Abstract
Background: In women with early ovarian cancer (EOC), comprehensive surgical staging is known to enhance ovarian cancer outcomes and requires specific surgical competence. Given that centralization of care remains a topic of continuing debate, a system of “guest operations” was introduced in the midwestern part of The Netherlands. During a guest operation a gynecologic oncologist participates in oncology surgery performed in the community hospital. Objective: This study was conducted to examine the effects of the presence of a gynecologic oncologist on the quality of staging, treatment, and survival in patients with EOC. Materials and Methods: All patients with a pathologically confirmed diagnosis of EOC between January 2000 and December 2009 were selected from a regional cancer registry. Surgical accuracy was checked on the basis of each patient's file, operative notes, and pathology report. Results: A total of 130 patients were included, of whom 15 were treated in the Leiden University Medical Center (LUMC) and 115 in eight regional community hospitals. If a gynecologic oncologist attended the operation, surgical staging was more often adequately performed, 81.1% versus 32.1% when a gynecologic oncologist was not present (p<0.001). Adherence to protocol was observed in 76.9% of operations when a gynecologic oncologist had been present, compared to 49.5% of patients who were treated by a general gynecologist alone (p=0.004). The 5-year disease-free survival was borderline significantly in favor of optimally staged patients, 75.1% in those who were not staged optimally versus 90.9% who were staged optimally (p=0.058). Conclusions: Guest operations deserve a distinguished place among the treatment modalities available to patients with EOC, because surgery by the most specialized and experienced surgeons contributes to better care. (J GYNECOL SURG 30:265)
Introduction
The issue of centralization of oncology treatment is a hot topic in the discussion on optimal health care patterns. Some oncology treatments represent high complex–low volume procedures, and it has been demonstrated that the risk of complications is inversely related to the volume of these procedures in a given hospital.1,2 Ovarian cancer has an incidence of ∼15 per 100,000 women per year,3 but only one-fourth of these cases are encountered in an early stage.4 Furthermore, a complete and comprehensive surgical staging procedure is an accurate and extensive task requiring specific expertise of the surgeon.5–8 This makes surgical management and staging of early ovarian cancer a high complex–low volume occurrence.
The need for centralization of surgical staging of early ovarian cancer (EOC) has been discussed in The Netherlands for some time now. Referral of all patients with (suspected) EOC to an oncology center has been considered impractical or undesirable for several reasons. EOC is not infrequently diagnosed unexpectedly when a patient is operated for an ovarian cyst that is sometimes complicated by acute clinical symptoms.9 Routine referral of all women with ovarian cysts to oncology centers meets reluctance among most gynecologists in community hospitals, because it narrows their range of clinical activities. In addition, the oncology centers might be confronted with a capacity problem if all patients with suspected ovarian cancer were to be referred to these centers even when discriminative guidelines, such as the risk of malignancy index (RMI), are used to diagnose these cases.10,11 Last but not least, sometimes financial considerations are involved.12
The Netherlands is a densely populated, small country with a population of ∼16.5 million people. The infrastructure for transportation is excellent, and virtually every part of the country is within a 15-minute distance from a hospital. These factors have contributed to an alternative pattern of care that is considered a compromise between centralization and noncentralization. This compromise means that gynecologic oncologists from the oncology centers travel to the community hospitals in the areas to perform cancer surgery there, together with the local gynecologists. This system of “guest-operations” is widely adopted in the regions of the nine oncology centers in the country.13,14
Like all compromises the system of guest operations also has weaknesses. One is travel time for the attending gynecologic oncologist and the other is lack of control, because the initiative to invite a gynecologic oncologist to attend an operation rests solely with the local gynecologist.
In this article, the current authors analyzed the recent practice of these guest operations for EOC in the community hospitals surrounding the oncology center of the Leiden University Medical Center (LUMC) in the Netherlands.
Materials and Methods
Patients and evaluation
The Comprehensive Cancer Center–The Netherlands location in Leiden (CCCN-L) is in the midwestern part of The Netherlands and comprises a partially urban, partially rural area. Cities, such as Leiden, The Hague, Gouda, and Delft, are part of the CCCN-L's sphere, and the area has nearly 1 million female inhabitants. The region has eight community hospitals and one university hospital (LUMC) acting as the oncology center for this region. All patients with a pathologically confirmed diagnosis of EOC (International Federation of Gynecology and Obstetrics [FIGO] stage Ia–IIa) in the period 2000–2009 were selected from the regional cancer registry of the CCCN-L. Data for patients with prior or concomitant second malignancies were excluded from the analysis.
At first, data from 139 women's records were considered for inclusion. Data on patient characteristics, tumor classification, pathology, surgical factors, and adjuvant treatment were extracted from the medical records. These data were initially provided by the CCCN-L, but, as substantial treatment considerations were lacking, they were complemented with data from the original patient files provided by the community hospitals. After revision, data from 9 patients were excluded because comprehensive study of these patient records revealed that these cases had to be considered advanced rather than early stage ovarian cancer. These determinations were made together with the general gynecologists in the community hospitals. Eventually, data from 130 patients who were diagnosed with EOC were included in the analysis. Tumors were staged according to FIGO guidelines.15
One of the issues in the gynecologic oncology protocol of the CCCN-L is that a gynecologic oncologist should preferably attend the surgical staging procedures in a community hospital if a patient is not referred for treatment to the university center. Data regarding the presence of a gynecologic oncologist were therefore also documented. Patients who were initially operated on in a community hospital but later referred to the LUMC for staging laparotomy were considered as being treated in the latter location.
The follow-up period lasted from the date of surgery to the end of the study in October 2012. Disease-free survival was defined as the period from the surgical intervention to cytologically or histologically proven evidence of recurrent disease. Salvage treatment of patients who had recurrences consisted of chemotherapy and/or radiotherapy, depending on each individual tumor's extent and location. Overall survival was defined as the time from date of operation to death or date last seen.
Surgical staging
The adequacy of each staging procedure was checked on the basis of the operative notes and the pathology reports. Surgery was considered adequate if all of the following staging requirements were met: inspection and palpation of all peritoneal surfaces; peritoneal washing for cytology analysis; removal of the affected and contralateral ovary; abdominal hysterectomy; biopsies of any suspect area for metastases; blind biopsies from the peritoneum in the pelvis (bladder, pelvic sidewalls, and pouch of Douglas); right and left paracolic gutters; right hemidiaphragm; and an infracolic omentectomy. In addition, iliac and periaortic lymph-node sampling had to be performed.15–19 The minimum number of lymph nodes removed to ensure adequate sampling had changed over time: before 2006, extirpating one periaortic lymph node was considered sufficient indication that the relevant lymph-node bearing tissue was at least inspected and assessed directly. From 2006 on, at least two periaortic lymph nodes and two iliac lymph nodes from different sites had to be removed, and, in 2009, a new guideline required that at least ten lymph nodes had to be removed.
In case of a stage Ia well-differentiated tumor, it was permissible to leave the uterus and the contralateral ovary in situ in order to preserve fertility.
Patients in whom surgical staging was carefully considered but eventually not performed were classified as “staging not intended” and distinguished from the patients in whom surgical staging was intended.
Treatment according to protocol
For this study, treatment was considered to be in accordance with the CCCN-L protocol if a patient was completely staged at the time of initial surgery or if inadequate surgery was followed by adjuvant chemotherapy.20–23 Adjuvant chemotherapy consisted of a platinum-based regimen. Generally, a combination regimen consisting of carboplatin and taxol was administered over six cycles. If patients suffered severely from toxic side-effects, the number of cycles was reduced or carboplatin monotherapy was given.
Treatment was considered not in accordance with the CCCN-L protocol if no adjuvant chemotherapy was provided following an inadequately performed surgical intervention or if patients received adjuvant chemotherapy despite optimal surgical staging.
Statistical analysis
Statistical analysis was performed, using the Statistical Package for the Social Sciences (SPSS) for the Mac, version 20.0. The Pearson Chi-square test and Fisher's exact test were used for group comparisons of categorical data, and statistical significance was assigned at the level of p<0.05. Survival rates were calculated according to the Kaplan-Meier method and compared with the log-rank test.
Results
Patients
This study included 130 patients; 15 of them were treated in the LUMC and 115 were treated in one of the eight community hospitals (A–H). Table 1 shows the clinical and tumor characteristics of the entire study population. The median age at time of surgery was 59 (range: 26–90). Tumors were graded using Silverberg's grading system. The majority of tumors were well-differentiated (grade I), and moderately and poorly differentiated tumors were found in 31.5% and 14.6% of the patients, respectively. In 16 patients, no grade was stated, and 9 of them had clear-cell tumors.
Table 1.
Clinical and Tumor Characteristics in Patients (N=130) with EOC
| Characteristic | n (%) |
|---|---|
| Age at time of surgery | 59 (26–90)a |
| <40 years | 10 (7.7) |
| 40–59 years | 62 (47.7) |
| 60–79 years | 44 (33.8) |
| >79 years | 14 (10.8) |
| Tumor localization | |
| Unilateral—left | 60 (46.2) |
| Unilateral— right | 64 (49.2) |
| Bilateral | 6 (4.6) |
| FIGO stage | |
| Ia | 73 (56.2) |
| Ib | 2 (1.5) |
| Ic | 53 (40.8) |
| IIa | 2 (1.5) |
| Frozen section results | |
| Not performed | 42 (32.3) |
| Benign | 5 (3.8) |
| Borderline | 9 (6.9) |
| At least borderline | 15 (11.5) |
| Malignant | 58 (44.6) |
| Other | 1 (0.8) |
| Histologic cell type | |
| Mucinous | 35 (26.9) |
| Papillary | 2 (1.5) |
| Serous | 16 (12.3) |
| Papillary mucinous | 1 (0.8) |
| Serous papillary | 25 (19.2) |
| Endometrioid | 30 (23.1) |
| Clear-cell | 16 (12.3) |
| Malignant Brenner tumor | 1 (0.8) |
| Undifferentiated carcinoma | 2 (1.5) |
| Adenocarcinoma (not specified further) | 2 (1.5) |
| Tumor grade | |
| I | 54 (41.5) |
| II | 41 (31.5) |
| III | 19 (14.6) |
| Unknown | 16 (12.3) |
| Attendance of gynecologic oncologist | |
| No | 81 (68.6) |
| Yes | 22 (18.6) |
| Gynecologic oncologist (LUMC) | 15 (12.7) |
| Surgical staging performance | |
| Adequate | 56 (43.1) |
| Inadequate | 62 (47.7) |
| Surgical staging not purposed | 12 (9.2) |
| Treatment according to protocol | |
| Yes | 75 (57.7) |
| No | 55 (42.3) |
Numbers indicate median (range).
EOC, early ovarian cancer; FIGO, International Federation of Gynecologic Oncologists; LUMC, Leiden University Medical Center.
In 57 patients (43.8%), frozen section was not performed or yielded results that were benign, borderline, or otherwise. Once the definitive pathology report revealed a malignancy, 34 patients (59.6%) were scheduled for staging laparotomy: 11 patients (32.4%) were treated by their general gynecologists alone; 15 patients (44.1%) in the presence of a gynecologic oncologist; and eight (23.5%) were referred to the LUMC. Twenty-seven of these 34 repeat laparotomies (79.4%) were complete. However, 22 women (38.6%) did not undergo a second surgery, and for 11 of these women no reason was specified for this decision. In the other 11 patients, surgical staging was considered but not performed because of a suboptimal health condition (4 patients), patient refusal (3 patients), or preference for chemotherapy over a repeat laparotomy after discussion with the multidisciplinary tumor board (4 patients). In 1 patient, a repeat laparotomy was performed without lymph-node dissection, because the pathology report initially showed a germ-cell tumor and the lymph-node sampling in this type of tumor is adequate. However, 3 years later, when it was noted that this patient developed metastases, the tumor then appeared to be of clear-cell origin. These 12 patients in whom surgical staging was carefully considered but not performed were classed as “staging not intended” and were distinguished from the patients in whom surgical staging was intended.
Among the 130 patients in this study, surgical staging appeared to be performed according to the criteria mentioned above (adequate) in 56 women (43.1%). Seventy-five patients (57.7%) were treated according to the regional protocol.
Table 2 shows the patients' characteristics for every single hospital. All patients in the LUMC were operated on by gynecologic oncologists. Among the patients being operated in the LUMC, 8 had been referred by community hospitals: hospital A, hospital D, hospital G, and hospital H each referred 1 patient; and hospital F sent 4 patients to the university hospital. In hospitals E and F, no gynecologic oncologist attended any surgery. The hospitals in which the largest number of patients was staged adequately were the LUMC and hospital C (both 80.0%). The percentage of patients who were treated according to protocol was greatest in the LUMC (93.3%), followed by hospital C (72.7%) and hospital D (68.8%).
Table 2.
Clinical Characteristics of Patients with EOC for the Eight Community Hospitals and the LUMC
| Hospital | Treated patients | Attendance of gynecologic oncologista | Adequately stageda | Treatment according to protocol | Recurrence | Deceased (all causes) | Deceased (from EOC) |
|---|---|---|---|---|---|---|---|
| A | 15/130 (11.5)b | 3/13 (23.1) | 7/13 (53.8) | 8/15 (53.3) | 6/15 (40.0) | 4/15 (26.7) | 2/15 (13.3) |
| B | 9/130 (6.9) | 2/7 (28.6) | 2/7 (28.6) | 4/9 (44.4) | 2/9 (22.2) | 2/9 (22.2) | 2/9 (22.2) |
| C | 11/130 (8.5) | 4/10 (40.0) | 8/10 (80.0) | 8/11 (72.7) | 0/11 (0.0) | 2/11 (18.2) | 0/11 (0.0) |
| D | 16/130 (12.3) | 6/15 (40.0) | 6/15 (40.0) | 11/16 (68.8) | 2/16 (12.5) | 2/16 (12.5) | 2/16 (12.5) |
| E | 27/130 (20.8) | 0/26 (0.0) | 6/26 (23.1) | 12/27 (44.4) | 5/27 (18.5) | 7/27 (25.9) | 4/27 (14.8) |
| F | 10/130 (7.7) | 0/10 (0.0) | 3/10 (30.0) | 2/10 (20.0) | 1/10 (10.0) | 0/10 (0.0) | 0/10 (0.0) |
| G | 19/130 (14.6) | 4/16 (25.0) | 9/16 (56.3) | 11/19 (57.9) | 5/19 (26.3) | 4/19 (21.1) | 4/19 (21.1) |
| H | 8/130 (6.2) | 3/6 (50.0) | 3/6 (50.0) | 5/8 (62.5) | 1/8 (12.5) | 3/8 (37.5) | 2/8 (25.0) |
| LUMC | 15/130 (11.5) | 15/15 (100.0) | 12/15 (80.0) | 14/15 (93.3) | 3/15 (20.0) | 1/15 (6.7) | 1/15 (6.7) |
Data for patients in whom surgical staging was not intended were omitted.
#/total # identified in registries (%) for all data in columns 2–7.
EOC, early ovarian cancer; LUMC, Leiden University Medical Center.
With respect to survival rates, no recurrence was detected in the patients from hospital C. Six of 15 patients (40.0%) treated in hospital A had recurrent disease. Twenty-five of 130 patients died; 17 of these deaths were from ovarian cancer. The highest percentage (25.0%; 2 of 8 patients) of patients was deceased in hospital H.
Effect of gynecologic oncologist presence on surgical staging performance
The attendance of a gynecologic oncologist had a positive effect on the surgical staging procedure (Table 3). Surgical staging was more often performed adequately if a gynecologic oncologist was present than if a gynecologic oncologist was not present, 81.1% versus 32.1% (p<0.001), respectively. If no gynecologic oncologist was present, 67.9% of 118 staging surgeries were incomplete.
Table 3.
Presence of Gynecologic Oncologist and Surgical Staging Performance for Patients with EOC
| Surgical staging performance | |||
|---|---|---|---|
| Gynecologic oncologist present | Adequate | Inadequate | Total (%) |
| GO in LUMCa | 12 (80.0) | 3 (20.0) | 15 (12.7) |
| Yes | 18 (81.8) | 4 (18.2) | 22 (18.6) |
| No | 26 (32.1) | 55 (67.9) | 81 (68.6) |
| Total (%) | 56 (47.5) | 62 (52.5) | 118 (100.0) |
Notes: Pearson Chi-Square: p<0.001. Data from patients in whom surgical staging was not intended were omitted.
GO in LUMC denotes gynecologic oncologist in the Leiden University Medical Center.
EOC, early ovarian cancer.
In 13 of 62 patients (21.0%) in whom staging performance was not complete, an explanation was provided in the files: in 5 patients, the procedure was hampered by gross obesity, extensive hemorrhage, adhesions, or comorbidity; in two cases, it was decided to abandon the surgery because of spill; in 2 cases, there was a lack of experience of the operating gynecologist reported; and, in 4 cases, no lymph node sampling was performed because of nonpalpable lymph nodes. In the remaining 79.0% of the patients, no reasons were specified.
Effect of the presence of a gynecologic oncologist on treatment according to protocol
Overall if a gynecologic oncologist was present, treatment was more often given following protocol, than if a gynecologic oncologist was not present: 76.9% versus 49.5%, respectively (p=0.004, Table 4). However, if a gynecologic oncologist did not attend the operation in the community hospital, 45 patients received treatment following protocol and 46 patients did not.
Table 4.
Presence of Gynecologic Oncologist and Treatment According to Protocol in Patients with EOC
| Treatment according to protocol | |||
|---|---|---|---|
| Gynecologic oncologist present | Yes | No | Total (%) |
| GO in LUMCa | 14 (93.3) | 1 (6.7) | 15 (11.5) |
| Yes | 16 (66.7) | 8 (33.3) | 24 (18.5) |
| No | 45 (49.5) | 46 (50.5) | 91 (70.0) |
| Total (%) | 75 (57.7) | 55 (42.3) | 130 (100) |
Note: Pearson Chi-Square: p=0.004.
GO in LUMC denotes gynecologic oncologist in the Leiden University Medical Center.
EOC, early ovarian cancer.
Reasons to renounce the protocol were related to a suboptimal health condition in 10 patients. One patient refused treatment. Seven patients (12.7%) underwent both adequate surgery and chemotherapy: 2 because of DNA aneuploidy and 1 because of a suspicion (but no proof) of pancreatic cancer. The reason for administering chemotherapy to the other 4 patients was unknown. In 11 patients (20.0%), an inappropriate treatment was pursued despite discussion with the existing multidisciplinary tumor board. The reasons for the remaining 26 patients (47.3%) in whom the protocol was not followed were unknown.
Effect of surgical staging performance on recurrent disease
A difference was shown in the effect of surgical staging on recurrent disease (Table 5). Recurrence was diagnosed in 15 patients (24.2%) who underwent inadequate surgery, while it was merely detected in 6 women (10.7%) who underwent a full staging procedure (p=0.090).
Table 5.
Surgical Staging Performance and Recurrent Disease in Patients With EOC
| Recurrent disease | |||
|---|---|---|---|
| Surgical staging performance | Yes | No | Total (%) |
| Adequate | 6 (10.7) | 50 (89.3) | 56 (47.5) |
| Inadequate | 15 (24.2) | 47 (75.8) | 62 (52.5) |
| Total (%) | 21 (17.8) | 97 (82.2) | 118 (100) |
Fisher's exact test: p=0.090.
Patients in whom surgical staging was not purposed were omitted.
EOC, early ovarian cancer.
Effect of treatment according to protocol on recurrent disease
Fifteen patients (20.0%) treated by adequate surgery or in whom incomplete surgery was followed by adjuvant chemotherapy developed recurrences versus 10 patients (18.2%) who were not treated in accordance with protocol. Overtreatment (i.e., an optimal staging procedure followed by adjuvant chemotherapy), might influence the survival rates inaccurately for treatment according to protocol analysis, but eliminating the data from patients who underwent both therapies from the calculations yielded equal results.
Survival rate
After surgery, patients were monitored over time. The mean duration of follow-up for the entire study population was 69 months (range: 1–128 months). Among the 25 women having recurrent disease, 16 patients died, with 15 (60.0%) of the deaths occurring as a result of ovarian cancer. The median time from surgical treatment to recurrence was 15 months (range: 2–97 months) and from recurrence to death 8 months (range: 0–38 months).
Twenty-five of 130 patients (19.2%) died: 17 from ovarian cancer; 3 from subsequently developed other carcinomas; 1 because of multiple ischemic cerebrovascular accidents; 2 from sepsis; one from a pulmonary embolism; and 1 from a pelvic fracture.
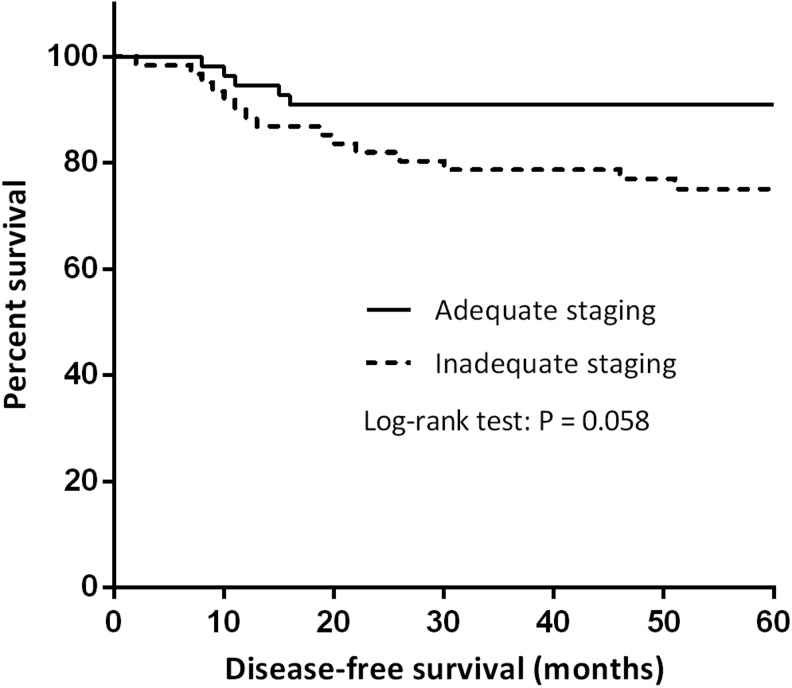
Figure 1 shows the effect of staging adequacy on disease-free survival in patients with early stage ovarian cancer. The mean disease-free duration for the adequately staged patients (n=56) was 70 months and for the inadequately staged patients (n=62), it was 65 months. A 5-year disease-free survival of 90.9% was noted for adequately staged patients and 75.1% for nonoptimally staged patients (p=0.058).
FIG. 1.
Surgical staging performance and disease-free survival in patients with early ovarian cancer.
Discussion
In this article, the current authors analyzed the recent practice of guest operations for EOC in the community hospitals surrounding the oncology center of the LUMC in The Netherlands. The population studied was restricted to patients diagnosed with FIGO stage Ia–IIa cancers, because it is particularly these patients in whom adequate management of the disease bears considerable potential for long-term survival.9,23 Undoubtedly, patients having advanced-stage ovarian cancer also deserve good treatment but, compared to patients with early stage disease, appropriateness of treatment is of less consequence given that these women already have poor prognoses.
This study had several limitations. It was retrospective and thus vulnerable to the effect of unappreciated bias in the coverage of data. Furthermore, patient files were reviewed in nine different hospitals, all with different systems of registration of clinical data. In addition, the accuracy of monitoring in these hospitals can be challenged in view of a mean follow-up duration in the study of 69 months in contrast to the study period of 9 years. To minimize these potential adverse effects, the current authors were as careful and cautious as possible to substantiate the data that were reviewed. The item of adequacy of surgical staging was not only decided on by the notes in the patients' files but also by careful analysis of the original operating notes and pathology reports in all cases.
The criteria for adequate staging that were followed can be criticized with respect to the required number of lymph nodes that were removed. Currently, a number of ten paraaortic and pelvic lymph nodes from specific and different sites is regarded as minimum.19 In the past, there had been considerable debate on the minimal requirements of adequate staging lymphadenectomy. The criteria used in the present study reflect this. The mean number of nodes removed in the 15 patients operated in the oncology center LUMC was 16.
A strong point of the current study may be that, in contrast to the situation that occurs in a prospective trial, the current study illustrated the real-life situation in all of the hospitals of a particular part of a densely populated Western European country during a specific period in time. In addition to this, the nine hospitals in the study followed the same oncology protocol for treating patients with EOC.
If a gynecologic oncologist attended the operation, surgical staging was more often adequately performed than if a gynecologic oncologist did not attend the operation, 81.1% versus 32.1% (p<0.001), respectively. Adherence to protocol was observed in 76.9% of patients treated in the presence of a gynecologic oncologist, compared to 49.5% of those treated by general gynecologists alone (p=0.004). The 5-year disease-free survival was in favor of optimally staged patients over nonoptimally staged patients, 90.9% versus 75.1%, respectively (p=0.058). Although this value did not meet statistical significance, it may possibly indicate a trend. A significant value (p=0.026) was found if all of the patients in the study (i.e., including the 12 patients who purposely did not undergo staging laparotomy) were included.
In 12 patients, a staging laparotomy was not performed on purpose because of a suboptimal health condition, patient refusal, chemotherapy being preferred over a repeat laparotomy, or an initially different pathology result obtained. A distinction between this group and the patients in whom staging laparotomy was intended was made in order to get the most accurate outcomes of the effect of the presence of a gynecologic oncologist on the quality of staging and survival. Although the patients did not undergo staging laparotomy, information about adjuvant chemotherapy and the follow-up thereafter was still considered to be important. These patients were therefore not excluded from the study.
A number of conclusions from the present study are sobering and clear. The attendance of a gynecologic oncologist in the operating room improved the rate of adequate surgical staging from a low 32% to a generally accepted level of 81%. This is important in view of the proven significance of adequate surgical staging for the prognosis of EOC. In the present study, this was confirmed by the finding that the number of recurrences was lower when the staging procedure had been adequate and complete. In addition, the adherence to the self-developed protocol was raised from 50% to 77%.
Interestingly, a difference was noted in surgical performance when the site of operation for the gynecologic oncologist was considered. In the on-site setting of the LUMC, with familiar operating-room personnel, instruments, anesthesiologists, and surgical consultants, treatment according to protocol occurred in 93% of the patients in contrast to 67% of the patients when the gynecologic oncologist performed guest surgery in a community hospital. However, this 67% was still significantly better than the 50% adherence to protocol when the gynecologic oncologist was not present. Regarding the adequacy of the surgical staging procedure, the differences between the LUMC setting and the guest operation setting were smaller, but these figures were again significantly higher than the 32% adequately staged patients when a gynecologic oncologist did not attend the operation in the community hospital.
Another interesting finding from this study was the wide range of performances among the different hospitals in the study. In the oncology center of the region, the rate of adequate staging was 80% and the percentage of adherence to the preset treatment protocol was 93%. This was in contrast with some of the other hospitals in the area with adequate staging rates of only 29% (hospital B), 23% (hospital E), and 30% (hospital F). Strikingly, hospitals E and F were also the hospitals with the lowest rates of performing the operation together with a gynecologic oncologist (both 0%). The same trend was seen for the adherence to protocol that was as low as 20% (hospital F) and 44% (hospitals B and E). These differences among patients from the same area and background suggest a remarkable variation in patterns of care despite universal and predefined regional guidelines for the treatment of these patients. One of the conclusions from this analysis may be that the phenomenon of guest operations is worthwhile but should be followed more strictly. During the study period, a gynecologic oncologist was asked to attend the staging operation in 22 of 103 cases (21%; range: 0%–50%) and the increase of these numbers should be subject to psychologic and logistic strategies.
One of the main reasons for revising all medical records was the fact that information regarding treatment considerations was lacking in the current authors' first analysis from the centralized database of the regional comprehensive cancer center. Therefore, the current authors asked the gynecologists from the community hospitals to allow access to the original patient files in order to gain better insight into the patient-care process. Remarkably, in 79% of the cases, no explanation was given for the inadequate staging procedure, and the lack of adherence to protocol remained unspecified in 47% of the cases.
From the information gathered three noteworthy factors came to light. First, in 4 cases no lymph-node sampling was performed because the lymph nodes were impalpable. Previous series in the literature showed that relying on palpable abnormalities of lymph nodes will result in missing approximately one-third of lymph-node metastases.17 Second, adjuvant chemotherapy was prescribed, despite optimal staging in 7 patients. A long-term analysis of the ACTION trial confirmed the original conclusion that the benefit of adjuvant chemotherapy appears to be limited to patients with more-substantial risk of unappreciated residual disease (i.e., inadequate staging).23 Hence, adjuvant chemotherapy seems to have no additional value in adequately staged patients. Third, in spite of a multidisciplinary approach, 11 patients who had been inadequately staged erroneously did not receive any adjuvant chemotherapy. The multidisciplinary tumor board consists of a general gynecologist from the community hospital, the local pathologist, a radiologist, and a medical oncologist, and preferably a gynecologic oncologist from the LUMC. Given that this study had a retrospective design, a definite cause cannot be defined, but one might suggest this protocol violation has its origins in a misunderstanding among the different physicians involved. To overcome these false interpretations, enclosing the operating notes and pathology reports might be helpful in the tumor-board conference.
In the literature, similar findings about guest operations have been described. In the area around Groningen in the northern part of The Netherlands, a comparable study was conducted based on data from 632 patients with ovarian cancer. The authors of that study reported that pelvic and/or para-aortic lymph-node sampling or lymphadenectomy was performed significantly more often in patients treated by a gynecologic oncologist than by a general gynecologist, in 61.5% of patients and 30.4% of patients, respectively. The performance of a (partial) omentectomy and peritoneal biopsies was also more frequently noted when patients were operated on by a gynecologic oncologist. Furthermore, a considerable 5-year survival benefit was demonstrated for patients who underwent surgery by a gynecologic oncologist, compared to patients treated by a general gynecologist.14
The results of that study were in agreement with a Dutch nationwide cohort study conducted by Vernooij et al. These authors evaluated the influence of hospital type on survival of patients with ovarian cancer and found that surgery by a consulting gynecologic oncologist was associated with significantly better survival than treatment by a general gynecologist.13 However, a population-based study from the University of Utah, in Salt Lake City, UT, showed that, in patients diagnosed with local and regional disease, no survival difference could be substantiated between those who did and those who did not undergo treatment by a gynecologic oncologist.24 According to the results obtained by Earle and colleagues, patients treated by a general gynecologist were more likely to have a second-look surgery.25 Goff et al. recently found elevated comprehensive surgery rates in teaching hospitals regardless of hospital volume. In nonteaching hospitals however, hospital volume proved to be predictive of surgical accuracy.4 In addition, Kumpulainen and colleagues suggested centralization of care to the university hospitals, because the number of staging biopsies and lymphadenectomies were higher in patients treated by multidisciplinary teams with physicians who specialized in treating ovarian cancer.26
Conclusions
With respect to these these literature findings and the data presented in this article, it seems clear that cancer surgery by the most specialized and experienced surgeons does contribute to better care for the patients. This seems to be also true for women with early stage ovarian cancer. Guest operations are beyond all the political arguments and represent a “Mohammad goes to the mountain” solution. When the logistics of the region are fit, guest operations deserve a distinguished place among the treatment modalities of patients with ovarian cancer.
Acknowledgments
This research was supported by a grant from the Zoleon Foundation (Grant Number 10-03). The authors thank Bart W.J. Hellebrekers, MD, PhD, Hélène T.C. Nagel, MD, PhD, Marjolein J. Kagie, MD, PhD, Lucien, C.F. Haans, MD, Astrid Baalbergen, MD, PhD, Hans C.M. van Huisseling, MD, PhD, Elvira M. Davelaar, MD, PhD, Fred Vork MD, and Oswald J.A. Mattheussens, MD, for making the patient records from the community hospitals available and for assessment of the this article.
Disclosure Statement
The authors declare that there is no conflict of interest associated with this article.
References
- 1.Shrag D, Earle C, Xu F, et al. Associations between hospital and surgeon procedure volumes and patient outcomes after ovarian cancer resection. J Natl Cancer Inst 2006;98:163. [DOI] [PubMed] [Google Scholar]
- 2.Vernooij F, Heintz APM, Coebergh JW, Massuger LFAG, Witteveen PO, Van der Graaf Y. Specialized and high-volume care leads to better outcomes of ovarian cancer treatment in The Netherlands. Gynecol Oncol 2009;112:455. [DOI] [PubMed] [Google Scholar]
- 3.Kristensen GB, Tropé C. Epithelial ovarian carcinoma. Lancet 1997;349:113. [DOI] [PubMed] [Google Scholar]
- 4.Goff BA, Matthews BJ, Larson EH, et al. Predictors of comprehensive surgical treatment in patients with ovarian cancer. Cancer 2007;109:2031. [DOI] [PubMed] [Google Scholar]
- 5.Giede KC, Kieser K, Dodge J, Rosen B. Who should operate on patients with ovarian cancer? An evidence-based review. Gynecol Oncol 2005;99:447. [DOI] [PubMed] [Google Scholar]
- 6.Mayer AR, Chambers SK, Graves E, et al. Ovarian cancer staging: Does it require a gynecologic oncologist? Gynecol Oncol 1992;47:223. [DOI] [PubMed] [Google Scholar]
- 7.Cress RD, Bauer K, O'Malley CD, et al. Surgical staging of early stage epithelial ovarian cancer: Results from the CDC-NPCR ovarian patterns of care study. Gynecol Oncol 2011;121:94. [DOI] [PubMed] [Google Scholar]
- 8.Chan JK, Kapp DS, Shin JY, et al. Influence of the gynecologic oncologist on the survival of ovarian cancer patients. Obstet Gynecol 2007;109:1342. [DOI] [PubMed] [Google Scholar]
- 9.Timmers PJ, Zwinderman AH, Coens C, Vergote I, Trimbos JB. Understanding the problem of inadequately staging early ovarian cancer. Eur J Cancer 2010;46:880. [DOI] [PubMed] [Google Scholar]
- 10.Van Trappen PO, Rufford BD, Mills TD, et al. Differential diagnosis of adnexal masses: Risk of malignancy index, ultrasonography, magnetic resonance imaging, and radioimmunoscintigraphy. Int J Gynecol Cancer 2007;17:61. [DOI] [PubMed] [Google Scholar]
- 11.Geomini P, Kruitwagen R, Bremer GL, Cnossen J, Mol BWJ. The accuracy of risk scores in predicting ovarian malignancy—a systematic review. Obstet Gynecol 2009;113:384. [DOI] [PubMed] [Google Scholar]
- 12.Greving JP, Vernooij F, Heintz APM, Van der Graaf Y, Buskens E. Is centralization of ovarian cancer care warranted? A cost-effectiveness analysis. Gynecol Oncol 2009;113:68. [DOI] [PubMed] [Google Scholar]
- 13.Vernooij F, Heintz APM, Witteveen PO, Van der Heiden-van der Loo M, Coebergh JW, Van der Graaf Y. Specialized care and survival of ovarian cancer patients in The Netherlands: Nationwide cohort study. J Natl Cancer Inst 2008;100:399. [DOI] [PubMed] [Google Scholar]
- 14.Engelen MJA, Kos HE, Willemse PHB, et al. Surgery by consultant gynecologic oncologists improves survival in patients with ovarian carcinoma. Cancer 2006;106:589. [DOI] [PubMed] [Google Scholar]
- 15.Heintz APM, Odicino F, Maisonneuve P, et al. Carcinoma of the ovary—FIGO 26th annual report on the results of treatment in gynecological cancer. Int J Gynaecol Obstet 2006;95(suppl1):S161. [DOI] [PubMed] [Google Scholar]
- 16.Trimbos JB, Bolis G. Guidelines for surgical staging of ovarian cancer. Obstet Gynecol Surv 1994;49:814. [DOI] [PubMed] [Google Scholar]
- 17.Trimbos JB. Staging of early ovarian cancer and the impact of lymph node sampling. Int J Gynecol Cancer 2000;10(suppl1):8. [DOI] [PubMed] [Google Scholar]
- 18.Timmers PJ, Zwinderman K, Coens C, Vergote I, Trimbos JB. Lymph node sampling and taking of blind biopsies are important elements of the surgical staging of early ovarian cancer. Int J Gynecol Cancer 2010;20:1142. [DOI] [PubMed] [Google Scholar]
- 19.Trimbos JB. Lymphadenectomy in ovarian cancer: Standard of care or unnecessary risk. Curr Opin Oncol 2011;23:507. [DOI] [PubMed] [Google Scholar]
- 20.Tropé C, Kaern J. Adjuvant chemotherapy for early-stage ovarian cancer: Review of the literature. J Clin Oncol 2007;25:2909. [DOI] [PubMed] [Google Scholar]
- 21.Trimbos JB, Vergote I, Bolis G, et al. Impact of adjuvant chemotherapy and surgical staging in early-stage ovarian carcinoma: European Organisation for Reseach and Treatment of Cancer-Adjuvant ChemoTherapy in Ovarian Neoplasm Trial. J Natl Cancer Inst 2003;95:113. [PubMed] [Google Scholar]
- 22.Trimbos JB, Parmar M, Vergote I, et al. International Collaborative Neoplasm Trial 1 and Adjuvant ChemoTherapy in Ovarian Neoplasm Trial: Two parallel randomized phase III trials of adjuvant chemotherapy in patients with early-stage ovarian carcinoma. J Natl Cancer Inst 2003;95:105. [PubMed] [Google Scholar]
- 23.Trimbos JB, Timmers P, Pecorelli S, et al. Surgical staging and treatment of early ovarian cancer: Long-term analysis from a randomized trial. J Natl Cancer Inst 2010;102:982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carney ME, Lancaster JM, Ford C, Tsodikov A, Wiggins CL. A population-based study of patterns of care for ovarian cancer: Who is seen by a gynecologic oncologist and who is not? Gynecol Oncol 2002;84:36. [DOI] [PubMed] [Google Scholar]
- 25.Earle CC, Shrag D, Neville BA, et al. The effect of surgeon specialty on processes of care and outcomes for ovarian cancer patients. J Natl Cancer Inst 2006;98:172. [DOI] [PubMed] [Google Scholar]
- 26.Kumpulainen S, Kuoppala T, Leminen A, et al. Surgical treatment of ovarian cancer in different hospital categories—a prospective nation-wide study in Finland. Eur J Cancer 2006;42:388. [DOI] [PubMed] [Google Scholar]