Abstract
Exposure to nerve agents induces prolonged status epilepticus (SE), causing brain damage or death. Diazepam (DZP) is the current US Food and Drug Administration–approved drug for the cessation of nerve agent–induced SE. Here, we compared the efficacy of DZP with that of UBP302 [(S)-3-(2-carboxybenzyl)willardiine; an antagonist of the kainate receptors that contain the GluK1 subunit] against seizures, neuropathology, and behavioral deficits induced by soman in rats. DZP, administered 1 hour or 2 hours postexposure, terminated the SE, but seizures returned; thus, the total duration of SE within 24 hours after soman exposure was similar to (DZP at 1 hour) or longer than (DZP at 2 hours) that in the soman-exposed rats that did not receive the anticonvulsant. Compared with DZP, UBP302 stopped SE with a slower time course, but dramatically reduced the total duration of SE within 24 hours. Neuropathology and behavior were assessed in the groups that received anticonvulsant treatment 1 hour after exposure. UBP302, but not DZP, reduced neuronal degeneration in a number of brain regions, as well as neuronal loss in the basolateral amygdala and the CA1 hippocampal area, and prevented interneuronal loss in the basolateral amygdala. Anxiety-like behavior was assessed in the open field and by the acoustic startle response 30 days after soman exposure. The results showed that anxiety-like behavior was increased in the DZP-treated group and in the group that did not receive anticonvulsant treatment, but not in the UBP302-treated group. The results argue against the use of DZP for the treatment of nerve agent–induced seizures and brain damage and suggest that targeting GluK1-containing receptors is a more effective approach.
Introduction
The devastating effects of the sarin attack against civilians in Syria that the world witnessed in August 2013 (Dolgin, 2013) brought again to the forefront questions regarding readiness and whether the existing medical countermeasures can save lives and protect against the long-term health consequences of exposure. The primary action of nerve agents is the inhibition of acetylcholinesterase (AChE). Without medical intervention, the ensuing cholinergic crisis can culminate in the collapse of the cardiorespiratory system and death (Bajgar, 2005). In addition to the peripheral effects, AChE inhibition in the brain produces convulsive seizures and status epilepticus (SE), which are initiated by the excessive stimulation of cholinergic receptors. If immediate death is prevented by adequate control of the peripheral symptoms, but SE is not controlled effectively, lives may still be lost from the prolonged SE, or severe brain damage can ensue with long-term behavioral consequences. The lasting behavioral deficits that follow nerve agent exposure are well known from experimental studies in animals (Filliat et al., 2007; Coubard et al., 2008; Langston et al., 2012; Prager et al., 2014) as well as from studies in the victims of the sarin attacks in Japan, who present neurologic and neuropsychiatric disturbances years after the exposure (Ohtani et al., 2004; Yanagisawa et al., 2006; Hoffman et al., 2007).
Because the initiation of seizures after nerve agent exposure is due to excessive elevation of acetylcholine acting primarily on muscarinic receptors, administration of muscarinic receptor antagonists can halt the development of SE, but only when administered within a short period of time after exposure (Lallement et al., 1998; Shih and McDonough, 1999; Skovira et al., 2010), suggesting that seizures are sustained and reinforced by glutamatergic, rather than cholinergic, mechanisms (McDonough and Shih, 1997). One way to suppress glutamatergic hyperactivity is by enhancing GABAergic inhibition. Benzodiazepines, which are positive allosteric modulators of GABAA receptors (Campo-Soria et al., 2006; Gielen et al., 2012), have long been the first line of treatment for the cessation of SE triggered by various etiologies (Shorvon, 2001). Accordingly, the benzodiazepine diazepam (DZP) is also currently the only US Food and Drug Administration–approved injectable drug for the control of seizures caused by nerve agents.
A number of concerns, however, are associated with the use of DZP for the cessation of nerve agent–induced SE. First, the efficacy of DZP decreases as the interval between the initiation of SE and the administration of DZP increases (Shih et al., 1999; McDonough et al., 2010; Todorovic et al., 2012). This has also been documented in the lithium-pilocarpine model of SE (Walton and Treiman, 1988; Jones et al., 2002; Goodkin et al., 2003), which has many similarities to the nerve agent–induced SE, in both the mechanisms of seizure initiation and the effects it produces (Tetz et al., 2006). The development of resistance to DZP is concerning because, in a terrorist attack with nerve agents, it may not be possible for medical assistance to arrive immediately; thus, when DZP is administered, the seizures may have already become resistant to benzodiazepines. Second, benzodiazepines are among the anticonvulsants with the most serious side effects (Mehta et al., 2007; Kellinghaus and Stögbauer, 2012). Third, seizures often recur after termination of the initial SE by benzodiazepines (Mayer et al., 2002; Singhi et al., 2002), and at least in the case of nerve agent–induced SE, it is unclear whether DZP compares favorably with other anticonvulsant treatments in the duration of its antiseizure effects. This is particularly significant considering that the duration and intensity of seizures clearly correlate with the severity of the resulting neuropathology (Shih et al., 2003; Prager et al., 2013).
We recently demonstrated that nerve agent–induced seizures can be effectively controlled by targeting the glutamatergic system. LY293558 [(3S,4aR,6R,8aR)-6-[2-(1(2)H-tetrazole-5-yl)ethyl]decahydroisoquinoline-3-carboxylic acid], which is an antagonist of both the AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) receptors and the kainate receptor subtype that contains the GluK1 subunit (GluK1R; formerly known as GluR5 kainate receptors or GLUK5 receptors; see Collingridge et al., 2009 and Jane et al., 2009) was very efficacious in stopping seizures induced by the nerve agent soman and protecting against neuronal damage (Figueiredo et al., 2011; Apland et al., 2013). In this study, we used a rat model of nerve agent exposure in which the anticonvulsant treatment was delayed to at least 1 hour, and compared the efficacy of DZP with that of another GluK1R antagonist UBP302 [(S)-3-(2-carboxybenzyl)willardiine], which selectively antagonizes the GluK1Rs (More et al., 2004), against soman-induced seizures, as well as acute and long-term neuropathology. We also examined the efficacy of DZP and UBP302 in preventing the development of anxiety, which is a prevalent behavioral deficit resulting from nerve agent–induced brain damage, in both animals (Coubard et al., 2008; Langston et al., 2012; Prager et al., 2014) and humans (Ohtani et al., 2004; Hoffman et al., 2007).
Materials and Methods
Animals.
Male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA), weighing 150–250 g (aged 6–8 weeks) at the start of the experiments, were individually housed in an environmentally controlled room (20–23°C, 12-hour light/dark cycle, lights on 6:00 AM), with food and water available ad libitum. The animal care and use programs at the US Army Medical Research Institute of Chemical Defense and the Uniformed Services University of the Health Sciences are accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. All animal experiments were conducted following the Guide for the Care and Use of Laboratory Animals by the Institute of Laboratory Animal Resources, National Research Council, and the Animal Welfare Act of 1966 (P.L. 89-544), as amended, and were approved by the Institutional Animal Care and Use Committees of the US Army Medical Research Institute of Chemical Defense and the Uniformed Services University of the Health Sciences.
Soman Administration and Drug Treatment.
Soman (pinacolyl methylphosphonofluoridate) was obtained from the US Army Edgewood Chemical Biologic Center (Aberdeen Proving Ground, MD). The agent was diluted in cold saline and was administered via a single subcutaneous injection (154 µg/kg, which is approximately 1.4× LD50; Jimmerson et al., 1989) to rats that were 7 to 8 weeks old. To increase the survival rate, rats were administered HI-6 [1-(2-hydroxyiminomethylpyridinium)-3-(4-carbamoylpyridinium)-2-oxapropane dichloride; 125 mg/kg i.p.; Starks Associates, Buffalo, NY] 30 minutes prior to soman exposure. HI-6 is a bispyridinium oxime that reactivates inhibited AChE, primarily in the periphery (Bajgar, 2005). Within 1 minute after soman exposure, rats also received an intramuscular injection of atropine sulfate (2 mg/kg; Sigma-Aldrich, St. Louis, MO) to minimize peripheral toxic effects. The soman-exposed rats were randomly divided into three groups: those that did not receive any further treatment (except for the oxime pretreatment and the atropine; soman group), those that received DZP (10 mg/kg i.m.) at 1 hour after exposure to soman (soman + DZP group), and those that received UBP302 (250 mg/kg i.p.) at 1 hour after exposure to soman (soman + UBP302 group). Some of the soman-exposed rats had been implanted with electrodes for electroencephalographic monitoring (see the following section for the implantation procedure), 10 days before exposure. From the implanted rats, some were administered DZP or UBP302 (doses same as above) at 1 hour or 2 hours after soman exposure; therefore, there were two soman + DZP groups and two soman + UBP302 groups for the electrode-implanted rats (for the two time points of anticonvulsant treatment; sample sizes are provided in Results). DZP and UBP302 were purchased from Hospira Inc. (Lake Forest, IL) and Tocris Bioscience (Bristol, UK), respectively. Control animals received HI-6 and atropine, but were injected with saline instead of soman (control group). For the soman + UBP302 groups, we had to decide on a dose based only on our own observations because there are no previous studies in which UBP302 has been injected systemically. First, we tested 100 mg/kg; this concentration suppressed seizures, but with a very slow time course (it took more than 3 hours to terminate seizure activity). We concluded with 250 mg/kg after also testing this concentration in control rats (rats not exposed to soman). Unlike DZP, which produces sedative effects even at 10 mg/kg, the 250 mg/kg UBP302 administered to control rats produced only a mild reduction in overall activity.
In the rats that were not implanted with electroencephalographic electrodes, the occurrence and the progression of seizures were monitored behaviorally and classified according to the Racine scale (Racine, 1972), with the following minor modifications: stage 0, no behavioral response; stage 1, behavioral arrest; stage 2, oral/facial movements, chewing, and head nodding; stage 3, unilateral/bilateral forelimb clonus without rearing, Straub tail, and extended body posture; stage 4, bilateral forelimb clonus plus rearing; stage 5, rearing and falling; and stage 6, full tonic-clonic seizures.
Electrode Implantation and Electroencephalographic Recordings.
Rats (aged 6 weeks) were anesthetized with isoflurane using a gas anesthesia system (Kent Scientific, Torrington, CT). Five stainless steel, cortical screw electrodes were stereotaxically implanted, as previously described (Figueiredo et al., 2011), using the following coordinates (from Paxinos and Watson, 2005): two frontal electrodes, 2.0 mm posterior from bregma and 2.5 mm lateral from the midline, and two parietal electrodes, 5.0 mm posterior from bregma and 2.5 mm lateral from the midline; and a cerebellar reference electrode was implanted 1.0 mm posterior to lambda. Each screw electrode (Plastics One Inc., Roanoke, VA) was placed in a plastic pedestal (Plastics One Inc.) and fixed to the skull with dental acrylic cement. For electroencephalographic recordings (obtained 10 days after electrode implantation), rats were placed in the electroencephalography (EEG) chamber and connected to the EEG system (200 Hz sampling rate; Stellate, Montreal, QC, Canada). Video-EEG recordings were performed in the freely moving rats, as previously described (Figueiredo et al., 2011). EEG was continuously recorded for 24 hours after soman injection; during that time, the animals had free access to food and water. The termination of the soman-induced SE was defined as the disappearance of large amplitude, repetitive discharges (>1 Hz with at least double the amplitude of the background activity).
Fixation and Tissue Processing.
Neuropathological analysis was performed in the amygdala, piriform cortex, entorhinal cortex, hippocampus, and a neocortical region of rats that were not implanted with EEG electrodes (the implantation procedure might cause some damage that could interfere with the neuropathology results). One day, 7 days, and 30 days after soman administration, rats were deeply anesthetized with pentobarbital (75–100 mg/kg i.p.) and transcardially perfused with phosphate-buffered saline (PBS; 100 ml) followed by 4% paraformaldehyde (200 ml). The brains were removed and postfixed overnight at 4°C, then transferred to a solution of 30% sucrose in PBS for 72 hours and frozen with dry ice before storage at –80°C until sectioning. A one-in-five series of sections from the rostral extent of the amygdala to the caudal extent of the entorhinal cortex was cut at 40 µm on a sliding microtome. One series of sections was mounted on slides (Superfrost Plus; Daigger, Vernon Hills, IL) in PBS for Nissl staining with cresyl violet. Two adjacent series of sections were mounted on slides for Fluoro-Jade C (FJC) staining or were stored at −20°C in a cryoprotectant solution for glutamic acid decarboxylase-67 (GAD-67) immunohistochemistry. All neuropathological analysis was done in a blind fashion.
FJC Staining and Analysis.
FJC (Histo-Chem, Jefferson, AR) was used to identify irreversibly degenerating neurons in all of the amygdalar nuclei and the piriform cortex (−2.04 mm to −3.36 mm from bregma), a neocortical area (−2.04 and −6.36 mm from bregma), the entorhinal cortex and the CA1, CA3, and hilar areas of the ventral hippocampus (−5.4 and −6.36 mm from bregma; all coordinates from Paxinos and Watson, 2005). We studied the ventral hippocampus because it displays significantly more severe neurodegeneration after soman exposure than the dorsal hippocampus (Apland et al., 2010). Mounted sections were air-dried overnight and then immersed in a solution of 1% sodium hydroxide in 80% ethanol for 5 minutes. The slides were then rinsed for 2 minutes in 70% ethanol and 2 minutes in distilled water (dH2O), and then incubated in 0.06% potassium permanganate solution for 10 minutes. After a 2-minute rinse in dH2O, the slides were transferred to a 0.0001% solution of FJC dissolved in 0.1% acetic acid for 10 minutes. After three 1-minute rinses in dH2O, the slides were dried on a slide warmer, cleared in xylene for at least 1 minute, and coverslipped with Distyrene Plasticizer Xylene (Sigma-Aldrich).
To assess the extent of neurodegeneration, we used a series of adjacent Nissl-stained sections to trace the regions of interest. The tracings from the Nissl-stained sections were superimposed on the FJC-stained sections, using the Stereo Investigator 9.0 (MicroBrightField, Williston, VT). The following rating system was used to score the extent of neuronal degeneration in each structure and substructures: 0, no damage; 1, minimal damage (1–10%); 2, mild damage (11–25%); 3, moderate damage (26–45%); and 4, severe damage (>45%). We previously showed that qualitative assessment using this scale produces results that are in agreement with quantitative assessments (Qashu et al., 2010). The scores for neurodegeneration present on FJC-stained sections were assessed considering the density of cells from Nissl-stained sections, along the anterior to posterior extent, at 600-μm intervals.
Stereological Quantification.
Design-based stereology was used to quantify the total number of neurons in Nissl-stained sections in the basolateral amygdala (BLA) and CA1 area. Sections were viewed with a Zeiss Axioplan 2ie (Carl Zeiss, Oberkochen, Germany) fluorescent microscope with a motorized stage, interfaced with a computer running StereoInvestigator 9.0 (MicroBrightField). The BLA and CA1 regions were identified on slide-mounted sections, and were delineated for each slide of each animal, under a 2.5× objective, based on the atlas of Paxinos and Watson (2005). All sampling was done under a 63× oil immersion objective. Nissl-stained neurons were distinguished from glial cells by their larger size and pale nuclei surrounded by darkly stained cytoplasm containing Nissl bodies. The total number of Nissl-stained neurons was estimated using the optical fractionator probe, and, along with the coefficient of error (CE), was calculated using Stereo Investigator 9.0 (MicroBrightField). The CE was calculated by the software according to Gundersen et al. (1999) (m = 1) and Schmitz and Hof (2000) (second estimation) equations.
For Nissl-stained neurons in the BLA, a one-in-five series of sections was analyzed (eight sections on average). The counting frame was 35 × 35 µm, the counting grid was 190 × 190 µm, and the dissector height was 12 µm. Nuclei were counted when the cell body came into focus within the dissector, which was placed 2 µm below the section surface. Section thickness was measured at every counting site, and the average mounted section thickness was 22.3 µm. An average of 365 neurons per rat was counted, and the average CE was 0.045 for both the Gunderson and Schmitz–Hof equations. For Nissl-stained neurons in the CA1 area, a 1-in-10 series of sections was analyzed (eight sections on average). The counting frame was 20 × 20 µm, the counting grid was 250 × 250 µm, and the dissector height was 10 µm. Nuclei were counted when the cell body came into focus within the dissector, which was placed 2 µm below the section surface. Section thickness was measured at every counting site, and the average mounted section thickness was 18.3 µm. An average of 253 neurons per rat was counted, and the CE was 0.065 for the Gunderson equation (m = 1) and 0.060 for the Schmitz–Hof equation (second estimation). For GABAergic interneurons immunolabeled for GAD-67 in the BLA (see the procedure below), a 1-in-10 series of sections was analyzed (six sections on average). The counting frame was 60 × 60 µm, the counting grid was 100 × 100 µm, and the dissector height was 20 µm. Nuclei were counted when the top of the nucleus came into focus within the dissector, which was placed 2 µm below the section surface. Section thickness was measured at every fifth counting site, and the average mounted section thickness was 30 µm. An average of 260 neurons per rat was counted, and the average CE was 0.07 for the Gunderson equation and 0.065 for the Schmitz–Hof equation.
GAD-67 Immunohistochemistry.
To label GAD-67 immunoreactive neurons, a one-in-five series of free-floating sections was collected from the cryoprotectant solution, washed three times for 5 minutes each in 0.1 M PBS, and then incubated in a blocking solution containing 10% normal goat serum (Chemicon International, Temecula, CA) and 0.5% Triton X-100 in PBS for 1 hour at room temperature. The sections were then incubated with mouse anti–GAD-67 serum (1:1000, MAB5406; Chemicon), 5% normal goat serum, 0.3% Triton X-100, and 1% bovine serum albumin, overnight at 4°C. After rinsing three times for 10 minutes each in 0.1% Triton X-100 in PBS, the sections were incubated with Cy3-conjugated goat anti-mouse antibody (1:1000; Jackson ImmunoResearch, West Grove, PA) and 0.0001% 4′,6-diamidino-2-phenylindole (Sigma-Aldrich) in PBS for 1 hour at room temperature. After a final rinse in PBS for 10 minutes, sections were mounted on slides, air-dried for at least 30 minutes, and coverslipped with ProLong Gold antifade reagent (Life Technologies, Grand Island, NY).
Behavioral Experiments.
Animals from the soman, soman + DZP, soman + UBP302, and control groups were tested in the open field and the acoustic startle apparatus, 30 days after soman administration. In the open field apparatus (40 × 40 × 30-cm clear Plexiglas arena), anxiety-like behavior was assessed as previously described (Aroniadou-Anderjaska et al., 2012; Prager et al., 2014), following the procedure used by Faraday et al. (2001). One day prior to testing (on day 29 after soman exposure), animals were acclimated to the apparatus for 20 minutes. On the test day, the rats were placed in the center of the open field, and activity was measured and recorded for 20 minutes, using an Accuscan Electronics infrared photocell system (Accuscan Instruments Inc., Columbus, OH). Data were automatically collected and transmitted to a computer equipped with “Fusion” software (Accuscan Electronics). Locomotion (distance traveled in centimeters), total movement time, and time spent in the center of the open field were analyzed. Anxiety behavior was measured as the ratio of the time spent in the center over the total movement time, expressed as a percentage of the total movement time. Subjects were exposed to an acclimation session on day 29 postexposure, and were tested on the next day.
Acoustic startle response (ASR) testing was conducted with the use of the Med Associates Acoustic Response Test System (Med Associates, Georgia, VT), which consists of weight-sensitive platforms inside individual sound-attenuating chambers. A ventilating fan built into the chamber provides background noise. Each rat was individually placed in a ventilated holding cage. The holding cages are small enough to restrict extensive locomotion, but large enough to allow the subject to turn around and make other small movements. Each cage was placed on a weight-sensitive platform. Subjects’ movements in response to stimuli were measured as a voltage change by a strain gauge inside each platform. All animals were acclimated to the apparatus in two sessions, on days 28 and 29 after soman exposure. Startle stimuli consisted of 120- or 110-db sound pressure level noise bursts of 20-millisecond duration. Each stimulus had a 2-millisecond rise and decay time, such that the onset and offset were abrupt, which is a primary requirement for startle. Each trial type (110- or 120-db stimulus) was presented eight times. Trial types were presented in random order to avoid effects and habituation, and intertrial intervals ranged randomly from 15 to 25 seconds. Responses were recorded by an interfaced Pentium computer as the maximum response occurring during the no-stimulus periods and during the startle period, and were assigned a value based on an arbitrary scale used by the software of the test system.
Statistical Analysis.
The Fisher exact test was used to compare the survival rate between the groups. Initial SE duration, total SE duration, number of convulsive seizures recurring during the 24-hour period after termination of the initial SE, stereological estimations of the number of neurons and interneurons, and results from behavioral tests were compared between the soman, the soman + DZP, and the soman + UBP302 groups using analysis of variance followed by post hoc tests as described in the figure legends. The statistical values are presented as the mean ± S.E.M. Neurodegeneration scores were compared between groups for each structure separately using the Kruskal–Wallis test followed by the Mann–Whitney U test for comparisons between pairs of groups. The statistical values are presented as the median and interquartile range (IQR; the difference between the 75th and the 25th percentiles). For all tests, differences were considered significant when P < 0.05. Sample sizes (n) refer to the number of animals.
Results
Behavioral SE (stage 3 seizures progressing to higher stages) developed within 5–15 minutes after soman injection. Eleven of 124 rats that were exposed to soman did not develop seizures and were not included in the study. The survival rate for the animals that were nonimplanted with EEG electrodes (92 rats) was 63% (22 of 34) for the soman group, which did not receive anticonvulsant treatment, 91% (21 of 23) for the soman + DZP group, which received DZP at 1 hour after soman challenge, and 96% (24 of 25) for the soman + UBP302 group, which received UBP302 at 1 hour after soman exposure. The higher survival rate of the DZP- and UBP302-treated rats, versus the soman group, was statistically significant (Fisher exact test, P = 0.018 and P = 0.003, respectively).
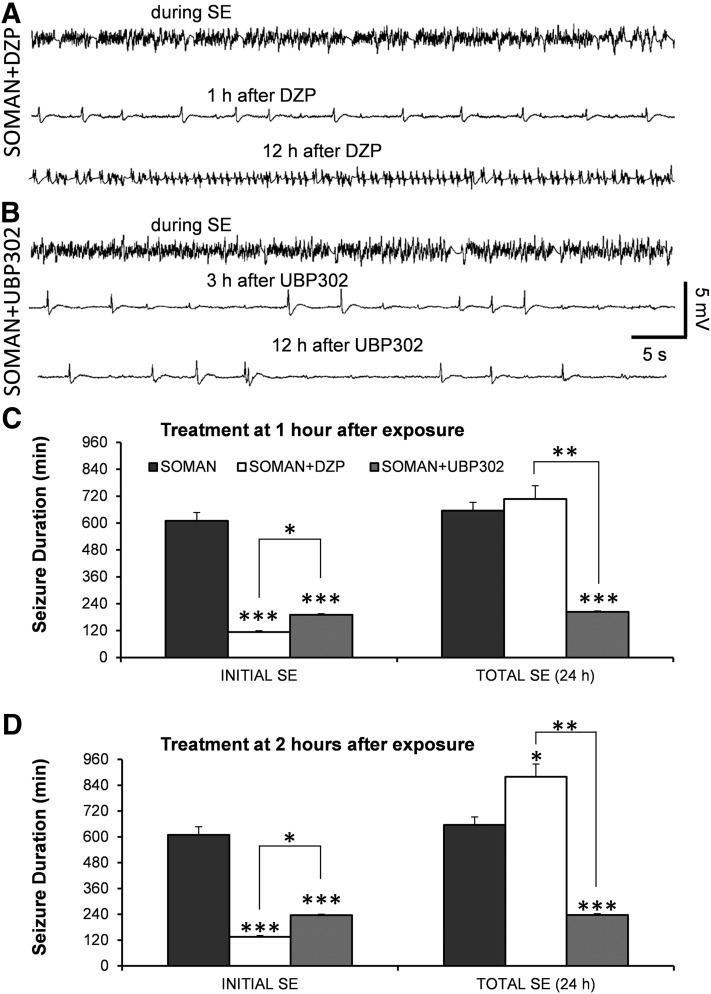
For the rats that were implanted with EEG electrodes (n = 31), the survival rate was 44% for the soman group (4 of 9) and 100% for the animals that were administered DZP at 1 hour (n = 6) or 2 hours (n = 4) after exposure, as well as for the animals administered UBP302 at 1 hour (n = 8) or 2 hours (n = 4) after exposure. In the soman + DZP group, the electrographic initial SE [i.e., the SE that started after soman injection and was terminated, at least temporarily, after anticonvulsant administration (Fig. 1A)] lasted for 113.6 ± 10.6 minutes (n = 6) when DZP was administered 1 hour after soman, and 133.7 ± 10.9 minutes (n = 4) when DZP was administered at 2 hours after soman. In the soman + UBP302 group, the electrographic initial SE (Fig. 1B) lasted for 189.9 ± 6.2 minutes (n = 8) when UBP302 was administered 1 hour after soman, and 236 ± 5.4 minutes (n = 4) when UBP302 was administered 2 hours after soman. Compared with the duration of the SE in the soman group (609.4 ± 37.3 minutes, n = 4), the initial SE duration in the soman + DZP and the soman + UBP302 groups was significantly lower (P < 0.001) whether the anticonvulsants were administered at 1 hour after soman exposure (Fig. 1C, left set of bars) or at 2 hours after soman exposure (Fig. 1D, left set of bars). The duration of the initial SE in the soman + UBP302 group was significantly greater than in the soman + DZP group (P < 0.05).
Fig. 1.
DZP terminates soman-induced SE, but does not reduce the total duration of SE within the 24-hour period after soman exposure, as seizures return; UBP302 reduces the total duration of SE within 24 hours. (A and B) Example traces from EEG recordings showing that both DZP and UBP302 (administered 1 hour after soman) terminated the SE induced by soman, but seizure activity returned after DZP administration. (C) Duration of initial SE and total duration of SE within 24 hours after soman exposure, when DZP and UBP302 were administered at 1 hour after soman injection. The three bars on the left show the duration of the initial SE (the SE that started 5–15 minutes after soman exposure and was terminated by DZP or UBP302, or spontaneously in the soman group), whereas the three bars on the right show the total duration of SE (soman: n = 4; soman + DZP: n = 6; soman + UBP302: n = 8). (D) Duration of initial SE and total duration of SE within 24 hours after soman exposure, when DZP and UBP302 were administered at 2 hours after soman injection. The three bars on the left show the duration of the initial SE, whereas the three bars to the right show the total duration of SE (soman: n = 4; soman + DZP: n = 4; soman + UBP302: n = 4). *P < 0.05; **P < 0.01; ***P < 0.001, compared with the soman group (analysis of variance [ANOVA] followed by Bonferroni post hoc test for the initial SE and ANOVA followed by the Games–Howell post hoc test for total SE). *P < 0.05; **P < 0.01 for the comparisons between the DZP-treated and the UBP302-treated groups (ANOVA followed by Fisher’s least significant difference test).
Seizures recurred in all of the rats that received DZP and in half of the rats that received UBP302. The total duration of electrographic seizures within the 24-hour period after soman exposure (initial SE + recurring seizures) was significantly lower in rats administered UBP302 at 1 hour (203.6 ± 4.4 minutes, n = 8; P < 0.001; Fig. 1C, right set of bars) or 2 hours (237 ± 3.2 minutes, n = 4; P = 0.003; Fig. 1D, right set of bars) after exposure, compared with the soman group (655.2 ± 36.9 minutes, n = 4). By contrast, rats treated with DZP at 1 hour after exposure had similar total duration of SE (707.4 ± 62.5 minutes, n = 6) to the untreated soman rats (Fig. 1C, right set of bars), whereas in rats treated with DZP at 2 hours postexposure, the total duration of SE (879 ± 59.2 minutes, n = 4) was significantly longer than that in the soman group (P < 0.05; Fig. 1D, right set of bars). The total duration of SE in the soman + UBP302 group was also significantly less than in the soman + DZP group (P < 0.01).
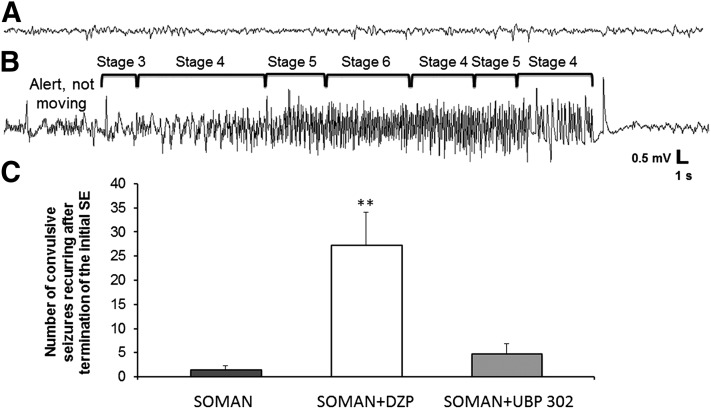
Using the video-EEG recording system, we also counted the number of convulsive seizures that recurred after termination of the initial SE and within the remaining time of the 24-hour period after the exposure, in the soman rats and in the rats that received anticonvulsant treatment at 1 hour after soman exposure. After the prolonged SE in the soman group, the number of recurring convulsive seizures was very low (1.5 ± 0.86, n = 4; Fig. 2). The number of recurring convulsive seizures in the DZP-treated group (27.2 ± 6.89, n = 6) was significantly greater than in the UBP302-treated group (4.72 ± 2.10, n = 8; P < 0.01; Fig. 2).
Fig. 2.
The number of convulsive seizures that recurred in the DZP-treated rats after termination of the initial SE was greater than in the UBP302-treated rats. (A) EEG baseline before soman exposure. (B) Representative recording of a convulsive seizure recurring after termination of the initial SE by DZP, and its correspondence with the behavioral seizure observations. (C) Number of convulsive seizures that occurred after cessation of the initial SE, within the remaining time of the 24-hour period after soman exposure (soman: n = 4; soman + DZP: n = 6; soman + UBP302: n = 8). **P < 0.01, significantly higher compared with the soman + UBP302 group and the soman group (analysis of variance followed by Holm–Sidak post hoc test).
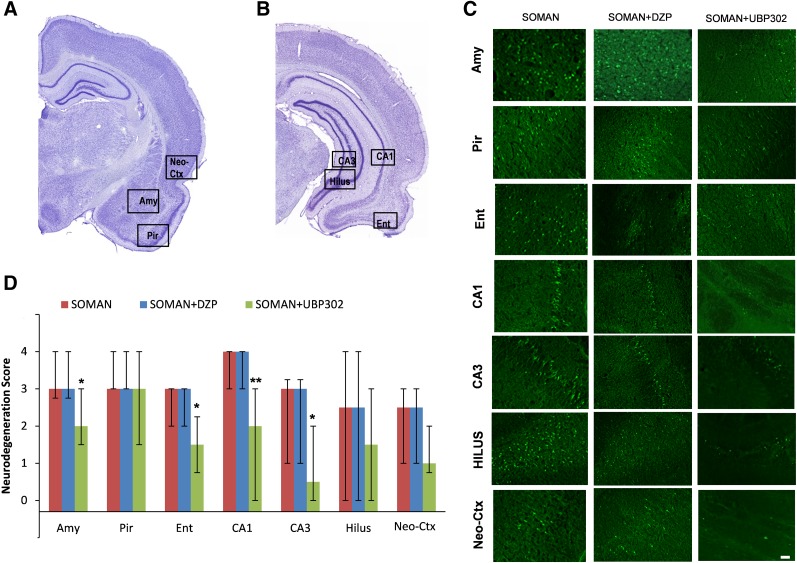
Neuronal Loss and Degeneration—1 Day after Soman Administration.
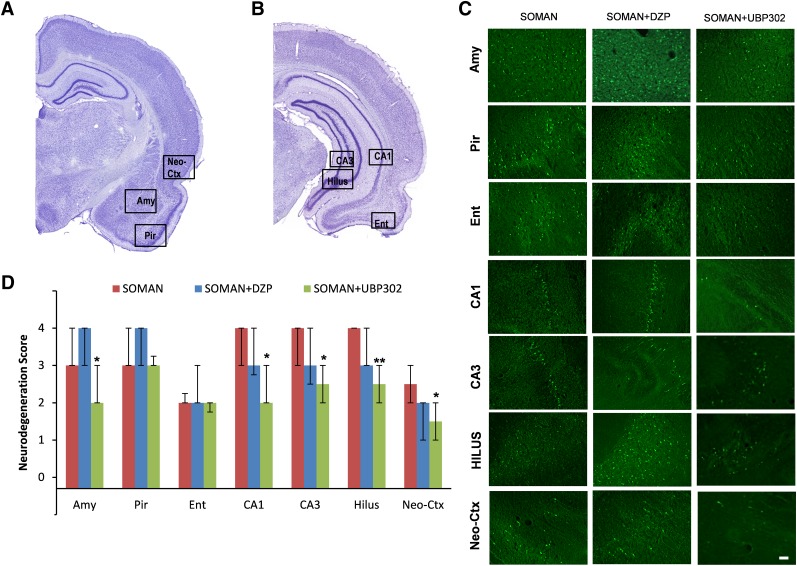
Neuropathological analysis was performed in the soman group and in the groups that received DZP or UBP302 at 1 hour after soman exposure; neuronal loss was determined based on comparisons with the control group. The neurodegeneration score for the amygdala was moderate in the soman group [median = 3 (IQR, 3–4), n = 6], severe in the soman + DZP group [median = 4 (IQR, 3–4), n = 6], and mild in the soman + UBP302 group [median = 2 (IQR, 2–3), n = 6; Fig. 3]. Neuronal degeneration in the CA1, CA3, and hilar regions of the ventral hippocampus was severe in the soman group [CA1: median = 4 (IQR, 3–4); CA3: median = 4 (IQR, 3–4); hilus: median = 4 (IQR, 4–4)], moderate in the soman + DZP group [CA1: median = 3 (IQR, 2.75–4); CA3: median = 3 (IQR, 2.5–4); hilus: median = 3 (IQR, 3–4)], and mild to moderate in the soman + UBP302 group [CA1: median =2 (IQR, 2–3); CA3: median = 2.5 (IQR, 2–3); hilus: median = 2.5 (IQR, 2–3)]. The neurodegeneration score for the neocortex was mild in the soman group [median = 2.5 (IQR, 2–3)] and the soman + DZP group [median = 2 (IQR, 1–2), and minimal to mild in the soman + UBP302 group [median = 1.5 (IQR, 1–2)]. The neuronal degeneration in the amygdala, ventral hippocampus, and neocortex of the soman + UBP302 group was significantly less extensive compared with the soman group (P = 0.026 for the amygdala, P = 0.041 for the CA1 area, P = 0.015 for the CA3 area, P = 0.002 for the hilus, P = 0.026 for the cortex; Fig. 3). By contrast, there were no significant differences between the soman + DZP group and the soman group in the neurodegeneration scores of the amygdala, hippocampus, and neocortex. In addition, neurodegeneration scores for the piriform cortex and entorhinal cortex did not differ significantly among the soman group [piriform cortex: median = 3 (IQR, 3–4); entorhinal cortex: median = 2 (IQR, 2–2.25)], the soman + DZP group [piriform cortex: median = 4 (IQR, 3–4); entorhinal cortex: median = 2 (IQR, 2–3)], and the soman + UBP302 group [piriform cortex: median = 3 (IQR, 3–3.25); entorhinal cortex: median = 2 (IQR, 1.75–2)]. The control group did not show any FJC-positive staining.
Fig. 3.
UBP302, but not DZP, administered 1 hour after soman exposure, reduced neuronal degeneration in the amygdala, hippocampus, and neocortex 1 day after the exposure. (A and B) Panoramic photomicrographs of Nissl-stained sections showing the brain regions evaluated by FJC staining. (C) Representative photomicrographs of FJC-stained sections from the brain regions where neuronal degeneration was evaluated, for the soman, soman + DZP, and soman + UBP302 groups. (D) Neuropathology scores (median and IQR) for the soman, soman + DZP, and soman + UBP groups (n = 6 for each group) for the amygdala (Amy), piriform cortex (Pir), entorhinal cortex (Ent), CA1, CA3, and hilar areas of the ventral hippocampus (HILUS), and neocortex (Neo-Ctx). *P < 0.05; **P < 0.01, compared with the soman group (Mann–Whitney U test). Original magnification, 100×. Bar, 50 μm.
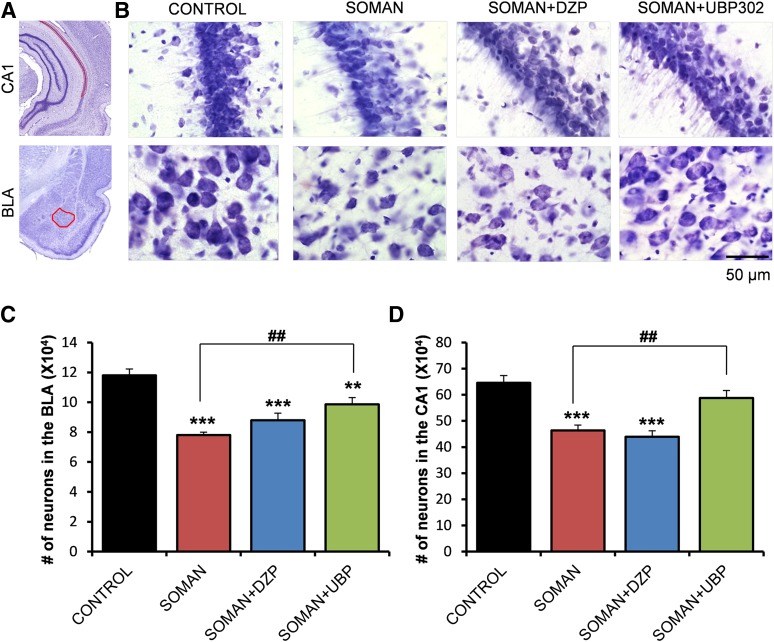
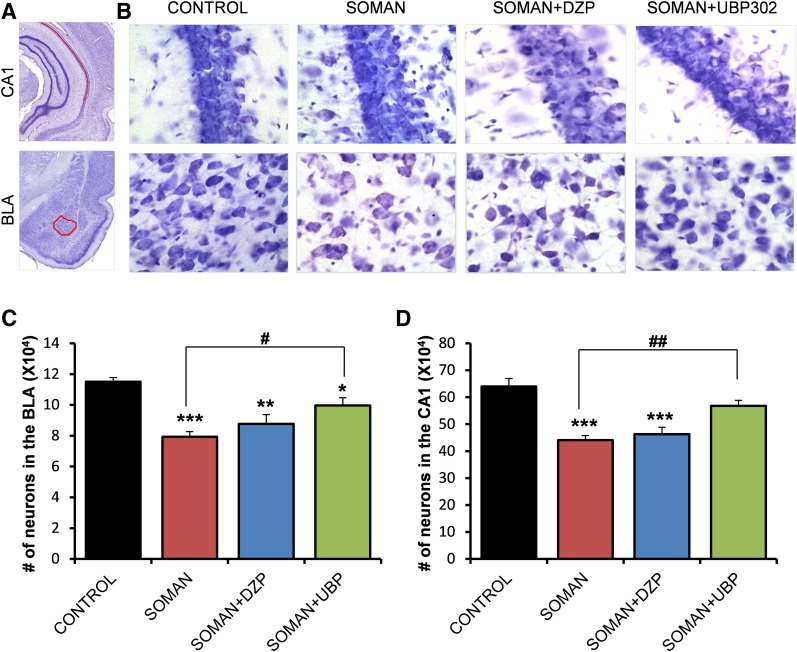
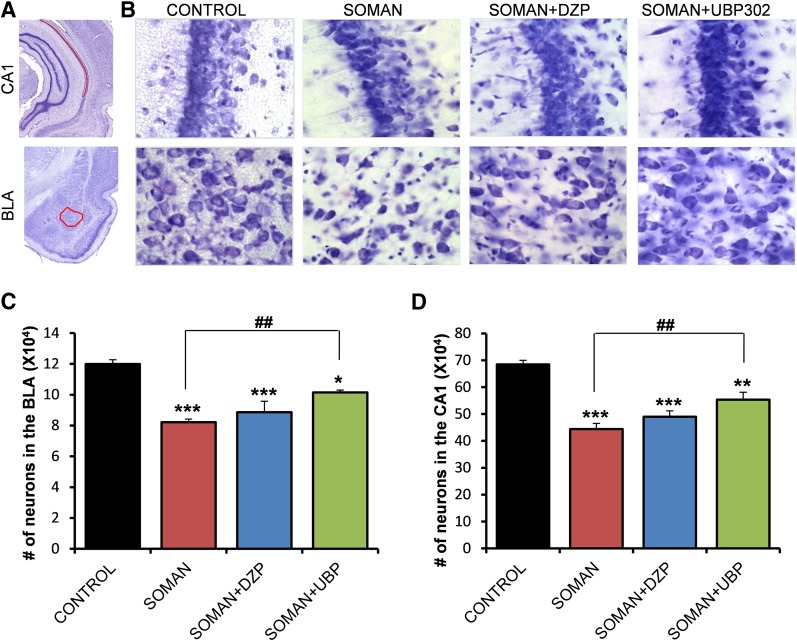
The total number of neurons in the BLA and the CA1 hippocampal area was estimated using an unbiased stereological method in Nissl-stained sections. The number of neurons in the soman group (BLA: 78,027 ± 1939; CA1: 463,976 ± 19,972; n = 6) was significantly lower than the number of neurons in the control group (BLA: 118,041 ± 4281; CA1: 645,450 ± 28,232; n = 6), in both brain regions (P < 0.001; Fig. 4). The number of neurons in the soman + DZP group (BLA: 87,893 ± 4814; CA1: 439,186 ± 23,211; n = 6) did not differ significantly from that in the soman group (P = 0.23 and P = 0.83 for the BLA and CA1, respectively; Fig. 4). In the soman + UBP302 group, the number of neurons in the BLA (98,648 ± 4513, n = 6) was significantly lower than in the controls (P = 0.008), but also significantly higher than in the soman group (P = 0.005; Fig. 4C). The number of neurons in the CA1 area of the soman + UBP302 group (588,100 ± 28,012, n = 6) did not differ from the control group (P = 0.276) and was significantly higher than in the soman group (P = 0.006; Fig. 4D).
Fig. 4.
UBP302, but not DZP, administered 1 hour after soman exposure, reduced neuronal loss in the BLA and the CA1 hippocampal area, 1 day after the exposure. (A) Panoramic photomicrographs of Nissl-stained half hemispheres outlining the amygdalar nucleus and the hippocampal subfield where stereological analysis was performed. (B) Representative photomicrographs of Nissl-stained sections showing BLA and CA1 cells from the control, soman, soman + DZP, and soman + UBP302 groups. (C and D) Group data (mean and S.E.; n = 6 for each group) of stereological estimation of the total number of Nissl-stained neurons in the BLA (left) and CA1 area (right). **P < 0.01; ***P < 0.001 in comparison with control; ##P < 0.01 in comparison with the soman group (analysis of variance, Dunnett post hoc test). Original magnification, 630×. Bar, 50 μm.
Neuronal Loss and Degeneration—7 Days after Soman Administration.
The neurodegeneration results at 7 days after soman exposure are shown in Fig. 5. The soman group (n = 6) had moderate to severe neurodegeneration in the amygdala [median = 3 (IQR, 2.75–4)], piriform cortex [median = 3 (IQR, 3–4)], entorhinal cortex [median = 3 (IQR, 2–3)], CA1 hippocampal area [median = 4 (IQR, 3–4)], and CA3 hippocampal area [median = 3 (IQR, 1–3.5)], and mild to moderate in the hilus [median = 2.5 (IQR, 0–4)] and neocortex [median = 2.5; (IQR, 1–3)]. Similarly, in the soman + DZP group (n = 6), neurodegeneration was moderate to severe in the amygdala [median = 3 (IQR, 0.75–3.25)], piriform cortex [median = 3.5; (IQR, 1.75–4)], entorhinal cortex [median = 3 (IQR, 1.5–3)], CA1 hippocampal area [median = 3.5; (IQR, 2.5–4)], and CA3 hippocampal area [median = 3 (IQR, 0.75–3.25)], and mild to moderate in the hilus [median = 2.5 (IQR, 1.75–3.25)] and neocortex [median = 2 (IQR, 1–3)]. In the soman + UBP302 group (n = 6), neurodegeneration was minimal to mild in the entorhinal cortex [median = 1.5 (IQR, 0.75–2.25)], CA3 hippocampal area [median = 0.5 (IQR, 0–2)], hilus [median = 1.5 (IQR, 0–3)], and neocortex [median = 1 (IQR, 0.75–2)], mild to moderate in the amygdala [median = 2 (IQR, 1.5–3)] and CA1 area [median = 2 (IQR, 0–3)], and moderate to severe in the piriform cortex [median = 3 (IQR, 1.5–4)]. Compared with the soman group, neurodegeneration in the soman + UBP302 group was significantly lower in the amygdala (P = 0.041), entorhinal cortex (P = 0.041), CA1 (P = 0.009), and CA3 (P = 0.041) hippocampal areas.
Fig. 5.
UBP302, but not DZP, administered 1 hour after soman exposure, reduced neuronal degeneration in the amygdala, CA1, and CA3 dorsal hippocampal areas, and entorhinal cortex, 7 days after the exposure. (A and B) Panoramic photomicrographs of Nissl-stained sections showing the brain regions evaluated by FJC staining. (C) Representative photomicrographs of FJC-stained sections from the brain regions where neuronal degeneration was evaluated, for the soman, soman + DZP, and soman + UBP302 groups. (D) Neuropathology scores (median and IQR) for the soman, soman + DZP, and soman + UBP groups (n = 6 for each group), for the amygdala (Amy), piriform cortex (Pir), entorhinal cortex (Ent), CA1, CA3, and hilar areas of the ventral hippocampus (HILUS), and neocortex (Neo-Ctx). *P < 0.05; **P < 0.01, compared with the soman group (Mann–Whitney U test). Original magnification, 100×. Bar, 50 μm.
The total number of neurons in the BLA and the CA1 hippocampal area was estimated 7 days after soman exposure; the results are shown in Fig. 6. The number of neurons in the soman group (BLA: 79,394 ± 3287; CA1: 440,698 ± 17,159; n = 6) was again significantly lower than the number of neurons in the control group (BLA: 115,108 ± 2,673; CA1: 639,701 ± 29,350; n = 6), in both brain regions (P < 0.001). The number of neurons in the soman + DZP group (BLA: 87,729 ± 6032; CA1: 463,196 ± 25,729; n = 6) did not differ significantly from those in the soman group (P = 0.424 and P = 0.841 for the BLA and CA1, respectively). The number of neurons in the soman + UBP302 group (BLA: 99,678 ± 4,947; CA1: 568,098 ± 20,386; n = 6) was significantly higher than in the soman group (P = 0.011 for the BLA and P = 0.003 for the CA1), but differed from the control group only in the BLA.
Fig. 6.
UBP302, but not DZP, administered 1 hour after soman exposure, reduced neuronal loss in the BLA and the CA1 hippocampal area, 7 days after the exposure. (A) Panoramic photomicrographs of Nissl-stained half hemispheres outlining the amygdalar nucleus and the hippocampal subfield where stereological analysis was performed. (B) Representative photomicrographs of Nissl-stained sections showing BLA and CA1 cells from the control, soman, soman + DZP, and soman + UBP302 groups. (C and D) Group data (mean and standard error; n = 6 for each group) of stereological estimation of the total number of Nissl-stained neurons in the BLA (left) and CA1 area (right). *P < 0.05; **P < 0.01; ***P < 0.001, compared with the control; #P < 0.05; ##P < 0.01, compared with the soman group (analysis of variance, Dunnett post hoc test). Original magnification, 630×. Bar, 50 μm.
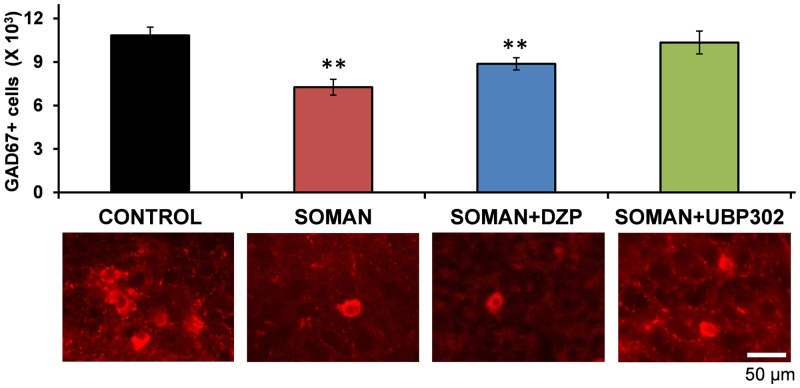
We previously showed that the number of GABAergic interneurons in the BLA is not altered 1 day after soman exposure, but it is significantly reduced 7 days after the exposure (Figueiredo et al., 2011; Prager et al., 2014). Therefore, we examined whether DZP or UBP302 protected against GABAergic interneuronal loss. Stereological estimation of GAD-67+ neurons in the BLA showed that compared with the control group (10,837 ± 565, n = 6), there was a significantly lower number of GABAergic interneurons in both the soman (7260 ± 367, n = 6; P = 0.001) and the soman + DZP (8875 ± 293, n = 6; P = 0.012) groups. By contrast, the number of GABAergic interneurons in the soman + UBP302 group (10,345 ± 633, n = 6) did not differ from the control group (P = 0.9; Fig. 7).
Fig. 7.
UBP302, but not DZP, administered 1 hour after soman exposure, prevented GABAergic interneuronal loss in the BLA, 7 days after the exposure. Group data (mean and standard error; n = 6 for the each group) of stereological estimation of the total number of GAD-67+ neurons in the BLA for the control, soman, soman + DZP, and soman + UBP302 groups. Representative photomicrographs of GAD-67+ interneurons are shown in the lower panel. **P < 0.01, compared with the control (analysis of variance, Dunnett post hoc test). Original magnification, 630×. Bar, 50 μm.
Neuronal Loss and Degeneration—30 Days after Soman Administration.
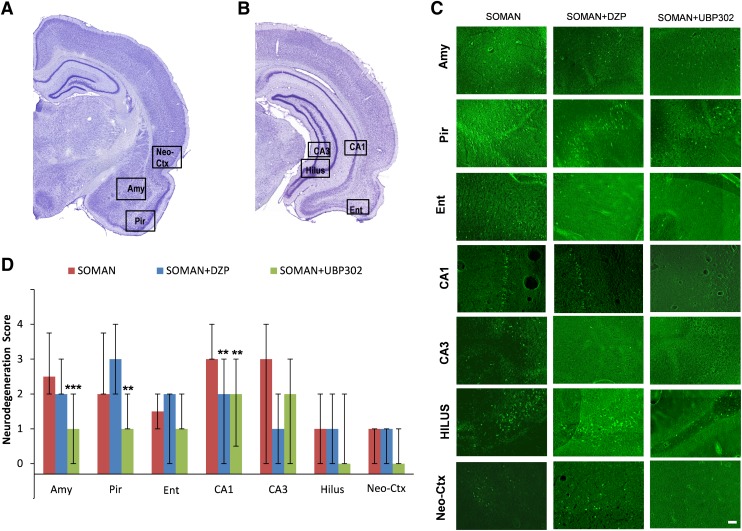
Thirty days after soman exposure, the analysis of ongoing neuronal degeneration showed that neurodegeneration in the amygdala was mild to moderate in the soman group [median = 2.5 (IQR, 2–3.75), n = 6] and the soman + DZP group [median = 2 (IQR, 2–3), n = 6], but only minimal in the soman + UBP302 [median = 1 (IQR, 0–2), n = 6; P < 0.001 compared with soman; Fig. 8). In the piriform cortex, neurodegeneration was mild to moderate in the soman group [median = 2 (IQR, 2–3.75)] and the soman + DZP group [median = 3 (IQR, 2–4)], but only minimal in the soman + UBP302 group [median = 1 (IQR, 1–2); P = 0.002 compared with soman; Fig. 8]. In the CA1 hippocampal area, neurodegeneration was moderate in the soman group [median = 3 (IQR, 3–4)], and mild in the soman + DZP group [median = 2 (IQR, 0–3); P = 0.002 compared with soman] and the soman + UBP302 groups [median = 2 (IQR, 0.5–3); P = 0.001]. There were no significant differences between the three groups in the neurodegeneration scores for the entorhinal cortex [soman group: median = 1.5 (IQR, 1–2); soman + DZP group: median = 2 (IQR, 0–2); soman + UBP302 group: median = 1 (IQR, 1–2)], CA3 hippocampal area [soman group: median = 3 (IQR, 0–3); soman + DZP group: median = 1 (IQR, 1–3); soman + UBP302 group: median = 2 (IQR, approximately 3)], the hilus [soman and soman + DZP groups: median = 1 (IQR, 0–2); soman + UBP302 group: median = 0 (IQR, 0–2)], and the neocortex [soman and soman + DZP groups: median = 1 (IQR, 0–1); soman + UBP302 group: median = 0 (IQR, 0–1)].
Fig. 8.
UBP302, administered 1 hour after soman exposure, reduces neuronal degeneration in the amygdala, piriform cortex, and CA1 hippocampal area, whereas DZP reduces neurodegeneration in the CA1 area, 30 days after the exposure. (A and B) Panoramic photomicrographs of Nissl-stained sections showing the brain regions evaluated by FJC staining. (C) Representative photomicrographs of FJC-stained sections from the brain regions where neuronal degeneration was evaluated, for the soman, soman + DZP, and soman + UBP302 groups. (D) Neuropathology scores (median and IQR) for the soman, soman + DZP, and soman + UBP groups (n = 6 for each group), for the amygdala (Amy), piriform cortex (Pir), entorhinal cortex (Ent), CA1, CA3, and hilar areas of the dorsal hippocampus (HILUS), and neocortex (Neo-Ctx). **P < 0.01; ***P < 0.001, compared with the soman group (Mann–Whitney U test). Original magnification, 100×. Bar, 50 μm.
Thirty days after soman exposure, neuronal loss was still present in the soman group (n = 6), in which the total number of neurons (BLA: 82,119 ± 2099; CA1: 444,356 ± 21,045) was significantly lower (P < 0.001) than in the control group (BLA: 119,860 ± 2898; CA1: 684,738 ± 15,340; n = 6; Fig. 9). In the soman + UBP302 group (n = 6), the total number of neurons (BLA: 101,412 ± 1535; CA1, 553,583 ± 27,489) was significantly lower than in the control group, both in the BLA (P = 0.012; Fig. 9C) and in the CA1 area (P = 0.006; Fig. 9D), but was also significantly higher than in the soman group (P = 0.001). The number of neurons in the soman + DZP group (BLA: 88,591 ± 7134; CA1: 463,211 ± 21,536; n = 6) was significantly lower than the control group (P < 0.001), and did not differ from the number of neurons in the soman group (P = 0.55 and P = 0.34 for the BLA and the CA1 area, respectively; Fig. 9).
Fig. 9.
UBP302, but not DZP, administered 1 hour after soman exposure, reduced neuronal loss in the BLA and the CA1 hippocampal area, 30 days after the exposure. (A) Panoramic photomicrographs of Nissl-stained half hemispheres outlining the amygdalar nucleus and the hippocampal subfield where stereological analysis was performed. (B) Representative photomicrographs of Nissl-stained sections showing BLA and CA1 cells from the control, soman, soman + DZP, and soman + UBP302 groups. (C and D) Group data (mean and standard error; n = 6 for each group) of stereological estimation of the total number of Nissl-stained neurons in the BLA (left) and CA1 area (right). *P < 0.05; **P < 0.01; ***P < 0.001, compared with the control; ##P < 0.01, compared with the soman group (analysis of variance, Dunnett post hoc test). Original magnification, 630×. Bar, 50 μm.
Behavioral Alterations—30 Days after Soman Administration.
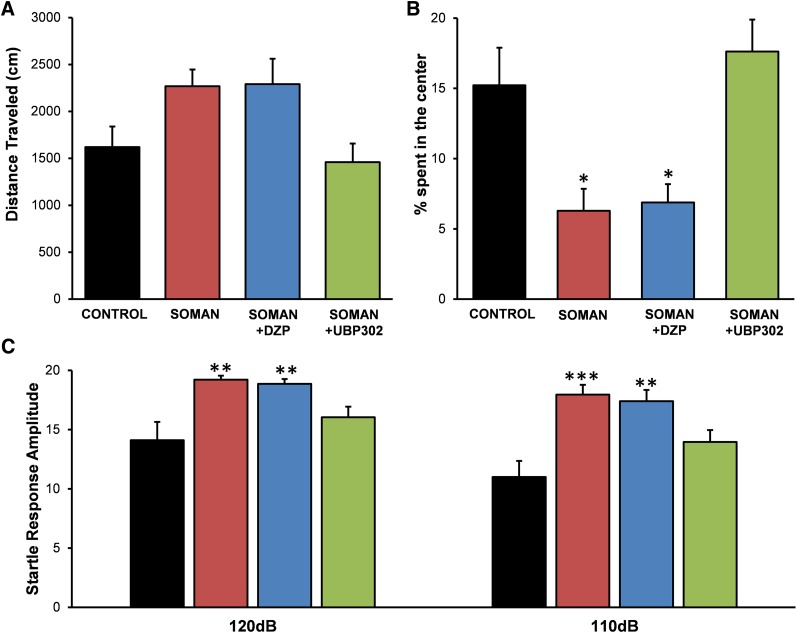
To investigate whether the long-term neuropathology observed 30 days after soman exposure had translated into behavioral deficits, we examined anxiety-like behavior in all experimental groups, using the open field and the ASR tests. The distance traveled in the open field by the groups exposed to soman (soman: 2270 ± 265 cm, n = 10; soman + DZP: 2290 ± 235 cm, n = 9; soman + UBP302: 1329 ± 279 cm, n = 12) was not significantly different from that in the control group (1620 ± 219 cm, n = 8; Fig. 10A). However, both the soman and the soman + DZP groups spent significantly less time in the center of the open field (soman group, 6.28% ± 1.56% of the total movement time, n = 10, P = 0.034; soman + DZP group, 6.88% ± 1.29% of the total movement time, n = 9, P = 0.027) compared with the time spent by the control rats (15.22% ± 2.67% of the total movement time, n = 8). The soman + UBP302 rats spent 17.61% ± 2.27% (n = 12) of the total movement time in the center of the open field, which was not different from the control group (P = 0.73; Fig. 10B).
Fig. 10.
UBP302, but not DZP, administered 1 hour after soman exposure, protected against the development of anxiety, 30 days after the exposure. (A) Distance traveled (mean ± standard error) in the open field. (B) Percentage of time spent in the center of the open field. (C) Amplitude of startle responses to 120- and 110-db acoustic stimuli (control: n = 8; soman: n = 10; soman + DZP: n = 9; soman + UBP302: n = 12). *P < 0.05; **P < 0.01; ***P < 0.001 (analysis of variance, Dunnett post hoc comparison with control).
In the ASR test, there was a significant increase in startle amplitude in the soman (110 db: 17.94 ± 0.83, n = 10, P < 0.001; 120 db: 19.20 ± 0.35, P = 0.001) and the soman + DZP (110 db: 17.38 ± 0.95, n = 9, P = 0.001; 120 db: 18.85 ± 0.41, P = 0.003) groups compared with the control group (110 db: 10.99 ± 1.35; 120 db: 14.09 ± 1.54, n = 8; Fig. 10C). Startle response amplitude in the soman + UBP302 group (110 db: 13.95 ± 0.99; 120 db: 16.03 ± 0.91, n = 12) did not differ from the control group (P = 0.123 and P = 0.291 for 110 and 120 db, respectively; Fig. 10C).
Discussion
Seizures induced by exposure to nerve agents require medical intervention, otherwise they can lead to severe brain damage or death. Administration of DZP is the current Food and Drug Administration–approved treatment of nerve agent–induced seizures. This study showed that if DZP is administered to soman-exposed rats at 1 hour postexposure, seizures are terminated effectively, but they soon return, resulting in a total duration of SE within 24 hours after exposure that is no different from the total SE duration in the soman-exposed rats that do not receive anticonvulsant treatment. Moreover, if DZP is administered at 2 hours after soman exposure, the total duration of SE in the DZP-treated rats is longer than in the rats that do not receive anticonvulsant treatment. The consequences of the return of seizures after DZP treatment were evident in the neuropathology analysis and the behavioral tests. Thus, DZP treatment provided no protection against neuronal degeneration and death, except for a lower number of degenerating neurons in the CA1 hippocampal area, 30 days after the exposure. By contrast, treatment with the GluK1 antagonist UBP302, which reduced the total duration of SE within 24 hours postexposure, protected against neuronal damage in most of the brain regions examined. The anxiety tests also revealed that UBP302, but not DZP treatment, prevented an increase in anxiety-like behavior, 30 days after soman exposure.
The Disadvantages of DZP Treatment as an Anticonvulsant.
From the clinical experience and studies in human patients, as well as from experimental studies in animals, the efficacy of benzodiazepines in terminating SE has long been recognized to decrease as the latency between the initiation of SE and the time point of drug administration increases (Walton and Treiman, 1988; Shorvon, 2001; Jones et al., 2002; Mayer et al., 2002; Goodkin et al., 2003; Naylor et al., 2005; Deeb et al., 2012). This is also the case with SE induced by nerve agents. Thus, in rats, Shih et al. (1999) found that DZP was very effective in stopping seizures when administered 5 minutes after the onset of soman-induced SE, but was virtually ineffective when administration was delayed to 40 minutes after seizure initiation. The mechanisms underlying the development of refractoriness to benzodiazepines are not fully understood, but may involve internalization and downregulation of GABAA receptors (Goodkin et al., 2005; Naylor et al., 2005; Deeb et al., 2012), or other dysfunctions in GABAergic synaptic transmission that manifest as the seizures progress. At least in hippocampal neurons, Goodkin et al. (2005) demonstrated that GABAergic inhibition displays a high degree of plasticity, in that, during prolonged epileptiform bursting, the rate of GABA receptor internalization increases rapidly, and the subunit composition of these receptors swiftly changes (as suggested by the changes in the kinetics of miniature inhibitory postsynaptic currents), perhaps due to movement of extrasynaptic GABAA receptors onto synaptic sites. Therefore, when DZP is administered at delayed time points, the function of the GABAergic system is already compromised; as a result, DZP may be ineffective. Nevertheless, the available evidence suggests that the progressive refractoriness to benzodiazepines is not an absolute phenomenon and can be partially overcome depending on the animal model used to induce SE, the dose of DZP, or other factors. For example, in a guinea pig model of nerve agent exposure, 10 mg/kg DZP was effective against soman-induced seizures in a sizeable proportion of the animals, even when administration was delayed beyond 1 hour after seizure onset, although its efficacy was still clearly reduced as the latency of administration increased (McDonough et al., 2010). In addition, kainic acid–induced SE can be stopped by 25 mg/kg DZP even when the drug is administered 3 hours after the onset of seizures (Qashu et al., 2010). In this study, 10 mg/kg DZP terminated SE at either the 1-hour or the 2-hour time point of administration.
Considering the above, one would conclude that DZP remains an effective anticonvulsant, but it is desirable that it be used at early time points after seizure initiation for greater effectiveness. However, the recurrence of intense seizures after cessation of the initial SE by DZP as well as the impact that such recurrence of SE has on the neuroprotective efficacy of DZP have not received enough attention. Shih et al. (1999) reported that 25% of the animals receiving 9 mg/kg DZP at 5 minutes after the onset of soman-induced seizures had seizures recurring within the 6-hour monitoring period. In this study, seizures recurred in all of the soman-exposed rats that received DZP, making the total duration of seizures within the 24-hour period of video-EEG monitoring no different from (Fig. 1C) or even longer than (Fig. 1D) in the soman-exposed rats that did not receive anticonvulsant treatment. The mechanisms underlying the recurrence of seizures after cessation of the initial SE by DZP probably involve a severely compromised GABAergic system, in combination with the pharmacokinetics of the drug. Thus, intense seizure activity impairs the function of GABAergic inhibition (Goodkin et al., 2005; Naylor et al., 2005; Deeb et al., 2012). The brain is then exposed to DZP, which enhances GABAA receptor activity and thereby stops the SE temporarily but may also contribute to more desensitization and downregulation of GABAA receptors (Uusi-Oukari and Korpi, 2010; Vinkers and Olivier, 2012), further impairing GABAergic synaptic transmission. As DZP is rapidly cleared from the animal’s system (Ramsay et al., 1979), the brain remains with severely compromised GABAergic inhibition, and thus neuronal networks intermittently enter into intense seizure activity every time the unopposed glutamatergic activity intensifies.
Targeting the GluK1Rs to Control Seizures.
Although inhibition progressively weakens during SE, glutamatergic excitation is reinforced, probably not only because of disinhibition. During SE, there is upregulation of AMPA receptors (Chen et al., 2007; Rajasekaran et al., 2013), as well as enhanced expression of the GluK1 subunit (Ullal et al., 2005), which could significantly contribute to worsening hyperexcitability and excitotoxicity. Therefore, suppressing glutamatergic hyperactivity can be a more effective way to control seizures, particularly if immediate treatment is not possible and the anticonvulsant is administered with a delay after seizure onset. Indeed, we previously showed that the GluK1/AMPA receptor antagonist LY293558, administered 1 hour after soman exposure, stops seizures and fully protects against neuronal damage (Figueiredo et al., 2011; Apland et al., 2013). In this study, we tested UBP302, which is selective for the GluK1 receptors (More et al., 2004). It is uncertain at what concentrations UBP302 reached the brain and whether other glutamatergic receptors were also affected; however, this compound also suppresses soman-induced seizure-like activity in vitro at relatively low concentrations that are considered selective for GluK1 antagonism (Apland et al., 2009). UBP302 blocked the seizures induced by soman, and protected against neuropathology and anxiety-related behavioral deficits.
The time course of termination of the initial SE by UBP302 was slow compared with that of DZP or LY293558. Although this may have to do with the type of receptors being targeted and their involvement in seizure activity, it is also possible that it relates to the pharmacokinetics of UBP302, which is not known. However, despite the apparently slow time course of action of UBP302, the total duration of SE within the 24-hour period after exposure was dramatically reduced in the UBP302-treated rats compared with either the soman-exposed rats that did not receive anticonvulsant treatment or the rats that received DZP. The mechanisms by which a GluK1 antagonist may suppress hyperexcitability and stop seizures were previously discussed (Figueiredo et al., 2011; Aroniadou-Anderjaska et al., 2012). GluK1Rs modulate both GABAergic and glutamatergic synaptic transmission in a number of brain regions (Jane et al., 2009). At least in the BLA and the hippocampus, the net effect of their activation is excitatory. This is suggested by the findings that GluK1R antagonists block epileptiform activity in hippocampal slices and limbic seizures in vivo (Smolders et al., 2002). In the BLA, GluK1Rs are present on postsynaptic (somatodendritic) sites of both principal cells (Gryder and Rogawski, 2003) and interneurons (Braga et al., 2003), as well as on the presynaptic terminals of both cell types, where they mediate facilitation of glutamate release (Aroniadou-Anderjaska et al., 2012) and either facilitation or inhibition of GABA release, depending on the concentration of the agonist (Braga et al., 2003). The net effect of their activation in the BLA network is an increased excitation and excitability, as suggested by a greater enhancement of spontaneous EPSCs versus inhibitory postsynaptic currents, the reduction of anxiety-like behavior when these receptors are blocked by microinjection of UBP302 selectively into the BLA, and the increased anxiety or the induction of seizures when a GluK1R agonist is injected into the BLA (Aroniadou-Anderjaska et al., 2012). Although it remains to be determined what role GluK1Rs play in the overall excitation of other neuronal networks, the fact that activation of these receptors increases the excitation state of the BLA and the hippocampus, two highly seizurogenic brain regions, may explain why blockade of these receptors can terminate seizures. It should also be noted that in contrast with the downregulation and internalization of GABAA receptors after excessive neuronal/seizure activity (Goodkin et al., 2005; Naylor et al., 2005; Deeb et al., 2012), which can render DZP ineffective, the upregulation of the GluK1 subunit (Ullal et al., 2005) may contribute to hyperexcitation, which further explains the efficacy of GluK1R antagonists.
Neuropathology and Associated Behavioral Deficits.
Depending on the extent of brain damage after prolonged SE, behavioral deficits may ensue. Increased anxiety is observed in animals that have been exposed to nerve agents (Coubard et al., 2008; Langston et al., 2012; Prager et al., 2014). Similarly, the human victims of the sarin terrorist attack in Japan report enduring symptoms of anxiety disorders, long after the exposure (Ohtani et al., 2004; Hoffman et al., 2007). The amygdala plays a central role in emotional behavior, and dysfunction with increased excitability of the BLA is associated with anxiety (Gonzalez et al., 1996; Shekhar et al., 2003; Zhou et al., 2010; Pidoplichko et al., 2014). The hippocampus is also significantly involved in the modulation of anxiety (Engin and Treit, 2007; Fournier and Duman, 2013). Both the amygdala and the hippocampus are severely damaged by nerve agent–induced SE, as shown in this study and previous studies (Shih et al., 2003; Aroniadou-Anderjaska et al., 2009; Apland et al., 2010). In the BLA, there is significant loss of GABAergic neurons by day 7 after exposure to soman (Figueiredo et al., 2011; Prager et al., 2014), and at 14 and 30 days after exposure the ratio of GABAergic interneurons to the total number of neurons in the BLA is significantly decreased (Prager et al., 2014). Reduction of inhibitory activity in the BLA, resulting from the interneuronal loss, will increase the excitability of the BLA network, which can lead to the development of anxiety.
In this study, treatment with UBP302 significantly reduced neuronal loss and degeneration in a number of brain regions, including the hippocampus and the amygdala, and prevented the loss of GABAergic interneurons in the BLA, as assessed 30 days after the exposure. The neuroprotection provided by UBP302, despite its being only partial, was sufficient to prevent the development of anxiety-like behavior, as assessed in the open field and the ASR tests. By contrast, DZP had no neuroprotective effects, except for reduced neurodegeneration in the CA1 area at 30 days after exposure, and did not prevent the development of anxiety. Since the extent of nerve agent–induced neuropathology is solely or largely determined by the intensity and duration of seizures (Shih et al., 2003; Prager et al., 2013), the failure of DZP to protect the brain can be attributed to the failure of this drug to reduce the total duration of seizures. Consistent with our findings revealing the inefficacy of DZP to reduce neuropathology and prevent behavioral deficits, previous studies in which 10 mg/kg DZP was administered at 30 minutes after soman-induced seizures show that this treatment did not prevent the development of anxiety-like behavior (Langston et al., 2012) or epileptogenesis (de Araujo Furtado et al., 2010).
Our data clearly argue against the use of DZP as a medical countermeasure for the treatment of SE induced by nerve agent exposure, at least when anticonvulsant treatment is delayed. DZP is likely to substantially increase the survival rate of exposed individuals; however, because seizures recur, there is virtually no protection against neuropathology and the resulting behavioral deficits. Administering DZP repeatedly, every time intense seizures recur, not only may not be feasible, but may also be detrimental to the victims’ health. The results suggest that targeting the glutamatergic system is a more effective approach to controlling nerve agent–induced SE, and antagonists of the GluK1Rs appear to be both safe and efficacious in this regard.
Abbreviations
- AChE
acetylcholinesterase
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- ASR
acoustic startle response
- BLA
basolateral amygdala
- CE
coefficient of error
- dH2O
distilled water
- DZP
diazepam
- EEG
electroencephalography
- FJC
Fluoro-Jade C
- GAD-67
glutamic acid decarboxylase-67
- GluK1R
kainate receptor containing the GluK1 subunit
- HI-6
1-(2-hydroxyiminomethylpyridinium)-3-(4-carbamoylpyridinium)-2-oxapropane dichloride
- IQR
interquartile range
- LY293558
(3S,4aR,6R,8aR)-6-[2-(1(2)H-tetrazole-5-yl)ethyl]decahydroisoquinoline-3-carboxylic acid
- PBS
phosphate-buffered saline
- SE
status epilepticus
- UBP302
(S)-3-(2-carboxybenzyl)willardiine
Authorship Contributions
Participated in research design: Apland, Aroniadou-Anderjaska, Braga.
Conducted experiments: Apland, Figueiredo, Rossetti, Miller.
Performed data analysis: Figueiredo, Apland, Rossetti, Aroniadou-Anderjaska, Braga.
Wrote or contributed to the writing of the manuscript: Aroniadou-Anderjaska, Apland, Figueiredo, Miller, Braga.
Footnotes
This work was supported by the National Institutes of Health Office of the Director and the National Institutes of Health National Institute of Neurologic Disorders and Stroke CounterACT Program [Grant 5U01-NS058162-07]. The views expressed in this article are those of the authors and do not reflect the official policy of the Department of the Army, the Department of Defense, or the US Government.
References
- Apland JP, Aroniadou-Anderjaska V, Braga MF. (2009) Soman induces ictogenesis in the amygdala and interictal activity in the hippocampus that are blocked by a GluR5 kainate receptor antagonist in vitro. Neuroscience 159:380–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apland JP, Aroniadou-Anderjaska V, Figueiredo TH, Green CE, Swezey R, Yang C, Qashu F, Braga MFM. (2013) Efficacy of the GluK1/AMPA receptor antagonist LY293558 against seizures and neuropathology in a soman-exposure model without pretreatment and its pharmacokinetics after intramuscular administration. J Pharmacol Exp Ther 344:133–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apland JP, Figueiredo TH, Qashu F, Aroniadou-Anderjaska V, Souza AP, Braga MF. (2010) Higher susceptibility of the ventral versus the dorsal hippocampus and the posteroventral versus anterodorsal amygdala to soman-induced neuropathology. Neurotoxicology 31:485–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroniadou-Anderjaska V, Figueiredo TH, Apland JP, Qashu F, Braga MF. (2009) Primary brain targets of nerve agents: the role of the amygdala in comparison to the hippocampus. Neurotoxicology 30:772–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroniadou-Anderjaska V, Pidoplichko VI, Figueiredo TH, Almeida-Suhett CP, Prager EM, Braga MF. (2012) Presynaptic facilitation of glutamate release in the basolateral amygdala: a mechanism for the anxiogenic and seizurogenic function of GluK1 receptors. Neuroscience 221:157–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajgar J. (2005) Complex view on poisoning with nerve agents and organophosphates. Acta Med (Hradec Kralove) 48:3–21 [PubMed] [Google Scholar]
- Braga MF, Aroniadou-Anderjaska V, Xie J, Li H. (2003) Bidirectional modulation of GABA release by presynaptic glutamate receptor 5 kainate receptors in the basolateral amygdala. J Neurosci 23:442–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campo-Soria C, Chang Y, Weiss DS. (2006) Mechanism of action of benzodiazepines on GABAA receptors. Br J Pharmacol 148:984–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JW, Naylor DE, Wasterlain CG. (2007) Advances in the pathophysiology of status epilepticus. Acta Neurol Scand Suppl 186:7–15 [PubMed] [Google Scholar]
- Collingridge GL, Olsen RW, Peters J, Spedding M. (2009) A nomenclature for ligand-gated ion channels. Neuropharmacology 56:2–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coubard S, Béracochéa D, Collombet JM, Philippin JN, Krazem A, Liscia P, Lallement G, Piérard C. (2008) Long-term consequences of soman poisoning in mice: part 2. Emotional behavior. Behav Brain Res 191:95–103 [DOI] [PubMed] [Google Scholar]
- de Araujo Furtado M, Lumley LA, Robison C, Tong LC, Lichtenstein S, Yourick DL. (2010) Spontaneous recurrent seizures after status epilepticus induced by soman in Sprague-Dawley rats. Epilepsia 51:1503–1510 [DOI] [PubMed] [Google Scholar]
- Deeb TZ, Maguire J, Moss SJ. (2012) Possible alterations in GABAA receptor signaling that underlie benzodiazepine-resistant seizures. Epilepsia 53 (Suppl 9):79–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolgin E. (2013) Syrian gas attack reinforces need for better anti-sarin drugs. Nat Med 19:1194–1195 [DOI] [PubMed] [Google Scholar]
- Engin E, Treit D. (2007) The role of hippocampus in anxiety: intracerebral infusion studies. Behav Pharmacol 18:365–374 [DOI] [PubMed] [Google Scholar]
- Faraday MM, Elliott BM, Grunberg NE. (2001) Adult vs. adolescent rats differ in biobehavioral responses to chronic nicotine administration. Pharmacol Biochem Behav 70:475–489 [DOI] [PubMed] [Google Scholar]
- Figueiredo TH, Qashu F, Apland JP, Aroniadou-Anderjaska V, Souza AP, Braga MF. (2011) The GluK1 (GluR5) Kainate/alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor antagonist LY293558 reduces soman-induced seizures and neuropathology. J Pharmacol Exp Ther 336:303–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filliat P, Coubard S, Pierard C, Liscia P, Beracochea D, Four E, Baubichon D, Masqueliez C, Lallement G, Collombet JM. (2007) Long-term behavioral consequences of soman poisoning in mice. Neurotoxicology 28:508–519 [DOI] [PubMed] [Google Scholar]
- Fournier NM, Duman RS. (2013) Illuminating hippocampal control of fear memory and anxiety. Neuron 77:803–806 [DOI] [PubMed] [Google Scholar]
- Gielen MC, Lumb MJ, Smart TG. (2012) Benzodiazepines modulate GABAA receptors by regulating the preactivation step after GABA binding. J Neurosci 32:5707–5715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez LE, Andrews N, File SE. (1996) 5-HT1A and benzodiazepine receptors in the basolateral amygdala modulate anxiety in the social interaction test, but not in the elevated plus-maze. Brain Res 732:145–153 [DOI] [PubMed] [Google Scholar]
- Goodkin HP, Liu X, Holmes GL. (2003) Diazepam terminates brief but not prolonged seizures in young, naïve rats. Epilepsia 44:1109–1112 [DOI] [PubMed] [Google Scholar]
- Goodkin HP, Yeh JL, Kapur J. (2005) Status epilepticus increases the intracellular accumulation of GABAA receptors. J Neurosci 25:5511–5520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryder DS, Rogawski MA. (2003) Selective antagonism of GluR5 kainate-receptor-mediated synaptic currents by topiramate in rat basolateral amygdala neurons. J Neurosci 23:7069–7074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen HJ, Jensen EB, Kiêu K, Nielsen J (1999) The efficiency of systematic sampling in stereology—reconsidered. J Microsc 193:199–211 [DOI] [PubMed] [Google Scholar]
- Hoffman A, Eisenkraft A, Finkelstein A, Schein O, Rotman E, Dushnitsky T. (2007) A decade after the Tokyo sarin attack: a review of neurological follow-up of the victims. Mil Med 172:607–610 [DOI] [PubMed] [Google Scholar]
- Jane DE, Lodge D, Collingridge GL. (2009) Kainate receptors: pharmacology, function and therapeutic potential. Neuropharmacology 56:90–113 [DOI] [PubMed] [Google Scholar]
- Jimmerson VR, Shih TM, Mailman RB. (1989) Variability in soman toxicity in the rat: correlation with biochemical and behavioral measures. Toxicology 57:241–254 [DOI] [PubMed] [Google Scholar]
- Jones DM, Esmaeil N, Maren S, Macdonald RL. (2002) Characterization of pharmacoresistance to benzodiazepines in the rat Li-pilocarpine model of status epilepticus. Epilepsy Res 50:301–312 [DOI] [PubMed] [Google Scholar]
- Kellinghaus C, Stögbauer F. (2012) Treatment of status epilepticus in a large community hospital. Epilepsy Behav 23:235–240 [DOI] [PubMed] [Google Scholar]
- Lallement G, Dorandeu F, Filliat P, Carpentier P, Baille V, Blanchet G. (1998) Medical management of organophosphate-induced seizures. J Physiol Paris 92:369–373 [DOI] [PubMed] [Google Scholar]
- Langston JL, Wright LK, Connis N, Lumley LA. (2012) Characterizing the behavioral effects of nerve agent-induced seizure activity in rats: increased startle reactivity and perseverative behavior. Pharmacol Biochem Behav 100:382–391 [DOI] [PubMed] [Google Scholar]
- Mayer SA, Claassen J, Lokin J, Mendelsohn F, Dennis LJ, Fitzsimmons BF. (2002) Refractory status epilepticus: frequency, risk factors, and impact on outcome. Arch Neurol 59:205–210 [DOI] [PubMed] [Google Scholar]
- McDonough JH, Jr, Shih TM. (1997) Neuropharmacological mechanisms of nerve agent-induced seizure and neuropathology. Neurosci Biobehav Rev 21:559–579 [DOI] [PubMed] [Google Scholar]
- McDonough JH, Jr, McMonagle JD, Shih TM. (2010) Time-dependent reduction in the anticonvulsant effectiveness of diazepam against soman-induced seizures in guinea pigs. Drug Chem Toxicol 33:279–283 [DOI] [PubMed] [Google Scholar]
- Mehta V, Singhi P, Singhi S. (2007) Intravenous sodium valproate versus diazepam infusion for the control of refractory status epilepticus in children: a randomized controlled trial. J Child Neurol 22:1191–1197 [DOI] [PubMed] [Google Scholar]
- More JC, Nistico R, Dolman NP, Clarke VR, Alt AJ, Ogden AM, Buelens FP, Troop HM, Kelland EE, Pilato F, et al. (2004) Characterisation of UBP296: a novel, potent and selective kainate receptor antagonist. Neuropharmacology 47:46–64 [DOI] [PubMed] [Google Scholar]
- Naylor DE, Liu H, Wasterlain CG. (2005) Trafficking of GABA(A) receptors, loss of inhibition, and a mechanism for pharmacoresistance in status epilepticus. J Neurosci 25:7724–7733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtani T, Iwanami A, Kasai K, Yamasue H, Kato T, Sasaki T, Kato N. (2004) Post-traumatic stress disorder symptoms in victims of Tokyo subway attack: a 5-year follow-up study. Psychiatry Clin Neurosci 58:624–629 [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. (2005) The Rat Brain in Stereotaxic Coordinates, 4th ed, Elsevier, New York [Google Scholar]
- Pidoplichko VI, Aroniadou-Anderjaska V, Prager EM, Figueiredo TH, Almeida-Suhett CP, Miller SL, Braga MF. (2014) ASIC1a activation enhances inhibition in the basolateral amygdala and reduces anxiety. J Neurosci 34:3130–3141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prager EM, Aroniadou-Anderjaska V, Almeida-Suhett CP, Figueiredo TH, Apland JP, Braga MF. (2013) Acetylcholinesterase inhibition in the basolateral amygdala plays a key role in the induction of status epilepticus after soman exposure. Neurotoxicology 38:84–90 [DOI] [PubMed] [Google Scholar]
- Prager EM, Aroniadou-Anderjaska V, Almeida-Suhett CP, Figueiredo TH, Apland JP, Rossetti F, Olsen CH, Braga MF. (2014) The recovery of acetylcholinesterase activity and the progression of neuropathological and pathophysiological alterations in the rat basolateral amygdala after soman-induced status epilepticus: relation to anxiety-like behavior. Neuropharmacology 81:64–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qashu F, Figueiredo TH, Aroniadou-Anderjaska V, Apland JP, Braga MF. (2010) Diazepam administration after prolonged status epilepticus reduces neurodegeneration in the amygdala but not in the hippocampus during epileptogenesis. Amino Acids 38:189–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine RJ. (1972) Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol 32:281–294 [DOI] [PubMed] [Google Scholar]
- Rajasekaran K, Joshi S, Kozhemyakin M, Todorovic MS, Kowalski S, Balint C, Kapur J. (2013) Receptor trafficking hypothesis revisited: plasticity of AMPA receptors during established status epilepticus. Epilepsia 54 (Suppl 6):14–16 [DOI] [PubMed] [Google Scholar]
- Ramsay RE, Hammond EJ, Perchalski RJ, Wilder BJ. (1979) Brain uptake of phenytoin, phenobarbital, and diazepam. Arch Neurol 36:535–539 [DOI] [PubMed] [Google Scholar]
- Schmitz C, Hof PR. (2000) Recommendations for straightforward and rigorous methods of counting neurons based on a computer simulation approach. J Chem Neuroanat 20:93–114 [DOI] [PubMed] [Google Scholar]
- Shekhar A, Sajdyk TJ, Gehlert DR, Rainnie DG. (2003) The amygdala, panic disorder, and cardiovascular responses. Ann N Y Acad Sci 985:308–325 [DOI] [PubMed] [Google Scholar]
- Shih T, McDonough JH, Jr, Koplovitz I. (1999) Anticonvulsants for soman-induced seizure activity. J Biomed Sci 6:86–96 [DOI] [PubMed] [Google Scholar]
- Shih TM, Duniho SM, McDonough JH., Jr (2003) Control of nerve agent-induced seizures is critical for neuroprotection and survival. Toxicol Appl Pharmacol 188:69–80 [DOI] [PubMed] [Google Scholar]
- Shih TM, McDonough JH., Jr (1999) Organophosphorus nerve agents-induced seizures and efficacy of atropine sulfate as anticonvulsant treatment. Pharmacol Biochem Behav 64:147–153 [DOI] [PubMed] [Google Scholar]
- Shorvon S. (2001) The management of status epilepticus. J Neurol Neurosurg Psychiatry 70 (Suppl 2):II22–II27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhi S, Murthy A, Singhi P, Jayashree M. (2002) Continuous midazolam versus diazepam infusion for refractory convulsive status epilepticus. J Child Neurol 17:106–110 [DOI] [PubMed] [Google Scholar]
- Skovira JW, McDonough JH, Jr, Shih TM. (2010) Protection against sarin-induced seizures in rats by direct brain microinjection of scopolamine, midazolam or MK-801. J Mol Neurosci 40:56–62 [DOI] [PubMed] [Google Scholar]
- Smolders I, Bortolotto ZA, Clarke VR, Warre R, Khan GM, O’Neill MJ, Ornstein PL, Bleakman D, Ogden A, Weiss B, et al. (2002) Antagonists of GLU(K5)-containing kainate receptors prevent pilocarpine-induced limbic seizures. Nat Neurosci 5:796–804 [DOI] [PubMed] [Google Scholar]
- Tetz LM, Rezk PE, Ratcliffe RH, Gordon RK, Steele KE, Nambiar MP. (2006) Development of a rat pilocarpine model of seizure/status epilepticus that mimics chemical warfare nerve agent exposure. Toxicol Ind Health 22:255–266 [DOI] [PubMed] [Google Scholar]
- Todorovic MS, Cowan ML, Balint CA, Sun C, Kapur J. (2012) Characterization of status epilepticus induced by two organophosphates in rats. Epilepsy Res 101:268–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullal G, Fahnestock M, Racine R. (2005) Time-dependent effect of kainate-induced seizures on glutamate receptor GluR5, GluR6, and GluR7 mRNA and Protein Expression in rat hippocampus. Epilepsia 46:616–623 [DOI] [PubMed] [Google Scholar]
- Uusi-Oukari M, Korpi ER. (2010) Regulation of GABA(A) receptor subunit expression by pharmacological agents. Pharmacol Rev 62:97–135 [DOI] [PubMed] [Google Scholar]
- Vinkers CH, Olivier B. (2012) Mechanisms underlying tolerance after long-term benzodiazepine use: A future for subtype-selective GABA(A) receptor modulators? Adv Pharmacol Sci 2012:416864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton NY, Treiman DM. (1988) Response of status epilepticus induced by lithium and pilocarpine to treatment with diazepam. Exp Neurol 101:267–275 [DOI] [PubMed] [Google Scholar]
- Yanagisawa N, Morita H, Nakajima T. (2006) Sarin experiences in Japan: acute toxicity and long-term effects. J Neurol Sci 249:76–85 [DOI] [PubMed] [Google Scholar]
- Zhou R, Wang S, Zhu X. (2010) Prenatal ethanol exposure attenuates GABAergic inhibition in basolateral amygdala leading to neuronal hyperexcitability and anxiety-like behavior of adult rat offspring. Neuroscience 170:749–757 [DOI] [PubMed] [Google Scholar]