Abstract
Alcohol drinking during adolescence is associated with increased alcohol drinking and alcohol dependence in adulthood. Research examining the biologic consequences of adolescent ethanol (EtOH) consumption on the response to EtOH in the neurocircuitry shown to regulate drug reinforcement is limited. The experiments were designed to determine the effects of periadolescent alcohol drinking on the reinforcing properties of EtOH within the posterior ventral tegmental area (pVTA) and the ability of EtOH microinjected into the pVTA to stimulate dopamine (DA) release in the nucleus accumbens shell (AcbSh). EtOH access (24-hour free-choice) by alcohol-preferring rats occurred during postnatal days (PND) 30–60. Animals were tested for their response to EtOH after PND 85. Intracranial self-administration techniques were performed to assess EtOH self-infusion into the pVTA. In the second experiment, rats received microinjections of EtOH into the pVTA, and dialysis samples were collected from the AcbSh. The results indicate that in rats that consumed EtOH during adolescence, the pVTA was more sensitive to the reinforcing effects of EtOH (a lower concentration of EtOH supported self-administration) and the ability of EtOH microinjected into the pVTA to stimulate DA release in the AcbSh was enhanced (sensitivity and magnitude). The data indicate that EtOH consumption during adolescence altered the mesolimbic DA system to be more sensitive and responsive to EtOH. This increase in the response to EtOH within the mesolimbic DA during adulthood could be part of biologic sequelae that are the basis for the deleterious effects of adolescent alcohol consumption on the rate of alcoholism during adulthood.
Introduction
For the vast majority of people in the United States, the initiation of alcohol use begins during adolescence. A high percentage (12%) of adolescents begin using alcohol during middle school (eighth grade), and before high school graduation, the vast majority of people in the United States (80–90%) have consumed alcohol (Johnston et al., 2004). In combination with moderate alcohol consumption, adolescents engage in binge drinking episodes. Self-reports have indicated that 22 and 28% of 10th and 12th grade students, respectively, reported an incident of binge drinking within the past 2 weeks (Johnston et al., 2004). College students report a previous high level of binge drinking during high school (70%) and frequent ongoing episodes of binge drinking during college (44%); a subset of college students are frequent binge drinkers (19–25% report more than three episodes of binge drinking per week) (Wechsler et al., 1995, 2000; SAMHSA, 2008).
Alcohol consumption during adolescence is associated with a number of deleterious consequences. Age of first drink and the propensity to have binge ethanol (EtOH) drinking episodes during adolescence are associated with increased alcohol involvement, heavier drinking bouts, arrests for driving with ability impaired, and an increased rate of alcohol dependence during adulthood (Chou and Pickering, 1992; Hingson et al., 2006, 2008). Epidemiologic studies have indicated a 1.3 to 1.6 times increased rate of alcohol dependence in individuals who initiate alcohol use before the age of 15 (Dawson et al., 2008). The deleterious effects of adolescent EtOH consumption on adult alcohol dependence are compounded in individuals with a family history of alcoholism (Agrawal et al., 2009; Jacobus et al., 2009).
The neurologic remodeling of the adolescent brain is extensive and includes cortical and limbic regions (Spear, 2000). In general, early adolescence is marked by an overproduction of axons and synapses, followed by a rapid pruning during late adolescence (Giedd, 2004). For example, glutamatergic projections into the prefrontal cortex (PFC) increase during early adolescence and subsequently significantly reduce by dendritic pruning and synaptic regression during late adolescence (Zecevic et al., 1989). Similar patterns of growth and pruning are observed in the hippocampus, nucleus accumbens (Acb), amygdala, hypothalamus, ventral tegmental area (VTA), and further cortical regions (c.f., Giedd, 2004; Gulley and Juraska, 2013). It is the interaction between adolescent EtOH drinking and the neurologic flux of adolescence that is thought to produce the enduring deleterious consequences observed in adult alcoholics.
Neurotransmitter systems are also altered through the transitional process of adolescence. Excitatory inputs into the Acb and VTA are pruned with an associated reduction in glutamate receptors in those areas during adolescence (Thomas et al., 2001). The glutamate system is involved in plasticity and learning and is directly modulated by EtOH. Thus, the glutamatergic system has been hypothesized to be a potential system that regulates the enduring effects of adolescent binge EtOH drinking (Carpenter-Hyland and Chandler, 2007; Crews et al., 2007). Similarly, serotonergic innervation of the mesocorticolimbic system peaks during adolescence and is subsequently reduced through dendritic pruning (Rosenberg and Lewis, 1994). In particular, the serotonin 5-HT2A receptor expression peaks during adolescence in the PFC and limbic regions (VTA) but are reduced during maturation (Morilak and Ciaranello, 1993). A consequence of adolescent EtOH binge drinking is an increase in adult levels of serotonin transporters, possibly indicating alterations in serotonin activity (Monti et al., 2005). The reorganization of the dopamine (DA) system during adolescence is regional and receptor subtype–specific (Tarazi and Baldessarini, 2000). In the mesocorticolimbic system (PFC, VTA, and Acb), the numbers of DA neurons peak during adolescence, and subsequent pruning in these areas is associated with maturation of reward and motor pathways (c.f., Crews et al., 2007). The glutamatergic and serotonergic systems directly activate VTA DA neurons in adults. Alterations in these neurotransmitter systems within the VTA by adolescent EtOH consumption may alter the response of the VTA to EtOH during adulthood (c.f., McBride et al., 2005).
Adolescent EtOH consumption can alter the mesolimbic DA system. Adolescent EtOH consumption (Sahr et al., 2004), as well as peripheral EtOH injections (Badanich et al., 2007), can increase basal DA levels or DA reuptake in the Acb shell (AcbSh) during adulthood. Similar results are not produced in adult rats with comparable EtOH exposure paradigms (Pascual et al., 2009). The mesolimbic DA system is altered in response to non-EtOH stimuli during adulthood after adolescent EtOH consumption. Consumption of EtOH during adolescence in rats results in greater DA release in the AcbSh after “risky”-choice responding compared with adolescent naïve controls (Nasrallah et al., 2009).
Periadolescent alcohol drinking by alcohol-preferring (P) rats has been reported to produce long-lasting alterations in the reinforcing effects of EtOH, as indicated by P rats with access to EtOH compared with the water control group, acquiring the acquisition of EtOH operant responding sooner, showing a greater resistance to extinguish responding, and having a more prolonged elevated level of relapse responding for EtOH (Rodd-Henricks et al., 2002a). However, central nervous system mechanisms underlying these long-lasting alterations have not yet been explored.
In naïve rats, EtOH is directly self-administered into the posterior, but not anterior, VTA (Rodd-Henricks et al., 2000). The reinforcing properties of EtOH within the posterior VTA (pVTA) are dependent on the activation of VTA DA neurons (Rodd et al., 2005a). Selective breeding for high P rats is associated with an increase in the sensitivity of the pVTA to the reinforcing properties of EtOH compared with Wistar rats (lower concentrations required to support self-administration (Rodd et al., 2004, 2005a). Chronic EtOH consumption (>10 weeks 24-hour free-choice access) can further reduce the concentration of EtOH required to support self-administration directly into the pVTA (Rodd et al., 2005b). Exposure to repeated periods of excessive EtOH consumption can enhance the magnitude of EtOH self-administration (higher rate of self-administration for equivalent concentration) in P rats (Rodd et al., 2005c). The biologic bases for these effects are unknown.
The current experiments were designed to determine the effects of adolescent EtOH consumption on the actions of EtOH within the mesolimbic DA system during adulthood. The first experiment determined whether adolescent EtOH consumption would alter the self-infusion of EtOH directly into the pVTA. The second experiment determined whether adolescent EtOH consumption would alter the effects of EtOH microinjected directly into the pVTA to stimulate DA release in the AcbSh. The overall hypothesis was that adolescent EtOH consumption should enhance the reinforcing properties of EtOH within the pVTA and promote EtOH’s ability to stimulate the mesolimbic DA system.
Materials and Methods
Subjects.
The adolescent EtOH access procedures used herein followed published procedures from our laboratory (Rodd-Henricks et al., 2002a; Bell et al., 2003, 2004, 2006; Sahr et al., 2004). Male P rats were chosen for use in the current study because their rapid growth postadolescence increases skull strength for earlier cannula placement surgery. Previous work (McKinzie et al., 1998; Bell et al., 2003) described the differences between male and female P rats in EtOH drinking at these ages as minimal. The main benefit of using the P rats is that they voluntarily consume pharmacologically relevant levels of EtOH during adolescence, thus avoiding the stress of other administration procedures (e.g., repeated injections).
Animals were maintained in facilities fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care. All research protocols were approved by the Indiana University School of Medicine (Indianapolis, IN) Institutional Animal Care and Use Committee and were in accordance with the guidelines of the Institutional Care and Use Committee of the National Institutes of Health National Institute on Drug Abuse and the 2011 Guide for the Care and Use of Laboratory Animals.
Periadolescent EtOH Exposure Procedure.
Pups were single-housed in hanging stainless steel cages (Allentown Caging Equipment Co, Allentown, NJ) on postnatal day (PND) 28. Subjects were initially maintained on a 12-hour light/dark cycle, lights on at 9:00 AM. On PND 30, subjects either received ad libitum water or had continuous access to 15% v/v EtOH and water, until PND 60 as previously described (Rodd-Henricks et al., 2002a). Food was available ad libitum. Bottle and body weights for all subjects were recorded every other day.
On PND 60, EtOH access ceased, and subjects were pair-housed in standard shoebox cages, within the same treatment conditions. Subjects were also immediately transferred to a 12-hour reverse dark/light cycle, lights off at 10:00 AM, to optimize rats’ nocturnal activity levels for later procedures. After PND 60, subjects received no further oral EtOH intake experience.
Intracranial Self-Administration Procedure.
Test chambers (Coulbourn Instruments, Allentown, PA) were situated in sound-attenuating cubicles, as described previously (Rodd-Henricks et al., 2000; Rodd et al., 2004). Intracranial self-administration (ICSA) was performed as previously described (Rodd-Henricks et al., 2000; Rodd et al., 2004). After PND 75, the rats were implanted under isoflurane anesthesia with a guide cannula (22-gauge; Plastics One, Roanoke, VA) stereotaxically aimed 1.0 mm above the pVTA. Coordinates were 5.8 to 6.1 mm posterior to bregma, 2.1 mm lateral, and 8.5 mm ventral from the surface of the skull at a 10-degree angle from the vertical (Paxinos and Watson, 1986). A place-holding stylet (28-gauge; Plastics One) extending 0.5 mm beyond the tip of the guide cannula was inserted at all times, except during test sessions. Subjects were single-housed postsurgery and allowed to recover for 7 days. Starting 3 days before testing, subjects were handled 5 minutes per day.
All infusates were prepared fresh on the day of the experiment. Artificial cerebrospinal fluid (aCSF) was used as the vehicle for ICSA infusions. This injection vehicle consisted of (in mM) 120.0 NaCl, 4.8 KCl, 1.2 KH2PO4, 1.2 MgSO4, 25.0 NaHCO3, 2.5 CaCl2, and 10.0 d-glucose, all filtered through a sterile filter (pore size, 0.2 µM) as previously described (Rodd-Henricks et al., 2000; Rodd et al., 2004). Ethyl EtOH (190 proof; McCormick Distilling Co., Weston, MO) was dissolved in the vehicle solution to the correct concentration. When necessary, 0.5 N HCl was added to adjust the pH to 7.4 (±0.1).
Intracranial self-administration was conducted similar to procedures previously described (Rodd-Henricks et al., 2000; Rodd et al., 2004). Briefly, subjects were brought to the testing room, the stylet was removed, and an injection cannula/infusate cylinder was affixed in place. The injection cannula extended 1.0 mm beyond the tip of the guide, into the pVTA. A single, noncontingent administration of infusate was given at the beginning of the session during this insertion procedure to prime the system. Test sessions occurred every other day. No operant shaping techniques were used. Active lever and inactive lever sides were counterbalanced between subjects, remaining the same for each individual rat. Within each 4-hour session, responses on the active lever resulted in 5-second infusions on a fixed-ratio-1 schedule of reinforcement. During infusion and timeout (5 seconds), responses on the active lever were recorded but did not produce further infusions. Responses on the inactive lever were recorded but did not result in infusions at any time; these responses were used to index nonspecific bar-pressing activity. During ICSA sessions 1 through 4 (acquisition), subjects received their respective dose of either the aCSF vehicle or EtOH. During ICSA sessions 5 and 6 (extinction), all subjects received aCSF vehicle only, and in session 7 (reinstatement), the original concentration was made available.
A total of 82 P males were used in the experiment (n = 41/41 adolescent naïve/adolescent drinkers). Rats from the adolescent groups were randomly assigned to groups that self-administered 0 (aCSF), 50, 75, 100, or 150 mg% EtOH directly into the pVTA (adolescent naïve, n = 6–10/group; adolescent drinkers, n = 7–10/group).
Microinjection-Microdialysis Procedure.
Experimental housing was composed of Plexiglas chambers (40 × 28 × 40 cm). Polyethylene tubing connected to a dedicated Harvard pump (Harvard Apparatus, Holliston, MA) was used to administer aCSF continuously throughout the experiment for microdialysis in the AcbSh. The connection to the electrolytic microinfusion transducer unit for microinjection administration to the pVTA was identical to that used in the ICSA experiment, although automated control of injections was programmed into the unit (Isolated Pulse Stimulator Model 2100; A-M Systems Inc., Sequim, WA), instead of the separate computer controlling and recording operant self-administration.
Microdialysis probes were prepared as approximately 2-mm loop style probes, as previously described (Perry and Fuller, 1992; Engleman et al., 2000; Melendez et al., 2002). Probes were manufactured in the laboratory with regenerated cellulose Spectra/Por hollow fiber microdialysis tubing, molecular weight cutoff of 13,000. In our laboratory, these have been previously found to have approximately 15% DA recovery, which is an average recovery rate for this design and probe length (Justice, 1993).
Adolescent EtOH treatment occurred as in the first experiment. Food and water were available ad libitum at all times, except during microinjection-microdialysis testing, which was performed as previously described (Ding et al., 2012). After PND 75, and under isoflurane anesthesia, a microinjection guide cannula (22-gauge; Plastics One) was implanted in the right hemisphere of each subject, stereotaxically aimed 1.0 mm above the pVTA. Coordinates were 5.8 to 6.1 mm posterior to bregma, 2.1 mm lateral, and 8.5 mm ventral from the surface of the skull at a 10-degree angle from the vertical (Paxinos and Watson, 1986). A place-holding stylet (28-gauge; Plastics One) extending 0.5 mm beyond the tip of the guide cannula was inserted at all times, except during final experimentation. Also in the right hemisphere, a guide cannula (18-gauge; Plastics One) was implanted, aimed 3.0 mm above the AcbSh. Coordinates were 1.7 mm anterior to bregma, 2.3 mm lateral, and 5.4 mm ventral to the surface of the skull at a 10-degree angle from the vertical (Paxinos and Watson, 1986). A place-holding stylet (Plastics One) extending 0.5 mm was inserted at all times, except during final experimentation.
Subjects were single-housed after surgery and allowed to recover for 6 days. Three days before testing, subjects were habituated to the testing chambers for 3 h/day. One day before testing, microdialysis probes were inserted into the AcbSh with the animals under isoflurane anesthesia. When inserted, the dialyzing loop was oriented in an anterior-posterior direction to maximize exposure to the AcbSh. The probe extended 3 mm below the guide cannula, into the AcbSh.
The next day, the microinjection-microdialysis procedure was performed. Subjects were placed in the experimental chambers, and the microdialysis probe tubing was connected to the pump for aCSF perfusion. The aCSF microdialysis perfusion medium was composed (in mM) as previously described (Melendez et al., 2002): 145.0 NaCl, 2.7 KCl, 1.0 MgCl2, 2.5 CaCl2, and 2.0 Na2HPO4, filtered through a sterile filter (pore size, 0.2 µM), always prepared fresh the day of the procedure. When necessary, pH was adjusted to 7.4 with 0.1 N acetic acid.
Flow speed of the perfusion medium was 1 µl/min. Microdialysis outflow was collected for 15 minutes per sample. After at least 90 minutes of washout flow, at least three baseline dialysate samples were collected, followed by one microinjection dialysate sample.
During the microinjection sample, the pVTA-cannula stylet was removed and the injection cannula/infusate cylinder affixed in place. The injection cannula extended 1.0 mm beyond the tip of the guide, into the pVTA. Administration of the infusate occurred automatically, as programmed into the electrolytic microinfusion transducer machine. Each subject received microinjections of one solution only, prepared identically to that in the ICSA experiment: either infusate vehicle aCSF or 50, 75, 100, or 200 mg% EtOH. All subjects received the same volume of microinjections. Microinjections were composed of a series of 100-nl boluses, each delivered over an interval of 5 seconds, repeated every 20 seconds for a total of 30 microinjections over a 10-minute period into the pVTA.
After the microinjection sample, 120 minutes of postinjection dialysate samples were collected. All samples were collected into tubes containing 5 µl of 0.1 N perchloric acid. After collection, samples were immediately frozen on dry ice and stored at −70°C until high-performance liquid chromatography (HPLC) analysis for DA content.
A total of 74 P males were used in the microdialysis experiment (n = 37/37 adolescent naïve/adolescent drinkers). Rats from the adolescent groups were randomly assigned to groups that microinjected with 0 (aCSF), 50, 75, 100, or 150 mg% EtOH directly into the pVTA (adolescent naïve, n = 6–10/group; adolescent EtOH drinkers, n = 6–9/group).
All microdialysis samples were analyzed with microbore HPLC with electrochemical detection to determine extracellular levels of DA. Samples were loaded into a 10-µl sample loop and injected onto an analytical column. Detector output ran to a computer program for analysis (ChromPerfect; Justice Innovations, Inc., Palo Alto, CA). DA levels were then determined by comparison with a standard curve. The lower sensitivity limit for DA was estimated to be 0.1 nM. Complete details of the HPLC procedure have been previously published (Sahr et al., 2004).
Histologies.
On termination of the experiments, a solution of 1% bromophenol blue dye was injected into the infusion site, and the animals were sacrificed. Brains were removed and immediately frozen at −70°C, for slicing into 40-μm sections with a cryostat microtome. Slides were stained with cresyl violet and examined for infusion site verification using the atlas of Paxinos and Watson (1986).
Statistical Analysis.
The EtOH consumption was analyzed as pure EtOH intake (g EtOH/kg body weight, g/kg). Change in preference ratio for EtOH intake versus water was calculated by plotting ratios each day of EtOH exposure as a percentage of 15% EtOH solution consumed in relation to total fluids consumed ([g ethanol solution intake/g ethanol solution intake + g water intake] × 100). The first day of access was compared with the last day of access. Intake and preference data were averaged in six blocks of 5 days, analyzed with a repeated measures analysis of variance (ANOVA) and linear regression between blocks.
The ICSA data were analyzed with a dose × session mixed ANOVA, with repeated measures on session performed on the number of active lever responses and the number of infusions separately. For each individual group, lever discrimination was determined by “lever (active versus inactive) × session” mixed ANOVA with repeated measures on session. Post hoc Tukey’s b tests were used when a significant main effect was found (P < 0.05). Extinction was determined by comparing the responses on the active lever during sessions 4–6; reinstatement was determined by comparing the responses on the active lever during sessions 5–7.
Microdialysis data were expressed as a percentage of basal DA values to correct for between-subject baseline variability, as previously described (Engleman et al., 2006). Basal dialysate values for each subject were calculated as the mean of three baseline samples before the injection sample. Also, absolute levels of baseline DA were calculated and compared as group means between the two periadolescent exposure groups. The effects of periadolescent EtOH administration on basal extracellular DA levels and maximal drug effect with each dose was calculated using the Student’s t test. The effects of EtOH microinjection administration on extracellular DA levels as a function of time and adolescent treatment condition were analyzed using a two-way adolescent group × time ANOVA, with repeated measures on time (15-minute samples), followed by the Tukey’s post hoc test. α was set at a P < 0.05 significance level.
Results
Adolescent EtOH Consumption.
Initial consumption for all periadolescent EtOH-exposed subjects was 5.17 ± 0.36 g/kg daily (data not shown). At the end of the access period, consumption was 7.80 ± 0.31 g/kg daily. A repeated measures within subjects ANOVA performed on the average intake in 5-day blocks revealed a significant main effect of day (F5,51 = 5.627, P < 0.001), indicating that EtOH consumption changed over time. There was no significant effect of study group (ICSA and microinjection-microdialysis groups consumed equivalent amounts of EtOH; F1,55 = 1.948, P = 0.168). The amount of EtOH consumed would be predicted to produce significant, pharmacologically relevant (>50 mg%) blood alcohol concentrations (Bell et al., 2003, 2004, 2006).
ICSA EtOH Dose Response.
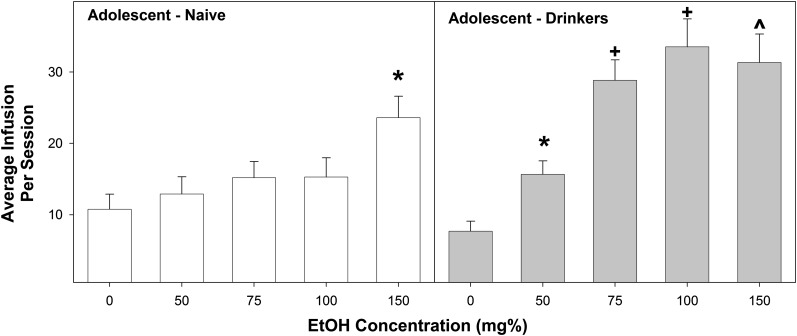
A multifactorial ANOVA was performed on the average number of infusions self-administered from sessions 1–4 (Fig. 1). The analysis indicated a significant adolescent group × EtOH concentration interaction (F4,72 = 2.85; P = 0.031). Reducing the interaction term was performed by examining the average number of infusions for each EtOH concentration. In rats self-administering aCSF, 50 mg%, or 150 mg%, there was no effect of adolescent group on infusions self-administered (F < 2.4; P > 0.14). In contrast, there was a significant difference between adolescent group (EtOH > naïve) in P rats self-infusing 75 or 100 mg% EtOH (F > 13.76; P < 0.003). In adolescent naïve rats, there was a significant effect of EtOH concentration on the number of self-infusions (F4,36 = 3.4; P = 0.019). Post hoc comparisons indicated that adolescent naïve rats received more self-infusions of 150 mg% EtOH than aCSF or 50 mg% EtOH. In the adolescent EtOH drinking rats, there was also a significant effect of EtOH concentration on the number of self-infusions (F4,36 = 14.44; P < 0.0001). Post hoc comparisons indicated that adolescent EtOH drinkers given 75, 100, or 150 mg% EtOH received more self-infusions than rats given aCSF or 50 mg% EtOH. In addition, adolescent EtOH drinkers given 50 mg% EtOH received more self-infusions than rats given aCSF.
Fig. 1.
Depicts the mean (±S.E.M.) average number of infusions self-administered directly into the pVTA during sessions 1–4 in adolescent naïve subjects (left) and adolescent drinkers (right). *Indicates significantly more infusions than aCSF controls. +Indicates significantly more infusions than aCSF controls and corresponding adolescent naïve group. ^Indicates significantly more infusions than aCSF controls and 50 mg% group (adolescent naïve, n = 6–10/group; adolescent drinkers, n = 7–10/group).
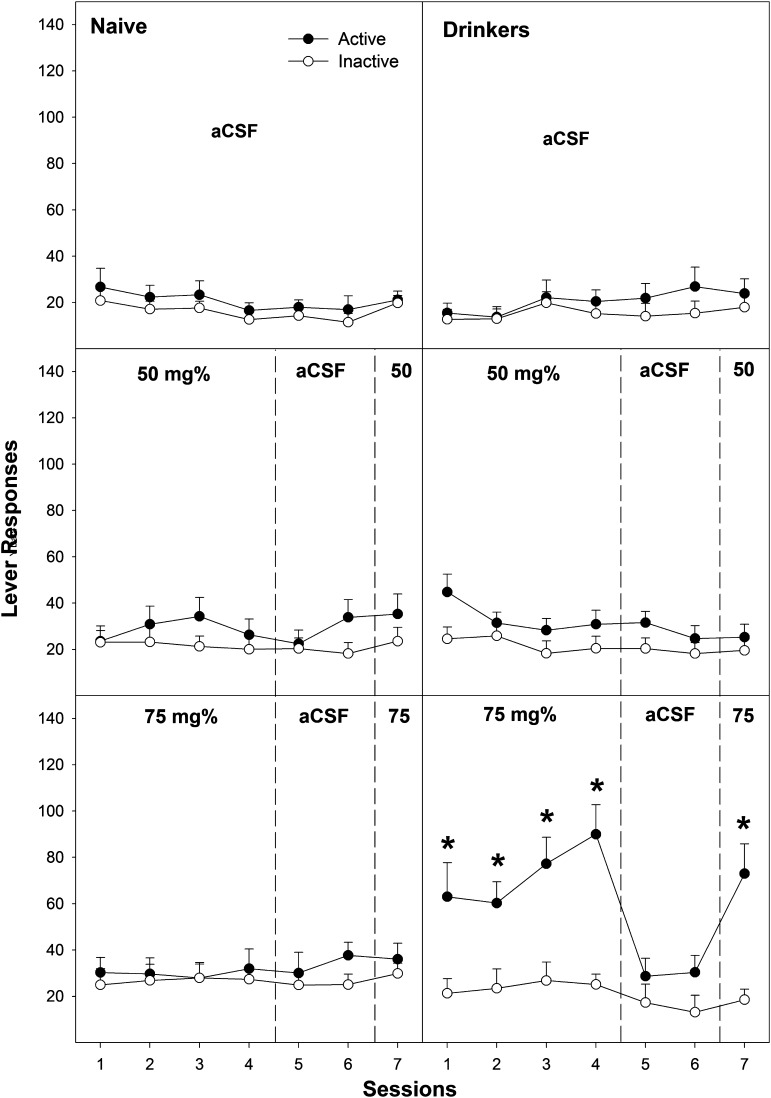
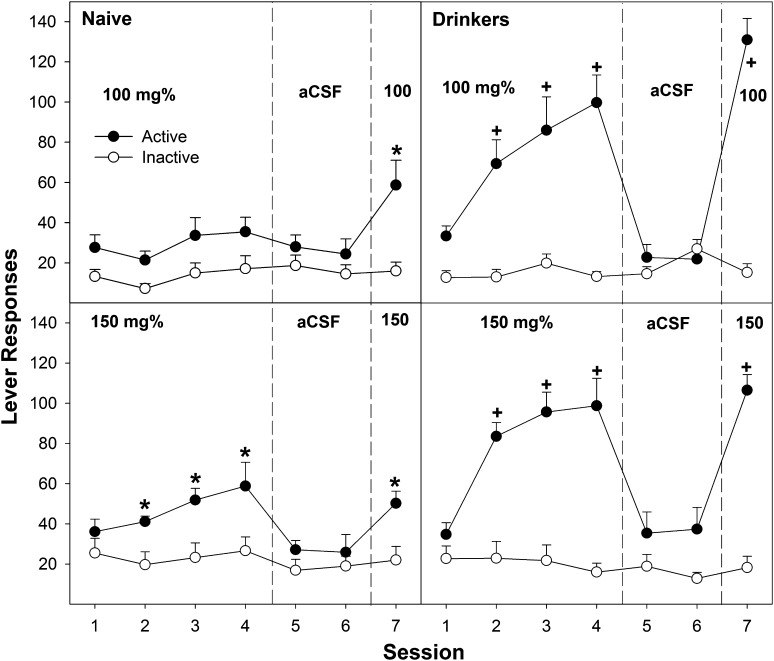
Examining the number of active and inactive lever responses across all seven sessions (Figs. 2 and 3) revealed a significant adolescent group × EtOH concentration × lever × session interaction term (F24,280 = 4.26; P < 0.001). In adolescent naïve rats, there was a significant EtOH concentration × lever × session interaction term (F24,136 = 2.82; P = 0.024). Performing individual ANOVAs on both the active and inactive lever responses for each session indicated only two significantly different sessions of responding. During the fourth operant session, there was a significant effect of EtOH concentration on active lever responding (F4,36 = 3.26; P = 0.022) with post hoc comparisons, indicating that the 150 mg% EtOH group was significantly different from all other groups (Fig. 3, bottom left). In the seventh session, there was also a significant effect of EtOH concentration on active lever responding (F4,36 = 3.52; P = 0.017), with post hoc comparisons indicating that the 100 and 150 mg% groups were significantly different from all other groups (Fig. 3, left). Analysis for lever discrimination indicated that the adolescent naïve P rats self-infusing 150 mg% EtOH discriminated between responding on the active and inactive lever during sessions 2–4 and 7 (P < 0.004). Adolescent naïve P rats given 100 mg% EtOH expressed lever discrimination only during the seventh session (P < 0.001). The 150 mg% EtOH group displayed extinction when aCSF was substituted (sessions 5 and 6), with a significant reduction from session 4 to sessions 5 (P = 0.003) and 6 (P = 0.02). The 100 and 150 mg% EtOH groups displayed reinstatement of EtOH self-infusions (significantly higher level of active lever responding during session 7 compared with session 6; P < 0.001).
Fig. 2.
Depicts the mean (±S.E.M.) number of lever responses in adolescent naïve subjects (left) and adolescent drinkers (right) given aCSF (top), 50 mg% (middle), or 75 mg% EtOH (bottom) to self-administer directly into the posterior VTA. *Indicates significantly more responding on the active lever than that observed in aCSF controls or 50 mg% groups and discrimination between levers.
Fig. 3.
Depicts the mean (± S.E.M.) number of lever responses in adolescent naïve subjects (left) and adolescent drinkers (right) given 100 mg% (top) or 150 mg% EtOH (bottom) to self-administer directly into the posterior VTA. *Indicates significantly more responding on the active lever than that observed in aCSF controls and discrimination between levers. +Indicates significantly more responding on the active lever than that observed in aCSF controls, discrimination between levers, and significantly more responding in the adolescent drinkers than adolescent naïve rats.
In adolescent EtOH drinkers, EtOH self-infusions occurred at lower concentrations and at higher levels (Figs. 2 and 3). In adolescent EtOH drinkers, there was a significant EtOH concentration × lever × session interaction term (F24,136 = 2.16; P = 0.03). During sessions 1–4 and 7, there was a significant effect of EtOH concentration on the number of active lever responses (F4,36 > 6.18; P < 0.001), but there were no significant effects on the inactive lever responding (F4,36 < 1.7; P ≥ 0.19). Post hoc comparisons indicated that during sessions 1–4, adolescent EtOH drinkers given 75, 100, and 150 mg% EtOH responded more on the active lever than did the aCSF and 50 mg% EtOH groups. During session 7, post hoc comparisons indicated that adolescent EtOH drinkers given 100 and 150 mg% EtOH responded more than the 75 mg% EtOH group, which was significantly higher than the aCSF and 50 mg% groups (100, 150 mg% > 75 mg% > aCSF, 50 mg%). During sessions 2–4, adolescent EtOH drinkers given 75, 100, and 150 mg% EtOH discriminated between the active and inactive levers (P < 0.014). These groups also reduced active lever responding during aCSF substitution (extinction, P < 0.025) and reinstated EtOH self-infusion during session 7 (P < 0.001).
Contrasting the adolescent naïve and EtOH drinkers at each individual EtOH concentration for active lever responding during each session provided evidence for enhanced responding by the adolescent EtOH drinkers. For rats self-infusing 75, 100, and 150 mg% EtOH directly into the pVTA, adolescent EtOH drinkers responded more on the active lever during sessions 2–4 and 7 than did adolescent naïve rats (all P < 0.013).
Microinjection-Microdialysis EtOH Dose Response.
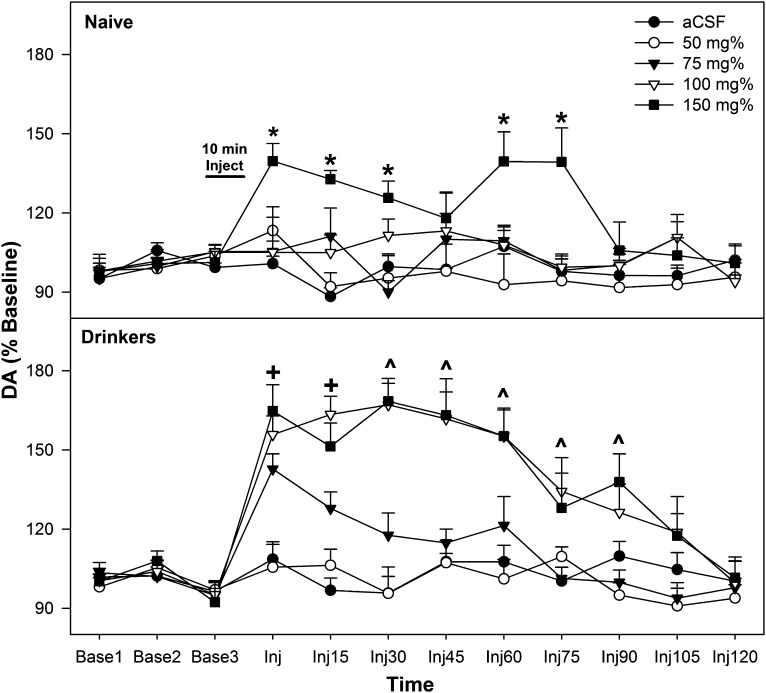
Similar to the ICSA data, the microdialysis experiment indicated that the pVTA of adolescent EtOH drinkers was more responsive than that of adolescent naïve rats to EtOH (Fig. 4). Overall, there was a significant adolescent group × EtOH concentration × time interaction term (F32,240 = 2.88; P = 0.009). The significant interaction term was initially decomposed by holding EtOH concentration constant. DA levels in the AcbSh did not differ in adolescent naïve and EtOH drinkers after microinjections of aCSF and 50 mg% EtOH into the pVTA (P > 0.186). In rats receiving microinjections of 75 mg% EtOH, DA levels in the AcbSh were significantly higher in the adolescent EtOH drinkers compared with adolescent naïve rats (F1,13 > 7.8; P < 0.015) during samples 1 and 2 after microinjection. Similar analysis revealed that DA levels in the AcbSh were elevated in adolescent EtOH drinkers compared with adolescent naïve rats during sample periods 1–6 after microinjection of 100 mg% EtOH (F1,14 > 6.7; P < 0.021). The increase in DA levels in the AcbSh after microinjection of 150 mg% EtOH were comparable between adolescent naïve and adolescent EtOH drinkers, except during the three sample periods immediately after the microinjections (EtOH > naïve; F1,12 = 4.7; P = 0.05).
Fig. 4.
Depicts the mean (±S.E.M.) percentage of change in DA levels in the AcbSh in adolescent naïve subjects (top) and adolescent drinkers (bottom) given 0, 50, 75, 100, or 150 mg% EtOH microinjected directly into the pVTA. *Indicates significantly higher level of DA in the AcbSh of 150 mg% group compared with all other groups. +Indicates significantly higher level of DA in the AcbSh of 75, 100, and 150 mg% group compared with aCSF and 50 mg% EtOH and 100 and 150 mg% adolescent drinkers are significantly higher than adolescent naïve rats. ^Indicates significantly higher level of DA in the AcbSh of 100 and 150 mg% group compared with aCSF and 50 mg% EtOH (adolescent naïve, n = 6–10/group; adolescent EtOH drinkers, n = 6–9/group).
In adolescent naïve rats (Fig. 4, top), there was a significant EtOH concentration × time interaction term (F24,192 = 3.16; P < 0.001). Performing individual ANOVAs for each sample period revealed significant EtOH concentration differences in the AcbSh during sample periods 1–3, 5, and 6 after microinjection (F4,32 > 3.8; P < 0.013). Post hoc comparisons indicated that for all these times points, adolescent naïve rats administered 150 mg% EtOH directly into the pVTA had significantly higher DA levels in the AcbSh than all other groups.
In adolescent EtOH drinkers (Fig. 4, bottom), there was also a significant EtOH concentration × time interaction term (F24,192 = 6.25; P < 0.001). Performing individual ANOVAs for each sample period revealed significant EtOH concentration differences in the AcbSh during sample periods 1–7 (Inj–Inj90) after microinjection (F4,32 > 5.3; P < 0.002). Post hoc comparisons indicated that during sample periods 1 and 2 after EtOH microinjection into the pVTA, the DA levels in the AcbSh of adolescent drinkers receiving 75, 100, and 150 mg% EtOH were higher than those in rats receiving microinjections of aCSF or 50 mg% EtOH. During sample periods 3–7 after EtOH microinjection into the pVTA, the DA levels in the AcbSh of adolescent EtOH drinkers receiving 100 and 150 mg% EtOH were higher than in subjects receiving microinjections of aCSF or 50 or 75 mg% EtOH.
Body Weights.
Adolescent EtOH consumption in P rats did not alter body weight growth (data not shown). Statistical analysis revealed a significant main effect of day (F7,96 = 1370.7, P < 0.001) but no significant main effect of the exposure group (F1,102= 0.257, P = 0.613) or day × exposure group interaction (F7,96 = 1.53, P = 0.167).
Discussion
The findings of the current experiments indicate that periadolescent EtOH consumption by male P rat pups produces neuroadaptations that result in individuals being more susceptible to the reinforcing and stimulatory actions of EtOH within the pVTA during adulthood. Specifically, adolescent EtOH consumption results in a greater sensitivity to EtOH (lower concentrations are required to establish intracranial self-administration) and a propensity to receive more self-infusions of EtOH at a given concentration within the pVTA (Figs. 1–3). The reinforcing properties of drugs of abuse within the pVTA are thought to be dependent on the stimulation of VTA DA neurons and DA release in the AcbSh (c.f., Deehan et al., 2013). The current data sets indicate a convergence of the effects of adolescent EtOH consumption on the self-infusion of EtOH directly into the pVTA and the ability of EtOH microinjected into the pVTA to stimulate DA release in the AcbSh. Paralleling the self-infusion data, adolescent EtOH consumption resulted in a lower concentration of EtOH required to be microinjected into the pVTA to increase DA release in the AcbSh (Fig. 4). In addition, in adolescent EtOH drinkers and naïve subjects given the same concentration of EtOH microinjected into the pVTA, the amount of DA released and the duration of the increase in DA release in the AcbSh were increased by adolescent EtOH consumption (Fig. 4).
The effect of EtOH consumption during adolescence on adult EtOH consumption is inconsistently reported in the literature. However, like every aspect of alcohol studies, the manner, amount, and duration of EtOH exposure are likely to affect the observed consequences of EtOH consumption. Nonphysiologically relevant levels of alcohol consumption (i.e., EtOH intake levels that would produce no significant blood EtOH concentration) during adolescence have been shown to have no effect on adult EtOH consumption (Slawecki and Betancourt, 2002; Slawecki, 2002; Slawecki et al., 2004; Siegmund et al., 2005). Significant consumption of sweetened EtOH in Sprague-Dawley adolescent rats increases adult consumption of sweetened EtOH but not unadulterated high EtOH concentration solutions (20%) (Broadwater et al., 2013). Injections of EtOH during adolescence can result in a conditioned taste aversion to sweetened solutions and indeterminate effects on adult EtOH consumption (Gilpin et al., 2012). In contrast, adolescents injected with EtOH in a manner that does not produce taste aversion can enhance adult EtOH consumption (Pascual et al., 2007, 2009; Maldonado-Devincci et al., 2010). Voluntary EtOH drinking during adolescence at a level that produces pharmacologically relevant levels of EtOH has been shown to enhance adult EtOH consumption (Walker and Ehlers, 2009; Strong et al., 2010; O’Tousa et al., 2013). In P rats, adolescent EtOH consumption (oral free-choice) increases the acquisition of EtOH self-administration (operant), decreases the rate of extinction of EtOH self-administration, enhances relapse drinking, and enhances the ability of a priming dose of EtOH to increase EtOH-seeking (Rodd-Henricks et al., 2002a). These effects of adolescent EtOH consumption in P rats were not observed in adult P rats given similar drinking exposure (Rodd-Henricks et al., 2002b). The current data are the first reported evidence that periadolescent EtOH consumption can increase adult EtOH self-infusions directly into a brain region that is a critical component of the neurocircuitry regulating drug reward.
In adults, chronic EtOH consumption (>8 weeks) enhances the reinforcing properties of EtOH within the pVTA (Rodd et al., 2005b). Exposure to repeated cycles of EtOH access and deprivation (>16 weeks of EtOH) further enhanced the alteration in the reinforcing properties of EtOH within the pVTA (Rodd et al., 2005c). The effects of adult EtOH consumption on the ability of EtOH microinjected into the pVTA to stimulate DA release in the AcbSh have not been examined. However, neuroadaptations produced by periadolescent EtOH consumption may not be identical to that observed after chronic adult EtOH consumption, but the overall impact is similar. Future studies need to determine whether these neuroadaptations are consistent or unique in periadolescent and adult EtOH consumption.
Adolescent exposure to EtOH has been shown to alter DA within the mesolimbic DA system. Multiple studies have indicated that systemic administration of EtOH during adolescence results in higher basal DA levels in the Acb (Badanich et al., 2007; Pascual et al., 2009). Comparable EtOH injections in adolescent and adult rats result in higher basal level of DA in the Acb in adolescent rats (Pascual et al., 2009). Adolescent EtOH consumption in P rats increases DA clearance without a change in the extracellular concentration of DA within the Acb, suggesting increased DA neurotransmission (Sahr et al., 2004). In addition, adolescent EtOH consumption in P rats enhances and prolongs DA release in the Acb (both shell and core were assessed) after systemic administration of EtOH during adulthood (Sahr et al., 2004). The current experiment did not empirically examine basal DA levels in the AcbSh (no-net-flux protocol), but an estimate of basal DA levels indicates that adolescent drinkers had higher basal DA levels in the AcbSh (approximately 22%). Therefore, the significant percentage of baseline enhancement of DA levels in the AcbSh after EtOH microinjection into the pVTA in adolescent EtOH drinkers was observed despite possible higher basal DA levels (Fig. 4). Similar to the findings of Sahr et al. (2004) with intraperitoneal EtOH injections, the ability of pVTA EtOH to stimulate DA release in the Acb was prolonged after adolescent EtOH consumption. Therefore, adolescent EtOH consumption in P rats results in a prolonged DA response in the Acb after both systemic and pVTA exposure to EtOH during adulthood.
Most research examining the effects of adolescent EtOH exposure has centered on the amygdala and hippocampus (learning associated effects; c.f. Spear and Varlinskaya, 2005; Gulley and Juraska, 2013). The biologic basis of the neuroadaptations in the pVTA produced by EtOH consumption during adolescence in the P rat was not directly examined in the current experiments. The data indicate three possibly distinct neuroadaptations (increased sensitivity to EtOH reinforcement in pVTA, increased pVTA DA neuronal response to EtOH, and a prolonged effect of EtOH on pVTA DA neurons) produced by adolescent EtOH consumption in the pVTA, which may involve unique, nonoverlapping neuronal systems. EtOH self-infusion directly into the pVTA requires DA neuronal activity (Rodd et al., 2004) and can be reduced by coadministration of serotonin 5-HT3 receptor antagonists (Rodd et al., 2005a). It is possible that these systems may be altered by adolescent EtOH consumption or exposure to other drugs of abuse during adolescence. Adolescent exposure to amphetamine (PND 30 to PND 50) results in an increase in basal firing rates of VTA DA neurons and 5-HT neurons in the dorsal raphe (Labonte et al., 2012). Comparable experiments have not been conducted with EtOH.
Overall, data indicate that adolescent EtOH consumption in the P rat, a rodent model of alcoholism, produced persistent alterations in the drug reward pathway that are indicative of increased sensitivity to EtOH and a potentiated or prolonged response to EtOH. The increase in reward sensitivity during adulthood after adolescent EtOH consumption may be the biologic basis for the deleterious effects that adolescent alcohol consumption has on adult alcoholism. Elucidating specific neuroadaptations within the mesolimbic DA system produced by adolescent EtOH consumption could lead to interventions or treatments to counter the consequences of this common human behavior.
Abbreviations
- Acb
nucleus accumbens
- AcbSh
nucleus accumbens shell
- aCSF
artificial cerebrospinal fluid
- ANOVA
analysis of variance
- DA
dopamine
- EtOH
ethanol
- HPLC
high-performance liquid chromatography
- ICSA
intracranial self-administration
- P
alcohol-preferring rats
- PFC
prefrontal cortex
- PND
postnatal day
- pVTA
posterior ventral tegmental area
- VTA
ventral tegmental area
Authorship Contributions
Participated in research design: Toalston, Bell, Murphy, McBride, Rodd.
Conducted experiments: Toalston, Deehan, Hauser, Engleman, Truitt, Rodd.
Performed data analysis: Toalston, Rodd.
Wrote or contributed to the writing of the manuscript: Toalston, McBride, Rodd.
Footnotes
These experiments were supported by grants obtained from the National Institutes of Health National Institute of Alcohol Abuse and Alcoholism [Grants P60-AA07611, T32-AA07462, R01-AA012262, R01-AA020396, and U01-AA013522].
References
- Agrawal A, Sartor CE, Lynskey MT, Grant JD, Pergadia ML, Grucza R, Bucholz KK, Nelson EC, Madden PA, Martin NG, et al. (2009) Evidence for an interaction between age at first drink and genetic influences on DSM-IV alcohol dependence symptoms. Alcohol Clin Exp Res 33:2047–2056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badanich KA, Maldonado AM, Kirstein CL. (2007) Chronic ethanol exposure during adolescence increases basal dopamine in the nucleus accumbens septi during adulthood. Alcohol Clin Exp Res 31:895–900 [DOI] [PubMed] [Google Scholar]
- Bell RL, Rodd-Henricks ZA, Kuc KA, Lumeng L, Li T-K, Murphy JM, McBride WJ. (2003) Effects of concurrent access to a single concentration or multiple concentrations of ethanol on the intake of ethanol by male and female periadolescent alcohol-preferring (P) rats. Alcohol 29:137–148 [DOI] [PubMed] [Google Scholar]
- Bell RL, Rodd ZA, Boutwell CL, Hsu CC, Lumeng L, Murphy JM, Li T-K, McBride WJ. (2004) Effects of long-term episodic access to ethanol on the expression of an alcohol deprivation effect in low alcohol-consuming rats. Alcohol Clin Exp Res 28:1867–1874 [DOI] [PubMed] [Google Scholar]
- Bell RL, Rodd ZA, Sable HJ, Schultz JA, Hsu CC, Lumeng L, Murphy JM, McBride WJ. (2006) Daily patterns of ethanol drinking in peri-adolescent and adult alcohol-preferring (P) rats. Pharmacol Biochem Behav 83:35–46 [DOI] [PubMed] [Google Scholar]
- Broadwater M, Varlinskaya EI, Spear LP. (2013) Effects of voluntary access to sweetened ethanol during adolescence on intake in adulthood. Alcohol Clin Exp Res 37:1048–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter-Hyland EP, Chandler LJ. (2007) Adaptive plasticity of NMDA receptors and dendritic spines: implications for enhanced vulnerability of the adolescent brain to alcohol addiction. Pharmacol Biochem Behav 86:200–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou SP, Pickering RP. (1992) Early onset of drinking as a risk factor for lifetime alcohol-related problems. Br J Addict 87:1199–1204 [DOI] [PubMed] [Google Scholar]
- Crews F, He J, Hodge C. (2007) Adolescent cortical development: a critical period of vulnerability for addiction. Pharmacol Biochem Behav 86:189–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson DA, Goldstein RB, Chou SP, Ruan WJ, Grant BF. (2008) Age at first drink and the first incidence of adult-onset DSM-IV alcohol use disorders. Alcohol Clin Exp Res 32:2149–2160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deehan GA, Jr, Hauser SR, Wilden JA, Truitt WA, Rodd ZA. (2013) Elucidating the biological basis for the reinforcing actions of alcohol in the mesolimbic dopamine system: the role of active metabolites of alcohol. Front Behav Neurosci 7:104–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding ZM, Oster SM, Hauser SR, Toalston JE, Bell RL, McBride WJ, Rodd ZA. (2012) Synergistic self-administration of ethanol and cocaine directly into the posterior ventral tegmental area: involvement of serotonin-3 receptors. J Pharmacol Exp Ther 340:202–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engleman EA, McBride WJ, Wilber AA, Shaikh SR, Eha RD, Lumeng L, Li T-K, Murphy JM. (2000) Reverse microdialysis of a dopamine uptake inhibitor in the nucleus accumbens of alcohol-preferring rats: effects on dialysate dopamine levels and ethanol intake. Alcohol Clin Exp Res 24:795–801 [PubMed] [Google Scholar]
- Engleman EA, Ingraham CM, McBride WJ, Lumeng L, Murphy JM. (2006) Extracellular dopamine levels are lower in the medial prefrontal cortex of alcohol-preferring rats compared to Wistar rats. Alcohol 38:5–12 [DOI] [PubMed] [Google Scholar]
- Giedd JN. (2004) Structural magnetic resonance imaging of the adolescent brain. Ann N Y Acad Sci 1021:77–85 [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Karanikas CA, Richardson HN. (2012) Adolescent binge drinking leads to changes in alcohol drinking, anxiety, and amygdalar corticotropin releasing factor cells in adulthood in male rats. PLoS ONE 7:e31466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulley JM, Juraska JM. (2013) The effects of abused drugs on adolescent development of corticolimbic circuitry and behavior. Neuroscience 249:3–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingson RW, Heeren T, Edwards EM. (2008) Age at drinking onset, alcohol dependence, and their relation to drug use and dependence, driving under the influence of drugs, and motor-vehicle crash involvement because of drugs. J Stud Alcohol Drugs 69:192–201 [DOI] [PubMed] [Google Scholar]
- Hingson RW, Heeren T, Winter MR. (2006) Age at drinking onset and alcohol dependence: age at onset, duration, and severity. Arch Pediatr Adolesc Med 160:739–746 [DOI] [PubMed] [Google Scholar]
- Jacobus J, McQueeny T, Bava S, Schweinsburg BC, Frank LR, Yang TT, Tapert SF. (2009) White matter integrity in adolescents with histories of marijuana use and binge drinking. Neurotoxicol Teratol 31:349–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. (2004) Secondary School Students (NIH Publication No. 04-5507) (Monitoring the future national survey results on drug use. 1975-2004.), vol. I. National Institute on Drug Abuse, Bethesda, MD [Google Scholar]
- Justice JB., Jr (1993) Quantitative microdialysis of neurotransmitters. J Neurosci Methods 48:263–276 [DOI] [PubMed] [Google Scholar]
- Labonte B, McLaughlin RJ, Dominguez-Lopez S, Bambico FR, Lucchino I, Ochoa-Sanchez R, Leyton M, Gobbi G. (2012) Adolescent amphetamine exposure elicits dose-specific effects on monoaminergic neurotransmission and behaviour in adulthood. Int J Neuropsychopharmacol 15:1319–1330 [DOI] [PubMed] [Google Scholar]
- Maldonado-Devincci AM, Alipour KK, Michael LA, Kirstein CL. (2010) Repeated binge ethanol administration during adolescence enhances voluntary sweetened ethanol intake in young adulthood in male and female rats. Pharmacol Biochem Behav 96:476–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride WJ, Bell RL, Rodd ZA, Strother WN, Murphy JM. (2005) Adolescent alcohol drinking and its long-range consequences. Studies with animal models. Recent Dev Alcohol 17:123–142 [DOI] [PubMed] [Google Scholar]
- McKinzie DL, Nowak KL, Murphy JM, Li T-K, Lumeng L, McBride WJ. (1998) Development of alcohol drinking behavior in rat lines selectively bred for divergent alcohol preference. Alcohol Clin Exp Res 22:1584–1590 [PubMed] [Google Scholar]
- Melendez RI, Rodd-Henricks ZA, Engleman EA, Li T-K, McBride WJ, Murphy JM. (2002) Microdialysis of dopamine in the nucleus accumbens of alcohol-preferring (P) rats during anticipation and operant self-administration of ethanol. Alcohol Clin Exp Res 26:318–325 [PubMed] [Google Scholar]
- Monti PM, Miranda R, Jr, Nixon K, Sher KJ, Swartzwelder HS, Tapert SF, White A, Crews FT. (2005) Adolescence: booze, brains, and behavior. Alcohol Clin Exp Res 29:207–220 [DOI] [PubMed] [Google Scholar]
- Morilak DA, Ciaranello RD. (1993) Ontogeny of 5-hydroxytryptamine2 receptor immunoreactivity in the developing rat brain. Neuroscience 55:869–880 [DOI] [PubMed] [Google Scholar]
- Nasrallah NA, Yang TW, Bernstein IL. (2009) Long-term risk preference and suboptimal decision making following adolescent alcohol use. Proc Natl Acad Sci USA 106:17600–17604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Tousa DS, Matson LM, Grahame NJ. (2013) Effects of intoxicating free-choice alcohol consumption during adolescence on drinking and impulsivity during adulthood in selectively bred high-alcohol preferring mice. Alcohol Clin Exp Res 37:141–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual M, Blanco AM, Cauli O, Miñarro J, Guerri C. (2007) Intermittent ethanol exposure induces inflammatory brain damage and causes long-term behavioural alterations in adolescent rats. Eur J Neurosci 25:541–550 [DOI] [PubMed] [Google Scholar]
- Pascual M, Boix J, Felipo V, Guerri C. (2009) Repeated alcohol administration during adolescence causes changes in the mesolimbic dopaminergic and glutamatergic systems and promotes alcohol intake in the adult rat. J Neurochem 108:920–931 [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. (1986) The rat brain in stereotaxic coordinates, Academic Press, New York, NY [Google Scholar]
- Perry KW, Fuller RW. (1992) Effect of fluoxetine on serotonin and dopamine concentration in microdialysis fluid from rat striatum. Life Sci 50:1683–1690 [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, McQueen VK, Davids MR, Hsu CC, Murphy JM, Li TK, Lumeng L, McBride WJ. (2005b) Chronic ethanol drinking by alcohol-preferring rats increases the sensitivity of the posterior ventral tegmental area to the reinforcing effects of ethanol. Alcohol Clin Exp Res 29:358–366 [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, McQueen VK, Davids MR, Hsu CC, Murphy JM, Li TK, Lumeng L, McBride WJ. (2005c) Prolonged increase in the sensitivity of the posterior ventral tegmental area to the reinforcing effects of ethanol following repeated exposure to cycles of ethanol access and deprivation. J Pharmacol Exp Ther 315:648–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, Zhang Y, Murphy JM, Goldstein A, Zaffaroni A, Li T-K, McBride WJ. (2005a) Regional heterogeneity for the intracranial self-administration of ethanol and acetaldehyde within the ventral tegmental area of alcohol-preferring (P) rats: involvement of dopamine and serotonin. Neuropsychopharmacology 30:330–338 [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Melendez RI, Bell RL, Kuc KA, Zhang Y, Murphy JM, McBride WJ. (2004) Intracranial self-administration of ethanol within the ventral tegmental area of male Wistar rats: evidence for involvement of dopamine neurons. J Neurosci 24:1050–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, Bell RL, Kuc KA, Murphy JM, McBride WJ, Lumeng L, Li T-K. (2002a) Effects of ethanol exposure on subsequent acquisition and extinction of ethanol self-administration and expression of alcohol-seeking behavior in adult alcohol-preferring (P) rats: I. Periadolescent exposure. Alcohol Clin Exp Res 26:1632–1641 [DOI] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, Bell RL, Kuc KA, Murphy JM, McBride WJ, Lumeng L, Li T-K. (2002b) Effects of ethanol exposure on subsequent acquisition and extinction of ethanol self-administration and expression of alcohol-seeking behavior in adult alcohol-preferring (P) rats: II. Adult exposure. Alcohol Clin Exp Res 26:1642–1652 [DOI] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, McKinzie DL, Crile RS, Murphy JM, McBride WJ. (2000) Regional heterogeneity for the intracranial self-administration of ethanol within the ventral tegmental area of female Wistar rats. Psychopharmacology (Berl) 149:217–224 [DOI] [PubMed] [Google Scholar]
- Rosenberg DR, Lewis DA. (1994) Changes in the dopaminergic innervation of monkey prefrontal cortex during late postnatal development: a tyrosine hydroxylase immunohistochemical study. Biol Psychiatry 36:272–277 [DOI] [PubMed] [Google Scholar]
- Sahr AE, Thielen RJ, Lumeng L, Li T-K, McBride WJ. (2004) Long-lasting alterations of the mesolimbic dopamine system after periadolescent ethanol drinking by alcohol-preferring rats. Alcohol Clin Exp Res 28:702–711 [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration Office of Applied Studies (SAMHSA) (2008) The NSDUH Report: Quantity and Frequency of Alcohol Use among Underage Drinkers SAMSHA, Rockville, Maryland. [Google Scholar]
- Siegmund S, Vengeliene V, Singer MV, Spanagel R. (2005) Influence of age at drinking onset on long-term ethanol self-administration with deprivation and stress phases. Alcohol Clin Exp Res 29:1139–1145 [DOI] [PubMed] [Google Scholar]
- Slawecki CJ. (2002) Altered EEG responses to ethanol in adult rats exposed to ethanol during adolescence. Alcohol Clin Exp Res 26:246–254 [PubMed] [Google Scholar]
- Slawecki CJ, Betancourt M. (2002) Effects of adolescent ethanol exposure on ethanol consumption in adult rats. Alcohol 26:23–30 [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Thorsell A, Ehlers CL. (2004) Long-term neurobehavioral effects of alcohol or nicotine exposure in adolescent animal models. Ann N Y Acad Sci 1021:448–452 [DOI] [PubMed] [Google Scholar]
- Spear LP. (2000) The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev 24:417–463 [DOI] [PubMed] [Google Scholar]
- Spear LP, Varlinskaya EI. (2005) Adolescence: alcohol sensitivity, tolerance, and intake. Recent Dev Alcohol 17:143–159 [PubMed] [Google Scholar]
- Strong MN, Yoneyama N, Fretwell AM, Snelling C, Tanchuck MA, Finn DA. (2010) “Binge” drinking experience in adolescent mice shows sex differences and elevated ethanol intake in adulthood. Horm Behav 58:82–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarazi FI, Baldessarini RJ. (2000) Comparative postnatal development of dopamine D(1), D(2) and D(4) receptors in rat forebrain. Int J Dev Neurosci 18:29–37 [DOI] [PubMed] [Google Scholar]
- Thomas MJ, Beurrier C, Bonci A, Malenka RC. (2001) Long-term depression in the nucleus accumbens: a neural correlate of behavioral sensitization to cocaine. Nat Neurosci 4:1217–1223 [DOI] [PubMed] [Google Scholar]
- Walker BM, Ehlers CL. (2009) Appetitive motivational experience during adolescence results in enhanced alcohol consumption during adulthood. Behav Neurosci 123:926–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler H, Dowdall GW, Davenport A, Castillo S. (1995) Correlates of college student binge drinking. Am J Public Health 85:921–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler H, Lee JE, Kuo M, Lee H. (2000) College binge drinking in the 1990s: a continuing problem. Results of the Harvard School of Public Health 1999 College Alcohol Study. J Am Coll Health 48:199–210 [DOI] [PubMed] [Google Scholar]
- Zecevic N, Bourgeois JP, Rakic P. (1989) Changes in synaptic density in motor cortex of rhesus monkey during fetal and postnatal life. Brain Res Dev Brain Res 50:11–32 [DOI] [PubMed] [Google Scholar]