Abstract
Alpha synuclein (αsyn) fibrils are found in the Lewy Bodies of patients with Parkinson’s disease (PD). The aggregation of the αsyn monomer to soluble oligomers and insoluble fibril aggregates is believed to be one of the causes of PD. Recently, the view of the native state of αsyn as a monomeric ensemble was challenged by a report suggesting that αsyn exists in its native state as a helical tetramer. This review reports on our current understanding of αsyn within the context of these recent developments and describes the work performed by a number of groups to address the monomer/tetramer debate. A number of in depth studies have subsequently shown that both non-acetylated and acetylated αsyn purified under mild conditions are primarily monomer. A description of the accessible states of acetylated αsyn monomer and the ability of αsyn to self-associate is explored.
Keywords: α-synuclein, Parkinson’s disease, Intrinsically Disordered Proteins, Acetylation, Monomer, Ensemble, Oligomer, Fibril, Aggregation, Fibril-resistance
1. Introduction
Parkinson’s disease (PD) research has sought to answer questions of alpha synuclein (αsyn) function and the mechanism of aggregation surrounding disease pathology. Both remain to be fully articulated today, but several observations have been established and a range of neurodegenerative diseases termed the “synucleinopathies” have been identified[1, 2]. PD in particular is the synucleinopathy characterized by the loss of dopaminergic neurons and is largely considered to be an age-related disease, accompanied in part by age-related deposition of αsyn[3]. αsyn, a major protein component of Lewy Bodies[4, 5] in patients with Parkinson’s, is a small primarily neuronal protein that is known to make a structural transition to amyloid fibrils[6-8]. αsyn is expressed abundantly in the nervous system and localizes near presynaptic nerve terminals[9-13]. It is also expressed at high levels in erythrocytes and platelets[14]. αsyn’s function is unknown, but there is strong evidence that it exhibits lipid binding in vesicles and synaptic membranes[15] and may somehow exert its pathology through this behavior[16]. There is evidence that αsyn functions in assembly of the SNARE complex involved in vesicle transport[17], that it may more generally be involved in synaptic vesicle trafficking and regulation and/or may play a key role in neuronal cell survival[18-22].
The deposition of αsyn has largely been thought to originate from an intrinsically disordered monomer ensemble that under fibril promoting conditions forms amyloid [7, 23, 24], but recently this view of αsyn’s native state was challenged[25]. Selkoe and colleagues pushed the biophysical community’s long-held view of αsyn as an intrinsically disordered monomer by suggesting that the protein exists in its native state as a fibril resistant helical tetramer. They purified the sample from human erythrocytes, opting to exclude a potentially “harsh” and commonly used boiling step from the purification. Based on this work several questions presented themselves. Do bacterial systems that are commonly used to obtain sample for biophysical characterization not possess the necessary machinery for tetramer assembly? Could the commonly used boiling step during purification denature some key native structure that promoted a helical tetramer of αsyn? Aside from these assembly and purification issues, there was also one molecular difference between the purified samples of Selkoe and colleagues and previous studies, indicative of modification to the monomer by an acetyl group (Ac-αsyn).
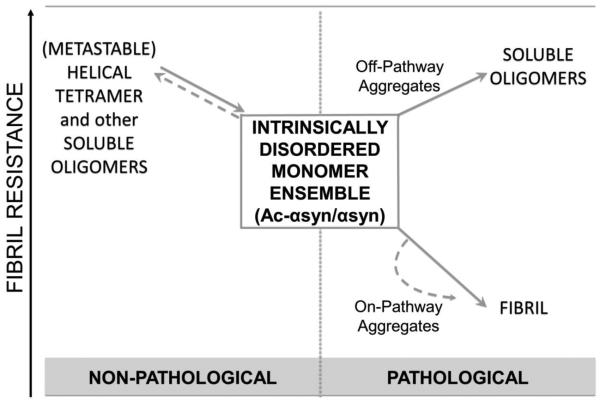
This review reports on our current understanding of αsyn within the context of these recent developments and describes the work performed by a number of groups to address the monomer/tetramer debate[25-33]. We summarize major shifts within recently published works addressing these issues in Table 1. Numerous studies indicate that αsyn, both acetylated and non-acetylated, exists as intrinsically disordered monomer conformational ensemble under mild purification conditions. We highlight that the ensemble of monomers is known to develop into a wide range of accessible conformations upon changes of environmental conditions, that it can populate many soluble oligomeric states of varying morphologies and toxicities, and settle into various insoluble fibril or amorphous aggregate morphologies[23], that have largely been studied in the context of PD-related pathology (Figure 1). We discuss the suggestion of a soluble fibril resistant helical tetramer that presumably represents a non-pathological aggregate of αsyn which may have to dissociate before fibril formation can proceed through the monomer (Figure 1). The potential that established methods might disrupt native-stabilizing interactions of a fibril-resistant helical tetramer of αsyn have heightened awareness to cell machinery, to αsyn purification methods, and to the difficulties in choosing appropriate methods of characterization. The extent to which N-terminal acetylation impacts upon the conformation and aggregation behavior of αsyn is discussed separately and it is shown that the acetyl group does not promote the formation of the helical tetramer under mild purification conditions.
Table 1.
Historical description of shifts in αsyn purification approaches and conformational properties.
| 1996- Dec. 2011 | > Dec 2011 | > May 2012 | |
|---|---|---|---|
| Source | Mostly Bacterial | Mammalian | Bacterial / Mammalian |
|
N-terminal
acetylation |
No | Yes | Yes |
| Purification protocol | Often denaturing | Non-denaturing | Denaturing and non-denaturing |
|
Average secondary
structure |
Primarily random coil | Primarily helical | Primarily random coil |
|
Transient initiating
N-terminal helix |
No | --- | Yes |
| Primary native state | Monomer | Tetramer | Primarily monomer |
| Fibril Prone | Yes | No | Yes |
|
Referring section
within text |
2-3 | 5, 7* | 6, 8 |
The most recent report by Selkoe and colleagues, suggest “metastability” of the tetramer (section 7).
Figure 1. A schematic diagram of the possible accessible states of non-acetylated and acetylated αsyn.
The right side represents two possible pathological aggregation pathways from the unfolded monomeric ensemble to 1) insoluble fibrils through on-pathway transient oligomeric intermediates and 2) to off-pathway soluble oligomers. Off-pathway soluble oligomers represent non-fibrillar end products of aggregation. The left side presents 1) the recent proposal that αsyn can exist as a soluble fibril resistant helical tetramer which is acetylated, and 2) other known oligomers that are not toxic such as methionine oxidized oligomers. It is proposed that the non-pathological tetramer needs to dissociate to the monomeric ensemble before pathological aggregation can occur (dark arrow).
2. Overview of non-acetylated αsyn ensemble: monomers and dimers
2.1. Biophysical characterization of the non-acetylated monomer ensemble
The native state of non-acetylated αsyn has been thought to originate from an ensemble of intrinsically disordered monomeric forms, with recognition that the monomers therein are capable of adopting a wide range of accessible conformations depending on solution and environmental conditions [34-38]. Uversky first spoke of αsyn as the “protein chameleon”[23] due to its ability to respond to its environment and binding partners by varying its foldedness and aggregation state. αsyn is often described as a 140 residue intrinsically disordered protein (IDP) characterized by three distinct regions of the protein: an N-terminal lipid binding repeat region that houses the mutations A30P, E46K, and A53T linked to early onset disease, a hydrophobic non-amyloid component (NAC) region implicated in fibril formation, and an acidic more proline-rich C-terminus suggested to have chaperone activity and possess some key role in modulating structure in the N-terminus[6, 39, 40]. As summarized in Table 1, to study PD related aggregation, αsyn has typically been obtained from overexpression in bacteria, yielding a non-acetylated IDP, as bacteria typically do not modify their proteins by acetylation (Figure 2A)[41-43]. Additionally, while boiling as part of the purification protocol would typically be considered to be harsh for a globular protein, IDP’s are in general characterized by thermostability[34]. Because of this heat stability, αsyn has often been boiled to achieve purity. In addition, IDP’s like αsyn are generally characterized by a highly charged sequence, a lack of stable secondary structure, and a larger than expected Stokes radius compared to spherical and folded proteins of the corresponding molecular weight[34, 44, 45].
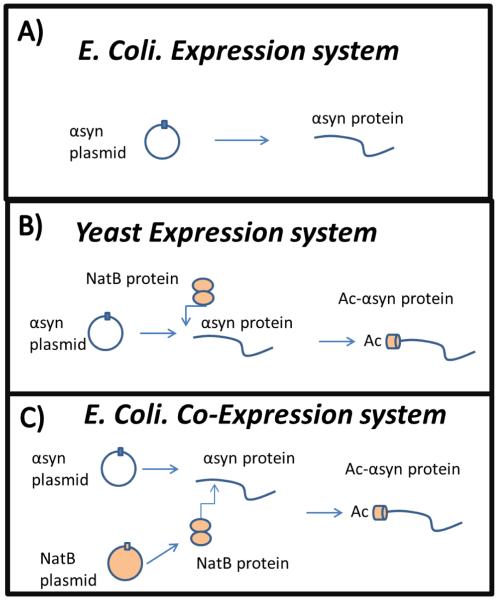
Figure 2. A pictoral representation of the co-expression system designed to generate Ac-αsyn in bacteria.
In this figure: ring-like circles represent plasmids, lines represent the αsyn protein, and two ellipses represent the NatB protein. A) Bacteria, lacking NatB, express non-acetylated protein. B) Yeast house yeast-NatB which acts upon αsyn and generates Ac-αsyn. C) To obtain Ac-αsyn within a bacterial expression system, plasmids encoding yeast-NatB can be co-expressed with the plasmid encoding for αsyn so that Ac-αsyn is obtained[164].
The αsyn monomer is both unfolded and extended, as it was first reported to have a larger Stokes radius than expected for globular protein of similar molecular weight and a primarily random coil circular dichroism (CD) spectrum[34, 36, 46]. However, the protein is not fully extended for a protein of its size, implying a slight compaction of the monomeric ensemble[36, 47]. Evidence for contact between the C-terminus and both the NAC and N-terminal regions of the protein from nuclear magnetic resonance (NMR) [38, 48-55], electron paramagnetic resonance[56] and molecular dynamic studies[52, 57] indicates a possible source of this compaction, as well as some transient secondary structure[55, 58-60]. The compaction may be at least be partially driven by hydrophobic patches located in the C-terminal (residues 115-119, and 125-129) associating with and shielding both the hydrophobic N-terminal and NAC regions[50, 61]. It should be emphasized that evidence for contact between the N- and C-termini does not imply a static closed picture of αsyn as an IDP[59, 62]. Rather, this is a dynamic interaction, and observation of slight compaction is the result of observations on a highly averaged bulk ensemble[48].
Under conditions promoting pathological aggregation of αsyn, conformational shifts in the ensemble are observed. There is evidence that these interactions may keep the N-terminus from pathological misfolding[39, 63], as their release is associated with increased fibril formation [38, 50, 52, 64, 65]. For example, when the solution pH is lowered, there is a structural rearrangement of the monomer ensemble with enhanced contacts NAC-C terminus and C-C contacts resulting from charge neutralization and compaction of the C-terminal region[38, 54, 58, 66]. Environmental or experimental shifts that reduce the net charge or increase hydrophobicity of the protein [67-69], or interaction with small molecules or metal ions[70-72] can change subtly, but significantly, both the long-range and short range contacts and conformations sampled in the monomeric ensemble.
Therefore, small changes to the αsyn monomer can potentiate big effects on aggregation behavior, yet only small differences to the monomer ensemble. For example, the familial mutations (A30P, E46K,A53T) of αsyn are structurally comparable, as they are similarly unfolded and have similar radii of gyration, but they have distinct kinetics of fibril formation[55, 73-79]. NMR spectroscopy has revealed that mutations affect chemical shifts surrounding the mutation site and that we can correlate these shifts to region-specific shifts in the population of transient secondary structure. These relatively small shifts in transient secondary structure populations can explain bulk differences in fibril formation rates[59, 80, 81].
The N-terminal region is also known to adopt helical structure upon binding lipids, representing a more dramatic conformational shift of the monomer ensemble[82-84]. NMR groups have demonstrated that αsyn displays chemical shifts characteristic of a mostly unfolded peptide, but that the first 100 residues transiently populate helical structure. When bound to lipids or micelles, however, chemical shifts of these residues indicate a structured helical environment.[85, 86]. Bax characterized the structure of the micelle bound form of the protein, and the fact that αsyn adopts helical structure at its N-termini through its repeat region upon binding lipids membranes and micelles has become well-known[82]. Because αsyn localizes near synaptic nerve termini[9], its lipid-induced helical structure[35, 87-89] may be crucial in understanding the protein’s function at the membrane, yet it is still unknown how this N-terminally helical monomer conformation is related to the trigger of fibril formation.
2.2. Dimers: equilibrium species and pathological intermediates
Dimers can exist in a small population in pseudo-equilibrium with the monomer ensemble. The Baum lab demonstrated that indeed antiparallel transient inter-chain contacts between the C-terminal hydrophobic patches and the N-terminal region (residues: 3-15 and 35-50) could be detected by using NMR paramagnetic relaxation enhancement experiments[54]. Electrospray ionization mass spectrometry (ESI-MS) and electrospray ionization-ion mobility spectrometry-mass spectrometry (ESI-IMS-MS) obtained under similar conditions has shown that the predominant oligomeric form we observe in αsyn is the dimer. This soft ionization technique has revealed the “conformational heterogeneity” of αsyn, where the monomers and dimers themselves exist in both extended and compact conformations[30, 90, 91], suggesting that the ensemble view of αsyn also extends into its higher oligomeric states.
It is unknown whether this pseudo-equilibrium anti-parallel dimer is on pathway to the fibril formation. It is reasonable to assume that inter-chain N-N species, which adopts the same parallel orientation as monomer units as in the core of the fibril, lies further along the pathway to fibril formation than anti-parallel oriented monomers[92]. This would imply reorientation of monomer units as an obligatory step before formation of the fibril. However, at least one report demonstrates that toxic prefibrillar amyloid aggregates adopt an antiparallel orientation[93], and in this sense we cannot draw any analogy to this pseudo-equilibrium dimer population that exists with the fibril accessible monomer ensemble, and how the monomers therein may interact with it.
As the monomeric ensemble is shifted towards more fibril prone conditions, previously described conformational changes (Section 2.1), as well as changes in population of oligomeric species occurs. Incubation at high temperature is one external factor inducing this shift[94]. Under these conditions, soluble oligomers of αsyn spontaneously associate and a dimer is the predominant oligomeric species of αsyn to appear alongside formation of the fibril, along with smaller populations of higher-order oligomers. Biophysical characterization of this intermediate which includes some dimer shows it has more hydrophobic patches exposed than the native monomer[36]. This on-pathway dimer that appears at the time of fibril formation may be conformationally dissimilar from the pseudo-equilibrium dimer population previously described, which may not necessarily be correlated with fibril formation. There is some evidence that formation of at least one species of dimer is the rate limiting step of fibril formation[95] and cysteine mutants have shown that particular dimer linkages accelerate fibril formation in vivo and in vitro[95]. This implies accessibility of many distinct conformations for the dimer, in the same way as the monomer, and presumably varying degrees of membrane affinity and cytotoxity Additionally, dimers are not the sole on-pathway oligomeric species that appear during events of PD pathology. Observations of higher order oligomers, that occur alongside fibril formation and in response to other events appear to include a large slew of different species, which we address in the upcoming section.
3. Heterogeneity of the αsyn oligomeric structures and their pathogenicity
3.1. The role of oligomers in in vivo pathology
The motivation to understand whether there is a helical tetramer of Ac-αsyn lies not only in desire to accurately portray the protein in vivo, but also to understand how oligomers in particular function in disease-related pathology of PD. There is ample evidence that soluble oligomers are the real pathogenic species of neurodegenerative disease, whereas fibrils serve as reservoirs of misfolded, irreversibly modified deposited protein better-off removed from solution[96-103]. Because amyloid deposits were first detected in brains of sick individuals, it was assumed that they were the neurotoxic species, but because amyloid is such a common structural motif, the ability to form amyloid is now considered a general property of a polypeptide in solution[104]. Over and over, conversion of IDPs into amyloid aggregates has not been observed to be a simple two-state transition. Oligomer formation has been established as an important mechanistic step in fibril formation, for example as in Alzheimer’s disease[102, 105]. As briefly described in αsyn, soluble oligomeric intermediates commonly appear as insoluble fibrils form[106-108] and the situation may be quite similar to that established for AD.
What determines if a protein will form soluble oligomeric species, or if an amyloid fibril will form? It seems that a polypeptide will sample many parallel or antiparallel conformations before a final structural state is preferred[109]. This arises from a competition between hydrophobic forces and side chain interactions, versus the propensity of the polypeptide chain to form β-sheet like hydrogen bonds[110]. The prefibrillar oligomer is thought to be the cytotoxic species, as toxic inter-chain associations are sampled that a monomer alone could not support. The fluorescent probe 8-anilinonaphthalene-1-sulfonic acid binds to exposed hydrophobic patches. Its binding demonstrates that the most toxic species are associated with greater overall surface hydrophobicity[94, 111-113]. In fact, overall greater hydrophobicity is associated with increased chance for exposed hydrophobic portions of the sequence to exhibit toxicity through interaction with the membrane. This may as well be the case for αsyn [112, 114].
Oligomeric PD pathology may be rooted in membrane association, where oligomers of αsyn can perturb membrane integrity and cause cell death by altering transport across the membrane [115-118]. At least one report demonstrates that in vivo membrane associated αsyn oligomers correlate with toxicity rather than inclusion formation[119] but also that the degree of oligomer toxicity is related to an array of structurally diverse morphologies that can form. Interestingly, of the familial mutants implicated in PD, A30P and A53T have different kinetics of fibril formation relative to the wild type monomer, but both share the property of an accelerated oligomerization[120, 121]. These mutants may exert their pathology through the formation of pore-like oligomers that form alongside fibril formation[122].
3.2. Oligomers associated with the fibril formation pathway are highly heterogeneous
During the time course of fibril formation early prefibrillar oligomers and late soluble oligomers, which are not a part of the fibril have been observed in αsyn[107, 123] Their isolation and structural characterization has been of great interest, and some shared features of fibril accelerating or inhibiting species have been characterized. It was postulated that an on-pathway amyloidogenic transition occurs through partially folded oligomeric species originating in the dimer[36]. Soluble aggregates first appear that maintain the helical character of the monomer, but lose some disorder in favor of β-rich structure as they age. β-rich intermediates build as fibril formation proceeds and begin to get consumed at the end of the lag phase[107, 111]. This conversion into more β-rich species may describe the formation of initial aggregates and their conversion into amyloid-like aggregates., The conformational conversion between oligomeric types observed by Dobson and colleagues is also accompanied by direct observations of conversion into a more toxic form and highlights that certain oligomer types can be either toxic or non-toxic. [106]. AFM has been used to observe β-rich spherical and annular oligomer morphologies prior to fibril formation of αsyn. The initial aggregates appear to be spherical aggregates. They have been shown to convert to more spherical compact species, and then into annular species upon further incubation[124]. Annular species of αsyn are known to induce membrane leakage[103, 125], but spheroidal species can bind brain-derived membranes quite tightly, as well[124]. Spherical morphologies seem to disappear once the fibril has formed, whereas annular species may sometimes coexist with the fibril[124]. Oligomer induced toxicity is relevant to the entire fibril formation process.
Soluble oligomers may appear after the fibril has formed, or their formation may instead be preferred. Late stage distinct oligomeric species appear once fibrils have formed and they are also β-rich [108, 111, 123, 126]. Some suggest they occur from dissociation of the fibril or that they represent end-products of a fibril resistant-soluble oligomerization pathway and may not be converted into fibril. At the end of fibril formation 10-20% of protein exists as such a non-fibrillar oligomer[127].
There are many pathways which have been identified toward soluble aggregates. Organic solvents have been used to model membranes, and it has been shown that a helical rich monomer will eventually associate into a helical rich oligomer that also appears stable [128]. Covalently cross-linked non-fibrillar oligomers are also well known to form under oxidative or nitrative stresses. Nitration, for example, inhibits fibril formation through the formation of inhibitory higher-order oligomers than the dimer [129]. This mix of species only further describes the range of the secondary structures, morphologies and pathologies that oligomers of αsyn are capable of populating[92, 94, 130-132]. Increased oxidative stresses and increased metal levels have been correlated with PD, so this class of stable non-fibrillar oligomers that form under stresses are potentially important players in the mechanism of aggregation as well[133].
Various pathways available to soluble oligomer, not surprisingly results in a very heterogeneous population of possible oligomers. Oligomer morphology has been shown to be highly dependent on solution conditions, including the presence of lipids[124, 134-137]. Also, incubation with different types of metals generates partially folded structures[138-140] that go on to form a variety of oligomeric structures. Whereas incubation with Cu2+/Fe3+/Ni2+ produce spherical oligomers of 0.8-4 nm particles, incubation with Co2+/Ca2+ produces yields pore-like annular rings 70-90 nm in diameter[141].
3.3. Stabilization of non-fibrillar oligomers that appear to be non-pathogenic
In previous paragraphs our focus was on oligomers more closely linked to pathology, but as mentioned, some oligomers can be stabilized in non-fibrillar forms not capable of adopting cross-β structure on their own and may not necessarily be linked to cytotoxicity (Figure 1). The αsyn monomer that has been modified by methionine oxidation of the αsyn monomer to the sulfoxides is one example of these non-toxic non-fibrillar species. This modification at methionine residues promotes the stabilization of an oligomer that appears slightly more unfolded than monomeric αsyn. While probably not covalently cross-linked, these oligomers exhibit stability and do not go on to form fibrils[142-144]. Furthermore, these oligomers do not exhibit toxicity toward dopaminergic neurons, suggesting that particular conformational features are indeed necessary to exert pathology as an oligomer (Figure 1)[144]. Interaction with small molecules like the flavonoid baicalein can also prevent formation of the fibril by stabilizing soluble oligomeric end products and these oligomers also do not disrupt membranes[145]. Structurally these species are spherical, have a well developed secondary structure, are relatively globular with a packing density intermediate between globular protein and pre-molten globule and very high thermodynamic stability. In contrast oligomers stabilized by modification of the monomer with 4-hydroxy-nonenal are non-fibrillar, but are also toxic[145]. Could a helical tetramer be similarly stabilized, such that the stabilization in the oligomer conformation is more favorable than in amyloid, and could it also share conformational features of non-pathology with aforementioned species? Descriptions of size, morphology or overall conformation may not be sufficient to describe the cytotoxicity of an oligomer of asyn. For example, in amyloid-β two oligomers of similar size but dissimilar toxicity have been identified, where the more toxic species adopts a conformation in which hydrophobic regions remain more exposed[146].
4. αsyn is N-terminally acetylated in vivo
Before attention was drawn to the possible role of the acetyl group by the recent report[25] of tetramer formation of αsyn, αsyn was studied from a variety of sources, some of which were mammalian and were likely to be N-terminally acetylated. Although the acetyl group had not previously warranted an explicit examination, drawing comparisons between in-cell work and in vitro work could be challenging. Therefore, the Bartels et al. report clearly suggested that co- or post-translational modifications (PTM’s), namely acetylation, may have significant influence on αsyn structure and aggregation properties so specific investigation of the acetyl group naturally followed in the reports we describe in this review(Table 1). PTM’s to αsyn are known to regulate/modify αsyn’s propensity to aggregate[132, 142, 147]. It has been known for some time that αsyn in human tissues is acetylated, but the role of N-terminal acetylation is unclear, as it is seen in both healthy and individuals sick with synucleinopathies. Two mass spectrometry (MS) studies of αsyn from human tissues, both report that the base mass of the protein before any other modifications is the acetylated form -- consistent with that reported by Selkoe and colleagues from red blood cells (RBCs)[25, 148, 149]. The report indicated that acetylation of αsyn was not limited to neuronal tissue; however, the site of acetylation was not identified.
4.1. Bacteria lack the machinery for N-terminal acetylation
Mammals modify the proteins they produce with many more PTM’s than yeast and bacteria, as these may play a role in more complex signaling pathways[150, 151]. N-terminal acetylation is a well-known modification in eukaryotic cells. Up to 80% of proteins are modified by N-terminal acetylation in mammals, whereas bacteria rarely acetylate their N-termini and if they do, by distinctly different mechanisms [43]. The aforementioned MS studies[148, 149] indicated that acetylation occurs at the N-terminus, where an acetyl group has removed the α-amino charge of the initiating amino acid by covalent modification at that site.
N-terminal acetylation is carried out mostly co-translationally by a group of enzymes known as N-acetyltransferases (Nat) in eukaryotes[152, 153]. Mammalian cells have these complexes, and yeast an analogous enzyme complex, but bacteria do not. Nat’s catalyze the transfer of an N-acetyl group from acetyl-coenzyme A to the N-termini of proteins with sequence specificity. Different Nat’s (types A-F in eukaryotes) work upon different initiating amino acid substrates, dependent upon the identity of the first two to three amino acids of the protein polypeptide[154]. Therefore, depending on the type of cell to synthesize αsyn, the protein may or may not be acetylated (Figure 2A, 2B). Specifically, N-acetyltransferase B (NatB) has αsyn as a substrate, producing acetylated αsyn (Ac-αsyn). NatB targets proteins beginning with Met-Asn-, Met-Glu- or as in the case of αsyn, Met-Asp-. Substrates of NatB are acetylated nearly 100% of the time, as the acidic amino acids in the second position are thought to stimulate the transfer of the acetyl group[151].
4.2. Possible roles of N-terminal acetylation in vivo
Recognizing that αsyn acetylation does indeed occur, one study prior to Bartels et al’s investigated the role of NatB activity in a yeast model by disrupting NatB activity[155]. They found NatB activity to be essential for proper membrane targeting of αsyn. Without NatB activity, non-acetylated αsyn is produced, and a much more diffuse cytoplasmic localization of αsyn compared to those with whole NatB activity (producing Ac-αsyn) was observed. While this in vivo effect was observed in this one instance for Ac-αsyn, the role of N-terminal acetylation is not generally well understood[156]. One study suggests that N-terminal acetylation represents an early sorting step, where acetylated proteins are targeted toward the endoplasmic reticulum, unless they remain non-acetylated and are kept localized to the cytosol instead[157]. N-terminal acetylation may also regulate degradation pathways[158] or be responsible for structural effects at the N-terminus[159]. Levels of acetylation may be related to regulation of other post translational modifications. NatB, specifically, has been shown to induce elevated phosphorylation levels in yeast[160] consistent with the aforementioned yeast study of αsyn, where decreased levels of phosphorylation were observed when the protein remained non-acetylated and localized in the cytosol[155]. Acetylation may not be necessary at all for proteins, but some examples do point to a necessity. For example, tropomyosin requires N-terminal acetylation so that it may bind to actin[161].
5. αsyn is proposed to be a helical tetramer in its native state
Selkoe and colleagues strived to isolate αsyn under physiological conditions and have challenged the existence of the αsyn monomeric ensemble[25] by proposing a fibril resistant helical tetramer form of the protein (Table 1). In contrast to the typical protocol in which αsyn has been derived from bacterial systems, overexpressed and denatured, they purified αsyn from gently-treated RBCs known to have a high endogenous expression level of human Ac-αsyn. From both RBC lysate and endogenously expressed αsyn from neuronal and non-neuronal cells lines, Selkoe and colleagues showed on Clear Native PAGE (CN-PAGE), that αsyn migrates near the tetramer position against folded, globular protein standards. The unusual migration of an IDP against globular standards was not unfamiliar. Native gels are unreliable objective determinants of molecular weight, as protein migration through the acrylamide matrix depends strongly on protein charge and shape. IDP’s typically display a Stokes radius of a much higher molecular weight species, and this has previously been attributed to enhanced interactions with the matrix[36], so that E. coli derived boiled αsyn, too, will migrate near the position of the tetramer at 58 kDa on a native gel[25].
Selkoe and colleagues’ report also stated that they obtained a CD spectrum that indicated largely helical structure that was sensitive to irreversible heat denaturation. Isolation from human cell line 3D5 (which are M17D cells stably expressing αsyn) yielded similar results to RBC derived αsyn, and they showed that αsyn derived from E. coli was random coil even after non-denaturing purification, consistent with previous reports[41]. Therefore Selkoe and colleagues implied that expression in human cell lines and a non-denaturing purification are necessary to “preserve” this native tetramer structure. If this is indeed the native form of αsyn, non-denaturing methods of purification and mammalian machinery may be necessary to observe it. When denatured, RBC αsyn became random coil, and migrated more similarly to E. coli derived αsyn on a native gel, rather than the mildly purified helical sample.
Helical structure of αsyn can be induced by its interaction with membranes. Therefore, it might logically follow that the milder purification did not fully remove helix inducing lipids. However, treatment with Lipidex and subsequent phosphate analysis indicated the sample was relatively pure in that regard (0.25 mol phosphate/αsyn monomer). At the same time, a higher lipid binding capacity for this “native” αsyn was demonstrated with surface plasmon resonance. They also employed some unbiased methods of MW determination including sedimentation equilibrium-analytical ultracentrifugation (SE-AUC) and scanning transmission electron microscopy both indicating a tetramer. Bartels et al. also observed one other unprecedented trait of the sample – that under standard fibril assay conditions, ThioflavinT (ThT) fluorescence did not indicate that RBC ac-αsyn formed any fibrils in vitro – clearly also in contrast to previously observed results and the in vivo condition (Table 1).
Not too long after, Wang et al.[135] similarly reported a dynamic tetramer form of the protein. The protein was obtained by recombinant expression methods and was modified by a 10 residue N-terminal tag left over from a glutathione S-transferase (GST) construct, making it difficult to compare directly with the tetramer obtained from RBCs. The purification method was similarly “non-denaturing” but included the non-physiological detergent beta-octyl glucopyranoside (BOG) typically used to purify membrane bound protein. Perhaps N-terminal acetylation was somehow mimicked by the cleaved GST-tag and would prove to be important in the context of a non-denaturing purification. Researchers now would interpret data in light of a greater possibility of the tetramer, and more carefully consider their assumption that their expression and purification methods did not preclude an accurate representation of the protein in vivo.
6. Subsequent studies indicate that αsyn exists as a primarily unfolded monomer
A rapid period of overlapping work began to determine the oligomeric state of αsyn from various cell sources, under non-denaturing conditions and to investigate the role of the acetyl group modification. Lashuel and colleagues examined αsyn from mouse, rat and human brains and addressed the issue of the source, the purification and the characterization methods of the protein and their impact on the oligomerization state (Table 1)[29].
In response to report by Bartels et al., Lashuel and colleagues determine that αsyn exists as an unfolded monomer within neuronal sources. Lashuel et al.[29] examined bacterial lysates under denaturing and non-denaturing conditions (with and without a boiling purification step respectively) against a range of non-globular standards, including: 1) E. coli derived unfolded monomeric αsyn 2) disulfide linked A140C αsyn including some dimer and 3) Ac-αsyn. Regardless of purification, samples from bacterial lysates elute and migrate at identical positions on a size exclusion chromatography (SEC) column or CN-PAGE. This indicated that the various samples are either all unfolded monomers, all more compact tetramers, or that coincidentally these structures migrate at identical positions. Coupled now with a far UV spectrum of a primarily random coil protein, rather than a helical spectrum observed by CD, however, Lashuel’s bacterial αsyn appears to be unfolded regardless of whether it has been boiled and it resembles unfolded monomeric αsyn. A random coil spectrum is not necessarily synonymous with a monomeric protein. Static light scattering (SLS) was used as a more unbiased molecular weight determinant alongside elution from SEC[162]. While data from size exclusion chromatography (SEC), indicated a Stokes radius close to a globular standard at 64 kDa, SLS indicates a protein of 14 kDa. Therefore bacteria, consistent with Selkoe and colleagues’ observations, do not assemble into a helical tetramer, even without boiling.
Lashuel and coworkers[29] demonstrated a sensitivity of CN-PAGE to small differences in the protein composition and went on to use CN-PAGE to explore the role of mammalian machinery and denaturation by boiling. Whether endogenous or overexpressed, whether boiled or not, whether isolated from bacteria or present in mouse, rat samples or HEK293,HeLa,SH-SY5Y,CHO,and COS-7 mammalian cell lines -- identical CN-page migration and sometimes SEC-SLS, repeatedly indicated the unfolded monomer. Across research groups, acrylamide percentages, purification protocols and the source, the samples of αsyn co-migrate with recombinant αsyn. To test whether factors present in cell could promote tetramerization, they examined fresh or aged samples, since aged samples are expected to be more oligomer-rich, along with a control of exogenously added recombinant protein. In vivo oligomer-specific enzyme-linked immunosorbent assay (ELISA) could not detect any other oligomers in the samples, confirming that purification has not disturbed this observation[29, 163]. In addition, the report explored the possibility that the tetramer population could be dynamic and unstable, so that if the protein for some fraction of the time populates a tetrameric state, it would have a different cross-linking profile than a protein that populates primarily the monomer state. They observed that no significant amount of oligomers beyond the dimer are observed, indicating that DSS could not effectively capture a tetramer either. This report additionally repeated the RBC purification procedure[29]. Unable to replicate the tetramer, it was still concluded that disordered monomer is isolated from RBC’s. It is not clear what Selkoe and colleagues[25] did differently, but Fauvet et al.[29] does note that samples of sufficient quantity and purity could not be obtained using this purification, even with another hydrophobic interaction chromatography (HIC) step, suggesting some complicating interactions in either sample. Fauvet et al. also attempted the GST-constructed αsyn protocol and cannot replicate the dynamic tetramer observations of Wang and colleagues.
Concurrently, Rhoades and colleagues sought to determine if the nature of the purification method[32] or N-terminal acetylation had enough biophysical consequence to promote the fibril resistant tetramer. They examined samples purified with and without BOG and the N-terminal acetyl-group. Rhoades is the first to use a bacterial co-expression system to generate Ac-αsyn (Figure 2C). In this co-expression system developed by Mulvihill et a[164], the yeast analog to NatB is cloned into a bacterial plasmid, allowing overexpression of αsyn into more unsophisticated expression systems. The yeast NatB is shown to function in bacteria to produce N-terminally acetylated proteins, and it seems to acetylate αsyn close to 100% of the time in E.coli. Rhoades finds that N-terminal acetylation and non-physiological purification including BOG were necessary for observation of helical oligomeric αsyn. Non-acetylated or BOG free αsyn was disordered and presumably monomeric, but the CD spectrum of Ac-αsyn purified in the presence of BOG was helical. Rhoades also encounters the complication that disordered monomer and helical tetramer have similar hydrodynamic radii, but coupled with SE-AUC, which is “independent” of molecular shape, Ac-αsyn(BOG) was shown to have a sedimentation curve that exchanged with an oligomer. That the sample was specifically tetrameric is not clear. While the report by Trexler et. al, does not exclude the possibility that N-terminally acetylated αsyn has a higher affinity for membranes and/or BOG itself, the work was further provocative towards the role of acetylation in helicity and oligomerization.
7. Continued discussion on the oligomerization state of αsyn
In response to the studies that indicate that cellular αsyn is an unfolded monomer[27, 29], Selkoe and coworkers[33],with an even further heightened awareness to experimental conditions, most recently reported that endogenous αsyn is predominantly tetramer. Using in vivo cross-linking as their primary tool, they identify several factors which might matter in terms of isolating the tetramer. During overexpression, particularly in protein derived from IPTG induction, more monomer is found. More monomer is also isolated when cross-linking is done at 4C° as opposed to 37C°. The tetramer is “preserved” in a concentration dependent manner at the time of lysis, where a higher concentration at the time of lysis favors the tetramer. This suggests that macromolecular crowding in cell may favor folding and stabilization of the native non-pathological tetramer. For this reason and for the fact that the Ac-αsyn level is endogenously high in erythrocytes, Selkoe and colleagues’ considers RBC’s an ideal system to obtain Ac-αsyn. These results may reflect of the experiments themselves, or may reflect the preference of the monomer to associate with itself, even in the presence of other binding partners, but that it is also stabilized in the monomeric form.
8. N-terminal acetylation of monomeric αsyn induces helix formation and affects lipid binding
Because Ac-αsyn is now recognized to be the physiologically relevant species in the brain, its biophysical characterization has been pursued. Questions that have been raised include the monomer or oligomeric preference of the Ac-αsyn species, its conformation, interactions with membranes and ability to form fibrils. Kang et al.[30], show that recombinant 100% acetylated Ac-αsyn purified under mild physiological conditions exists primarily as a monomeric protein. ESI-IMS-MS experiments indicate a small population (5-10%) of dimer that is consistent with previous observations of dimer species in solution. Lashuel and colleagues[28] use similar techniques as in their first report and again do not observe any higher-order oligomers, now in the acetylated protein. This suggests that acetylation by itself is not sufficient to favor a helical tetramer. Selenko and colleagues [27] show by in-cell NMR that non-acetylated αsyn is a disordered monomer in the macromolecular environment of the cell. Lashuel’s group[28] additionally compares Ac-αsyn and αsyn with in-cell NMR and draws similar conclusions. Although the possibility of exchange with higher-order oligomers cannot be ruled out, the predominant cellular form indicated by these experiments of Ac-asyn is unfolded monomer (Table 1).
The conformational properties of Ac-αsyn have also thus far been investigated and it is suggested that there is minimal change in the hydrodynamic radius and intra-chain long-range interactions, if any[28, 31]. However, the N-terminal acetyl group affects the transient secondary structure as observed by NMR[28, 30, 31]. Residue-specific NMR chemical shift analysis shows that there is an increase in the transient helical propensity at the initiating N-terminus[28, 30, 31] that may arise as the acetyl group masks the alpha-amino positive charge and interacts favorably with the helix dipole moment. Additionally, the acetyl group itself is a good N-cap, favoring hydrogen bonding for an N-terminal alpha helix at the initiating residues[165, 166] .
At least one report suggests the membrane binding properties of Ac-αsyn are strongly altered by acetylation and indicates a two- fold higher lipid affinity. It is suggested that the increase in N-terminal transient helix may be critical to initiating membrane binding. Preformed transient helix at the N-terminus may therefore play an important role in the recognition of binding partners, may be important for membrane recognition, or may imply that lipid mediated association of the hydrophobic surfaces of helices may relevant to routes of self-association of the acetylated monomer.
Fibril formation of Ac-αsyn was investigated by measuring the fibrillation kinetics using ThT fluorescence. While groups of Lashuel[28] and Bax[31] found no significant differences in fibril formation rate, Kang et al.[30] found that N-terminal acetylation slows the rates of fibril formation by approximately a factor of two. Clearly the acetyl group alone cannot inhibit fibril formation, but it does impart a small inhibitory effect, which may arise from a redistribution of the monomeric protein ensemble.
9. Conclusions
Recent studies that suggested that asyn exists as a soluble, tetrameric, fibril-resistant form of αsyn were provocative, and a monomer/tetramer debate followed. The discussions about the accessible states of αsyn have raised many important questions related to cellular machinery, αsyn purification methods and the extent to which acetylation impacts on a monomer-oligomer equilibrium. A number of in depth studies have subsequently revealed that both non-acetylated and acetylated αsyn purified under mild or harsh conditions is primarily a monomer.
Despite the controversy surrounding the notion of a helical non-pathological tetramer, the concept of a soluble, non-pathological αsyn oligomer was perhaps not new. Biophysical studies have shown that αsyn can be induced to self-associate into a heterogeneous variety of soluble oligomers, some of which may be beneficial, or non-pathological. For example, methionine oxidation, arising from conditions of oxidative stress, stabilizes a fibril resistant oligomer of αsyn that is non-toxic to dopaminergic cells[144]. This may be consistent with the regulatory role methionine oxidation is suggested to have, sometimes being beneficial.
In order to understand the interplay between aggregation prone and aggregation resistant kinetic pathways from the unfolded monomer, the initial interchain associations between monomers within the starting ensemble and associations present in already-isolated stable soluble oligomers may need to be considered further. Defining the properties that drive these different species may lend to our understanding of how to enhance fibril resistant, fibril prone and/or non-toxic pathways in vivo. Because of the great ability of αsyn to adopt many conformations in a variety of oligomeric states, working from the monomer ensemble of Ac-αsyn we may (again) isolate stabilizing interactions of a helical oligomeric species that does not tend toward fibril and we may begin to better elucidate shared features of non-pathology and fibril resistance amidst the entirety of the currently known heterogeneous oligomer population of αsyn.
Acknowledgments
This work was supported by NIH grant GM087012.
Abbreviations
- PD
Parkinson’s disease
- αsyn
α-synuclein
- Ac-αsyn
Acetylated α-synuclein
- IDP
intrinsically disordered protein
- BOG
beta-octyl glucopyranoside
- GST
glutathione S-transferase
- CD
circular dichroism
- CN-PAGE
clear native PAGE
- NAC
non-amyloid component region
- PTM
post-translational modifications
- RBC
red blood cells
- Nat
N-acetyltransferase
- NatB
N-acetyltransferase B
- SE-AUC
sedimentation equilibrium-analytical ultracentrifugation
- ELISA
enzyme-linked immunosorbent assay
- ThT
Thioflavin T
- SEC
Size exclusion chromatography
- SLS
static light scattering
- NMR
nuclear magnetic resonance
- ESI-MS
electrospray ionization-mass spectrometry
- ESI-IMS-MS
electrospray ionization-ion mobility spectrometry-mass spectrometry
References
- 1.Spillantini MG, Goedert M. The alpha-synucleinopathies: Parkinson's disease, dementia with Lewy bodies, and multiple system atrophy. Ann N Y Acad Sci. 2000;920:16–27. doi: 10.1111/j.1749-6632.2000.tb06900.x. [DOI] [PubMed] [Google Scholar]
- 2.Stefanis L. alpha-Synuclein in Parkinson's Disease. Cold Spring Harb Perspect Med. 2012;2:a009399. doi: 10.1101/cshperspect.a009399. doi: 10.1101/cshperspect.a009399 a009399 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hindle JV. Ageing, neurodegeneration and Parkinson's disease. Age and ageing. 2010;39:156–161. doi: 10.1093/ageing/afp223. doi: 10.1093/ageing/afp223. [DOI] [PubMed] [Google Scholar]
- 4.Spillantini MG, Crowther RA, Jakes R, Cairns NJ, Lantos PL, Goedert M. Filamentous alpha-synuclein inclusions link multiple system atrophy with Parkinson's disease and dementia with Lewy bodies. Neurosci Lett. 1998;251:205–208. doi: 10.1016/s0304-3940(98)00504-7. [DOI] [PubMed] [Google Scholar]
- 5.Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M. alpha-synuclein in filamentous inclusions of Lewy bodies from Parkinson's disease and dementia with Lewy bodies. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:6469–6473. doi: 10.1073/pnas.95.11.6469. doi: DOI 10.1073/pnas.95.11.6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Breydo L, Wu JW, Uversky VN. A-synuclein misfolding and Parkinson's disease. Biochimica et biophysica acta. 2012;1822:261–285. doi: 10.1016/j.bbadis.2011.10.002. doi: 10.1016/j.bbadis.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Fink AL. The aggregation and fibrillation of alpha-synuclein. Accounts Chem Res. 2006;39:628–634. doi: 10.1021/ar050073t. doi: Doi 10.1021/Ar050073t. [DOI] [PubMed] [Google Scholar]
- 8.Lashuel HA, Overk CR, Oueslati A, Masliah E. The many faces of alpha-synuclein: from structure and toxicity to therapeutic target. Nat Rev Neurosci. 2013;14:38–48. doi: 10.1038/nrn3406. doi: Doi 10.1038/Nrn3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maroteaux L, Campanelli JT, Scheller RH. SYNUCLEIN - A NEURON-SPECIFIC PROTEIN LOCALIZED TO THE NUCLEUS AND PRESYNAPTIC NERVE-TERMINAL. Journal of Neuroscience. 1988;8:2804–2815. doi: 10.1523/JNEUROSCI.08-08-02804.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shibayamaimazu T, Okahashi I, Omata K, Nakajo S, Ochiai H, Nakai Y, Hama T, Nakamura Y, Nakaya K. Cell and Tissue Distribution and Developmental-Change of Neuron-Specific 14 Kda Protein (Phosphoneuroprotein-14) Brain Res. 1993;622:17–25. doi: 10.1016/0006-8993(93)90796-p. doi: Doi 10.1016/0006-8993(93)90796-P. [DOI] [PubMed] [Google Scholar]
- 11.Jakes R, Spillantini MG, Goedert M. Identification of 2 Distinct Synucleins from Human Brain. FEBS letters. 1994;345:27–32. doi: 10.1016/0014-5793(94)00395-5. doi: Doi 10.1016/0014-5793(94)00395-5. [DOI] [PubMed] [Google Scholar]
- 12.Iwai A, Masliah E, Yoshimoto M, Ge N, Flanagan L, de Silva HA, Kittel A, Saitoh T. The precursor protein of non-A beta component of Alzheimer's disease amyloid is a presynaptic protein of the central nervous system. Neuron. 1995;14:467–475. doi: 10.1016/0896-6273(95)90302-x. doi: 0896-6273(95)90302-X [pii] [DOI] [PubMed] [Google Scholar]
- 13.Bottner M, Zorenkov D, Hellwig I, Barrenschee M, Harde J, Fricke T, Deuschl G, Egberts JH, Becker T, Fritscher-Ravens A, et al. Expression pattern and localization of alpha-synuclein in the human enteric nervous system. Neurobiol Dis. 2012;48:474–480. doi: 10.1016/j.nbd.2012.07.018. doi: 10.1016/j.nbd.2012.07.018 S0969-9961(12)00266-5 [pii] [DOI] [PubMed] [Google Scholar]
- 14.Barbour R, Kling K, Anderson JP, Banducci K, Cole T, Diep L, Fox M, Goldstein JM, Soriano F, Seubert P, et al. Red blood cells are the major source of alpha-synuclein in blood. Neurodegener Dis. 2008;5:55–59. doi: 10.1159/000112832. doi: 10.1159/000112832 000112832 [pii] [DOI] [PubMed] [Google Scholar]
- 15.Davidson WS, Jonas A, Clayton DF, George JM. Stabilization of alpha-synuclein secondary structure upon binding to synthetic membranes. Journal of Biological Chemistry. 1998;273:9443–9449. doi: 10.1074/jbc.273.16.9443. doi: DOI 10.1074/jbc.273.16.9443. [DOI] [PubMed] [Google Scholar]
- 16.Bisaglia M, Tessari I, Mammi S, Bubacco L. Interaction Between alpha-Synuclein and Metal Ions, Still Looking for a Role in the Pathogenesis of Parkinson's Disease. Neuromol Med. 2009;11:239–251. doi: 10.1007/s12017-009-8082-1. doi: DOI 10.1007/s12017-009-8082-1. [DOI] [PubMed] [Google Scholar]
- 17.Chandra S, Gallardo G, Fernandez-Chacon R, Schluter OM, Sudhof TC. Alpha-synuclein cooperates with CSPalpha in preventing neurodegeneration. Cell. 2005;123:383–396. doi: 10.1016/j.cell.2005.09.028. doi: S0092-8674(05)01022-6 [pii] 10.1016/j.cell.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 18.da Costa CA, Ancolio K, Checler F. Wild-type but not Parkinson's disease-related ala-53 --> Thr mutant alpha -synuclein protects neuronal cells from apoptotic stimuli. J Biol Chem. 2000;275:24065–24069. doi: 10.1074/jbc.M002413200. doi: 10.1074/jbc.M002413200 M002413200 [pii] [DOI] [PubMed] [Google Scholar]
- 19.Murphy DD, Rueter SM, Trojanowski JQ, Lee VMY. Synucleins are developmentally expressed, and alpha-synuclein regulates the size of the presynaptic vesicular pool in primary hippocampal neurons. Journal of Neuroscience. 2000;20:3214–3220. doi: 10.1523/JNEUROSCI.20-09-03214.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dev KK, Hofele K, Barbieri S, Buchman VL, van der Putten H. Part II: alpha-synuclein and its molecular pathophysiological role in neurodegenerative disease. Neuropharmacology. 2003;45:14–44. doi: 10.1016/s0028-3908(03)00140-0. doi: S0028390803001400 [pii] [DOI] [PubMed] [Google Scholar]
- 21.Maries E, Dass B, Collier TJ, Kordower JH, Steece-Collier K. The role of alpha-synuclein in Parkinson's disease: Insights from animal models. Nat Rev Neurosci. 2003;4:727–738. doi: 10.1038/nrn1199. doi: Doi 10.1038/Nrn1199. [DOI] [PubMed] [Google Scholar]
- 22.Jin H, Kanthasamy A, Ghosh A, Yang Y, Anantharam V, Kanthasamy AG. alpha-Synuclein negatively regulates protein kinase Cdelta expression to suppress apoptosis in dopaminergic neurons by reducing p300 histone acetyltransferase activity. J Neurosci. 2011;31:2035–2051. doi: 10.1523/JNEUROSCI.5634-10.2011. doi: 10.1523/JNEUROSCI.5634-10.2011 31/6/2035 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uversky VN. A protein-chameleon: Conformational plasticity of alpha-synuclein, a disordered protein involved in neurodegenerative disorders. Journal of Biomolecular Structure & Dynamics. 2003;21:211–234. doi: 10.1080/07391102.2003.10506918. [DOI] [PubMed] [Google Scholar]
- 24.Uversky VN, Fink AL. Conformational constraints for amyloid fibrillation: the importance of being unfolded. Bba-Proteins Proteom. 2004;1698:131–153. doi: 10.1016/j.bbapap.2003.12.008. doi: DOI 10.1016/j.bbapap.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 25.Bartels T, Choi JG, Selkoe DJ. α-Synuclein occurs physiologically as a helically folded tetramer that resists aggregation. Nature. 2011;477:107–110. doi: 10.1038/nature10324. doi: 10.1038/nature10324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang W, Perovic I, Chittuluru J, Kaganovich a, Nguyen LTT, Liao J, Auclair JR, Johnson D, Landeru a, Simorellis aK, et al. A soluble α-synuclein construct forms a dynamic tetramer. Proceedings of the National Academy of Sciences. 2011 doi: 10.1073/pnas.1113260108. doi: 10.1073/pnas.1113260108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Binolfi A, Theillet FX, Selenko P. Bacterial in-cell NMR of human alpha-synuclein: a disordered monomer by nature? Biochem Soc T. 2012;40:950–U292. doi: 10.1042/BST20120096. doi: Doi 10.1042/Bst20120096. [DOI] [PubMed] [Google Scholar]
- 28.Fauvet B, Fares MB, Samuel F, Dikiy I, Tandon A, Eliezer D, Lashuel HA. Characterization of Semisynthetic and Naturally N-alpha-Acetylated alpha-Synuclein in Vitro and in Intact Cells IMPLICATIONS FOR AGGREGATION AND CELLULAR PROPERTIES OF alpha-SYNUCLEIN. Journal of Biological Chemistry. 2012;287:28243–28262. doi: 10.1074/jbc.M112.383711. doi: DOI 10.1074/jbc.M112.383711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fauvet B, Mbefo MK, Fares M-B, Desobry C, Michael S, Ardah MT, Tsika E, Coune P, Prudent M, Lion N, et al. Alpha-synuclein in the central nervous system and from erythrocytes, mammalian cells and E. coli exists predominantly as a disordered monomer. The Journal of biological chemistry. 2012;287:15345–15364. doi: 10.1074/jbc.M111.318949. doi: 10.1074/jbc.M111.318949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kang LJ, Moriarty GM, Woods LA, Ashcroft AE, Radford SE, Baum J. N-terminal acetylation of alpha-synuclein induces increased transient helical propensity and decreased aggregation rates in the intrinsically disordered monomer. Protein Sci. 2012;21:911–917. doi: 10.1002/pro.2088. doi: Doi 10.1002/Pro.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maltsev AS, Ying JF, Bax A. Impact of N-Terminal Acetylation of alpha-Synuclein on Its Random Coil and Lipid Binding Properties. Biochemistry. 2012;51:5004–5013. doi: 10.1021/bi300642h. doi: Doi 10.1021/Bi300642h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trexler AJ, Rhoades E. N-terminal acetylation is critical for forming a-helical oligomer of a-synuclein. Protein Sci. 2012;21:601–605. doi: 10.1002/pro.2056. doi: Doi 10.1002/Pro.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dettmer U, Newman AJ, Luth ES, Bartels T, Selkoe D. In vivo crosslinking reveals principally oligomeric forms of alpha-synuclein and beta-synuclein in neurons and non-neural cells. Journal of Biological Chemistry. 2013 doi: 10.1074/jbc.M112.403311. doi: M112.403311 [pii] 10.1074/jbc.M112.403311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weinreb PH, Zhen W, Poon AW, Conway KA, Lansbury PT., Jr. NACP, a protein implicated in Alzheimer's disease and learning, is natively unfolded. Biochemistry. 1996;35:13709–13715. doi: 10.1021/bi961799n. doi: 10.1021/bi961799n bi961799n [pii] [DOI] [PubMed] [Google Scholar]
- 35.Bussell R, Eliezer D. A structural and functional role for 11-mer repeats in alpha-synuclein and other exchangeable lipid binding proteins. Journal of Molecular Biology. 2003;329:763–778. doi: 10.1016/s0022-2836(03)00520-5. doi: Doi 10.1016/S0022-2836(03)00520-5. [DOI] [PubMed] [Google Scholar]
- 36.Uversky VN, Li J, Fink AL. Evidence for a partially folded intermediate in alpha-synuclein fibril formation. Journal of Biological Chemistry. 2001;276:10737–10744. doi: 10.1074/jbc.M010907200. doi: DOI 10.1074/jbc.M010907200. [DOI] [PubMed] [Google Scholar]
- 37.Munishkina LA, Fink AL, Uversky VN. Accelerated Fibrillation of alpha-Synuclein Induced by the Combined Action of Macromolecular Crowding and Factors Inducing Partial Folding. Curr Alzheimer Res. 2009;6:252–260. doi: 10.2174/156720509788486491. [DOI] [PubMed] [Google Scholar]
- 38.Wu KP, Weinstock DS, Narayanan C, Levy RM, Baum J. Structural Reorganization of alpha-Synuclein at Low pH Observed by NMR and REMD Simulations. Journal of Molecular Biology. 2009;391:784–796. doi: 10.1016/j.jmb.2009.06.063. doi: DOI 10.1016/j.jmb.2009.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hong D-P, Xiong W, Chang J-Y, Jiang C. The role of the C-terminus of human α-synuclein: intra-disulfide bonds between the C-terminus and other regions stabilize non-fibrillar monomeric isomers. FEBS letters. 2011;585:561–566. doi: 10.1016/j.febslet.2011.01.009. doi: 10.1016/j.febslet.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 40.Park SM, Jung HY, Kim TD, Park JH, Yang CH, Kim J. Distinct roles of the N-terminal-binding domain and the C-terminal-solubilizing domain of alpha-synuclein, a molecular chaperone. J Biol Chem. 2002;277:28512–28520. doi: 10.1074/jbc.M111971200. doi: 10.1074/jbc.M111971200 M111971200 [pii] [DOI] [PubMed] [Google Scholar]
- 41.Weinreb PH, Zhen WG, Poon AW, Conway KA, Lansbury PT. NACP, a protein implicated in Alzheimer's disease and learning, is natively unfolded. Biochemistry. 1996;35:13709–13715. doi: 10.1021/bi961799n. doi: 10.1021/bi961799n. [DOI] [PubMed] [Google Scholar]
- 42.Giasson BI, Uryu K, Trojanowski JQ, Lee VMY. Mutant and wild type human alpha-synucleins assemble into elongated filaments with distinct morphologies in vitro. Journal of Biological Chemistry. 1999;274:7619–7622. doi: 10.1074/jbc.274.12.7619. doi: 10.1074/jbc.274.12.7619. [DOI] [PubMed] [Google Scholar]
- 43.Soppa J. Protein acetylation in archaea, bacteria, and eukaryotes. Archaea (Vancouver, BC) 2010;2010 doi: 10.1155/2010/820681. doi: 10.1155/2010/820681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Uversky VN. Natively unfolded proteins : A point where biology waits for physics. Protein science : a publication of the Protein Society. 2002;11:739–756. doi: 10.1110/ps.4210102. doi: 10.1110/ps.4210102.matic. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Uversky VN. Use of fast protein size-exclusion liquid chromatography to study the unfolding of proteins which denature through the molten globule. Biochemistry. 1993;32:13288–13298. doi: 10.1021/bi00211a042. [DOI] [PubMed] [Google Scholar]
- 46.Dunker AK, Lawson JD, Brown CJ, Williams RM, Romero P, Oh JS, Oldfield CJ, Campen AM, Ratliff CR, Hipps KW, et al. Intrinsically disordered protein. Journal of Molecular Graphics & Modelling. 2001;19:26–59. doi: 10.1016/s1093-3263(00)00138-8. doi: 10.1016/s1093-3263(00)00138-8. [DOI] [PubMed] [Google Scholar]
- 47.Eliezer D, Kutluay E, Bussell R, Browne G. Conformational properties of alpha-synuclein in its free and lipid-associated states. Journal of Molecular Biology. 2001;307:1061–1073. doi: 10.1006/jmbi.2001.4538. [DOI] [PubMed] [Google Scholar]
- 48.Allison JR, Varnai P, Dobson CM, Vendruscolo M. Determination of the Free Energy Landscape of alpha-Synuclein Using Spin Label Nuclear Magnetic Resonance Measurements. Journal of the American Chemical Society. 2009;131:18314–18326. doi: 10.1021/ja904716h. doi: Doi 10.1021/Ja904716h. [DOI] [PubMed] [Google Scholar]
- 49.Salmon L, Nodet G, Ozenne V, Yin G, Jensen MR, Zweckstetter M, Blackledge M. NMR Characterization of Long-Range Order in Intrinsically Disordered Proteins. Journal of the American Chemical Society. 2010;132:8407–8418. doi: 10.1021/ja101645g. doi: 10.1021/ja101645g. [DOI] [PubMed] [Google Scholar]
- 50.Bertoncini CW, Jung YS, Fernandez CO, Hoyer W, Griesinger C, Jovin TM, Zweckstetter M. Release of long-range tertiary interactions potentiates aggregation of natively unstructured alpha-synuclein. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:1430–1435. doi: 10.1073/pnas.0407146102. doi: DOI 10.1073/pnas.0407146102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bernado P, Bertoncini CW, Griesinger C, Zweckstetter M, Blackledge M. Defining long-range order and local disorder in native alpha-synuclein using residual dipolar couplings. Journal of the American Chemical Society. 2005;127:17968–17969. doi: 10.1021/ja055538p. doi: Doi 10.1021/Ja055538p. [DOI] [PubMed] [Google Scholar]
- 52.Dedmon MM, Lindorff-Larsen K, Christodoulou J, Vendruscolo M, Dobson CM. Mapping long-range interactions in alpha-synuclein using spin-label NMR and ensemble molecular dynamics simulations. Journal of the American Chemical Society. 2005;127:476–477. doi: 10.1021/ja044834j. doi: 10.1021/ja044834j. [DOI] [PubMed] [Google Scholar]
- 53.Cho MK, Kim HY, Bernado P, Fernandez CO, Blackledge M, Zweckstetter M. Amino acid bulkiness defines the local conformations and dynamics of natively unfolded alpha-synuclein and tau. Journal of the American Chemical Society. 2007;129:3032. doi: 10.1021/ja067482k. +, doi: Doi 10.1021/Ja067482k. [DOI] [PubMed] [Google Scholar]
- 54.Wu KP, Baum J. Detection of Transient Interchain Interactions in the Intrinsically Disordered Protein alpha-Synuclein by NMR Paramagnetic Relaxation Enhancement. Journal of the American Chemical Society. 2010;132:5546. doi: 10.1021/ja9105495. +, doi: Doi 10.1021/Ja9105495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kang L, Wu K-P, Vendruscolo M, Baum J. The A53T mutation is key in defining the differences in the aggregation kinetics of human and mouse α-synuclein. Journal of the American Chemical Society. 2011;133:13465–13470. doi: 10.1021/ja203979j. doi: 10.1021/ja203979j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rao JN, Jao CC, Hegde BG, Langen R, Ulmer TS. A Combinatorial NMR and EPR Approach for Evaluating the Structural Ensemble of Partially Folded Proteins. Journal of the American Chemical Society. 2010;132:8657–8668. doi: 10.1021/ja100646t. doi: 10.1021/ja100646t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee JC, Lai BT, Kozak JJ, Gray HB, Winkler JR. alpha-Synuclein tertiary contact dynamics. Journal of Physical Chemistry B. 2007;111:2107–2112. doi: 10.1021/jp068604y. doi: 10.1021/jp068604y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cho MK, Nodet G, Kim HY, Jensen MR, Bernado P, Fernandez CO, Becker S, Blackledge M, Zweckstetter M. Structural characterization of alpha-synuclein in an aggregation prone state. Protein Science. 2009;18:1840–1846. doi: 10.1002/pro.194. doi: Doi 10.1002/Pro.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bussell R, Eliezer D. Residual structure and dynamics in Parkinson's disease-associated mutants of alpha-synuclein. Journal of Biological Chemistry. 2001;276:45996–46003. doi: 10.1074/jbc.M106777200. doi: DOI 10.1074/jbc.M106777200. [DOI] [PubMed] [Google Scholar]
- 60.Sung Y-H, Eliezer D. Residual structure, backbone dynamics, and interactions within the synuclein family. 2008;372:689–707. doi: 10.1016/j.jmb.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Narayanan C, Weinstock DS, Wu KP, Baum J, Levy RM. Investigation of the Polymeric Properties of alpha-Synuclein and Comparison with NMR Experiments: A Replica Exchange Molecular Dynamics Study. J Chem Theory Comput. 2012;8:3929–3942. doi: 10.1021/ct300241t. doi: Doi 10.1021/Ct300241t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dyson HJ, Wright PE. Intrinsically unstructured proteins and their functions. Nature Reviews Molecular Cell Biology. 2005;6:197–208. doi: 10.1038/nrm1589. doi: 10.1038/nrm1589. [DOI] [PubMed] [Google Scholar]
- 63.Hoyer W, Cherny D, Subramaniam V, Jovin TM. Impact of the acidic C-terminal region comprising amino acids 109-140 on alpha-synuclein aggregation in vitro. Biochemistry. 2004;43:16233–16242. doi: 10.1021/bi048453u. doi: 10.1021/bi048453u. [DOI] [PubMed] [Google Scholar]
- 64.Bertoncini CW, Jung Y-S, Fernandez CO, Hoyer W, Griesinger C, Jovin TM, Zweckstetter M. Release of long-range tertiary interactions potentiates aggregation of natively unstructured alpha-synuclein. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:1430–1435. doi: 10.1073/pnas.0407146102. doi: 10.1073/pnas.0407146102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yap TL, Pfefferkorn CM, Lee JC. Residue-specific fluorescent probes of alpha-synuclein: detection of early events at the N- and C-termini during fibril assembly. Biochemistry. 2011;50:1963–1965. doi: 10.1021/bi2000824. doi: 10.1021/bi2000824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McClendon S, Rospigliosi CC, Eliezer D. Charge neutralization and collapse of the C-terminal tail of alpha-synuclein at low pH. Protein Sci. 2009;18:1531–1540. doi: 10.1002/pro.149. doi: Doi 10.1002/Pro.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Uversky VN, Li J, Fink aL. Evidence for a partially folded intermediate in alpha-synuclein fibril formation. The Journal of biological chemistry. 2001;276:10737–10744. doi: 10.1074/jbc.M010907200. doi: 10.1074/jbc.M010907200. [DOI] [PubMed] [Google Scholar]
- 68.Uversky VN, Li J, Souillac P, Millett IS, Doniach S, Jakes R, Goedert M, Fink AL. Biophysical properties of the synucleins and their propensities to fibrillate: inhibition of alpha-synuclein assembly by beta- and gamma-synucleins. The Journal of biological chemistry. 2002;277:11970–11978. doi: 10.1074/jbc.M109541200. doi: 10.1074/jbc.M109541200. [DOI] [PubMed] [Google Scholar]
- 69.Uversky VN, Lee H-JJ, Li J, Fink AL, Lee S-JJ. Stabilization of partially folded conformation during alpha-synuclein oligomerization in both purified and cytosolic preparations. The Journal of biological chemistry. 2001;276:43495–43498. doi: 10.1074/jbc.C100551200. doi: 10.1074/jbc.C100551200. [DOI] [PubMed] [Google Scholar]
- 70.Uversky VN, Li J, Fink aL. Metal-triggered structural transformations, aggregation, and fibrillation of human alpha-synuclein. A possible molecular NK between Parkinson's disease and heavy metal exposure. The Journal of biological chemistry. 2001;276:44284–44296. doi: 10.1074/jbc.M105343200. doi: 10.1074/jbc.M105343200. [DOI] [PubMed] [Google Scholar]
- 71.Uversky VN, Li J, Fink AL. Pesticides directly accelerate the rate of alpha-synuclein fibril formation: a possible factor in Parkinson's disease. Febs Letters. 2001;500:105–108. doi: 10.1016/s0014-5793(01)02597-2. doi: Doi 10.1016/S0014-5793(01)02597-2. [DOI] [PubMed] [Google Scholar]
- 72.Uversky VN, Li J, Bower K, Fink AL. Synergistic effects of pesticides and metals on the fibrillation of alpha-synuclein: Implications for Parkinson's disease. Neurotoxicology. 2002;23:527–536. doi: 10.1016/s0161-813x(02)00067-0. doi: Pii S0161-813x(02)00067-0 Doi 10.1016/S0161-813x(02)00067-0. [DOI] [PubMed] [Google Scholar]
- 73.Bertoncini CW, Fernandez CO, Griesinger C, Jovin TM, Zweckstetter M. Familial mutants of alpha-synuclein with increased neurotoxicity have a destabilized conformation. Journal of Biological Chemistry. 2005;280:30649–30652. doi: 10.1074/jbc.C500288200. doi: DOI 10.1074/jbc.C500288200. [DOI] [PubMed] [Google Scholar]
- 74.Conway KA, Harper JD, Lansbury PT. Accelerated in vitro fibril formation by a mutant alpha-synuclein linked to early-onset Parkinson disease. Nature Medicine. 1998;4:1318–1320. doi: 10.1038/3311. doi: 10.1038/3311. [DOI] [PubMed] [Google Scholar]
- 75.Li J, Uversky VN, Fink AL. Effect of familial Parkinson's disease point mutations A30P and A53T on the structural properties, aggregation, and fibrillation of human alpha-synuclein. Biochemistry. 2001;40:11604–11613. doi: 10.1021/bi010616g. doi: Doi 10.1021/Bi010616g. [DOI] [PubMed] [Google Scholar]
- 76.Li J, Uversky VN, Fink AL. Conformational behavior of human alpha-synuclein is modulated by familial Parkinson's disease point mutations A30P and A53T. Neurotoxicology. 2002;23:553–567. doi: 10.1016/s0161-813x(02)00066-9. doi: Pii S0161-813x(02)00066-9 Doi 10.1016/S0161-813x(02)00066-9. [DOI] [PubMed] [Google Scholar]
- 77.El-Agnaf OM, Jakes R, Curran MD, Wallace A. Effects of the mutations Ala30 to Pro and Ala53 to Thr on the physical and morphological properties of alpha-synuclein protein implicated in Parkinson's disease. Febs Letters. 1998;440:67–70. doi: 10.1016/s0014-5793(98)01419-7. doi: S0014-5793(98)01419-7 [pii] [DOI] [PubMed] [Google Scholar]
- 78.Greenbaum EA, Graves CL, Mishizen-Eberz AJ, Lupoli MA, Lynch DR, Englander SW, Axelsen PH, Giasson BI. The E46K mutation in alpha-synuclein increases amyloid fibril formation. Journal of Biological Chemistry. 2005;280:7800–7807. doi: 10.1074/jbc.M411638200. doi: 10.1074/jbc.M411638200. [DOI] [PubMed] [Google Scholar]
- 79.Wu KP, Kim S, Fela DA, Baum J. Characterization of conformational and dynamic properties of natively unfolded human and mouse alpha-synuclein ensembles by NMR: Implication for aggregation. Journal of Molecular Biology. 2008;378:1104–1115. doi: 10.1016/j.jmb.2008.03.017. doi: DOI 10.1016/j.jmb.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fredenburg RA, Rospigliosi C, Meray RK, Kessler JC, Lashuel HA, Eliezer D, Lansbury PT. The impact of the E46K mutation on the properties of alpha-synuclein in its monomeric and oligomeric states. Biochemistry. 2007;46:7107–7118. doi: 10.1021/bi7000246. doi: Doi 10.1021/Bi7000246. [DOI] [PubMed] [Google Scholar]
- 81.Rospigliosi CC, McClendon S, Schmid AW, Ramlall TF, Barre P, Lashuel HA, Eliezer D. E46K Parkinson's-Linked Mutation Enhances C-Terminal-to-N-Terminal Contacts in alpha-Synuclein. Journal of Molecular Biology. 2009;388:1022–1032. doi: 10.1016/j.jmb.2009.03.065. doi: 10.1016/j.jmb.2009.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ulmer TS, Bax A, Cole NB, Nussbaum RL. Structure and dynamics of micelle-bound human alpha-synuclein. The Journal of biological chemistry. 2005;280:9595–9603. doi: 10.1074/jbc.M411805200. doi: 10.1074/jbc.M411805200. [DOI] [PubMed] [Google Scholar]
- 83.Eliezer D, Kutluay E, Bussell R, Browne G. Conformational properties of alpha-synuclein in its free and lipid-associated states. Journal of molecular biology. 2001;307:1061–1073. doi: 10.1006/jmbi.2001.4538. doi: 10.1006/jmbi.2001.4538. [DOI] [PubMed] [Google Scholar]
- 84.Bartels T, Ahlstrom LS, Leftin A, Kamp F, Haass C, Brown MF, Beyer K. The N-terminus of the intrinsically disordered protein alpha-synuclein triggers membrane binding and helix folding. Biophys J. 2010;99:2116–2124. doi: 10.1016/j.bpj.2010.06.035. doi: 10.1016/j.bpj.2010.06.035 S0006-3495(10)00778-2 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Eliezer D, Kutluay E, Bussell R, Jr., Browne G. Conformational properties of alpha-synuclein in its free and lipid-associated states. J Mol Biol. 2001;307:1061–1073. doi: 10.1006/jmbi.2001.4538. doi: 10.1006/jmbi.2001.4538 S0022-2836(01)94538-3 [pii] [DOI] [PubMed] [Google Scholar]
- 86.Croke RL, Sallum CO, Watson E, Watt ED, Alexandrescu AT. Hydrogen exchange of monomeric alpha-synuclein shows unfolded structure persists at physiological temperature and is independent of molecular crowding in Escherichia coli. Protein Sci. 2008;17:1434–1445. doi: 10.1110/ps.033803.107. doi: 10.1110/ps.033803.107 ps.033803.107 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Trexler AJ, Rhoades E. alpha-Synuclein Binds Large Unilamellar Vesicles as an Extended Helix. Biochemistry. 2009;48:2304–2306. doi: 10.1021/bi900114z. doi: Doi 10.1021/Bi900114z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jao CC, Hegde BG, Chen J, Haworth IS, Langen R. Structure of membrane-bound alpha-synuclein from site-directed spin labeling and computational refinement. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:19666–19671. doi: 10.1073/pnas.0807826105. doi: 10.1073/pnas.0807826105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ulmer TS, Bax A, Cole NB, Nussbaum RL. Structure and dynamics of micelle-bound human alpha-synuclein. Journal of Biological Chemistry. 2005;280:9595–9603. doi: 10.1074/jbc.M411805200. doi: DOI 10.1074/jbc.M411805200. [DOI] [PubMed] [Google Scholar]
- 90.Bernstein SL, Liu DF, Wyttenbach T, Bowers MT, Lee JC, Gray HB, Winkler JR. alpha-synuclein: Stable compact and extended monomeric structures and pH dependence of dimer formation. J Am Soc Mass Spectr. 2004;15:1435–1443. doi: 10.1016/j.jasms.2004.08.003. doi: DOI 10.1016/j.jasms.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 91.Frimpong AK, Abzalimov RR, Uversky VN, Kaltashov IA. Characterization of intrinsically disordered proteins with electrospray ionization mass spectrometry: conformational heterogeneity of alpha-synuclein. Proteins-Structure Function and Bioinformatics. 2010;78:714–722. doi: 10.1002/prot.22604. doi: 10.1002/prot.22604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pivato M, De Franceschi G, Tosatto L, Frare E, Kumar D, Aioanei D, Brucale M, Tessari I, Bisaglia M, Samori B, et al. Covalent α-Synuclein Dimers: Chemico-Physical and Aggregation Properties. PloS one. 2012;7:e50027. doi: 10.1371/journal.pone.0050027. doi: 10.1371/journal.pone.0050027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Celej MS, Sarroukh R, Goormaghtigh E, Fidelio GD, Ruysschaert JM, Raussens V. Toxic prefibrillar alpha-synuclein amyloid oligomers adopt a distinctive antiparallel beta-sheet structure. Biochemical Journal. 2012;443:719–726. doi: 10.1042/BJ20111924. doi: BJ20111924 [pii] 10.1042/BJ20111924. [DOI] [PubMed] [Google Scholar]
- 94.Uversky VN, Lee HJ, Li J, Fink AL, Lee SJ. Stabilization of partially folded conformation during alpha-synuclein oligomerization in both purified and cytosolic preparations. Journal of Biological Chemistry. 2001;276:43495–43498. doi: 10.1074/jbc.C100551200. doi: DOI 10.1074/jbc.C100551200. [DOI] [PubMed] [Google Scholar]
- 95.Zhou W, Freed CR. Tyrosine-to-cysteine modification of human alpha-synuclein enhances protein aggregation and cellular toxicity. Journal of Biological Chemistry. 2004;279:10128–10135. doi: 10.1074/jbc.M307563200. doi: 10.1074/jbc.M307563200 M307563200 [pii] [DOI] [PubMed] [Google Scholar]
- 96.Goldberg MS, Lansbury PT. Is there a cause-and-effect relationship between alpha-synuclein fibrillization and Parkinson's disease? Nat Cell Biol. 2000;2:E115–E119. doi: 10.1038/35017124. [DOI] [PubMed] [Google Scholar]
- 97.Conway KA, Lee SJ, Rochet JC, Ding TT, Harper JD, Williamson RE, Lansbury PT. Accelerated oligomerization by Parkinson's disease linked alpha-synuclein mutants. Ann Ny Acad Sci. 2000;920:42–45. doi: 10.1111/j.1749-6632.2000.tb06903.x. [DOI] [PubMed] [Google Scholar]
- 98.Masliah E, Rockenstein E, Veinbergs I, Mallory M, Hashimoto M, Takeda A, Sagara Y, Sisk A, Mucke L. Dopaminergic loss and inclusion body formation in alpha-synuclein mice: Implications for neurodegenerative disorders. Science. 2000;287:1265–1269. doi: 10.1126/science.287.5456.1265. doi: 10.1126/science.287.5456.1265. [DOI] [PubMed] [Google Scholar]
- 99.Amer DAM, Irvine GB, El-Agnaf OMA. Inhibitors of alpha-synuclein oligomerization and toxicity: a future therapeutic strategy for Parkinson's disease and related disorders. Exp Brain Res. 2006;173:223–233. doi: 10.1007/s00221-006-0539-y. doi: DOI 10.1007/s00221-006-0539-y. [DOI] [PubMed] [Google Scholar]
- 100.Kayed R, Head E, Thompson JL, McIntire TM, Milton SC, Cotman CW, Glabe CG. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science. 2003;300:486–489. doi: 10.1126/science.1079469. doi: 10.1126/science.1079469. [DOI] [PubMed] [Google Scholar]
- 101.Wolfe KJ, Cyr DM. Amyloid in neurodegenerative diseases: friend or foe? Semin Cell Dev Biol. 2011;22:476–481. doi: 10.1016/j.semcdb.2011.03.011. doi: 10.1016/j.semcdb.2011.03.011 S1084-9521(11)00060-7 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid beta-peptide. Nat Rev Mol Cell Biol. 2007;8:101–112. doi: 10.1038/nrm2101. doi: nrm2101 [pii] 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- 103.Caughey B, Lansbury PT. Protofibrils, pores, fibrils, and neurodegeneration: Separating the responsible protein aggregates from the innocent bystanders. Annu Rev Neurosci. 2003;26:267–298. doi: 10.1146/annurev.neuro.26.010302.081142. doi: DOI 10.1146/annurev.neuro.26.010302.081142. [DOI] [PubMed] [Google Scholar]
- 104.Dobson CM. Protein misfolding, evolution and disease. Trends Biochem Sci. 1999;24:329–332. doi: 10.1016/s0968-0004(99)01445-0. doi: S0968-0004(99)01445-0 [pii] [DOI] [PubMed] [Google Scholar]
- 105.Ollis Aa, Postle K. ExbD Mutants Define Initial Stages in TonB Energization. Journal of molecular biology. 2011 doi: 10.1016/j.jmb.2011.11.005. doi: 10.1016/j.jmb.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cremades N, Cohen SIA, Deas E, Abramov AY, Chen AY, Orte A, Sandal M, Clarke RW, Dunne P, Aprile FA, et al. Direct Observation of the Interconversion of Normal and Toxic Forms of alpha-Synuclein. Cell. 2012;149:1048–1059. doi: 10.1016/j.cell.2012.03.037. doi: DOI 10.1016/j.cell.2012.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Apetri MM, Maiti NC, Zagorski MG, Carey PR, Anderson VE. Secondary structure of alpha-synuclein oligomers: characterization by raman and atomic force microscopy. Journal of Molecular Biology. 2006;355:63–71. doi: 10.1016/j.jmb.2005.10.071. doi: S0022-2836(05)01331-8 [pii] 10.1016/j.jmb.2005.10.071. [DOI] [PubMed] [Google Scholar]
- 108.Lee J-H, Lee I-H, Choe Y-J, Kang S, Kim HY, Gai W-P, Hahn J-S, Paik SR. Real-time analysis of amyloid fibril formation of alpha-synuclein using a fibrillation-state-specific fluorescent probe of JC-1. The Biochemical journal. 2009;418:311–323. doi: 10.1042/BJ20081572. doi: 10.1042/BJ20081572. [DOI] [PubMed] [Google Scholar]
- 109.Gsponer J, Haberthur U, Caflisch A. The role of side-chain interactions in the early steps of aggregation: Molecular dynamics simulations of an amyloid-forming peptide from the yeast prion Sup35. Proc Natl Acad Sci U S A. 2003;100:5154–5159. doi: 10.1073/pnas.0835307100. doi: 10.1073/pnas.0835307100 0835307100 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cheon M, Chang I, Mohanty S, Luheshi LM, Dobson CM, Vendruscolo M, Favrin G. Structural reorganisation and potential toxicity of oligomeric species formed during the assembly of amyloid fibrils. Plos Comput Biol. 2007;3:1727–1738. doi: 10.1371/journal.pcbi.0030173. doi: 07-PLCB-RA-0148 [pii] 10.1371/journal.pcbi.0030173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dusa A, Kaylor J, Edridge S, Bodner N, Hong DP, Fink AL. Characterization of oligomers during alpha-synuclein aggregation using intrinsic tryptophan fluorescence. Biochemistry. 2006;45:2752–2760. doi: 10.1021/bi051426z. doi: Doi 10.1021/Bi051426z. [DOI] [PubMed] [Google Scholar]
- 112.Bolognesi B, Kumita JR, Barros TP, Esbjorner EK, Luheshi LM, Crowther DC, Wilson MR, Dobson CM, Favrin G, Yerbury JJ. ANS binding reveals common features of cytotoxic amyloid species. ACS Chem Biol. 2010;5:735–740. doi: 10.1021/cb1001203. doi: 10.1021/cb1001203. [DOI] [PubMed] [Google Scholar]
- 113.Outeiro TF, Putcha P, Tetzlaff JE, Spoelgen R, Koker M, Carvalho F, Hyman BT, McLean PJ. Formation of toxic oligomeric alpha-synuclein species in living cells. PloS one. 2008;3:e1867. doi: 10.1371/journal.pone.0001867. doi: 10.1371/journal.pone.0001867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Campioni S, Mannini B, Zampagni M, Pensalfini A, Parrini C, Evangelisti E, Relini A, Stefani M, Dobson CM, Cecchi C, et al. A causative link between the structure of aberrant protein oligomers and their toxicity. Nat Chem Biol. 2010;6:140–147. doi: 10.1038/nchembio.283. doi: 10.1038/nchembio.283 nchembio.283 [pii] [DOI] [PubMed] [Google Scholar]
- 115.Kim HY, Cho MK, Kumar A, Maier E, Siebenhaar C, Becker S, Fernandez CO, Lashuel HA, Benz R, Lange A, et al. Structural Properties of Pore-Forming Oligomers of alpha-Synuclein. Journal of the American Chemical Society. 2009;131:17482–17489. doi: 10.1021/ja9077599. doi: Doi 10.1021/Ja9077599. [DOI] [PubMed] [Google Scholar]
- 116.Tsigelny IF, Sharikov Y, Wrasidlo W, Gonzalez T, Desplats PA, Crews L, Spencer B, Masliah E. Role of alpha-synuclein penetration into the membrane in the mechanisms of oligomer pore formation. Febs J. 2012;279:1000–1013. doi: 10.1111/j.1742-4658.2012.08489.x. doi: DOI 10.1111/j.1742-4658.2012.08489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Danzer KM, Haasen D, Karow AR, Moussaud S, Habeck M, Giese A, Kretzschmar H, Hengerer B, Kostka M. Different species of alpha-synuclein oligomers induce calcium influx and seeding. Journal of Neuroscience. 2007;27:9220–9232. doi: 10.1523/JNEUROSCI.2617-07.2007. doi: Doi 10.1523/Jneurosci.2617-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cole NB, Murphy DD, Grider T, Rueter S, Brasaemle D, Nussbaum RL. Lipid droplet binding and oligomerization properties of the Parkinson's disease protein alpha-synuclein. Journal of Biological Chemistry. 2002;277:6344–6352. doi: 10.1074/jbc.M108414200. doi: 10.1074/jbc.M108414200. [DOI] [PubMed] [Google Scholar]
- 119.Winner B, Jappelli R, Maji SK, Desplats Pa, Boyer L, Aigner S, Hetzer C, Loher T, Vilar M, Campioni S, et al. In vivo demonstration that alpha-synuclein oligomers are toxic. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:4194–4199. doi: 10.1073/pnas.1100976108. doi: 10.1073/pnas.1100976108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Conway KA, Lee SJ, Rochet JC, Ding TT, Harper JD, Williamson RE, Lansbury PT., Jr. Accelerated oligomerization by Parkinson's disease linked alpha-synuclein mutants. Ann N Y Acad Sci. 2000;920:42–45. doi: 10.1111/j.1749-6632.2000.tb06903.x. [DOI] [PubMed] [Google Scholar]
- 121.Conway KA, Lee SJ, Rochet JC, Ding TT, Williamson RE, Lansbury PT., Jr. Acceleration of oligomerization, not fibrillization, is a shared property of both alpha-synuclein mutations linked to early-onset Parkinson's disease: implications for pathogenesis and therapy. Proc Natl Acad Sci U S A. 2000;97:571–576. doi: 10.1073/pnas.97.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lashuel HA, Petre BM, Wall J, Simon M, Nowak RJ, Walz T, Lansbury PT. alpha-synuclein, especially the Parkinson's disease-associated mutants, forms pore-like annular and tubular protofibrils. Journal of Molecular Biology. 2002;322:1089–1102. doi: 10.1016/s0022-2836(02)00735-0. doi: Doi 10.1016/S0022-2836(02)00735-0. [DOI] [PubMed] [Google Scholar]
- 123.Kaylor J, Bodner N, Edridge S, Yamin G, Hong D-p, Fink AL. Characterization of Oligomeric Intermediates in a-Synuclein Fibrillation : FRET Studies of Y125W/Y133F/Y136F alpha-synuclein. Journal of molecular biology. 2005;353:357–372. doi: 10.1016/j.jmb.2005.08.046. doi: 10.1016/j.jmb.2005.08.046. [DOI] [PubMed] [Google Scholar]
- 124.Ding TT, Lee SJ, Rochet JC, Lansbury PT. Annular alpha-synuclein protofibrils are produced when spherical protofibrils are incubated in solution or bound to brain-derived membranes. Biochemistry. 2002;41:10209–10217. doi: 10.1021/bi020139h. doi: Doi 10.1021/Bi020139h. [DOI] [PubMed] [Google Scholar]
- 125.Pountney DL, Voelcker NH, Gai WP. Annular alpha-synuclein oligomers are potentially toxic agents in alpha-synucleinopathy. Hypothesis. Neurotoxicity Research. 2005;7:59–67. doi: 10.1007/BF03033776. [DOI] [PubMed] [Google Scholar]
- 126.Zerovnik E. Oligomerization preceding amyloid fibril formation: a process in common to intrinsically disordered and globular proteins. Network. 2011;22:154–161. doi: 10.3109/0954898X.2011.639842. doi: 10.3109/0954898X.2011.639842. [DOI] [PubMed] [Google Scholar]
- 127.Hong DP, Han S, Fink AL, Uversky VN. Characterization of the non-fibrillar alpha-synuclein oligomers. Protein Pept Lett. 2011;18:230–240. doi: 10.2174/092986611794578332. doi: BSP/ PPL/ E pub/0214 [pii] [DOI] [PubMed] [Google Scholar]
- 128.Munishkina LA, Phelan C, Uversky VN, Fink AL. Conformational behavior and aggregation of alpha-synuclein in organic solvents: modeling the effects of membranes. Biochemistry. 2003;42:2720–2730. doi: 10.1021/bi027166s. doi: 10.1021/bi027166s. [DOI] [PubMed] [Google Scholar]
- 129.Souza JM, Giasson BI, Chen QP, Lee VMY, Ischiropoulos H. Dityrosine cross-linking promotes formation of stable alpha-synuclein polymers - Implication of nitrative and oxidative stress in the pathogenesis of neurodegenerative synucleinopathies. Journal of Biological Chemistry. 2000;275:18344–18349. doi: 10.1074/jbc.M000206200. doi: 10.1074/jbc.M000206200. [DOI] [PubMed] [Google Scholar]
- 130.Krishnan S, Chi EY, Wood SJ, Kendrick BS, Li C, Garzon-Rodriguez W, Wypych J, Randolph TW, Narhi LO, Biere AL, et al. Oxidative dimer formation is the critical rate-limiting step for Parkinson's disease alpha-synuclein fibrillogenesis. Biochemistry. 2003;42:829–837. doi: 10.1021/bi026528t. doi: 10.1021/bi026528t. [DOI] [PubMed] [Google Scholar]
- 131.Norris EH, Giasson BI, Ischiropoulos H, Lee VMY. Effects of oxidative and nitrative challenges on alpha-synuclein fibrillogenesis involve distinct mechanisms of protein modifications. Journal of Biological Chemistry. 2003;278:27230–27240. doi: 10.1074/jbc.M212436200. doi: 10.1074/jbc.M212436200. [DOI] [PubMed] [Google Scholar]
- 132.Hodara R, Norris EH, Giasson BI, Mishizen-Eberz AJ, Lynch DR, Lee VM-YM-Y, Ischiropoulos H. Functional consequences of alpha-synuclein tyrosine nitration: diminished binding to lipid vesicles and increased fibril formation. The Journal of biological chemistry. 2004;279:47746–47753. doi: 10.1074/jbc.M408906200. doi: 10.1074/jbc.M408906200. [DOI] [PubMed] [Google Scholar]
- 133.Yamin G, Glaser CB, Uversky VN, Fink AL. Certain metals trigger fibrillation of methionine-oxidized alpha-synuclein. Journal of Biological Chemistry. 2003;278:27630–27635. doi: 10.1074/jbc.M303302200. doi: DOI 10.1074/jbc.M303302200. [DOI] [PubMed] [Google Scholar]
- 134.Jo EJ, McLaurin J, Yip CM, St George-Hyslop P, Fraser PE. alpha-synuclein membrane interactions and lipid specificity. Journal of Biological Chemistry. 2000;275:34328–34334. doi: 10.1074/jbc.M004345200. doi: 10.1074/jbc.M004345200. [DOI] [PubMed] [Google Scholar]
- 135.Lee HJ, Choi C, Lee SJ. Membrane-bound alpha-synuclein has a high aggregation propensity and the ability to seed the aggregation of the cytosolic form. Journal of Biological Chemistry. 2002;277:671–678. doi: 10.1074/jbc.M107045200. doi: DOI 10.1074/jbc.M107045200. [DOI] [PubMed] [Google Scholar]
- 136.Wright JA, Wang X, Brown DR. Unique copper-induced oligomers mediate alpha-synuclein toxicity. Faseb Journal. 2009;23:2384–2393. doi: 10.1096/fj.09-130039. doi: 10.1096/fj.09-130039. [DOI] [PubMed] [Google Scholar]
- 137.Haque F, Pandey AP, Cambrea LR, Rochet J-C, Hovis JS. Adsorption of alpha-Synuclein on Lipid Bilayers: Modulating the Structure and Stability ofProtein Assemblies. Journal of Physical Chemistry B. 2010;114:4070–4081. doi: 10.1021/jp1006704. doi: 10.1021/jp1006704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Munishkina LA, Fink AL, Uversky VN. Concerted Action of Metals and Macromolecular Crowding on the Fibrillation of alpha-Synuclein. Protein and Peptide Letters. 2008;15:1079–1085. doi: 10.2174/092986608786071102. doi: 10.2174/092986608786071102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Uversky VN, Li J, Fink AL. Metal-triggered structural transformations, aggregation, and fibrillation of human alpha-synuclein - A possible molecular link between Parkinson's disease and heavy metal exposure. Journal of Biological Chemistry. 2001;276:44284–44296. doi: 10.1074/jbc.M105343200. doi: DOI 10.1074/jbc.M105343200. [DOI] [PubMed] [Google Scholar]
- 140.Santner A, Uversky VN. Metalloproteomics and metal toxicology of alpha-synuclein. Metallomics. 2010;2:378–392. doi: 10.1039/b926659c. doi: 10.1039/b926659c. [DOI] [PubMed] [Google Scholar]
- 141.Lowe R, Pountney DL, Jensen PH, Gai WEIP. Calcium ( II ) selectively induces α-synuclein annular oligomers via interaction with the C-terminal domain. 2004. pp. 3245–3252. doi: 10.1110/ps.04879704.its. [DOI] [PMC free article] [PubMed]
- 142.Uversky VN, Yamin G, Souillac PO, Goers J, Glaser CB, Fink AL. Methionine oxidation inhibits fibrillation of human alpha-synuclein in vitro. Febs Letters. 2002;517:239–244. doi: 10.1016/s0014-5793(02)02638-8. doi: Pii S0014-5793(02)02638-8 Doi 10.1016/S0014-5793(02)02638-8. [DOI] [PubMed] [Google Scholar]
- 143.Glaser CB, Yamin G, Uversky VN, Fink AL. Methionine oxidation, alpha-synuclein and Parkinson's disease. Bba-Proteins Proteom. 2005;1703:157–169. doi: 10.1016/j.bbapap.2004.10.008. doi: DOI 10.1016/j.bbapap.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 144.Zhou W, Long C, Reaney SH, Di Monte DA, Fink AL, Uversky VN. Methionine oxidation stabilizes non-toxic oligomers of alpha-synuclein through strengthening the auto-inhibitory intra-molecular long-range interactions. Biochimica Et Biophysica Acta-Molecular Basis of Disease. 2010;1802:322–330. doi: 10.1016/j.bbadis.2009.12.004. doi: 10.1016/j.bbadis.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hong DP, Fink AL, Uversky VN. Structural Characteristics of alpha-Synuclein Oligomers Stabilized by the Flavonoid Baicalein. Journal of Molecular Biology. 2008;383:214–223. doi: 10.1016/j.jmb.2008.08.039. doi: DOI 10.1016/j.jmb.2008.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ladiwala AR, Litt J, Kane RS, Aucoin DS, Smith SO, Ranjan S, Davis J, Van Nostrand WE, Tessier PM. Conformational differences between two amyloid beta oligomers of similar size and dissimilar toxicity. J Biol Chem. 2012;287:24765–24773. doi: 10.1074/jbc.M111.329763. doi: 10.1074/jbc.M111.329763 M111.329763 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lee KW, Chen W, Junn E, Im JY, Grosso H, Sonsalla PK, Feng XY, Ray N, Fernandez JR, Chao Y, et al. Enhanced Phosphatase Activity Attenuates alpha-Synucleinopathy in a Mouse Model. Journal of Neuroscience. 2011;31:6963–6971. doi: 10.1523/JNEUROSCI.6513-10.2011. doi: Doi 10.1523/Jneurosci.6513-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ohrfelt A, Zetterberg H, Andersson K, Persson R, Secic D, Brinkmalm G, Wallin A, Mulugeta E, Francis PT, Vanmechelen E, et al. Identification of Novel α-Synuclein Isoforms in Human Brain Tissue by using an Online NanoLC-ESI-FTICR-MS Method. Neurochemical research. 2011 doi: 10.1007/s11064-011-0527-x. doi: 10.1007/s11064-011-0527-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Anderson JP, Walker DE, Goldstein JM, de Laat R, Banducci K, Caccavello RJ, Barbour R, Huang JP, Kling K, Lee M, et al. Phosphorylation of Ser-129 is the dominant pathological modification of alpha-synuclein in familial and sporadic Lewy body disease. Journal of Biological Chemistry. 2006;281:29739–29752. doi: 10.1074/jbc.M600933200. doi: DOI 10.1074/jbc.M600933200. [DOI] [PubMed] [Google Scholar]
- 150.Polevoda B, Norbeck J, Takakura H, Blomberg a, Sherman F. Identification and specificities of N-terminal acetyltransferases from Saccharomyces cerevisiae. The EMBO journal. 1999;18:6155–6168. doi: 10.1093/emboj/18.21.6155. doi: 10.1093/emboj/18.21.6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Polevoda B, Sherman F. N-terminal Acetyltransferases and Sequence Requirements for N-terminal Acetylation of Eukaryotic Proteins. Journal of Molecular Biology. 2003;325:595–622. doi: 10.1016/s0022-2836(02)01269-x. doi: 10.1016/S0022-2836(02)01269-X. [DOI] [PubMed] [Google Scholar]
- 152.Starheim KK, Gromyko D, Velde R, Varhaug JE, Arnesen T. Composition and biological significance of the human Nalpha-terminal acetyltransferases. BMC Proc. 2009;3(Suppl 6):S3. doi: 10.1186/1753-6561-3-S6-S3. doi: 10.1186/1753-6561-3-S6-S3 1753-6561-3-S6-S3 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Van Damme P, Arnesen T, Gevaert K. Protein alpha-N-acetylation studied by N-terminomics. The FEBS journal. 2011;278:3822–3834. doi: 10.1111/j.1742-4658.2011.08230.x. doi: 10.1111/j.1742-4658.2011.08230.x. [DOI] [PubMed] [Google Scholar]
- 154.Helbig AO, Gauci S, Raijmakers R, van Breukelen B, Slijper M, Mohammed S, Heck AJR. Profiling of N-acetylated protein termini provides in-depth insights into the N-terminal nature of the proteome. Molecular & cellular proteomics : MCP. 2010;9:928–939. doi: 10.1074/mcp.M900463-MCP200. doi: 10.1074/mcp.M900463-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zabrocki P, Bastiaens I, Delay C, Bammens T, Ghillebert R, Pellens K, De Virgilio C, Van Leuven F, Winderickx J. Phosphorylation, lipid raft interaction and traffic of alpha-synuclein in a yeast model for Parkinson. Biochimica et biophysica acta. 2008;1783:1767–1780. doi: 10.1016/j.bbamcr.2008.06.010. doi: 10.1016/j.bbamcr.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 156.Arnesen T. Towards a functional understanding of protein N-terminal acetylation. PLoS biology. 2011;9:e1001074. doi: 10.1371/journal.pbio.1001074. doi: 10.1371/journal.pbio.1001074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Forte GM, Pool MR, Stirling CJ. N-terminal acetylation inhibits protein targeting to the endoplasmic reticulum. Plos Biol. 2011;9:e1001073. doi: 10.1371/journal.pbio.1001073. doi: 10.1371/journal.pbio.1001073 PBIOLOGY-D-10-01235 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hwang C-S, Shemorry A, Varshavsky A. N-terminal acetylation of cellular proteins creates specific degradation signals. Science (New York, NY) 2010;327:973–977. doi: 10.1126/science.1183147. doi: 10.1126/science.1183147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Jornvall H. Acetylation of Protein N-terminal amino groups structural observations on alpha-amino acetylated proteins. J Theor Biol. 1975;55:1–12. doi: 10.1016/s0022-5193(75)80105-6. [DOI] [PubMed] [Google Scholar]
- 160.Helbig AO, Rosati S, Pijnappel PWWM, van Breukelen B, Timmers MHTH, Mohammed S, Slijper M, Heck AJR. Perturbation of the yeast N-acetyltransferase NatB induces elevation of protein phosphorylation levels. BMC genomics. 2010;11:685. doi: 10.1186/1471-2164-11-685. doi: 10.1186/1471-2164-11-685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Greenfield NJ, Stafford WF, Hitchcock-DeGregori SE. The effect of N-terminal acetylation on the structure of an N-terminal tropomyosin peptide and alpha alpha-tropomyosin. Protein science : a publication of the Protein Society. 1994;3:402–410. doi: 10.1002/pro.5560030304. doi: 10.1002/pro.5560030304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Gast K, Fiedler C. Dynamic and static light scattering of intrinsically disordered proteins. Methods Mol Biol. 2012;896:137–161. doi: 10.1007/978-1-4614-3704-8_9. doi: 10.1007/978-1-4614-3704-8_9. [DOI] [PubMed] [Google Scholar]
- 163.El-Agnaf OMA, Salem SA, Paleologou KE, Curran MD, Gibson MJ, Court JA, Schlossmacher MG, Allsop D. Detection of oligomeric forms of alpha-synuclein protein in human plasma as a potential biomarker for Parkinson's disease. Faseb Journal. 2006;20:419–425. doi: 10.1096/fj.03-1449com. doi: DOI 10.1096/fj.03-1449com. [DOI] [PubMed] [Google Scholar]
- 164.Johnson M, Coulton AT, Geeves Ma, Mulvihill DP. Targeted amino-terminal acetylation of recombinant proteins in E. coli. PloS one. 2010;5:e15801. doi: 10.1371/journal.pone.0015801. doi: 10.1371/journal.pone.0015801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Aurora R, Rose GD. Helix capping. Protein Sci. 1998;7:21–38. doi: 10.1002/pro.5560070103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Chakrabartty A, Doig AJ, Baldwin RL. Helix Capping Propensities in Peptides Parallel Those in Proteins. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:11332–11336. doi: 10.1073/pnas.90.23.11332. doi: DOI 10.1073/pnas.90.23.11332. [DOI] [PMC free article] [PubMed] [Google Scholar]