Abstract
Gut is very sensitive to hypoperfusion and hypoxia, and deranged gastrointestinal barrier is implicated in systemic failure of various organs. We recently demonstrated that diphenyldihaloketone EF24 [3,5-bis(2-fluorobenzylidene)piperidin-4-one] improves survival in a rat model of hemorrhagic shock. In this study, we tested EF24 and its other analog CLEFMA (4-[3,5-bis(2-chlorobenzylidene)-4-oxo-piperidine-1-yl]-4-oxo-2-butenoic acid) for their effect on intestinal barrier dysfunction in hypovolemic shock. Hypovolemia was induced in rats by withdrawing 50% of blood. EF24 or CLEFMA (0.4 mg/kg i.p.) treatment was provided, without volume resuscitation, after 1 hour of hemorrhage. Ileum was collected 5 hours after the treatment to investigate the expression of tight junction proteins (zonula occludens, claudin, and occludin) and epithelial injury markers [myeloperoxidase, ileal lipid-binding protein (ILBP), CD163, and plasma citrulline]. The ileal permeability for dextran-fluoroisothiocyanate and Evan’s blue dye was determined. EF24 and CLEFMA reduced the hypovolemia-induced plasma citrulline levels and the ileal expression of myeloperoxidase, ILBP, and CD163. The drugs also restored the basal expression levels of zonula occludens, claudin, and occludin, which were substantially deranged by hypovolemia. In ischemic ileum, the expression of phospho(tyrosine)-zonula occludens-1 was reduced, which was reinstated by EF24 and CLEFMA. In contrast, the drug treatments maintained the hypovolemia-induced expression of phospho(threonine)-occludin, but reduced that of phospho(tyrosine)-occludin. Both EF24 and CLEFMA treatments reduced the intestinal permeability enhanced by hypovolemia. EF24 and CLEFMA attenuate hypovolemic gut pathology and protect barrier function by restoring the status of tight junction proteins. These effects were observed in unresuscitated shock, implying the benefit of EF24 and CLEFMA in prehospital care of shock.
Introduction
Moderate to severe blood loss is accompanied by a systemic compensation to maintain cardiac output. This compensation is characterized by increased sympathetic outflow that results in an increase in heart rate and vasoconstriction in nonessential tissues. The resultant hypoperfusion causes a disproportionate decrease in portal blood flow that adversely affects the barrier and absorptive functions of the intestine (Rhodes et al., 1973; Fink and Delude, 2005). The physical boundary of the intestinal barrier is maintained by a columnar epithelium characterized by the presence of adherens junctions and tight junctions (TJ) (Groschwitz and Hogan, 2009). Whereas adherens junctions are responsible for maintaining cell-cell contacts, the TJs control the paracellular movement of ions and solutes. The TJs consist of the transmembrane proteins occludin and claudins and the cytoplasmic scaffolding proteins zonula occludens (ZO) (Hartsock and Nelson, 2008). At steady state, gut barrier is maintained by optimal turnover of these molecular components of TJs. Therapeutic interventions that assist in maintaining or restoring gut homeostasis may be of immense benefit as adjunct treatment in hemorrhagic shock and ischemia/reperfusion injury. The genesis of barrier dysfunction is not limited to hemorrhagic shock, because the same is also observed in the victims of burn, trauma, sepsis, and radiation injury.
Because gut dysfunction is a trigger in the pathogenesis of multiple organ failure (Moore, 1999; Rotstein, 2000; Senthil et al., 2006; Hauer-Jensen et al., 2007; Groschwitz and Hogan, 2009), there exists a need to address loss of barrier function early after traumatic injury. Apart from prompt and adequate resuscitation (Shi et al., 2002; Vega et al., 2008), no one specific medication alone is known to support circulatory deficit in hemorrhagic shock. Therefore, pharmacologic agents have been investigated as part of a comprehensive resuscitation regimen (Kao and Fink, 2010; Cotton, 2011). For instance, ethyl pyruvate, a free radical scavenger, has been shown to improve survival and/or reduce organ dysfunction in a wide variety of preclinical models of critical illnesses (Fink and Delude, 2005; Fink, 2007). It has been shown to ameliorate barrier dysfunction in lipopolysaccharide (LPS)-treated Caco-2 monolayers in vitro (Sappington et al., 2003).
Recently, we reported that a diphenyldihaloketone, EF24 [3,5-bis(2-fluorobenzylidene)piperidin-4-one] (Fig. 1A), suppresses inflammatory phenotype in lung of rats hemorrhaged by 50% of circulating blood (Yadav et al., 2013). In the process, EF24 also significantly improved the survival in this preclinical model of hemorrhagic shock (Yadav et al., 2013). We have also shown that EF24 inhibits LPS-induced nuclear factor κB (NF-κB) and reduces secretion of proinflammatory cytokines in LPS-stimulated dendritic cells used as an in vitro model of sterile inflammation (Vilekar et al., 2012). Another potent analog of EF24 is CLEFMA (4-[3,5-bis(2-chlorobenzylidene)-4-oxo-piperidine-1-yl]-4-oxo-2-butenoic acid) (Fig. 1A), which has bis-2-chloro in place of bis-2-fluoro functional groups and carries an additional maleic acid chain at the piperidonyl nitrogen (Lagisetty et al., 2010). In this study, we hypothesized that the salutary effects of diphenyldihaloketones in unresuscitated hemorrhagic shock would involve remediation of intestinal barrier function loss. The putative mechanism of EF24 action is based on its ability to suppress NF-κB activation by inhibiting the catalytic activity of inhibitory NF-κB kinase (Kasinski et al., 2008). The results unraveled for the first time that EF24 and CLEFMA administration dramatically improved histologic, functional, and molecular signatures of intestinal barrier in a rat model of fixed (50%) volume hemorrhagic shock. Significantly, these effects were observed without any accompanying volume resuscitation.
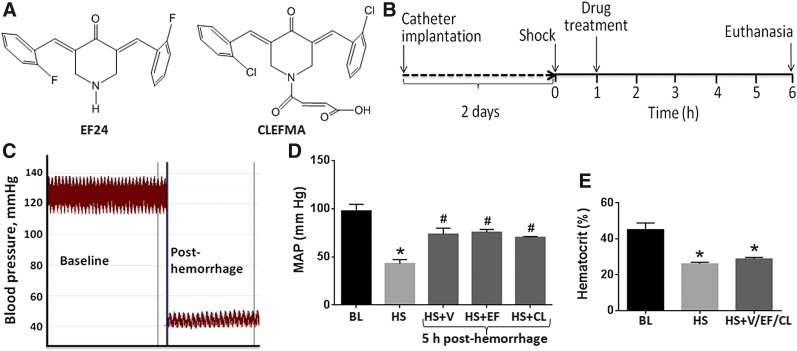
Fig. 1.
Structures of (A) EF24 and CLEFMA. (B) Experimental design: Hemorrhagic shock was induced by withdrawing 50% of blood through indwelling femoral artery catheter. The hemorrhaged rats were treated with EF24 or CLEFMA (abbreviated as EF and CL) after 1 hour (0.4 mg/kg b.wt. i.p.). The untreated hemorrhaged rats (HS + V group) received equal volume of vehicle (100 µl). The control group consisted of normal rats subjected to catheter implantation, but no hemorrhage or drug treatment was provided. At 6 hours, the rats were euthanized and ileum was collected for evaluations described in this article. (C) A representative blood pressure profile at baseline and after hemorrhagic shock. (D) Mean arterial pressure (MAP) in various groups of rats. Blood pressure was recorded at baseline (BL, n = 12), immediately after hemorrhage (HS, n = 12), and after 5 hours of treatment with EF24 (n = 4), CLEFMA (n = 4), or vehicle (V, n = 4). (E) Mean hematocrit values at baseline (BL, n = 12), immediately after shock (HS, n = 12), and after 5 hours of treatment with EF24, CLEFMA, or vehicle (n = 12). The hematocrit values of rats belonging to the three treatment groups were combined to obtain a composite value for HS + EF/CL/V group (*P < 0.05 versus BL; #P < 0.05 versus HS).
Materials and Methods
EF24 and CLEFMA were synthesized in-house by the procedures published elsewhere (Lagisetty et al., 2009, 2010). Sterile solutions of these drugs were prepared in normal saline using poly(ethylene glycol) (PEG)-400 as a cosolvent (3 parts saline + 1 part PEG400). The primary rabbit antibodies against rat antigens were obtained from Santa Cruz Biotechnology (Dallas, TX). Horseradish peroxidase (HRP)–conjugated secondary goat anti-rabbit IgG was from Sigma-Aldrich (St. Louis, MO). All other chemicals were obtained from diverse vendors represented by VWR (Radnor, PA).
Rat Model of Hypovolemic Shock.
The animal experiments were performed according to the National Institutes of Health Animal Use and Care Guidelines and were approved by the Institutional Animal Care and Use Committee of the University of Oklahoma Health Sciences Center. Male Sprague Dawley rats (250–300 g) were purchased from Harlan Laboratories (Indianapolis, IN). The rats were housed in regular 12-hour light/dark cycles. Before initiating the experiment, we allowed the rats to acclimatize for at least 5 days. The method of femoral artery catheterization in rats has been described elsewhere (Awasthi et al., 2007). Briefly, left femoral artery was cannulated with a Teflon-tipped catheter and the catheter was subcutaneously tunneled and secured to the nape; the rats were allowed 2 days to recover from surgery. The cannulated rats were clustered a priori in four groups (n = 4–6/group), as follows: control (CTRL), hemorrhagic shock + vehicle (HS + V), hemorrhagic shock + EF24 (HS + EF), and hemorrhagic shock + CLEFMA (HS + CL). On the day of the experiment, the rats were handled under isoflurane (2–3%) anesthesia in medical air stream (2 l/min). The rats were heparinized with 100 U heparin to prevent catheter blockade. Hemorrhagic shock was induced by withdrawing approximately 50% of circulating blood at the rate of 1.0 ml/min. The total volume of blood was estimated approximately 6% of the total body weight (Weiss et al., 2000). The hypovolemic rats were allowed to wake up and freely compensate for 1 hour, before drug solution was administered intraperitoneally. The drug treatment consisted of approximately 0.4 mg/kg bodyweight, whereas HS + V and CTRL groups received equivalent amounts of vehicle (25% PEG400 in saline) or 0.9% saline, respectively, in an identical fashion. The treatment volume was approximately 100 µl for all groups, irrespective of small differences in body weight. Blood pressure was digitally monitored by instrumenting the rats to an iWorx data acquisition system (Dover, NH). After 6 hours of hemorrhage, the surviving rats were euthanized with an overdose of SOMNASOL, Euthanasia-III Solution (Butler Schein Animal Health, Dublin, OH). Small intestine was immediately isolated and cleaned of luminal debris with ice-cold saline.
Real-Time Polymerase Chain Reaction.
The total RNA was extracted using RNA-STAT60 (TEL-TEST, Friendswood, TX) and quantified by absorbance values at 260 nm. The reverse-transcriptase reaction was performed for 1 hour at 42°C using 2 μg total RNA, 1 μg oligo(dT), 200 U Moloney murine leukemia virus reverse-transcriptase enzyme, 500 μM dNTP mix, and 25 U RNase inhibitor (Promega, Madison, WI). The resultant cDNA was used to carry out 40 polymerase chain reaction (PCR) cycles consisting of 15 seconds at 95°C, 30 seconds at 58°C, and 30 seconds at 72°C on an ABIPrism 7000 sequence detection system (Applied Biosystems, Foster City, CA). The reactions were performed using SybrGreen II (Qiagen, Valencia, CA) and Go Taq Colorless master mix (Promega). Each PCR was set up in triplicate wells in a total volume of 25 μl, containing cDNA equivalent of 20 ng total RNA. The quantitative values of the genes of interest were normalized using β-actin as the endogenous reference, and fold increase over control values was calculated using the relative quantification method of 2-ΔΔ cycle threshold. All the primers were of 20 base pairs (Supplemental Table 1).
Immunohistochemistry.
The protein expression levels of ZO-1, ZO-2, claudin, and occludin were assessed in formalin-fixed ileal tissues by using an immunohistochemistry (IHC) kit from DakoCytomation (Carpinteria, CA). In brief, the tissue samples were fixed with paraformaldehyde and embedded in paraffin. The tissues were sectioned and processed in the imaging core facility of the Oklahoma Medical Research Foundation (Oklahoma City, OK). Briefly, the slides were blocked with a protein-block solution for 20 minutes and incubated overnight with primary antibody dilutions (Supplemental Table 2). The slides were washed and incubated with biotinylated link universal antiserum, followed by HRP-streptavidin conjugate. The slides were rinsed, and the color was developed using 3,3-diaminobenzidine hydrochloride as a chromogen. Finally, the sections were rinsed in distilled water, counterstained with Mayer’s hematoxylin solution, and mounted with DPX-mounting medium for evaluation. For histopathological examination, the paraffin-embedded tissues were sectioned, stained with H&E stain, observed with Olympus microscope IX701, and digitally recorded using an Olympus DP70 camera (Olympus, Center Valley, PA).
Immunoblotting.
The isolated frozen tissues were minced and incubated on ice for 30 minutes in ice-cold whole-cell lysate buffer consisting of 10% Nonidet P-40, 5 M NaCl, 1 M HEPES, 0.1 M ethyleneglycoltetraacetic acid, 0.5 M EDTA, 0.1 M phenylmethylsulfonyl fluoride, 0.2 M NaOV, 1 M NaF, 2 μg/ml aprotinin, and 2 μg/ml leupeptin. The protein was extracted by homogenization using a dounce homogenizer and centrifugation at 14,000 rpm for 10 minutes. The proteins were fractionated by SDS-PAGE, electrotransferred onto nitrocellulose membranes, blotted with primary antibodies, followed by HRP-conjugated secondary antibody. The primary antibodies were obtained from Santa Cruz Biotechnology and used at a dilution of 1:1000 (Supplemental Table 2). The immunoreactive bands were detected by SuperSignal West Femto detection reagent (Thermo Fischer Scientific, Rockford, IL). The blots were imaged using Ultraquant image acquisition machine (Claremont, CA), and the densitometric readings for proteins were normalized with those of actin.
Immunoprecipitation.
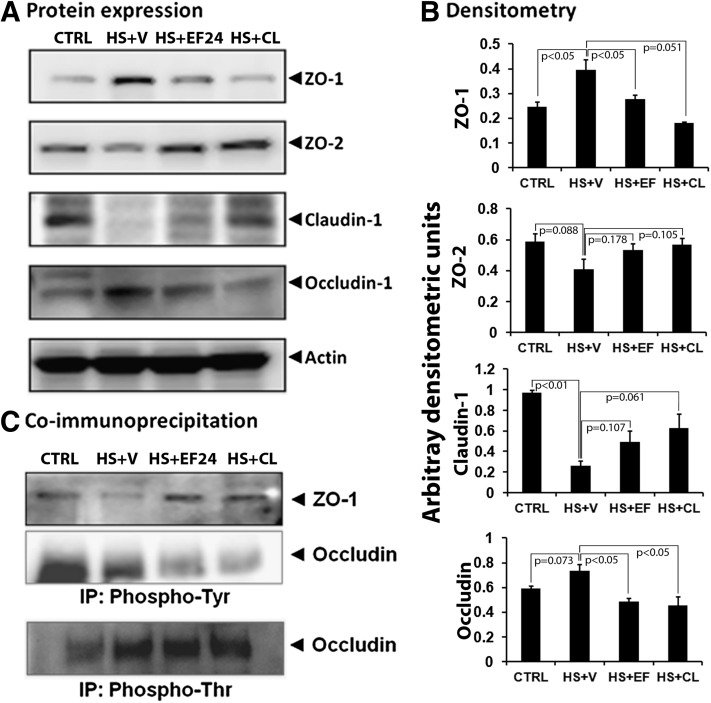
Ileal tissues were lysed in ice-cold homogenization buffer containing 25 mM Tris-HCl (pH 7.4), 2 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 0.2 mM NaOV, 50 mM NaF, 150 mM NaCl, and 1 μg/ml each of leupeptin and pepstatin. After a 10-minute spin at 20,000g, 4°C, the supernatant was collected and protein concentration was determined (Bio-Rad Bradford protein assay; Bio-Rad, Hercules, CA). Homogenate volumes corresponding to 0.2 mg total protein were incubated overnight with 2 μg monoclonal anti–phospho-Tyr or anti–phospho-Thr antibodies (Supplemental Table 2) at 4°C and then with 20 µl bead-immobilized protein A (GBiosciences, St. Louis, MO) for 2 hours to collect the immunocomplex. The beads were washed three times, and the bound complex was eluted from the agarose beads with SDS-PAGE sample buffer for electrophoresis and immunoblotting with rabbit anti–ZO-1 and anti-occludin antibodies at 1:1000 dilution in TBST.
Intestinal Permeability.
We assessed intestinal permeability by two methods, as follows: one in situ and the other in vitro. For in situ assessment of intestinal permeability in hypovolemic rats, the rats were injected with 50 µl solution of Evan’s blue dye (EBD) in saline (30 mg/kg via the arterial catheter) 1 hour prior to euthanasia. The accumulation of dye in intestinal tissue was quantitated by determining the absorbance of tissue lysate at 620 nm. Briefly, approximately 100 mg ileal tissue was extracted in dimethylformamide (500 µl). The extracts were centrifuged at 12,000g, and the absorbance values of supernatants were determined. The amount of EBD accumulated in intestinal tissue was normalized with the concentration of EBD in plasma for each individual rat. The EBD concentration in plasma was estimated by comparing the absorbance (620 nm) of appropriate dilutions of plasma with a standard curve.
The in situ method was accompanied by a determination of permeability in isolated ileal segments following a method described elsewhere (Cruz et al., 2011). Briefly, the harvested small intestine was carefully cleaned of luminal debris with ice-cold saline and inverted over a plastic pipette. Approximately 5-cm segments were cut and tied on one end to create a pouch. After filling the pouch with 1 ml saline, the other end was also tied and the segments were immersed in a saline bath containing 0.1 mg/ml fluoroisothiocyanate (FITC)-dextran (mol. wt. 4000). The bath was kept at 37°C, with constant bubbling of oxygen and gentle mixing. After allowing 45 minutes of incubation, the external surface of intestinal pouches was thoroughly washed with saline, and the internal content was collected for fluorescence reading. The fluorescence values were calculated on per unit volume per hour basis and expressed as percentage of that in the external bath. At least three intestinal segments from each rat (n = 4 per group) were assayed to determine the mean ± S.E.M. values.
Myeloperoxidase Assay.
The frozen ileal tissue samples were thawed, diced with a razor blade, and homogenized in 50 mM potassium phosphate buffer (pH 6.0). The homogenate was centrifuged at 10,000 rpm for 15 minutes, and the pellet was resuspended in 0.3 ml phosphate buffer containing 50 mM hexadecyltrimethylammonium bromide. The mixture was subjected to three cycles of bath sonication (20 seconds), snap-freezing in liquid nitrogen, and thawing to room temperature. The supernatants were collected by centrifuging the mixture at 10,000 rpm for 10 minutes. The aliquots of supernatants were diluted 1:2 and 1:10 with the hexadecyltrimethylammonium bromide phosphate buffer and added with 30 times the volume of phosphate buffer containing 0.167 mg/ml O-dianisidine dihydrochloride and 0.0005% hydrogen peroxide. The absorbance was monitored at 460 nm. At least four ileal tissues per group were assayed, each in triplicate.
Plasma Citrulline Enzyme-Linked Immunosorbent Assay.
Concentration of citrulline in plasma samples was determined by using a commercial sandwich enzyme-linked immunosorbent assay test kit (MyBioSource, San Diego, CA). The manufacturer-recommended method was followed.
Data Analysis.
The results were analyzed by one-way analysis of variance applying the Bonferroni post-test using Prism 6 software (GraphPad, San Diego, CA). A value P < 0.05 was considered statistically significant. The densitometry of immunoreactive bands was performed from three replicates using Image J 1.46r freeware (National Institutes of Health, Bethesda, MD).
Results
The gut barrier dysfunction stimulates the activation of gut-liver-lung axis, and it often precedes multiple organ dysfunction in severe hemorrhagic shock. In our previous study, we had found that administration of a diphenyldihaloketone EF24 improved the survival rate in a rat fixed-volume hemorrhagic shock model (Yadav et al., 2013). CLEFMA, an analog of EF24, was not tested in the same manner. To substantiate the therapeutic effect of these diphenyldihaloketones, we now present the histologic, molecular, and functional evidences demonstrating the improvement of gut barrier function in fixed-volume hemorrhagic shock. The structure of EF24 and CLEFMA work is provided in Fig. 1A, whereas the experimental design is depicted in Fig. 1B. The characteristics of this rat model are summarized in Fig. 1, C–E. The blood withdrawal caused a drop in mean arterial pressure (mm Hg) from 97.5 ± 6.9 to 43 ± 4.1. After 6 hours of the treatment with EF24, CLEFMA, or vehicle control (saline), mean arterial pressure was significantly increased, but remained well below the basal level (Fig. 1D). The corresponding mean hematocrit value at 6 hours after hemorrhage was 26 ± 0.9 as compared with 45 ± 3.7 of control rats (Fig. 1E).
EF24 and CLEFMA Treatments Ameliorate Hemorrhage-Induced Ileal Injury.
H&E-stained pictures of ileum at low original magnification (100×) showing global injury induced by hemorrhagic shock are provided in Supplemental Fig. 1, whereas high original magnification pictures (400×) are shown in Fig. 2A. The control rats showed a normal ileal structure, characterized by intact villi and a normal epithelial cell lining. Rats sacrificed 6 hours after shock showed substantial extension of the subepithelial space, edema, and denudation as well as massive lifting and sloughing of the villi. The villi heights were considerably shorter in the case of untreated rats as compared with the control rats, and the columnar architecture of ileal barrier was completely lost in untreated rats. There appeared to be increased number and activity of goblet cells in ileal epithelium of hemorrhaged rats. Treatments with EF24 and CLEFMA considerably reduced this injury, with ileal epithelium showing close to normal architecture.
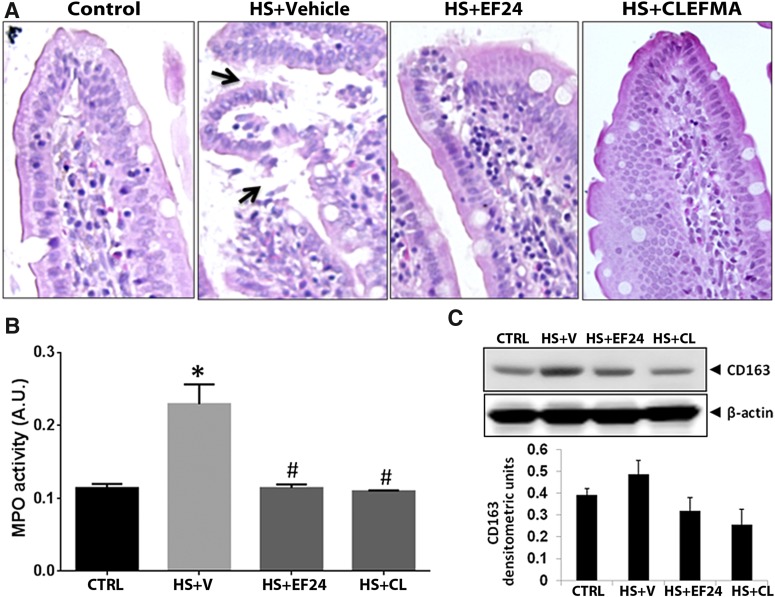
Fig. 2.
(A) A representative set of H&E-stained ileal tissues of rats subjected to hemorrhagic shock. The arrows point to the disruption of epithelial integrity. (B) Effect of EF24 and CLEFMA on HS-induced myeloperoxidase (MPO) activity in ileal tissue (*P < 0.05 versus CTRL; #P < 0.05 versus HS, n = 4/group). The groups are defined in Fig. 1. (C) CD163 expression in ileal tissue of rats subjected to hemorrhagic shock (upper panel). CD163 expression was quantitated by densitometry (lower panel; P = 0.24, CTRL versus HS + V; P = 0.13, HS + V versus HS + EF; and P = 0.07, HS + V versus HS + CL, n = 3/group). CL, CLEFMA; CTRL, control; HS, hemorrhagic shock; V, vehicle.
EF24 and CLEFMA Treatments Reduce Myeloperoxidase Activity and CD163 Expression in Ileal Tissue.
The influx of neutrophils in the ileal epithelium was determined by estimating the activity of myeloperoxidase, an enzyme released during degranulation of neutrophils and monocytes. Treatments with EF24 and CLEFMA markedly ameliorated the hemorrhage-induced increase in ileal myeloperoxidase activity (Fig. 2B). We also estimated cytokine-induced neutrophil chemoattractant-1, the rat equivalent of interleukin (IL)-8, which is a powerful neutrophil chemoattractant expressed by macrophages in inflamed intestinal tissue (MacDermott, 1999). There was no change in ileal IL-8 levels after hemorrhage or drug treatment (Supplemental Fig. 2), suggesting a possibility of some other chemoattractant for neutrophil accumulation in ileal tissue. CD163 is a hemoglobin-heptaglobin scavenger receptor, which marks the presence of monocytes/macrophages in the subepithelial lamina propria; it is highly expressed on resident tissue macrophages and to a lesser extent on monocytes (Fabriek et al., 2005). Figure 2C shows that the expression of CD163 in ileal tissue was increased in rats subjected to hemorrhagic shock. This hemorrhagic shock–induced expression of CD163 was suppressed by EF24 and CLEFMA treatments.
EF24 and CLEFMA Treatments Restore the Expression of TJ and Antimicrobial Proteins in Ileal Tissue.
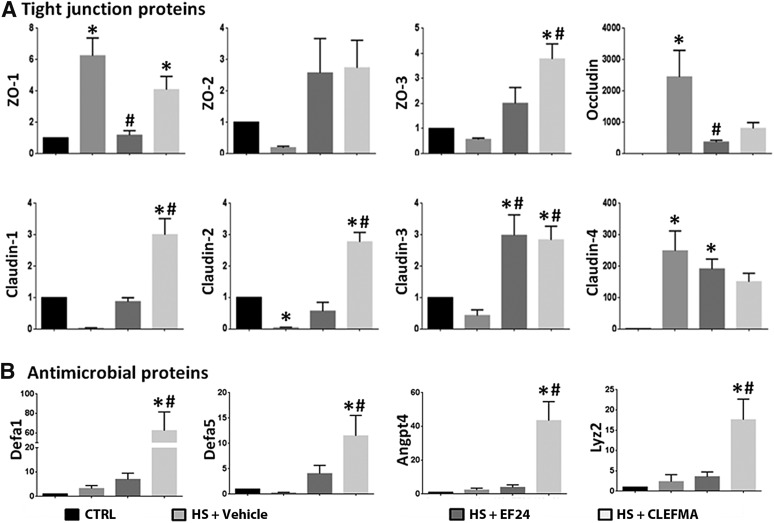
One of the early events in the failure of gut wall integrity is the TJ loss. We found that the mRNA expression of ZO-1, occludin, and claudin-4 was increased, but that of ZO-2, ZO-3, claudin-1, claudin-2, and claudin-3 was suppressed (Fig. 3A). In general, the mRNAs that were reduced by hemorrhagic shock showed large increase by EF24 and CLEFMA treatments, whereas the effect of these drugs was moderately suppressive on the mRNAs that were increased by hemorrhage.
Fig. 3.
Messenger RNA levels of (A) tight junction proteins (ZO-1, ZO-2, ZO-3, occludin, claudin-1, claudin-2, claudin-3, and claudin-4) and (B) antimicrobial proteins Defa-1 (defensin α-1), Defa-5 (defensin α-5), angiopoietin 4 (Angpt-4), and lysozyme-2 (Lyz-2) in ileal tissues. At least three tissues per group were assayed, each in triplicate (*P < 0.05 versus control [CRTL]; #P < 0.05 versus hemorrhagic shock [HS]).
Among the major defenses against potential posthemorrhage infectious insult, the secretions of Paneth cells play an important role (Gunther et al., 2013). They contribute to the intestinal antibacterial defense by releasing antibacterial factors stored in their cytoplasmic granules. Lysozyme is an early marker of Paneth cells’ health. We estimated the status of the rat homologs of defensins lysozyme, α-defensin–related sequence-1, angiopoetin-4, and defensin-5 by real-time PCR, and found that, although hemorrhage did not significantly alter the mRNA levels of antimicrobial proteins, CLEFMA treatment increased the expression levels of all four antimicrobial proteins in a significant manner; the effect of EF24 treatment on their mRNA levels was not significant (Fig. 3B).
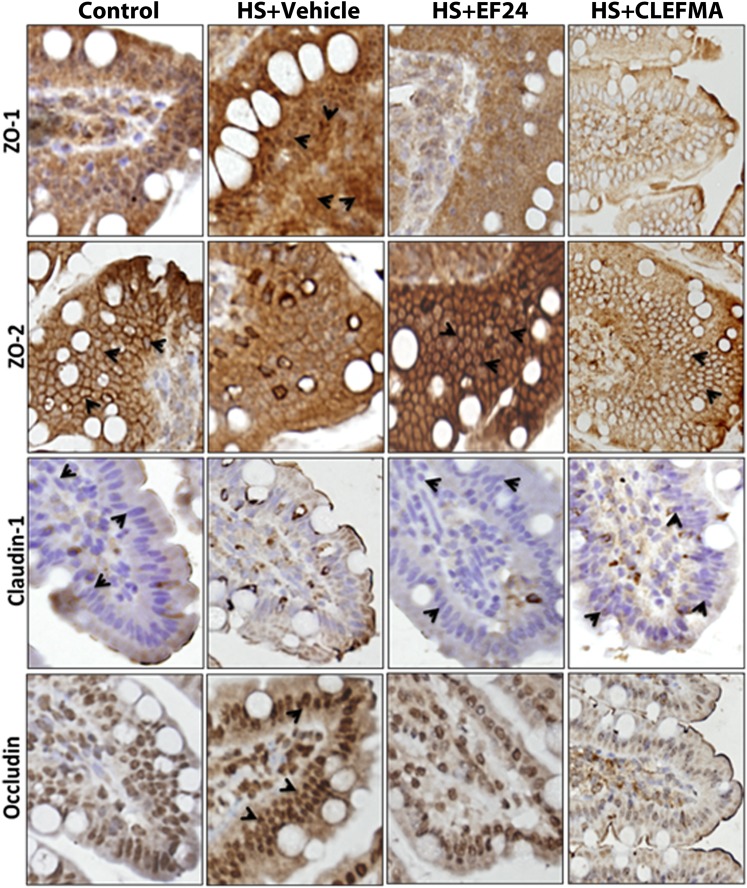
The protein expression of ZO-1, ZO-2, occludin, and claudin-1 was first assessed by IHC (Fig. 4). At protein levels also, the expression of ZO-1 and occludin was enhanced, whereas that of ZO-2 and claudin-1 was reduced by hypovolemia. These effects of blood loss on the expression of various TJ proteins were reversed when the hypovolemic rats were treated with EF24 or CLEFMA (Fig. 4). The results from IHC analyses of tissues were confirmed by immunoblotting of ileal lysates (Fig. 5, A and B). The expression-inducing effect of hypovolemic shock on ZO-1 and occludin was contrary to what was expected in a deranged ileal epithelium. Because ZO-1 is post-transcriptionally regulated by tyrosine (Tyr) phosphorylation, we investigated whether hypovolemia and EF24 treatment have any effect on the expression of phosphorylated form of ZO-1. To this end, we immunoprecipitated the tissue lysate with anti-phosphotyrosine antibody and probed the blot with anti–ZO-1 antibody. We found that hypovolemia significantly reduced the Tyr-phosphorylated ZO-1. EF24 as well as CLEFMA treatment recovered the phospho(Tyr)–ZO-1 to its basal levels (Fig. 5C). In case of occludin, the Tyr phosphorylation was not much affected by hemorrhagic shock, but it was substantially reduced by EF24 and CLEFMA treatments. At the same time, threonine (Thr) phosphorylation of occludin was found to be increased by hemorrhagic shock. Treatments with EF24 and CLEFMA appeared to have maintained the hemorrhage-induced Thr phosphorylation of occludin (Fig. 5C).
Fig. 4.
Immunohistochemical staining (original magnification, 400×) for the expression of tight junction proteins, ZO-1, ZO-2, claudin-1, and occludin, in ileal tissue from hemorrhaged rats treated with EF24 or CLEFMA. The pictures are representative of at least three tissues stained from each group. ZO-1, ZO-2, and occludin expression are indicated by brown stain associated with membranous structures (arrowheads), whereas the claudin-1 is stained blue (arrowheads). HS, hemorrhagic shock.
Fig. 5.
(A) Expression of tight junction proteins ZO-1, ZO-2, claudin, and occludin in ileal tissue from various groups (n ≥ 3 per group). (B) The immunoblots were analyzed by densitometry. Actin expression was probed to ensure equal loading of total protein in each well and to normalize the densitometry values. (C) The effect of hemorrhagic shock and drug treatments on the phosphorylation status of ZO-1 and occludin proteins. Tyrosine phosphorylation was probed for both ZO-1 and occludin, whereas threonine phosphorylation was examined only for occludin. The ileal tissue homogenate was immunoprecipitated by anti-phosphotyrosine or anti-phosphothreonine antibodies, and the blots were probed with anti–ZO-1 and anti-occludin antibodies. The immunoprecipitation was performed on two separate tissues from each group. CL, CLEFMA; CTRL, control; HS, hemorrhagic shock; V, vehicle.
EF24 and CLEFMA Treatments Diminish Epithelial Cell Injury.
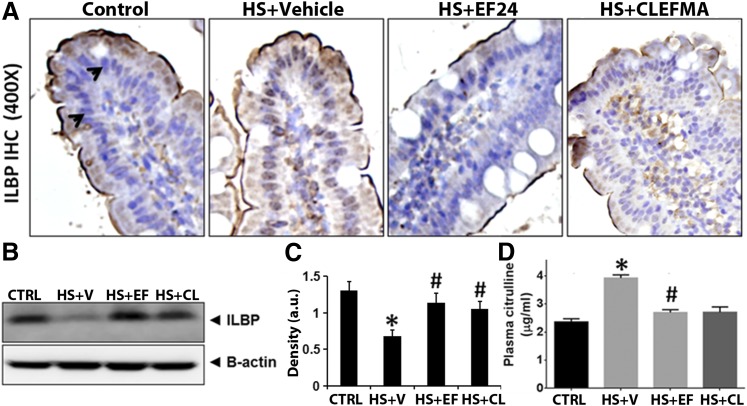
Injury to intestinal epithelia is marked by an increased shedding of ILBP, resulting in lowered detection of cell-associated ILBP. ILBP is a small cytosolic protein (15 kDa) belonging to a family of fatty acid–binding proteins. We examined ILBP levels in ileal tissue by IHC and immunoblotting. As shown in Fig. 6, A and B, hemorrhagic shock substantially decreased the expression of ILBP in ileum, but EF24 and CLEFMA treatments recovered the basal level of ILBP expression.
Fig. 6.
(A) A representative immunohistochemical staining of ILBP expression (blue stain, arrowheads) in rat ileum. (B) The ileal tissue lysates were immunoblotted with anti-rat ILBP antibody. (C) The immunoblots were analyzed by densitometry. At least three randomly selected ileal tissues per group were assayed. Actin expression was probed to ensure equal loading of total protein in each well and to normalize the ILBP densitometry values. (D) Plasma levels of citrulline (n = 4 per group). CL, CLEFMA; EF, EF24; HS, hemorrhagic shock; V, vehicle (*P < 0.05 versus control [CRTL]; #P < 0.05 versus HS).
Systemic citrulline almost exclusively originates from the intestine, where enterocytes synthesize it from either arginine or glutamine. As such, the estimation of plasma citrulline levels is being debated as a clinical marker of acute intestinal failure in critically ill patients (Piton et al., 2011). It is also a surrogate marker for estimating nitric oxide production by nitric oxide synthase activity. As shown in Fig. 6D, hemorrhagic shock significantly increased the plasma citrulline levels. Both EF24 and CLEFMA treatments reduced this hemorrhage-induced citrulline concentration in plasma, but only the EF24 effect was statistically significant.
EF24 and CLEFMA Treatments Reinstate Intestinal Barrier.
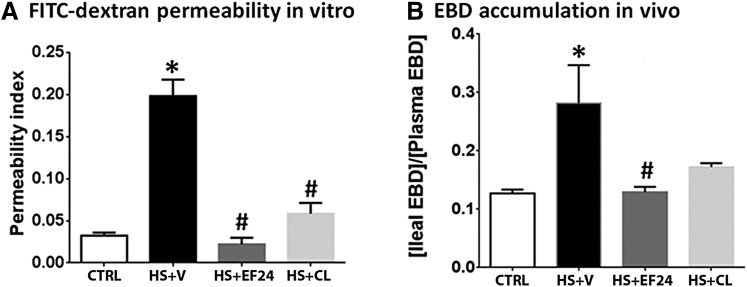
To assess the functional changes in intestinal barrier function, we performed two separate evaluations, as follows: in vitro transmembrane permeability of FITC-dextran and in situ EBD method. The results of both the permeability tests showed a significant increase in permeability after hemorrhagic shock (Fig. 7). The flux of FITC-dextran in isolated inverted sacs of ileal segments (Fig. 7A) was significantly increased in hemorrhaged rats. Both EF24 and CLEFMA reduced the hemorrhage-induced flux of FITC-dextran. The in situ accumulation of EBD also demonstrated the same scenario. Both EF24 and CLEFMA treatments reduced the levels of transmembrane localization of EBD induced by hypovolemia (Fig. 7B).
Fig. 7.
Effect of EF24 and CLEFMA on hemorrhagic shock-induced increase in intestinal permeability for dextran-FITC4000 and EBD. (A) Approximately 5-cm saline-filled inverted sacs of ileum (n = 4 segments per rat, 4 rats per group) were allowed to equilibrate with dextran-FITC solution at 37°C. After 45 minutes, the inner contents of the segments were assayed for accumulation of fluorescent dextran. Permeability index was expressed as fluorescence units/cm per hour. (B) EBD was injected 1 hour before euthanasia, and its accumulation in the ileal tissues was estimated by colorimetry (n = 3 per group). The EBD accumulation in ileal tissue was quantitated with respect to the plasma EBD concentration. *P < 0.05 versus control (CTRL); #P < 0.05 versus HS. CL, CLEFMA; HS, hemorrhagic shock; V, vehicle.
Discussion
Shock is a multifactorial syndrome characterized by an inadequate tissue perfusion and cellular hypoxia affecting multiple organ systems. Gut is one of the major target organs of the systemic inflammatory response syndrome after hemorrhagic shock, and, from a prognostic viewpoint, the status of intestinal barrier function appears to be the most important determinant of clinical outcome (Moore, 1999). One of the early events in severe blood loss is the disintegration of gut wall integrity characterized by marked TJ loss and increased permeability, allowing luminal bacteria, toxins, and other macromolecules to become systemic. The present study focused on the health of intestinal barrier after hemorrhagic shock and treatment with two diphenyldihaloketones, EF24 and CLEFMA. Earlier, we have shown that EF24 treatment suppresses hemorrhage-induced pulmonary markers of inflammation, namely NF-κB, Toll-like receptor 4, cyclooxygenase-2, and IL-1 receptor 1, as well as systemic proinflammatory cytokines, such as tumor necrosis factor-α and IL-6 (Yadav et al., 2013, 2014). We also reported that EF24 potently suppressed NF-κB activation, modulated dendritic cell phenotype, and reduced secretion of proinflammatory cytokines (Vilekar et al., 2012). These initial observations led us to hypothesize that EF24 and CLEFMA will protect ileum from barrier function loss in hemorrhagic shock.
The TJ proteins belonging to the ZO family not only provide scaffolding for the assembly of other TJ proteins via their PDZ domains, but also act as membrane-associated guanylate kinase-like signaling proteins in cellular growth pathways (Bauer et al., 2010). We found that hemorrhage affected the expression of ZO-1 and ZO-2 differently—whereas ZO-1 was upregulated, ZO-2 showed downregulation after hemorrhagic shock; EF24 and CLEFMA both recovered ZO-1 and ZO-2 expressions to their basal levels. The reasons for differential response of ZO-1 and ZO-2 to hemorrhagic shock are not clear, but literature provides some clues about the behavior of ZO-1. It turns out that appropriate localization of these PDZ-containing proteins close to the plasma membrane in the enterocytes is more important than mere cellular expression levels for effective barrier function. This restricted localization is dependent on post-translational modification, such as tyrosine phosphorylation (Rao et al., 2002). Even when the expression levels of ZO-1 remain unaltered, the tyrosine phosphorylation and relocalization from apical membrane of intestinal villi could occur in breached intestinal barrier (Hamada et al., 2010). Phosphorylation also affects mutual interaction among TJ proteins. For instance, the interaction between ZO-1 and occludin is phosphorylation-dependent (Tash et al., 2012). Dephosphorylated ZO-1 has been shown to cause absence of membranous localization of occludin in active celiac disease (Ciccocioppo et al., 2006). Our observation that hemorrhage induces the expression of ZO-1, but decreases the phosphorylated form of ZO-1, also points to the importance of phospho–ZO-1 in the formation of intestinal barrier.
The claudins and occludins are two major transmembrane proteins that interact with ZO proteins and directly determine the paracellular permeability to different ions and large molecules (Hu et al., 2013). The function of claudins in epithelial barrier is also subject to modulation by Ser/Thr phosphorylation and interaction with PDZ-binding domains (Groschwitz and Hogan, 2009). We found that hemorrhage downregulated the expression of claudins 1, 2, and 3, but upregulated the expression of claudin 4. Even though such differential expression of various claudin proteins in response to ischemia/reperfusion injury of the intestine has also been noted by others (Takizawa et al., 2012), the reasons are not clearly understood. Among 27 known members of claudin family, claudins-1 and -3 have sealing functions, claudin-2 forms a channel providing selectivity to the cations, whereas the role of claudin 4 in barrier function is not known (Gunzel and Fromm, 2012). Recent reports have associated claudin-4 expression with epithelial malignancies and premalignant precursor lesions (Neesse et al., 2012). Interestingly, transforming growth factor-β has been reported to transcriptionally upregulate claudin-4 expression via a Smad-4–dependent pathway (Kotler et al., 2013). Because transforming growth factor-β is increased in hemorrhagic shock (Ayala et al., 1993), its role in hypovolemia-induced claudin-4 expression could not be ruled out.
Unlike claudins, occludin protein has the greatest effect on the flux of large macromolecules (Al-Sadi et al., 2011). The expression of occludin is markedly decreased in intestinal permeability disorders, such as Crohn's disease and ulcerative colitis (Gassler et al., 2001), suggesting that decreased occludin expression is associated with an increase in intestinal permeability. Our finding is contrary to this conjecture, and we found that hemorrhagic shock increased occludin expression at both mRNA and protein levels. The effect of phosphorylation status of occludin in maintaining ileal homeostasis has been elegantly investigated by Rao’s group (Seth et al., 2007; Rao, 2009). The phosphorylation of occludin at Ser/Thr helps, whereas phosphorylation at Tyr residue deters its interaction with ZO-1 (Seth et al., 2007; Rao, 2009). Our results from phospho-Tyr coimmunoprecipitation show that the ileum-preserving EF24 and CLEFMA treatments reduce ileal phospho(Tyr)-occludin in hypovolemic rats and support these in vitro findings in Caco-2 monolayers. We also found that hemorrhagic shock induced Thr phosphorylation of occludin, and treatments with EF24 and CLEFMA preserved this modification. The occludin-specific effect of EF24 and CLEFMA may be explained by their ability to reduce Tyr phosphorylation and maintain Thr phosphorylation of occludin, thereby aiding its interaction with ZO-1. Earlier, Seth et al. (2007) predicted that the net role of occludin is determined by the relative levels of Tyr and Thr phosphorylation. It is noteworthy that the Tyr/Thr phosphorylation ratio of occludin is regulated by protein phosphatases 2A and 1 (Seth et al., 2007; Sheth et al., 2009). Overall, these results imply that mere increased expression of occludin may not be sufficient, but phosphorylation at appropriate amino acid residue may be important in regulating its interaction with other TJ proteins.
The remarkable restoration of TJ proteins and accompanying histology by treatments with EF24 and CLEFMA was manifested in the resurrection of ileal barrier function. The treatments significantly reduced hemorrhagic shock-induced increase in the intestinal permeability. We also found that only CLEFMA treatment upregulated mRNA levels of antimicrobial proteins; neither hypovolemia nor EF24 significantly altered the antimicrobial protein mRNA levels. The hypothesis that the variation in chemical structure of EF24 and CLEFMA results in these differences is a subject of further investigation. Not much is known about the changes in antimicrobial proteins secreted by Paneth cells in hemorrhagic shock, but in one report rat enteric α-defensin gene was found to be upregulated immediately after termination of shock (Condon et al., 1999). Because no long-term assessment was performed in the published study (Condon et al., 1999), our results could be reconciled by speculating that the defense mechanisms originating from Paneth cells are progressively salvaged over time. Paneth cells develop from epithelial progenitor cells and are restricted to crypt base in the gut epithelium (Gunther et al., 2013).
The above-described salutary effects of EF24 and their time course pointed toward a rather rapid localization in the intestinal tissue. We examined the biodisposition of EF24 by labeling it with imageable radionuclide technetium-99m (140 KeV γ ray, 6-hour decay half-life) for γ camera imaging. For this we synthesized a N-HYNIC conjugate of EF24 by employing a procedure described elsewhere (Lagisetty et al., 2012). As shown in Supplemental Fig. 3, intravenously-injected technetium-99m–EF24 rapidly accumulated in rat liver and intestine. The first sign of EF24 localization in gut was observed within 5 minutes of injection. The accumulation increased over time, and EF24 appeared to be retained in intestinal tissue up to 6 hours after injection. No other organ except liver and intestines accumulated significant radioactivity, suggesting that its clearance depended on hepatobiliary route. The image-derived knowledge of drug accumulation suggests that EF24 is naturally cleared from circulation into the intestine, which might explain the remarkable effects of EF24 and CLEFMA on intestinal integrity in hemorrhagic shock. What molecular pathways are involved in these actions remains a subject of continued investigation in our laboratory.
Conclusions.
Besides hemorrhagic shock, dysregulated barrier function is a hallmark in many other disorders, such as inflammatory bowel diseases, food allergy, celiac disease, type I diabetes, etc. (Groschwitz and Hogan, 2009; Salim and Soderholm, 2011). The intestinal pathology of hemorrhagic shock is also similar to that observed in victims of burn, sepsis, radiation exposure (MacNaughton, 2000; Hauer-Jensen et al., 2007), or drug toxicities (Meng et al., 2013; Russo et al., 2013). Despite such widespread impact, the current therapies for the management of barrier function loss remain inadequate. The results of this study show that the administration of EF24 or CLEFMA significantly improved the intestinal integrity of rats subjected to hemorrhage. These encouraging observations led us to speculate about the additive benefits of EF24 or CLEFMA when administered in combination with conventional resuscitation fluids used to treat hemorrhagic shock.
Supplementary Material
Acknowledgments
The authors thank Andria Hedrick, research associate in the Department of Pharmaceutical Sciences, University of Oklahoma Health Sciences Center, for technical help.
Abbreviations
- CLEFMA
4-[3,5-bis(2-chlorobenzylidene)-4-oxo-piperidine-1-yl]-4-oxo-2-butenoic acid
- EBD
Evan’s blue dye
- EF24
3,5-bis(2-fluorobenzylidene)piperidin-4-one
- FITC
fluoroisothiocyanate
- HRP
horseradish peroxidase
- IHC
immunohistochemistry
- IL
interleukin
- ILBP
Ileal lipid-binding protein
- LPS
lipopolysaccharide
- NF-κB
nuclear factor κB
- PCR
polymerase chain reaction
- PEG
poly(ethylene glycol)
- TJ
tight junction
- ZO
zonula occludens
Authorship Contributions
Participated in research design: Awasthi.
Conducted experiments: Yadav, Hussain, Sahoo, Awasthi.
Contributed new reagents or analytic tools: Awasthi.
Performed data analysis: Yadav, Awasthi.
Wrote or contributed to the writing of the manuscript: Yadav, Hussain, Awasthi.
Footnotes
This work was supported by the National Institutes of Health National Heart, Lung, and Blood Institute [Grant R01-HL104286].
 This article has supplemental material available at jpet.aspetjournals.org.
This article has supplemental material available at jpet.aspetjournals.org.
References
- Al-Sadi R, Khatib K, Guo S, Ye D, Youssef M, Ma T. (2011) Occludin regulates macromolecule flux across the intestinal epithelial tight junction barrier. Am J Physiol Gastrointest Liver Physiol 300:G1054–G1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awasthi V, Yee SH, Jerabek P, Goins B, Phillips WT. (2007) Cerebral oxygen delivery by liposome-encapsulated hemoglobin: a positron-emission tomographic evaluation in a rat model of hemorrhagic shock. J Appl Physiol (1985) 103:28–38 [DOI] [PubMed] [Google Scholar]
- Ayala A, Meldrum DR, Perrin MM, Chaudry IH. (1993) The release of transforming growth factor-beta following haemorrhage: its role as a mediator of host immunosuppression. Immunology 79:479–484 [PMC free article] [PubMed] [Google Scholar]
- Bauer H, Zweimueller-Mayer J, Steinbacher P, Lametschwandtner A, Bauer HC. (2010) The dual role of zonula occludens (ZO) proteins. J Biomed Biotechnol 2010:402593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Finamore A, Ara C, Di Sabatino A, Mengheri E, Corazza GR. (2006) Altered expression, localization, and phosphorylation of epithelial junctional proteins in celiac disease. Am J Clin Pathol 125:502–511 [DOI] [PubMed] [Google Scholar]
- Condon MR, Viera A, D’Alessio M, Diamond G. (1999) Induction of a rat enteric defensin gene by hemorrhagic shock. Infect Immun 67:4787–4793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton BA. (2011) Alternative fluids for prehospital resuscitation: “pharmacological” resuscitation fluids. J Trauma 70:S30–S31 [DOI] [PubMed] [Google Scholar]
- Cruz RJ, Jr, Harada T, Sasatomi E, Fink MP. (2011) Effects of ethyl pyruvate and other α-keto carboxylic acid derivatives in a rat model of multivisceral ischemia and reperfusion. J Surg Res 165:151–157 [DOI] [PubMed] [Google Scholar]
- Fabriek BO, Dijkstra CD, van den Berg TK. (2005) The macrophage scavenger receptor CD163. Immunobiology 210:153–160 [DOI] [PubMed] [Google Scholar]
- Fink MP. (2007) Ethyl pyruvate: a novel anti-inflammatory agent. J Intern Med 261:349–362 [DOI] [PubMed] [Google Scholar]
- Fink MP, Delude RL. (2005) Epithelial barrier dysfunction: a unifying theme to explain the pathogenesis of multiple organ dysfunction at the cellular level. Crit Care Clin 21:177–196 [DOI] [PubMed] [Google Scholar]
- Gassler N, Rohr C, Schneider A, Kartenbeck J, Bach A, Obermüller N, Otto HF, Autschbach F. (2001) Inflammatory bowel disease is associated with changes of enterocytic junctions. Am J Physiol Gastrointest Liver Physiol 281:G216–G228 [DOI] [PubMed] [Google Scholar]
- Groschwitz KR, Hogan SP. (2009) Intestinal barrier function: molecular regulation and disease pathogenesis. J Allergy Clin Immunol 124:3–20, quiz 21–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Günther C, Neumann H, Neurath MF, Becker C. (2013) Apoptosis, necrosis and necroptosis: cell death regulation in the intestinal epithelium. Gut 62:1062–1071 [DOI] [PubMed] [Google Scholar]
- Günzel D, Fromm M. (2012) Claudins and other tight junction proteins. Compr Physiol 2:1819–1852 [DOI] [PubMed] [Google Scholar]
- Hamada K, Shitara Y, Sekine S, Horie T. (2010) Zonula Occludens-1 alterations and enhanced intestinal permeability in methotrexate-treated rats. Cancer Chemother Pharmacol 66:1031–1038 [DOI] [PubMed] [Google Scholar]
- Hartsock A, Nelson WJ. (2008) Adherens and tight junctions: structure, function and connections to the actin cytoskeleton. Biochim Biophys Acta 1778:660–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauer-Jensen M, Kumar KS, Wang J, Berbee M, Fu Q, Boerma M. (2007) Intestinal toxicity in radiation- and combined injury: significance, mechanisms, and countermeasures, in Global Terrorism Issues and Developments (Larche RA. ed) pp 1–40, Nova Science Publishers, New York [Google Scholar]
- Hu YJ, Wang YD, Tan FQ, Yang WX. (2013) Regulation of paracellular permeability: factors and mechanisms. Mol Biol Rep 40:6123–6142 [DOI] [PubMed] [Google Scholar]
- Kao KK, Fink MP. (2010) The biochemical basis for the anti-inflammatory and cytoprotective actions of ethyl pyruvate and related compounds. Biochem Pharmacol 80:151–159 [DOI] [PubMed] [Google Scholar]
- Kasinski AL, Du Y, Thomas SL, Zhao J, Sun SY, Khuri FR, Wang CY, Shoji M, Sun A, Snyder JP, et al. (2008) Inhibition of IkappaB kinase-nuclear factor-kappaB signaling pathway by 3,5-bis(2-flurobenzylidene)piperidin-4-one (EF24), a novel monoketone analog of curcumin. Mol Pharmacol 74:654–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotler BM, Kerstetter JE, Insogna KL. (2013) Claudins, dietary milk proteins, and intestinal barrier regulation. Nutr Rev 71:60–65 [DOI] [PubMed] [Google Scholar]
- Lagisetty P, Powell DR, Awasthi V. (2009) Synthesis and structural determination of 3 3,5-bis(2-fluorobenzylidene)-4-piperidone analogs of curcumin. J Mol Struct 936:23–28 [Google Scholar]
- Lagisetty P, Subramaniam D, Sahoo K, Anant S, Awasthi V. (2012) Anticancer activity of an imageable curcuminoid 1-[2-aminoethyl-(6-hydrazinopyridine-3-carbamidyl)-3,5-bis-(2-fluorobenzylidene)-4-piperidone] (EFAH). Chem Biol Drug Des 79:194–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagisetty P, Vilekar P, Sahoo K, Anant S, Awasthi V. (2010) CLEFMA - an anti-proliferative curcuminoid from structure-activity relationship studies on 3,5-bis(benzylidene)-4-piperidones. Bioorg Med Chem 18:6109–6120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDermott RP. (1999) Chemokines in the inflammatory bowel diseases. J Clin Immunol 19:266–272 [DOI] [PubMed] [Google Scholar]
- MacNaughton WK. (2000) Review article: new insights into the pathogenesis of radiation-induced intestinal dysfunction. Aliment Pharmacol Ther 14:523–528 [DOI] [PubMed] [Google Scholar]
- Meng J, Yu H, Ma J, Wang J, Banerjee S, Charboneau R, Barke RA, Roy S. (2013) Morphine induces bacterial translocation in mice by compromising intestinal barrier function in a TLR-dependent manner. PLoS One 8:e54040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore FA. (1999) The role of the gastrointestinal tract in postinjury multiple organ failure. Am J Surg 178:449–453 [DOI] [PubMed] [Google Scholar]
- Neesse A, Griesmann H, Gress TM, Michl P. (2012) Claudin-4 as therapeutic target in cancer. Arch Biochem Biophys 524:64–70 [DOI] [PubMed] [Google Scholar]
- Piton G, Manzon C, Cypriani B, Carbonnel F, Capellier G. (2011) Acute intestinal failure in critically ill patients: is plasma citrulline the right marker? Intensive Care Med 37:911–917 [DOI] [PubMed] [Google Scholar]
- Rao R. (2009) Occludin phosphorylation in regulation of epithelial tight junctions. Ann N Y Acad Sci 1165:62–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao RK, Basuroy S, Rao VU, Karnaky KJ, Jr, Gupta A. (2002) Tyrosine phosphorylation and dissociation of occludin-ZO-1 and E-cadherin-beta-catenin complexes from the cytoskeleton by oxidative stress. Biochem J 368:471–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes RS, Depalma RG, Robinson AV. (1973) Intestinal barrier function in hemorrhagic shock. J Surg Res 14:305–312 [DOI] [PubMed] [Google Scholar]
- Rotstein OD. (2000) Pathogenesis of multiple organ dysfunction syndrome: gut origin, protection, and decontamination. Surg Infect (Larchmt) 1:217–223 [DOI] [PubMed] [Google Scholar]
- Russo F, Linsalata M, Clemente C, D’Attoma B, Orlando A, Campanella G, Giotta F, Riezzo G. (2013) The effects of fluorouracil, epirubicin, and cyclophosphamide (FEC60) on the intestinal barrier function and gut peptides in breast cancer patients: an observational study. BMC Cancer 13:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salim SY, Söderholm JD. (2011) Importance of disrupted intestinal barrier in inflammatory bowel diseases. Inflamm Bowel Dis 17:362–381 [DOI] [PubMed] [Google Scholar]
- Sappington PL, Han X, Yang R, Delude RL, Fink MP. (2003) Ethyl pyruvate ameliorates intestinal epithelial barrier dysfunction in endotoxemic mice and immunostimulated caco-2 enterocytic monolayers. J Pharmacol Exp Ther 304:464–476 [DOI] [PubMed] [Google Scholar]
- Senthil M, Brown M, Xu DZ, Lu Q, Feketeova E, Deitch EA. (2006) Gut-lymph hypothesis of systemic inflammatory response syndrome/multiple-organ dysfunction syndrome: validating studies in a porcine model. J Trauma 60:958–965. [DOI] [PubMed] [Google Scholar]
- Seth A, Sheth P, Elias BC, Rao R. (2007) Protein phosphatases 2A and 1 interact with occludin and negatively regulate the assembly of tight junctions in the CACO-2 cell monolayer. J Biol Chem 282:11487–11498 [DOI] [PubMed] [Google Scholar]
- Sheth P, Samak G, Shull JA, Seth A, Rao R. (2009) Protein phosphatase 2A plays a role in hydrogen peroxide-induced disruption of tight junctions in Caco-2 cell monolayers. Biochem J 421:59–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi HP, Deitch EA, Da Xu Z, Lu Q, Hauser CJ. (2002) Hypertonic saline improves intestinal mucosa barrier function and lung injury after trauma-hemorrhagic shock. Shock 17:496–501 [DOI] [PubMed] [Google Scholar]
- Takizawa Y, Kishimoto H, Kitazato T, Tomita M, Hayashi M. (2012) Changes in protein and mRNA expression levels of claudin family after mucosal lesion by intestinal ischemia/reperfusion. Int J Pharm 426:82–89 [DOI] [PubMed] [Google Scholar]
- Tash BR, Bewley MC, Russo M, Keil JM, Griffin KA, Sundstrom JM, Antonetti DA, Tian F, Flanagan JM. (2012) The occludin and ZO-1 complex, defined by small angle X-ray scattering and NMR, has implications for modulating tight junction permeability. Proc Natl Acad Sci USA 109:10855–10860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega D, Badami CD, Caputo FJ, Watkins AC, Lu Q, Xu da Z, Berezina TL, Zaets SB, Feketeova E, Deitch EA. (2008) The influence of the type of resuscitation fluid on gut injury and distant organ injury in a rat model of trauma/hemorrhagic shock. J Trauma 65:409–414. [DOI] [PubMed] [Google Scholar]
- Vilekar P, Awasthi S, Natarajan A, Anant S, Awasthi V. (2012) EF24 suppresses maturation and inflammatory response in dendritic cells. Int Immunol 24:455–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss J, Taylor GR, Zimmermann F, Nebendahl K. (2000) Collection of body fluids, in The Laboratory Rat (Krinke GJ. ed) pp 485–510, Academic Press, London [Google Scholar]
- Yadav VR, Sahoo K, Roberts PR, Awasthi V. (2013) Pharmacologic suppression of inflammation by a diphenyldifluoroketone, EF24, in a rat model of fixed-volume hemorrhage improves survival. J Pharmacol Exp Ther 347:346–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav VR, Vilekar P, Awasthi S, Awasthi V. (2014) Hemorrhage-induced interleukin-1 receptor pathway in lung is suppressed by 3,5-bis(2-fluorobenzylidene)-4-piperidone in a rat model of hypovolemic shock. Artif Organs 38:675–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.