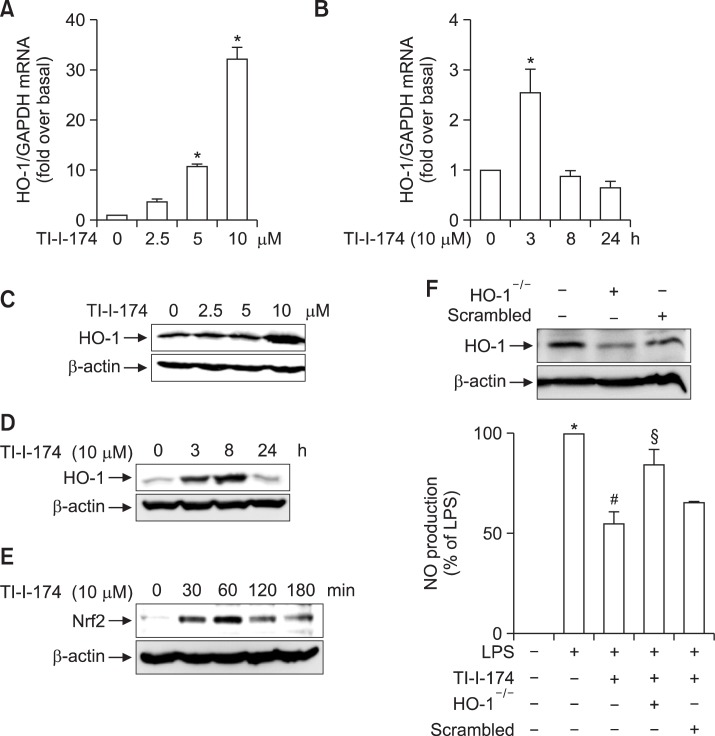
Role of HO-1 in TI-I-174-mediated suppression of NO production in LPS-stimulated RAW264.7 macrophages
HO-1 is widely known to modulate inflammatory response in various experimental conditions. We next examined whether HO-1 induction is involved in suppression of NO production by TI-I-174. To test this hypothesis, we first examined if TI-I-174 induced HO-1 expression. As shown in Fig. 8, treatment of RAW 264.7 macrophages with TI-I-174 led to a marked increase in HO-1 mRNA expression in a dose (Fig. 8A) and time dependent manner (Fig. 8B), showing a maximal increase after 3 h treatment with TI-I-174 and returned to the baseline level after 24 h of treatment (Fig. 8B). TI-I-174 treatment also caused significant increase in HO-1 protein expression level in a dose (Fig. 8C) and time dependent manner (Fig. 8D). TII-174 treatment also transiently increased HO-1 protein expression. It caused a maximal increase at 8 h treatment and returned to the almost normal level at 24 h, showing a pattern similar to that of mRNA expression.
Fig. 8.
Role of HO-1 induction in the suppression of LPS-stimulated nitrite production in RAW 264.7 macrophages. (A) and (B) Cells were pretreated with TI-I-174 for the indicated concentrations (A) or time periods (B). HO-1 mRNA level was measured by qRT-PCR and normalized to GAPDH mRNA as described previously. Values are presented as mean ± SEM (n=3). *p<0.05 compared with control cells. (C) and (D) Cells were treated with the TI-I-174 for the indicated concentrations (C) or time periods (D). HO-1 expression was determined by Western blot analysis as described in material and methods. Representative image from three independent experiments is shown along with β-actin for internal loading control. (E) Cells were treated with TI-I-174 for the indicated time periods. Nrf2 protein expression in total cellular lysates was measured by Western analysis. β-actin was used for internal control. Images are representative of three independent experiments that showed similar results. (F) (Upper panel) Cells were transfected with siRNA targeting HO-1 or scrambled (25 nM). After 48 h incubation, expression level of HO-1 was determined by Western blot analysis. (Lower panel) Cells were transfected with siRNA targeting HO-1(25 nM) or scrambled siRNA. After 6 h incubation, media were replaced with DMEM containing 10% FBS and then further incubated for 22 h. Cells were incubated with TI-I-174 (10 μM) for 1 h followed by stimulation of LPS (100 ng/ml) for additional 24 h. NO production was determined as described previously and values are presented as fold change relative to control cells and expressed as mean ± SEM (n=4). *p<0.05 compared with control group; #p<0.05 compared with cells treated with only LPS; §p<0.05 compared with the cells treated with TI-I-174 and LPS.
Nuclear factor-erythroid 2 (Nrf2) is well known as a key transcription factor responsible for various anti-oxidative proteins, including HO-1 (Na and Surh, 2014). To further elucidate the mechanisms underlying HO-1 expression by TI-I-174, we examined whether Nrf2 signaling plays a role in the induction of HO-1 by TI-I-174. For this, we investigated the effect of TII-174 on Nrf2 expression. As shown in Fig. 8E, treatment with TI-I-174 rapidly increased Nrf2 protein expression.
Finally, to verify that HO-1 induction is involved in TI-I-174-mediated suppression of nitrite production, we investigated whether gene silencing of HO-1 is sufficient to rescue nitrite production. As shown in Fig. 8F, transfection with siRNA targeting HO-1 efficiently suppressed HO-1 expression (Upper panel). In addition, inhibitory effect of TI-I-174 on LPS-induced nitrite production was partially restored by transfection with siRNA targeting HO-1 (compare 3th and 4th column), while scramble siRNA transfection did not significantly affect LPS-induced nitrite production (5th column), suggesting that suppression of LPS-induced nitrite production by TI-I-174 is mediated, at least in part, by HO-1 induction.
DISCUSSION
Nitric oxide (NO), a gaseous cellular signaling molecule, plays an important role in a number of normal physiological processes, such as vasodilation and neurotransmission. However, NO is also considered as a pro-inflammatory mediator and overproduction of NO is involved in the pathogenesis of inflammatory of disorders in many tissues (Ruan, 2002). Therefore, development of pharmacological agent inhibiting NO production would be a promising strategy for the management of inflammatory diseases. Chalcones, a group of phenolic compounds, exhibited potent anti-oxidant and anti-inflammatory properties in diverse in vitro and in vivo conditions (Berger et al., 2006; Kontogiorgis et al., 2008). In the present study, we prepared synthetic chalcone derivatives in an attempt to develop optimal anti-inflammatory agents. Among these chalcone derivatives, TI-I-174 was highly effective at inhibiting NO production in macrophages stimulated with LPS, and we further demonstrated that TI-I-174 mediated its effects by inducing HO-1 expression and blockade of AP-1 activation.
Chalcone (1,3-diaryl-2-propen-1-ones) has been shown to exhibit a variety of pharmacological activities. In addition to pharmacological responses by natural chalcone, its structural modifications on propenone moiety by introduction and/or substitution of aromatic rings exhibited a wide range of pharmacological activities, including anti-oxidant and anti-inflammatory activities. In this study, we incorporated the hydroxyl group on phenyl moiety in phenyl-3-thienylpropenones at different positions (none, ortho, meta, or para) (Fig. 1). We demonstrated that introduction of hydroxyl group at ortho position produced more potent suppressive effect on nitrite production, whereas introducing at meta and para positions are toxic to the macrophages (Fig. 2). It seems ortho-hydroxy would a beneficial moiety to suppress NO production without producing cytotoxic effects in macrophages. Although it is not sufficient to demonstrate structure activity relationship (SAR) at this stage, it would be interesting to further investigate SAR of the chalcone derivatives on suppressing NO production for future study.
Chalcones are known as potent inducers of phase II detoxifying enzymes, and a number of previous studies suggest that beneficial actions of chalcones are derived from induction of endogenous cytoprotective pathways, such as HO-1 (Foresti et al., 2005; Abuarqoub et al., 2006), in addition to direct inhibition of inflammation-associated transcription factors, such as AP-1 and NF-κB. In this study, we explored the potential involvement of HO-1 in the anti-inflammatory activity elicited by TI-I-174. We found that TI-I-174 induced HO-1 expression both at mRNA and protein level, and further gene silencing of HO-1 reversed inhibitory effect of TI-I-174 on NO production (Fig. 8), suggesting that anti-inflammatory property of TII-174 involve the dynamic action of HO-1. HO-1 expression can be modulated by various transcription factors depending on experimental conditions. Among them, nuclear factor-erythroid 2-related factor 2 (Nrf2) is known to play a crucial role in the HO-1 induction (Na and Surh, 2014). With respect to the regulation of Nrf2 activity, two different mechanisms have been proposed. In response to certain stimuli, Nrf2 is phosphorylated and released from its inhibitor keap1 (Cullinan et al., 2003; Numazawa et al., 2003), resulting in translocation into the nucleus and binding with antioxidant response element (ARE) in promoter region of HO-1. On the other hand, a number of natural anti-oxidant agents caused increase in cellular expression level of Nrf2 (Kwak et al., 2002; Nguyen et al., 2003). In this present study, we have demonstrated that TII-174 enhanced Nrf2 protein expression in whole cell lysates (Fig. 8E), suggesting a possible role of Nrf2 in HO-1induction by TI-I-174. We could not thoroughly address the mechanisms underlying HO-1 induction elicited by TI-I-174 in the current study, investigating the effects of TI-I-174 on regulation of Nrf2 and its role in HO-1 expression would be worth pursuing for future study to unravel molecular mechanisms underlying HO-1 expression by TI-I-174.
NO production is regulated by inflammation-related transcription factors. Previous studies have shown that anti-inflammatory activities of chalcones are derived from inhibition of transcription factors that regulate inflammatory gene expressions, such as AP-1 and NF-κB (Madan et al., 2000; Ban et al., 2004), as wells as from their induction of cytoprotective genes such as HO-1. It is well known that HO-1 induction and by-products of heme catabolism, in particular carbon monoxide (CO), generate anti-inflammatory responses via regulation of inflammation-related transcription factors, including AP-1 and/or NF-κB. In the present study, we found that HO-1 induction by TI-I-174 treatment significantly suppressed AP-1-dependent reporter gene expression and activation of JNK, which is an upstream signaling molecule for AP-1 activation (Fig. 6A), while it did not affect transcriptional activity of NF-κB (Fig. 6B). While it is widely accepted that HO-1induction suppresses NF-κB signaling, modulation of NF-κB activity by HO-1 signaling would be depending on experimental conditions. It has been shown that HO-1 induction or CO production negatively regulates NF-κB signaling in macrophages (Ashino et al., 2008). However, many other studies have also reported that HO-1 induction does not affect NF-κB activity, even if it suppresses inflammatory response, indicating that HO-1 can inhibit inflammatory response through the mechanisms independent of NF-κB regulation. For example, it has been shown that a synthetic chalcone compound induces HO-1 expression and suppresses NO production, but HO-1 induction does not mediate suppression of NF-κB signaling (Park et al., 2009). In addition, HO-1 induction by a synthetic chalcone derivative (3′, 4′, 5′, 3, 4, 5-hexamethoxy-chalcone, CH) did not mediate inhibition of IκB-α phosphorylation and IKK activity (Alcaraz et al., 2004). Furthermore, HO-1 induction by statins further significantly increased phosphorylation of IκB-α and translocation of p65 into the nucleus in the presence of LPS (Hsieh et al., 2008). These reports further indicate that HO-1 induction modulates NF-κB signaling in a context-dependent manner. At this stage, even though we cannot identify the detailed mechanisms underlying no significant effect on NF-κB transcriptional activity by HO-1 induction, data demonstrated in the present study imply that AP-1, rather than NF-κB, would be the key target by which TI-I-174 inhibits NO production.
In the present study, we demonstrated that TI-I-174 treatment caused increase in HO-1 expression and blockade of AP-1 activation. Since previous studies have also shown that HO-1 induction negatively regulates AP-1 activation (Dijkstra et al., 2004; Sasaki et al., 2006; Yasui et al., 2007), we further investigated whether HO-1 induction is required for blockade of AP-1 activation by TI-I-174. However, gene silencing of HO-1 by siRNA transfection did not restore TI-I-174-mediated suppression of AP-1 activation and iNOS mRNA accumulation (data not shown). These results are not supportive to the notion that HO-1 induction inhibits AP-1 activation and iNOS transcription, but in accordance with the previous observations showing that CO, a by-product of heme catabolism by HO-1, inhibited LPS-stimulated nitrite production without affecting iNOS expression (Sawle et al., 2005) and HO-1 induction by a synthetic chalcone derivative (3′,4′,5′,3,4,5-hexamethoxychalcone, CH) did not mediate suppression of transcriptional activity by CH (Alcaraz et al., 2004). Taken together, data presented here imply that TI-I-174 suppress NO production via two individual pathways (HO-1 induction and AP-1 inhibition), and also suggest that inhibition of NO production by TI-I-174 is a product of multiple pathways acting in concert.
In conclusion, we demonstrated that TI-I-174, a synthetic chalcone derivative, potently suppressed LPS-stimulated NO production in macrophages. This effect was mediated via induction of HO-1 and inhibition of AP-1 activation. Based on these findings, we propose that TI-I-174 would be a promising agent for the treatment of diseases associated with inflammation. Further studies are now required to validate the therapeutic effects of TI-I-174 in an in vivo model.
Acknowledgments
This research was supported by the Yeungnam University research grant in 2012.
REFERENCES
- Abuarqoub H, Foresti R, Green CJ, Motterlini R. Heme oxygenase-1 mediates the anti-inflammatory actions of 2′-hydroxychalcone in RAW 264.7 murine macrophages. Am J Physiol Cell Physiol. 2006;290:C1092–1099. doi: 10.1152/ajpcell.00380.2005. [DOI] [PubMed] [Google Scholar]
- Alcaraz MJ, Vicente AM, Araico A, Dominguez JN, Terencio MC, Ferrandiz ML. Role of nuclear factor-kappaB and heme oxygenase-1 in the mechanism of action of an anti-inflammatory chalcone derivative in RAW 264.7 cells. Br J Pharmacol. 2004;142:1191–1199. doi: 10.1038/sj.bjp.0705821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashino T, Yamanaka R, Yamamoto M, Shimokawa H, Sekikawa K, Iwakura Y, Shioda S, Numazawa S, Yoshida T. Negative feedback regulation of lipopolysaccharide-induced inducible nitric oxide synthase gene expression by heme oxygenase-1 induction in macrophages. Mol Immunol. 2008;45:2106–2115. doi: 10.1016/j.molimm.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Avila HP, Smania Ede F, Monache FD, Smania A., Jr Structure-activity relationship of antibacterial chalcones. Bioorg Med Chem. 2008;16:9790–9794. doi: 10.1016/j.bmc.2008.09.064. [DOI] [PubMed] [Google Scholar]
- Ban HS, Suzuki K, Lim SS, Jung SH, Lee S, Ji J, Lee HS, Lee YS, Shin KH, Ohuchi K. Inhibition of lipopolysaccharide-induced expression of inducible nitric oxide synthase and tumor necrosis factor-alpha by 2′-hydroxychalcone derivatives in RAW 264.7 cells. Biochem Pharmacol. 2004;67:1549–1557. doi: 10.1016/j.bcp.2003.12.016. [DOI] [PubMed] [Google Scholar]
- Batovska D, Parushev S, Slavova A, Bankova V, Tsvetkova I, Ninova M, Najdenski H. Study on the substituents’ effects of a series of synthetic chalcones against the yeast Candida albicans. Eur J Med Chem. 2007;42:87–92. doi: 10.1016/j.ejmech.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Berger B, Rothmaier AK, Wedekind F, Zentner J, Feuerstein TJ, Jackisch R. Presynaptic opioid receptors on noradrenergic and serotonergic neurons in the human as compared to the rat neocortex. Br J Pharmacol. 2006;148:795–806. doi: 10.1038/sj.bjp.0706782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullinan SB, Zhang D, Hannink M, Arvisais E, Kaufman RJ, Diehl JA. Nrf2 is a direct PERK substrate and effector of PERK-dependent cell survival. Mol Cell Biol. 2003;23:7198–7209. doi: 10.1128/MCB.23.20.7198-7209.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkstra G, Blokzijl H, Bok L, Homan M, van Goor H, Faber KN, Jansen PL, Moshage H. Opposite effect of oxidative stress on inducible nitric oxide synthase and haem oxygenase-1 expression in intestinal inflammation: anti-inflammatory effect of carbon monoxide. J Pathol. 2004;204:296–303. doi: 10.1002/path.1656. [DOI] [PubMed] [Google Scholar]
- Dinkova-Kostova AT, Massiah MA, Bozak RE, Hicks RJ, Talalay P. Potency of Michael reaction acceptors as inducers of enzymes that protect against carcinogenesis depends on their reactivity with sulfhydryl groups. Proc Natl Acad Sci USA. 2001;98:3404–3409. doi: 10.1073/pnas.051632198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foresti R, Hoque M, Monti D, Green CJ, Motterlini R. Differential activation of heme oxygenase-1 by chalcones and rosolic acid in endothelial cells. J Pharmacol Exp Ther. 2005;312:686–693. doi: 10.1124/jpet.104.074153. [DOI] [PubMed] [Google Scholar]
- Gibaldi M. What is nitric oxide and why are so many people studying it? J Clin Pharmacol. 1993;33:488–496. doi: 10.1002/j.1552-4604.1993.tb04694.x. [DOI] [PubMed] [Google Scholar]
- Hsieh CH, Jeng SF, Hsieh MW, Chen YC, Rau CS, Lu TH, Chen SS. Statin-induced heme oxygenase-1 increases NF-kappaB activation and oxygen radical production in cultured neuronal cells exposed to lipopolysaccharide. Toxicol Sci. 2008;102:150–159. doi: 10.1093/toxsci/kfm298. [DOI] [PubMed] [Google Scholar]
- Karki R, Thapa P, Kang MJ, Jeong TC, Nam JM, Kim HL, Na Y, Cho WJ, Kwon Y, Lee ES. Synthesis, topoisomerase I and II inhibitory activity, cytotoxicity, and structure-activity relationship study of hydroxylated 2,4-diphenyl-6-aryl pyridines. Bioorg Med Chem. 2010;18:3066–3077. doi: 10.1016/j.bmc.2010.03.051. [DOI] [PubMed] [Google Scholar]
- Karki R, Thapa P, Yoo HY, Kadayat TM, Park PH, Na Y, Lee E, Jeon KH, Cho WJ, Choi H, Kwon Y, Lee ES. Dihydroxylated 2,4,6-triphenyl pyridines: synthesis, topoisomerase I and II inhibitory activity, cytotoxicity, and structure-activity relationship study. Eur J Med Chem. 2012;49:219–228. doi: 10.1016/j.ejmech.2012.01.015. [DOI] [PubMed] [Google Scholar]
- Kim SH, Lee E, Baek KH, Kwon HB, Woo H, Lee ES, Kwon Y, Na Y. Chalcones, inhibitors for topoisomerase I and cathepsin B and L, as potential anti-cancer agents. Bioorg Med Chem Lett. 2013;23:3320–3324. doi: 10.1016/j.bmcl.2013.03.106. [DOI] [PubMed] [Google Scholar]
- Kontogiorgis C, Mantzanidou M, Hadjipavlou-Litina D. Chalcones and their potential role in inflammation. Mini Rev Med Chem. 2008;8:1224–1242. doi: 10.2174/138955708786141034. [DOI] [PubMed] [Google Scholar]
- Kwak MK, Itoh K, Yamamoto M, Kensler TW. Enhanced expression of the transcription factor Nrf2 by cancer chemopreventive agents: role of antioxidant response element-like sequences in the nrf2 promoter. Mol Cell Biol. 2002;22:2883–2892. doi: 10.1128/MCB.22.9.2883-2892.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madan B, Batra S, Ghosh B. 2′-hydroxychalcone inhibits nuclear factor-kappaB and blocks tumor necrosis factor-alpha- and lipopolysaccharide-induced adhesion of neutrophils to human umbilical vein endothelial cells. Mol Pharmacol. 2000;58:526–534. doi: 10.1124/mol.58.3.526. [DOI] [PubMed] [Google Scholar]
- Maines MD. The heme oxygenase system: a regulator of second messenger gases. Annu Rev Pharmacol Toxicol. 1997;37:517–554. doi: 10.1146/annurev.pharmtox.37.1.517. [DOI] [PubMed] [Google Scholar]
- Moncada S, Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med. 1993;329:2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- Motterlini R, Foresti R, Bassi R, Green CJ. Curcumin, an antioxidant and anti-inflammatory agent, induces heme oxygenase-1 and protects endothelial cells against oxidative stress. Free Radic Biol Med. 2000;28:1303–1312. doi: 10.1016/s0891-5849(00)00294-x. [DOI] [PubMed] [Google Scholar]
- Na HK, Surh YJ. Oncogenic potential of Nrf2 and its principal target protein heme oxygenase-1. Free Radic Biol Med. 2014;67:353–365. doi: 10.1016/j.freeradbiomed.2013.10.819. [DOI] [PubMed] [Google Scholar]
- Nguyen T, Sherratt PJ, Huang HC, Yang CS, Pickett CB. Increased protein stability as a mechanism that enhances Nrf2-mediated transcriptional activation of the antioxidant response element. Degradation of Nrf2 by the 26 S proteasome. J Biol Chem. 2003;278:4536–4541. doi: 10.1074/jbc.M207293200. [DOI] [PubMed] [Google Scholar]
- Numazawa S, Ishikawa M, Yoshida A, Tanaka S, Yoshida T. Atypical protein kinase C mediates activation of NF-E2-related factor 2 in response to oxidative stress. Am J Physiol Cell Physiol. 2003;285:C334–342. doi: 10.1152/ajpcell.00043.2003. [DOI] [PubMed] [Google Scholar]
- Nussler AK, Billiar TR. Inflammation, immunoregulation, and inducible nitric oxide synthase. J Leukoc Biol. 1993;54:171–178. [PubMed] [Google Scholar]
- Onyiah JC, Sheikh SZ, Maharshak N, Steinbach EC, Russo SM, Kobayashi T, Mackey LC, Hansen JJ, Moeser AJ, Rawls JF, Borst LB, Otterbein LE, Plevy SE. Carbon monoxide and heme oxygenase-1 prevent intestinal inflammation in mice by promoting bacterial clearance. Gastroenterology. 2013;144:789–798. doi: 10.1053/j.gastro.2012.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otterbein LE, Soares MP, Yamashita K, Bach FH. Heme oxygenase-1: unleashing the protective properties of heme. Trends Immunol. 2003;24:449–455. doi: 10.1016/s1471-4906(03)00181-9. [DOI] [PubMed] [Google Scholar]
- Park PH, Kim HS, Jin XY, Jin F, Hur J, Ko G, Sohn DH. KB-34, a newly synthesized chalcone derivative, inhibits lipopolysaccharide-stimulated nitric oxide production in RAW 264.7 macrophages via heme oxygenase-1 induction and blockade of activator protein-1. Eur J Pharmacol. 2009;606:215–224. doi: 10.1016/j.ejphar.2008.12.034. [DOI] [PubMed] [Google Scholar]
- Ruan RS. Possible roles of nitric oxide in the physiology and pathophysiology of the mammalian cochlea. Ann N Y Acad Sci. 2002;962:260–274. doi: 10.1111/j.1749-6632.2002.tb04073.x. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Takahashi T, Maeshima K, Shimizu H, Toda Y, Morimatsu H, Takeuchi M, Yokoyama M, Akagi R, Morita K. Heme arginate pretreatment attenuates pulmonary NF-kappaB and AP-1 activation induced by hemorrhagic shock via heme oxygenase-1 induction. Med Chem. 2006;2:271–274. doi: 10.2174/157340606776930781. [DOI] [PubMed] [Google Scholar]
- Sawle P, Foresti R, Mann BE, Johnson TR, Green CJ, Motterlini R. Carbon monoxide-releasing molecules (CORMs) attenuate the inflammatory response elicited by lipopolysaccharide in RAW264.7 murine macrophages. Br J Pharmacol. 2005;145:800–810. doi: 10.1038/sj.bjp.0706241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- True AL, Olive M, Boehm M, San H, Westrick RJ, Raghavachari N, Xu X, Lynn EG, Sack MN, Munson PJ, Gladwin MT, Nabel EG. Heme oxygenase-1 deficiency accelerates formation of arterial thrombosis through oxidative damage to the endothelium, which is rescued by inhaled carbon monoxide. Circ Res. 2007;101:893–901. doi: 10.1161/CIRCRESAHA.107.158998. [DOI] [PubMed] [Google Scholar]
- Wang WP, Guo X, Koo MW, Wong BC, Lam SK, Ye YN, Cho CH. Protective role of heme oxygenase-1 on trinitrobenzene sulfonic acid-induced colitis in rats. Am J Physiol Gastrointest Liver Physiol. 2001;281:G586–594. doi: 10.1152/ajpgi.2001.281.2.G586. [DOI] [PubMed] [Google Scholar]
- Wu J, Li J, Cai Y, Pan Y, Ye F, Zhang Y, Zhao Y, Yang S, Li X, Liang G. Evaluation and discovery of novel synthetic chalcone derivatives as anti-inflammatory agents. J Med Chem. 2011;54:8110–8123. doi: 10.1021/jm200946h. [DOI] [PubMed] [Google Scholar]
- Yasui Y, Nakamura M, Onda T, Uehara T, Murata S, Matsui N, Fukuishi N, Akagi R, Suematsu M, Akagi M. Heme oxygenase-1 inhibits cytokine production by activated mast cells. Biochem Biophys Res Commun. 2007;354:485–490. doi: 10.1016/j.bbrc.2006.12.228. [DOI] [PubMed] [Google Scholar]