Abstract
Investigations of plausible prebiotic chemistry on early Earth must consider not only chemical reactions to form more complex products such as proto-biopolymers but also reversible, molecular self-assembly that would influence the availability, organization, and sequestration of reactant molecules. The self-assembly of guanosine compounds into higher-order structures and lyotropic liquid crystalline “gel” phases through formation of hydrogen-bonded guanine tetrads (G-tetrads) is one such consideration that is particularly relevant to an RNA-world scenario. G-tetrad-based gelation has been well studied for individual guanosine compounds and was recently observed in mixtures of guanosine with 5′-guanosine monophosphate (GMP) as well. The present work investigates the self-assembly of GMP in the presence of the other RNA nucleotides. Effects of the total concentration and relative proportion of the nucleotides in the mixtures, the form (disodium salt vs. free acid) of the nucleotides, temperature, pH, and salt concentration were determined by visual observations and circular dichroism (CD) spectroscopy. The results show that formation of cholesteric G-tetrad phases is influenced by interactions with other nucleotides, likely through association (e.g., intercalation) of the nucleotides with the G-tetrad structures. These interactions affect the structure and stability of the G-tetrad gel phase, as well as the formation of alternate self-assembled GMP structures such as a continuous, hydrogen-bonded GMP helix or dimers and aggregates of GMP. These interactions and multiple equilibria are influenced by the presence of cations, especially in the presence of K+. This work could have important implications for the emergence of an RNA or proto-RNA world, which would require mixtures of nucleotides at sufficiently high, local concentrations for abiotic polymerization to occur. Key Words: RNA world—Prebiotic chemistry—RNA polymerization—Guanosine monophosphate—Molecular self-assembly. Astrobiology 14, 876–886.
1. Introduction
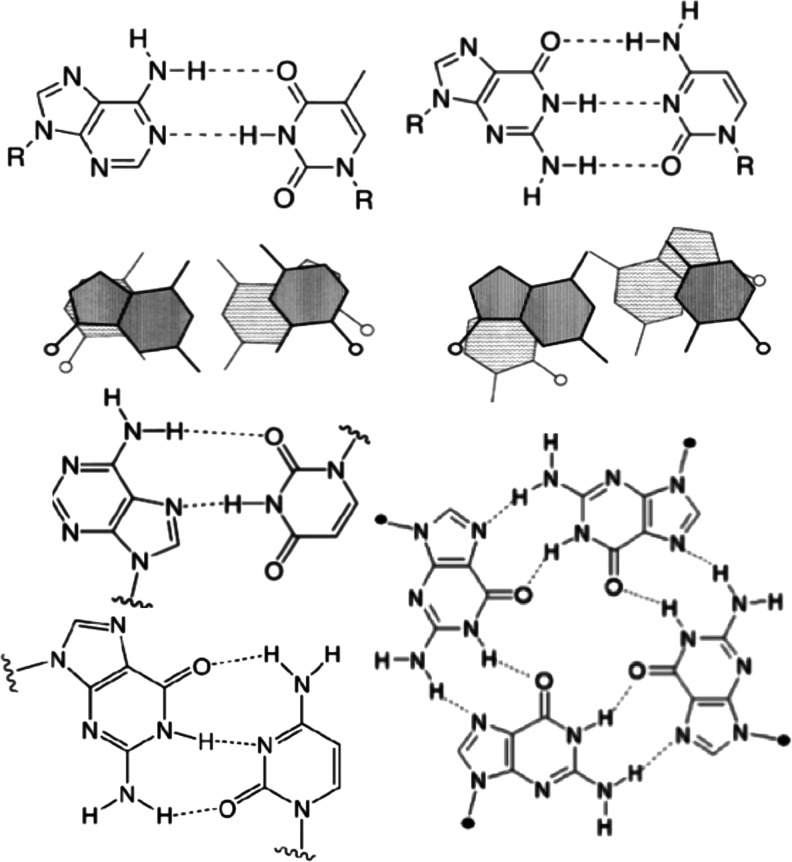
Investigations of prebiotic chemistry on early Earth must consider not only chemical reactions to form more complex products such as proto-biopolymers but also reversible molecular self-assembly that can influence the availability, organization, and sequestration of the monomeric reactants. Specifically, in an RNA-world scenario (Gilbert, 1986), abiotic polymerization of RNA would have occurred in complex mixtures of nucleotides (along with a multitude of other chemical species) that are capable of other, reversible modes of interaction such as base stacking through pi-pi interactions and base pairing through Watson-Crick or Hoogsteen hydrogen bonding. Here, we focus on a different interaction, namely, the reversible self-assembly of 5′-guanosine monophosphate (GMP) into planar, “G-tetrad” structures through Hoogsteen hydrogen bonding between four nucleotides, each having hydrogen bonds to its two nearest neighbors (Chantot et al., 1971; Guschlbauer et al., 1990; Pieraccini et al., 2003; Davis, 2004; Davis and Spada, 2007). These various interactions are depicted in Fig. 1.
FIG. 1.
Examples of interactions of nucleotides. Top: Watson-Crick base pairing of A:U (left) and G:C (right). Center: examples of base stacking through pi-pi interactions. Bottom: Hoogsteen base pairing of A:U (left, top) and G:C (left, bottom), and tetrad formation by G (right).
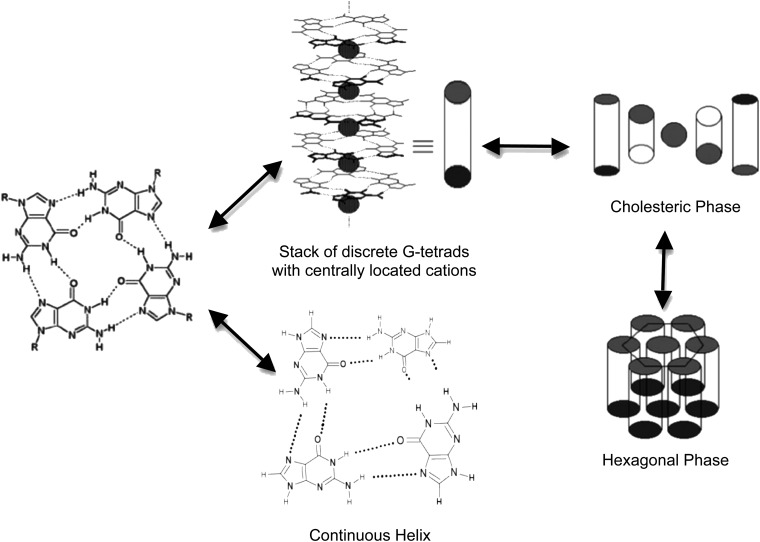
While base pairing between complementary nucleotides does not generally lead to higher-order assemblies in solutions of monomeric nucleotides, the planar G-tetrads can self-assemble into helical stacks that further organize with increasing concentration into lyotropic, liquid crystalline “gel” phases that can progress from cholesteric to hexagonal organization (Chantot et al., 1971; Guschlbauer et al., 1990; Pieraccini et al., 2003; Davis, 2004; Wong et al., 2005; Davis and Spada, 2007). In the case of GMP, an alternate structure in which the guanines are associated with each other in a continuous helix rather than discrete tetrads has been reported to form in the presence of K+ (Walmsley and Burnett, 1999). Higher-order G-tetrad assemblies and gel phases have also been observed in solutions containing mixtures of G-tetrad-forming compounds such as GMP and guanosine (Yu et al., 2008; Novotná et al., 2012; Lokesh and Suryaprakash, 2013) and GMP and isoguanosine phosphate (Roberts et al., 1997). Formation of self-assembled, organized phases (depicted for GMP in Fig. 2) is completely reversible at every stage, from monomer to gel.
FIG. 2.
GMP self-assembly, progressing from individual G-tetrads to either stacks of G-tetrads or a continuous Hoogsteen H-bonded helical structure, to cholesteric and then hexagonal liquid crystalline phases.
Any RNA or proto-RNA scenario that includes guanine nucleobase must take into consideration the reversible self-assembly of guanine compounds through Hoogsteen hydrogen bonding that could compete with its incorporation into oligomers. Formation of G-tetrad-based, lyotropic liquid crystalline “gel” phases by GMP at high concentrations might be encountered, for example, in shallow surface pools during the dry phase of a wet-dry cycle or during low tide in a tidal cycle of concentration/dilution. Reports of formation of G-tetrad structures at mica surfaces (Samori et al., 2002; Kunstelj et al., 2007) support the potential influence of GMP self-assembly on prebiotic chemistry in early Earth environments.
Because of this competition between GMP self-assembly and addition of GMP monomers into growing RNA strands, GMP (or a related guanine compound) would seem to be an unlikely candidate for inclusion in prebiotic RNA or proto-RNA, yet there are compelling reasons that argue for its inclusion. First, G-rich genomic sequences that form G-tetrad-based structures are widespread throughout living organisms (Simonsson, 2005), from “evolutionarily distant genomes of prokaryotes” (Fry, 2007) to modern mammalian species (Rawal et al., 2006; Todd et al., 2006; Fry, 2007), and these sequences exhibit selective interactions with biomolecules such as proteins (Wang et al., 2009; Xiao et al., 2009; Zhang et al., 2012). They are abundant in telomeres, gene promoter regions, rDNA gene clusters, and recombination “hotspots” (Gatto et al., 2009). The prevalence of G-tetrad-forming sequences dating back to primitive terrestrial life suggests a driving force to select G-rich sequences at an early point in biological evolution and, possibly, even earlier in prebiotic selection. Additionally, intramolecular G-quadruplex structures comprising two or more G-tetrads are common structural motifs in RNA and DNA aptamers (Gatto et al., 2009) selected through combinatorial “molecular evolution” for highly selective, high-affinity binding to molecular and macromolecular targets. Selective affinity interactions of prebiotic, RNA-like oligomers with amino acids, peptides, primitive cofactors, and inorganic cations would serve to facilitate and regulate catalysis and autocatalysis.
Previous studies of interactions between different nucleotides have included considerations of G-tetrad formation of GMP (Eimer and Dorfmuller, 1992), but systematic studies focusing on the self-assembly of GMP in the presence of other, non-G-tetrad-forming nucleotides, especially in the context of prebiotic chemistry, are unexplored. It is essential to address this significant gap in knowledge, since these interactions could collectively affect the outcome (e.g., polymer yields, sequence space, and length) of RNA polymerization, particularly in prebiotic environments that lack the biological tools responsible for regulation of RNA polymerization in modern biology. This line of inquiry complements recent explorations by others on molecular self-assembly in prebiotic environments (Deamer et al., 2006), nucleotide aggregation (e.g., Raszka and Kaplan, 1972; Rymden and Stilbs, 1985; Eimer and Dorfmuller, 1992), and self-assembly of nucleobase analogues (Cafferty et al., 2013).
Here, we describe the effects of AMP, CMP, and UMP on the self-assembly of GMP in water. Although only GMP is able to form tetrad structures, the other RNA nucleotides may influence GMP self-assembly and the structure and stability of the resulting phases through other interactions such as base stacking, hydrophobic forces, electrostatic interactions, and hydrogen bonding. We studied the effects of the total concentration and relative proportion of GMP and XMP, disodium salt versus free acid form of the nucleotides, temperature, pH, and salt content using visual observations and circular dichroism (CD) spectroscopy. For comparison, we also studied mixtures of guanosine with guanosine 5′-monophosphate disodium salt (GMPdss), and mixtures of GMPdss with sodium or potassium phosphate salts or phosphoric acid in place of the non-guanine nucleotide.
2. Materials and Methods
Chemicals including GMPdss, guanosine (Guo), adenosine 5′-monophosphate free acid (AMPfa), cytidine 5′-monophosphate disodium salt (CMPdss), cytidine 5′-monophosphate free acid (CMPfa), sodium hydroxide (NaOH), sodium chloride (NaCl), potassium chloride (KCl), sodium and potassium phosphate salts, and magnesium chloride (MgCl2) were purchased from Sigma-Aldrich. Hydrochloric acid (HCl) and phosphoric acid (H3PO4) were purchased from Baker Chemicals. Adenosine 5′-monophosphate disodium salt (AMPdss) and uridine 5′-monophosphate disodium salt (UMPdss) were purchased from Acros Organics. GMP and UMP were not commercially available in the free acid form, so the disodium salt forms were passed through a column of 32g Dowex 50WX8 hydrogen form, 200–400 mesh, from Sigma-Aldrich, and titrated with 3 M HCl to pH 5.
All solutions were prepared in ultrapure water from a Barnstead Nanopure System (Thermo Scientific, Waltham, MA). As indicated for the specific experiments, some solutions were pH adjusted with NaOH or HCl. Others contained NaCl, KCl, or MgCl2.
The solutions were prepared in glass vials. Unless otherwise noted, the solutions were heated to 50–90°C to dissolve the compounds and then allowed to cool to room temperature. The solutions were stored in the refrigerator when not in use.
Circular dichroism (CD) measurements were performed with a Jasco J-715 spectrophotometer (JASCO, Easton, MD). Samples were contained in a 0.01 mm pathlength, dismountable quartz cuvette (Starna Cells, Inc., Atascadero, CA). Cuvettes were filled at an elevated temperature to ensure uniform sample distribution. CD spectra were measured from 200 to 350 nm, at 5°C and 20°C, allowing 15 min equilibration between temperature changes. Thermal melt experiments were monitored at 275 nm. The temperature was changed at a rate of 150°C/min. Thermal response was measured first for increasing temperature and then for decreasing temperature to obtain both melting and cooling curves. Unless otherwise indicated, each spectrum and thermal melt curve is the average of three replicates performed on the same sample, generally on the same day.
3. Results
3.1. Visual observations
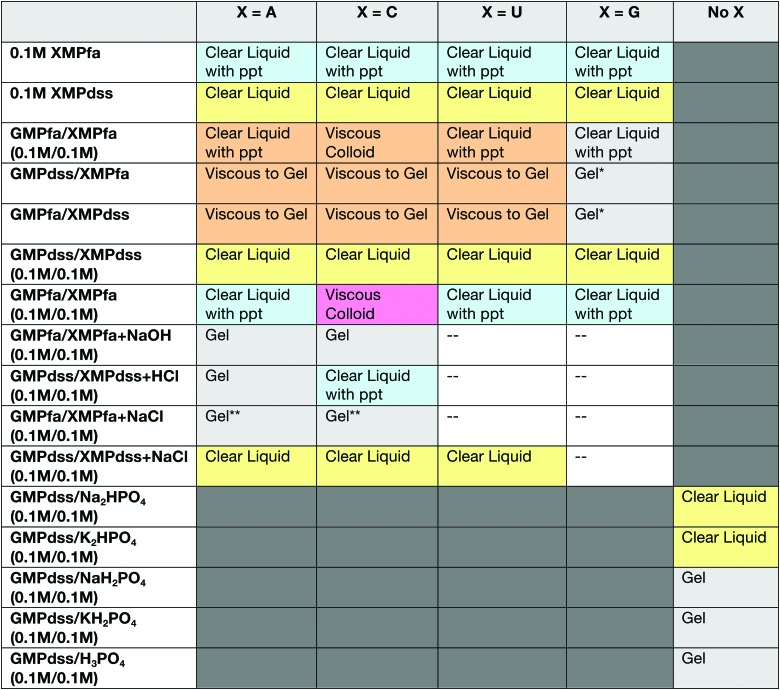
Solutions of the individual nucleotides (XMP, where X=A, G, C, or U) and mixtures of two nucleotides in various combinations of disodium salt (XMPdss) and free acid (XMPfa) forms were visually classified as “liquid,” “viscous,” or “gel.” Solutions containing GMPdss with sodium or potassium phosphate salts or phosphoric acid were also examined. Results are summarized in Fig. 3. The term “liquid” describes a solution with waterlike viscosity, the term “viscous” describes more viscous solutions that can still move when the container is inverted, and the term “gel” describes firm gels that maintain their shape with no evidence of flowing when the container is inverted. Within these three groups, we also noted whether the solutions showed evidence of precipitation or turbidity.
FIG. 3.
Summary of visual observations of nucleotides alone and in mixtures. *Unusually firm and ductile, forms threads. **Up to 1 M salt, then precipitates. (Color graphics are available online at www.liebertonline.com/ast)
Reports of the solubilities of the nucleosides and nucleotides are given in Table 1 (Windholz, 1976; Wishart et al., 2013; Santa Cruz Biotechnology, 2014). It is interesting that GMPdss and GMPfa are at least as soluble as AMPdss and AMPfa, respectively, considering the low solubility of Guo compared to Ade. It is likely that GMP self-assembly into organized G-tetrad “G4” structures with the phosphate groups extending into the aqueous solution plays a role in its solubilization.
Table 1.
Reported Solubilities of Nucleosides and Nucleotides
Mixtures containing GMPdss and XMPdss (X=A, C, U) were clear liquids, which is consistent with the good solubility of XMP disodium salts. The mixtures of the less soluble, free acid forms (GMPfa/XMPfa) contained precipitate for all X except CMPfa, which was a viscous, colloidal suspension, indicating some degree of GMP self-assembly. Solutions of GMPdss/XMPfa (X=A, C, U) ranged from viscous solution to gel depending upon concentration. Based on visual observation, the gelation behavior of solutions of GMPfa/XMPdss (X=A, C, U) was generally similar to that of their GMPdss/XMPfa counterparts.
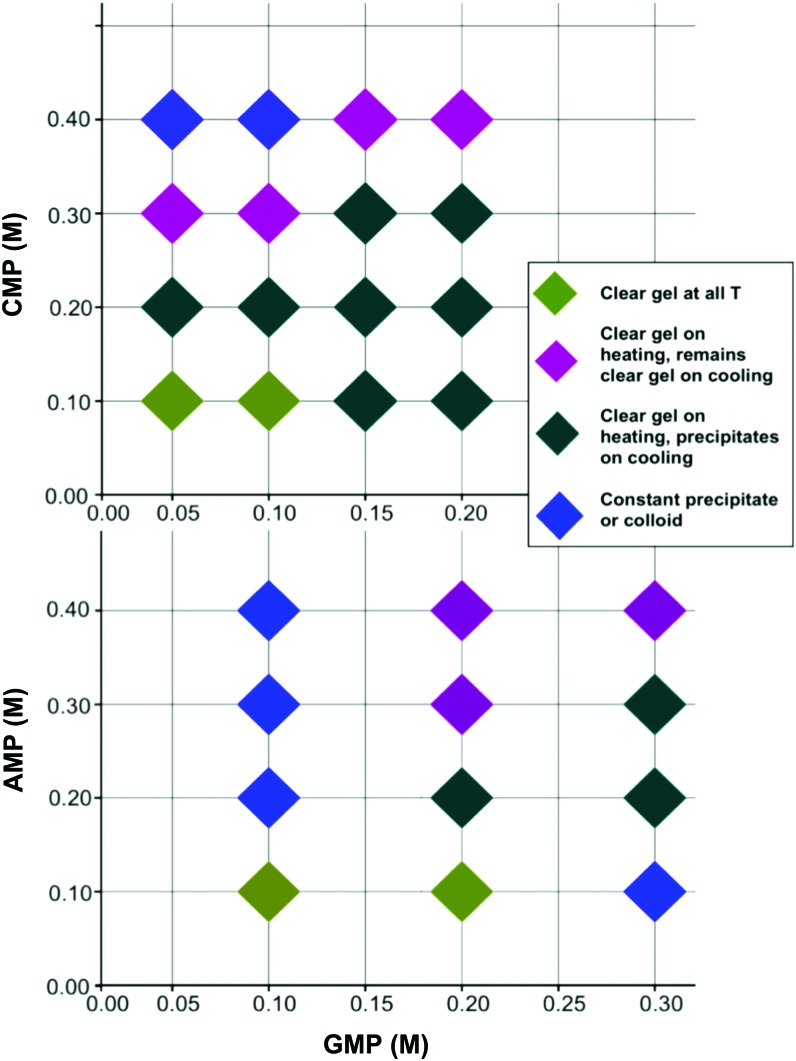
We next studied GMPdss/AMPfa and GMPdss/CMPfa in greater detail, first, in order to compare a purine to a pyrimidine, and second, because CMP is the complementary nucleotide to GMP and has also been reported to associate with GMP aggregates (Eimer and Dorfmuller, 1992). The results, summarized in Fig. 4, show that, for a given concentration of XMP, it takes more GMP to solubilize and form stable gels with AMP than with CMP. This may be due to higher solubility of CMPfa or greater uptake into the GMP gel.
FIG. 4.
Thermoresponsiveness of GMPdss/CMPfa and GMPdss/AMPfa in water as a function of concentration. (Color graphics are available online at www.liebertonline.com/ast)
We also examined mixtures of the two forms of GMP (GMPdss/GMPfa). These solutions were firm, clear gels at all concentrations studied (0.025–0.10 M each). The gels were unusual compared to the others in that they melted at much higher temperatures and reformed gels immediately after heating. Moreover, the gels were ductile and could be pulled into “threads.” This has also been observed for mixtures of GMP/Guo gels at certain concentrations (Cassidy, 2014).
Although solutions of like forms of nucleotides (GMPfa/XMPfa and GMPdss/XMPdss) did not form gels, gelation could in most cases be achieved by adjusting the pH of the solutions to ∼6, which is the approximate pH of the mixtures of unlike forms (GMPdss/XMPfa and GMPfa/XMPdss). This was demonstrated for solutions of GMP/AMP and GMP/CMP. GMPfa/XMPfa solutions started out at pH ∼2 and were adjusted to pH ∼6 with NaOH. The GMPfa/AMPfa began as a clear liquid with a precipitate. Upon pH adjustment and heating, the precipitate dissolved, and the solution formed a clear gel upon cooling. The GMPfa/CMPfa solution began as a viscous, colloidal suspension and formed a clear gel immediately upon addition of NaOH without heating, again indicating greater ease of solubilization and/or association of CMPfa with the GMP gel.
For GMPdss/XMPdss (X=A, C), the solutions started out at pH ∼8 and were adjusted to pH ∼6 with HCl. The GMPdss/AMPdss solution began as a clear liquid and gelled immediately upon addition of HCl without heating. The GMPdss/CMPdss solution is the only solution tested that did not form a clear gel upon pH adjustment. It began as a clear liquid, and upon addition of HCl, it formed a precipitate that persisted even upon heating.
We also investigated the effects of NaCl at concentrations up to 1 M on the solutions of GMPdss/XMPdss, GMPfa/XMPfa, and GMPdss/XMPfa (X=A, C). The GMPfa/XMPfa solutions formed clear gels even though the pH remained ∼2, while the GMPdss/XMPdss solutions, which were clear liquids with pH ∼8, remained as clear liquids. For GMPdss/XMPfa mixtures, which in the absence of salt were clear and ranged from viscous to gel, the solutions remained clear and became increasingly firm with increasing salt concentrations up to 0.5 M for monoprotic salts (NaCl, KCl) or 0.25 M for diprotic MgCl2. Above these salt concentrations, the solutions remained gels but became cloudy and eventually opaque due to suspension of insoluble material.
Finally, we examined mixtures of 0.1 M GMPdss with 0.1 M of monobasic or dibasic sodium and potassium phosphate salts and with phosphoric acid in order to compare GMPdss self-assembly in mixtures with XMP to its self-assembly in mixtures with phosphate salts or phosphoric acid, which do not contain the nucleoside moiety (X). These solutions were liquids for the dibasic salts (which is similar to the observations for GMPdss/XMPdss) and firm gels for the monobasic salts and phosphoric acid (which is similar to the observations for GMPdss/XMPfa at the same concentrations).
3.2. CD analysis
To gain additional insight into the effects of the other nucleotides on GMP self-assembly, we examined the CD spectra and thermal responses of the solutions. CD spectra are highly dependent upon the degree of molecular self-assembly and the higher-order structure of organized media. We collected CD spectra in the 200–350 nm range, which corresponds to the CD spectra of G-tetrad-based structures. Spectra were measured at 5°C and 20°C. Thermal melt curves were obtained at 275 nm, which corresponds to the cholesteric G-tetrad (G4) gel phase formed by guanosine compounds (Panda and Walmsley, 2011) and therefore does not necessarily report on other coexisting or alternate structures in the solutions. Because the thermal melt curves varied in shape among different samples and did not always have the typical S shape associated with melt curves, we did not attempt to identify curve midpoints to calculate melting temperatures. Instead, we compared the temperatures at which the 275 nm CD signal returned to zero, indicating essentially complete melting of the gel phase. Experiments were performed at least two to three times on different solutions and at different times after solution preparation to make sure that the results were reproducible.
3.2.1. CD of individual nucleotides
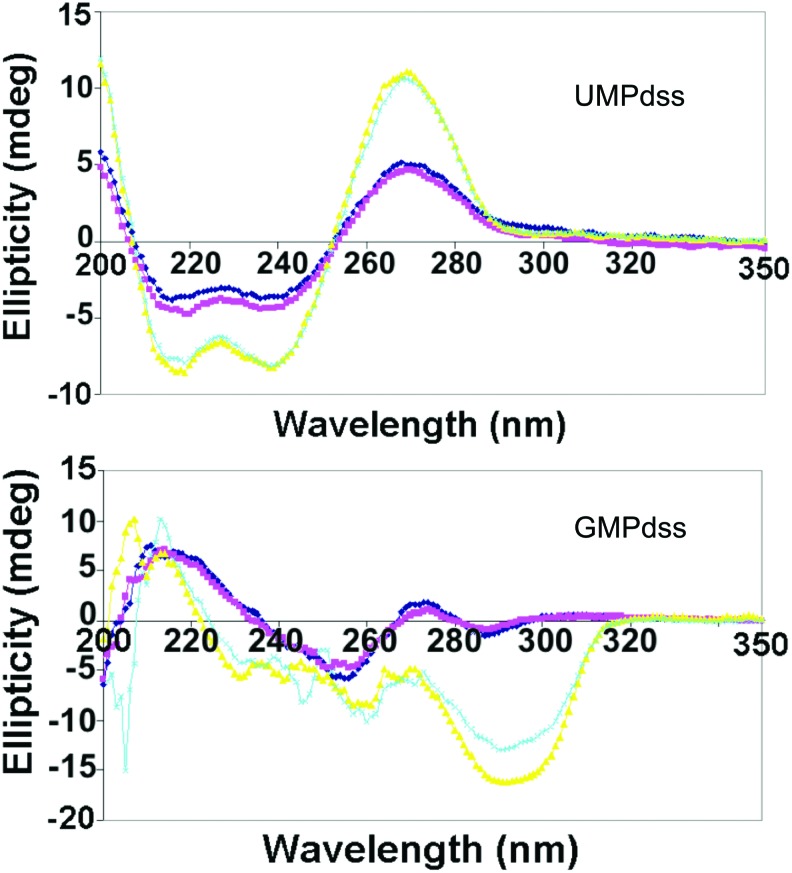
Solutions of the nucleotides in the free acid form (XMPfa) could not be measured due to turbidity, and those of AMPdss and CMPdss showed no significant CD features. The CD spectra of UMPdss and of GMPdss in water at concentrations of 0.1 and 0.2 M are shown in Fig. 5. Both solutions were clear liquids. This is expected, since UMPdss does not exhibit organized self-assembly, and GMPdss does not form gels in water at pH close to neutral. The CD spectral profiles of UMPdss are independent of concentration and correspond to the spectrum of the monomeric nucleotide. In contrast, the CD spectrum of GMPdss at 0.1 M corresponds to monomeric GMP, while at 0.2 M, the spectrum exhibits a (+)220 nm shoulder, a (−)260 nm peak, and a (−)295 nm peak that indicate a different self-assembled structure, possibly due to base stacking (Panda and Walmsley, 2011). There is no clear evidence of higher-order, G-tetrad assemblies.
FIG. 5.
CD spectra of UMPdss (top) and GMPdss (bottom) in water. Dark blue: 0.1 M XMPdss at 5°C; pink: 0.1 M XMPdss at 20°C; yellow: 0.2 M XMPdss at 5°C; light blue: 0.2 M XMPdss at 20°C. (Color graphics are available online at www.liebertonline.com/ast)
3.2.2. CD of mixtures
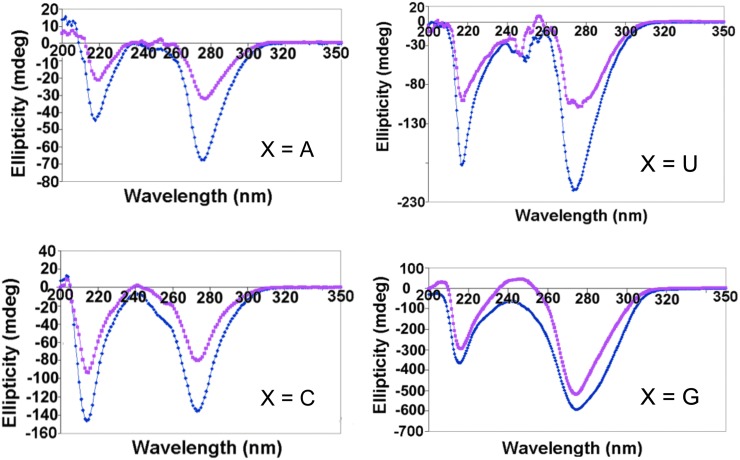
Our next experiments looked at mixtures of unlike forms of nucleotides. The final concentration of each nucleotide in the mixtures was 0.1 M, which was chosen because the mixtures at these concentrations are clear gels at room temperature. The solutions of GMPdss/XMPfa exhibited large, negative peaks at 220 nm and 275–280 nm (Fig. 6) that are indicative of a cholesteric G4 gel phase (Panda and Walmsley, 2011). There are differences among the different XMPfa with respect to both peak magnitudes and relative magnitudes of the two peaks. Not surprisingly, X=G gives the largest magnitude gel peaks. It is also the most stable gel, as shown in the corresponding thermal melt curves (Fig. 7 and Table 2).
FIG. 6.
CD spectra of GMPdss (0.1 M)/XMPfa (0.1 M) mixtures in water at 5°C (blue) and 20°C (pink). (Color graphics are available online at www.liebertonline.com/ast)
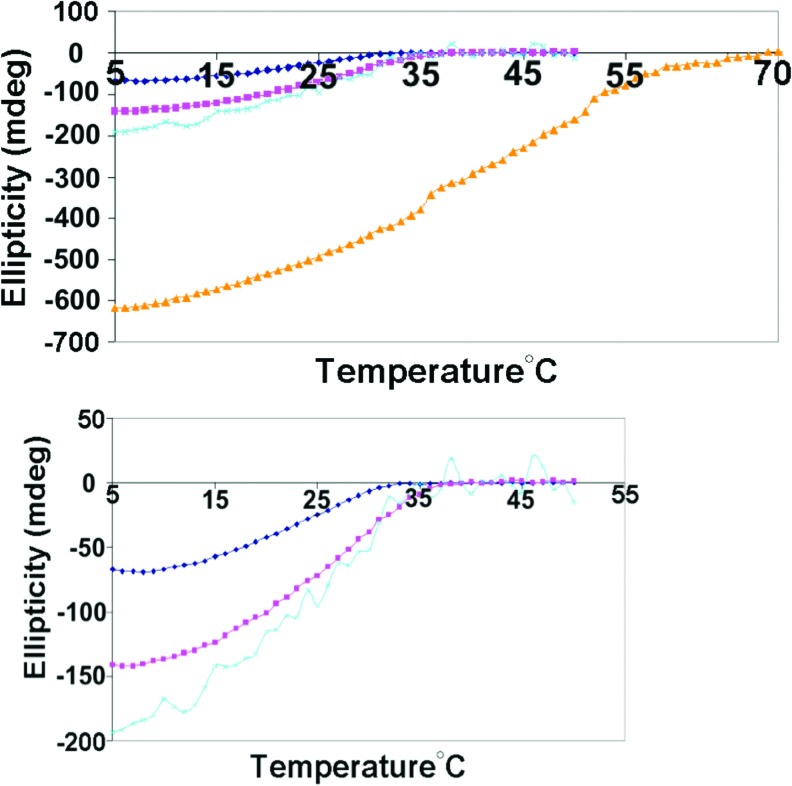
FIG. 7.
Summary of CD thermal melt results for GMPdss (0.1 M)/XMPfa (0.1 M) mixtures in water. Top: X=A (dark blue), C (pink), U (light blue), G (yellow). Bottom graph is expanded scale showing curves for X=A, C, and U. (Color graphics are available online at www.liebertonline.com/ast)
Table 2.
Thermal Stabilities of Cholesteric G4 Phases, Determined by CD Thermal Melt Experiments and Expressed as the Temperature at Which the (−)275 nm Peak Corresponding to the Cholesteric G4 Gel Phase Returned to Zero, Indicating Complete Melting (Dissociation) of the Gel
| Melting temperature (°C) | |||||
|---|---|---|---|---|---|
| X=A | X=C | X=G | X=U | No X | |
| GMPdss/XMPfa | 33 | 38 | ∼70 | 37 | |
| GMPfa/XMPdss | 36 | 38 | – | – | |
| GMPdss/XMPdss+HCl | 35 | – | – | – | |
| GMPfa/XMPfa+NaOH | 33 | 37 | – | – | |
| GMPfa/XMPfa+NaCl | 33 | 27 | – | – | |
| GMPdss/KH2PO4 | 40 | ||||
| GMPdss/NaH2PO4 | 30 | ||||
| GMPdss/H3PO4 | 30 | ||||
For X=C and, to a lesser extent, X=U, there is an increase in the magnitude of the (−)220 nm peak relative to the (−)275 nm peak that is accompanied by the appearance of a shoulder at (−)250 nm. This is likely due to the presence of a second structure that also contributes to the (−)220 nm peak. Its presence does not appear to affect the stability of the cholesteric G4 phase, which decreases as G >> C ∼ U>A.
When the temperature ramps were run in reverse (50°C to 5°C), the results (not shown) indicated thermal reversibility, although the curves were slightly shifted to cooler temperatures. This was observed for all thermal melts and is attributed to the rate of the temperature ramp relative to the kinetics of melting versus re-gelation. There were no significant differences between the CD spectra of solutions that were equilibrated at 5°C before and after a thermal melt experiment, confirming reversibility.
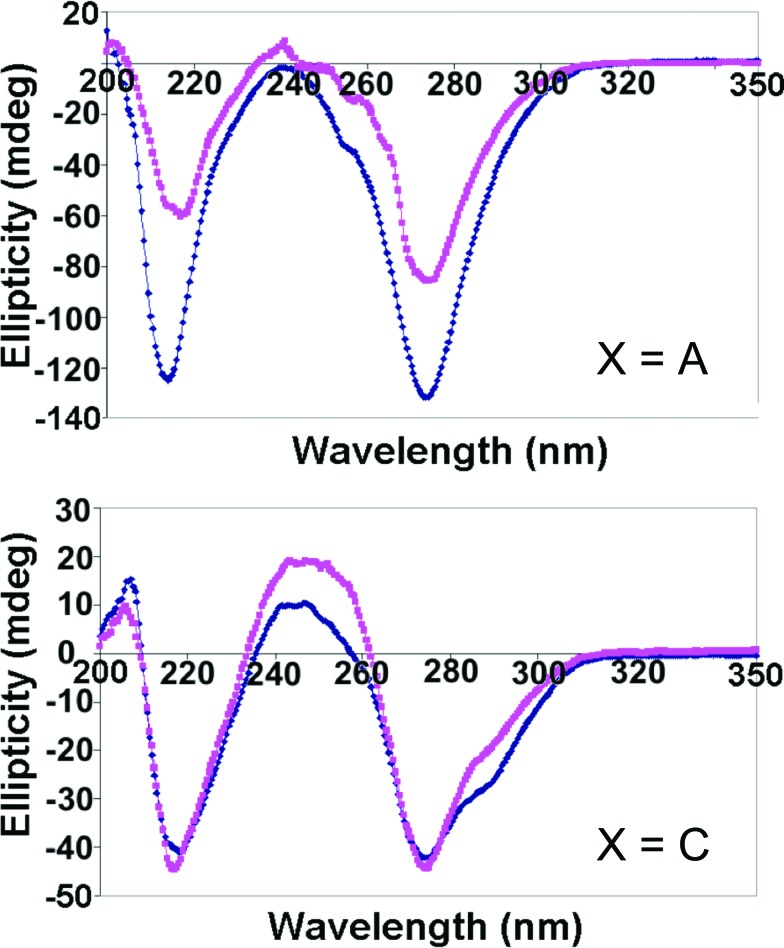
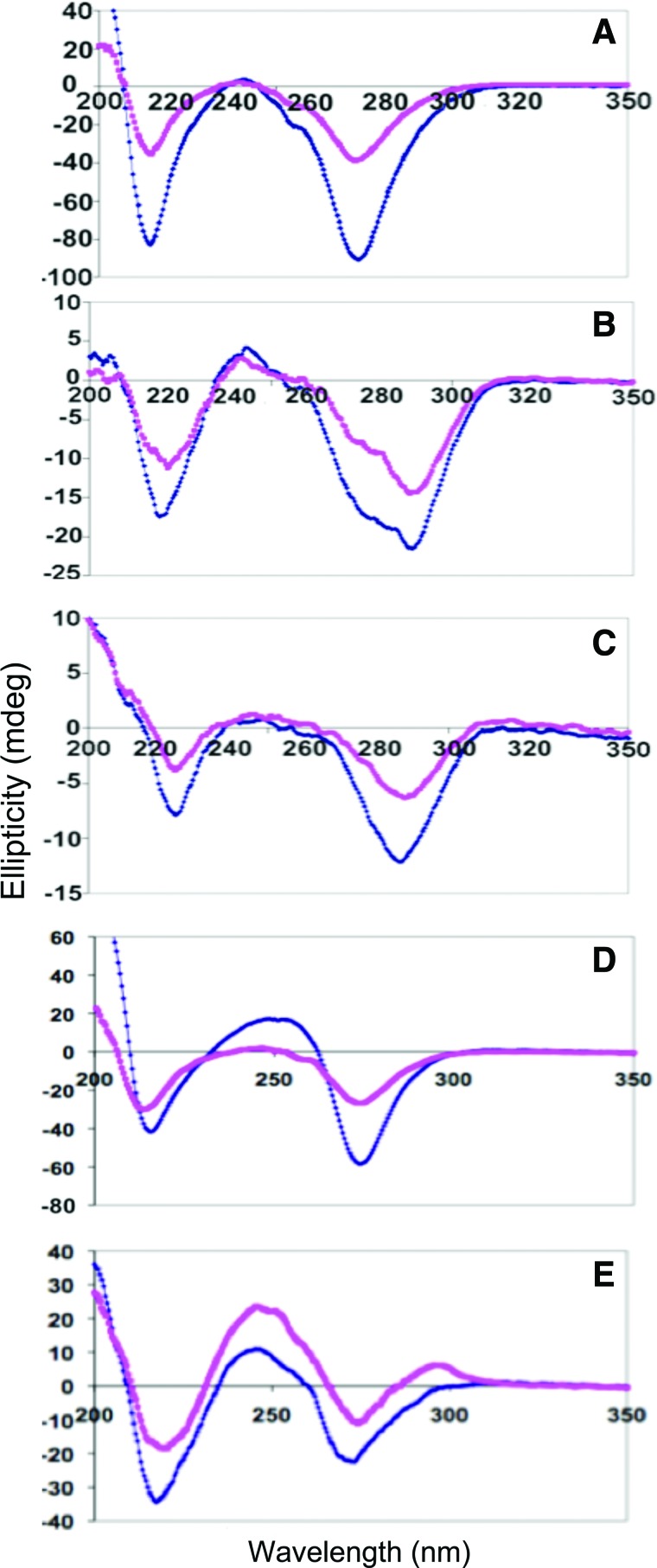
The CD spectra and melting temperatures of GMPfa/XMPdss (X=A, C), which behaved similarly to the corresponding GMPdss/XMPfa solutions based on visual observation, are shown in Fig. 8 and Table 2, respectively. Interestingly, comparison with Fig. 6 shows that the spectrum of GMPfa/AMPdss looks more like that of GMPdss/CMPfa than GMPdss/AMPfa, and that of GMPfa/CMPdss looks more like that of GMPdss/GMPfa than GMPdss/CMPfa. For GMPfa/AMPdss, there is also an increase in peak magnitude and thermal stability, which becomes similar to that of the mixtures (dss/fa and fa/dss) of GMP with CMP or UMP (Table 2). Although in most cases the solutions are heated to aid in dissolution of the nucleotides (see Materials and Methods), it is possible that not all the G-tetrads are disrupted during heating, especially for the GMPdss form that would have stabilizing Na+ centrally located in the tetrad. Were this the case, GMPdss might interact with XMPfa as a stable G-tetrad, while GMPfa might interact with XMPdss as individual or stacked monomers. These differences would affect the interactions of XMP with GMP during the gelation process, and the resulting structures could be “locked” into place if gelation were fast.
FIG. 8.
CD spectra of GMPfa (0.1 M)/XMPdss (0.1 M) mixtures in water at 5°C (blue) and 20°C (pink). Top: X=A; bottom: X=C. (Color graphics are available online at www.liebertonline.com/ast)
Solutions in which a sodium or potassium phosphate salt or phosphoric acid was used in place of XMPfa in mixtures with GMPdss were also examined. As described above, GMPdss formed clear liquids with the dibasic salts (Na2HPO4 or K2HPO4) and clear, firm gels with the monobasic salts (NaH2PO4 or KH2PO4) and phosphoric acid. The CD spectra (Supplementary Fig. S1; Supplementary Data are available online at www.liebertonline.com/ast) and thermal melt results (Table 2) indicate cholesteric G4 phases for GMPdss/Na2HPO4 and GMPdss/H3PO4 that were completely melted by 30°C. A different, highly organized phase was observed for GMPdss/K2HPO4 that had much greater peak magnitudes, including the appearance of the (−)250 nm peak that dominates the spectrum and is indicative of a second organized phase in addition to the cholesteric G4 phase [indicated by the shoulder at (−)275 nm] and was completely melted by 40°C. These results show that the stability of the cholesteric G4 phase is increased in the presence of the nucleotides relative to phosphoric acid or monobasic sodium phosphate even though the latter solutions are more acidic, which promotes GMP self-assembly, while the presence of K+ in monobasic potassium phosphate has a stabilizing effect and also introduces a second, organized structure that is favored over the cholesteric G4 structure.
As discussed above, although the GMPfa/XMPfa and GMPdss/XMPdss solutions do not form clear gels in plain water, gelation could be achieved in all cases studied (X=A, C) except GMPdss/CMPdss by adjusting the pH of the solutions or by adding a suitable salt containing a G-tetrad-stabilizing cation. The CD spectra and melt temperatures of the solutions with pH adjusted to 6 are shown in Fig. 9 and Table 2, respectively. The spectrum (Fig. 9A) and thermal stability for GMPfa/AMPfa after pH adjustment with NaOH are similar to those for GMPdss/AMPfa (Fig. 6 and Table 2). The spectral peaks for GMPdss/AMPdss after pH adjustment with HCl (Fig. 9C) are significantly red-shifted and much smaller in magnitude than those of GMPdss/AMPfa (Fig. 6) or GMPfa/AMPdss (Fig. 8), and the thermal stability is slightly higher. This suggests a modification of the cholesteric G4 structure by AMP.
FIG. 9.
CD spectra of like forms of GMP (0.1 M)/XMP (0.1 M) in water with pH adjusted to 6, or with added NaCl, at 5°C (blue) and 20°C (pink): (A) GMPfa/AMPfa+NaOH; (B) GMPfa/CMPfa+NaOH; (C) GMPdss/AMPdss+HCl; (D) GMPfa/AMPfa+NaCl; (E) GMPfa/CMPfa+NaCl. (Color graphics are available online at www.liebertonline.com/ast)
The spectrum for GMPfa/CMPfa after pH adjustment with NaOH (Fig. 9B) shows the development of the (−)290 nm peak that is also present in GMPfa/CMPdss (Fig. 8), and the intensity of the (−)290 nm peak relative to the (−)275–280 nm peak is greater at 20°C than at 5°C. This suggests that the alternate structure corresponding to the (−)290 nm peak becomes more dominant at the higher temperature, which is interesting in light of the report that CMP interaction with G4 structures increases with increasing temperature (Eimer and Dorfmuller, 1992).
We next examined the effects of added NaCl instead of acid or base to the solutions of GMPfa/XMPfa (since the GMPdss/XMPdss mixtures with added NaCl remained clear liquids with no sign of gelation, they were not examined by CD). As discussed above, the GMPfa/XMPfa solutions in plain water, which had pH ∼2, were a clear liquid with precipitate for X=A and a colloidal suspension for X=C. Both solutions became clear gels upon addition of NaCl, even though their pH was unchanged. Spectra (Fig. 9D, 9E) and melting temperatures (Table 2) were obtained for solutions to which NaCl was added just to the point of gelation. For X=A (Fig. 9D), the spectrum shows weak peaks indicative of the onset of the cholesteric G4 phase. For X=C (Fig. 9E), the cholesteric G4 peaks also appear to be present, albeit even weaker than for X=A, and there is a small (+)295 nm peak of unknown origin that does not appear in any of the other spectra. The thermal stability of GMPfa/AMPfa+NaCl is slightly lower than that of GMPdss/AMPfa, while for GMPfa/CMPfa, the gel phase is much less stable than the gel formed by GMPdss/CMPfa.
To further examine the effects of pH, we collected the spectra of GMPdss/XMPfa (X=A, C) at different pH (Supplementary Fig. S2). The pH was adjusted with HCl or NaOH. When X=C, pH has little effect on the spectral profiles, and the peak magnitudes of the cholesteric G4 phase increase at acidic pH. In contrast, when X=A, the cholesteric G4 peaks decrease with decreasing pH, and there is a significant red shift at acidic pH. This suggests that AMP has a significant effect on the structure of the cholesteric G4 phase at acidic pH, possibly due to strong pi-pi interactions between the two purine bases upon intercalation of AMP into the G4 gel structure.
4. Discussion
Previous studies (Yu et al., 2008; Novotná et al., 2012; Lokesh and Suryaprakash, 2013) have shown that the combination of the soluble GMPdss with the relatively insoluble Guo in aqueous solution promotes the formation of cholesteric G4 gels. The present work shows that cholesteric G4 phases are also formed by mixtures of GMP with XMP (X=A, C, G, U) when the two are in different forms (GMPdss/XMPfa or GMPfa/XMPdss). Gelation is not observed in mixtures of poorly soluble free acids (GMPfa/XMPfa) or highly soluble disodium salts (GMPdss/XMPdss), but gelation generally could be induced by pH adjustment to create a mixture of the two forms of each nucleotide, or by addition of NaCl to GMPfa/XMPfa, even though the pH remained at 2. In the case of GMPdss/XMPfa, visual observation showed that gelation was promoted by addition of NaCl, KCl, or MgCl2.
The different types of interactions available to the nucleotides are key to interpretation of these results. Adenine and guanine are both purines, and their nucleotides have a strong tendency toward base stacking with themselves and with each other (Rymden and Stilbs, 1985). It has been shown that GMP forms larger stacks than AMP at a given concentration, most likely due to G-tetrad formation (Eimer and Dorfmuller, 1992). The pyrimidine nucleotides CMP and UMP, on the other hand, have minimal tendency to self-associate through base stacking or other interactions, although CMP has been shown to interact with G4 structures (Eimer and Dorfmuller, 1992). Previous studies that used dynamic light scattering indicate that G-tetrad formation predominates over complementary base pairing in mixtures of GMP with CMP but that the G4 aggregates were larger in the presence of CMP, indicating that CMP was in some way associated with the aggregates, possibly through intercalation into the G-tetrad stacks (Eimer and Dorfmuller, 1992). They also observed that incorporation of CMP increased with increasing temperature. Given the propensity of purines toward base stacking, it is even more likely that AMP would be integrated into the G-tetrad structures. The need for higher concentrations of GMPdss to solubilize a given concentration of AMPfa compared to CMPfa (Fig. 4) is therefore attributed to the higher aqueous solubility of the latter. The lower melting temperature for X=A compared to X=C or U suggests strong base stacking of AMP in the G4 gel structure, although the G4 gel is still more stable with AMP than in the mixtures with NaH2PO4 or H3PO4, suggesting an overall stabilizing effect of base stacking in the gel. The results for the phosphate salts and phosphoric acid also show that pH is not the only factor that determines the stability of the G4 gels, which is supported by the results in Supplementary Fig. S2.
Cations clearly play an important role in GMP self-assembly, as indicated by the promotion of gelation in solutions of GMPfa/XMPfa upon addition of NaCl and on the differences between the effects of monobasic sodium and potassium phosphate salts on the self-assembly of GMPdss. In the latter case, the melting temperature of GMPdss was significantly higher (Table 2), and the CD spectral peaks had dramatically larger magnitudes (Fig. S1) in the presence of KH2PO4 compared to NaH2PO4. Additionally, the CD spectrum in the presence of KH2PO4 was dominated by the (−)250 nm peak that corresponds to a second, noncholesteric phase. It is not surprising that Na+ and K+ would favor different structures. Na+ is smaller than K+ and fits into the center of a single tetrad, whereas the larger K+ occupies the space between two tetrads (Walmsley and Burnett, 1999; Panda and Walmsley, 2011). Another consideration is the competition between G-tetrads and the continuous helix, which is promoted by K+ (Walmsley and Burnett, 1999), although it may also occur in the presence of Na+ (Sasisekharan et al., 1975). It has been observed that the proportion of helix to G4 increases with increasing K+ (Hightower et al., 2009). Additionally, the structure of the tetrads in the two salts is different (Hightower et al., 2009); in Na+ the tetrads alternate between C3′-endo (anti) and C2′-endo (syn) sugar conformations (Wu and Kwan, 2009), while in K+ they are all anti, consistent with a parallel arrangement or a continuous helix (Walmsley and Burnett, 1999). Interestingly, the solutions of GMPdss with NaH2PO4 and KH2PO4 tend to establish the low and high ends, respectively, of thermal stability and CD shape and peak magnitude, which bracket the solutions of GMPdss/XMPfa (with the exception of X=G).
5. Conclusions
This work establishes that GMP can form cholesteric G4 gels in the presence of other nucleotides, which can associate with the self-assembled GMP structures and affect their organization and stability. The gelation itself as well as the interactions with nucleotides are affected by pH and cations. These results could be important for understanding the early stages of an RNA world, when mixtures of nucleobase compounds at high local concentrations would have presumably been required for polymerization to occur by influencing the availability of the various monomeric nucleotides for incorporation into growing RNA polymers. The liquid crystalline structure of the cholesteric G4 phase might also serve as a scaffold for arrangement of associated nucleotides that could serve as a template for polymerization. There are differences among the nucleotides in terms of their solubility in the G4 phase, their effects on stabilization and organization of the G4 phase, and their effects on the coexistence of alternative GMP structures such as the continuous helix, dimers, and base-stacked monomers. Further study is needed to understand how these effects influence the outcome of abiotic RNA polymerization in terms of polymer yields, lengths, and sequence space.
Supplementary Material
Acknowledgment
This research was funded by the National Aeronautics and Space Administration Astrobiology Institute through the New York Center for Astrobiology at Rensselaer Polytechnic Institute (Grant NNA09DA80A).
Author Disclosure Statement
No competing financial interests exist.
Abbreviations
AMPdss, adenosine 5′-monophosphate disodium salt; AMPfa, adenosine 5′-monophosphate free acid; CD, circular dichroism; CMPdss, cytidine 5′-monophosphate disodium salt; CMPfa, cytidine 5′-monophosphate free acid; GMP, 5′-guanosine monophosphate; GMPdss, guanosine 5′-monophosphate disodium salt; Guo, guanosine; UMPdss, uridine 5′-monophosphate disodium salt.
References
- Cafferty B.J., Gallego I., Chen M.C., Farley K.I., Eritja R., and Hud N.V. (2013) Efficient self-assembly in water of long noncovalent polymers by nucleobase analogues. J Am Chem Soc 135:2447–2450 [DOI] [PubMed] [Google Scholar]
- Cassidy L.C. (2014) Astrobiological implications of guanosine gels and analysis of abiotic RNA polymerization. PhD dissertation, Rensselaer Polytechnic Institute, Troy, NY [Google Scholar]
- Chantot J.F., Sarocchi M.-T., and Guschlbauer W. (1971) Physico-chemical properties of nucleosides: 4. Gel formation by guanosine and its analogues. Biochimie 53:347–354 [DOI] [PubMed] [Google Scholar]
- Davis J.T. (2004) G-Quartets forty years later: from 5′ GMP to molecular biology and supramolecular chemistry. Angew Chem Int Ed Engl 43:668–698 [DOI] [PubMed] [Google Scholar]
- Davis J.T. and Spada G.P. (2007) Supramolecular architectures generated by self-assembly of guanosine derivatives. Chem Soc Rev 36:296–313 [DOI] [PubMed] [Google Scholar]
- Deamer D., Singaram S., Rajamani S., Kompanichenko V., and Guggenheim S. (2006) Self assembly processes in the prebiotic environment. Philos Trans R Soc Lond B Biol Sci 361:1809–1818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eimer W. and Dorfmuller Th. (1992) Interaction of the complementary mononucleotides in aqueous solution. J Phys Chem 96:6801–6804 [Google Scholar]
- Fry M. (2007) Tetraplex DNA and its interacting proteins. Front Biosci 12:4336–4351 [DOI] [PubMed] [Google Scholar]
- Gatto B., Palumbo M., and Sissi C. (2009) Nucleic acid aptamers based on the G-quadruplex structure: therapeutic and diagnostic potential. Curr Med Chem 16:1248–1265 [DOI] [PubMed] [Google Scholar]
- Gilbert W. (1986) The RNA world. Nature 319:618 [Google Scholar]
- Guschlbauer W., Chantot J.-F., and Thiele D. (1990) Four-stranded nucleic acid structures 25 years later: from guanosine gels to telomere DNA. J Biomol Struct Dyn 8:491–511 [DOI] [PubMed] [Google Scholar]
- Hightower J.B., Olmos D.R., and Walmsley J.A. (2009) Supramolecular structure and polymorphism of alkali metal salts of guanosine 5′-monophosphate: SEM and NMR study. J Phys Chem B 113:12214–12219 [DOI] [PubMed] [Google Scholar]
- Kunstelj K., Federiconi F., Spindler L., and Drevensek-Olenik I. (2007) Self-organization of guanosine 5′-monophosphate on mica. Colloids Surf B Biointerfaces 59:120–127 [DOI] [PubMed] [Google Scholar]
- Lokesh and Suryaprakash N. (2013) Weakly ordered chiral alignment medium derived from 5′-GMP:guanosine. Chem Commun (Camb) 49:2049–2051 [DOI] [PubMed] [Google Scholar]
- Novotná J., Goncharova I., and Urbanová M. (2012) Supramolecular arrangement of guanosine/5-guanosine monophosphate binary mixtures studied by methods of circular dichroism. Chirality 24:432–438 [DOI] [PubMed] [Google Scholar]
- Panda M. and Walmsley J.A. (2011) Circular dichroism study of supramolecular assemblies of guanosine 5′-monophosphate. J Phys Chem B 115:6377–6383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieraccini S., Giorgi T., Gottarelli G., Masiero S., and Spada G.P. (2003) Guanosine derivatives: self-assembly and lyotropic liquid crystal formation. Molecular Crystals and Liquid Crystals 398:57–73 [Google Scholar]
- Raszka M. and Kaplan N.O. (1972) Association by hydrogen bonding of mononucleotides in aqueous solution. Proc Natl Acad Sci USA 69:2025–2029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawal P., Kummarasetti B.B., Ravindran J., Kumar N., Halder K., Sharma R., Mukerji M., Das S.K., and Chowdhury S. (2006) Genome-wide prediction of G4 DNA as regulatory motifs: role in Escherichia coli global regulation. Genome Res 16:644–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts C., Chaput J.C., and Switzer C. (1997) Beyond guanine quartets: cation-induced formation of homogeneous and chimeric DNA tetraplexes incorporating iso-guanine and guanine. Chem Biol 4:899–908 [DOI] [PubMed] [Google Scholar]
- Rymden R. and Stilbs P. (1985) Nucleotide aggregation in aqueous solution. A multicomponent self-diffusion study. Biophys Chem 21:145–156 [DOI] [PubMed] [Google Scholar]
- Samori P., Pieraccini S., Masiero S., Spada G.P., Gottarelli G., and Rabe J.P. (2002) Controlling the self-assembly of deoxiguaninosine on mica. Colloids Surf B Biointerfaces 23:283–288 [Google Scholar]
- Santa Cruz Biotechnology (2014) Santa Cruz Biotechnology. Available online at http://www.scbt.com
- Sasisekharan V., Zimmerman S., and Davies D.R. (1975) The structure of helical 5′-guanosine monophosphate. J Mol Biol 92:171–179 [DOI] [PubMed] [Google Scholar]
- Simonsson T. (2005) G-quadruplex structures—variations on a theme. Biol Chem 382:621–628 [DOI] [PubMed] [Google Scholar]
- Todd A.K., Johnston M., and Neidle S. (2006) Highly prevalent putative quadruplex sequence motifs in human DNA. Nucleic Acids Res 33:2901–2907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walmsley J.A. and Burnett J.F. (1999) A new model for the K+-induced macromolecular structure of guanosine-5′-monophosphate in solution. Biochemistry 38:14063–14068 [DOI] [PubMed] [Google Scholar]
- Wang Y., Zhang H., Ligon L.A., and McGown L.B. (2009) Association of insulin-like growth factor 2 with the insulin-linked polymorphic region in cultured fetal thymus cells. Biochemistry 48:8189–8194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windholz M., editor. (1976) The Merck Index: An Encyclopedia of Chemicals and Drugs, 9th ed., Merck, Rahway, NJ [Google Scholar]
- Wishart D.S., Jewison T., Guo A.C., Wilson M., Knox C., Liu Y., Djoumbou Y., Mandal R., Aziat F., Dong E., Bouatra S., Sinelnikov I., Arndt D., Xia J., Liu P., Yallou F., Bjorndahl T., Perez-Pineiro R., Eisner R., Allen F., Neveu V., Greiner R., and Scalbert A. (2013) HMDB 3.0—The Human Metabolome Database in 2013. Nucleic Acids Res 41(D1):D801–D807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong A., Ida R., Spindler L., and Wu G. (2005) Disodium guanosine 5′-monophosphate self-associates into nanoscale cylinders at pH 8: a combined diffusion NMR spectroscopy and dynamic light scattering study. J Am Chem Soc 127:6990–6998 [DOI] [PubMed] [Google Scholar]
- Wu G. and Kwan I.C.M. (2009) Helical structure of disodium 5′-guanosine monophosphate self-assembly in neutral solution. J Am Chem Soc 131:3180–3182 [DOI] [PubMed] [Google Scholar]
- Xiao J.F., Carter J., Frederick K.A., and McGown L.B. (2009) A genome-inspired DNA ligand for affinity capture of insulin and insulin-like growth factor-2. J Sep Sci 32:1654–1664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y., Nakamura D.T., DeBoyace K., Neisius A.W., and McGown L.B. (2008) Tunable thermoassociation of binary guanosine gels. J Phys Chem B 112:1130–1134 [DOI] [PubMed] [Google Scholar]
- Zhang T., Zhang H., Wang Y., and McGown L.B. (2012) Capture and identification of proteins that bind to a GGA-rich sequence from the ERBB2 gene promoter region. Anal Bioanal Chem 404:1867–1876 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.