Abstract
Prostate cancer is the most frequently diagnosed cancer. Although prostate tumors respond to androgen ablation therapy at an early stage, they often acquire the potential of androgen-independent growth. Elevated transcriptional activity of androgen receptor (AR) and/or signal transducer and activator of transcription-3 (STAT3) contributes to the proliferation of prostate cancer cells. In the present study, we examined the effect of resveratrol, a phytoalexin present in grapes, on the reporter gene activity of AR and STAT3 in human prostate cancer (LNCaP-FGC) cells stimulated with interleukin-6 (IL-6) and/or dihydrotestosterone (DHT). Our study revealed that resveratrol suppressed the growth of LNCaP-FGC cells in a time- and concentration-dependent manner. Whereas the AR transcriptional activity was induced by treatment with either IL-6 or DHT, the STAT3 transcriptional activity was induced only by treatment with IL-6 but not with DHT. Resveratrol significantly attenuated IL-6-induced STAT3 transcriptional activity, and DHT- or IL-6-induced AR transcriptional activity. Treatment of cells with DHT plus IL-6 significantly increased the AR transcriptional activity as compared to DHT or IL-6 treatment alone and resveratrol markedly diminished DHT plus IL-6-induced AR transcriptional activity. Furthermore, the production of prostate-specific antigen (PSA) was decreased by resveratrol in the DHT-, IL-6- or DHT plus IL-6-treated LNCaP-FGC cells. Taken together, the inhibitory effects of resveratrol on IL-6- and/or DHT-induced AR transcriptional activity in LNCaP prostate cancer cells are partly mediated through the suppression of STAT3 reporter gene activity, suggesting that resveratrol may be a promising therapeutic choice for the treatment of prostate cancer.
Keywords: Resveratrol, AR transcriptional activity, STAT3 transcriptional activity, Prostate cancer
INTRODUCTION
Prostate cancer is the most frequently diagnosed cancer among the American men and is the second largest cause of cancer-related mortality in the United States (Siegel et al., 2014). While the radical prostatectomy and radiotherapy are the potential therapies for localized prostate tumors, success of these therapeutic modalities in malignant prostate cancer is poor (Ratan et al., 2002). Although prostate tumors respond to hormone ablation therapy at early stage, they often acquire the potential of hormone-independent growth (Laufer et al., 2000; Walsh et al., 2007; Bommareddy et al., 2013).
One of the most exciting strategies to reduce the burden of prostate cancer is the use of chemopreventive agents (Ratan et al., 2002; Bommareddy et al., 2013; Wang et al., 2013). Resveratrol (3,5,4′-trihydroxy-trans-stilbene), a phytoalexin ab undantly present in grapes and other medicinal plants, has been extensively investigated for its chemopreventive and chemotherapeutic potential (Shankar et al., 2007; Kundu and Surh, 2008). Previous studies have shown that resveratrol inhibits the androgen-dependent or -independent growth of prostate cancer cells (Mitchell et al., 1999; Harada et al., 2007). Kotha et al. reported that resveratrol induced apoptosis in human prostate cancer (DU145) cells by blocking STAT3 signaling pathway (Kotha et al., 2006). Others have shown that resveratrol attenuates the proliferation of LNCaP prostate cancer cells by downregulating AR protein expression following transcriptional (Mitchell et al., 1999) or post-translational (Harada et al., 2007) mechanisms. However, a molecular link between the inhibitory effect of resveratrol on STAT3 signaling and the downregulation of AR has not been established. Since the cytokine interleukin-6 (IL-6) promotes tumor cell proliferation by activating STAT3 signaling (Guo et al., 2012), we sought to examine whether resveratrol can attenuate IL-6-induced STAT3 transcriptional activity in LNCaP-FGC cells. Moreover, the role of IL-6 in AR activation has not been reported yet. We, therefore, examined the IL-6, in presence or absence of AR ligand dihydrotestosterone (DHT), in inducing the AR transcriptional activity, and its possible modulation by resveratrol in LNCaP-FGC cells.
MATERIALS AND METHODS
Chemicals and reagents
Resveratrol (purity >99%), dimethylsulfoxide and MTT [3-(4, 5-dimethyl-thiazolyl-2)-2, 5-diphephenyl-tetrazolium bromide] were procured from Sigma (St. Louis, MO, USA). IL-6 and DHT were purchased from R&D systems (Minneapolis, MN, USA) and TCI (Tokyo, Japan), respectively. RPMI-1640 and fetal bovine serum (FBS) were purchased from Invitrogen (GIBCO, Grand Island, NY, USA).
Cell culture
LNCaP-FGC (human prostate cancer) was purchased from American Type Culture Collection (ATCC, Manassas, VA, USA) and were cytogenetically tested and authenticated before the cells were frozen. Each vial of frozen cells was thawed and maintained in culture for a maximum of 8 weeks. LNCaPFGC cells were cultured in RPMI 1640 containing penicillin (100 units/mL), streptomycin (100 μg/mL), and 10% FBS or Dextran charcoal coated FBS (DCC-FBS, Clontech). Cells were maintained at 37°C in a 5% CO2 incubator.
Cell proliferation assay
LNCaP-FGC cells (5,000 cells per well) were plated in 96-well plates and treatment were started after 24 h. Cells were treated with 0, 12.5, 25, 50 or 100 μM resveratrol (dimethylsulfoxide as a vehicle) for 12, 24, 48, 72 h. At the time point of the incubation, 10 μL of MTT (5 mg/mL) was added to each well. After 2 h of incubation, 100 μl of DMSO was added and the optical density (O.D.) was read with Microplate reader (Molecular Devices, USA) at 570 nm.
Luciferase-reporter assay
LNCaP-FGC cells permanently transfected with AR-luc or STAT3-luc constructs were seeded at 20,000 cells in each well of a 96-well plate in RPMI containing 3% DCC-FBS. After 24 h incubation, cells were treated with IL-6, DHT, resveratrol separately or their combination for 12 h. The supernatant was discarded and passive lysis buffer was added and incubated for 10 min in orbital shaker. The Luciferase activity was measured by luminometer (Berthold corp.).
Human Prostate Specific Antigen (PSA) ELISA assay
For quantitative determination of human PSA in culture supernatant, human PSA ELISA kit (Alpha Diagnostic International, San Antonio, Texas, USA) was used according to the manufacturer’s instructions. In brief, before addition of the samples, strip wells were washed with 200 μl of 1X wash buffer and then 25 μl of standards, control, and serum samples were added into wells. Then, 100 μl of Ab-enzyme conjugate was given into each well followed by gentle mixing for 10 seconds and incubation for 30 min at room temperature. Each well was aspirated and washed 3 times with 300 μl of 1X wash buffer. TMB substrate (100 μl per well) was added and mixed gently. Plates were then incubated for 15 min at room temperature and the reaction was stopped by adding 50 μl of stopping solution. Upon gentle mixing the absorbance was measured at 450 nm using a microplate reader.
Statistical analysis
Values were expressed as the mean ± S.E.M. of at least three independent experiments. Statistical significance was determined by Student’s t-test and a p-value of less than 0.05 was considered to be statistically significant.
RESULTS
Effects of resveratrol on LNCaP-FGC cell growth
Resveratrol has been reported to inhibit the growth of various cancer cells. In the present study, we attempted to examine the effects of resveratrol on the growth of LNCaP-FGC human prostate cancer cells. Treatment of these cells with resveratrol (12.5, 25, 50 or 100 μM) resulted in decreased cell viability in a concentration- and time-dependent fashion (Fig. 1).
Fig. 1.
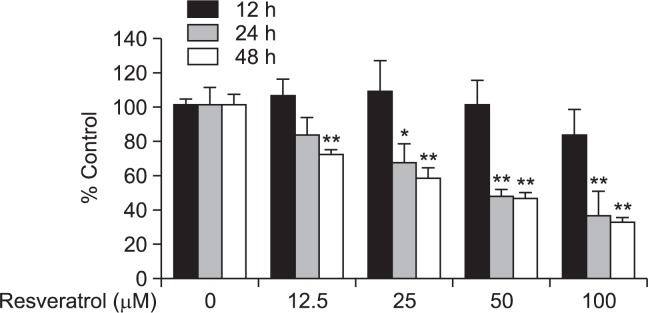
Effect of resveratrol on the viability of LNCaP-FGC cells. Cells were treated with resveratrol (12.5, 25, 50 or 100 μM) for 24, 48, or 72 h and cell viability was analyzed using the MTT assay. The asterisk(s) indicate a significant (*p<0.05; **p<0.01) inhibitory effect of resveratrol on cancer cell proliferation.
Resveratrol inhibited DHT-induced AR transcriptional activity in LNCaP-FGC cells
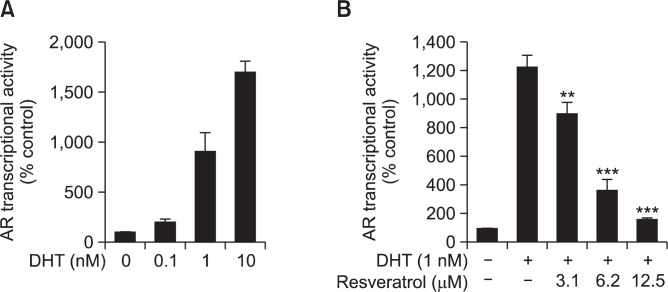
Since resveratrol suppressed the growth of LNCaP-FGC prostate cancer cells, we sought to examine whether resveratrol can suppress the transcriptional activity of AR in LNCaP cells. We transfected LNCaP-FGC cells with luciferase reporter gene construct containing AR response element and cells were then stimulated with dihydrotestosterone (DHT) (Fig. 2A). We optimized the concentration of DHT to 1 nM. Treatment with DHT (1 nM) induced the AR transcriptional activity, which was attenuated by incubation of cells with resveratrol (Fig. 2B). The transcriptional activity of AR was assessed by corresponding luciferase reporter gene analysis.
Fig. 2.
Effects of resveratrol on DHT-induced androgen receptor transcriptional activity. (A) LNCaP-FGC cells stably transfected with AR-luc vector were incubated with DHT (0.1, 1 or 10 nM) and the AR transcriptional activity was measured as described in Materials and methods. (B) Cells stably were transfected with reporter genes harboring the AR binding site. Cells were treated for 12 h with the indicated concentrations of resveratrol and the luciferase activity was measured. The transcriptional activity of AR was assessed by corresponding luciferase reporter gene analysis. The asterisk(s) indicate a significant (**p<0.01; ***p<0.001) inhibitory effect of resveratrol on AR transcriptional activity.
Effect of resveratrol on IL-6-induced STAT3 transcriptional activity in LNCaP-FGC cells
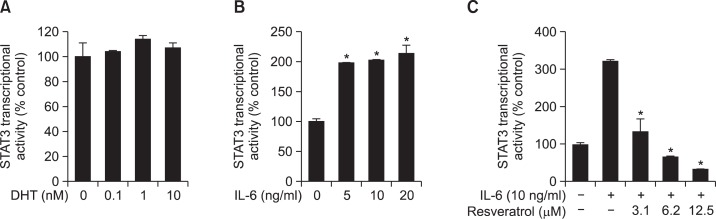
We next examined the effects of resveratrol on the transcriptional activity of STAT3, which is known to augment prostate cancer cell proliferation. While treatment of cells with DHT failed to induce STAT3 luciferase activity in LNCaP-FGC cells stably transfected with a STAT3-luc construct (Fig. 3A), stimulation with IL-6 caused significant induction of STAT3 transcriptional activity (Fig. 3B), which was attenuated by incubation of cells with resveratrol (Fig. 3C).
Fig. 3.
Effects of resveratrol on the STAT3 transcriptional activity. Cells stably transfected with reporter genes harboring the STAT3 binding sequences were treated for 12 h with the indicated concentration of DHT, IL-6 or resveratrol. (A) DHT-induced STAT3 transcriptional activity. (B) IL-6-induced STAT3 transcriptional activity. The asterisk(s) indicate a significant (*p<0.01) induction of the STAT3 transcriptional activity in response to IL-6 treatment. (C) Cells transfected with STAT3 reporter gene construct were treated with resveratrol in presence or absence of IL-6 and the STAT3 reporter gene activity assay was performed. The asterisk(s) indicate a significant (*p<0.01) inhibitory effect of resveratrol on STAT3 transcriptional activity.
Effect of resveratrol on DHT, IL-6 or DHT plus IL-6 induced AR transcriptional activity in LNCaP-FGC prostate cancer cells
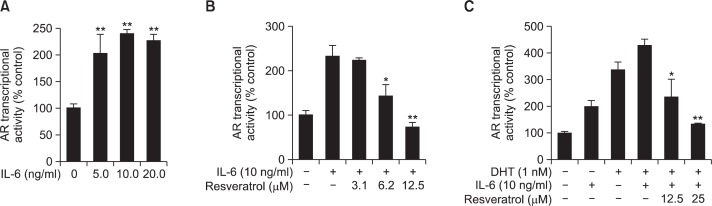
To investigate whether IL-6 activates the AR transcriptional activity, we incubated LNCaP-FGC cells stably transfected with AR-luc constructs with different concentrations of IL-6. As shown in Fig. 4A, incubation of cells with IL-6 induced the AR transcriptional activity. Treatment with resveratrol inhibited IL-6-induced AR transcriptional activity (Fig. 4B). Since DHT is a known inducer of the AR activity, we treated cells with IL-6 in presence or absence of DHT. Combined treatment with IL-6 and DHT showed synergistic increase in the AR transcriptional activity as compared to treatment of cells with IL-6 or DHT alone (Fig. 4C). Moreover, resveratrol treatment attenuated the AR transcriptional activity in cells stimulated DHT plus IL-6 (Fig. 4C).
Fig. 4.
Effects of resveratrol on the DHT or IL-6- or IL-6 plus DHT-induced AR transcriptional activity. (A) The AR transcriptional activity was induced by treatment of cells harboring AR binding site with indicated concentrations of IL-6. The asterisk(s) indicate a significant (**p<0.01) induction of IL-6 on AR transcriptional activity. (B) Inhibitory effect of resveratrol on IL-6-induced AR transcriptional activity in LNCaP-FGC cells. (C) Inhibitory effect of resveratrol on IL-6 plus DHT-induced AR transcriptional activity in LNCaP-FGC cells. The asterisk(s) indicate a significant (*p<0.05; **p<0.01) inhibitory effect of resveratrol on the AR transcriptional activity.
Effect of resveratrol on DHT, IL-6 or DHT plus IL-6 induced PSA expression in LNCaP-FGC cells
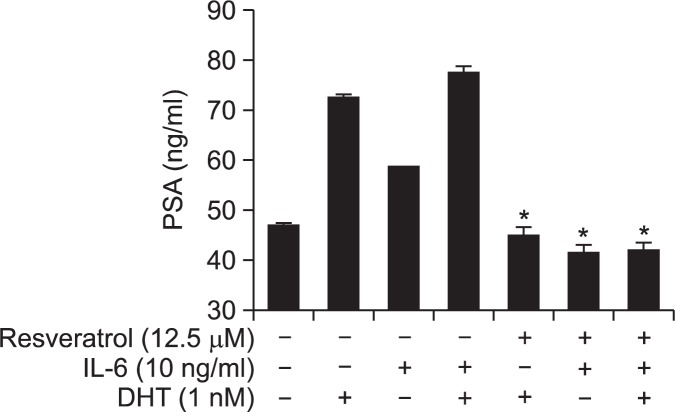
Since treatment of LNCaP-FGC cells with DHT, IL-6 or DHT plus IL-6 showed elevated AR transcriptional activity, we examined whether stimulation of cells with androgen or IL-6 or their combination can affect PSA production and whether resveratrol can modulate that. The PSA ELISA assay revealed that treatment of cells with either DHT or IL-6 or their combination increased the PSA level and that resveratrol significantly attenuated PSA production in LNCaP cells stimulated with DHT and/or IL-6 (Fig. 5).
Fig. 5.
Effects of resveratrol on PSA production. LNCaP-FGC prostate cancer cells were incubated with resveratrol with or without IL-6 and DHT and the PSA level was measured by using PSA-ELISA kit as described in Materials and methods. The asterisk(s) indicate a significant (*p<0.01) inhibitory effect of resveratrol on PSA production.
DISCUSSION
The anticancer effects of resveratrol have been extensively invested. Till to date, multidirectional studies attempting to unravel the biochemical basis of chemoprevention and chemotherapy with resveratrol have revealed that the compound (i) prevents the activation of various carcinogens, (ii) protects cellular macromolecules from oxidative damage, (iii) activates the carcinogen detoxification pathways, (iv) attenuates inflammatory responses, (v) inhibits proliferation of cancer cells, (vi) induces apoptosis selectively in cancer cells, (vii) impedes the angiogenic and metastatic progression, and (viii) enhances the sensitivity of cancer cells to conventional chemotherapy and radiotherapy (Kundu and Surh, 2008). Previous studies have demonstrated that resveratrol inhibits the proliferation and induces apoptosis in various prostate cancer cells (Mitchell et al., 1999; Narayanan et al., 2003; Harada et al., 2007). Resveratrol attenuated the growth of prostate tumors in a transgenic rat adenocarcinoma of prostate (TRAMP) model. It has been shown that the antiproliferative and apoptosis inducing effect of resveratrol in prostate cancer cells are mediated through multiple mechanisms including the activation of p53-mdiated expression of apoptotic gene products (Narayanan et al., 2003), inhibition of STAT3 signaling pathway (Kotha et al., 2006) and the downregulation of the expression and activity of AR (Shi et al., 2009; Harada et al., 2011). However, the effects of resveratrol on androgen receptor positive LNCaP-FGC prostate cancer cells have not been reported yet. Although the activation of both STAT3- and AR-mediated signaling pathways have been implicated in prostate carcinogenesis (Kotha et al., 2006; Shi et al., 2009), a possible cross-talk between these two signaling cascades are yet to be established. Moreover, the effect of IL-6, a cytokine that stimulates STAT3 activation, on the AR activity remains elusive. Since IL-6 plays a key role in various cancers, we sought to examine whether IL-6 stimulation can lead to increased AR transcriptional activity and its modulation by resveratrol.
Our finding that resveratrol reduced the cell viability of LNCaP-FGC cells in a concentration- and time-dependent manner is well correlated with the antiproliferative effects of this compound on other prostate cancer cells (Mitchell et al., 1999; Narayanan et al., 2003; Harada et al., 2007). Also the inhibitory effect of resveratrol on DHT-induced AR transcriptional activity in LNCaP-FGC cells is in good agreement with the previous studies reporting the suppression of AR expression and activity by this compound in PC3 and DU145 prostate cancer cells (Kai and Levenson, 2011). Harada et al. demonstrated that resveratrol inhibited AR binding to PSA promoter by decreasing the acetylation and nuclear translocation of AR (Harada et al., 2011).
Kotha et al. have reported that resveratrol inhibited STAT3 activation in DU145 prostate cancer cells. In accordance with this report, we have also found that resveratrol treatment attenuated IL-6-induced STAT3 transcriptional activity in LNCaP-FGC cells. Since androgen stimulation plays a decisive role in prostate carcinogenesis, we examined whether stimulation of LNCaP-FGC cells with DHT can induce the STAT3 activity. However, we noticed that DHT treatment failed to induce the STAT3 transcriptional activity in LNCaP-FGC cells. As the induction of the AR transcriptional activity is a key molecular event in prostate carcinogenesis, we examined whether stimulation of LNCaP-FGC cells with IL-6 can induce the AR activity. Our study revealed for the first time that stimulation of LNCaP-FGC cells with IL-6 markedly induced the AR transcriptional activity and that resveratrol attenuated IL-6-induced AR activity. Furthermore, incubation of cells with a combination of IL-6 and DHT synergistically induced the AR activity at a level higher than that observed upon treatment of cells with DHT or IL-6 alone. Resveratrol not only inhibited DHT- or IL-6-induced AR activity but also markedly diminished the transcriptional activation of AR and the production of PSA in cells stimulated with DHT plus IL-6.
It was reported that IL-6 stimulation LNCaP cells increased the level of androgen (Chun et al., 2009), which may contribute to the IL-6-induced AR activation. While IL-6 treatment of cancer cells (LNCaP and PC3) increased the expression of a serine-threonine kinase Pim1, the overexpression of Pim1 induced the AR transcriptional activity (van der Poel et al., 2010). Moreover, Pim1 was shown to phosphorylate AR at serine-213 residue, thereby allowing prostate cancer cells to be more aggressive and refractory to androgen ablation therapy (Ha et al., 2013). Thus, activation of Pim1 may underlie the IL-6-induced AR transcriptional activity in LNCaP-FGC cells. It would be worthwhile to examine if resveratrol could inhibit the activation of Pim1 in LNCaP cells. Since the expression of Pim1 is transcriptionally regulated by STAT3 (Zemskova et al., 2008), it is plausible that IL-6-mediated STAT3 activation may lead to the Pim1 expression, which in turn can induce the AR activity. However, the detailed mechanisms underlying IL-6-induced AR activation and the molecular basis of a possible cross-talk between STAT3 and AR signaling merit further investigation. Taken together, our study provides the new insight into the role of IL-6 in AR activation in prostate cancer cells and its inhibition by resveratrol, indicating a new molecular aspect of its prostate cancer chemopreventive effects.
Acknowledgments
The work was supported by grants from Bio-Theme Cluster program of Korea Industrial Complex corp (KICOX) [2013055-1]. We thank to Hyung-Jo Moon, Mi-Sun Kim, Il-Hyun Han for supporting experiments, C&C Research Labs, Hwasung City, Gyeonggi-do 445-970.
REFERENCES
- Bommareddy A, Eggleston W, Prelewicz S, Antal A, Witczak Z, McCune DF, Vanwert AL. Chemoprevention of prostate cancer by major dietary phytochemicals. Anticancer Res. 2013;33:4163–4174. [PubMed] [Google Scholar]
- Chun JY, Nadiminty N, Dutt S, Lou W, Yang JC, Kung HJ, Evans CP, Gao AC. Interleukin-6 regulates androgen synthesis in prostate cancer cells. Clin Cancer Res. 2009;15:4815–4822. doi: 10.1158/1078-0432.CCR-09-0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Xu F, Lu T, Duan Z, Zhang Z. Interleukin-6 signaling pathway in targeted therapy for cancer. Cancer Treat Rev. 2012;38:904–910. doi: 10.1016/j.ctrv.2012.04.007. [DOI] [PubMed] [Google Scholar]
- Ha S, Iqbal NJ, Mita P, Ruoff R, Gerald WL, Lepor H, Taneja SS, Lee P, Melamed J, Garabedian MJ, Logan SK. Phosphorylation of the androgen receptor by PIM1 in hormone refractory prostate cancer. Oncogene. 2013;32:3992–4000. doi: 10.1038/onc.2012.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada N, Atarashi K, Murata Y, Yamaji R, Nakano Y, Inui H. Inhibitory mechanisms of the transcriptional activity of androgen receptor by resveratrol: Implication of DNA binding and acetylation of the receptor. J Steroid Biochem Mol Biol. 2011;123:65–70. doi: 10.1016/j.jsbmb.2010.11.002. [DOI] [PubMed] [Google Scholar]
- Harada N, Murata Y, Yamaji R, Miura T, Inui H, Nakano Y. Resveratrol down-regulates the androgen receptor at the post-translational level in prostate cancer cells. J. Nutr. Sci. Vitaminol. (Tokyo) 2007;53:556–560. doi: 10.3177/jnsv.53.556. [DOI] [PubMed] [Google Scholar]
- Kai L, Levenson AS. Combination of resveratrol and antiandrogen flutamide has synergistic effect on androgen receptor inhibition in prostate cancer cells. Anticancer Res. 2011;31:3323–3330. [PubMed] [Google Scholar]
- Kotha A, Sekharam M, Cilenti L, Siddiquee K, Khaled A, Zervos AS, Carter B, Turkson J, Jove R. Resveratrol inhibits Src and Stat3 signaling and induces the apoptosis of malignant cells containing activated Stat3 protein. Mol Cancer Ther. 2006;5:621–629. doi: 10.1158/1535-7163.MCT-05-0268. [DOI] [PubMed] [Google Scholar]
- Kundu JK, Surh YJ. Cancer chemopreventive and therapeutic potential of resveratrol: mechanistic perspectives. Cancer Lett. 2008;269:243–261. doi: 10.1016/j.canlet.2008.03.057. [DOI] [PubMed] [Google Scholar]
- Laufer M, Denmeade SR, Sinibaldi VJ, Carducci MA, Eisenberger MA. Complete androgen blockade for prostate cancer: what went wrong? J Urol. 2000;164:3–9. [PubMed] [Google Scholar]
- Mitchell SH, Zhu W, Young CY. Resveratrol inhibits the expression and function of the androgen receptor in LNCaP prostate cancer cells. Cancer Res. 1999;59:5892–5895. [PubMed] [Google Scholar]
- Narayanan BA, Narayanan NK, Re GG, Nixon DW. Differential expression of genes induced by resveratrol in LNCaP cells: P53-mediated molecular targets. Int. J. Cancer. 2003;104:204–212. doi: 10.1002/ijc.10932. [DOI] [PubMed] [Google Scholar]
- Ratan HL, Steward WP, Gescher AJ, Mellon JK. Resveratrol--a prostate cancer chemopreventive agent? Urol Oncol. 2002;7:223–227. doi: 10.1016/s1078-1439(02)00194-1. [DOI] [PubMed] [Google Scholar]
- Shankar S, Singh G, Srivastava RK. Chemoprevention by resveratrol: molecular mechanisms and therapeutic potential. Front Biosci. 2007;12:4839–4854. doi: 10.2741/2432. [DOI] [PubMed] [Google Scholar]
- Shi WF, Leong M, Cho E, Farrell J, Chen HC, Tian J, Zhang D. Repressive effects of resveratrol on androgen receptor transcriptional activity. PLoS One. 2009;4:e7398. doi: 10.1371/journal.pone.0007398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- van der Poel HG, Zevenhoven J, Bergman AM. Pim1 regulates androgen-dependent survival signaling in prostate cancer cells. Urol Int. 2010;84:212–220. doi: 10.1159/000277601. [DOI] [PubMed] [Google Scholar]
- Walsh PC, DeWeese TL, Eisenberger MA. Clinical practice. Localized prostate cancer. N Engl J Med. 2007;357:2696–2705. doi: 10.1056/NEJMcp0706784. [DOI] [PubMed] [Google Scholar]
- Wang Z, Fan J, Liu M, Yeung S, Chang A, Chow MS, Pon D, Huang Y. Nutraceuticals for prostate cancer chemoprevention: from molecular mechanisms to clinical application. Expert Opin. Investig. Drugs. 2013;22:1613–1626. doi: 10.1517/13543784.2013.833183. [DOI] [PubMed] [Google Scholar]
- Zemskova M, Sahakian E, Bashkirova S, Lilly M. The PIM1 kinase is a critical component of a survival pathway activated by docetaxel and promotes survival of docetaxel-treated prostate cancer cells. J Biol Chem. 2008;283:20635–20644. doi: 10.1074/jbc.M709479200. [DOI] [PMC free article] [PubMed] [Google Scholar]