Abstract
Background: Children with severe chronic pancreatitis may undergo total pancreatectomy with islet autotransplantation (TPIAT) to relieve pain while minimizing the risk of postsurgical diabetes. Because overstimulation of transplanted islets by hyperglycemia can result in β-cell loss, we developed a specialized intravenous insulin infusion protocol (IIP) for pediatric TPIAT recipients to maintain euglycemia or near-euglycemia posttransplant.
Subjects and Methods: Our objective was to review glucose control using an IIP specific for TPIAT recipients at a single institution. We reviewed postoperative blood glucose (BG) levels for 32 children 4–18 years old with chronic pancreatitis who underwent TPIAT between July 2011 and June 2013. We analyzed the proportion of BG values in the range of 70–140 mg/dL, mean glucose, glucose variability, and occurrence of hypoglycemia during the IIP; we also evaluated the transition to subcutaneous therapy (first 72 h with multiple daily injections [MDI]).
Results: During IIP, the mean patient BG level was 116±27 mg/dL, with 83.1% of all values in the range of 70–140 mg/dL. Hypoglycemia was rare, with only 2.5% of values <70 mg/dL. The more recent era (n=16) had a lower mean BG and less variability than the early era (first 16 patients) (P≤0.004). Mean glucose level (116 vs. 128 mg/dL) and glucose variability were significantly lower during the IIP compared with MDI therapy (P<0.0001).
Conclusions: Tight glycemic control without excessive severe hypoglycemia was achieved in children undergoing TPIAT using an IIP specifically designed for this population; the ability to maintain BG in target range improved with experience with the protocol.
Introduction
Chronic pancreatitis is associated with incapacitating pain, frequent hospitalizations, and a high risk of narcotic dependence. Although rare in children, the disease can be debilitating for those afflicted. In children with severe chronic pancreatitis, total pancreatectomy (TP) may be considered to relieve pain, with simultaneous islet autotransplantation (IAT) performed to minimize the risk for postsurgical diabetes mellitus. In this procedure, the islets are isolated from the remainder of the pancreas, infused back into the portal vein, and subsequently engraft in the liver sinusoids where they release insulin in response to ambient blood glucose (BG).1
Approximately 40% of children discontinue insulin therapy after TPIAT, with islet mass being the most important measurable predictor of subsequent diabetes risk.2–6 During isolation, islets are stripped of their native arteriolar blood supply. Once transplanted, islets are reliant on diffusion of nutrients and oxygen to the islet core until neovascularization is complete, a process that takes weeks to months.7,8 During this period of engraftment, the islets are particularly vulnerable to overstimulation by hyperglycemia in an anoxic environment, which contributes to β-cell loss.9,10 In animal models, hyperglycemia increases β-cell apoptosis, while maintenance of euglycemia reduces the islet mass required to reverse diabetes.11–16 Although such studies are difficult to perform in human recipients, data from a large TPIAT cohort at our institution further suggest that small differences in mean BG level in the first week posttransplant correlate with later insulin independence.17 For these reasons, TPIAT recipients are carefully managed with insulin therapy, targeting glucose levels as close to physiologic as possible to protect the engrafting islet mass, while minimizing the risk of severe hypoglycemia. In the first week posttransplant (when islets are most hypoxic), insulin is administered primarily by an intravenous insulin infusion protocol (IIP).
Development of any IIP involves certain key elements: selecting a target BG level appropriate for the patient population, establishing a schedule for frequent BG monitoring, adjusting insulin infusion rate based on BG values and the rate of BG change, and establishing policies to minimize hypoglycemia.18–22 After development, novel IIPs require a period of implementation during which there is established staff acceptance and comfort with nurse-driven management changes.18 Although early clinical trials favored strict BG targets (80–110 mg/dL) in postsurgical intensive care unit (ICU) patients,23–26 more recent trials have favored moderate glycemic goals (conventionally approximately 140 mg/dL) to reduce hypoglycemia-associated mortality.27–29 These findings have led to subsequent efforts to develop hospital-wide insulin infusion protocols that tolerate moderate hyperglycemia as a trade-off for reduced ICU mortality.18–20,22,30 However, these general ICU protocols do not take into consideration the specific needs of the islet autotransplant population. Protocols that tolerate hyperglycemia may not be optimal for the management of TPIAT recipients, for whom mortality is rare and nearer- normal glycemic targets are desired to protect the engrafting islets. For this reason, we implemented a novel “pediatric TPIAT” IIP, favoring more physiologic glucose targets than those used in the conventional pediatric IIP protocol, for targeted use only in TPIAT recipients. Herein, we describe a retrospective cohort of 32 children who underwent TPIAT at a single institution treated with the novel TPIAT IIP. We hypothesized that the adapted IIP would produce near-normal glycemia with a majority of measured values in a physiologic range (70–140 mg/dL) without severe hypoglycemia.
Research Design and Methods
We studied glycemic control on the IIP and during transition to subcutaneous insulin (first 72 h on subcutaneous insulin) in a cohort of 32 children (4–18 years of age) who underwent TPIAT at the University of Minnesota between July 2011 and June 2013. All patients were enrolled in an Institutional Review Board–approved prospective cohort study following outcomes after TPIAT. Informed consent or assent was obtained from parents and patients as appropriate.
Procedure of TPIAT
The surgical procedure of TPIAT was performed as previously described,1 with minor modifications to the gastrointestinal reanastamosis. In brief, a TP, partial duodenectomy, Roux-en-Y duodenojejunostomy, choledochojejunostomy, and splenectomy were performed. Islet isolation and purification were performed in the University of Minnesota Molecular and Cellular Therapeutics GMP Facility. The pancreas was distended with cold enzyme solution using a pressure-controlled pump system31 and then digested using the semiautomated method of Ricordi et al.32 The islets were infused into a tributary of the portal vein; if elevated portal pressures prevented infusion of all islets intraportally, the remaining islets were transplanted into the peritoneal cavity.
IIP
All patients are placed on insulin therapy after TPIAT, adjusted to maintain the majority of glucose levels in the range of 80–125 mg/dL for the first month after TPIAT. Insulin is administered as an intravenous infusion for approximately 1 week and then transitioned to subcutaneous insulin.
We designed a novel pediatric TPIAT intravenous IIP to maintain physiologic glucose targets (insulin adjusted to target 100–120 mg/dL) (Fig. 1), using an electronic medical record–based protocol with pediatric endocrinology consultation. This protocol was adapted from the hospital's standard pediatric IIP protocol (implemented in 2011, with a higher glucose target of approximately 130 mg/dL [range, 100–160 mg/dL]) developed by committee at the University of Minnesota and based on published ICU intensive insulin infusion guidelines.18–21 Prior to implementation in 2011, there was no standard IIP for children undergoing TPIAT (weighing <45 kg), and insulin infusion rates were adjusted on a case-by-case basis, also involving electronic medical record–based ordering with endocrinology consultation. The pediatric endocrinology group was familiarized with the IIP through presentation at division conferences.
FIG. 1.
University of Minnesota pediatric total pancreatectomy with islet autotransplantation (TPIAT) insulin infusion protocol. BG, blood glucose; ENDO, endocrinologist.
The pediatric TPIAT IIP is used only within this prespecified population, recognizing that these children have a different risk–benefit ratio that favors maintaining tighter glycemic control compared with the conventional pediatric ICU patient. Differences from the hospital standard IIP include lower target range, greater adjustments in insulin infusion rates for mild hyperglycemia, tolerating lower glucose values (infusion rate is reduced but not always stopped for physiologically normal glucose values of 80–99 mg/dL), and different threshold for hypoglycemia treatment (<80 mg/dL vs. <100 mg/dL). To minimize risk of hypoglycemia, the IIP is designed to reduce or temporarily halt insulin infusion when the glucose level is <100 mg/dL (for potential impending hypoglycemia), although glucose values of 80–99 mg/dL are considered acceptable. This protocol addresses the critical elements of insulin adjustments based on the following: frequent glucose checks, current as well as previous glucose values, rate of change in glucose level, protocolized nurse-directed decisions, and minimizing hypoglycemia. The IIP allows nursing discretion around the target range (titrate by 0–10%) based on their experience and taking into account BG trend.
Subcutaneous insulin injections
At our institution, all patients were placed on continuous enteral feeds via J-tube and slowly advanced to goal volumes postoperatively. Thus, introduction of subcutaneous insulin therapy via multiple daily injections (MDI) was delayed until feeds were at full enteral requirements (typically postoperative Day 5–7). Long-acting insulin (glargine or detemir) was calculated at 100–120% of the total daily intravenous insulin dose, based on the rate at stable enteral feeds, and often administered as a divided twice-daily dose. BG values were monitored every 4 h, with rapid-acting insulin (aspart) administered as needed for glucose levels >125 mg/dL; rapid-acting insulin was generally initiated at 1 unit per 25 or per 50 mg/dL (based on total daily insulin use >30 or <30 units/day, respectively). The basal insulin analog was adjusted to maintain the majority of daily glucose values in a target range of 80–125 mg/dL. Once the patient is eating (typically approximately 2–3 weeks postoperatively), an insulin-to-carbohydrate ratio was added at 0.5–1 unit per 15 g.
Although beyond the scope of this report, long-term glucose targets are more lenient, targeting 80–125 mg/dL fasting and 80–150 mg/dL nonfasting (postprandial), and with hemoglobin A1c≤6.5%.
Data collection
Demographic data, BG levels, and insulin administration data were collected retrospectively from the electronic medical record. A BG level of <70 mg/dL was defined as hypoglycemia, and one of <40 mg/dL was defined as severe hypoglycemia. We analyzed the proportion of BG values <70 mg/dL and >140 mg/dL (considered meaningful hyperglycemia) and the number of patients with any severe hypoglycemic episode. Glycemic control achieved while the patient was on the IIP was evaluated longitudinally to look for a learning curve among the physician and nursing care providers. We evaluated the same parameters for the first 72 h immediately after transition to MDI therapy and compared the glycemic parameters for the IIP and MDI periods.
BG levels were measured with a standard bedside hospital glucose meter (SureStep® Pro® meters [LifeScan, Milpitas, CA] prior to October 2012 and StatStrip® glucose meters [Nova Biomedical, Waltham, MA] after October 2012). BG values were obtained every 1–2 h while the patient was on the intravenous IIP and every 4 h after transition to subcutaneous therapy.
Statistical analysis
Data are expressed as mean±SD values or as a percentage. The first 16 patients treated with the IIP were considered Era 1 (early era), and the latter 16 patients were treated as Era 2 (later era). BG values between the eras were compared by two-tailed Student's t tests, and the IIP and MDI groups were compared using two-tailed Student's t test in a paired fashion, in which patients served as their own controls. An adjusted comparison for the IIP and MDI was performed controlling for the potential confounding variables of gender, body mass index, age, and islet mass transplanted using a mixed-model analysis, with a Type 3 test of fixed effects to control for the four covariates regardless of ordering. General linear mixed models were used to compare the trajectory of change in glucose levels using the insulin drip versus subcutaneous injection. The adjusted differences for glucose levels within subjects were expressed as least squares mean by group and time. The independent effects of time on treatment, the adapted protocol, and the interaction were tested using a Type 3 test. Homogeneity of variances (glucose variability) was compared between groups for all subjects and each subject separately using Levene's homogeneity of variance test. All analyses were performed using the software from SAS (Cary, NC). Values of P≤0.05 were considered statistically significant.
Results
Patients
Patients had a mean age of 13±3.7 years, 59% were female, and most had genetic disease (Table 1). Patients received 4,830±3,692 islet equivalents (IEQ)/kg of body weight transplanted.
Table 1.
Patient Demographics
| Characteristic | TPIAT patients |
|---|---|
| Number of patients | 32 |
| Age (years) | 13±3.7 |
| Male sex | 41% |
| Weight (kg) | 50±20 |
| BMI (kg/m2) | 20.57±5.075 |
| Duration on intravenous insulin infusion (days) | 6.9±2.4 |
| Primary etiology of pancreatitis | |
| PRSS1 (autosomal dominant, hereditary) | 59% |
| Cystic fibrosis (two CFTR mutations or positive sweat chloride test) | 16% |
| Other genetic mutation(s) (SPINK1, CTRC, CFTR carrier) | 16% |
| Other (pancreatic divisum, idiopathic) | 9% |
| Transplanted IEQ/kg of body weight | 4,830±3,692 |
Data are mean±SD values or percentages as indicated.
BMI, body mass index; CFTR, cystic fibrosis transmembrane conductance regulator; IEQ, islet equivalents; TPIAT, total pancreatectomy with islet autotransplantation.
Glycemic targets are achieved on the intravenous IIP
Patients were maintained on the intravenous IIP for a mean of 6.9±2.4 days prior to initiation of subcutaneous injections (Table 2). Overall, patients on the IIP had a mean BG level of 116±27 mg/dL, with 83.1% of all values in the range of 70–140 mg/dL. Hypoglycemia was rare, with only 2.5% of values <70 mg/dL. Only two patients had any severe hypoglycemia (<40 mg/dL), representing 0.1% of all values; no patient experienced adverse sequelae from hypoglycemia.
Table 2.
Blood Glucose Values Comparisons
| Averages | Era | |||||
|---|---|---|---|---|---|---|
| IIP | 72-h MDI | P value | Era 1 (first 16 patients) | Era 2 (latter 16 patients) | P value | |
| Blood glucose (mg/dL) | ||||||
| Average | 116 | 128 | 0.003a | 118 | 114 | 0.005a |
| SD | 27 | 38 | 0.0001a | 31 | 23 | 0.0007a |
| Minimum | 56 | 67 | 0.003a | 52 | 61 | 0.02a |
| Maximum | 216 | 220 | 0.64 | 241 | 190 | 0.002a |
| % >140 mg/dL | 14.4 | 31.2 | <0.0001a | 17.7 | 11.1 | 0.001a |
| % <70 mg/dL | 2.5 | 4.5 | 0.098 | 3.4 | 1.6 | 0.02a |
| % in target range 70–140 mg/dL | 83.1 | 64.4 | <0.0001a | 78.9 | 87.3 | 0.0005a |
| % values <40 mg/dL | 0.1 | 0.1 | 0.68 | 0.2 | 0.0 | 0.24 |
| % of patients with a value | ||||||
| <70 mg/dL | 90.6 | 50.0 | 0.000273a | 100.0 | 81.3 | 0.08253 |
| <40 mg/dL | 6.3 | 6.3 | 1.00 | 12.5 | 0.0 | 0.16388 |
| Insulin received during period (average±SD) | ||||||
| Per day (U/day) | 43±29 | 62±38 | 0.000239a | 45±27 | 40±31 | 0.64189 |
| Per kg/day (U/kg/day) | 0.96±0.77 | 1.33±0.56 | 0.00299a | 0.96±0.55 | 0.96±0.47 | 0.99 |
Mean values for glycemic variables in the 32 patients while on the insulin infusion protocol (IIP) and during the first 72 h on multiple daily injections (MDI) therapy are compared on the left and glycemic parameters for the initial 16 patients (Era 1) versus the latter 16 patients (Era 2) during the intravenous IIP are compared on the right. P values are based on paired Student's t tests.
Significant difference.
Time spent in target range and glycemic variability improved over time
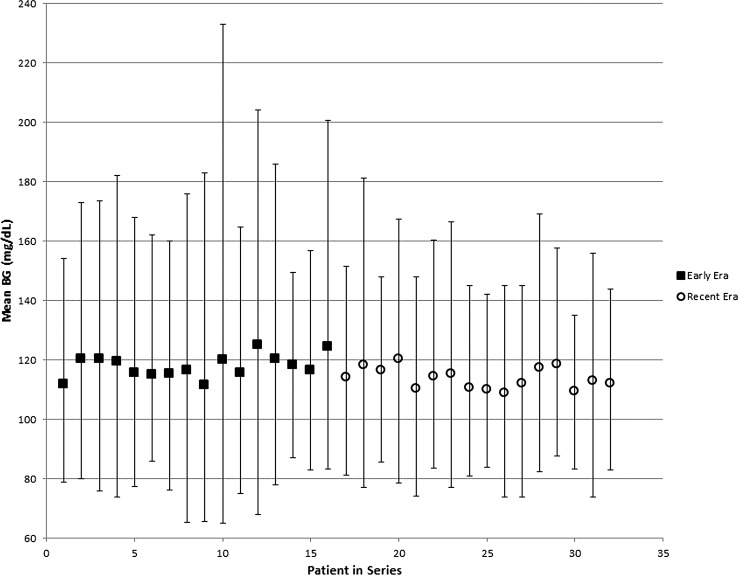
As with any new protocol, it was expected that there would be a gradual learning curve for healthcare providers prescribing or administering the IIP. To evaluate effect of experience, we compared the first 16 patients (Era 1) and the latter 16 patients (Era 2) managed on the IIP (Table 2). Era 1 and Era 2 did not differ in transplanted IEQ/kg (4,956±4,703 vs. 4,167±3,282, respectively; P=0.85), time on the IIP (6.7±2.0 vs. 7.1±2.7 days, respectively; P=0.64), postsurgical length of stay (17.9±5.3 days vs. 18.8±7.9 days, respectively; P=0.65), or surgical compilations (12.5% vs. 25.0%, respectively; P=0.33). The mean BG level in Era 2 was 4 mg/dL lower, a small but statistically significant difference, which remained significant when adjusted for gender, age, body mass index, and transplanted IEQ/kg (P=0.004). Glucose variance was less in Era 2 (Levene's test for homogeneity, P<0.0001). Mean and range between the 5th and 95th percentile BG values for each patient sequentially are displayed in Figure 2. In addition, Era 2 patients, compared with Era 1 patients, had less hyperglycemia (11.1% vs. 17.7% of readings >140 mg/dL), less hypoglycemia (1.6% vs. 3.4% <70 mg/dL), and a greater percentage of readings in the range of 70–140 mg/dL (87.3% vs. 78.9%) (P<0.05 for all). The rate of severe hypoglycemia was low; although this did not differ significantly across the eras (P>0.05), the only two patients with glucose levels <40 mg/dL on the IIP were in Era 1.
FIG. 2.
Comparison of early (Era 1) and recent (Era 2) era mean blood glucose (BG) levels. Values displayed are mean BG (mg/dL) for Era 1 (frst 16 patients) and Era 2 latter 16 patients). Error bars represent the 5th and 95th percentile values.
Transition to subcutaneous insulin therapy
Per our institutional protocol, patients were transitioned to subcutaneous MDI insulin once on full enteral feeds. Glycemic control on MDI was reasonable, based on a near-target mean BG level (128±38 mg/dL), but was limited by a greater proportion of values >140 mg/dL (31%; Table 2).
To account for the correlated nature of repeated BG testing and differences in time on the insulin infusion, a mixed-models approach (Type 3 test of fixed effects) was used to compare mean glucose levels on the IIP versus MDI therapy. The mean glucose level was significantly lower while patients were on the intravenous IIP versus the subcutaneous MDI therapy (P<0.0001) when controlling for gender, age, body mass index, and IEQ/kg.
Glucose variance was significantly less during the IIP protocol compared with the MDI therapy (by Levene's homogeneity of variance test, P<0.0001). It is notable that when glucose variability was analyzed within each individual patient, half of the patients (16 of 32) had significantly less glucose variability on the IIP (P<0.05, favoring IIP over MDI), whereas the other half had statistically similar glycemic variability on both regimens.
Discussion
Children undergoing TP and IAT for chronic pancreatitis represent a unique patient population for which a highly specialized IIP may be desired. Current hospital standard IIPs are adapted from the medical literature focused on primarily adult ICU and cardiac ICU populations, where the impact of hyperglycemia on surgical recovery, wound healing, and infection must be balanced with the mortality risks that are incumbent in this population and increased by occurrence of severe hypoglycemia.18–20,22–28,30 In contrast, in the pediatric TPIAT population, mortality is rare, and maintenance of euglycemia is essential to reduce glycemic stress on the transplanted pancreatic islets and thereby improve engraftment and long-term diabetes outcomes.9,11–13 For this population, at our institution, we have adopted a modified IIP to target a lower and narrower target BG range, designed to minimize both significant hypoglycemic and hyperglycemic events.
We hypothesized that the use of a specialized IIP in our pediatric TPIAT recipients would result in the majority of glucose values maintained in the range of 70–140 mg/dL without severe hypoglycemia. Overall, the TPIAT IIP was highly successful in achieving strict glycemic control in postsurgical TPIAT patients. The mean glucose level for all patients while on the IIP was within the goal range (116±27 mg/dL; range of mean BG levels for all patients, 109–125 mg/dL), and more than 80% of all glucose values remained within an acceptable range of 70–140 mg/dL, with minimal hypoglycemia and no serious adverse events. It should be noted that the islet yield for many patients was moderate to high (4,830±3,692 IEQ/kg), and these transplanted islets may contribute to better glycemic control.1–5
The rationale for targeting a physiologic glucose range in our transplant recipients is to avoid hyperglycemia-induced β-cell loss. This concept is supported by a large body of animal studies as well as ongoing work in clinical islet transplant recipients at our institution.2–17 Although some conflicting studies have suggested that glucose is a stimulus for the expansion of β-cell mass or survival in the vascularized pancreases,33,34 one should be cautious in applying these same principles to newly transplanted devascularized islets. Even so, the IIP at our institution does not keep islets devoid of glucose; although physiologic BG is the goal, all patients experience some supraphysiologic excursions (nearly 15% of BG values are >140 mg/dL), and patients are never maintained intentionally in hypoglycemia.
Both mean glucose and glucose variability were lower during the intravenous insulin infusion period than at the time of transition to subcutaneous insulin MDI. Although this may be expected, given limitations of subcutaneous insulin analogs and the need for titration over several days, it does suggest an opportunity to improve during this period of transition from the IIP to MDI therapy. Because islet revascularization is occurring and animal studies have demonstrated β-cell apoptosis (under conditions of hyperglycemia) still present at 30 days posttransplant, we believe BG control in this time period remains important for islet engraftment and β-cell survival.11–13 Emerging diabetes technologies, as they become available, may benefit this population.
Severe hypoglycemic events (BG level <40 mg/dL) were an extremely rare occurrence overall, observed in only two patients on intravenous insulin. In both cases (patients 2 and 8 in this series) precipitating events were noted: in one case, feeds were held for a procedure; in the second, the insulin infusion was not reduced per protocol for mild hypoglycemia. Neither patient had sequelae as a result of severe hypoglycemia. It is notable that most patients experienced at least one moderate hypoglycemic episode (BG <70 mg/dL) related to the IIP, including 80% of patients in Era 2, a rate comparable to or slightly higher than the 74% rate observed in the NICE-SUGAR Study.27 Although such events were easily treated and without evident long-term impact, this emphasizes the critical importance of frequent BG sampling within individual patients and the necessity of ongoing safety assessments of the protocol in general as patient volume increases.
A key aspect of development of an IIP policy is training of nursing staff, residents, and physicians.18 As such, we expected that glycemic control with our IIP would improve with time as nursing staff and physicians became more comfortable with the protocol and expertise with its use improves. In comparing the initial 16 and latter 16 patients (Era 1 and Era, respectively), we observed statistically significant improvements in mean glucose and glucose variability, with fewer hyperglycemic and hypoglycemic excursions in the more recent era. Thus, in this highly specialized population of children undergoing TPIAT, nursing and physician experience with insulin management after TPIAT may play a role in the better glucose control observed in our analysis. As a large referral center for this procedure, medical providers at our institution have received extensive training and experience in the postoperative management of these patients. Such specialized IIPs may be best restricted to tertiary referral centers with sufficient patient volume to maintain physician and nursing familiarity with the protocol.
There are several steps not displayed in the written IIP but that are taken at our institution to ensure patient safety and minimize variability. The patient population is homogeneous in regard to postoperative management, particularly nutritional management (administered as continuous enteral feeds and sugar free oral feeds only while the patient is on the IIP). As per standard care for patients on intravenous insulin infusions, patients have a dedicated nurse in the pediatric ICU to facilitate frequent glucose monitoring. In addition, we are cautious to avoid unnecessary boluses of dextrose in medications, using instead a normal saline carrier when feasible.
Although this study is clearly promising in the ability to achieve a high proportion of BG values in the target range intended, this study is limited to a retrospective analysis of a single IIP. Because there was no standard protocol for insulin infusion in patients weighing <45 kg prior to 2011 and therefore insulin drips were individualized for each child, we are not able to compare our current drip protocol with another preexisting standard. Of note is that prior to 2011 most insulin drips were written with a glycemic target of 80–110 mg/dL, consistent with the old standards for “tight” control in the ICU setting, and thus the glycemic goals historically were very similar to those targeted with this current protocol. Following a hypoglycemic event, BG was remeasured after 30 min; this may bias the data toward a lower mean BG level but would also bias away from the target range (over-represent BG levels <70 mg/dL). As most low BG values corrected easily, this bias is likely small. Because of the rarity of chronic pancreatitis in children, a relatively small number of patients is included. Although we hypothesize that improvement in glucose control in the later era (the most recent 16 patients) is due to a learning curve, there are other factors that could have influenced this, including small adjustments to the infusion protocol with the first five patients and more caution given by providers to eliminating antibiotics in dextrose carriers.
In conclusion, we observed that tight glycemic control can be safely achieved in children undergoing TP and islet autotransplant using an intravenous IIP specifically designed for this population and that the ability to maintain BG levels in target range improved with experience with the protocol. Special consideration should be given to this population and its unique set of risks, including postoperative diabetes mellitus, when implementing insulin management protocols.
Acknowledgments
This work was supported in part by the National Institute of Diabetes and Digestive and Kidney Diseases (grant K23DK084315, to M.B.). We would like to acknowledge the contributions of the physicians, nursing staff, and all other medical staff involved in the care of the participants.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Bellin MD, Sutherland DE: Pediatric islet autotransplantation: indication, technique, and outcome. Curr Diab Rep 2010;10:326–331 [DOI] [PubMed] [Google Scholar]
- 2.Blondet JJ, Carlson AM, Kobayashi T, Jie T, Bellin M, Hering BJ, Freeman ML, Beilman GJ, Sutherland DE: The role of total pancreatectomy and islet autotransplantation for chronic pancreatitis. Surg Clin North Am 2007;87:1477–1501, x. [DOI] [PubMed] [Google Scholar]
- 3.Sutherland DE, Radosevich DM, Bellin MD, Hering BJ, Beilman GJ, Dunn TB, Chinnakotla S, Vickers SM, Bland B, Balamurugan AN, Freeman ML, Pruett TL: Total pancreatectomy and islet autotransplantation for chronic pancreatitis. J Am Coll Surg 2012;214:409–424; discussion 424–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sutherland DE, Gruessner AC, Carlson AM, Blondet JJ, Balamurugan AN, Reigstad KF, Beilman GJ, Bellin MD, Hering BJ: Islet autotransplant outcomes after total pancreatectomy: a contrast to islet allograft outcomes. Transplantation 2008;86:1799–1802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmad SA, Lowy AM, Wray CJ, D'Alessio D, Choe KA, James LE, Gelrud A, Matthews JB, Rilo HL: Factors associated with insulin and narcotic independence after islet autotransplantation in patients with severe chronic pancreatitis. J Am Coll Surg 2005;201:680–687 [DOI] [PubMed] [Google Scholar]
- 6.Wilson GC, Sutton JM, Salehi M, Schmulewitz N, Smith MT, Kucera S, Choe KA, Brunner JE, Abbott DE, Sussman JJ, Ahmad SA: Surgical outcomes after total pancreatectomy and islet cell autotransplantation in pediatric patients. Surgery 2013;154:777–783; discussion 783–754. [DOI] [PubMed] [Google Scholar]
- 7.Hathout E, Chan NK, Tan A, Sakata N, Mace J, Pearce W, Peverini R, Chinnock R, Sowers L, Obenaus A: In vivo imaging demonstrates a time-line for new vessel formation in islet transplantation. Pediatr Transplant 2009;13:892–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Speier S, Nyqvist D, Cabrera O, Yu J, Molano RD, Pileggi A, Moede T, Kohler M, Wilbertz J, Leibiger B, Ricordi C, Leibiger IB, Caicedo A, Berggren PO: Noninvasive in vivo imaging of pancreatic islet cell biology. Nat Med 2008;14:574–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finzi G, Davalli A, Placidi C, Usellini L, La Rosa S, Folli F, Capella C: Morphological and ultrastructural features of human islet grafts performed in diabetic nude mice. Ultrastruct Pathol 2005;29:525–533 [DOI] [PubMed] [Google Scholar]
- 10.Nacher V, Merino JF, Raurell M, Soler J, Montanya E: Normoglycemia restores beta-cell replicative response to glucose in transplanted islets exposed to chronic hyperglycemia. Diabetes 1998;47:192–196 [DOI] [PubMed] [Google Scholar]
- 11.Biarnes M, Montolio M, Nacher V, Raurell M, Soler J, Montanya E: Beta-cell death and mass in syngeneically transplanted islets exposed to short- and long-term hyperglycemia. Diabetes 2002;51:66–72 [DOI] [PubMed] [Google Scholar]
- 12.Davalli AM, Scaglia L, Zangen DH, Hollister J, Bonner-Weir S, Weir GC: Vulnerability of islets in the immediate posttransplantation period. Dynamic changes in structure and function. Diabetes 1996;45:1161–1167 [DOI] [PubMed] [Google Scholar]
- 13.Juang JH, Bonner-Weir S, Wu YJ, Weir GC: Beneficial influence of glycemic control upon the growth and function of transplanted islets. Diabetes 1994;43:1334–1339 [DOI] [PubMed] [Google Scholar]
- 14.Jimbo T, Inagaki A, Imura T, Sekiguchi S, Nakamura Y, Fujimori K, Miyagawa J, Ohuchi N, Satomi S, Goto M: A novel resting strategy for improving islet engraftment in the liver. Transplantation 2014;97:280–286 [DOI] [PubMed] [Google Scholar]
- 15.Merino JF, Nacher V, Raurell M, Biarnes M, Soler J, Montanya E: Optimal insulin treatment in syngeneic islet transplantation. Cell Transplant 2000;9:11–18 [DOI] [PubMed] [Google Scholar]
- 16.Ferrer-Garcia JC, Merino-Torres JF, Perez Bermejo G, Herrera-Vela C, Ponce-Marco JL, Pinon-Selles F: Insulin-induced normoglycemia reduces islet number needed to achieve normoglycemia after allogeneic islet transplantation in diabetic mice. Cell Transplant 2003;12:849–857 [PubMed] [Google Scholar]
- 17.Bellin MD, Sutherland DE, Chinnakotla S, Dunn T, Kim Y, Johnson J, Saeed A, Manchester C, Balamurugan AN, Hering BJ, Pruett TL, Beilman GJ: Insulin independence after islet autotransplant is associated with subtle differences in early post-transplant glycemic control [abstract]. Diabetes 2012;61(Suppl 1):A43 [Google Scholar]
- 18.Ahmann AJ, Maynard G: Designing and implementing insulin infusion protocols and order sets. J Hosp Med 2008;3(5 Suppl):42–54 [DOI] [PubMed] [Google Scholar]
- 19.Dilkhush D, Lannigan J, Pedroff T, Riddle A, Tittle M: Insulin infusion protocol for critical care units. Am J Health Syst Pharm 2005;62:2260–2264 [DOI] [PubMed] [Google Scholar]
- 20.Wilson M, Weinreb J, Hoo GW: Intensive insulin therapy in critical care: a review of 12 protocols. Diabetes Care 2007;30:1005–1011 [DOI] [PubMed] [Google Scholar]
- 21.Moghissi ES, Korytkowski MT, DiNardo M, Einhorn D, Hellman R, Hirsch IB, Inzucchi SE, Ismail-Beigi F, Kirkman MS, Umpierrez GE; American Association of Clinical Endocrinologists, American Diabetes Association: American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Diabetes Care 2009;32:1119–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meijering S, Corstjens AM, Tulleken JE, Meertens JH, Zijlstra JG, Ligtenberg JJ: Towards a feasible algorithm for tight glycaemic control in critically ill patients: A systematic review of the literature. Crit Care 2006;10:R19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Furnary AP, Zerr KJ, Grunkemeier GL, Starr A: Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures. Ann Thorac Surg 1999;67:352–360; discussion 360–362. [DOI] [PubMed] [Google Scholar]
- 24.Furnary AP, Gao G, Grunkemeier GL, Wu Y, Zerr KJ, Bookin SO, Floten HS, Starr A: Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting. J Thorac Cardiovasc Surg 2003;125:1007–1021 [DOI] [PubMed] [Google Scholar]
- 25.Krinsley JS: Effect of an intensive glucose management protocol on the mortality of critically ill adult patients. Mayo Clin Proc 2004;79:992–1000 [DOI] [PubMed] [Google Scholar]
- 26.van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R: Intensive insulin therapy in critically ill patients. N Engl J Med 2001;345:1359–1367 [DOI] [PubMed] [Google Scholar]
- 27.NICE-SUGAR Study Investigators, Finfer S, Liu B, Chittock DR, Norton R, Myburgh JA, McArthur C, Mitchell I, Foster D, Dhingra V, Henderson WR, Ronco JJ, Bellomo R, Cook D, McDonald E, Dodek P, Hebert PC, Heyland DK, Robinson BG: Hypoglycemia and risk of death in critically ill patients. N Engl J Med 2012;367:1108–1118 [DOI] [PubMed] [Google Scholar]
- 28.Magaji V, Nayak S, Donihi AC, Willard L, Jampana S, Nivedita P, Eder R, Johnston J, Korytkowski MT: Comparison of insulin infusion protocols targeting 110–140 mg/dL in patients after cardiac surgery. Diabetes Technol Ther 2012;14:1013–1017 [DOI] [PubMed] [Google Scholar]
- 29.Vlasselaers D, Milants I, Desmet L, Wouters PJ, Vanhorebeek I, van den Heuvel I, Mesotten D, Casaer MP, Meyfroidt G, Ingels C, Muller J, Van Cromphaut S, Schetz M, Van den Berghe G: Intensive insulin therapy for patients in paediatric intensive care: A prospective, randomised controlled study. Lancet 2009;373:547–556 [DOI] [PubMed] [Google Scholar]
- 30.Goldberg PA, Siegel MD, Sherwin RS, Halickman JI, Lee M, Bailey VA, Lee SL, Dziura JD, Inzucchi SE: Implementation of a safe and effective insulin infusion protocol in a medical intensive care unit. Diabetes Care 2004;27:461–467 [DOI] [PubMed] [Google Scholar]
- 31.Lakey JR, Warnock GL, Shapiro AM, Korbutt GS, Ao Z, Kneteman NM, Rajotte RV: Intraductal collagenase delivery into the human pancreas using syringe loading or controlled perfusion. Cell Transplant 1999;8:285–292 [DOI] [PubMed] [Google Scholar]
- 32.Ricordi C, Lacy PE, Scharp DW: Automated islet isolation from human pancreas. Diabetes 1989;38(Suppl 1):140–142 [DOI] [PubMed] [Google Scholar]
- 33.Alonso LC, Yokoe T, Zhang P, Scott DK, Kim SK, O'Donnell CP, Garcia-Ocana A: Glucose infusion in mice: A new model to induce beta-cell replication. Diabetes 2007;56:1792–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martens GA, Pipeleers D: Glucose, regulator of survival and phenotype of pancreatic beta cells. Vitam Horm 2009;80:507–539 [DOI] [PubMed] [Google Scholar]