Abstract
Chronic mild stress (CMS) has been reported to induce an anhedonic-like state in mice that resembles some of the symptoms of human depression. In the present study, we used a chronic mild stress animal model of depression and anxiety to examine the responses of two strains of mice that have different behavioral responsiveness. An outbred ICR and an inbred C57BL/6 strain of mice were selected because they are widely used strains in behavioral tests. The results showed that the inbred C57BL/6 and outbred ICR mice were similarly responsive to CMS treatment in sucrose intake test (SIT) and open field test (OFT). However, the two strains showed quite different responses in forced swimming test (FST) and novelty-suppressed feeding (NSF) test after 3 weeks of CMS treatment. Only C57BL/6 mice displayed the depression- and anxiety-like behavioral effects in response to CMS treatment in FST and NSF test. Our results suggest that there are differences in responsiveness to CMS according to the different types of strain of mice and behavioral tests. Therefore, these results provide useful information for the selection of appropriate behavioral methods to test depression- and anxiety-like behaviors using CMS in ICR and C57BL/6 mice.
Keywords: Chronic mild stress, Depression, Anxiety, Behavior, Mice
INTRODUCTION
Depression is a serious disorder characterized by either a depressed mood or anhedonia, weight changes, sleep disturbances, psychomotor agitation, fatigue, feelings of worthlessness, diminished cognitive functioning, or recurrent thoughts of death. Stressful experiences have been reported to favor the development of depression in humans (Kessler, 1997; Kendler et al., 1999; Kim et al., 2013). Therefore, stress-based animal models were developed to explore depressive phenomena. Depression is thought to result from interactions between the effects of environmental stress and genetic predisposition. In the present study, we used a stress procedure that included most of the commonly used “unpredictable, mild” stressors (Willner et al., 1992; Willner, 1997). The CMS procedure has been shown to decrease rodents’ preference for sweet solutions, which is considered to represent anhedonia, a core symptom of major depression (Willner, 2005). Also, the CMS procedure mimics the role of chronic stress in precipitating depression and induces various physical, behavioral, and neurochemical alterations that resemble those observed in depressed patients.
There has been an increasing number of studies on CMS effects in mice although most studies were previously performed in rats. It has been reported that the CMS paradigm in mice elicits a decrease in responsiveness to rewards, as revealed by decreased palatable sucrose consumption (Pothion et al., 2004), deteriorates their physical state, and induces changes in emotion-related behaviors. In addition, a difference in sensitivity to chronic mild stress has been shown according to the strain of mice. Furthermore, mouse strain differences have been reported in response to antidepressant drugs in depression-related behavior models (Yalcin et al., 2008). These reports suggest that genetic factors, such as strain differences, may contribute to the behavioral effects of mice in models of depression.
Previous studies showed that there are differences between strains of mice in the sucrose consumption test (Pothion et al., 2004) and depression-related behavior models such as the forced swimming test and the tail suspension test (Mineur et al., 2006). For instance, C57BL/6 mice show a decrease in immobility duration in the tail suspension task after CMS, whereas BALB/c mice show increased immobility. On the other hand, C57BL/6 mice show increased immobility in the forced swimming test after CMS. However, reports are still controversial, making it difficult to establish a clear behavioral profile of genetically/phenotypically different mouse strains after stress.
Generally, high comorbidity rates of depression and anxiety are observed in depressed patients, and the margins between depression and anxiety become blurred, which suggests that these disorders may not be entirely distinct conditions in humans or animals (Kaufman and Charney, 2000). Increases in anxiety-related behavior and locomotor disturbances were found to be induced by the stress procedure (Strekalova et al., 2004; Schweizer et al., 2009). These behavioral changes were considered to reflect additional depression-related symptoms (Willner, 2005). However, very little is known about how strain differences in mice impact stress-induced behavioral changes in behavior models including the open field test and novelty-suppressed feeding test.
In the present study, we examined the impact of differences in responsiveness to CMS on “anhedonia” (sucrose consumption) and depression- and anxiety-like behaviors in different strains of mice. We investigated the effects of CMS on different tests measuring anxiety- and depression-like behaviors using the sucrose intake test, forced swimming test, open field test, and novelty-suppressed feeding test. C57BL/6 and ICR strains of mice were selected because they are representative of inbred and outbred strains, respectively. Also, they are the most widely used strains in various behavioral tests. The purpose of this study was to identify potential behavioral differences in depression and anxiety between C57BL/6 and ICR strains after CMS treatment.
MATERIALS AND METHODS
Animals
Male C57BL/6 (J) and ICR mice weighing 22–30 g were purchased from Dae Han Biolink, Co., Ltd (Eumseong, Korea). The animals were housed 4–5 mice per cage in a temperature-and humidity-controlled room (23 ± 1°C, 55 ± 5%) under a 12-h light/dark cycle (light on 07:00–19:00) and allowed access to food and water ad libitum. After arrival, mice were acclimatized for 1 week prior to use in experimental procedures. Mice were introduced to the experiment room at least 1 h before the behavioral tests. All animal care procedures were conducted in accordance with the U.S. National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of Sungkyunkwan University.
Chronic mild stress schedule
The CMS procedure was performed as described (Elizalde et al., 2010; Briones et al., 2012) with slight modifications. The mice in the experiment were subjected to CMS for 3 weeks. The stressors were applied twice per day, in the morning (9:00) and the evening (19:00). The CMS procedure consists of a variety of unpredictable mild stressors including two periods of confinement (1 h), one period of paired housing (2 h), one period of white noise (85 dB, 4 h), one period of tilted cage (45°, overnight), one period of nesting materials removal (overnight), one period of water deprivation (overnight), one period of exposure to an empty bottle (1 h), one period of soiled bedding (overnight), one period of overnight illumination, and one period of reversed light/dark cycle (24 h). The weekly CMS schedules are listed in Table 1. The stressors were randomly scheduled over a 1-week period and repeated throughout the 3 weeks. The non-stressed control mice were housed in groups (4–5 per cage), and the stressed mice were singly housed. After 3 weeks of CMS, behavioral tests were carried out in the order presented in Fig. 1. The sucrose intake tests were performed at baseline, 7, 14, and 21 days along with body weight measurement. The open field test, forced swimming test, and novelty-suppressed feeding test were performed at 22, 23, and 25 days, respectively. All behavioral tests were conducted between 9 h and 13 h.
Table 1.
Weekly CMS schedule
| Mon | Tue | Wed | Thu | Fri | Sat | Sun | |
|---|---|---|---|---|---|---|---|
| Morning | Confinement (1 h) | Weekly sucrose intake test (9:00–10:00) | Paired housing (2 h) | White noise (4 h) | Confinement (1 h) | Empty bottle (1 h) | Reversed light/dark cycle |
| Evening | Food and water deprivation (15 h) | Removal of nesting materials | Overnight illumination | Soiled bedding | Water deprivation | Reversed light/dark cycle | Tilt cage (45°) |
Fig. 1.
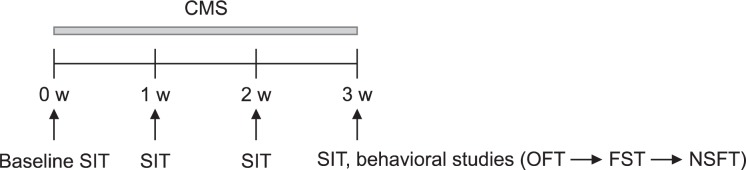
Experimental schedule. The chronic mild stress (CMS) protocol lasted 3 weeks. Before the CMS procedure, baseline sucrose intake was measured. Once a week, sucrose intake and body weight were measured. After 3 weeks of CMS, behavioral tests were carried out in order as follows.
Sucrose intake test
The SIT was conducted as described (Gronli et al., 2005; Schweizer et al., 2009; Ma et al., 2011; Lee et al., 2013) with slight modifications. Following acclimation, mice were first trained to consume a 1% (w/v) sucrose solution for 48 h. After consumption stabilization, mice were divided into two groups and matched for sucrose intake baseline and body weight baseline. Once a week, sucrose intake (1% sucrose solution) and body weight of all animals were measured during a 1 h window (Tuesday 9:00–10:00) after 15 h of food and water deprivation in both the CMS and control groups. The food and water deprivation period preceding sucrose intake measurement may be considered to be a further stress applied on top of the CMS protocol. However, control mice were also exposed to food and water deprivation as a part of the test (Stedenfeld et al., 2011; Zhang et al., 2013). Consumption was measured by weighing the pre-weighed bottle at the end of the test. The relative sucrose intake was expressed as absolute intake (g) per mouse body weight (g).
Open field test
Locomotor activity was assessed by OFT after 3 weeks of CMS exposure. The open field consisted of an opaque plastic box (30×30×30 cm) divided into 16 (4×4) identical sectors (7.5×7.5 cm). The field was subdivided into peripheral and central sectors, where the central sector included 4 central squares (2×2) and the peripheral sector was the remaining squares (Li et al., 2008; Sakata et al., 2010). The animals were placed into the center of an open field and allowed to explore for 10 min under dim light. The open field arena was thoroughly cleaned with 70% ethanol between each test. A video tracking system (NeuroVision, Pusan, Korea) was used to record the distance traveled as a measure of locomotor activity. The time spent in and entries into the center were measured as an anxiolytic indicator (Prut and Belzung, 2003; Grivas et al., 2013).
Forced swimming test
The FST was carried out on mice according to the method of Porsolt (Porsolt et al., 1977). Briefly, mice were individually placed into a glass cylinder (20 cm in height, 14 cm in diameter) filled 16 cm high with water (25 ± 1°C). A styrofoam divider separated the cylinders so that the mice could not see each other during the trials. After a 6-min swim test session, the immobility time during the final 4-min interval of the test was measured using the video tracking system (EthoVision, Noldus, Wageningen, Netherlands). Immobility time was considered to be a mouse floating passively, making only small movements to keep its nose above the surface.
Novelty-suppressed feeding test
The NSF test was performed according to a modified procedure, as described previously (Jang et al., 2013). Twenty-four hours after food deprivation, mice were placed in a plastic box, where they were subjected to the NSF test for 5 min. At the beginning of the test, a single food pellet was placed in the center of the box, and mice were placed in one corner. Latencies to approach and to begin eating were recorded. Scoring for the measure of interest did not begin until the mouse reached for the food with its forepaws and began eating. Immediately after the test, the mouse was transferred to its home cage and the amount of food consumed in the next 5 min was measured (home-cage food consumption).
Statistical analysis
Data are expressed as the means ± SEMs. The data were analyzed by two-way ANOVA followed by the Bonferroni post hoc test using Prism 5.0 (GraphPad Software, Inc., USA). Significant differences were set at the p<0.05 level.
RESULTS
Sucrose intake test
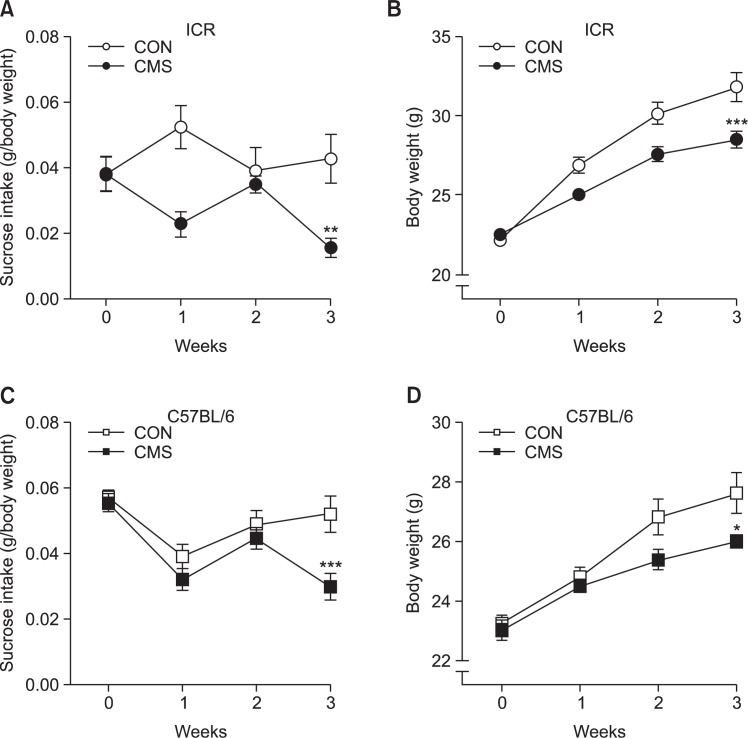
Before the onset of CMS, sucrose intake was similar in both the control group and in the group of mice that were to undergo stress. In ICR mice, there was a significant interaction between stress and time (F1,80=3.897, p<0.05) (two-way ANOVA). The post hoc test revealed that exposure to CMS produced a significant decrease in the sucrose intake (absolute intake per body weight) in the third week, compared to the control mice (t=3.327, p<0.01, Fig. 2A). In C57BL/6 mice, a significant interaction between stress and time was found in the group exposed to CMS (F1,72=3.026; p<0.05) (two-way ANOVA). The post hoc test revealed that exposure to CMS produced a significant decrease in sucrose intake after 3 weeks, compared to the control mice (t=4.190, p<0.001, Fig. 2C). Moreover, the stressed mice showed significant decreases of body weight compared to the control groups in ICR and C57BL/6 mice (p<0.001, p<0.05, Fig. 2B, 2D).
Fig. 2.
Effects of CMS on sucrose intake test and body weight in ICR (A and B) and C57BL/6 (C and D) mice. Once a week, sucrose intake and body weight of all animals were measured during a 1-h window after 15 h food and water deprivation in both control and CMS group. The relative sucrose intake was expressed as absolute intake (g) per mouse body weight (g). Data are presented as means ± SEMs (n=10–12/group). *p<0.05, **p<0.01, ***p<0.001 compared to the corresponding control (two-way ANOVA, Bonferroni post-test).
Open field test
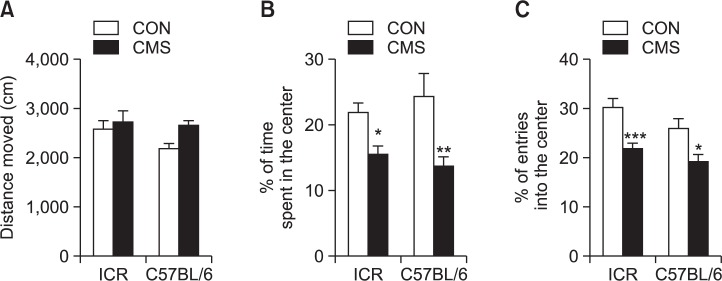
After 3 weeks of CMS exposure, the open field test was performed to evaluate locomotion and anxiety-like behaviors. The stressed mice showed increased movement without significant changes compared to the control mice in both strains (Fig. 3A). In addition, the two strains of ICR and C57BL/6 mice caused significant central activities in the open field test. Both strains, ICR and C57BL/6 mice, showed significant decreases in time spent in the center after CMS (ICR: t=2.438, p<0.05; C57BL/6: t=3.672, p<0.01, Fig. 3B). Furthermore, entries into the center decreased significantly in stressed mice of both strains (ICR: t=3.996, p<0.001; C57BL/6: t=2.923, p<0.05, Fig. 3C).
Fig. 3.
Effects of CMS on locomotion and central activity in the open field test. The animals were allowed to explore the open field arena for 10 min. (A) Total distance moved (cm), (B) % of time spent in the center, (C) % of entries into the center in ICR and C57BL/6 mice. Data are presented as means ± SEMs (n=10–12/group). *p<0.05, **p<0.01 and ***p<0.001 compared to the corresponding control (two-way ANOVA, Bonferroni post-test).
Forced swimming test
Depression-like behavior of the different strains in the FST is shown in Fig. 4. The stressed C57BL/6 mice significantly showed depressive behavior by displaying increased immobility compared to the non-stressed mice after CMS (t=2.548, p<0.05). On the other hand, ICR mice did not show a significant change after the stress exposure (t=1.002, p>0.05). With regard to significant effect across strains, we have performed further statistical analysis. When CMS group was compared between ICR and C57BL/6 mice, the results showed a significant effect (p<0.05, Fig. 4).
Fig. 4.
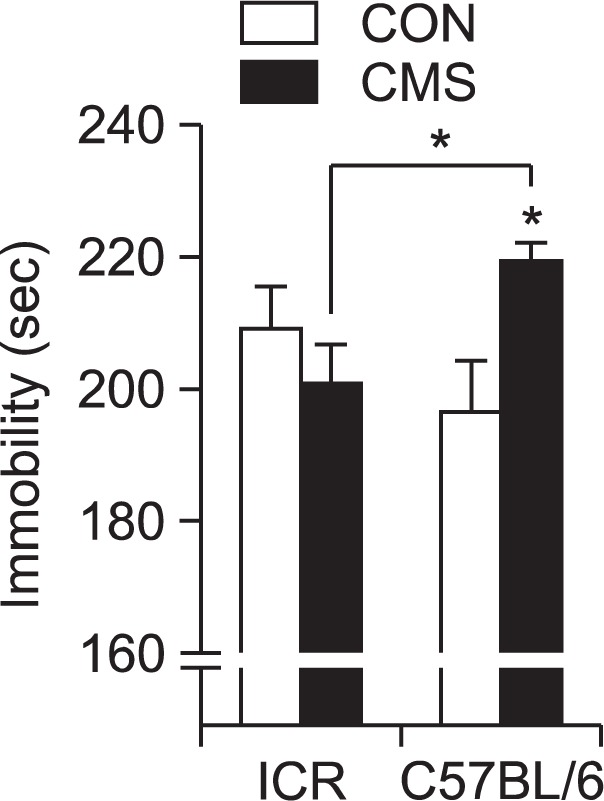
Effects of CMS on immobility in the forced swimming test. Immobility was analyzed by final 4 min of total 6 min test. Data are presented as means ± SEMs (n=10–12/group). *p<0.05 compared to the corresponding control (two-way ANOVA, Bonferroni post-test).
Novelty-suppressed feeding test
The NSF test measures food consumption in a novel environment under food deprivation, where reduction is interpreted as anxiety-like behavior as well as depression-related behavior (Bodnoff et al., 1988). Depression- and anxiety-like behavior of the different strains during the NSF test is illustrated in Fig. 5. In ICR mice, the latency of eating in the NSF test was not affected by 3 weeks of CMS experience (Fig. 5A). On the other hand, C57BL/6 mice showed a significant increase in latency of eating after CMS (t=4.200, p<0.001, Fig. 5A). We also tested home-cage food consumption to assess whether the increased feeding latency of stressed C57BL/6 mice could be attributed to a change in appetite. However, there were no differences in home-cage food consumption among groups in either the ICR and C57BL/6 strains (Fig. 5B).
Fig. 5.
Effects of CMS on depression- and anxiety-like behavior in the novelty suppressed feeding test. (A) Latency for eating (sec), (B) Home-cage food consumption (mg). Twenty-four hours after food deprivation, mice were subjected to the NSF test for 5 min. Immediately after the test, the mouse was transferred to its home cage and the amount of food consumed in the next 5 min was measured. Data are presented as means ± SEMs (n=10–12/group). ***p<0.001 compared to the corresponding control (two-way ANOVA, Bonferroni post-test).
DISCUSSION
In the present study, we assessed potential differences between one outbred (ICR) and one inbred (C57BL/6) mouse strain in response to different types of behavioral tests in the CMS model. The present data showed marked differences between the two mouse strains in their responsiveness to the CMS procedure. The C57BL/6 mice showed depression- and anxiety-like behavioral effects in the SIT, OFT, FST, and NSF tests after 3 weeks of CMS treatment. However, ICR mice failed to display depression-like behaviors in the FST and NSF tests.
CMS is generally thought to be the most promising and valuable rodent model for studying depression in animals because it mimics several human depressive symptoms. Moreover, genetic factors, such as strain differences, may contribute to the behavioral performance of mice in models of depression. Previous studies reported that there were differences between strains of mice in sucrose intake (Pothion et al., 2004) or in some of the CMS-induced behavioral alterations (Mineur et al., 2003, 2006).
Reduced consumption of sucrose solutions induced by CMS has been used as a measure of anhedonia, a major symptom of depression (Willner et al., 1992). As weight loss following CMS may influence sucrose intake and could be a confounding variable, we measured relative sucrose intake as absolute intake (g) per mouse body weight (g), which is considered to be an index for anhedonia (Matthews et al., 1995). In our experiment, a variety of mild stressors induced similar significant effects in sucrose intake in both strains. Stressed ICR mice showed a 63.3% decrease in sucrose intake compared to the non-stressed control group, whereas stressed C57BL/6 mice displayed a 42.6% decrease in the 3rd week of CMS. In this study, we applied a food and water deprivation schedule for sucrose intake measurement. The food and water deprivation period preceding sucrose intake measurement may be considered to be a stressor for the CMS group but as a part of the test for the control group. However, we cannot exclude the possibility that an overnight food and water deprivation period influences other behaviors besides depressive behaviors. Moreover, we observed fluctuations in the consummatory data in both strains. These might be a consequence of the short measurement interval and the consequential low intake during test time.
When testing anxiety-related behaviors in the open field, similar effects on CMS were obtained in both strains. When the mice were placed in a novel environment, increased tendencies of locomotor activity in the stressed mice were observed. A previous study reported that CMS-treated mice showed more activity than control mice in the open field (Mineur et al., 2006). Moreover, Strekalova and colleagues (2009) showed that chronic stress caused hyperactivity in mice, when tested under a light of modest brightness. These results are in accordance with our data that show increased tendency of movement after chronic stress. In this study, the CMS procedure significantly decreased preference for the central area in both strains. A decrease in these parameters could be indicative of anxiogenic behaviors (Prut and Belzung, 2003). These results suggest that anxiety-related behaviors in the OFT may be relatively unaffected by genetic susceptibility to CMS.
Some strain differences have been described previously for the tail suspension test (Liu et al., 2001) and forced swimming test in mice (Lucki et al., 2001). However, we chose only the FST because C57BL/6 mice have a strong tendency to climb up their tails, which would have defeated the purpose of the TST (Mayorga and Lucki, 2001). In our experiment, C57BL/6 mice showed increased immobility after CMS, whereas ICR mice did not show a significant change in the FST (Fig. 4). These results suggest that ICR mice are inappropriate for evaluating stress-induced depressive behavior in the FST. These results also underscore the strong influence of genetic background on susceptibility to CMS. Similarly, Lucki et al. (2001) reported that the C57BL/6 mice with the highest immobility values may be more vulnerable to stress-evoked depressive behavior.
The NSF test is often used as an additional measurement of depressive behavior and is also used to assess chronic antidepressant efficacy in rodents (Santarelli et al., 2003; Bessa et al., 2009; Zhu et al., 2010). The NSF test is more sensitive to chronic depression-like responses that are correlated to anxiety, whereas the FST evaluates severe depression-like responses in desperate situations. In this study, C57BL/6 mice showed significantly increased latency in eating compared to the control group. Our results also support the idea that mice exposed to chronic stress respond more sensitively to the NSF test (p<0.001) than to the FST (p<0.05). On the other hand, ICR mice failed to display depression-like behavior in the NSF test.
In this study, we found that the C57BL/6 and ICR strains of mice displayed different responsiveness to CMS through depression- and anxiety-like behaviors. It should be mentioned that there might be relevant neurochemical differences between two strains that could influence behavioral responsiveness. Previously, it has been reported that a specific and regionally differentiated serotonin-dopamine interaction is directly related to the observed stress-induced anhedonia in different rat strains (Bekris et al., 2005). Moreover, Wu and Wang (2010) reported that there were differences in HPA axis responsiveness between inbred and outbred strains of rats, thereby affecting the manifestation of depressive behavior. The evaluation of neurochemical alterations following behavioral changes may help to elucidate clear differences of responsiveness in different mouse strains.
In summary, this study showed that different strains of mice exhibited different behavioral patterns in various animal models including the SIT, OFT, FST, and NSF tests. The inbred C57BL/6 and outbred ICR mice were similarly responsive to CMS treatment in SIT and OFT. However, two strains showed quite different responses in FST and NSF test. Only C57BL/6 mice displayed depression- and anxiety-like behavioral effects to CMS treatment in FST and NSF test. Our results suggest that there are differences in responsiveness to CMS according to the different types of strain of mice and behavioral tests. Therefore, these results provide useful information for the selection of appropriate behavioral methods to test depression-and anxiety-like behaviors using CMS in ICR and C57BL/6 mice.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2012R1A5A2A28671860).
REFERENCES
- Bekris S, Antoniou K, Daskas S, Papadopoulou-Daifoti Z. Behavioural and neurochemical effects induced by chronic mild stress applied to two different rat strains. Behav Brain Res. 2005;161:45–59. doi: 10.1016/j.bbr.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Bessa JM, Mesquita AR, Oliveira M, Pego JM, Cerqueira JJ, Palha JA, Almeida OF, Sousa N. A trans-dimensional approach to the behavioral aspects of depression. Front Behav Neurosci. 2009;3:1. doi: 10.3389/neuro.08.001.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnoff SR, Suranyi-Cadotte B, Aitken DH, Quirion R, Meaney MJ. The effects of chronic antidepressant treatment in an animal model of anxiety. Psychopharmacology. 1988;95:298–302. doi: 10.1007/BF00181937. [DOI] [PubMed] [Google Scholar]
- Briones A, Gagno S, Martisova E, Dobarro M, Aisa B, Solas M, Tordera R, Ramirez M. Stress-induced anhedonia is associated with an increase in Alzheimer’s disease-related markers. Br J Pharmacol. 2012;165:897–907. doi: 10.1111/j.1476-5381.2011.01602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elizalde N, Garcia-Garcia AL, Totterdell S, Gendive N, Venzala E, Ramirez MJ, Del Rio J, Tordera RM. Sustained stress-induced changes in mice as a model for chronic depression. Psychopharmacology. 2010;210:393–406. doi: 10.1007/s00213-010-1835-6. [DOI] [PubMed] [Google Scholar]
- Grivas V, Markou A, Pitsikas N. The metabotropic glutamate 2/3 receptor agonist LY379268 induces anxiety-like behavior at the highest dose tested in two rat models of anxiety. Eur J Pharmacol. 2013;715:105–110. doi: 10.1016/j.ejphar.2013.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronli J, Murison R, Fiske E, Bjorvatn B, Sorensen E, Portas CM, Ursin R. Effects of chronic mild stress on sexual behavior, locomotor activity and consumption of sucrose and saccharine solutions. Physiol Behav. 2005;84:571–577. doi: 10.1016/j.physbeh.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Jang CG, Whitfield T, Schulteis G, Koob GF, Wee S. A dysphoric-like state during early withdrawal from extended access to methamphetamine self-administration in rats. Psychopharmacology. 2013;225:753–763. doi: 10.1007/s00213-012-2864-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Charney D. Comorbidity of mood and anxiety disorders. Depress Anxiety. 2000;12:69–76. doi: 10.1002/1520-6394(2000)12:1+<69::AID-DA9>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Prescott CA. Causal relationship between stressful life events and the onset of major depression. Am J Psychiatry. 1999;156:837–841. doi: 10.1176/ajp.156.6.837. [DOI] [PubMed] [Google Scholar]
- Kessler RC. The effects of stressful life events on depression. Annu Rev Psychol. 1997;48:191–214. doi: 10.1146/annurev.psych.48.1.191. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Lee MS, Kim JH, Lee TH, Shim I. Antidepressant-like effects of Lycii Radicis Cortex and betaine in the forced swimming test in rats. Biomol Ther. 2013;21:79–83. doi: 10.4062/biomolther.2012.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B, Sur B, Kwon S, Yeom M, Shim I, Lee H, Hahm DH. Chronic administration of catechin decreases depression and anxiety-like behaviors in a rat model using chronic corticosterone injections. Biomol Ther. 2013;21:313–322. doi: 10.4062/biomolther.2013.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Wang C, Wang W, Dong H, Hou P, Tang Y. Chronic mild stress impairs cognition in mice: From brain homeostasis to behavior. Life Sci. 2008;82:934–942. doi: 10.1016/j.lfs.2008.02.010. [DOI] [PubMed] [Google Scholar]
- Liu X, Gershenfeld HK. Genetic differences in the tail-suspension test and its relationship to imipramine response among 11 inbred strains of mice. Biol Psychiatry. 2001;49:575–581. doi: 10.1016/s0006-3223(00)01028-3. [DOI] [PubMed] [Google Scholar]
- Lucki I, Dalvi A, Mayorga AJ. Sensitivity to the effects of pharmacologically selective antidepressants in different strains of mice. Psychopharmacology. 2001;155:315–322. doi: 10.1007/s002130100694. [DOI] [PubMed] [Google Scholar]
- Ma XC, Jiang D, Jiang WH, Wang F, Jia M, Wu J, Hashimoto K, Dang YH, Gao Cg. Social isolation-induced aggression potentiates anxiety and depressive-like behavior in male mice subjected to unpredictable chronic mild stress. PLoS ONE. 2011;6:e20955. doi: 10.1371/journal.pone.0020955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews K, Forbes N, Reid IC. Sucrose consumption as an hedonic measure following chronic unpredictable mild stress. Physiol Behav. 1995;57:241–248. doi: 10.1016/0031-9384(94)00286-e. [DOI] [PubMed] [Google Scholar]
- Mayorga AJ, Lucki I. Limitations on the use of the C57BL/6 mouse in the tail suspension test. Psychopharmacology. 2001;155:110–112. doi: 10.1007/s002130100687. [DOI] [PubMed] [Google Scholar]
- Mineur YS, Belzung C, Crusio WE. Effects of unpredictable chronic mild stress on anxiety and depression-like behavior in mice. Behav Brain Res. 2006;175:43–50. doi: 10.1016/j.bbr.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Mineur YS, Prasol DJ, Belzung C, Crusio WE. Agonistic behavior and unpredictable chronic mild stress in mice. Behav Genet. 2003;33:513–519. doi: 10.1023/a:1025770616068. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Pothion S, Bizot JC, Trovero F, Belzung C. Strain differences in sucrose preference and in the consequences of unpredictable chronic mild stress. Behav Brain Res. 2004;155:135–146. doi: 10.1016/j.bbr.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: A review. Eur J Pharmacol. 2003;463:3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- Sakata K, Jin L, Jha S. Lack of promoter IV-driven BDNF transcription results in depression-like behavior. Genes Brain Behav. 2010;9:712–721. doi: 10.1111/j.1601-183X.2010.00605.x. [DOI] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Schweizer MC, Henniger MSH, Sillaber I. Chronic mild stress (CMS) in mice: Of anhedonia, ‘anomalous anxiolysis’ and activity. PLoS ONE. 2009;4:e4326. doi: 10.1371/journal.pone.0004326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stedenfeld KA, Clinton SM, Kerman IA, Akil H, Watson SJ, Sved AF. Novelty-seeking behavior predicts vulnerability in a rodent model of depression. Physiol Behav. 2011;103:210–216. doi: 10.1016/j.physbeh.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strekalova T, Spanagel R, Bartsch D, Henn FA, Gass P. Stress-induced anhedonia in mice is associated with deficits in forced swimming and exploration. Neuropsychopharmacology. 2004;29:2007–2017. doi: 10.1038/sj.npp.1300532. [DOI] [PubMed] [Google Scholar]
- Willner P. Validity, reliability and utility of the chronic mild stress model of depression: A 10-year review and evaluation. Psychopharmacology. 1997;134:319–329. doi: 10.1007/s002130050456. [DOI] [PubMed] [Google Scholar]
- Willner P. Chronic mild stress (CMS) revisited: Consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology. 2005;52:90–110. doi: 10.1159/000087097. [DOI] [PubMed] [Google Scholar]
- Willner P, Muscat R, Papp M. Chronic mild stress-induced anhedonia: A realistic animal model of depression. Neurosci Biobehav Rev. 1992;16:525–534. doi: 10.1016/s0149-7634(05)80194-0. [DOI] [PubMed] [Google Scholar]
- Wu HH, Wang S. Strain differences in the chronic mild stress animal model of depression. Behav Brain Res. 2010;213:94–102. doi: 10.1016/j.bbr.2010.04.041. [DOI] [PubMed] [Google Scholar]
- Yalcin I, Belzung C, Surget A. Mouse strain differences in the unpredictable chronic mild stress: a four-antidepressant survey. Behav Brain Res. 2008;193:140–143. doi: 10.1016/j.bbr.2008.04.021. [DOI] [PubMed] [Google Scholar]
- Zhang K, Song X, Xu Y, Li X, Liu P, Sun N, Zhao X, Liu Z, Xie Z, Peng J. Continuous GSK-3β overexpression in the hippocampal dentate gyrus induces prodepressant-like effects and increases sensitivity to chronic mild stress in mice. J Affect Disord. 2013;146:45–52. doi: 10.1016/j.jad.2012.08.033. [DOI] [PubMed] [Google Scholar]
- Zhu XH, Yan HC, Zhang J, Qu HD, Qiu XS, Chen L, Li SJ, Cao X, Bean JC, Chen LH, Qin XH, Liu JH, Bai XC, Mei L, Gao TM. Intermittent hypoxia promotes hippocampal neurogenesis and produces antidepressant-like effects in adult rats. J Neurosci. 2010;30:12653–12663. doi: 10.1523/JNEUROSCI.6414-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]