Abstract
Early-onset pre-eclampsia is characterized by decreased placental perfusion, new-onset hypertension, angiogenic imbalance, and endothelial dysfunction associated with excessive activation of the innate immune complement system. Although our previous studies demonstrated that inhibition of complement activation attenuates placental ischemia–induced hypertension using the rat reduced uterine perfusion pressure (RUPP) model, the important product(s) of complement activation has yet to be identified. We hypothesized that antagonism of receptors for complement activation products C3a and C5a would improve vascular function and attenuate RUPP hypertension. On gestational day (GD) 14, rats underwent sham surgery or vascular clip placement on ovarian arteries and abdominal aorta (RUPP). Rats were treated once daily with the C5a receptor antagonist (C5aRA), PMX51 (acetyl-F-[Orn-P-(D-Cha)-WR]), the C3a receptor antagonist (C3aRA), SB290157 (N2-[(2,2-diphenylethoxy)acetyl]-l-arginine), or vehicle from GD 14–18. Both the C3aRA and C5aRA attenuated placental ischemia–induced hypertension without affecting the decreased fetal weight or decreased concentration of free circulating vascular endothelial growth factor (VEGF) also present in this model. The C5aRA, but not the C3aRA, attenuated placental ischemia–induced increase in heart rate and impaired endothelial-dependent relaxation. The C3aRA abrogated the acute pressor response to C3a peptide injection, but it also unexpectedly attenuated the placental ischemia–induced increase in C3a, suggesting nonreceptor-mediated effects. Overall, these results indicate that both C3a and C5a are important products of complement activation that mediate the hypertension regardless of the reduction in free plasma VEGF. The mechanism by which C3a contributes to placental ischemia–induced hypertension appears to be distinct from that of C5a, and management of pregnancy-induced hypertension is likely to require a broad anti-inflammatory approach.
Introduction
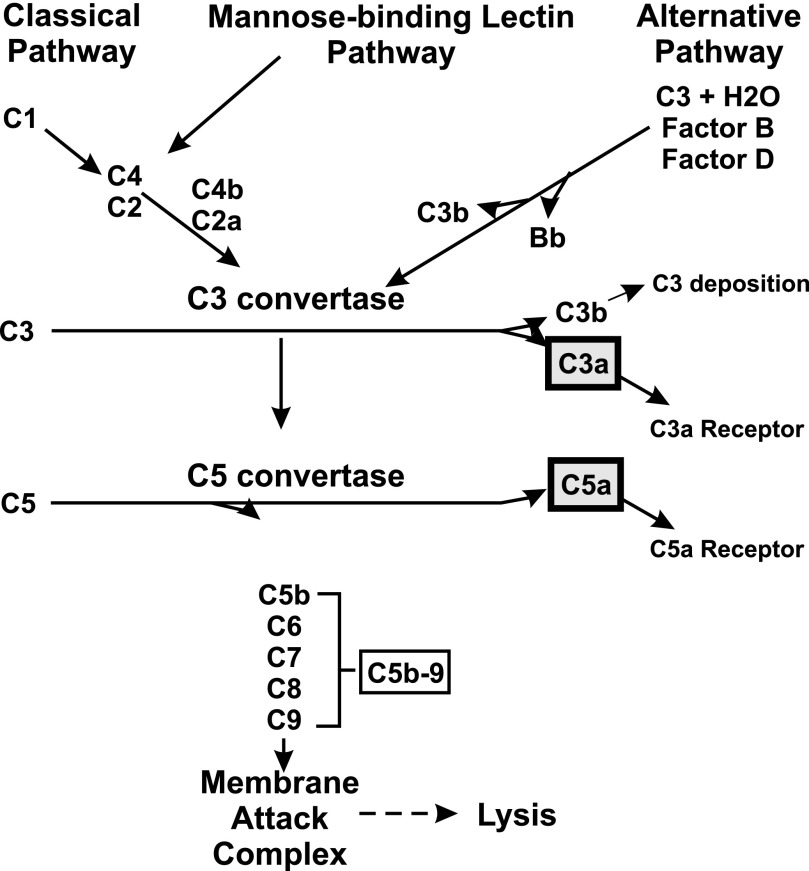
Pre-eclampsia is a pregnancy-specific condition characterized by decreased placental perfusion, new-onset hypertension, intrauterine growth restriction, and endothelial dysfunction. Affecting up to 10% of pregnancies in the United States, pre-eclampsia and hypertensive disorders of pregnancy are a leading cause of maternal and perinatal morbidity and mortality with few effective treatment options (American College of Obstetricians and Gynecologists, 2013; Ananth et al., 2013). The complement system, part of the innate immune response, is an enzymatic amplification system composed of plasma proteins essential for host defense and inflammation. The complement system can be activated by multiple pathways, with a central event being formation of the enzyme C3 convertase, which cleaves C3 to generate fluid-phase C3a as well as C3b, which covalently binds to an invader or self, i.e., C3 deposition (Fig. 1). C3b serves as a nucleus for formation of C5 convertase, which cleaves C5 into two fragments: C5a and the larger fragment C5b, which inserts into membranes. Continuation of the cascade results in formation of membrane attack complex C5b-9, which either forms a pore in the membrane or is released as a soluble complex into circulation. Complement activation products include C3a, C5a, and C5b-9. Although the complement system is essential for fetal survival (Usami et al., 2010) and normal placental development (Singh et al., 2011), excessive complement activation is associated with recurrent miscarriages, preterm labor, and pre-eclampsia (Lynch and Salmon, 2010). During pregnancies complicated with pre-eclampsia, there is strong evidence of excessive complement activation in the circulation, with a decrease in C3 concentration and an increase in C5a, soluble C5b-9, and the ratio of C3a to C3 (Derzsy et al., 2010; Soto et al., 2010), as well as excessive complement activation in placenta (Buurma et al., 2012; Gilbert et al., 2012c) and urine (Burwick et al., 2013). A recent case report suggests that complement contributes to pre-eclamptic symptoms because depletion of complement component C5 in a severely pre-eclamptic woman was able to prolong pregnancy by >2 weeks (Burwick and Feinberg, 2013).
Fig. 1.
Major pathways of complement activation leading to generation of anaphylatoxins C3a and C5a.
Mechanistic studies in clinically relevant animal models are clearly needed to identify critical products of complement activation contributing to pre-eclamptic symptoms. Using the reduced uterine perfusion pressure (RUPP) rat model of placental ischemia–induced hypertension and soluble complement receptor 1 as an inhibitor of complement activation, our studies were the first to describe a link between complement system activation and the hypertension resulting from third-trimester placental ischemia in pregnancy (Lillegard et al., 2013b). We also demonstrated that angiogenic imbalance as well as impaired endothelial-dependent relaxation in isolated blood vessels occur in this model (Gilbert et al., 2010). Inhibiting complement activation attenuated hypertension in the RUPP model, but angiogenic imbalance, i.e., decreased vascular endothelial growth factor (VEGF), was still evident, suggesting that the role of complement in placental ischemia–induced hypertension was independent of VEGF. Complement activation products C3a and C5a are potent (∼10-kDa) fluid-phase inflammatory mediators that interact with G protein–coupled receptors on many cell types to elicit biologic activities including smooth muscle contraction and changes in blood pressure (Regal et al., 1980; Regal, 1982; Proctor et al., 2009), activation and chemotaxis of immune cells, and increased vascular permeability (Klos et al., 2013). In addition, excessive complement activation can adversely affect endothelial function (Collard et al., 2000). With the realization of the importance of immune inflammatory pathways in numerous disease states, C3a and C5a receptor antagonists are being developed for potential clinical use and have also been used in numerous animal models to investigate the role of C3a and C5a in disease models (Ricklin and Lambris, 2013). Using antagonists of the C3a and C5a receptors, the present study is the first to test the hypothesis that C3a and C5a are the complement activation products that mediate endothelial dysfunction and hypertension following placental ischemia.
Materials and Methods
RUPP Procedure and Antagonist Administration.
The RUPP procedure was performed on gestational day (GD) 14 to achieve chronic placental ischemia and hypertension in pregnant rats on GD 19 (see Supplemental Methods for details) (Lillegard et al., 2013b). All animal experiments were approved by the University of Minnesota Institutional Animal Care and Use Committee and conformed to the National Institutes of Health Guide for Care and Use of Laboratory Animals. Animals were treated with either the C3a receptor antagonist (C3aRA), SB290157 (N2-[(2,2-diphenylethoxy)acetyl]-l-arginine; Merck KGaA, Darmstadt, Germany) (Ames et al., 2001), at 5 mg/kg i.v., the C5a receptor antagonist (C5aRA), PMX53 (acetyl-F-[Orn-P-(D-Cha)-WR]; synthesized by Atlantic Peptides, Lewisburg, PA) (Finch et al., 1999), at 3 mg/kg s.c., or 10% ethanol/saline vehicle (Veh). Once-daily treatments began 10–15 minutes before RUPP or sham surgery (Sham) on GD 14 and ended on GD 18. Animals were randomly assigned to the following treatment groups: 1) RUPP surgery with intravenous Veh; 2) RUPP surgery with subcutaneous Veh; 3) RUPP surgery with C3aRA (RUPP C3aRA); 4) RUPP surgery with C5aRA (RUPP C5aRA); 5) RUPP surgery with C3aRA and C5aRA; 6) sham surgery with intravenous Veh; 7) sham surgery with subcutaneous Veh; 8) sham surgery with C3aRA (Sham C3aRA); or 9) sham surgery with C5aRA (Sham C5aRA). The vehicle for both antagonists was 10% ethanol/saline and was administered either intravenously or subcutaneously. With statistical analysis, no differences in outcomes were detected with administration of Veh by either the subcutaneous or intravenous route, so groups 1 (n = 10) and 2 (n = 13) were combined as RUPP Veh and groups 6 (n = 10) and 7 (n = 9) combined as Sham Veh in the final data presentation and analysis.
Mean Arterial Pressure Measurements and Tissue Collection.
Mean arterial pressure (MAP) and heart rate were measured via intra-arterial catheter in unanesthetized, restrained animals on GD 19, and serum, plasma, and fetal and placental tissue were harvested as described previously (Lillegard et al., 2013b). Circulating white blood cells (WBCs) in EDTA blood were counted by standard methods in a hemacytometer. Blood smears were stained with a modified Wright’s stain (Diff-Quik; American Scientific Products, McGraw Park, IL), and at least 400 cells were counted and categorized as neutrophils, eosinophils, monocytes, or lymphocytes as determined by their morphology. Myeloperoxidase in homogenized lung was determined as an indicator of the number of neutrophils in the lung (Greene et al., 2005) (details in Supplemental Methods). Circulating free VEGF in EDTA plasma collected on GD 19 was measured using a commercially available kit for mouse VEGF (R&D Systems, Minneapolis, MN).
C3a Pressor Response in Nonpregnant and Pregnant Rats.
To test the efficacy of C3aRA, we used C3a peptide, an analog of C3a described by Ember et al. (1991) (WWGKKYRASKLGLAR; AnaSpec, Fremont, CA), to acutely increase blood pressure. Pregnant and nonpregnant female rats were anesthetized intraperitoneally with 90 mg/kg ketamine plus 2.5 mg/kg xylazine for placement of a jugular catheter (used for intravenous administration of C3a peptide and C3aRA) and a carotid catheter (MAP measurements). Blood pressure was allowed to stabilize for 15 minutes, and either 100 μg/kg C3aRA or Veh was administered. After 15 minutes, C3a peptide (30 μg/kg) was delivered and increase in MAP monitored until it returned to baseline within 10 minutes. Norepinephrine (80 μg/kg; Sigma-Aldrich, St. Louis, MO) was then administered to verify responsiveness.
Complement Measurements.
C3a serum concentration was measured by Western immunoblot as described previously (Lillegard et al., 2013b) and is expressed as C3a units per microliter relative to the signal intensity of 1 μl of standard pool of yeast-activated rat serum. Total complement hemolytic activity was measured as previously described and expressed as inverse dilution of serum causing 50% hemolysis of sensitized sheep erythrocytes (CH50) (Lillegard et al., 2013b). The ability of C3aRA or C5aRA to inhibit complement activation in vitro was also assessed by incubation of antagonist or Veh with a pool of nonpregnant rat serum prior to determination of CH50.
Mesenteric Artery Myography.
Third-order mesenteric arteries were cleaned of adipose tissue and 2-mm segments mounted on 40-μm-thick wires for myography as previously described (Gilbert et al., 2012a). Buffers and vessel normalization were performed according to DMT Multi Wire Myograph System User Manual (Model 610M; Danish Myo Technology, Aarhus, Denmark). Vessels were normalized to mimic a transmural pressure of 100 mm Hg and equilibrated for 30–60 minutes with three or four buffer washes. Diameters ranged from 200–350 μm, and vessels were precontracted with the thromboxane mimetic U46619 (9α,11α-methanoepoxy-PGF2α) (5.7 × 10−7 M) followed by half-log increments of acetylcholine (Ach) (0.03 × 10−6 to 33 × 10−6 M) to assess endothelial-dependent relaxation. After washing, vessels were contracted again with U46619 and relaxation to increasing concentrations of sodium nitroprusside (SNP; 0.01 × 10−6 to 333 × 10−6 M) was measured as an indicator of endothelial-independent vasodilation. Finally, a cumulative concentration-response curve to U46619 confirmed vessel reactivity.
Statistical Analyses.
Data were analyzed using three-way analysis of variance using JMP and SAS software (SAS Institute, Cary, NC) and presented as geometric mean or mean ± S.E. Differences were considered significant when P < 0.05. Specific individual contrasts evaluated and presented in figures were 1) Sham Veh versus RUPP Veh, 2) RUPP versus RUPP C3aRA, 3) RUPP versus RUPP C5aRA, 4) Sham versus Sham C3aRA, and 5) Sham versus Sham C5aRA.
Results
Receptor Antagonists Attenuate Placental Ischemia–Induced Hypertension.
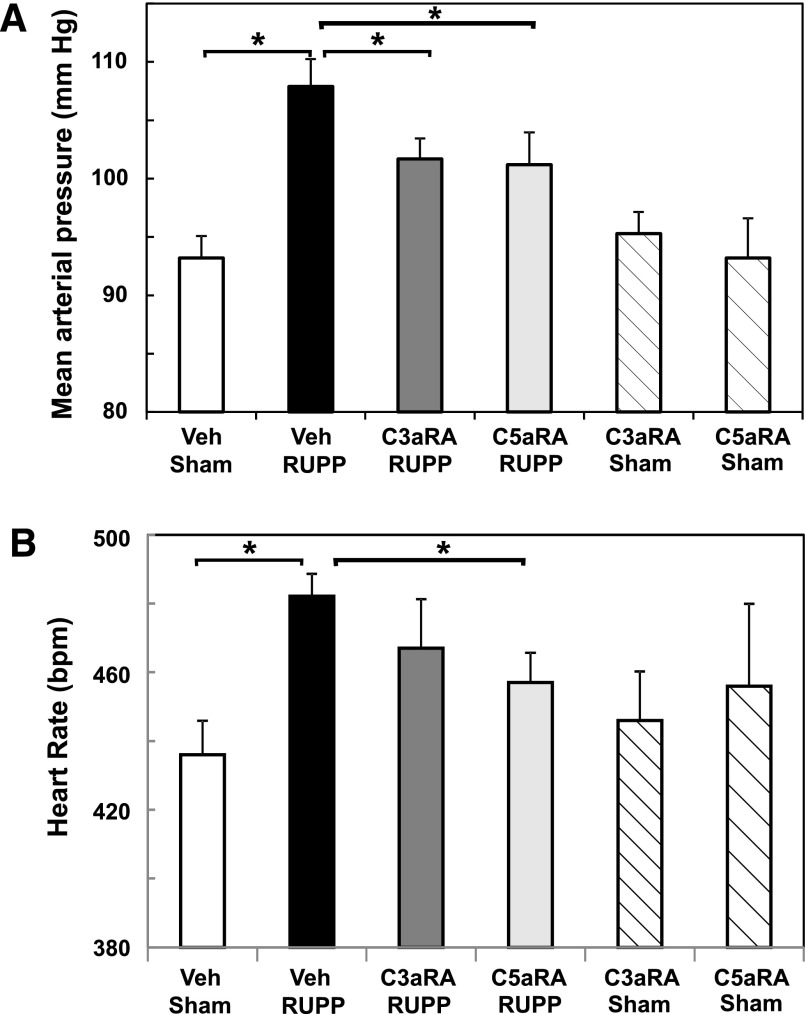
To determine if the complement activation products C3a and/or C5a were important in mediating placental ischemia–induced hypertension, we evaluated the effect of treatment with C3aRA or C5aRA. Chronic placental ischemia caused a significant increase in MAP by GD 19 (Fig. 2A). Clearly, treatment with either the C3aRA or C5aRA significantly inhibited RUPP-induced increase in MAP without altering MAP in Sham animals. Treatment of animals with a combination of C3aRA and the C5aRA did not result in greater attenuation of MAP than treatment with either antagonist alone (104 ± 3 mm Hg; n = 9; data not shown). As seen in Fig. 2B, heart rate in RUPP rats was increased as previously described (Gilbert et al., 2012e) and was significantly decreased by treatment with the C5aRA (P < 0.05) but not the C3aRA (P = 0.11).
Fig. 2.
C3a and C5a receptor antagonists differentially attenuate placental ischemia–induced hypertension and heart rate. Sham or RUPP animals were treated with Veh, C3aRA, or C5aRA from GD 14–18. Values represent mean ± S.E. of MAP or heart rate measured on GD 19. (A) Increase in MAP in the RUPP Veh group (n = 23) was significantly inhibited by the C3aRA (n = 12) or C5aRA (n = 11). MAP did not differ between Sham animals treated with Veh (n = 19), C3aRA (n = 6), or C5aRA (n = 5). (B) Increased heart rate in RUPP Veh (n = 23) versus Sham Veh (n = 19) animals was significantly inhibited by the C5aRA (n = 11; P < 0.05) but not the C3aRA (n = 12; P = 0.11). Heart rate did not differ between Sham animals treated with Veh, C3aRA (n = 6), or C5aRA (n = 5). *P < 0.05 for indicated comparisons.
Placental Ischemia–Induced Decrease in Free Plasma VEGF, Fetal Weight, and Resorptions Is Unaffected by Receptor Antagonists.
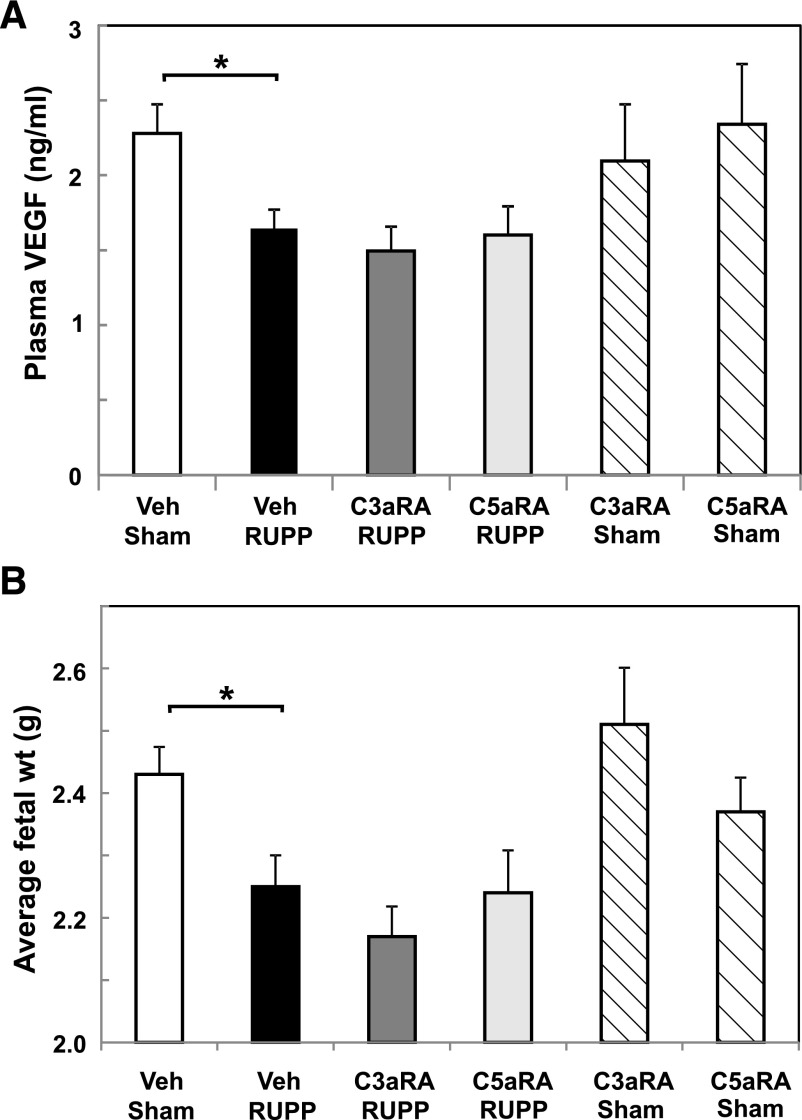
As previously shown, placental ischemia resulted in decreased free plasma VEGF (Fig. 3A) and intrauterine growth restriction in RUPP compared with Sham controls (Fig. 3B). Treatment with either the C3aRA or C5aRA did not alter VEGF or fetal weight in RUPP rats (Fig. 3). Combined treatment with both antagonists (n = 9) also did not affect RUPP-induced decrease in either outcome (fetal weight, 2.1 ± 0.8 g; VEGF, 1679 ± 220 pg/ml). Antagonist treatment of Sham animals did not significantly alter circulating free plasma VEGF or fetal weight compared with Sham Veh animals. As previously reported, no increase in soluble fms-like tyrosine kinase-1 (soluble VEGF receptor 1), as measured by the commercially available mouse kit from R&D Systems, was detected in EDTA plasma collected on GD 19 from RUPP versus Sham animals in our experiments (Lillegard et al., 2013b). In addition, a significant increase in protein in urine was also not detected between RUPP and Sham animals in these studies, consistent with previous reports regarding the inconsistency of this measure in the RUPP model (Granger et al., 2006). Fetal resorptions were also assessed, and as previously shown (Lillegard et al., 2013b), RUPP surgery increased fetal resorptions (Supplemental Fig. 1). Treatment of RUPP or Sham animals with the C3aRA, the C5aRA, or the combination did not change the number of resorptions (Supplemental Fig. 1).
Fig. 3.
C3a and C5a receptor antagonists do not reverse decreased free plasma VEGF or average fetal weight following placental ischemia. Sham or RUPP animals were treated with Veh, C3aRA, or C5aRA from GD 14–18. Values represent geometric mean ± S.E. of VEGF in GD 19 plasma or fetal weight measured at GD 19. (A) Decrease in VEGF in the RUPP Veh group (n = 23) was not affected by the C3aRA (n = 12) or C5aRA (n = 11). Free plasma VEGF at GD 19 did not differ between Sham animals treated with Veh (n = 19), C3aRA (n = 6), or C5aRA (n = 5). (B) Decrease in fetal weight in the RUPP Veh group (n = 22) was not affected by the C3aRA (n = 11) or C5aRA (n = 10). Fetal weight did not differ between Sham animals treated with Veh (n = 19), C3aRA (n = 6), or C5aRA (n = 5). *P < 0.05 for indicated comparisons.
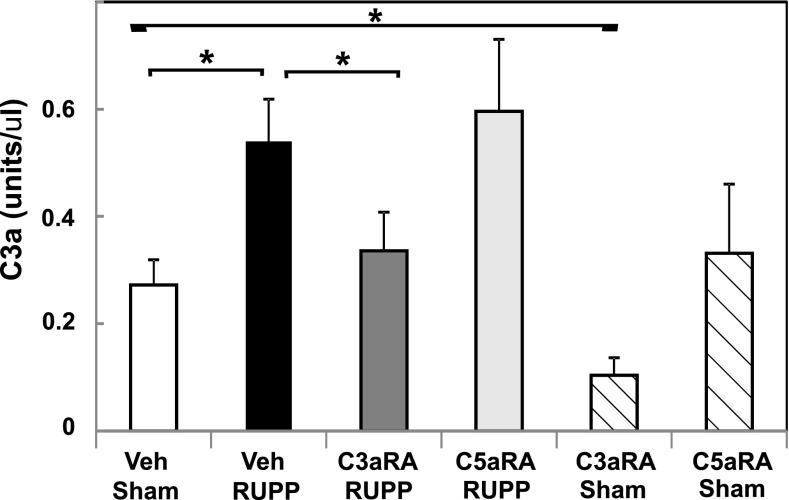
C3aRA, but Not C5aRA, Inhibits Increased C3a In Vivo Following Placental Ischemia.
Relative serum C3a concentrations were used as an indicator of complement activation. Rat C5a was not detectable by current assay methods. The C3aRA and C5aRA were not expected to inhibit complement activation, but only antagonize actions of cleavage fragments C3a and C5a at their receptors. As seen in Fig. 4, our data confirm our previous studies (Lillegard et al., 2013b), demonstrating that RUPP significantly increases C3a in the circulation. Treatment with the C5aRA altered neither RUPP-induced increase in C3a nor baseline C3a concentration in Sham animals. Surprisingly, treatment with the C3aRA in vivo significantly inhibited C3a generation in both RUPP and Sham groups. CH50 measures the ability of the classical complement pathway components in serum to react sequentially and lyse the red blood cell coated with antibody. The receptor antagonists are not expected to affect CH50 because they prevent the action of C3a and C5a on their receptors without affecting activation of the complement cascade. Neither C3aRA nor C5aRA treatment in vivo, either alone or in combination, significantly affected total hemolytic complement activity (CH50; Supplemental Fig. 2), indicating that they were not inhibiting classical pathway complement activation or appreciably depleting total complement activity in vivo. In addition, in vitro studies demonstrated that incubating the C3aRA (≤390 μg/ml) or C5aRA (≤100 μg/ml) with normal rat serum did not inhibit the CH50 of the serum (data not shown), indicating that the antagonists were not altering classical hemolytic complement pathway activity in vitro.
Fig. 4.
The C3aRA, but not the C5aRA, attenuates increased serum C3a following placental ischemia. Sham or RUPP animals were treated with Veh, C3aRA, or C5aRA from GD 14–18. The increase in C3a in RUPP Veh animals (n = 23) was significantly inhibited by the C3aRA (n = 12) but not the C5aRA (n = 11). Compared with Sham Veh animals (n = 19), the C3a concentration was significantly decreased by the C3aRA (n = 6) but not the C5aRA (n = 5). Values represent mean ± S.E. of serum C3a measured on GD 19. *P < 0.05 for indicated comparisons.
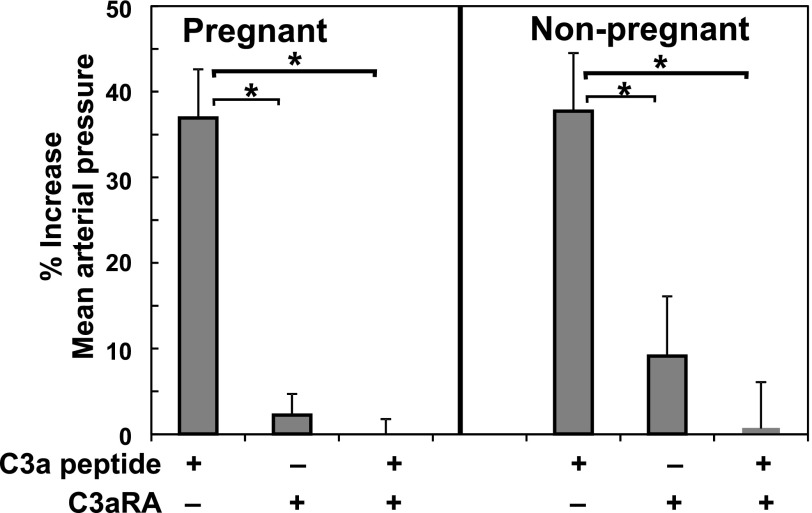
C3aRA Inhibits C3a-Induced Pressor Response.
Previous studies of others indicated differential vascular reactivity in pregnant versus nonpregnant rats (Paller, 1987). Thus, the ability of C3a to cause a pressor response in the pregnant rat was evaluated. An intravenous injection of 30 μg/kg C3a peptide in anesthetized pregnant dams on GD 19 resulted in a rapid transient pressor response within minutes (Fig. 5), similar to the response in nonpregnant rats of a comparable age and size. Injection of the C3aRA (100 μg/kg) itself did not significantly alter MAP in nonpregnant or pregnant rats at GD 19 (Fig. 5), but it significantly inhibited the pressor response to 30 μg/kg C3a peptide in both nonpregnant and pregnant rats.
Fig. 5.
The C3aRA prevents increased MAP induced by C3a peptide in pregnant (GD 19) and nonpregnant females. C3a peptide increased MAP in GD 19 animals or nonpregnant females. The C3aRA did not increase MAP but inhibited the C3a peptide–induced increase in MAP. Values represent mean ± S.E. of percent increase in MAP in 4–8 animals per treatment group. *P < 0.05 versus C3a peptide injection alone.
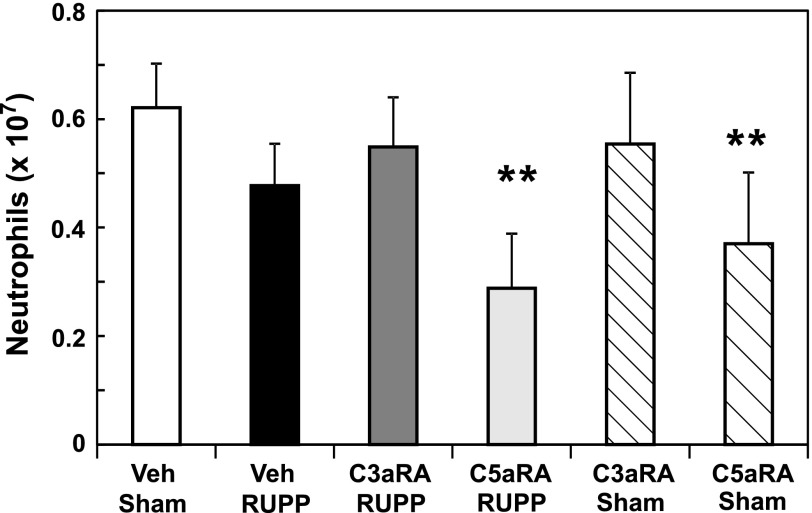
C5aRA Reduces Circulating Neutrophils.
Because studies of Proctor et al. (2004) indicated that the C3aRA exhibits partial agonist activity and affects circulating neutrophils in the nonpregnant rat, we determined the effect of both the C3aRA and C5aRA on circulating WBCs. Although RUPP did not alter total WBCs and neutrophils in circulation, analysis of variance indicated that the C5aRA decreased circulating WBCs and neutrophils, regardless of Sham or RUPP surgery (Fig. 6). Chronic C3aRA administration had no significant effect on circulating WBCs or neutrophils. Total lymphocytes were not affected by RUPP surgery, C3aRA, or C5aRA (data not shown). Previous studies of others have clearly demonstrated an important role for lymphocytes in the RUPP model (LaMarca et al., 2013). However, our studies were not designed to detect changes in lymphocyte subpopulations. Analysis of neutrophils in the lung demonstrated that neutrophils were not being sequestered in the lung by any of the treatments (Supplemental Fig. 3).
Fig. 6.
The C5aRA, but not the C3aRA, attenuates numbers of circulating neutrophils. Sham or RUPP animals were treated with Veh, C3aRA, or C5aRA from GD 14–18. A significant decrease in circulating neutrophils occurred with C5aRA treatment. Values represent mean ± S.E. of circulating neutrophils measured on GD 19. **P < 0.05 for C5aRA effect by analysis of variance.
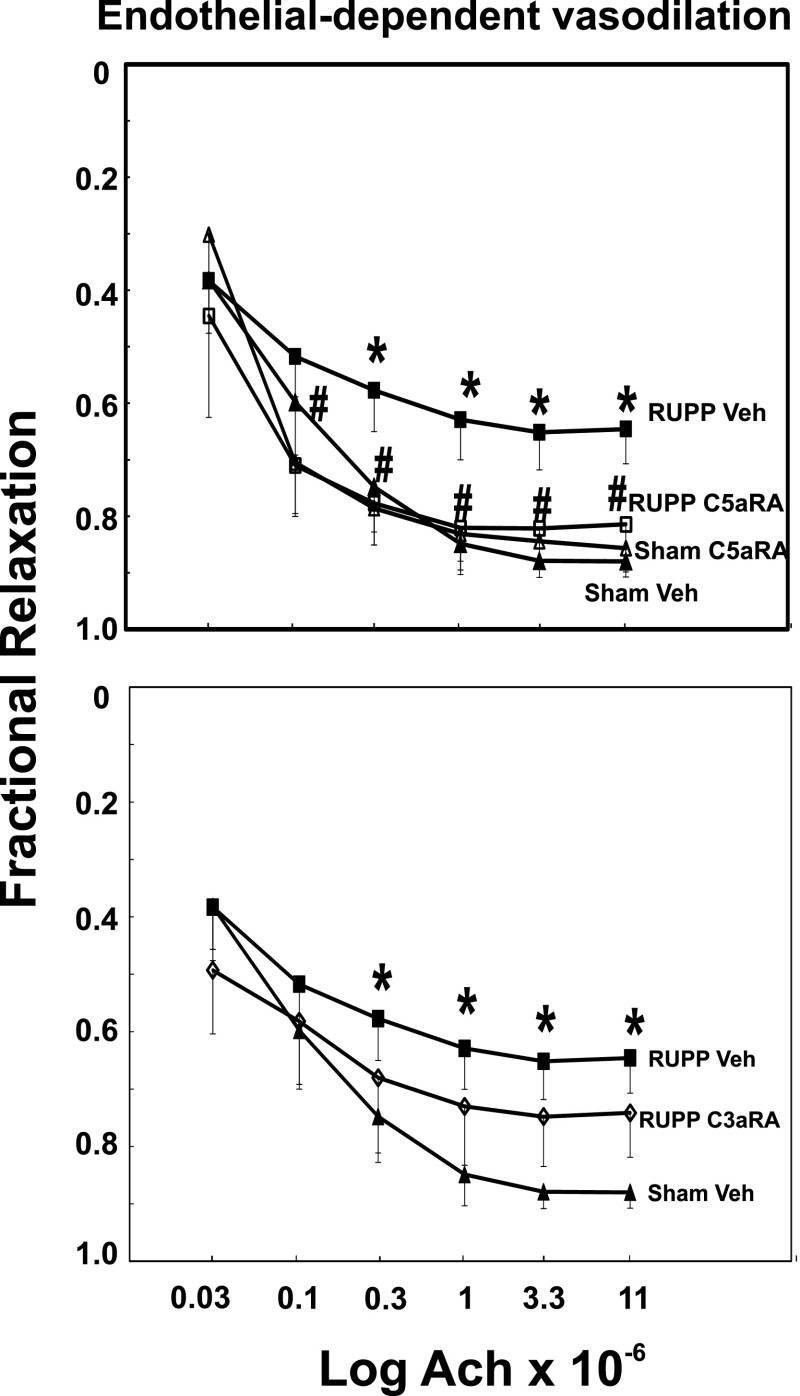
C5aRA, but Not C3aRA, Attenuates Endothelial Dysfunction Following Placental Ischemia.
As previously demonstrated in this model, vasodilation in response to Ach was attenuated in mesenteric arteries from rats with placental ischemia (Fig. 7). In contrast, endothelial-independent vasodilation in response to SNP was not affected by placental ischemia (Supplemental Fig. 4). The C5aRA, but not the C3aRA, preserved endothelial function following placental ischemia, as indicated by Ach-induced vasodilation equivalent to that seen in rats undergoing Sham Veh treatment. Combination treatment with both the C3aRA and C5aRA affected Ach and SNP relaxation in the same way as the C5aRA alone (data not shown). Both C3a and C5a have been reported to contract isolated blood vessels (Regal, 1982; Bjork et al., 1985), including microvessels in the hamster cheek pouch (Bjork et al., 1985), but neither C3a peptide nor recombinant human C5a contracted the isolated rat mesenteric arteries (data not shown). In addition, neither the C3aRA nor the C5aRA significantly relaxed isolated mesenteric arteries whether at normal resting tension or when precontracted with the thromboxane mimetic U46619.
Fig. 7.
The C5aRA, but not the C3aRA, prevents placental ischemia–induced endothelial dysfunction. Sham or RUPP animals were treated with Veh, C3aRA, or C5aRA from GD 14–18. Mesenteric arteries were isolated on GD 19 and precontracted with the thromboxane mimetic U46619, and relaxation to Ach (endothelial-dependent vasodilation) was assessed. Relaxation to Ach was reduced in RUPP Veh (n = 14) compared with Sham Veh (n = 10) animals. The C5aRA (n = 9), but not the C3aRA (n = 6), prevented the RUPP-induced change in Ach vasodilation. Values represent mean ± S.E. of fractional relaxation in precontracted arteries. *P < 0.05, RUPP versus Sham; #P < 0.05, RUPP Veh versus RUPP C3aRA or RUPP C5aRA. No difference was detected between the Sham Veh and Sham C5aRA groups.
Discussion
Using soluble complement receptor 1, an established inhibitor of complement activation, our previous studies demonstrated a critical role for complement activation in placental ischemia–induced hypertension in rat (Lillegard et al., 2013b), suggesting that manipulation of the complement system is a viable therapeutic strategy for management of pregnancy-induced hypertension. Given the importance of complement in host defense and immune complex clearance, defining specific product(s) of complement activation responsible for placental ischemia–induced hypertension is an important step toward defining therapeutic targets that minimally compromise the protective role of complement. Because complement activation products C3a and C5a are well known for their role as inflammatory mediators through interaction with G protein–coupled receptors on multiple cell types (Klos et al., 2013), we used receptor antagonists to assess the contribution of C3a and C5a to placental ischemia–induced hypertension. Our studies are the first to demonstrate that both C3a and C5a contribute to placental ischemia–induced hypertension, and inhibiting both receptors was no more effective than inhibiting each receptor alone.
By design, multiple inflammatory mediators can be evoked by any given perturbation, thus promoting survival of the organism but also contributing to difficulty in treating inflammatory disease. If the action or synthesis of one mediator (i.e., C3a or C5a) is prevented, another mediator may assume a primary role in uncontrolled inflammation. Our studies, and those of others, suggest that increased inflammation in pre-eclampsia involves cytokines, T cells, autoantibodies, and complement, along with pro- and antiangiogenic factors (LaMarca et al., 2013). This complex interplay of mediators suggests that therapy for pregnancy-induced hypertension will be a challenge similar to treatment of general inflammatory disease.
Increasing evidence supports a role for angiogenic imbalance in pregnancy-induced hypertension (Gilbert et al., 2012d). Our current studies demonstrated a decrease in circulating free VEGF but no increase in soluble fms-like tyrosine kinase-1 in the RUPP model. Further, neither C3aRA nor C5aRA affected the decrease in circulating VEGF concentrations, suggesting that this effect mechanistically precedes increased C3a and/or C5a action or that VEGF mediates hypertension via a parallel pathway. In the RUPP model, decreased VEGF could also potentially result in decreased complement control proteins, allowing increased complement activation as demonstrated in kidney endothelium (Mason et al., 2004; Kerr and Richards, 2012). Although a recent study by Weissgerber et al. (2014) questions the validity of the enzyme-linked immunosorbent assay for measurement of VEGF in rat plasma and serum, our previous studies have reported decreased angiogenic potential via endothelial tube formation assays in RUPP compared with normal pregnant controls (Gilbert et al., 2012b; Bauer et al., 2013). Clearly, a re-evaluation of the methodology for assessing angiogenic factors in the rat circulation is needed, with additional studies to understand the relationship between angiogenic imbalance and complement activation/control proteins following placental ischemia.
Both endothelial dysfunction and increased heart rate may contribute to cardiovascular abnormalities observed in pre-eclampsia and the RUPP rat model (Gilbert et al., 2012e). The C5aRA prevented endothelial dysfunction and increased heart rate associated with RUPP hypertension, suggesting that C5a substantially contributes to both in the RUPP model. We have previously shown that C5a has positive chronotropic effects on isolated male guinea pig atria (Regal, 1985). Thus, it is possible that C5aRA prevents a direct action of C5a on cardiomyocytes to increase heart rate in the RUPP model. Alternatively, C5aRA treatment may decrease sympathetic tone reportedly elevated in RUPP rats (Hines et al., 2007). However, the relevance of this to cardiovascular function in pre-eclampsia remains unclear (Greenwood et al., 2001). C5aRA also decreased circulating neutrophils in our study, consistent with recent data indicating that C5a stimulates production of granulocyte colony-stimulating factor (Bosmann et al., 2013), a substance known to play a role in regulating the number of neutrophils in circulation. C5a is also a potent neutrophil activator. The difficulty in detecting increases in C5a in circulation may be due to binding of any C5a generated in the circulation to the very-high-affinity receptor for C5a on neutrophils. However, our data demonstrate no evidence that the RUPP procedure nor the C3aRA or C5aRA results in neutrophil activation with resultant pulmonary sequestration. Our preliminary studies indicate that depletion of neutrophils also attenuates placental ischemia–induced hypertension (Lillegard et al., 2013a), suggesting that C5aRA could be limiting placental ischemia–induced hypertension by a neutrophil-dependent effect.
Clearly, a limitation of our study lies in inherent limitations of receptor antagonists. PMX53, the C5aRA chosen for this study, has been used in numerous animal models in both mice and rats to assess the importance of C5a in mediating a response through the C5a receptor (CD88; C5aR; C5aR1). It is important to recognize that C5a can interact with another receptor (C5L2; C5aR2), but PMX53 does not alter C5a action at this second C5a receptor (Klos et al., 2013). Both the C5aR and C5L2 mediate production of granulocyte colony-stimulating factor by C5a (Bosmann et al., 2013), and C5aR and C5L2 can form heteromers to regulate C5a function, with heteromer formation being inhibited by C5aRA (Croker et al., 2013). Our studies do not provide information regarding the importance of C5L2 or the cell or tissue location of the important C5aR for mediating hypertension. Further studies are needed to investigate these possibilities.
Both C3a and C5a have distinct acute hemodynamic effects in the anesthetized rat, with C3a increasing blood pressure transiently and C5a causing a more prolonged decrease in blood pressure (Proctor et al., 2009). In the guinea pig, we previously demonstrated that C5a increases blood pressure via thromboxane and the acute increase in blood pressure is dependent on WBCs and platelets (Fraser and Regal, 1990). Vascular reactivity to agonists can vary during pregnancy when compared with the nonpregnant state (Paller, 1987), but our current studies indicate that a pregnant rat reacts to C3a peptide with a similar acute pressor response as a nonpregnant rat (Proctor et al., 2006). The C3aRA prevents the increase in blood pressure, indicating that it is an effective antagonist of the C3a receptor (C3aR) in the pregnant and nonpregnant rat. The reduction in circulating C3a following administration of the C3aRA SB290157 suggests that it has other effects and may exert its inhibitory effect on placental ischemia–induced hypertension by non-C3aR actions, such as interfering with generation of C3a or complement activation in general. SB290157 in vitro has demonstrated high efficacy and specificity for the C3aR in rat and human cells, and it does not disrupt C5a-mediated cellular responses in vitro (Ames et al., 2001). However, Proctor et al. (2004) noted that SB290157 in vivo caused an acute transient increase in blood pressure and decrease in circulating neutrophils with intravenous injection, suggesting that it was also acting as a C3aR agonist. They concluded that the favorable effect of SB290157 in intestinal ischemia/reperfusion was likely due to its agonist action on neutrophils rather than its antagonism of C3aR (Wu et al., 2013). Mathieu et al. (2005) also described agonist activity of SB290157 in systems with high C3aR density, and others observed similar agonist properties in Chinese hamster ovary cells and human U937 cells (Scully et al., 2010). In our study, the most perplexing limitation of the C3aRA was the fact that at 5 mg/kg it inhibited the RUPP-induced increase in C3a itself, and an explanation for this is not readily apparent. These data temper conclusions regarding the importance of C3a in our studies and suggest that the C3aRA is affecting hypertension by both C3a receptor and nonreceptor pathways. In addition, our studies temper a conclusive role for C3aR in hypertension and proteinuria in pregnancy following adoptive transfer of angiotensin II type 1 receptor into third-trimester mice (Wang et al., 2012), in which doses up to 30 mg/kg per day of SB290157 were used to provide evidence for an important role of C3a. In our studies, the C3aRA SB290157 definitely inhibits increases in blood pressure following either C3a administration or placental ischemia, suggesting that C3a is an important mediator of placental ischemia–induced hypertension. However, non-C3aR effects of SB290157 may also be operating in vivo to reduce placental ischemia–induced hypertension.
In a recently published case report (Burwick and Feinberg, 2013), eculizumab, an antibody to C5, favorably extended pregnancy in a woman with severe pre-eclampsia. Whether eculizumab was effective in prolonging pregnancy because of its ability to limit C5a or C5b-9 formation or both is unknown. However, our studies in the rat model suggest that eculizumab may attenuate the severity of pre-eclampsia in part through its ability to limit formation of C5a, and further studies are under way to evaluate this possibility. Our studies to date have also not addressed how complement is activated following placental ischemia or which pathway(s) of complement activation is leading to the increase in circulating C3a. In a pregnant mouse model, studies by Wang et al. (2012) indicate that infusion of autoantibodies to the angiotensin II type 1 receptor lead to complement activation and hypertension, so a role for autoantibodies in RUPP-induced complement activation is possible. In addition, future immunohistochemistry studies are warranted to determine the site of complement activation by determining if C3 deposition is occurring in the vasculature and/or placenta and is associated with the increased blood pressure seen following placental ischemia.
Overall, our studies suggest an important role for both C3aR and C5aR in mediating placental ischemia–induced hypertension. Differential effects of the C3a and C5a receptor antagonists on endothelial cell–dependent vascular relaxation, cardiovascular function, and circulating neutrophil count suggest that C5a contributes to hypertension by increasing heart rate and impairing endothelial-dependent relaxation of blood vessels, whereas the C3aRA likely affects placental ischemia–induced hypertension by both C3aR and non-C3aR pathways. Previous studies of others have also demonstrated an important role for angiotensin II type 1 receptor agonistic autoantibodies, lymphocytes, endothelin, interleukin-10, interleukin-6, tumor necrosis factor, and angiogenic imbalance in placental ischemia–induced hypertension, with partial attenuation of the hypertension as each of these pathways is manipulated. The interrelationship of complement activation with these mediator pathways is unexplored, as is the mechanism of complement activation following placental ischemia. Clearly, a complex array of mediators and pathways, including the complement activation products C3a and C5a, are involved, and data suggest that therapeutic strategies for management of placental ischemia–induced hypertension will require a broad anti-inflammatory approach including therapy targeted at limiting the production or activity of complement activation products.
Supplementary Material
Acknowledgments
The authors thank Megan Strehlke and Courtney Bauer for excellent technical assistance in the completion of these studies.
Abbreviations
- Ach
acetylcholine
- C3aR
C3a receptor
- C3aRA
C3a receptor antagonist
- C5aR
C5a receptor
- C5aRA
C5a receptor antagonist
- CH50
inverse dilution of serum causing 50% hemolysis of sensitized sheep erythrocytes
- GD
gestational day
- MAP
mean arterial pressure
- PMX53
acetyl-F-[Orn-P-(D-Cha)-WR]
- RUPP
reduced uterine perfusion pressure
- SB290157
N2-[(2,2-diphenylethoxy)acetyl]-l-arginine
- Sham
sham surgery
- SNP
sodium nitroprusside
- U46619
9α,11α-methanoepoxy-PGF2α
- VEGF
vascular endothelial growth factor
- Veh
10% ethanol/saline vehicle
- WBC
white blood cell
Authorship Contributions
Participated in research design: Lillegard, R. Regal, Gilbert, J. Regal.
Conducted experiments: Lillegard, Loeks-Johnson, Opacich, Peterson, Bauer, Elmquist, Gilbert, J. Regal.
Performed data analysis: R. Regal, J. Regal.
Wrote or contributed to the writing of the manuscript: Lillegard, Loeks-Johnson, Opacich, Peterson, Bauer, Elmquist, R. Regal, Gilbert, J. Regal.
Footnotes
This work was supported by the National Institutes of Health National Heart, Lung, and Blood Institute [Grants R15-HL109843 (to J.F.R. and J.S.G.) and R01-HL114096 to (J.S.G.)].
Some of the data presented in this article were published in abstract form as part of conference proceedings: Peterson JM, Lillegard KE, Elmquist BE, Gilbert JS, and Regal JF (2014) Complement C5a receptor antagonist attenuates placental ischemia-induced hypertension in rat (Abstract). FASEB J 28:1084.7; Opacich JW, Johnson AC, Bauer AJ, Gilbert JS, and Regal JF (2014) Antagonism of complement C5a receptor but not C3a receptor attenuates placental ischemia-induced endothelial dysfunction in rat (Abstract). FASEB J 28:1084.8; and Regal JF, Lillegard KE, Elmquist BJ, Bauer AJ, Johnson AC, Opacich JW, Peterson JM, and Gilbert JS (2013) Complement C3a receptor antagonist attenuates placental ischemia-induced hypertension in rat (Abstract). Hypertension 62:A10.
 This article has supplemental material available at jpet.aspetjournals.org.
This article has supplemental material available at jpet.aspetjournals.org.
References
- American College of Obstetricians and Gynecologists. Task Force on Hypertension in Pregnancy (2013) Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol 122:1122–1131 [DOI] [PubMed] [Google Scholar]
- Ames RS, Lee D, Foley JJ, Jurewicz AJ, Tornetta MA, Bautsch W, Settmacher B, Klos A, Erhard KF, Cousins RD, et al. (2001) Identification of a selective nonpeptide antagonist of the anaphylatoxin C3a receptor that demonstrates antiinflammatory activity in animal models. J Immunol 166:6341–6348 [DOI] [PubMed] [Google Scholar]
- Ananth CV, Keyes KM, Wapner RJ. (2013) Pre-eclampsia rates in the United States, 1980-2010: age-period-cohort analysis. BMJ 347:f6564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer AJ, Banek CT, Needham K, Gillham H, Capoccia S, Regal JF, Gilbert JS. (2013) Pravastatin attenuates hypertension, oxidative stress, and angiogenic imbalance in rat model of placental ischemia-induced hypertension. Hypertension 61:1103–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björk J, Hugli TE, Smedegård G. (1985) Microvascular effects of anaphylatoxins C3a and C5a. J Immunol 134:1115–1119 [PubMed] [Google Scholar]
- Bosmann M, Haggadone MD, Zetoune FS, Sarma JV, Ward PA. (2013) The interaction between C5a and both C5aR and C5L2 receptors is required for production of G-CSF during acute inflammation. Eur J Immunol 43:1907–1913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burwick RM, Feinberg BB. (2013) Eculizumab for the treatment of preeclampsia/HELLP syndrome. Placenta 34:201–203 [DOI] [PubMed] [Google Scholar]
- Burwick RM, Fichorova RN, Dawood HY, Yamamoto HS, Feinberg BB. (2013) Urinary excretion of C5b-9 in severe preeclampsia: tipping the balance of complement activation in pregnancy. Hypertension 62:1040–1045 [DOI] [PubMed] [Google Scholar]
- Buurma A, Cohen D, Veraar K, Schonkeren D, Claas FH, Bruijn JA, Bloemenkamp KW, Baelde HJ. (2012) Preeclampsia is characterized by placental complement dysregulation. Hypertension 60:1332–1337 [DOI] [PubMed] [Google Scholar]
- Collard CD, Väkevä A, Morrissey MA, Agah A, Rollins SA, Reenstra WR, Buras JA, Meri S, Stahl GL. (2000) Complement activation after oxidative stress: role of the lectin complement pathway. Am J Pathol 156:1549–1556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croker DE, Halai R, Fairlie DP, Cooper MA. (2013) C5a, but not C5a-des Arg, induces upregulation of heteromer formation between complement C5a receptors C5aR and C5L2. Immunol Cell Biol 91:625–633 [DOI] [PubMed] [Google Scholar]
- Derzsy Z, Prohászka Z, Rigó J, Jr, Füst G, Molvarec A. (2010) Activation of the complement system in normal pregnancy and preeclampsia. Mol Immunol 47:1500–1506 [DOI] [PubMed] [Google Scholar]
- Ember JA, Johansen NL, Hugli TE. (1991) Designing synthetic superagonists of C3a anaphylatoxin. Biochemistry 30:3603–3612 [DOI] [PubMed] [Google Scholar]
- Finch AM, Wong AK, Paczkowski NJ, Wadi SK, Craik DJ, Fairlie DP, Taylor SM. (1999) Low-molecular-weight peptidic and cyclic antagonists of the receptor for the complement factor C5a. J Med Chem 42:1965–1974 [DOI] [PubMed] [Google Scholar]
- Fraser DG, Regal JF. (1990) C5a/C5ades-Arg-induced increase in blood pressure in the guinea pig: role of thromboxane. Immunopharmacology 19:59–68 [DOI] [PubMed] [Google Scholar]
- Gilbert JS, Banek CT, Bauer AJ, Gingery A, Dreyer HC. (2012a) Placental and vascular adaptations to exercise training before and during pregnancy in the rat. Am J Physiol Regul Integr Comp Physiol 303:R520–R526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert JS, Banek CT, Bauer AJ, Gingery A, Needham K. (2012b) Exercise training attenuates placental ischemia-induced hypertension and angiogenic imbalance in the rat. Hypertension 60:1545–1551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert JS, Banek CT, Katz VL, Babcock SA, Regal JF. (2012c) Complement activation in pregnancy: too much of a good thing? Hypertension 60:1114–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert JS, Bauer AJ, Gilbert SA, Banek CT. (2012d) The opposing roles of anti-angiogenic factors in cancer and preeclampsia. Front Biosci (Elite Ed) 4:2752–2769 [DOI] [PubMed] [Google Scholar]
- Gilbert JS, Bauer AJ, Gingery A, Banek CT, Chasson S. (2012e) Circulating and utero-placental adaptations to chronic placental ischemia in the rat. Placenta 33:100–105 [DOI] [PubMed] [Google Scholar]
- Gilbert JS, Verzwyvelt J, Colson D, Arany M, Karumanchi SA, Granger JP. (2010) Recombinant vascular endothelial growth factor 121 infusion lowers blood pressure and improves renal function in rats with placental ischemia-induced hypertension. Hypertension 55:380–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger JP, LaMarca BB, Cockrell K, Sedeek M, Balzi C, Chandler D, Bennett W. (2006) Reduced uterine perfusion pressure (RUPP) model for studying cardiovascular-renal dysfunction in response to placental ischemia. Methods Mol Med 122:383–392 [DOI] [PubMed] [Google Scholar]
- Greene AL, Rutherford MS, Regal RR, Flickinger GH, Hendrickson JA, Giulivi C, Mohrman ME, Fraser DG, Regal JF. (2005) Arginase activity differs with allergen in the effector phase of ovalbumin- versus trimellitic anhydride-induced asthma. Toxicol Sci 88:420–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood JP, Scott EM, Stoker JB, Walker JJ, Mary DA. (2001) Sympathetic neural mechanisms in normal and hypertensive pregnancy in humans. Circulation 104:2200–2204 [DOI] [PubMed] [Google Scholar]
- Hines T, Beauchamp D, Rice C. (2007) Baroreflex control of sympathetic nerve activity in hypertensive pregnant rats with reduced uterine perfusion. Hypertens Pregnancy 26:303–314 [DOI] [PubMed] [Google Scholar]
- Kerr H, Richards A. (2012) Complement-mediated injury and protection of endothelium: lessons from atypical haemolytic uraemic syndrome. Immunobiology 217:195–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klos A, Wende E, Wareham KJ, Monk PN. (2013) International Union of Basic and Clinical Pharmacology. [corrected]. LXXXVII. Complement peptide C5a, C4a, and C3a receptors. Pharmacol Rev 65:500–543 [DOI] [PubMed] [Google Scholar]
- LaMarca B, Cornelius D, Wallace K. (2013) Elucidating immune mechanisms causing hypertension during pregnancy. Physiology (Bethesda) 28:225–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillegard KE, Bauer AJ, Johnson AC, Lojovich SJ, Gilbert JS, Regal JF. (2013a) Neutrophil depletion attenuates placental ischemia-induced hypertension in rat. FASEB J 27:907.423180826 [Google Scholar]
- Lillegard KE, Johnson AC, Lojovich SJ, Bauer AJ, Marsh HC, Gilbert JS, Regal JF. (2013b) Complement activation is critical for placental ischemia-induced hypertension in the rat. Mol Immunol 56:91–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch AM, Salmon JE. (2010) Dysregulated complement activation as a common pathway of injury in preeclampsia and other pregnancy complications. Placenta 31:561–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason JC, Steinberg R, Lidington EA, Kinderlerer AR, Ohba M, Haskard DO. (2004) Decay-accelerating factor induction on vascular endothelium by vascular endothelial growth factor (VEGF) is mediated via a VEGF receptor-2 (VEGF-R2)- and protein kinase C-alpha/epsilon (PKCalpha/epsilon)-dependent cytoprotective signaling pathway and is inhibited by cyclosporin A. J Biol Chem 279:41611–41618 [DOI] [PubMed] [Google Scholar]
- Mathieu MC, Sawyer N, Greig GM, Hamel M, Kargman S, Ducharme Y, Lau CK, Friesen RW, O’Neill GP, Gervais FG, et al. (2005) The C3a receptor antagonist SB 290157 has agonist activity. Immunol Lett 100:139–145 [DOI] [PubMed] [Google Scholar]
- Paller MS. (1987) Decreased pressor responsiveness in pregnancy: studies in experimental animals. Am J Kidney Dis 9:308–311 [DOI] [PubMed] [Google Scholar]
- Proctor LM, Arumugam TV, Shiels I, Reid RC, Fairlie DP, Taylor SM. (2004) Comparative anti-inflammatory activities of antagonists to C3a and C5a receptors in a rat model of intestinal ischaemia/reperfusion injury. Br J Pharmacol 142:756–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor LM, Moore TA, Monk PN, Sanderson SD, Taylor SM, Woodruff TM. (2009) Complement factors C3a and C5a have distinct hemodynamic effects in the rat. Int Immunopharmacol 9:800–806 [DOI] [PubMed] [Google Scholar]
- Proctor LM, Strachan AJ, Woodruff TM, Mahadevan IB, Williams HM, Shiels IA, Taylor SM. (2006) Complement inhibitors selectively attenuate injury following administration of cobra venom factor to rats. Int Immunopharmacol 6:1224–1232 [DOI] [PubMed] [Google Scholar]
- Regal JF. (1982) C5a-induced aortic contraction: effect of an antihistamine and inhibitors of arachidonate metabolism. J Pharmacol Exp Ther 220:102–107 [PubMed] [Google Scholar]
- Regal JF. (1985) Effect of C5a on isolated guinea pig atria. Immunopharmacology 9:27–31 [DOI] [PubMed] [Google Scholar]
- Regal JF, Eastman AY, Pickering RJ. (1980) C5a induced tracheal contraction: a histamine independent mechanism. J Immunol 124:2876–2878 [PubMed] [Google Scholar]
- Ricklin D, Lambris JD. (2013) Complement in immune and inflammatory disorders: therapeutic interventions. J Immunol 190:3839–3847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scully CC, Blakeney JS, Singh R, Hoang HN, Abbenante G, Reid RC, Fairlie DP. (2010) Selective hexapeptide agonists and antagonists for human complement C3a receptor. J Med Chem 53:4938–4948 [DOI] [PubMed] [Google Scholar]
- Singh J, Ahmed A, Girardi G. (2011) Role of complement component C1q in the onset of preeclampsia in mice. Hypertension 58:716–724 [DOI] [PubMed] [Google Scholar]
- Soto E, Romero R, Richani K, Espinoza J, Chaiworapongsa T, Nien JK, Edwin SS, Kim YM, Hong JS, Goncalves LF, et al. (2010) Preeclampsia and pregnancies with small-for-gestational age neonates have different profiles of complement split products. J Matern Fetal Neonatal Med 23:646–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usami M, Mitsunaga K, Miyajima A, Sunouchi M, Doi O. (2010) Complement component C3 functions as an embryotrophic factor in early postimplantation rat embryos. Int J Dev Biol 54:1277–1285 [DOI] [PubMed] [Google Scholar]
- Wang W, Irani RA, Zhang Y, Ramin SM, Blackwell SC, Tao L, Kellems RE, Xia Y. (2012) Autoantibody-mediated complement C3a receptor activation contributes to the pathogenesis of preeclampsia. Hypertension 60:712–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissgerber TL, McConico A, Knudsen BE, Butters KA, Hayman SR, White WM, Milic N, Miller VM, Garovic VD. (2014) Methodological differences account for inconsistencies in reported free VEGF concentrations in pregnant rats. Am J Physiol Regul Integr Comp Physiol 306:R796–R803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MC, Brennan FH, Lynch JP, Mantovani S, Phipps S, Wetsel RA, Ruitenberg MJ, Taylor SM, Woodruff TM. (2013) The receptor for complement component C3a mediates protection from intestinal ischemia-reperfusion injuries by inhibiting neutrophil mobilization. Proc Natl Acad Sci USA 110:9439–9444 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.