Abstract
Nicotine addiction is a worldwide problem. However, previous studies characterizing the rewarding and reinforcing effects of nicotine in animal models have reported inconsistent findings. It was observed that the addictive effects are variable on different factors (e.g. route, dose, and age). Here, we evaluated the rewarding and reinforcing effects of nicotine in different routes of administration, across a wide dose range, and in different age groups. Two of the most widely used animal models of drug addiction were employed: the conditioned place preference (CPP) and self-administration (SA) tests. Nicotine CPP was evaluated in different routes [intraperitoneal (i.p.) and subcutaneous (s.c.)], doses (0.05 to 1.0 mg/kg) and age [adolescent and adult rats]. Similarly, intravenous nicotine SA was assessed in different doses (0.01 to 0.06 mg/kg/infusion) and age (adolescent and adult rats). In the CPP test, s.c. nicotine produced greater response than i.p. The 0.2 mg/kg dose produced highest CPP response in adolescent, while 0.6 mg/kg in adult rats; which were also confirmed in 7 days pretreated rats. In the SA test, adolescent rats readily self-administer 0.03 mg/kg/infusion of nicotine. Doses that produced nicotine CPP and SA induced blood nicotine levels that corresponded well with human smokers. In conclusion, we have demonstrated that nicotine produces reliable CPP [0.2 mg/kg dose (s.c.)] in adolescents and [0.6 mg/kg dose (s.c.)] in adults, and SA [0.03 mg/kg/infusion] in adolescent rats. Both tests indicate that adolescent rats are more sensitive to the rewarding and reinforcing effects of nicotine.
Keywords: Nicotine, Conditioned place preference, Self-administration, Adolescent, Adults, Addiction
INTRODUCTION
Tobacco/cigarette smoking causes 6 million deaths every year (World Health Organization, 2013). Adding alarm is the consistent increase in the number of smokers around the world (Lantz, 2003). Because of this immense threat to public health, various efforts have been made to curb this problem (Gilpin et al., 1999; Stanton et al., 1996). One vital step is to understand why tobacco/cigarettes are so addictive. Substantial evidences have implicated that nicotine is the primary addictive component in tobacco (Dani and De Biasi, 2001). Nicotine is an alkaloid found in abundant amounts in tobacco leaf (Rogge et al., 1994; Seeman et al., 1999). Nicotine produces strong positive effects on mood and cognition, which directly contribute to the development of addiction (Le Houezec, 2003; Meyer and Quenzer, 2005).
Although nicotine is generally acknowledged to produce positive rewarding and reinforcing, demonstrating these effects in animal models has proven to be difficult (Hyman et al., 2006). Animal models are essential in understanding and managing addiction because it bypasses the ethical and methodological concerns associated with human studies (Jain, 2003; Benowitz et al., 2009). Two of the most widely used and accepted animal models of drug addiction are the conditioned place preference (CPP) and self-administration (SA) tests (Torres et al., 2008; Shram and Le, 2010). The CPP test has been used for assessing addiction-related behaviors and considered to quantify the rewarding effects (hedonic value) of a substance (Carboni et al., 1989) whereas the SA test, believed to be the most valid animal model of addiction, measures drug-seeking and drug taking behavior (reinforcing effects) (Vastola et al., 2002). When a substance produces both CPP and SA, then it is likely that the substance may have high addictive liability.
Previous nicotine CPP and SA studies have reported somewhat inconsistent results. Some have successfully produced nicotine CPP and SA while others did not (Le Foll and Goldberg, 2005; Benowitz et al., 2009). Those who have established nicotine CPP and SA also have discrepant findings, mostly associated with the methodological differences (e.g. route of administration, dosages used, and age of the subjects) (Le Foll and Goldberg, 2005). Because of these, there are no standard or established protocol for nicotine CPP and SA.
Thus, in the present study we sought to characterize the rewarding and reinforcing effects of nicotine through the CPP and the SA tests. Nicotine CPP and SA were evaluated across a wide dose range, in both adolescent and adult rats. The difference between the i.p. and the s.c. route was also tested for nicotine CPP.
MATERIALS AND METHODS
Subjects
Male Sprague-Dawley adolescent [postnatal day (PND) 21] and adult rats (PND 56), were obtained from Hanlim Animal Corporation (Hwasung, Korea). They were caged in groups (conditioned place preference) or individually (self-administration) in temperature- (22 ± 2°C) and humidity- (55 ± 5%) controlled animal room on a 12 h/12 h light/dark (7 AM–7 PM) schedule. Food and water were freely accessible, except on days when they undergone lever training. All rats were acclimated to their home cages for at least 3 days before the commencement of any tests. Eight to ten rats were chosen for each group. Animal treatment and maintenance were carried out in accordance with the Principles of Laboratory Animal Care (NIH publication no. 85-23 revised 1985) and the Animal Care and Use Guidelines of Sahmyook University, Korea.
Drugs
(−)Nicotine hydrogen tartrate salt (Sigma) was dissolved in physiologic saline, with pH adjusted to 7.3 ± 0.1, then administered at 1 ml/kg of body weight. Nicotine dosages are reported as the free base.
Conditioned place preference (CPP) test
A two-compartment place preference apparatus made of polyvinylchloride was used. Each of the compartments (measuring 47 cm×47 cm×47 cm) had unique visual and tactile cues, that is, one side was dark with a smooth floor and the other side had white dotted walls and a rough, black floor. A guillotine door divided the apparatus and served as a partition between the compartments during the conditioning phase. Ethovision (Noldus, Netherlands) system was used to record animal movement and behavior.
The CPP test was performed in accordance to previous studies with slight modification (Benowitz, 2010; de la Peña et al., 2012; de la Peña et al., 2013a; de la Pena et al., 2013b). A biased CPP design was employed based on the recommendation of (Le Foll and Goldberg, 2005). The test is comprised of three phases: (1) habituation and preconditioning, (2) conditioning, and (3) post-conditioning. During the first (preconditioning) phase, the guillotine door was opened to allow each rat free access to both compartments for 15 minutes, once a day for three days. On third day, the time spent by rats in both sides of the box was measured using an automated video tracking system [EthoVision (Noldus IT b.v. Netherlands)] to determine their preferred and non-preferred sides. During the second (conditioning) phase, the guillotine door was closed. The initially preferred side was paired with saline and non-preferred side was paired with nicotine. Rats were injected with the drug (s.c. or i.p.) and contained in the non-preferred side for 30 minutes, and on alternate days saline was administered. This procedure was repeated for three cycles (6 days). In the post-conditioning phase, rats were drug-free and were allowed free access to both compartments for 15 min. The time spent in each compartment was recorded. To further confirm our findings, we also evaluated nicotine CPP in pretreated rats [0.5 mg/kg (s.c) for 7 days (twice a day)].
Self-administration (SA) test
Rats were contained in standard sound attenuating operant chambers with ventilation fans to reduce external noise (Coulbourn Instruments, Allen-town, Pennsylvania, USA). There was a food pellet dispenser and two response levers of 4.5 cm width in one wall of each operant chamber. Then, there were stimulus lights positioned 6 cm above each lever and a house light (2.5-W, 24-V) was sited centrally at the top of the opposite wall to illuminate the chamber. A minimal downward pressure (approximately 25 g) on a lever could cause an automated consequence. Outside the chamber, there was a motor-driven syringe pump (Coulbourn) that served to supply solution at a rate of 0.01 ml/sec, through a liquid swivel via Teflon tubing. The swivel that was positioned at the top of the chamber was mounted on a counter-balanced arm which would allow free movement of the rats. For intravenous delivery of nicotine, the tubing was connected to the catheter system of the rats. Software (Graphic State Notation, Coulbourn) was installed on a computer and used in lever training and self-administration experiment. This system controlled experimental parameters, such as schedule of reinforcements, time periods, and data collection.
Rats were trained to press a lever for a contingent pellet reward on a continuous schedule of reinforcement (30 mins/day for 3 days). Only those rats which acquired at least 100 pellets during the last session were selected and prepared for surgery. Before the surgery, they were given anesthesia. Surgical procedures and post-surgical care are described in our previous studies (de la Peña et al., 2012; de la Pena et al., 2013b). After a 3 days recovery period, the 2-hour per day nicotine self-administration test commenced. During the first five days of self-administration, rats were placed under FR1 schedule. Subsequently, FR schedule was adjusted to FR2 for the next 3 days (6th–8th day) and FR3 for the last 2 days (9th and 10th day). During the session, two levers were present and a press on the left lever would create a cascade response: initiation of infusion pump for 10 seconds resulting to a delivery of 0.1 ml drug (0.01, 0.03, and 0.06 mg/kg nicotine), setting of a stimulus light above the left lever which remained lit for 20 seconds after the end of the infusion. Responses were also recorded during time-outs, but did not have any automated consequence. As a control for general activity, presses on the left lever were noted but not reinforced with drug infusions. To prevent from drug intoxication, rats were only allowed 3 ml infusions per session; although, lever responses were still recorded for the rest of the 2-hour session.
Determination of blood nicotine levels
HPLC system of the Nanospace SI-2 series (Shiseido, Tokyo, Japan) consisted of two 3001 Binary pumps, a 3002 UV-vis detector, a 3003 autosampler, a 3004 column oven, a 3012 high switching valve was used. The signals were processed by dsChrom-I (Donam Instrument Inc., Seoul, Korea). 200 μL serum of calibrators, controls, or samples, 50 μL trichloroacetic acid (10 g/100 mL) was vortexed for 30 seconds. Then 150 μL of washing solvent, 5% (v/v) acetonitrile in 20 mM phosphate buffer (pH 5.1) was added and vortexed for 30 s. The tubes were centrifuged at 12000 rpm for 5 min. 40 μL of the clear supernatants were injected into the HPLC for on-line solid phase extraction and analysis with an autosampler. The serum sample was pre-separated on the pre-column, Capcell Pak MF ph column (10 mm×2.0 mm I.d., Shiseido, Tokyo, Japan) with a washing solvent at flow-rate of 0.5 mL/min to remove proteins and concentrate nicotine from serum sample. The nicotine molecule fractions from first separation were transferred to a concentration column, Capcell Pak UG 120 U column (35 mm×2.0 mm I.D., Shiseido, Tokyo, Japan) and then the final separation was performed on an analytical column, Capcell Pak UG 120 U column (250 mm×1.5 mm I.D., Shiseido, Tokyo, Japan) with a mobile phase at a flow rate of 100 μL/min. The mobile phase was 10% methanol, 5% acetonitrile, 0.05% diethyl amine, 1 mM heptane sulfonate sodium in 20 mM phosphate buffer (pH 5.1). The column was maintained at 30°C and the eluent was monitored at 260 nm with UV-vis detector.
Data analysis
All results were presented as means and standard error of the mean (±S.E.M.). The conditioned place preference (CPP) data was presented as the difference in time spent in the nicotine- or saline- (for control group) paired compartment during the post-conditioning and preconditioning phases (Fig. 1, 2). One way analysis of variance (ANOVA) was applied to measure effects of drug-pretreatment followed by Dunnett’s posttest which compare the effects of each group versus the control group. The locomotor activity was expressed as the distanced moved, in centimeters, of the animals during the nicotine-conditioning phase of the CPP. Two-way ANOVA was used to identify effect of route, douse or interaction between the two factors. In the self-administration test the number of lever responses/presses both in the active (left) and inactive (right) lever and number of infusions obtained, during the 2-hours self-administration period, were recorded. Two-way ANOVA was employed to determine variations in lever response, day or interaction between the two factors (Fig. 3A). The different phases of the self-administration test were analyzed separately. If significant results were obtained, post-hoc comparisons were made using the Bonferroni’s test. The accepted level of significance was set as p<0.05. All statistical analyses were conducted using GraphPad Prism Version 4.01 software (California, USA).
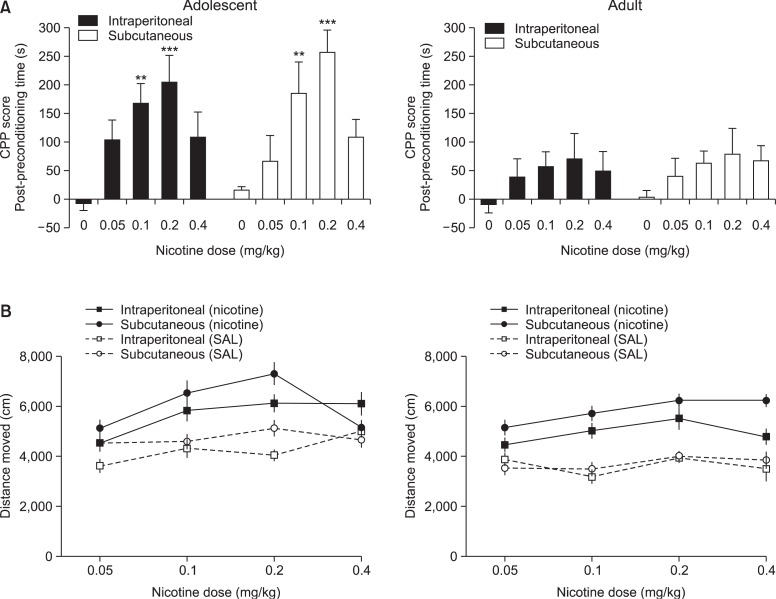
Fig. 1.
CPP route determination. Effects of subcutaneously (s.c.) or intraperitoneally (i.p.) injected nicotine on the CPP test. (A) Both s.c and i.p. injected nicotine produced CPP at 0.1 and 0.2 mg/kg dose, but only in adolescent rats. Although not significant, the CPP induced by s.c. injected nicotine is somewhat higher than i.p. (B) The locomotor activity during the conditioning phase of CPP. Although not significant, rats injected with s.c. nicotine showed higher distance moved than those injected by i.p. The values are presented means and S.E.M. **p<0.01, ***p<0.001 statistically significant vs. the control group (Dunnet’s posttest).
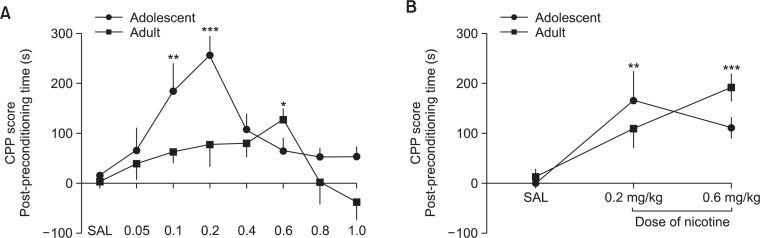
Fig. 2.
CPP dose determination. (A) Nicotine (s.c.) induced CPP in adolescent and adult rats across a wide dose range. Adolescent rats showed substantially higher CPP relevant to adult rats, particularly at 0.1 and 0.2 mg/kg nicotine. Adult rats also showed CPP at 0.6 mg/kg dose. (B) As confirmation, nicotine pretreated rats (0.5 mg/kg s.c., 7 days, 2 times a day) adolescent rats still showed higher nicotine CPP score at 0.2 mg/kg while pretreated adult rats at 0.6 mg/kg. The values are presented as means and S.E.M. *p<0.05, **p<0.01, ***p<0.001 statistically significant vs. the control group (Dunnet’s posttest).
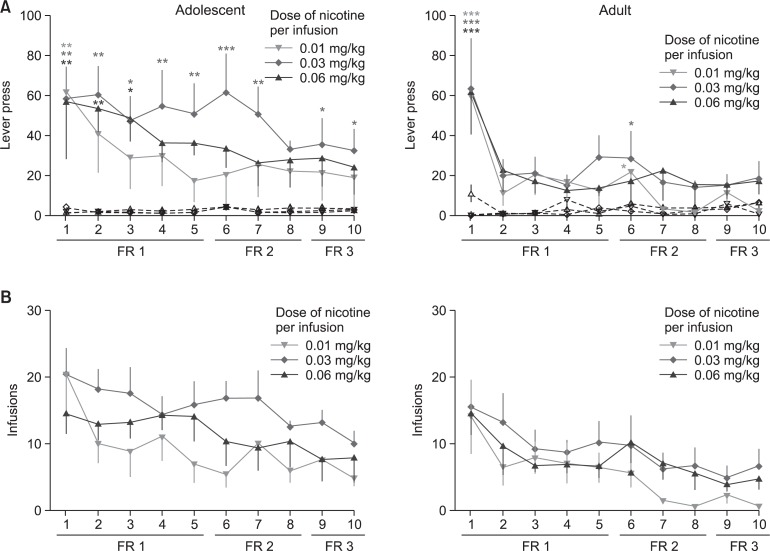
Fig. 3.
Self-administration data. Nicotine self-administration in adolescent and adult rats. (A) The number of lever responses made or (B) infusions obtained by adolescent and adult rats during the 2 h/day, 10-days nicotine SA sessions under the FR1, FR2, and FR3 schedules. Filled symbols indicate lever responses on the active lever while unfilled symbols show responses for the inactive lever. The values are presented means and S.E.M. *p<0.05, **p<0.01, ***p<0.001 statistically significant vs. the control group (Bonferroni’s posttest).
RESULTS
Route determination for the CPP test
Fig. 1 shows the behavior induced by, intraperitoneally or subcutaneously injected, nicotine in adolescent and adult rats. Although not significant, it can be gleaned from (Fig. 1A) that s.c. nicotine induced slightly higher CPP than i.p. nicotine. In addition, s.c. nicotine produced somewhat greater locomotor activity than i.p. injection (Fig. 1B).
Dose determination for the CPP test
Fig. 2A shows the CPP score produced by different dosages of nicotine (s.c.) in adolescent and adult rats. ANOVA showed that response to different doses of nicotine vary in adolescent [F(7, 58)=6.009, p<0.001] but not in adult rats [F (7, 56)=1.604, <0.05]. Among the dosages used, only 0.1 (q=3.941, p<0.01) and 0.2 mg/kg (q=5.225, p<0.001) produced significant nicotine CPP in adolescent and 0.6 mg/kg (q=2.754, p<0.05) in adult rats. Similar results were observed in pretreated rats, such that 0.2 mg/kg (q=3.281, p<0.01) of nicotine induced CPP in adolescent and 0.6 mg/kg (q=4.315, p<0.001) in adult rats.
Dose determination for the SA test
Fig. 3A shows the number of lever responses made by adolescent or adult rats during the 2 h/day, 10 day SA sessions under the FR1, FR2 and FR3 schedules. It can be observed that adolescent rats, especially those that are self-administering 0.03 mg/kg/infusion, significantly acquired and maintained nicotine self-administration. Under the FR1 schedule, two-way ANOVA showed significant difference in responses on the active and inactive levers [F (1, 70)=59.04, p<0.001]. This was also observed under the FR2 [F (1, 42)=32.64, p<0.001] and FR3 [F(1, 28)=14.31, p<0.001] schedules. No significant variation in days or lever response x day interaction.
Determination of blood nicotine levels
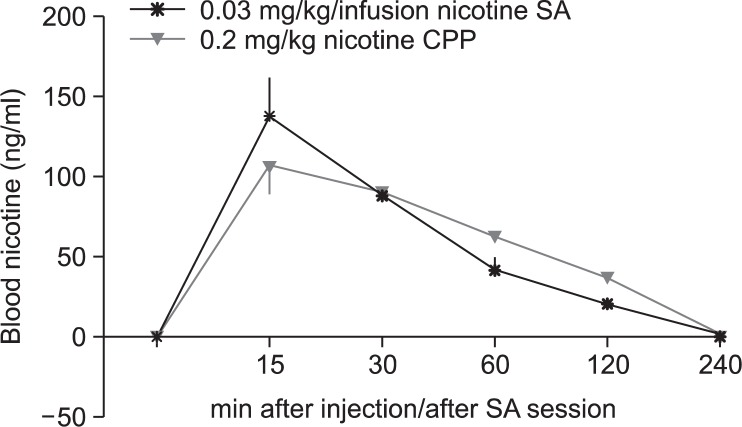
Fig. 4 shows the blood nicotine levels induced by dosages that produced nicotine CPP and SA. A single injection of 0.2 mg/kg nicotine s.c. or a session of 0.03 mg/kg/infusion nicotine SA (with a mean of 10 nicotine infusions) induced blood nicotine levels that correspond well with that of an average human smoker.
Fig. 4.
Blood nicotine level. Blood nicotine levels induced by dosages that produced nicotine CPP and SA. Blood nicotine levels in adolescent rats after single subcutaneous injection of 0.2 mg/kg of nicotine (CPP) or immediately after a 0.03 mg/kg/infusion nicotine SA session (mean of 10 infusions).
DISCUSSION
In the present we have demonstrated that nicotine can produce significant CPP, at dosages of 0.1 and 0.2 mg/kg (s.c.), in adolescent and 0.6 mg/kg (s.c.), in adult rats. These results were also observed in the 7 days pretreated rats. Nicotine also induced SA at 0.03 mg/kg/infusion dose, but only in adolescent rats.
For more than thirty years, CPP remains a common choice in evaluating the rewarding effects of drugs (van der Kooy, 1987; Bardo et al., 1995; Tzschentke, 1998). This might be because the test is comparatively simple and easy to perform. The CPP test measures a drug’s rewarding effect by analyzing it’s capability to produce a change in the animal’s behavior (Le Foll and Goldberg, 2005). Numerous studies have performed nicotine CPP; however, these studies greatly differ in methodology. Other studies have reported and tested factors that can influence the expression of nicotine CPP, such as the time of injection of the drug before putting the rats to the CPP apparatus. It was observed that rats tended to give affirmative response when nicotine is injected just before putting them in the apparatus (Papp et al., 2002; Le Foll and Goldberg, 2005). Accordingly, nicotine injection 20 minutes before the test would result in a weak reaction (Fudala and Iwamoto, 1986; Vastola et al., 2002; Le Foll and Goldberg, 2005). In addition, nicotine’s rewarding effects could be highly influenced by the conditioning method used (biased vs. unbiased). Most studies which used unbiased procedure have failed to observe nicotine CPP, which was attributed to the weak rewarding effects of nicotine (Clarke and Fibiger, 1987; Jorenby et al., 1990). A study by (Le Foll and Goldberg, 2005), have presented that the biased method is more efficient in tracing even minute behavioral changes caused by nicotine. These reports were considered in performing the CPP procedure in this study.
One important difference that has not yet been addressed by previous studies is the route of administration. Two of the most common routes used in previous nicotine CPP studies are subcutaneous (Vastola et al., 2002; Torres et al., 2008; Thiel et al., 2009; Natarajan et al., 2011) and intraperitoneal (Biala and Weglinska, 2004; Kota et al., 2007; Brunzell et al., 2009). The intraperitoneal route was commonly used in mice (Walters et al., 2006) while the subcutaneous route was usually used in rats (Vastola et al., 2002; Torres et al., 2008; Thiel et al., 2009; Natarajan et al., 2011). However, there are no definite reasons/explanations behind this practice. Although not significant, here we have observed that subcutaneously injected nicotine was better in inducing CPP and locomotor activation than intraperitoneal injection (Fig. 1). This observation might probably be due to pharmacokinetic differences, pharmacological effects of nicotine and absorption of the drug for both routes (O’Dell and Khroyan, 2009; Small et al., 2010). To our knowledge, this is the first study to directly compare the rewarding effects of nicotine in these two commonly used routes.
Another important factor that could influence the expression of CPP is the dosage of the drug. Several studies presented various dosages of nicotine believed to induce place preference. For instance, Torres and his colleagues (2008) described that at 0.4 and 0.6 mg/kg of nicotine could significantly stimulate place preference. Other evidences reported that an intermediate dose of nicotine (0.5–0.8 mg/kg) could initiate CPP response (Vastola et al., 2002; Shram et al., 2006). On the contrary, (Shoaib et al., 1994) stated that nicotine (0.06 mg/kg) failed to cause place preference. These varying results created puzzling information as to the definite nicotine dose which could significantly support place preference. In this study, we also tried to determine the nicotine dose that would effectively produce place preference by using a wide range of dose (0.05–1 mg/kg), based on dosages reported to induce CPP by previous studies (Donny et al., 1995; Shoaib et al., 1997; Levin et al., 2003; Le Foll and Goldberg, 2005). The subcutaneous route of administration was used. Results revealed that, in adolescents, 0.1 and 0.2 mg/kg dose of nicotine induced highly significant place preference. This result coincides with other published reports (Torres et al., 2008; Yararbas et al., 2010). On the other hand, adult rats showed place preference for nicotine but only in the higher dose of 0.6 mg/kg. This result is similar to that of (Vastola et al., 2002). However, when compared to adolescent rats, nicotine CPP in adult rats is noticeably lesser. These results highlight the disparity in response to nicotine between adolescent and adult rats, which will be further discussed below. To further confirm our CPP findings, rats were pretreated with nicotine for 7 days and then subsequent nicotine CPP was evaluated. This was based on the observation that CPP to psychostimulants are better expressed in pretreated rats (Schoffelmeer et al., 2002). Indeed, we have confirmed that the dose of 0.2 mg/kg is rewarding for adolescents while the 0.6 mg/kg is rewarding for adult rats.
Previous nicotine self-administration studies examined different nicotine doses and reported varying results. For instance, nicotine SA was observed at 0.03 and 0.06 mg/kg/infusion while negligible response was noticed at 0.003 mg/kg/infusion (Donny et al., 1995). Contrarily, another experiment failed to produce significant nicotine SA at 0.03 mg/kg/infusion (Levin et al., 2003). Hence, here we evaluated nicotine SA across a wide dose range (0.01 to 0.03 mg/kg/infusion). In line with the findings of (Donny et al., 1995), here we have demonstrated that at 0.03 mg/kg/infusion rats significantly acquired and maintained nicotine SA. However, this was only observed in adolescent rats; adult rats failed and to acquire significant nicotine SA. This result supports the growing evidence that adolescents are more responsive to the reinforcing effect of nicotine.
Epidemiological studies have raised concerns that young individuals are more likely to develop nicotine addiction/dependence (Prokhorov et al., 1996). These were further supported by preclinical studies reporting that adolescents are more sensitive to the addictive effects of nicotine, but not all have observed this effect (Shram et al., 2008). The present findings add to the growing evidence that adolescents are indeed more sensitive to the rewarding and reinforcing effects of nicotine. However, the exact neurobiological mechanism underlying this phenomenon is still unknown (Kota et al., 2007).
Doses that produce nicotine CPP and SA in the present study produced blood nicotine levels that correspond well with human smokers (Fig. 4). Blood nicotine levels of smokers usually range from 10–60 ng/ml (Rose et al., 1999; Lunell et al., 2000), with some reaching up to 100 ng/ml (Matta et al., 2007). Within this range, nicotine was shown to sufficiently increase dopamine in the brain’s reward system, consequently inducing reward and reinforcement (Brielmaier et al., 2012). This might explain the observed rewarding and reinforcing effects in these dosages.
In conclusion, these present findings extend and corroborate previous studies by demonstrating that nicotine exposure or pre-exposure lead to a steadfast CPP [0.2 mg/kg dose (s.c.)] in adolescent and [0.6 mg/kg dose (s.c.)] adult rats. Nicotine also supports reliable self-administration (0.03 mg/kg/infusion), but only in adolescents. These rewarding and reinforcing effects may contribute to addiction/dependence towards this drug. Moreover, the results highlight that adolescents respond more sensitively to nicotine’s addictive liability. Anyhow, these findings may serve as a promising basis for future experiments on nicotine addiction. Importantly, it encourages careful monitoring of nicotine and its related substances.
Acknowledgments
This study was supported by the Ministry of Food and Drug Safety of Korea (13182담배안759).
REFERENCES
- Bardo M, Rowlett J, Harris M. Conditioned place preference using opiate and stimulant drugs: a meta-analysis. Neurosci Biobehav Rev. 1995;19:39–51. doi: 10.1016/0149-7634(94)00021-r. [DOI] [PubMed] [Google Scholar]
- Benowitz NL. Nicotine addiction. N Engl J Med. 2010;362:2295–2303. doi: 10.1056/NEJMra0809890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL, Hukkanen J, Jacob P., 3rd Nicotine chemistry, metabolism, kinetics and biomarkers. Handb Exp Pharmacol. 2009:29–60. doi: 10.1007/978-3-540-69248-5_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biala G, Weglinska B. Calcium channel antagonists attenuate cross-sensitization to the rewarding and/or locomotor effects of nicotine, morphine and MK-801. J Pharm Pharmacol. 2004;56:1021–1028. doi: 10.1211/0022357043888. [DOI] [PubMed] [Google Scholar]
- Brielmaier J, McDonald CG, Smith RF. Effects of acute stress on acquisition of nicotine conditioned place preference in adolescent rats: a role for corticotropin-releasing factor 1 receptors. Psychopharmacology (Berl) 2012;219:73–82. doi: 10.1007/s00213-011-2378-1. [DOI] [PubMed] [Google Scholar]
- Brunzell DH, Mineur YS, Neve RL, Picciotto MR. Nucleus accumbens CREB activity is necessary for nicotine conditioned place preference. Neuropsychopharmacology. 2009;34:1993–2001. doi: 10.1038/npp.2009.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carboni E, Acquas E, Leone P, Di Chiara G. 5HT3 receptor antagonists block morphine-and nicotine-but not amphetamine-induced reward. Psychopharmacology (Berl) 1989;97:175–178. doi: 10.1007/BF00442245. [DOI] [PubMed] [Google Scholar]
- Clarke PB, Fibiger HC. Apparent absence of nicotine-induced conditioned place preference in rats. Psychopharmacology (Berl) 1987;92:84–88. doi: 10.1007/BF00215484. [DOI] [PubMed] [Google Scholar]
- Dani JA, De Biasi M. Cellular mechanisms of nicotine addiction. Pharmacol Biochem Behav. 2001;70:439–446. doi: 10.1016/s0091-3057(01)00652-9. [DOI] [PubMed] [Google Scholar]
- de la Peña JBI, Lee HC, de la Peña IC, Woo TS, Yoon SY, Lee HL, Han JS, Lee JI, Cho YJ, Shin CY, Cheong JH. Rewarding and reinforcing effects of the NMDA receptor anta gonist-benzodiazepine combination, zoletil®: Difference between acute and repeated exposure. Behav Brain Res. 2012;233:434–442. doi: 10.1016/j.bbr.2012.05.038. [DOI] [PubMed] [Google Scholar]
- de la Peña JB, dela Peña IJ, Lee HL, dela Peña I, Shin CY, Sohn AR, Cheong JH. Pre-exposure to ethanol, but not to caffeine and nicotine, induced place preference and self-administration of the NMDA receptor antagonist-benzodiazepine combination, Zoletil®. Pharmacol Biochem Behav. 2013a;110:231–237. doi: 10.1016/j.pbb.2013.07.016. [DOI] [PubMed] [Google Scholar]
- de la Peña JB, Yoon SY, de la Peña IC, Lee HL, I de la Peña IJ, Cheong JH. Pre-exposure to related substances induced place preference and self-administration of the NMDA receptor antagonist-benzodiazepine combination, zoletil. Behav Pharmacol. 2013b;24:20–28. doi: 10.1097/FBP.0b013e32835cf442. [DOI] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Knopf S, Brown C. Nicotine self-administration in rats. Psychopharmacology (Berl) 1995;122:390–394. doi: 10.1007/BF02246272. [DOI] [PubMed] [Google Scholar]
- Fudala PJ, Iwamoto ET. Further studies on nicotine-induced conditioned place preference in the rat. Pharmacol Biochem Behav. 1986;25:1041–1049. doi: 10.1016/0091-3057(86)90083-3. [DOI] [PubMed] [Google Scholar]
- Gilpin EA, Choi WS, Berry C, Pierce JP. How many adolescents start smoking each day in the United States? J Adolesc Health. 1999;25:248–255. doi: 10.1016/s1054-139x(99)00024-5. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Jain A. Extracts from “BestTreatments”: Treating nicotine addiction. BMJ. 2003;327:1394. doi: 10.1136/bmj.327.7428.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorenby DE, Steinpreis RE, Sherman JE, Baker TB. Aversion instead of preference learning indicated by nicotine place conditioning in rats. Psychopharmacology (Berl) 1990;101:533–538. doi: 10.1007/BF02244233. [DOI] [PubMed] [Google Scholar]
- Kota D, Martin BR, Robinson SE, Damaj MI. Nicotine dependence and reward differ between adolescent and adult male mice. J Pharmacol Exp Ther. 2007;322:399–407. doi: 10.1124/jpet.107.121616. [DOI] [PubMed] [Google Scholar]
- Lantz PM. Smoking on the rise among young adults: implications for research and policy. Tob Control. 2003;12:i60–i70. doi: 10.1136/tc.12.suppl_1.i60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Foll B, Goldberg SR. Nicotine induces conditioned place preferences over a large range of doses in rats. Psychopharmacology (Berl) 2005;178:481–492. doi: 10.1007/s00213-004-2021-5. [DOI] [PubMed] [Google Scholar]
- Le Houezec J. Role of nicotine pharmacokinetics in nicotine addiction and nicotine replacement therapy: a review. Int J Tuberc Lung Dis. 2003;7:811–819. [PubMed] [Google Scholar]
- Levin ED, Rezvani AH, Montoya D, Rose JE, Swartz-welder HS. Adolescent-onset nicotine self-administration modeled in female rats. Psychopharmacology (Berl) 2003;169:141–149. doi: 10.1007/s00213-003-1486-y. [DOI] [PubMed] [Google Scholar]
- Lunell E, Molander L, Ekberg K, Wahren J. Site of nicotine absorption from a vapour inhaler-comparison with cigarette smoking. Eur J Clin Pharmacol. 2000;55:737–741. doi: 10.1007/s002280050007. [DOI] [PubMed] [Google Scholar]
- Matta SG, Balfour DJ, Benowitz NL, Boyd RT, Buccafusco JJ, Caggiula AR, Craig CR, Collins AC, Damaj MI, Donny EC, Gardiner PS, Grady SR, Heberlein U, Leonard SS, Levin ED, Lukas RJ, Markou A, Marks MJ, McCallum SE, Parameswaran N, Perkins KA, Picciotto MR, Quik M, Rose JE, Rothenfluh A, Schafer WR, Stolerman IP, Tyndale RF, Wehner JM, Zirger JM. Guidelines on nicotine dose selection for in vivo research. Psychopharmacology (Berl) 2007;190:269–319. doi: 10.1007/s00213-006-0441-0. [DOI] [PubMed] [Google Scholar]
- Meyer JS, Quenzer LF. Psychopharmacology: Drugs, the brain, and behavior. Sinauer Associates, Inc. Publishers; Sunderland, Massachusetts USA: 2005. [Google Scholar]
- Natarajan R, Wright JW, Harding JW. Nicotine-induced conditioned place preference in adolescent rats. Pharmacol Biochem Behav. 2011;99:519–523. doi: 10.1016/j.pbb.2011.05.004. [DOI] [PubMed] [Google Scholar]
- O’Dell LE, Khroyan TV. Rodent models of nicotine reward: what do they tell us about tobacco abuse in humans? Pharmacol Biochem Behav. 2009;91:481–488. doi: 10.1016/j.pbb.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp M, Gruca P, Willner P. Selective blockade of drug-induced place preference conditioning by ACPC, a functional NDMA-receptor antagonist. Neuropsychopharmacology. 2002;27:727–743. doi: 10.1016/S0893-133X(02)00349-4. [DOI] [PubMed] [Google Scholar]
- Prokhorov AV, Pallonen UE, Fava JL, Ding L, Niaura R. Measuring nicotine dependence among high-risk adolescent smokers. Addict Behav. 1996;21:117–127. doi: 10.1016/0306-4603(96)00048-2. [DOI] [PubMed] [Google Scholar]
- Rogge WF, Hildemann LM, Mazurek MA, Cass GR, Simoneit BR. Sources of fine organic aerosol. 6. Cigaret smoke in the urban atmosphere. Environ Sci Technol. 1994;28:1375–1388. doi: 10.1021/es00056a030. [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm FM, Westman EC, Coleman RE. Arterial nicotine kinetics during cigarette smoking and intravenous nicotine administration: implications for addiction. Drug Alcohol Depend. 1999;56:99–107. doi: 10.1016/s0376-8716(99)00025-3. [DOI] [PubMed] [Google Scholar]
- Schoffelmeer AN, De Vries TJ, Wardeh G, van de Ven HW, Vanderschuren LJ. Psychostimulant-induced behavioral sensitization depends on nicotinic receptor activation. J Neurosci. 2002;22:3269–3276. doi: 10.1523/JNEUROSCI.22-08-03269.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman JI, Fournier JA, Paine JB, 3rd, Waymack BE. The form of nicotine in tobacco. Thermal transfer of nicotine and nicotine acid salts to nicotine in the gas phase. J Agric Food Chem. 1999;47:5133–5145. doi: 10.1021/jf990409b. [DOI] [PubMed] [Google Scholar]
- Shoaib M, Schindler CW, Goldberg SR. Nicotine self-administration in rats: strain and nicotine pre-exposure effects on acquisition. Psychopharmacology (Berl) 1997;129:35–43. doi: 10.1007/s002130050159. [DOI] [PubMed] [Google Scholar]
- Shoaib M, Stolerman IP, Kumar RC. Nicotine-induced place preferences following prior nicotine exposure in rats. Psychopharmacology (Berl) 1994;113:445–452. doi: 10.1007/BF02245221. [DOI] [PubMed] [Google Scholar]
- Shram MJ, Funk D, Li Z, Le AD. Periadolescent and adult rats respond differently in tests measuring the rewarding and aversive effects of nicotine. Psychopharmacology (Berl) 2006;186:201–208. doi: 10.1007/s00213-006-0373-8. [DOI] [PubMed] [Google Scholar]
- Shram MJ, Funk D, Li Z, Le AD. Nicotine self-administration, extinction responding and reinstatement in adolescent and adult male rats: evidence against a biological vulnerability to nicotine addiction during adolescence. Neuropsychopharmacology. 2008;33:739–748. doi: 10.1038/sj.npp.1301454. [DOI] [PubMed] [Google Scholar]
- Shram MJ, Le AD. Adolescent male Wistar rats are more responsive than adult rats to the conditioned rewarding effects of intravenously administered nicotine in the place conditioning procedure. Behav Brain Res. 2010;206:240–244. doi: 10.1016/j.bbr.2009.09.018. [DOI] [PubMed] [Google Scholar]
- Small E, Shah HP, Davenport JJ, Geier JE, Yavarovich KR, Yamada H, Sabarinath SN, Derendorf H, Pauly JR, Gold MS, Bruijnzeel AW. Tobacco smoke exposure induces nicotine dependence in rats. Psychopharmacology (Berl) 2010;208:143–158. doi: 10.1007/s00213-009-1716-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton WR, McClelland M, Elwood C, Ferry D, Silva PA. Prevalence, reliability and bias of adolescents’ reports of smoking and quitting. Addiction. 1996;91:1705–1714. [PubMed] [Google Scholar]
- Thiel KJ, Sanabria F, Neisewander JL. Synergistic interaction between nicotine and social rewards in adolescent male rats. Psychopharmacology (Berl) 2009;204:391–402. doi: 10.1007/s00213-009-1470-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres OV, Tejeda HA, Natividad LA, O’Dell LE. Enhanced vulnerability to the rewarding effects of nicotine during the adolescent period of development. Pharmacol Biochem Behav. 2008;90:658–663. doi: 10.1016/j.pbb.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Prog Neurobiol. 1998;56:613–672. doi: 10.1016/s0301-0082(98)00060-4. [DOI] [PubMed] [Google Scholar]
- van der Kooy D. Place conditioning: a simple and effective method for assessing the motivational properties of drugs. In: Bozarth Michael A., editor. In Methods of assessing the reinforcing properties of abused drugs. Springer; New York: 1987. pp. 229–240. [Google Scholar]
- Vastola BJ, Douglas LA, Varlinskaya EI, Spear LP. Nicotine-induced conditioned place preference in adolescent and adult rats. Physiol Behav. 2002;77:107–114. doi: 10.1016/s0031-9384(02)00818-1. [DOI] [PubMed] [Google Scholar]
- Walters CL, Brown S, Changeux JP, Martin B, Damaj MI. The β2 but not α7 subunit of the nicotinic acetylcholine receptor is required for nicotine-conditioned place preference in mice. Psychopharmacology (Berl) 2006;184:339–344. doi: 10.1007/s00213-005-0295-x. [DOI] [PubMed] [Google Scholar]
- World Health Organization . Report on the global tobacco epidemic. WHO Press, World Health Organization; Geneva, Switzerland: 2013. [Google Scholar]
- Yararbas G, Keser A, Kanit L, Pogun S. Nicotine-induced conditioned place preference in rats: sex differences and the role of mGluR5 receptors. Neuropharmacology. 2010;58:374–382. doi: 10.1016/j.neuropharm.2009.10.001. [DOI] [PubMed] [Google Scholar]