Abstract
Experiments determined whether the combination of endothelin A (ETA) receptor antagonist [ABT-627, atrasentan; (2R,3R,4S)-4-(1,3-benzodioxol-5-yl)-1-[2-(dibutylamino)-2-oxoethyl]-2-(4-methoxyphenyl)pyrrolidine-3-carboxylic acid] and a thiazide diuretic (chlorthalidone) would be more effective at lowering blood pressure and reducing renal injury in a rodent model of metabolic syndrome compared with either treatment alone. Male Dahl salt-sensitive rats were fed a high-fat (36% fat), high-salt (4% NaCl) diet for 4 weeks. Separate groups of rats were then treated with vehicle (control), ABT-627 (ABT; 5 mg/kg per day, in drinking water), chlorthalidone (CLTD; 5 mg/kg per day, in drinking water), or both ABT plus CLTD. Mean arterial pressure (MAP) was recorded continuously by telemetry. After 4 weeks, both ABT and CLTD severely attenuated the development of hypertension, whereas the combination further reduced MAP compared with ABT alone. All treatments prevented proteinuria. CLTD and ABT plus CLTD significantly reduced nephrin (a podocyte injury marker) and kidney injury molecule-1 (a tubulointerstitial injury marker) excretion. ABT, with or without CLTD, significantly reduced plasma 8-oxo-2′-deoxyguanosine, a measure of DNA oxidation, whereas CLTD alone had no effect. All treatments suppressed the number of ED1+ cells (macrophages) in the kidney. Plasma tumor necrosis factor receptors 1 and 2 were reduced only in the combined ABT and CLTD group. These results suggest that ABT and CLTD have antihypertensive and renal-protective effects in a model of metabolic syndrome that are maximally effective when both drugs are administered together. The findings support the hypothesis that combined ETA antagonist and diuretic treatment may provide therapeutic benefit for individuals with metabolic syndrome consuming a Western diet.
Introduction
Metabolic syndrome (metS) is a combination of risk factors that predispose to cardiovascular disease and the development of type 2 diabetes mellitus. The syndrome is complex, with significant variability among individuals, and remains an area of intense research in both the basic and clinical sciences. Hypertension is a common feature of patients with metabolic syndrome and is the leading cause of various cardiovascular diseases and the second leading cause of end-stage renal disease.
Endothelin (ET)-1 is an important contributor to blood pressure regulation and may be a possible target for the treatment and prevention of hypertension. ET-1 acts through ETA and ETB receptors found primarily on vascular smooth muscle and endothelial cells, respectively. An emerging hypothesis is that reduced ETB receptor function leads to an imbalance such that ETA receptors become overstimulated and contribute to reduced salt and water excretion, increased vascular resistance, and a proinflammatory condition (Stuart et al., 2013). Atrasentan [ABT-627; (2R,3R,4S)-4-(1,3-benzodioxol-5-yl)-1-[2-(dibutylamino)-2-oxoethyl]-2-(4-methoxyphenyl)pyrrolidine-3-carboxylic acid] is an ETA-selective antagonist that has been proposed for the treatment of nephropathy and possibly hypertension. Preclinical studies have indicated that atrasentan reduces diabetic renal injury via an anti-inflammatory mechanism (Sasser et al., 2007). This has led to ongoing clinical trials for the treatment of diabetic nephropathy (Andress et al., 2012). However, ETA receptor antagonists can produce fluid retention and may be better suited for use in combination with other antihypertensive medications, especially diuretics that are actually first-line treatment of hypertension in the United States.
Chlorthalidone (CLTD) is a thiazide diuretic drug that is commonly used for the treatment of hypertension. This compound has long-acting duration (Sica et al., 2011) and is more likely to maintain net negative sodium (Na+) balance, thereby lowering blood pressure. Major findings from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial concluded that thiazide diuretics are equal or superior to other antihypertensive drugs in reducing cardiovascular events (ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group, 2002), yet the problem of hypertension-induced complications remains.
In the current study, we used a new, clinically relevant rodent model of the metS that has the unique feature of developing hypertension while consuming a high-fat, high-salt (HF/HS) diet, referred to as a Western diet. The model uses the Dahl salt-sensitive (DS) rat that develops hypertension and chronic kidney disease when fed diets with high salt (Tobian et al., 1979; Rostand et al., 1982; Mattson et al., 2006) and induces insulin resistance (Ogihara et al., 2002; Shehata, 2008). Moreover, fat loading accelerates salt-induced hypertension and renal damage in DS rats (Nagae et al., 2009). Thus, the aim of this study was to determine the effect of atrasentan, CLTD, and the combination of each on blood pressure and renal injury in a rodent model of the metS.
Materials and Methods
Animals and Experimental Procedures.
All procedures were performed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved and monitored by the Georgia Resents University Institutional Animal Care and Use Committee. Male DS (Charles River Laboratories, Wilmington, MA) rats were obtained at 8 weeks of age and maintained on a standard rat chow containing 0.4% NaCl with free access to water. Rats were housed in temperature and humidity-controlled, light-cycled quarters. After allowing 3–5 days for acclimatization, rats were instrumented with telemetry transmitters (PAC-40; Data Sciences, St. Paul, MN) placed in the abdominal aorta while under isoflurane anesthesia, according to the manufacturer’s specifications, as previously described (Pollock and Pollock, 2001). After 10 days of recovery from surgery, rats were randomly assigned to four groups according to the following treatment regimens: 1) combined HF/HS diet (as control; n = 7); 2) HF/HS diet plus ABT-627 [5 mg/kg per day, in drinking water; n = 7]; 3) HF/HS plus chlothalidone [(RS)-2-chloro-5-(1-hydroxy-3-oxo-2,3-dihydro-1H-isoindol-1-yl)benzene-1-sulfonamide; 5 mg/kg per day, in drinking water; n = 6]; 4) HF/HS plus ABT plus CLTD (ABT; 5 mg/kg per day, in drinking water, CLTD; 5 mg/kg per day, in drinking water; n = 6). The dose of ABT was chosen based on previous pharmacologic studies in rats demonstrating that 5 mg/kg per day produced a maximal inhibition of ETA-dependent responses when given orally (Wessale et al., 2002). The CLTD dose was chosen based on estimating full efficacy from a previous in vivo study (Zhou et al., 2008). The rats were then monitored for 4 weeks, and mean arterial pressure (MAP) was recorded continuously. Body weight was measured weekly in all rats. At the end of the 4-week treatment, rats were placed in metabolic cages for 24-hour urine collection. Urine volume was measured gravimetrically, and urine was centrifuged to remove particulates and stored at −80°C. At the end of the treatment period, rats were anesthetized with isoflurane and blood was collected by cardiac puncture. The kidneys were removed and fixed in 10% neutral buffered formalin, embedded in paraffin, and sectioned for histologic analysis. The left epididymal fat pad was also carefully dissected and weighed.
The HF/HS diet was prepared by adding NaCl to a commercially available high-fat diet (Bio-Serv F2685; Flemington, NJ) to make the final 4% NaCl included with the high-fat diet. The high-fat diet contains 36% fat and a total of 5.4 kcal/g.
Analytical Methods.
Plasma glucose (Accu-Check glucometer; Roche Diagnostics), insulin (EMD Millipore, Billerica, MA), total cholesterol (Wako Life Sciences, Richmond, VA), triglycerides (Cayman Chemical, Ann Arbor, MI), and free fatty acids (Zen-Bio, Durham, NC) were measured as markers of the metS. Plasma and urinary sodium concentrations were determined by ion selective electrode (Medica Easylyte, Bedford, MA). Creatinine was measured using the picric acid method (Allcock et al., 1998). Creatinine clearance was determined using the standard clearance equation, as follows: urine creatinine concentration (mg/dl) × urine flow rate (ml/day) divided by plasma creatinine (mg/dl). Glomerular and podocyte injury was assessed by measuring urinary protein (Bradford colorimetric method; Bio-Rad, Hercules, CA) and urinary nephrin [enzyme-linked immunosorbent assay (ELISA); Exocell, Philadelphia, PA]. Tubulointerstitial injury was assessed by measuring kidney injury molecule-1 (ELISA; R&D Systems, Minneapolis, MN). The inflammatory cytokine tumor necrosis factor (TNF)-α and its soluble receptors [TNF receptor (TNF-R)1 and TNF-R2] were measured (ELISA; R&D Systems). Overall oxidative stress was assessed by measuring plasma 8-hydroxy-2′deoxyguanosine (8-OHdG, a marker of DNA oxidation) (enzyme immunoassay; Cayman Chemical). All measurements were performed according to the manufacturer’s directions.
Renal Histology.
For histologic assessment of renal injury, kidneys were processed, sectioned, and stained using standard techniques. Vascular injury was assessed in H&E-stained sections. Necrotic glomeruli were evaluated in a periodic acid-Schiff–stained section, and tubulointerstitial injury was analyzed using a blue trichrome-stained section from each rat by a board-certified renal pathologist in a blinded manner. The severity of changes was graded with an arbitrary score of 0–4 in which 0 is nil, 1 is mild, 2 is moderate, 3 is severe, and 4 is very severe.
Immunohistochemical stains were performed on harvested renal tissue and evaluated for ED-1 and CD3 positivity for determining the degree of infiltration with macrophages and T cells, respectively, as described previously (Saleh et al., 2010).
Statistical Analysis.
Data are expressed as the mean ± S.E. Telemetry data were analyzed by two-way repeated-measures analysis of variance to determine the significance of differences between groups. Between-group comparisons for other measurements were performed using a one-way analysis of variance. P < 0.05 was considered statistically significant.
Results
Characteristics of Experimental Animals.
As shown in Table 1, there were no significant intergroup differences in body weight, food and water intake, urine volume, sodium excretion, creatinine clearance, and plasma sodium at the end of the 4-week treatment period. The metabolic parameters associated with the metS are given in Table 2. Overall, there were no significant differences between groups, although there was a high degree of variability in plasma insulin levels and improvement in glucose and lipid parameters in the various drug treatment groups.
TABLE 1.
General characteristics of DS rats on a HF/HS diet following 4 weeks of treatment with vehicle control, the ETA receptor antagonist, ABT-627, and/or the diuretic, CLTD
| Control | ABT | CLTD | ABT + CLTD | |
|---|---|---|---|---|
| Food intake (g/day) | 14 ± 2 | 14 ± 1 | 15 ± 1 | 17 ± 2 |
| Water intake (ml/day) | 75 ± 9 | 70 ± 7 | 60 ± 4 | 62 ± 3 |
| Urine volume (ml/day) | 72 ± 10 | 65 ± 9 | 50 ± 2 | 48 ± 2 |
| Sodium excretion (mmol/day) | 24.5 ± 3.3 | 25.1 ± 3.4 | 24.8 ± 2.2 | 21.3 ± 0.6 |
| Creatinine clearance (ml/min) | 0.67 ± 0.15 | 0.90 ± 0.24 | 0.64 ± 0.20 | 0.33 ± 0.10 |
| Plasma sodium (mmol/l) | 135 ± 2 | 140 ± 1 | 140 ± 1 | 142 ± 1 |
| Body weight (g) | 349 ± 10 | 369 ± 12 | 374 ± 5 | 385 ± 13 |
| Kidney weight (g/100 g b.wt.) | 0.57 ± 0.04 | 0.60 ± 0.02 | 0.49 ± 0.01† | 0.47 ± 0.01† |
| Heart weight (g/100 g b.wt.) | 0.42 ± 0.01 | 0.403 ± 0.004* | 0.29 ± 0.06† | 0.35 ± 0.01 |
| Number of rats | 7 | 7 | 6 | 6 |
P < 0.05 versus control; †P < 0.05 versus ABT.
TABLE 2.
Metabolic characteristics of DS rats on a HF/HS diet following 4 weeks of treatment with vehicle control, the ETA receptor antagonist, ABT-627, and/or the diuretic, CLTD
| Control | ABT | CLTD | ABT + CLTD | |
|---|---|---|---|---|
| Plasma glucose (mg/dl) | 355 ± 35 | 292 ± 51 | 273 ± 30 | 242 ± 32 |
| Plasma insulin (ng/ml) | 0.86 ± 22 | 1.53 ± 0.44 | 1.76 ± 0.34 | 1.90 ± 0.20 |
| Total cholesterol (mg/dl) | 129 ± 18 | 109 ± 9 | 82 ± 1 | 95 ± 9 |
| Triglyceride (mg/dl) | 75 ± 16 | 67 ± 23 | 66 ± 19 | 107 ± 23 |
| Free fatty acid (mmol/l) | 164 ± 34 | 139 ± 52 | 103 ± 20 | 190 ± 45 |
| Epididymal fat (g/100 g b.wt.) | 0.41 ± 0.03 | 0.46 ± 0.03 | 0.50 ± 0.03 | 0.48 ± 0.03 |
Hemodynamics.
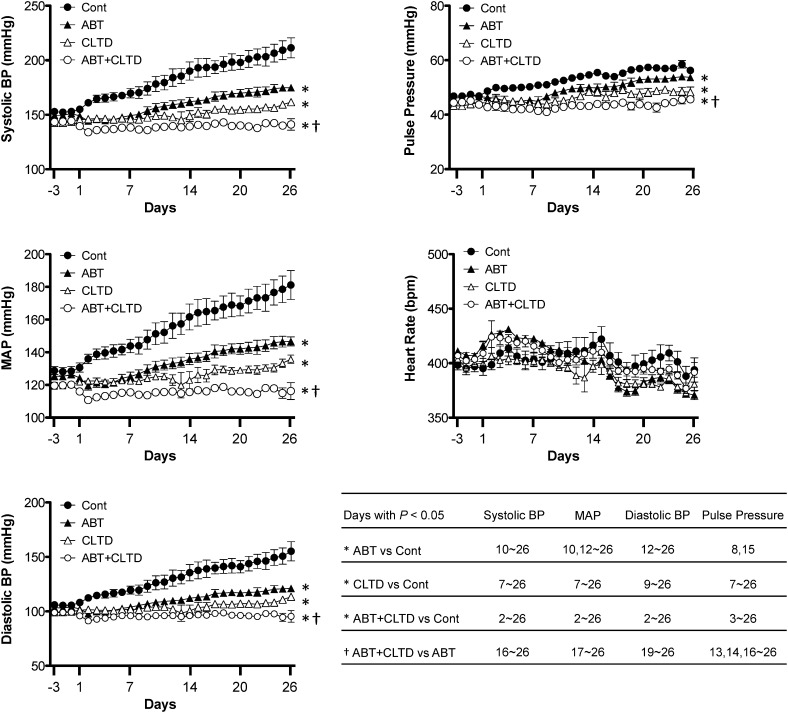
Figure 1 illustrates 24-hour hemodynamic data in control, ABT, CLTD, and ABT plus CLTD rats during a 3-day baseline period and the 4-week treatment period. The average 24-hour MAP in the control group progressively increased to 181 ± 9 mm Hg during the 4-week experimental period. MAP in the ABT, CLTD, and ABT plus CLTD groups (147 ± 3, 136 ± 3, and 115 ± 5 mm Hg, respectively) was significantly lower than the control group at 4 weeks. Furthermore, MAP was lowest in the ABT plus CLTD group and significantly lower than CLTD alone. A similar pattern was observed with systolic blood pressure, diastolic blood pressure, and pulse pressure. There were no significant differences in heart rate among groups.
Fig. 1.
Systolic blood pressure (BP), mean arterial pressure, diastolic BP, heart rate, and pulse pressure measured by telemetry in conscious DS rats maintained on a HF/HS diet during a 4-week course of study. Separate groups were given vehicle, the ETA receptor antagonist, ABT-627 (ABT), or the diuretic, CLTD. Group effects are indicated as *P < 0.05 versus control; †P < 0.05 versus ABT alone. n = 6 each group. Comparisons between individual groups on individual days are indicated in the inset table (P < 0.05 for specific days indicated).
Assessment of Renal Injury.
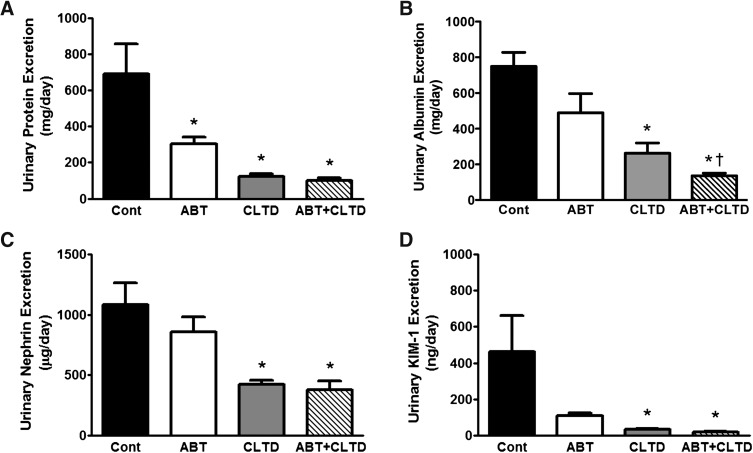
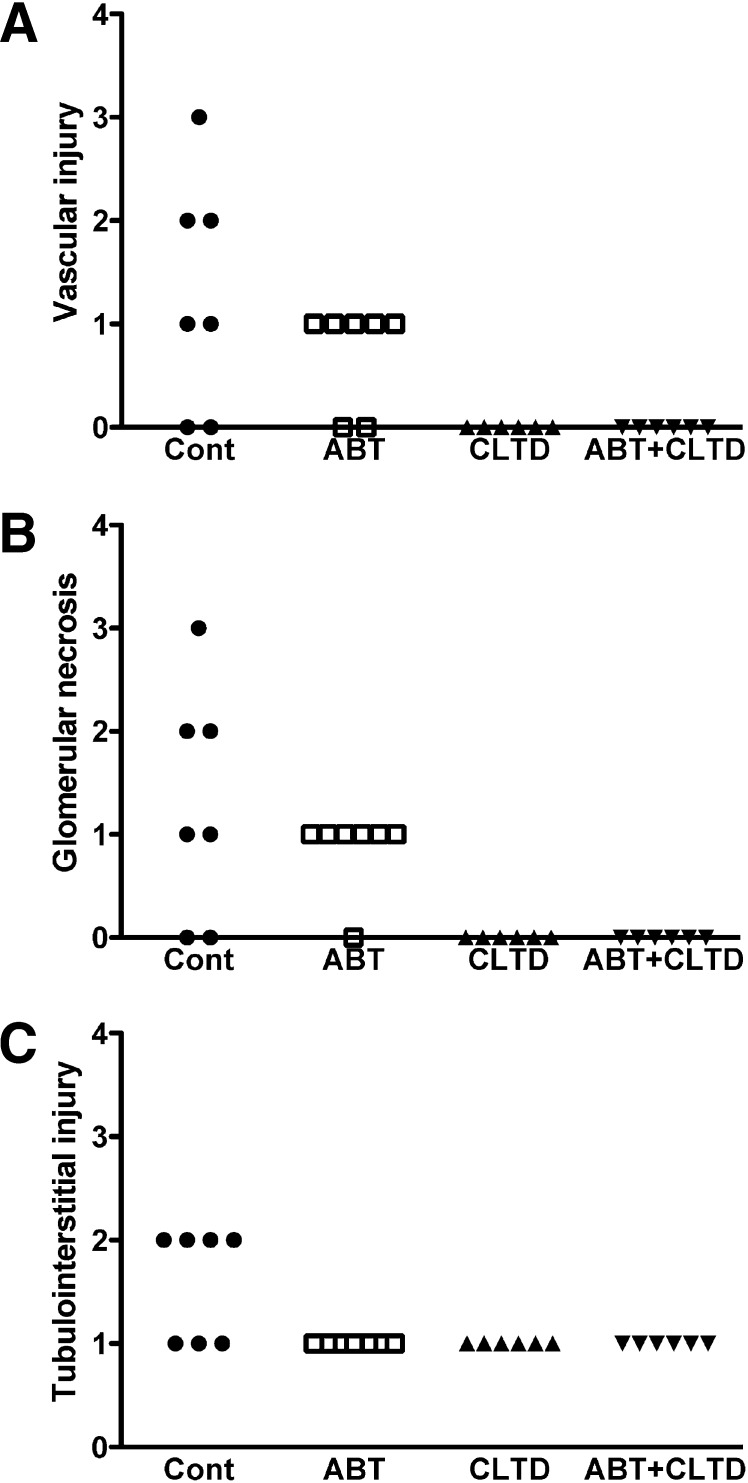
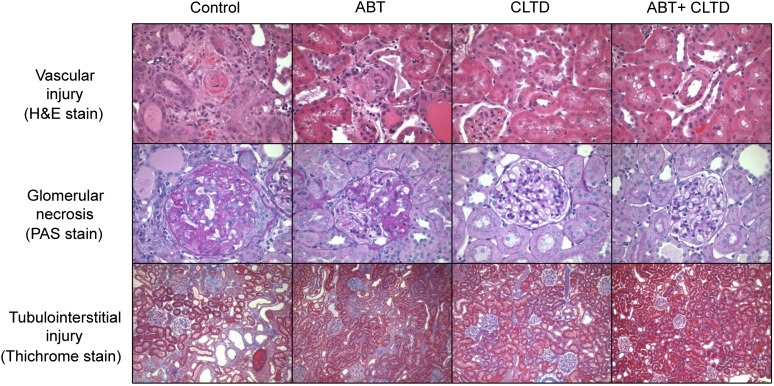
As shown in Fig. 2A, compared with control rats, all treatment groups were effective at significantly lowering proteinuria. A similar pattern was observed with albumin excretion, although the effect of ABT alone was not statistically significant (Fig. 2B). All treatments lowered the excretion rate of the podocyte injury marker nephrin and the tubulointerstitial injury marker kidney injury molecule-1. However, this difference was statistically significant only in the groups treated with CLTD (CLTD and ABT plus CLTD) (Fig. 2, C and D). Similarly, qualitative assessment of vascular, glomerular, and tubulointerstitial injury in the kidney followed a similar pattern in which CLTD and ABT plus CLTD treatment reduced the extent of injury observed in the DS rat on a HF/HS diet (Fig. 3). Representative histologic stains are presented in Fig. 4.
Fig. 2.
Determination of glomerular injury as assessed by measurement of proteinuria (A), albuminuria (B), and nephrinuria (C) in DS rats on a HF/HS diet treated with vehicle, ABT, CLTD, and ABT + CLTD for 4 weeks. Tubulointerstitial injury was assessed by measuring urinary KIM-1 excretion (D). *P < 0.05 versus control; †P < 0.05 versus ABT alone.
Fig. 3.
Qualitative assessment of vascular injury (A), glomerular necrosis (B), and tubulointerstitial injury (C) in control, ABT-, CLTD-, and ABT + CLTD–treated rats after 4 weeks of treatment.
Fig. 4.
Histologic images depicting vascular injury (A) (H&E, 400×), glomerular necrosis (B) (periodic acid-Schiff [PAS], 400×), and tubulointerstitial injury (C) (Trichrome, 100×) in control, ABT-, CLTD-, and ABT + CLTD–treated rats.
Systemic Inflammation and Oxidative Stress.
As shown in Table 3, there were no significant intergroup differences in plasma TNF-α levels; however, plasma TNF-R1 and TNF-R2 were significantly reduced in the ABT plus CLTD group compared with control group. The plasma concentration of 8-OHdG was significantly decreased in ABT and ABT plus CLTD groups compared with the control group, whereas 8-OHdG was not significantly reduced by CLTD alone.
TABLE 3.
Plasma measurement of inflammatory and oxidative stress markers from DS rats on a HF/HS diet following 4 weeks of treatment with vehicle control, the ETA receptor antagonist, ABT-627, and/or the diuretic, CLTD
| Control | ABT | CLTD | ABT + CLTD | |
|---|---|---|---|---|
| TNF-α (pg/ml) | 0.105 ± 0.001 | 0.103 ± 0.001 | 0.105 ± 0.005 | 0.098 ± 0.002 |
| TNF-R1 (pg/ml) | 943 ± 169 | 536 ± 64 | 538 ± 128 | 412 ± 35* |
| TNF-R2 (pg/ml) | 1571 ± 119 | 1254 ± 50 | 1155 ± 175 | 975 ± 79* |
| 8-OHdG (ng/ml) | 3.6 ± 0.8 | 1.5 ± 0.2* | 2.1 ± 0.2 | 1.6 ± 0.2* |
P < 0.05 versus control.
Immunohistochemical Analysis of the Kidney.
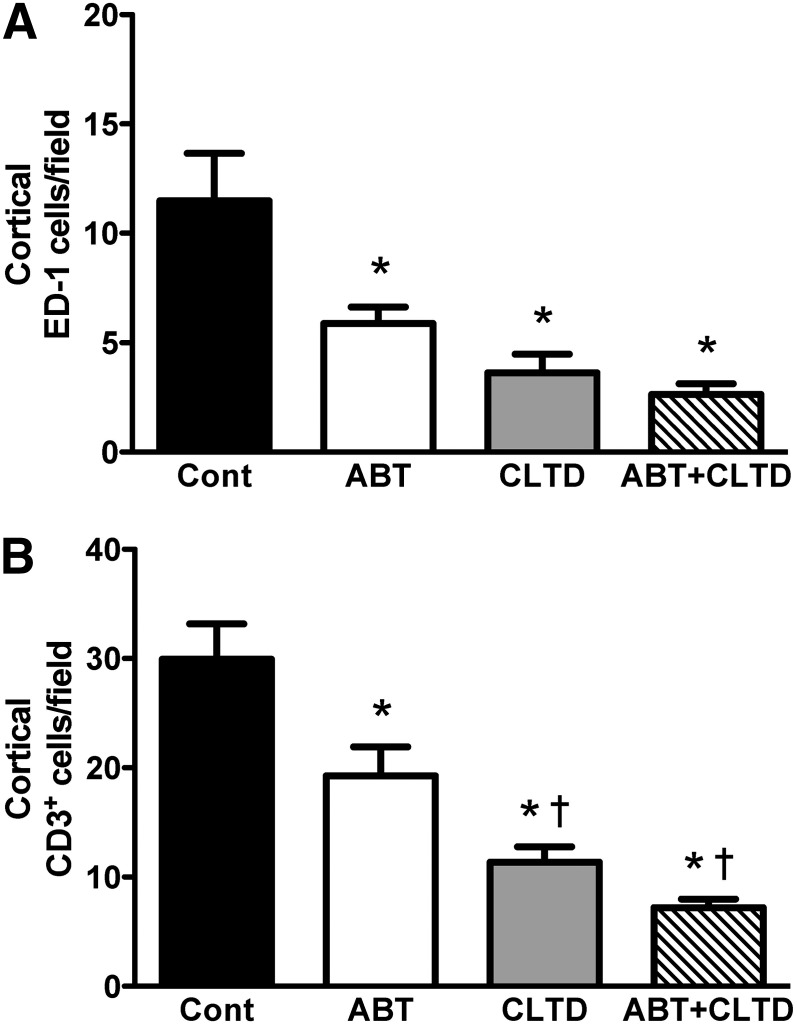
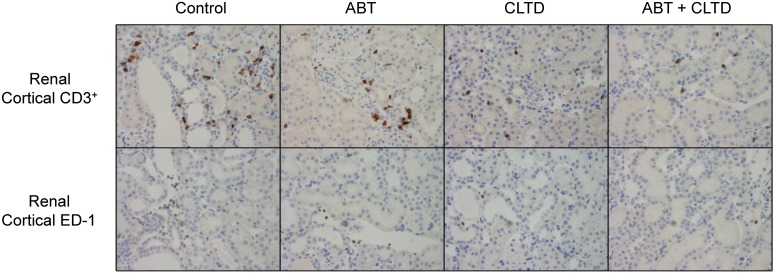
Quantitative assessment of the immunohistochemistry results following 4-week treatment is shown in Fig. 5. ED-1–expressing macrophages accumulated in the renal cortex in the control group, which was significantly suppressed in the ABT, CLTD, and ABT plus CLTD groups. CD3-positive T cells in the ABT, CLTD, and ABT plus CLTD groups were significantly lower than in the control group. Moreover, the combination of ABT and CLTD further attenuated the number of CD3-positive T cells in the kidney. Representative images from these experiments are depicted in Fig. 6.
Fig. 5.
Quantification of ED-1 (A) and CD3 (B)-positive staining cells in the renal cortex of control, ABT-, CLTD-, and ABT + CLTD–treated rats (field: 400 × 400 µm). *P < 0.05 versus control; †P < 0.05 versus ABT alone.
Fig. 6.
Representative immunostaining images of T cells (CD3+) and macrophages (ED-1) in kidney cortical sections from control, ABT-, CLTD-, and ABT + CLTD–treated rats.
Discussion
In the present study, DS rats given a HF/HS diet exhibited features of metabolic syndrome, including significant high blood pressure and renal damage. Treatment with ABT-627, CLTD, and the combination effectively lowered blood pressure and attenuated renal injury. Combination therapy was most effective, although diuretic treatment alone appeared to be the primary driver in terms of reducing the detrimental effects of this diet. It is important to note that the doses used in this study are based on maximal efficacy in a number of previous studies conducted in rat models. ABT-627 and CLTD significantly attenuated the development of hypertension, but, in this particular case, CLTD was slightly more effective at reducing the consequences of metS compared with ABT-627 alone, even though the difference in blood pressure between these groups was not statistically significant. CLTD was more effective at reducing indices of renal injury and measures of inflammation, but this could be due to the slight difference in blood pressure lowering. Clearly, the combination of both drugs was more effective at reducing blood pressure, albuminuria, and renal cortical T-cell infiltration. This supplemental renal protection again could be due to the significantly lower blood pressure in the combined treatment group. Nonetheless, these findings provide a rational argument that the use of combined diuretic and ETA receptor blockade is more protective than either treatment alone.
There is a wide variety of animal models used to mimic the metabolic syndrome, but surprisingly, few studies have used the DS rat maintained on a HF/HS diet. The metabolic profile is remarkably similar to the common human condition that is characterized by hyperlipidemia, hyperinsulinemia, and hypertension. Furthermore, consumption of a HF/HS diet is similar to what is often referred to as a Western diet. Although most studies have focused on salt-induced hypertension in the DS rat, we know that other diets can elevate blood pressure in this model. It is also important to note that not all humans who consume diets rich in salt and fat develop metabolic syndrome, so the genetic propensity to complications in this diet is indeed relevant to humans suffering from this frequent disorder.
Although blood pressure reduction could account for many of the beneficial effects observed in the current study with these two types of drugs, there is additional evidence in the literature to suggest that ET-1 may contribute to inflammation in hypertension and diabetes (Saleh and Pollock, 2011). Using a dose of ET-1 that does not increase blood pressure, we have previously observed significant increases in renal T-cell and macrophage infiltration (Saleh et al., 2010). Chronic ET-1 administration was associated with increases in early inflammatory markers such as monocyte chemoattractant protein-1 and soluble intercellular adhesion molecule-1, both within the plasma as well as the kidney (Saleh et al., 2010). Likewise, in a rat model of type 1 diabetes, we observed increased renal inflammation despite animals maintaining normal blood pressure throughout the study (Saleh et al., 2011).
ETA-selective and combined ETA/ETB receptor antagonists have been approved for use in pulmonary hypertension (Rubin et al., 2002; Blalock et al., 2010), but there is a wide variety of cardiovascular-related diseases in which preclinical studies have suggested efficacy (Krum and Liew, 2003; Vatter and Seifert, 2006; Prasad et al., 2009). In recent years, considerable attention has been paid to the possibility of using this class of drugs for the treatment of resistant hypertension and diabetic nephropathy. The ETA-selective antagonist, darusentan, has been studied in a series of phase 2 and phase 3 clinical trials for blood pressure lowering in patients with resistant hypertension (Weber et al., 2009; Bakris et al., 2010). Ambrisentan significantly reduced ambulatory blood pressure in subjects already being treated with an average of five distinct antihypertensive medications. However, because clinic blood pressure was the end point and because there was a significant reduction in clinic blood pressure in the placebo group, the company terminated their efforts at approval. In a phase 3 trial for diabetic nephropathy, the modestly selective ETA antagonist, avosentan, effectively reduced proteinuria when given on top of standard medication (Mann et al., 2010). However, this trial was terminated early because of potentially life-threatening fluid retention. In each of these trials, a key element of pharmacology was overlooked. The doses used were derived from a phase 2 trial that did not demonstrate dose dependency, that is, the lower doses produced the same degree of efficacy as the higher doses. Yet, the side effect profile was dose-dependent with more fluid retention at the higher doses. It is unclear why and unfortunate that phase 3 trials, ASCEND and DORADO, moved forward with the higher doses (Bakris et al., 2010; Mann et al., 2010). Learning from these mistakes, investigators sponsored by AbbVie tested the influence of the selective ETA antagonist, atrasentan, in diabetic patients with nephropathy, but used much lower doses and supplemented with more aggressive diuretic treatment to manage fluid retention (Kohan et al., 2011b). There is some evidence that ETA receptor blockade may contribute to fluid retention by the kidney (Stuart et al., 2013), but it is not clear whether this accounts for the fluid retention issues in human trials. It is certainly possible that these drugs have some unexpected degree of ETB receptor blockade in humans, especially those with susceptibility to fluid retention, because the ETB receptor clearly facilitates sodium excretion (Kohan et al., 2011a). They have now conducted two successful phase 2 studies that demonstrate manageability of the fluid retention issue (Kohan et al., 2011b; de Zeeuw et al., 2014). Findings in our current study provide preclinical support for the idea that this combined treatment may be useful in subjects with metabolic syndrome who could evolve into resistant hypertension and diabetic nephropathy.
Acknowledgments
The authors thank Hiram Ocasio for expert assistance with the telemetry procedures.
Abbreviations
- ABT-627
(2R,3R,4S)-4-(1,3-benzodioxol-5-yl)-1-[2-(dibutylamino)-2-oxoethyl]-2-(4-methoxyphenyl)pyrrolidine-3-carboxylic acid
- CLTD
chlorthalidone
- DS
Dahl salt-sensitive
- ELISA
enzyme-linked immunosorbent assay
- ET
endothelin
- HF/HS
high fat, high salt
- MAP
mean arterial pressure
- metS
metabolic syndrome
- 8-OHdG
8-oxo-2′-deoxyguanosine
- TNF
tumor necrosis factor
- TNF-R
TNF receptor
Authorship Contributions
Participated in research design: Jin, J. S. Pollock, White, D. M. Pollock.
Conducted experiments: Jin, Jeon, Kleven.
Performed data analysis: Jin, Kleven, White.
Wrote or contributed to the writing of the manuscript: Jin, J. S. Pollock, White, D. M. Pollock.
Footnotes
This work was supported by an unrestricted educational grant from Abbvie (Abbott Park, IL); and the National Institutes of Health National Heart, Lung, and Blood Institute [Grants P01-HL095499 and P01-HL069999].
References
- Allcock GH, Venema RC, Pollock DM. (1998) ETA receptor blockade attenuates the hypertension but not renal dysfunction in DOCA-salt rats. Am J Physiol 275:R245–R252 [DOI] [PubMed] [Google Scholar]
- Andress DL, Coll B, Pritchett Y, Brennan J, Molitch M, Kohan DE. (2012) Clinical efficacy of the selective endothelin A receptor antagonist, atrasentan, in patients with diabetes and chronic kidney disease (CKD). Life Sci 91:739–742 [DOI] [PubMed] [Google Scholar]
- Bakris GL, Lindholm LH, Black HR, Krum H, Linas S, Linseman JV, Arterburn S, Sager P, Weber M. (2010) Divergent results using clinic and ambulatory blood pressures: report of a darusentan-resistant hypertension trial. Hypertension 56:824–830 [DOI] [PubMed] [Google Scholar]
- Blalock SE, Matulevicius S, Mitchell LC, Reimold S, Warner J, Peshock R, Torres F, Chin KM. (2010) Long-term outcomes with ambrisentan monotherapy in pulmonary arterial hypertension. J Card Fail 16:121–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Zeeuw D, Coll B, Andress D, Brennan JJ, Tang H, Houser M, Correa-Rotter R, Kohan D, Lambers Heerspink HJ, Makino H, et al. (2014) The endothelin antagonist atrasentan lowers residual albuminuria in patients with type 2 diabetic nephropathy. J Am Soc Nephrol 25:1083–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohan DE, Inscho EW, Wesson D, Pollock DM. (2011a) Physiology of endothelin and the kidney. Compr Physiol 1:883–919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohan DE, Pritchett Y, Molitch M, Wen S, Garimella T, Audhya P, Andress DL. (2011b) Addition of atrasentan to renin-angiotensin system blockade reduces albuminuria in diabetic nephropathy. J Am Soc Nephrol 22:763–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krum H, Liew D. (2003) Current status of endothelin blockade for the treatment of cardiovascular and pulmonary vascular disease. Curr Opin Investig Drugs 4:298–302 [PubMed] [Google Scholar]
- Mann JF, Green D, Jamerson K, Ruilope LM, Kuranoff SJ, Littke T, Viberti G, ASCEND Study Group (2010) Avosentan for overt diabetic nephropathy. J Am Soc Nephrol 21:527–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson DL, James L, Berdan EA, Meister CJ. (2006) Immune suppression attenuates hypertension and renal disease in the Dahl salt-sensitive rat. Hypertension 48:149–156 [DOI] [PubMed] [Google Scholar]
- Nagae A, Fujita M, Kawarazaki H, Matsui H, Ando K, Fujita T. (2009) Effect of high fat loading in Dahl salt-sensitive rats. Clin Exp Hypertens 31:451–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group; The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (2002) Major outcomes in moderately hypercholesterolemic, hypertensive patients randomized to pravastatin vs usual care: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT-LLT). JAMA 288:2998–3007 [DOI] [PubMed] [Google Scholar]
- Ogihara T, Asano T, Ando K, Sakoda H, Anai M, Shojima N, Ono H, Onishi Y, Fujishiro M, Abe M, et al. (2002) High-salt diet enhances insulin signaling and induces insulin resistance in Dahl salt-sensitive rats. Hypertension 40:83–89 [DOI] [PubMed] [Google Scholar]
- Pollock DM, Pollock JS. (2001) Evidence for endothelin involvement in the response to high salt. Am J Physiol Renal Physiol 281:F144–F150 [DOI] [PubMed] [Google Scholar]
- Prasad VS, Palaniswamy C, Frishman WH. (2009) Endothelin as a clinical target in the treatment of systemic hypertension. Cardiol Rev 17:181–191 [DOI] [PubMed] [Google Scholar]
- Rostand SG, Kirk KA, Rutsky EA, Pate BA. (1982) Racial differences in the incidence of treatment for end-stage renal disease. N Engl J Med 306:1276–1279 [DOI] [PubMed] [Google Scholar]
- Rubin LJ, Badesch DB, Barst RJ, Galie N, Black CM, Keogh A, Pulido T, Frost A, Roux S, Leconte I, et al. (2002) Bosentan therapy for pulmonary arterial hypertension. N Engl J Med 346:896–903 [DOI] [PubMed] [Google Scholar]
- Saleh MA, Boesen EI, Pollock JS, Savin VJ, Pollock DM. (2010) Endothelin-1 increases glomerular permeability and inflammation independent of blood pressure in the rat. Hypertension 56:942–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh MA, Pollock DM. (2011) Endothelin in renal inflammation and hypertension. Contrib Nephrol 172:160–170 [DOI] [PubMed] [Google Scholar]
- Saleh MA, Pollock JS, Pollock DM. (2011) Distinct actions of endothelin A-selective versus combined endothelin A/B receptor antagonists in early diabetic kidney disease. J Pharmacol Exp Ther 338:263–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasser JM, Sullivan JC, Hobbs JL, Yamamoto T, Pollock DM, Carmines PK, Pollock JS. (2007) Endothelin A receptor blockade reduces diabetic renal injury via an anti-inflammatory mechanism. J Am Soc Nephrol 18:143–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shehata MF. (2008) Genetic and dietary salt contributors to insulin resistance in Dahl salt-sensitive (S) rats. Cardiovasc Diabetol 7:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sica DA, Carter B, Cushman W, Hamm L. (2011) Thiazide and loop diuretics. J Clin Hypertens 13:639–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart D, Chapman M, Rees S, Woodward S, Kohan DE. (2013) Myocardial, smooth muscle, nephron, and collecting duct gene targeting reveals the organ sites of endothelin A receptor antagonist fluid retention. J Pharmacol Exp Ther 346:182–189 [DOI] [PubMed] [Google Scholar]
- Tobian L, Lange J, Iwai J, Hiller K, Johnson MA, Goossens P. (1979) Prevention with thiazide of NaCl-induced hypertension in Dahl “S” rats: evidence for a Na-retaining humoral agent in “S” rats. Hypertension 1:316–323 [DOI] [PubMed] [Google Scholar]
- Vatter H, Seifert V. (2006) Ambrisentan, a non-peptide endothelin receptor antagonist. Cardiovasc Drug Rev 24:63–76 [DOI] [PubMed] [Google Scholar]
- Weber MA, Black H, Bakris G, Krum H, Linas S, Weiss R, Linseman JV, Wiens BL, Warren MS, Lindholm LH. (2009) A selective endothelin-receptor antagonist to reduce blood pressure in patients with treatment-resistant hypertension: a randomised, double-blind, placebo-controlled trial. Lancet 374:1423–1431 [DOI] [PubMed] [Google Scholar]
- Wessale JL, Adler AL, Novosad EI, Calzadilla SV, Dayton BD, Marsh KC, Winn M, Jae HS, von Geldern TW, Opgenorth TJ, et al. (2002) Pharmacology of endothelin receptor antagonists ABT-627, ABT-546, A-182086 and A-192621: ex vivo and in vivo studies. Clin Sci 103 (Suppl 48):112S–117S [DOI] [PubMed] [Google Scholar]
- Zhou MS, Schulman IH, Jaimes EA, Raij L. (2008) Thiazide diuretics, endothelial function, and vascular oxidative stress. J Hypertens 26:494–500 [DOI] [PubMed] [Google Scholar]