Abstract
l-Dopa (l-3,4-dihydroxyphenylalanine) is the precursor to dopamine and has become the mainstay therapeutic treatment for Parkinson’s disease. Chronic l-dopa is administered to recover motor function in Parkinson’s disease patients. However, drug efficacy decreases over time, and debilitating side effects occur, such as dyskinesia and mood disturbances. The therapeutic effect and some of the side effects of l-dopa have been credited to its effect on serotonin (5-HT) neurons. Given these findings, it was hypothesized that chronic l-dopa treatment decreases 5-HT neurons in the dorsal raphe nucleus (DRN) and the content of 5-HT in forebrain regions in a manner that is mediated by oxidative stress. Rats were treated chronically with l-dopa (6 mg/kg; twice daily) for 10 days. Results indicated that the number of 5-HT neurons was significantly decreased in the DRN after l-dopa treatment compared with vehicle. This effect was more pronounced in the caudal-extent of the dorsal DRN, a subregion found to have a significantly higher increase in the 3,4-dihydroxyphenylacetic acid/dopamine ratio in response to acute l-dopa treatment. Furthermore, pretreatment with ascorbic acid (400 mg/kg) or deprenyl (2 mg/kg) prevented the l-dopa–induced decreases in 5-HT neurons. In addition, 5-HT content was decreased significantly in the DRN and prefrontal cortex by l-dopa treatment, effects that were prevented by ascorbic acid pretreatment. Taken together, these data illustrate that chronic l-dopa causes a 5-HT neuron loss and the depletion of 5-HT content in a subregion of the DRN as well as in the frontal cortex through an oxidative-stress mechanism.
Introduction
l-Dopa (l-3,4-dihydroxyphenylalanine), the main pharmacologic treatment of Parkinson’s disease, improves motor symptoms associated with the disease by restoring striatal dopamine content (Birkmayer and Hornykiewicz, 1962; Carlsson, 2002). Unlike dopamine, l-dopa crosses the blood-brain barrier and reaches all areas of the central nervous system (Bertler et al., 1963). Acute l-dopa administration at a therapeutic dose increases dopamine concentrations in the striatum as well as in extrastriatal areas of the brain that contain little or no dopamine cell bodies or terminals (Lindgren et al., 2010; Navailles et al., 2010a). The increases in dopamine are due in large part to serotonin (5-HT) neurons (Navailles et al., 2011b). 5-HT neurons have the ability to synthesize dopamine in the presence of l-dopa through the enzymatic decarboxylation of l-dopa to dopamine by amino-acid decarboxylase, the enzyme that also converts 5-hydroxytryptophan to 5-HT (Ng et al., 1970; Arai et al., 1994). Dopamine produced from l-dopa can also be exocytosed by 5-HT neurons in an impulse-dependent manner (Miller and Abercrombie, 1999; Tanaka et al., 1999; Navailles et al., 2010b), thus accounting for its therapeutic efficacy.
Although the ability of 5-HT neurons to synthesize and release dopamine as a “false neurotransmitter” within the striatum may explain the therapeutic efficacy of the drug, chronic treatment with high doses of l-dopa has negative side effects, such as dyskinesias and mood disturbances (Bezard et al., 2004; Carlsson et al., 2007; Carta et al., 2007; Eskow Jaunarajs et al., 2011; Porras et al., 2014). Furthermore, chronic administration of l-dopa leads to a loss of drug efficacy (Marsden, 1994; Hauser, 2009), an observation possibly attributable to decreased efficiency of l-dopa–induced dopamine synthesis and release from 5-HT neurons (Navailles et al., 2011a). Given that the mechanisms underlying the side-effect liability of l-dopa remains unclear, further investigation into the effects of l-dopa on the cellular biology of the 5-HT system is warranted.
The toxicity profile of dopamine is well known such that cytosolic, nonvesicular dopamine is subject to enzymatic degradation via monoamine-oxidase to form the byproduct hydrogen peroxide. Dopamine is also susceptible to auto-oxidation with the subsequent formation of highly reactive quinone molecules that produce oxidative stress (Graham, 1978; Mena et al., 1992; Hastings et al., 1996). Previous studies have shown dopamine degradation and oxidation are toxic to serotonergic cells in culture (Stansley and Yamamoto, 2013) and to tryptophan hydroxylase (TPH), the rate-limiting enzyme for 5-HT synthesis (Kuhn and Arthur, 1998). Furthermore, rats treated chronically with l-dopa exhibit decreases in 5-HT tissue content in several brain regions (Borah and Mohanakumar, 2007; Eskow Jaunarajs et al., 2012) and decreased l-dopa–induced dopamine release by 5-HT terminals (Navailles et al., 2010a, 2011a). These reports suggest that chronic l-dopa leads to the accumulation of dopamine within 5-HT neurons that is detrimental to 5-HT system physiology and function.
Despite these findings, direct evidence for a possible loss of 5-HT neurons in vivo has not been shown. Our study conducted experiments to directly test the hypothesis that chronic l-dopa at a therapeutically relevant dose decreases 5-HT neurons in the rat dorsal raphe nucleus (DRN) and brain 5-HT tissue content through oxidative stress.
Materials and Methods
Animals.
Male Sprague-Dawley rats (175–199 g; Harlan, Indianapolis, IN) were used in all experiments. Rats were housed two per cage in clear plastic containers (45 × 24 × 20 cm) and were allowed to acclimate for 1 week to the vivarium before experimentation. The rats were housed under a 12-hour light/dark cycle in a temperature (∼24°C) and humidity (∼40%) controlled environment. Rats had ad libitum access to food and water. All procedures were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the University of Toledo Institutional Animal Care and Use Committee.
6-Hydroxydopamine Lesion Surgery.
Unilateral 6-hydroxydopamine (6-OHDA) or sham injections were made into the medial forebrain bundle (anteroposterior: −4.3 mm; mediolateral: +1.5 mm; dorsoventral: −7.5 mm relative to bregma with the nose bar positioned −3.3 mm from horizontal). Side of lesion was counterbalanced within groups. Before stereotaxic surgery, desipramine HCL (25 mg/kg i.p.) (Sigma-Aldrich, St. Louis, MO) was administered to protect noradrenergic neurons. Rats were anesthetized with ketamine (75 mg/kg; Hospira, Lake Forest, IL) and xylazine (5 mg/kg; Lloyd Laboratories, Shenandoah, IA). 6-OHDA HBr (17 µg of free base; Sigma-Aldrich) was prepared in 0.9% saline containing 0.01% ascorbic acid and infused at a rate of 1 µl/min for a total volume of 4 µl. After infusion, the infusion needle remained in position for 10 minutes to allow for the 6-OHDA to completely diffuse. The needle was then retracted slowly to minimize mechanical damage. Lesion severity was confirmed by tyrosine hydroxylase staining of the substantia nigra pars compacta, and only rats with tyrosine hydroxylase + loss >95% were included in the analysis.
Lesion rats were only used in experiments related to the results illustrated in Figs. 1 and 2. All other subsequent experiments were performed with nonlesioned rats because the 6-OHDA lesion itself did not affect the TPH+ neuron number in the DRN.
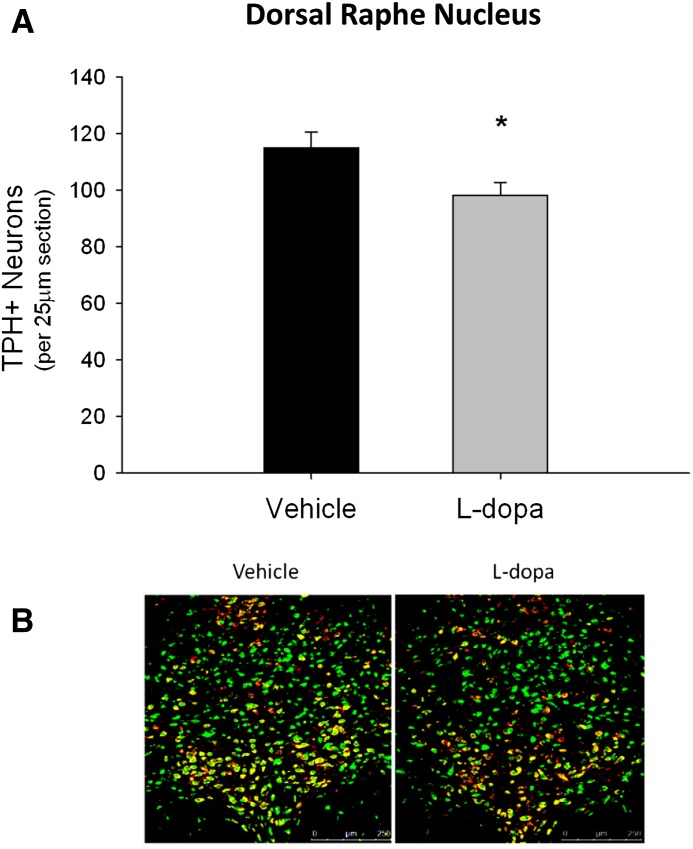
Fig. 1.
Effects of l-dopa on TPH+/NeuN+ cell counts in the DRN. Rats received 10 days of l-dopa or vehicle treatment. (A) Chronic l-dopa treatment significantly decreased TPH+/NeuN+ cell bodies (*P < 0.05 compared with vehicle, Student’s t test). (B) Representative DRN images (original magnification: 20×) illustrating TPH+/NeuN+ colabeling (green = NeuN+, red = TPH+, yellow = TPH+/NeuN+ colabel) (n = 9–11 per group).
Fig. 2.
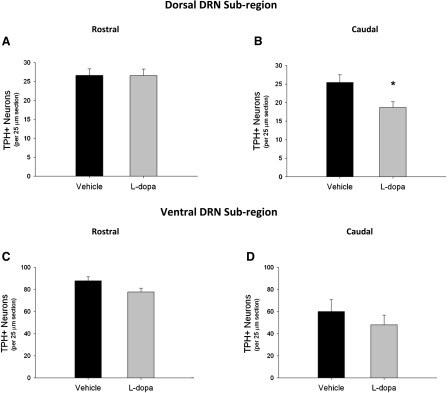
Subregional effects of l-dopa on TPH+ cell counts in the DRN. (A) Chronic l-dopa treatment had no effect on TPH+/NeuN+ cell bodies compared with vehicle in the rostral-extent of the dorsal subregion. (B) l-Dopa treatment significantly decreased TPH+/NeuN+ cell bodies in the caudal-extent of the dorsal subregion (*P < 0.05 compared with vehicle, Student’s t test). (C and D) l-Dopa treatment had no effect on TPH+/NeuN cell bodies compared with vehicle in the either rostral or caudal-extent of the ventral subregion (n = 9–11 per group).
Pharmacologic Treatments.
Two weeks after surgery, the rats were treated twice daily with either vehicle saline (0.9% NaCl) or l-dopa (6 mg/kg i.p.; Sigma-Aldrich) and benserazide [Dl-serine 2-(2,3,4-trihydroxybenzyl) hydrazide hydrochloride, 12 mg/kg i.p.; Sigma-Aldrich] for 10 days. This dosing regimen was chosen because it restores therapeutic concentrations of striatal dopamine to near physiologic concentrations in 6-OHDA lesioned rats (Navailles et al., 2010b). Four groups were evaluated: 1) sham lesion, vehicle-treated, 2) sham lesion, l-dopa–treated, 3) 6-OHDA lesion, vehicle-treated, and 4) 6-OHDA lesion, l-dopa–treated.
To assess the roles of oxidative stress, ascorbic acid (sodium l-ascorbate, 400 mg/kg i.p.; Sigma-Aldrich) or deprenyl [(R)-(−)-N,α-dimethyl-N-(2-propynyl) phenethylamine hydrochloride; 2 mg/kg i.p.; Tocris, Minneapolis, MN] was administered before each l-dopa dose. These drugs were chosen because drug interactions that might prevent l-dopa accumulation in the brain are unlikely because l-dopa is taken up into the brain by the large neutral amino acid transporter (Yee et al., 2001), whereas deprenyl readily crosses the blood-brain barrier and ascorbic acid is transported into the brain in its oxidized form, dehydroascorbic acid, via glucose transporter type-1 (Agus et al., 1997). Rats that received an acute injection of l-dopa were killed 45 minutes after drug administration. Rats treated chronically with l-dopa were killed 48 hours after the last drug injection.
5-HT Cell Counts.
Phosphate-buffered saline (PBS; 1600 ml) containing 4% paraformaldehyde was perfused intracardially at a rate of 5 ml/min under ketamine (75 mg/kg i.m.) + xylazine (5 mg/kg i.m.) anesthesia subsequent to the injection of heparin (35 USP units; APP Pharmaceuticals, Schaumburg, IL) into the left ventricle. Brains were removed from the skull and placed in 4% paraformaldehyde overnight at 4°C, followed by 10% glycerol and 20% glycerol, each for 24 hours. Brains were then flash frozen in 2-methylbutane over dry ice.
Brains were serially sectioned through the DRN (−7.3 to −8.3 mm from bregma) (Paxinos and Watson, 1997) at a thickness of 25 µm using a cryostat microtome (Microm HM550; Thermo Scientific, Waltham, MA). Sections were mounted onto gelatin-coated slides and blocked for 1 hour at room temperature with 10% normal goat serum in 0.1 N PBS containing 0.25% Triton X-100. Sections were incubated with primary antibodies for TPH2 (NB100-74555 xRb, 1:1000; Novus Biologicals, Oakville, ON, Canada) and NeuN (MAB377 xMs, 1:1000; Millipore, Billerica, MA) overnight at 4°C. Subsequently, sections were washed three times in PBS and then incubated with Alexa Fluor secondary antibodies (Alexa Fluor 688, goat anti-rabbit; Alexa Fluor 488 goat anti-mouse, 1:2000) for 30 minutes at room temperature. After three additional washes with PBS, sections were cover-slipped with Fluoromount and stored in the dark before imaging.
Sections were imaged using a Leica SP5 confocal microscope and the standard Leica Applications Suite Advanced Fluorescence software (Leica Microsystems, Buffalo Grove, IL). The parameters used to image included an excitation wavelength of 488 and 633 nm, and collection wavelengths of 504–556 and 690–740 nm, respectively. Exposure, gain, and offset remained constant for all images. All sections were imaged at original magnification 20× to include the midline DRN structures within at least six slices per rat; each rat yielded n = 1. The investigator was blinded to the treatment conditions during imaging and analysis.
To identify 5-HT neurons in the DRN, images were loaded into the ImageJ (http://imagej.nih.gov/ij/) software program. The overlay of the emissions from the secondary Alexa Fluor that labeled tryptophan hydroxylase 2-Red and NeuN-Green yielded a yellow colabel that signified 5-HT soma. Cell bodies were counted in serial sections to give the number of serotonin neurons in DRN per 25 µm section. For further analysis, the DRN was divided into rostral (−7.3 to −7.8 mm from bregma) and caudal (−7.8 to −8.3 mm from bregma) subregions. In addition, midline structures of the DRN were subdivided into dorsal and ventral regions (Paxinos and Watson, 1997). These divisions formed four DRN subregions, 1) dorsal rostral-extent, 2) ventral rostral-extent, 3) dorsal caudal-extent, and 4) ventral caudal-extent.
High-Performance Liquid Chromatography for Monoamine Tissue Content.
Brain monoamine content was measured in non–6-OHDA lesioned rats. Brain tissue was micropunched or microdissected according to rat stereotactic coordinates (Paxinos and Watson, 1997). The DRN subregions (−7.3 to −8.3 mm from bregma) rectangular dissection with boundaries consisted of the cerebral aqueduct on top and the decussation of the superior cerebellar peduncle on bottom and 1 mm to either side of the midline; the top half was considered dorsal DRN, and the bottom half was ventral DRN. The hippocampus (−3.3 to −3.8 mm from bregma; dorsal hippocampus), striatum (1.5 to −0.5 mm from bregma; dorsal caudate/putamen), and prefrontal cortex (PFC) (2.7 to 2.2 mm from bregma; prelimbic/cingulate cortex) were micropunched with a cylindrical flat needle (14.5 gauge; 1.6 mm internal diameter). The tissue was then homogenized in cold 0.25 M perchloric acid, and centrifuged at 14,000g for 15 minutes.
Monoamines were measured by high-performance liquid chromatography with electrochemical detection. The supernatant (20 µl) was injected onto a C18 column (100 × 2.0 mm; Phenomenex, Torrance, CA). The mobile phase consisted of 21 g/l citric acid anhydrous, 10.65 g/l disodium phosphate, 380 mg/l octyl sodium sulfate, 3% acetonitrile, and 15% methanol, with a pH 4.0. An LC-4B amperometric detector (BAS Bioanalytical Systems, West Lafayette, IN) was used, and data were analyzed with EZChrom_software (Scientific Software Inc., Pleasanton, CA). The limit of detection for dopamine and 5-HT in DRN microdissections was 5.4 and 2.3 pg/20 µl, respectively, with signal to noise ratio of 3:1.
The protein content of the samples was determined using a Bradford assay (Bio-Rad Laboratories, Hercules, CA) after resuspending the pellet in 1 N NaOH. The monoamine content per sample was normalized to protein and expressed as picograms per microgram of protein.
Statistical Analysis.
Statistical comparison of cell counts or monoamine content was performed using Student’s t test or two-way analysis of variance (ANOVA) with post-hoc Tukey’s multiple comparison test where appropriate. Analyses were performed using SigmaPlot 11.0 software (SigmaPlot for Windows; Systat Software, San Jose, CA). All data are presented as mean ± S.E.M. P ≤ 0.05 was considered statistically significant.
Results
Effects of l-Dopa on TPH-Positive Cell Counts in the DRN.
The initial experiments employed unilateral 6-OHDA or sham lesions of the medial-forebrain bundle before l-dopa treatment to investigate the possible combined effects of nigrostriatal dopamine degeneration with l-dopa treatment on TPH+ cell body number in the DRN. Two weeks after surgery, l-dopa (6 mg/kg i.p. + benserazide, 12 mg/kg i.p.) was injected into rats twice daily for 10 days. Rats were killed 48 hours after the last injection. A two-way ANOVA revealed the lesion had no effect on the number of TPH+ cell bodies in the DRN in the presence or absence of l-dopa [F(1,18) = 0.55, P = 0.47]. Therefore, the lesioned groups were combined with the unlesioned groups in subsequent analyses (Figs. 1 and 2).
l-Dopa treatment alone caused a statistically significant decrease in the average number of TPH+ cell bodies per 25-µm slice in the DRN when compared with vehicle controls (t = 2.33, P < 0.05) (Fig. 1A).
Subregional Effects of l-Dopa on TPH-Positive Cell Counts in the DRN.
Given that the DRN is known to contain distinct anatomic subregions that have different afferent and efferent projections and cell types, TPH+ cell counts in subregions were conducted to determine whether the effect of l-dopa was subregion-specific. The midline DRN was subdivided into 1) a dorsal subregion containing both rostral and caudal aspects and 2) a ventral subregion containing both rostral and caudal aspects. Of the four subregions analyzed, l-dopa caused a 28% decrease in TPH+ cell bodies within the dorsal subregion in the caudal-extent (t = 2.62, P < 0.05) (Fig. 2B). In contrast, there were no statistically significant effects of l-dopa treatment within the rostral portion of dorsal subregion (t = 0.02, P = 0.98) (Fig. 2A) or throughout the entire rostral-to-caudal extent of the ventral subregions (rostral: t = 2.01, P = 0.06) (caudal: t = 0.87, P = 0.39) (Fig. 2, C and D).
Effects of Ascorbic Acid and Deprenyl on l-Dopa–Induced Decreases in TPH-Positive Cell Counts.
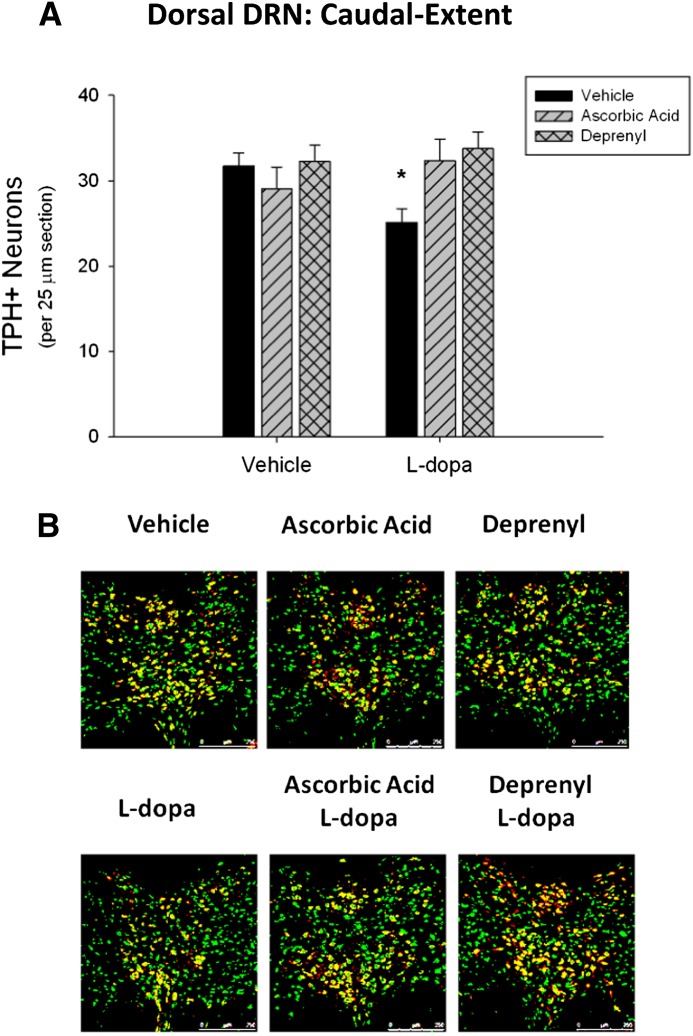
To elucidate a mechanism by which l-dopa treatment decreases TPH+ neurons, an antioxidant (ascorbic acid, 400 mg/kg i.p.) or monoamine-oxidase type B (MAO-B)–specific inhibitor (deprenyl, 2 mg/kg i.p.) was administered before each l-dopa injection. A two-way ANOVA revealed a statistically significant interaction between l-dopa and ascorbic acid treatment [F(1,16) = 4.55, P < 0.05] on the number of TPH+ cell bodies in the caudal-extent of the dorsal subregion of the DRN, indicating that the effects of l-dopa treatment on TPH+ cell counts varied according to whether the rats were treated with ascorbic acid. Furthermore, Tukey’s post-hoc analysis revealed a statistically significant effect of l-dopa treatment (q = 3.19, P < 0.05). Ascorbic acid treatment alone had no statistically significant effect on TPH+ cell counts (q = 1.13, P = 0.44); however, ascorbic acid treatment blocked the l-dopa–induced decreases in TPH+ cells (q = 3.13, P < 0.05) (Fig. 3A).
Fig. 3.
Effects of ascorbic acid and deprenyl on l-dopa induced decreases in TPH+ cell counts. TPH+/NeuN+ cell bodies were counted within the caudal-extent of the dorsal DRN subregion. (A) Both ascorbic acid (400 mg/kg) and deprenyl (2 mg/kg) prevented the effects of l-dopa at decreasing TPH+/NeuN+ cell bodies (*P < 0.05 compared with vehicle, two-way ANOVA and Tukey’s post-hoc test). (B) Representative DRN images (original magnification: 20×) illustrating TPH+/NeuN+ colabeling (green = NeuN+, red = TPH+, yellow = TPH+/NeuN+ colabel) (n = 4–6 per group).
A separate group of rats was injected with the MAO-B inhibitor deprenyl before each l-dopa injection. A two-way ANOVA revealed a statistically significant interaction between l-dopa and deprenyl treatment [F(1,16) = 5.18, P < 0.05]. A Tukey’s post-hoc test revealed that deprenyl had no effect on TPH+ cells alone (q = 0.31, P = 0.83); however, deprenyl treatment statistically significantly blocked the l-dopa–induced decreases in TPH+ cells (q = 4.86, P < 0.05).
Acute l-Dopa and Dopamine Tissue Content in the DRN.
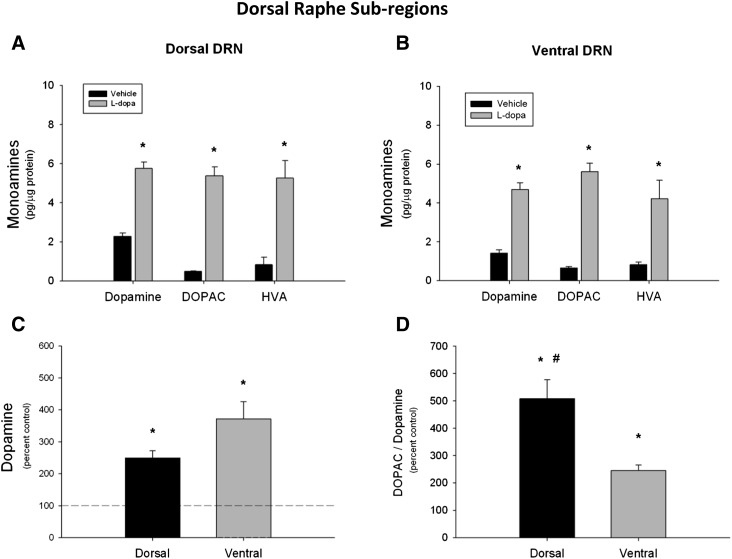
To investigate the effects of l-dopa on dopamine content in the DRN subregions, acute l-dopa was administered and the DRN was microdissected into raphettes, consisting of dorsal and ventral subregions to parallel the TPH+ cell count analyses. Dopamine content within the dorsal DRN was statistically significantly increased by more than 2.5-fold in rats that received l-dopa (t = −10.09, P < 0.05) relative to the rats that received saline vehicle (Fig. 4A). Similarly, acute l-dopa caused a statistically significant increase in dopamine content within the ventral DRN (t = −9.25, P < 0.05) (Fig. 4B). Acute treatment also statistically significantly increased the dopamine metabolites 3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA) in both dorsal (DOPAC: t = −14.74, P < 0.05; HVA: t = −5.17, P < 0.05) and ventral (DOPAC: t = −14.62, P < 0.05; HVA: t = −5.04, P < 0.05) subregions. The magnitude of dopamine elevation above control levels did not differ when the dorsal and ventral subregions were compared (t = −2.08, P < 0.06) (Fig. 4C). However, there was a greater increase in the ratio of DOPAC to dopamine in the dorsal (saline: 0.19 ± 0.02; l-dopa: 0.98 ± 0.14) compared with the ventral DRN (saline: 0.47 ± 0.05; l-dopa: 1.15 ± 0.10) after acute l-dopa when normalized to saline controls (t = 3.60, P < 0.05) (Fig. 4D).
Fig. 4.
Acute l-dopa and dopamine tissue content in the DRN. Dopamine, DOPAC, and HVA tissue content in the DRN was measured 45 minutes after l-dopa injection. (A and B) Acute l-dopa significantly elevated dopamine and its metabolites in both the dorsal and ventral DRN compared with vehicle. (C) The magnitude of dopamine increase after acute l-dopa did not differ between dorsal and ventral DRN. (D) The DOPAC/dopamine ratio was significantly greater in the dorsal compared with ventral DRN after acute l-dopa. (*P < 0.05 compared with vehicle controls; #P < 0.05 compared with ventral DRN, Student’s t test) (n = 6 per group).
Chronic l-Dopa and 5-HT Tissue Content.
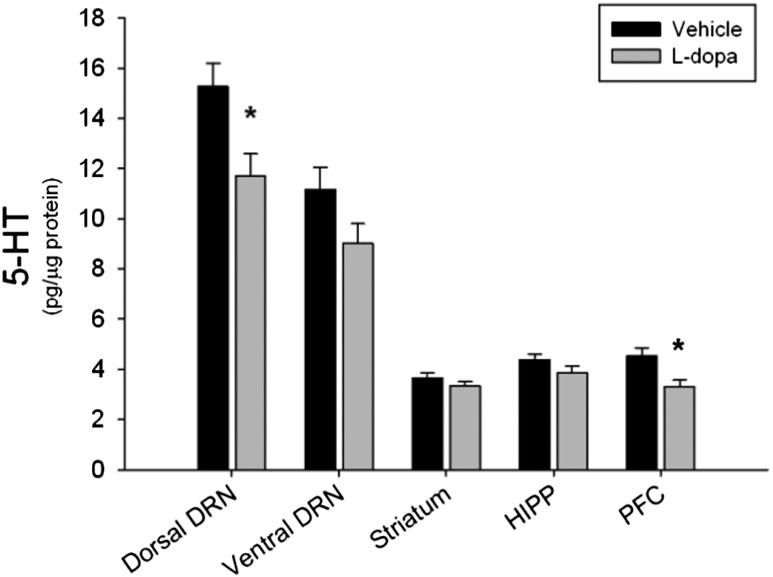
To evaluate the possible consequences of l-dopa–induced decreases in TPH+ neurons, the amount of 5-HT within the DRN and forebrain regions was measured. Nonlesioned rats were treated for 10 days with l-dopa and killed 48 hours after the last injection. Chronic l-dopa treatment statistically significantly lowered 5-HT concentrations in both the DRN and the PFC. More specifically, the dorsal subregion of the DRN was statistically significantly depleted of 5-HT and 5-hydroxyindoleacetic acid (5-HIAA) content by 23% and 29% (data not shown), respectively, after l-dopa treatment (5-HT: t = 2.55, P < 0.05; 5-HIAA: t = 2.48, P < 0.05). 5-HT content in the PFC was statistically significantly decreased by 28% in rats treated with l-dopa (t = 2.87, P < 0.05) (Fig. 5) whereas 5-HIAA content was unaffected (t = 1.61, P = 0.12); however, the ratio of 5HIAA to 5-HT was statistically significantly elevated after l-dopa compared with vehicle (t = −2.36, P < 0.05) (data not shown). There was no effect of l-dopa treatment on 5-HT tissue content of the ventral DRN, hippocampus, or striatum (DRV: t = 1.84, P = 0.09; HIPP: t = 1.38, P = 0.19; STR: t = 1.16, P = 0.27, respectively) (Fig. 5).
Fig. 5.
Chronic l-dopa and 5-HT tissue content. Rats received 10 days of l-dopa or vehicle treatment. Chronic l-dopa significantly reduced 5-HT tissue content in the dorsal DRN and the PFC (*P < 0.05 compared with vehicle, Student’s t test). l-Dopa treatment did not affect the ventral DRN, striatum, or hippocampus (n = 8–12 per group).
Effects of Ascorbic Acid on l-Dopa–Induced Decreases in 5-HT Tissue Content.
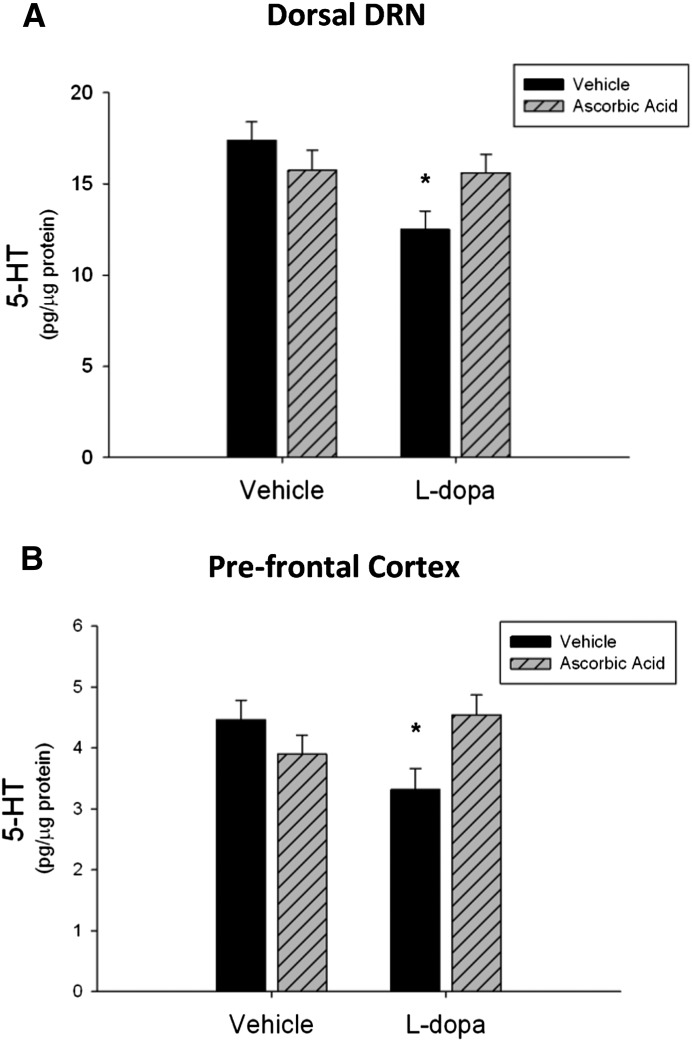
To examine the effects of an antioxidant on chronic l-dopa induced decreases in 5-HT content within the dorsal DRN and PFC, ascorbic acid was administered before each l-dopa dose twice daily for 10 days. Rats were killed 48 hours after the last injection. There was a statistically significant interaction between l-dopa and ascorbic acid on 5-HT in the dorsal DRN subregion [F(1,38) = 5.18, P < 0.05], as the effect of l-dopa on 5-HT tissue content depended on ascorbic acid cotreatment. Ascorbic acid alone had no effect on 5-HT content but blocked the decreases in 5-HT content when administered with l-dopa (q = 3.06, P < 0.05) (Fig. 6A). In the PFC, there was a statistically significant interaction between l-dopa and ascorbic acid [F(1,36) = 7.48, P < 0.05]. Whereas l-dopa alone decreased 5-HT tissue content significantly (q = 3.42, P < 0.05), this effect was blocked by ascorbic acid cotreatment (q = 3.66, P < 0.05) (Fig. 6B).
Fig. 6.
Effects of ascorbic acid on l-dopa induced decreases in 5-HT tissue content. (A) Ascorbic acid prevented chronic l-dopa–induced decreases in 5-HT tissue content in the dorsal DRN. (B) Ascorbic acid pretreatment also prevented the 5-HT tissue content decreases in the PFC (*P < 0.05 compared with vehicle, two-way ANOVA and Tukey’s post-hoc test) (n = 9–12 per group).
Discussion
The effects of chronic l-dopa on TPH+ neuron cell bodies in the dorsal raphe and 5-HT tissue content in the rat brain were investigated. Chronic l-dopa decreased TPH+ cell bodies within the caudal-extent of the dorsal DRN and decreased 5-HT tissue content in the dorsal DRN as well as the PFC. Ascorbic acid and deprenyl prevented the decreases in TPH+ cell bodies when administered before l-dopa. Ascorbic acid pretreatment also blocked the decreases in 5-HT tissue content caused by l-dopa.
Chronic l-dopa decreased the number of TPH+ cell bodies throughout the entire rostral-caudal extent of the DRN (Fig. 1), but a 6-OHDA lesion had no effect on cell body number in the presence or absence of l-dopa. When the DRN was analyzed subregionally, the caudal-extent of the dorsal DRN was specifically susceptible to l-dopa (Fig. 2B). This is the first direct evidence for decreased TPH+ cell bodies after l-dopa treatment in vivo. The lack of a 6-OHDA lesion effect on 5-HT neurons is in contrast to a previous report (Eskow Jaunarajs et al., 2012) that found that bilateral 6-OHDA lesions but not chronic l-dopa decreased 5-HT immunoreactive cell bodies throughout the entire DRN. In our current study, no effect of unilateral 6-OHDA lesion on TPH+ was noted but l-dopa alone differentially decreased TPH+ cell bodies in a DRN subregion-dependent manner. These seemingly contradictory results between Eskow Jaunarajs et al. (2012) and the current study may be explained by the differences in whether bilateral lesions or unilateral lesions (current study) were made. Moreover, the current study provides a more detailed assessment of the DRN by considering possible subregional variations in susceptibility to l-dopa, the effects of which might be otherwise masked if the DRN was analyzed as a homogeneous structure.
Previous in vitro studies using 5-HT neurons in culture showed that l-dopa can be toxic to 5-HT neurons through a pro-oxidant mechanism that is dependent on the intracellular synthesis of dopamine and its metabolism (Stansley and Yamamoto, 2013). The current study extends these findings to demonstrate that chronic l-dopa administered in vivo to intact rats decreases TPH+ cell bodies in the DRN by similar mechanisms as that demonstrated in vitro. The finding that the antioxidant ascorbate blocked the decrease in TPH+ neurons within the DRN is consistent with a pro-oxidant effect of l-dopa on TPH+ neurons (Fig. 3A) demonstrated in vitro with cultured 5-HT cells (Stansley and Yamamoto, 2013).
In addition to antioxidant protection, rats pretreated with deprenyl before each l-dopa dose also blocked the decreases in TPH+ cell number within the DRN (Fig. 3A), suggesting that the dopamine formed from l-dopa is metabolized by MAO-B to a pro-oxidant species that damages TPH+ neurons. These data contrast with Camp et al. (2000), who found that l-dopa, acutely or chronically (5 days), did not elevate extracellular hydroxyl radicals in the rat striatum (Camp et al., 2000). This difference may be due to a longer duration of treatment in the present study (10 days) and the finding that a separate group showed that when l-dopa was given for an even longer duration (16 days), hydroxyl radical production was significantly increased (Ishida et al., 2000). Regardless, the current study is consistent with the finding that l-dopa can produce intracellular reactive oxygen species and cell death to cultured 5-HT neurons in a manner that is dependent on the formation of dopamine and the degradation of dopamine by MAO (Stansley and Yamamoto, 2013).
It is well known that non-neuronal cells, such as glia, can decarboxylate l-dopa to dopamine (Juorio et al., 1993) but glial cells have a limited ability to exocytose dopamine formed from l-dopa due to the lack the vesicular monoamine transporter, a necessary component of l-dopa induced dopamine release (Kannari et al., 2000). Therefore, it is likely that the decreases in 5-HT are direct effects of dopamine within 5-HT neurons and not an indirect effect of glial-derived dopamine.
The role of dopamine and dopamine metabolism in mediating the differential effects of l-dopa on subregions within the DRN was also determined. Acute l-dopa increased dopamine and its metabolites to a similar magnitude in both the dorsal and ventral subregions (Fig. 4, A–C). Although no subregional differences in dopamine concentration after acute l-dopa were noted, chronic treatment may result in a greater increase in dopamine, its degradation, and thus oxidative stress. Moreover, there may be a persistent conversion of l-dopa to dopamine within 5-HT neurons during chronic treatment because 5-HT neurons lack the autoregulatory mechanisms of dopamine production inherent to dopaminergic neurons such as the dopamine receptor-mediated reduction in aromatic amino acid decarboxylase activity and dopamine synthesis (Zhu et al., 1994). Another factor contributing to DRN subregional differences after l-dopa may be a greater degradation of dopamine and thus increased oxidative stress. Indeed, there was a greater increase in the DOPAC to dopamine ratio after l-dopa in the dorsal compared with the ventral subregion (Fig. 4D), indicating that dopamine is degraded by MAO to a greater extent in the dorsal subregion. The higher dopamine turnover in the dorsal subregion likely results in increased hydroxyl radicals formed by the MAO-dependent production of hydrogen peroxide (Spina and Cohen, 1989). This higher turnover rate and oxidation of dopamine by MAO-B in the dorsal subregion could account for the subregional effects of chronic l-dopa on 5-HT neurons within the DRN.
In addition to the decrease in TPH+ cell bodies after chronic l-dopa, there was a parallel decrease in 5-HT tissue content in the dorsal but not ventral subregion of the DRN (Fig. 5). Interestingly, only the PFC exhibited significantly decreased 5-HT tissue content after l-dopa compared with vehicle injected rats, while no changes were observed in the striatum or hippocampus. These forebrain region-specific decreases may be explained by the fact that TPH+ cells are decreased only in the dorsal DRN subregion. In fact, the PFC not only receives dense 5-HT innervations from the DRN, the majority of those innervations emanate from 5-HT neurons in the caudal extent of the dorsal DRN (Van Bockstaele et al., 1993). Thus, decreases in 5-HT tissue content within the PFC and dorsal DRN may be a consequence of TPH+ cell body loss within the caudal extent of the dorsal DRN. Furthermore, pretreatment with ascorbic acid prevented TPH+ cell body loss (Fig. 3) and decreases in 5-HT tissue content in the dorsal DRN and PFC (Fig. 6) and supports the interpretation that decreased 5-HT tissue content is a consequence of TPH+ cell body loss through oxidative mechanisms.
The chronic l-dopa–induced 5-HT decreases in the present study were restricted to dorsal DRN and PFC, in contrast to the study by Navailles et al. (2011a) which demonstrated a more homogenous decrease in 5-HT tissue content and release throughout the rat brain after chronic l-dopa (Navailles et al., 2011a). These differences may be attributable to when 5-HT was measured. Indeed, in the present study 5-HT was measured at 48 hours after the last injection when exogenous l-dopa is no longer elevated, suggesting that these longer lasting decreases are likely due to 5-HT cell loss or terminal damage as opposed to competition between l-dopa and 5-hydroxytryptophan at the amino acid decarboxylase enzyme. Although it is possible that a higher l-dopa dose may have produced a 5-HT deficit in other brain regions, it is noteworthy that chronic low-dose l-dopa results in persistent decreases in concentrations of 5-HT.
The current findings have implications for the non-motor symptoms of Parkinson’s disease. Chronic l-dopa specifically affected the caudal extent of the dorsal DRN and the PFC, two brain regions involved in affective behaviors (Lowry et al., 2005, 2008; Warden et al., 2012; Albert et al., 2014). The cross-talk between these two regions is involved in fear and anxiety responses in rats (Hashimoto et al., 1999; Bishop et al., 2004; Amat et al., 2005; Forster et al., 2006) and several preclinical and clinical studies propose that chronic l-dopa therapy for Parkinson’s disease could result in mood disturbances, such as anxiety and depression (Damasio et al., 1971; Choi et al., 2000; Borah and Mohanakumar, 2007; Eskow Jaunarajs et al., 2010, 2012). These mood disturbances may be attributable to an underlying dysregulation of the monoaminergic systems within limbic regions as demonstrated in rats (Eskow Jaunarajs et al., 2012) and more recently in monkeys (Engeln et al., 2014). Additionally, the current findings support the use of selective MAO-B inhibitors in the treatment of Parkinson’s disease, as deprenyl prevented the toxicity of l-dopa to 5-HT neurons. Furthermore, MAO-B inhibitors have been clinically proven to be an effective adjunct therapy to l-dopa for the treatment of motor-symptoms associated with Parkinson’s disease and the depression associated with the disease (Mendlewicz and Youdim, 1983; Youdim and Bakhle, 2006; Fernandez and Chen, 2007; Riederer and Laux, 2011). Thus, the underlying the efficacy of MAO-B inhibitors and perhaps the potential use of antioxidants in this context may be related to the amelioration of l-dopa–induced dopamine and dopamine-dependent reactive oxygen species that would otherwise lead to 5-HT neuron loss.
Abbreviations
- ANOVA
analysis of variance
- DOPAC
3,4-dihydroxyphenylacetic acid
- DRN
dorsal raphe nucleus
- 5-HIAA
5-hydroxyindoleacetic acid
- 5-HT
serotonin
- HVA
homovanillic acid
- l-dopa
l-3,4-dihydroxyphenylalanine
- MAO-B
monoamine oxidase type B
- 6-OHDA
6-hydroxydopamine
- PBS
phosphate-buffered saline
- PFC
prefrontal cortex
- TPH
tryptophan hydroxylase
Authorship Contributions
Participated in research design: Stansley, Yamamoto.
Conducted experiments: Stansley.
Contributed new reagents or analytic tools: Yamamoto.
Performed data analysis: Stansley, Yamamoto.
Wrote or contributed to the writing of the manuscript: Stansley, Yamamoto.
Footnotes
This research was supported by the National Institutes of Health National Institute on Drug Abuse [Grant R01-DA07606].
References
- Agus DB, Gambhir SS, Pardridge WM, Spielholz C, Baselga J, Vera JC, Golde DW. (1997) Vitamin C crosses the blood-brain barrier in the oxidized form through the glucose transporters. J Clin Invest 100:2842–2848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert PR, Vahid-Ansari F, Luckhart C. (2014) Serotonin-prefrontal cortical circuitry in anxiety and depression phenotypes: pivotal role of pre- and post-synaptic 5-HT1A receptor expression. Front Behav Neurosci 8:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF. (2005) Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat Neurosci 8:365–371 [DOI] [PubMed] [Google Scholar]
- Arai R, Karasawa N, Geffard M, Nagatsu T, Nagatsu I. (1994) Immunohistochemical evidence that central serotonin neurons produce dopamine from exogenous L-DOPA in the rat, with reference to the involvement of aromatic l-amino acid decarboxylase. Brain Res 667:295–299 [DOI] [PubMed] [Google Scholar]
- Bertler A, Falck B, Rosengren E. (1963) The Direct Demonstration of a Barrier Mechanism in the Brain Capillaries. Acta Pharmacol Toxicol (Copenh) 20:317–321 [DOI] [PubMed] [Google Scholar]
- Bezard E, Hill MP, Crossman AR, Brotchie JM, Michel A, Grimée R, Klitgaard H. (2004) Levetiracetam improves choreic levodopa-induced dyskinesia in the MPTP-treated macaque. Eur J Pharmacol 485:159–164 [DOI] [PubMed] [Google Scholar]
- Birkmayer W, Hornykiewicz O. (1962) The l-dihydroxyphenylalanine (L-DOPA) effect in Parkinson’s syndrome in man: on the pathogenesis and treatment of Parkinson akinesis. [article in German] Arch Psychiatr Nervenkr Z Gesamte Neurol Psychiatr 203:560–574 [DOI] [PubMed] [Google Scholar]
- Bishop S, Duncan J, Brett M, Lawrence AD. (2004) Prefrontal cortical function and anxiety: controlling attention to threat-related stimuli. Nat Neurosci 7:184–188 [DOI] [PubMed] [Google Scholar]
- Borah A, Mohanakumar KP. (2007) Long-term L-DOPA treatment causes indiscriminate increase in dopamine levels at the cost of serotonin synthesis in discrete brain regions of rats. Cell Mol Neurobiol 27:985–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camp DM, Loeffler DA, LeWitt PA. (2000) L-DOPA does not enhance hydroxyl radical formation in the nigrostriatal dopamine system of rats with a unilateral 6-hydroxydopamine lesion. J Neurochem 74:1229–1240 [DOI] [PubMed] [Google Scholar]
- Carlsson A. (2002) Treatment of Parkinson’s with L-DOPA. The early discovery phase, and a comment on current problems. J Neural Transm 109:777–787 [DOI] [PubMed] [Google Scholar]
- Carlsson T, Carta M, Winkler C, Björklund A, Kirik D. (2007) Serotonin neuron transplants exacerbate L-DOPA-induced dyskinesias in a rat model of Parkinson’s disease. J Neurosci 27:8011–8022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carta M, Carlsson T, Kirik D, Björklund A. (2007) Dopamine released from 5-HT terminals is the cause of L-DOPA-induced dyskinesia in parkinsonian rats. Brain 130:1819–1833 [DOI] [PubMed] [Google Scholar]
- Choi C, Sohn YH, Lee JH, Kim J. (2000) The effect of long-term levodopa therapy on depression level in de novo patients with Parkinson’s disease. J Neurol Sci 172:12–16 [DOI] [PubMed] [Google Scholar]
- Damãsio AR, Lobo-Antunes J, Macedo C. (1971) Psychiatric aspects in Parkinsonism treated with l-dopa. J Neurol Neurosurg Psychiatry 34:502–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engeln M, De Deurwaerdère P, Li Q, Bezard E, Fernagut PO. (2014) Widespread monoaminergic dysregulation of both motor and non-motor circuits in parkinsonism and dyskinesia. Cereb Cortex DOI: 10.1093/cercor/bhu076 [published ahead of print]. [DOI] [PubMed] [Google Scholar]
- Eskow Jaunarajs KL, Angoa-Perez M, Kuhn DM, Bishop C. (2011) Potential mechanisms underlying anxiety and depression in Parkinson’s disease: consequences of l-DOPA treatment. Neurosci Biobehav Rev 35:556–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskow Jaunarajs KL, Dupre KB, Ostock CY, Button T, Deak T, Bishop C. (2010) Behavioral and neurochemical effects of chronic L-DOPA treatment on nonmotor sequelae in the hemiparkinsonian rat. Behav Pharmacol 21:627–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskow Jaunarajs KL, George JA, Bishop C. (2012) L-DOPA-induced dysregulation of extrastriatal dopamine and serotonin and affective symptoms in a bilateral rat model of Parkinson’s disease. Neuroscience 218:243–256 DOI: 10.1016/j.neuroscience.2012.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez HH, Chen JJ. (2007) Monoamine oxidase-B inhibition in the treatment of Parkinson’s disease. Pharmacotherapy 27:174S–185S [DOI] [PubMed] [Google Scholar]
- Forster GL, Feng N, Watt MJ, Korzan WJ, Mouw NJ, Summers CH, Renner KJ. (2006) Corticotropin-releasing factor in the dorsal raphe elicits temporally distinct serotonergic responses in the limbic system in relation to fear behavior. Neuroscience 141:1047–1055 [DOI] [PubMed] [Google Scholar]
- Graham DG. (1978) Oxidative pathways for catecholamines in the genesis of neuromelanin and cytotoxic quinones. Mol Pharmacol 14:633–643 [PubMed] [Google Scholar]
- Hashimoto S, Inoue T, Koyama T. (1999) Effects of conditioned fear stress on serotonin neurotransmission and freezing behavior in rats. Eur J Pharmacol 378:23–30 [DOI] [PubMed] [Google Scholar]
- Hastings TG, Lewis DA, Zigmond MJ. (1996) Role of oxidation in the neurotoxic effects of intrastriatal dopamine injections. Proc Natl Acad Sci USA 93:1956–1961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser RA. (2009) Levodopa: past, present, and future. Eur Neurol 62:1–8 [DOI] [PubMed] [Google Scholar]
- Ishida Y, Hashiguchi H, Todaka K, Ishizuka Y, Mitsuyama Y. (2000) Repeated administration of high dose levodopa enhances hydroxyl radical production in the rat striatum denervated with 6-hydroxydopamine. Neurosci Lett 290:33–36 [DOI] [PubMed] [Google Scholar]
- Juorio AV, Li XM, Walz W, Paterson IA. (1993) Decarboxylation of l-dopa by cultured mouse astrocytes. Brain Res 626:306–309 [DOI] [PubMed] [Google Scholar]
- Kannari K, Tanaka H, Maeda T, Tomiyama M, Suda T, Matsunaga M. (2000) Reserpine pretreatment prevents increases in extracellular striatal dopamine following L-DOPA administration in rats with nigrostriatal denervation. J Neurochem 74:263–269 [DOI] [PubMed] [Google Scholar]
- Kuhn DM, Arthur R., Jr (1998) Dopamine inactivates tryptophan hydroxylase and forms a redox-cycling quinoprotein: possible endogenous toxin to serotonin neurons. J Neurosci 18:7111–7117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren HS, Andersson DR, Lagerkvist S, Nissbrandt H, Cenci MA. (2010) L-DOPA-induced dopamine efflux in the striatum and the substantia nigra in a rat model of Parkinson’s disease: temporal and quantitative relationship to the expression of dyskinesia. J Neurochem 112:1465–1476 [DOI] [PubMed] [Google Scholar]
- Lowry CA, Hale MW, Evans AK, Heerkens J, Staub DR, Gasser PJ, Shekhar A. (2008) Serotonergic systems, anxiety, and affective disorder: focus on the dorsomedial part of the dorsal raphe nucleus. Ann N Y Acad Sci 1148:86–94 [DOI] [PubMed] [Google Scholar]
- Lowry CA, Johnson PL, Hay-Schmidt A, Mikkelsen J, Shekhar A. (2005) Modulation of anxiety circuits by serotonergic systems. Stress 8:233–246 [DOI] [PubMed] [Google Scholar]
- Marsden CD. (1994) Problems with long-term levodopa therapy for Parkinson’s disease. Clin Neuropharmacol 17 (Suppl 2):S32–S44 [PubMed] [Google Scholar]
- Mena MA, Pardo B, Casarejos MJ, Fahn S, García de Yébenes J. (1992) Neurotoxicity of levodopa on catecholamine-rich neurons. Mov Disord 7:23–31 [DOI] [PubMed] [Google Scholar]
- Mendlewicz J, Youdim MB. (1983) l-Deprenil, a selective monoamine oxidase type B inhibitor, in the treatment of depression: a double blind evaluation. Br J Psychiatry 142:508–511 [DOI] [PubMed] [Google Scholar]
- Miller DW, Abercrombie ED. (1999) Role of high-affinity dopamine uptake and impulse activity in the appearance of extracellular dopamine in striatum after administration of exogenous L-DOPA: studies in intact and 6-hydroxydopamine-treated rats. J Neurochem 72:1516–1522 [DOI] [PubMed] [Google Scholar]
- Navailles S, Benazzouz A, Bioulac B, Gross C, De Deurwaerdère P. (2010a) High-frequency stimulation of the subthalamic nucleus and l-3,4-dihydroxyphenylalanine inhibit in vivo serotonin release in the prefrontal cortex and hippocampus in a rat model of Parkinson’s disease. J Neurosci 30:2356–2364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navailles S, Bioulac B, Gross C, De Deurwaerdère P. (2010b) Serotonergic neurons mediate ectopic release of dopamine induced by L-DOPA in a rat model of Parkinson’s disease. Neurobiol Dis 38:136–143 [DOI] [PubMed] [Google Scholar]
- Navailles S, Bioulac B, Gross C, De Deurwaerdère P. (2011a) Chronic L-DOPA therapy alters central serotonergic function and L-DOPA-induced dopamine release in a region-dependent manner in a rat model of Parkinson’s disease. Neurobiol Dis 41:585–590 [DOI] [PubMed] [Google Scholar]
- Navailles S, Carta M, Guthrie M, De Deurwaerdère P. (2011b) L-DOPA and serotonergic neurons: functional implication and therapeutic perspectives in Parkinson’s disease. Cent Nerv Syst Agents Med Chem 11:305–320 [DOI] [PubMed] [Google Scholar]
- Ng KY, Chase TN, Colburn RW, Kopin IJ. (1970) l-Dopa-induced release of cerebral monoamines. Science 170:76–77 [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. (1997) The Rat Brain in Stereotaxic Coordinates, 3rd ed Academic Press, San Diego, CA [Google Scholar]
- Porras G, De Deurwaerdere P, Li Q, Marti M, Morgenstern R, Sohr R, Bezard E, Morari M, Meissner WG. (2014) l-Dopa-induced dyskinesia: beyond an excessive dopamine tone in the striatum. Sci Rep 4:3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riederer P, Laux G. (2011) MAO-inhibitors in Parkinson’s disease. Exp Neurobiol 20:1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spina MB, Cohen G. (1989) Dopamine turnover and glutathione oxidation: implications for Parkinson disease. Proc Natl Acad Sci USA 86:1398–1400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stansley BJ, Yamamoto BK. (2013) l-Dopa-induced dopamine synthesis and oxidative stress in serotonergic cells. Neuropharmacology 67:243–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka H, Kannari K, Maeda T, Tomiyama M, Suda T, Matsunaga M. (1999) Role of serotonergic neurons in L-DOPA-derived extracellular dopamine in the striatum of 6-OHDA-lesioned rats. Neuroreport 10:631–634 [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Biswas A, Pickel VM. (1993) Topography of serotonin neurons in the dorsal raphe nucleus that send axon collaterals to the rat prefrontal cortex and nucleus accumbens. Brain Res 624:188–198 [DOI] [PubMed] [Google Scholar]
- Warden MR, Selimbeyoglu A, Mirzabekov JJ, Lo M, Thompson KR, Kim SY, Adhikari A, Tye KM, Frank LM, Deisseroth K. (2012) A prefrontal cortex-brainstem neuronal projection that controls response to behavioural challenge. Nature 492:428–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee RE, Cheng DW, Huang SC, Namavari M, Satyamurthy N, Barrio JR. (2001) Blood-brain barrier and neuronal membrane transport of 6-[18F]fluoro-L-DOPA. Biochem Pharmacol 62:1409–1415 [DOI] [PubMed] [Google Scholar]
- Youdim MB, Bakhle YS. (2006) Monoamine oxidase: isoforms and inhibitors in Parkinson’s disease and depressive illness. Br J Pharmacol 147 (Suppl 1):S287–S296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu MY, Juorio AV, Paterson IA, Boulton AA. (1994) Regulation of aromatic l-amino acid decarboxylase in rat striatal synaptosomes: effects of dopamine receptor agonists and antagonists. Br J Pharmacol 112:23–30 [DOI] [PMC free article] [PubMed] [Google Scholar]