Abstract
The unicellular photosynthetic freshwater flagellate Euglena gracilis is a promising candidate as an oxygen producer in biological life-support systems. In this study, the capacity of Euglena gracilis to cope with different light regimes was determined. Cultures of Euglena gracilis in closed bioreactors were exposed to different dark-light cycles (40 W/m2 light intensity on the surface of the 20 L reactor; cool white fluorescent lamps in combination with a 100 W filament bulb): 1 h–1 h, 2 h–2 h, 4 h–4 h, 6 h–6 h, and 8 h–16 h, respectively. Motility and oxygen development in the reactors were measured constantly. It was found that, during exposure to light-dark cycles of 1 h–1 h, 2 h–2 h, 4 h–4 h, and 6 h–6 h, precision of gravitaxis as well as the number of motile cells increased during the dark phase, while velocity increased in the light phase. Oxygen concentration did not yet reach a plateau phase. During dark-light cycles of 8 h–16 h, fast changes of movement behavior in the cells were detected. The cells showed an initial decrease of graviorientation after onset of light and an increase after the start of the dark period. In the course of the light phase, graviorientation increased, while motility and velocity decreased after some hours of illumination. In all light profiles, Euglena gracilis was able to produce sufficient oxygen in the light phase to maintain the oxygen concentration above zero in the subsequent dark phase. Key Words: Euglena gracilis—Bioreactor—Light-dark cycle—Motility—Gravitaxis. Astrobiology 14, 848–858.
1. Introduction
In contrast to higher plants and, to a smaller extent, higher algae such as seaweeds, the various microalgal groups scarcely play a role in food production or feedstock supplementation. Nevertheless, microalgae have an enormous potential because of their fast growth rate. For several decades, there have been significant technical efforts to make use of the mass production of microalgae, but so far these have only met with limited success (Sanderson et al., 1978; Mata et al., 2010; Ahmad et al., 2011; Committee on the Sustainable Development of Algal Biofuels, 2012). Currently, because of shrinking supplies of fossil fuels and the limited amount of agricultural land that supplies the competing needs of food production and cultivation of plants for technical products such as biodiesel, ethanol, and so on, culturing of microalgae has become a subject of interest. In addition to the production of those products, microalgae offer very interesting possibilities with regard to sewage and wastewater treatment and CO2 sequestration (Douskova et al., 2009). Currently, many research groups and companies are committing research to microalgae (http://www.oilgae.com). Different types of algal reactors and housing facilities are being designed with the intent to breed different microalgae in mass culture (Carvalho et al., 2006).
Because of the advantages associated with microalgae, such as their small size, fast growth, and robustness, they are organisms of interest for spaceflight campaigns. Recently, microalgae were employed as test objects in spaceflight experiments (physiology and molecular biology) as well as in biological regenerative life-support systems, where they served as oxygen producers and general recyclers of oxidized organic matter (Blüm, 2003; Strauch, 2009).
Algal reactor systems on Earth are designed to produce large amounts of biomass and have a partially open flux of material. To achieve maximal biomass production, carbon dioxide and nutrients are supplied at the optimal level. In contrast, algal reactors in the limited environment of a space mission form a closed system with very limited exchange of matter. Closed ecosystems are characterized by a closed cycle of substances. Externally supplied energy in the form of light, heat, and eventually electrical/mechanical energy to drive the internal components of the system (e.g., pumps or filters) is necessary to maintain the function of such a system. The cycle of material in almost all systems is driven by the interplay of photosynthesis and dissimilation processes. Using the energy of light photosynthesis produces organic products rich in energy (e.g., sugar) by reduction of highly oxidized compounds such as carbon dioxide or water. Consumers (e.g., animals) and destruents (fungi and bacteria) use the reduced organic substances as stored energy, which is released by oxidation of these organic products to carbon dioxide and water. Reduction of nitrate to ammonia, or of sulfate to sulfide, is also driven by energy primarily produced in photosynthesis.
Closed ecological systems provide a variety of subjects for scientific research. On the one hand, they can be seen as a simplified representation of a natural ecosystem and allow for the study of the interdependences of the components. On the other, they can be used as life-support systems, if one of the components is considered the supporter or producer while others serve as consumers. The inherent elegance of biological life-support systems makes them of interest for space missions with humans as consumers (Blüm, 2003; Häder et al., 2006). Photosynthetic organisms require only light as their energy source (at temperature conditions that also serve well for humans) to produce oxygen and remove carbon dioxide. They can be used to treat wastewater and produce carbohydrates, which is not possible with any other system. The freshly produced biomass is ideal with regard to enrichment of astronaut's diet during a long voyage or extended stay on a space station or Moon or Mars base. Furthermore, water-based systems offer the opportunity to grow fish, the only economic source of fresh animal protein under the limited conditions in space (Blüm et al., 1995). Many different approaches to the establishment of closed ecosystems or closed biological regenerative life-support systems are currently under development. Larger systems that include humans are, for example, BION 3 in Krasnoyarsk or Biosphere 2 in Arizona. While the latter was not very successful, BION 3 had a recycle rate of biomass of more than 90%. MELiSSA (Micro-Ecological Life Support System Alternative) or C.E.B.A.S. (Closed Equilibrated Biological Aquatic System) were developed as smaller-scale projects. The latter was successfully flown on shuttle missions (Gòdia et al., 2004).
An alga strain that has been frequently employed in space missions is the photosynthetic freshwater flagellate Euglena gracilis. In recent years, it has become a model organism for gravitational research. This organism is believed to have a high potential as an oxygen producer in biological life-support systems either alone or in combination with higher plants. The cells show an interesting movement behavior toward light, gravity, and oxygen (Häder, 1987; Porterfield, 1997). In darkness, the cells swim upward in the water column, which is referred to as negative gravitaxis. They display positive phototaxis toward weak light sources (<10 W/m2) but negative phototaxis at higher irradiances. In addition, the cells react with a phobic tumbling movement at sudden changes of ambient light intensity (step-up or step-down photophobic reaction, respectively). Exposure to high light switches negative to positive gravitaxis and results, upon prolonged exposure, in the loss of phototaxis (Gerber and Häder, 1995).
Phototaxis, gravitaxis, and the general effects of microgravity have been investigated during various space experiments. In addition, Euglena gracilis was successfully flown on several long-term missions (Häder, 1996; Häder et al., 2006; Nasir et al., 2014). A fully autonomous reactor with Euglena cells was flown on a 12-day mission on board the Foton M-2 satellite. The cells provided sufficient oxygen for fish in a tank that was coupled to the algal compartment (Strauch, 2009).
Lebert et al. (1999) employed a fully automatic sample and image analysis device to analyze the movement behavior of cells in a closed reactor with a high temporal resolution. This device was modified to allow constant observation of cells at regular time intervals. Movement was automatically analyzed by an attached microscope device that consisted of pumps and valves as well as a microscope equipped with a CCD camera. The camera signal was analyzed with customized software located on a Pentium PC. The PC also sampled data from the sensors and operated the image analysis (pumping of cells, etc.). Significant changes in movement of the cells during light and dark periods (10 h–14 h) were detected. Like all organisms, Euglena gracilis possesses internal clocks that drive a variety of cellular rhythms at different timescales (from seconds to monthly cycles) (Anderson et al., 1985; Carre and Edmunds, 1992; Mohabir and Edmunds, 1999).
The goal of the experiments in the present study was to detect possible impacts of irregular light exposure on photosynthetic performance of the Euglena gracilis. A producer with low sensitivity to irregular illumination would be more advantageous in life-support systems. In addition, although less important for life-support systems, the effect of different day-night cycles on movement behavior was observed to provide information concerning entrainable internal rhythms in Euglena gracilis.
The measurements elucidated very interesting and significant effects, which are presented in this report.
2. Materials and Methods
2.1. Organisms and culture conditions
Euglena gracilis Z was obtained from the collection of algal cultures at the University of Göttingen (Schlösser, 1994). The cells were grown in a mineral medium as described earlier (Starr, 1964; Benedetti et al., 1976; Checcucci, 1976) in stationary cultures in 100 mL Erlenmeyer flasks at about 20°C under continuous light of about 18 W/m2 from mixed cool white and warm tone fluorescent lamps.
2.2. Euglena gracilis bioreactor
Five hundred milliliters of static stock cultures of Euglena gracilis were transferred into a fermenter (Biostat, Braun Biotech GmbH, Melsungen) filled with mineral medium. The end volume was 11 L. The fermenter was illuminated with cool white fluorescent lamps and a 100 W filament bulb that reached 40 W/m2 at the surface of the reactor. The temperature was maintained at 20°C with a thermostat. Two closed-loop systems were installed in which cell suspension could be transported into a microscope cuvette or through a chamber with different sensors (oxygen, pH, temperature). The liquid was pumped by way of peristaltic pumps. The light-dark cycles were triggered by a time switch. A photodiode registered the timing of the light to enable exact comparison with the other measurement data. The data were recorded with computers that were equipped with suitable I/O cards or frame grabbers, respectively. Oxygen, pH, temperature, and light were recorded with custom-made software.
Image analysis was recorded with a separate computer system. The system times of the different computers were regularly controlled and adjusted to avoid time shifts between the recordings. The oxygen measurement was based on the Clark principle or on a newly developed fluorimetric system (Presens, Regensburg, Germany). While the former has a limited lifetime and consumes oxygen during the process, the latter is stable for longer periods and does not influence oxygen concentrations. It excites a fluorescent dye immobilized in a polymer matrix by way of a blue light flash and measures the subsequent fluorescence lifetime of this substance. The fluorescence lifetime strongly depends on the oxygen concentration, since excitation energy is transferred to oxygen upon molecule collision, which quenches the fluorescence. The data presented were obtained in the course of different independent experiment runs. Light-dark cycles always lead to pronounced changes in movement behavior, as described in this report.
2.3. Image analysis
As described above, the cells were pumped into an observation cuvette with a chamber diameter of about 2 cm and a depth of 200 μm. The cells were observed with an attached microscope, under IR-LED illumination (invisible for the cells). The cuvette was oriented vertically to allow the cells to demonstrate gravitactic upward swimming. An analog CCD camera (European video norm CCIR) was mounted on top of the microscope, and the video stream was transferred to a computer, which was equipped with a frame-grabber card (Matrox Meteor PPB, Quebec, Canada). The software, written in Visual Basic by Harald Tahedl, employed the ancient but very reliable and robust DOS versions of Wintrack (Vogel and Häder, 1990; Häder and Vogel, 1991; Häder et al., 1991). By way of RS-232 trigger signals, the software could control the pump and valves. The valves were necessary to prevent streaming inside the observation cuvette. Measurements were performed in desired time intervals. Cells were pumped into the cuvette, and subsequently streaming was blocked upon locking of the valves. Thereafter, five subsequent measurements were performed, each of which took exactly 1 min. The shift of the visible object in subsequent video frames was analyzed and used to construct movement vectors. Because the timing is correlated with the video frequency, the velocity of each object could also be calculated. For each measurement, the data for all recorded movement vectors were analyzed and movement parameters calculated. The results of these calculations were stored digitally and the following parameters reported.
r Value: indicates precision of orientation (Batschelet, 1981), calculating the sines and cosines of all movement vectors detected (values toward 1 stand for precise orientation, while values close to 0 indicate low or no orientation of a cell culture):
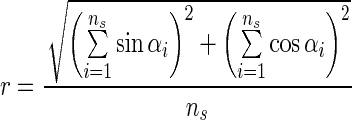 |
Motility (percentage of motile organisms): where ns=number of vectors for which the calculated velocity is higher than a predefined threshold value and n=the total number of calculated vectors:
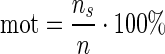 |
Theta Φ: average movement vector of all single tracks (°; 0°/360° is upward). Provides the angle of the main distribution (Häder et al., 2005).
Upward: percentage of upward-swimming cells (300° to 60°).
Velocity: average velocity of the detected objects (μm/s).
2.4. Statistical analysis
Movement data obtained in the different illumination phases were pooled and analyzed with the Student t test.
3. Results
The following dark-light cycles were established: 1 h–1 h, 2 h–2 h, 4 h–4 h, 6 h–6 h, and 8 h–16 h. Motility parameters of Euglena gracilis were influenced in the course of all light profiles.
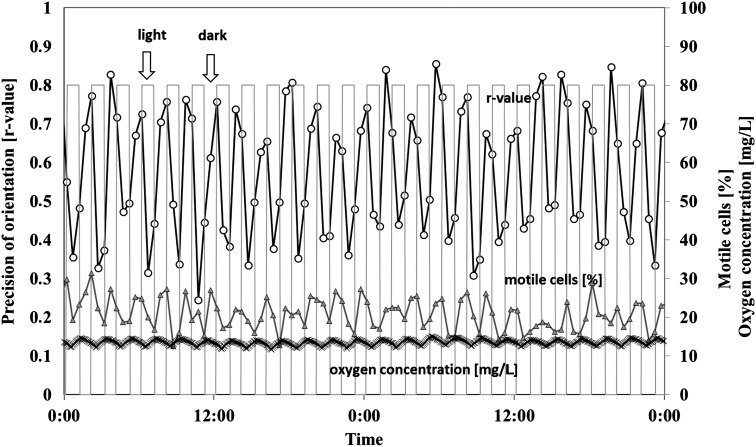
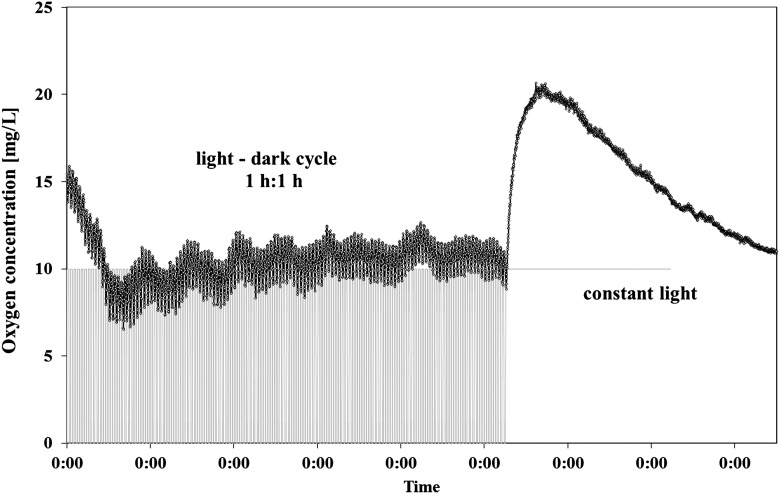
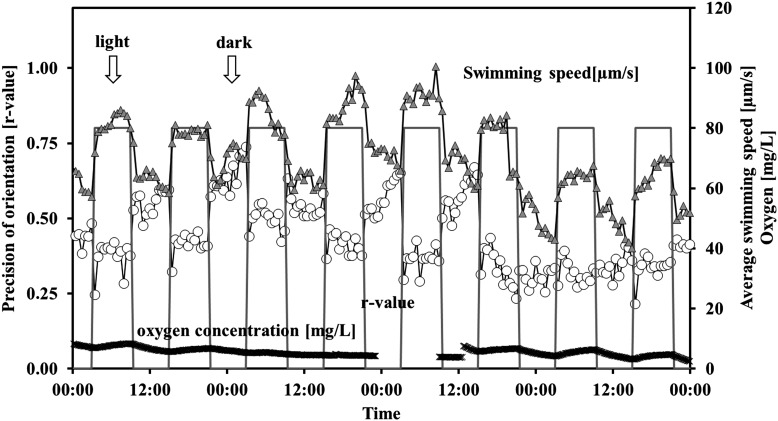
For a 1 h–1 h dark-light cycle, gravitactic orientation and motility were better in the dark phase than in the light phase while velocity increased in the light compared to the dark (Table 1). Figure 1 shows the changes in the r values, motility, and oxygen concentration in the course of 2 representative days. While average r values were about 0.7 in darkness, the r values in light were only 0.44. In subsequent constant light, the r values were on average only 0.33. In darkness, 86.9% of the cells swam upward; in the light phase, 71.73% swam upward. Under constant light, only 66.17% of the cells swam upward. The differences were significant (p<0.001). The speed increased significantly from 71.2 μm/s in darkness to 75.8 μm/s in light (p<0.001). The detected motility dropped from 24.02% in darkness to 17.54% in light and was 16.16% in constant light (p<0.001 in each comparison). The oxygen production was high during this illumination profile, with an average oxygen level of 10 mg/L, that is, close to saturation. Under constant light, the oxygen level increased initially to more than 20 mg/mL then began to drop (Fig. 2).
Table 1.
A Bioreactor with Euglena gracilis Cells under Alternating Illumination (1 h Dark–1 h Light)
| Light profile | r Value | Theta | Up (%) | Motile (%) | Velocity (μm/s) | Counts |
|---|---|---|---|---|---|---|
| DP | 0.71 | 353.58 | 86.86 | 24.02 | 71.21 | 327.64 |
| DP stdev | 0.10 | 5.09 | 5.93 | 4.52 | 2.29 | 74.45 |
| LP | 0.44a | 337.98 | 71.74a | 17.55a | 75.81a | 214.35a |
| LP stdev | 0.13 | 13.41 | 7.51 | 3.78 | 2.40 | 62.23 |
| CL | 0.33a,b | 336.64 | 66.17a,b | 16.17a,b | 68.89a,b | 219.76a |
| CL stdev | 0.06 | 12.53 | 3.87 | 2.29 | 2.36 | 42.07 |
Mean values of movement parameters at different illumination phases. The table shows average and standard deviation (stdev) of all recorded movement parameters of the dark and light cycles as well as during subsequent constant illumination.
r Value: precision of orientation. Theta: mean direction of movement (upward=0° or 360°, respectively, downward=180°). Up: upward-swimming cells (%). Motile: fraction of motile cells (%). Velocity: mean velocity of cell culture (μm/s). Counts: average of analyzed cells per minute (for detailed description of movement parameters, see the Materials and Methods section).
DP, movement of the cells in the dark phase; LP, movement of the cells in the light phase of the cycle; CL, movement of the cells under constant illumination (no light-dark cycle).
Significant differences of movement behavior between (i) dark phase and light phase and (ii) dark phase and constant light.
Significant differences between constant light and light period during light-dark cycles.
FIG. 1.
Influence of 1 h–1 h dark-light cycles on gravitactic orientation (r value) and motility of Euglena gracilis, as well as the oxygen concentration in a closed bioreactor. Cells were measured every 30 min. The bars indicate the light period. The r value is a parameter of the precision of the movement direction (0: no orientation, 1: all cells swim in the same direction). Open circles: precision of orientation. Triangles: motile cells (%). Crosses: oxygen concentration. For details, see the text.
FIG. 2.
Oxygen concentrations in a closed bioreactor with Euglena gracilis during 1 h–1 h dark-light cycles. The gray line indicates the light status. After 12 days, the illumination was switched to constant light.
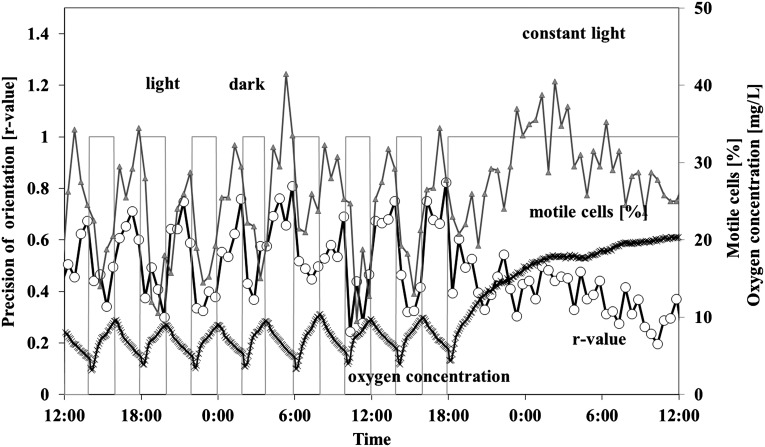
The results in 2 h–2 h dark-light cycles are comparable to the 1 h–1 h treatment. The cells were precisely oriented in the dark phase, less so in the subsequent light period (Fig. 3 and Table 2). The average r value was 0.55 in darkness and decreased significantly to 0.34 in the light phase; in a subsequent constant light regime, the r value was 0.32. The fraction of upward-swimming cells was 78.32% in darkness and 63.84% in light or 63.36% in constant light, respectively. Detected motility dropped from 28.96% in darkness to 20.27% in light (Fig. 3). Under subsequent constant illumination, the average motility was 25.65%. Speed, in contrast, increased slightly in light. Swimming speed was 63 μm/s in darkness, 67 μm/s in the light period, and 71.5 μm/s in constant light.
FIG. 3.
Influence of 2 h–2 h dark-light cycles on gravitactic orientation (r value) and swimming speed of Euglena gracilis, as well as the oxygen concentration in a closed bioreactor. Cells were measured every 30 min. The bars indicate the light period. The figure shows representative dark-light cycles with subsequent constant illumination. The r value is a parameter of the precision of the movement direction (0: no orientation, 1: all cells swim in the same direction). Open circles: precision of orientation. Triangles: motile cells (%). Crosses: oxygen concentration. For details, see the text.
Table 2.
A Bioreactor with Euglena gracilis Cells under Alternating Illumination (2 h Dark–2 h Light)
| Light profile | r Value | Theta | Up (%) | Motile (%) | Velocity (μm/s) | Counts |
|---|---|---|---|---|---|---|
| DP | 0.56 | 258.24 | 78.33 | 28.97 | 63.28 | 356.74 |
| DP stdev | 0.14 | 56.66 | 8.31 | 6.35 | 10.11 | 106.92 |
| LP | 0.35a | 239.76a | 63.84a | 20.27a | 67.65a | 201.51a |
| LP stdev | 0.13 | 45.38 | 9.06 | 5.83 | 10.92 | 98.54 |
| CL | 0.32a | 235.76a | 63.36a | 25.66a,b | 71.55a,b | 268.46a,b |
| CL stdev | 0.10 | 41.17 | 6.54 | 5.76 | 7.52 | 78.83 |
Mean values of movement parameters at different illumination phases. The table shows average and standard deviation (stdev) of all recorded movement parameters of the dark and light cycles as well as during subsequent constant illumination. For detailed information, see the caption to Table 1.
Immediately after closure of the reactor, no net photosynthetic oxygen production could be observed. After about 5 days, photosynthetic oxygen evolution was detected. After 3 days, the dark-light fluctuation reached a constant level where maximum oxygen values between about 8 and 10 mg/L were measured. In constant light, the oxygen level rose up to 25 mg/L within 1 day (Fig. 3).
In a 4 h–4 h dark-light cycle, the effect on cell motility was similar as during the previously described illumination profiles (Table 3). The average r value was 0.5 in the dark and 0.38 in light (Fig. 4). Constant light was not applied in this experiment. About 75.37% of the cells swam upward in darkness, and 68.88% swam upward in the light (differences are significant). Speed increased slightly, but significantly, from 62.69 to 71.33 μm/s (Fig. 4), and motility increased in light from 19.59% to 23.8% (significant as well). Motility did not increase directly after the onset of light but only after a lag of between 1 and 2 h. Oxygen production was pronounced, but oxygen consumption decreased the oxygen concentration considerably within 4 h (Fig. 4). Overall, both the oxygen concentration and amplitude of its fluctuation decreased during the course of the experiment. While initially the difference between lowest value (end of dark period) and highest value (end of light period) was about 6.4 mg/L, the range of the fluctuation in oxygen concentration decreased to 4.3 mg/L in later cycles.
Table 3.
A Bioreactor with Euglena gracilis Cells under Alternating Illumination (4 h Dark–4 h Light)
| Light profile | r Value | Theta | Up (%) | Motile (%) | Velocity (μm/s) | Counts |
|---|---|---|---|---|---|---|
| DP | 0.50 | 346.48 | 75.37 | 19.59 | 62.70 | 181.96 |
| DP stdev | 0.13 | 9.04 | 8.00 | 2.91 | 3.49 | 43.43 |
| LP | 0.38a | 336.90a | 68.88a | 23.81a | 71.33a | 252.40a |
| LP stdev | 0.09 | 12.21 | 5.90 | 4.63 | 3.29 | 82.73 |
Mean values of movement parameters at different illumination phases. The table shows average and standard deviation (stdev) of all recorded movement parameters of the dark and light cycles. For detailed information, see the caption to Table 1. Effects of constant light were not determined.
FIG. 4.
Influence of 4 h–4 h dark-light cycles on gravitactic orientation (r value) and swimming speed of Euglena gracilis, as well as the oxygen concentration in a closed bioreactor. Cells were measured every 30 min. The bars indicate the light period. The figure shows representative dark-light cycles with subsequent constant illumination. The r value is a parameter of the precision of the movement direction (0: no orientation, 1: all cells swim in the same direction). Open circles: precision of orientation. Triangles: swimming speed (μm/s). Crosses: oxygen concentration. Gap in oxygen measurement: malfunction of oxygen measurement device. For details, see the text.
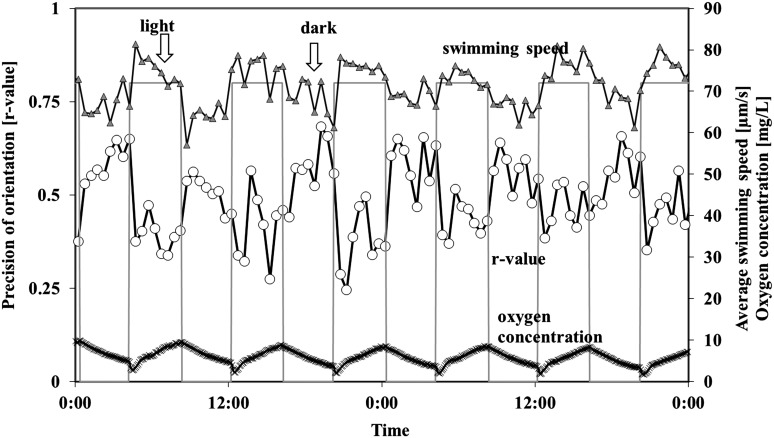
In a 6 h–6 h dark-light cycle, gravitactic orientation was significantly increased in the darkness (Table 4). The r value decreased from 0.48 to 0.38, and the amount of upward-swimming cells decreased from 80.22% to 74.04% in the light period (Fig. 5). Motility was slightly, but significantly, lower in light (66.58% vs. 70.38%) while the average speed was considerably increased (61.09 μm/s in darkness, 76.59 μm/s in light, Fig. 5). The response to the changing light regime was remarkably fast in this experiment. The oxygen concentration was in a range of 1.4 mg/L (darkness) and between 5 and 6 mg/L in the light.
Table 4.
A Bioreactor with Euglena gracilis Cells under Alternating Illumination (6 h Dark–6 h Light)
| Light profile | r Value | Theta | Up (%) | Motile (%) | Velocity (μm/s) | Counts |
|---|---|---|---|---|---|---|
| DP | 0.49 | 295.19 | 80.23 | 70.39 | 61.09 | 1675.29 |
| DP stdev | 0.12 | 62.63 | 4.51 | 7.49 | 4.16 | 226.32 |
| LP | 0.38a | 283.16 | 74.04a | 66.59a | 76.59a | 1505.53a |
| LP stdev | 0.07 | 56.84 | 6.09 | 8.11 | 4.58 | 199.17 |
Mean values of movement parameters at different illumination phases. The table shows average and standard deviation (stdev) of all recorded movement parameters of the dark and light cycles. For detailed information, see the caption to Table 1. Effects of constant light were not determined.
FIG. 5.
Influence of 6 h–6 h dark-light cycles on gravitactic orientation (r value) and swimming speed of Euglena gracilis, as well as the oxygen concentration in a closed bioreactor. Cells were measured every 30 min. The bars indicate the light period. The figure shows representative dark-light cycles. The r value is a parameter of the precision of the movement direction (0: no orientation, 1: all cells swim in the same direction). Open circles: precision of orientation. Triangles: swimming speed (μm/s). Crosses: oxygen concentration. Gap in oxygen measurement: malfunction of oxygen measurement device. For details, see the text.
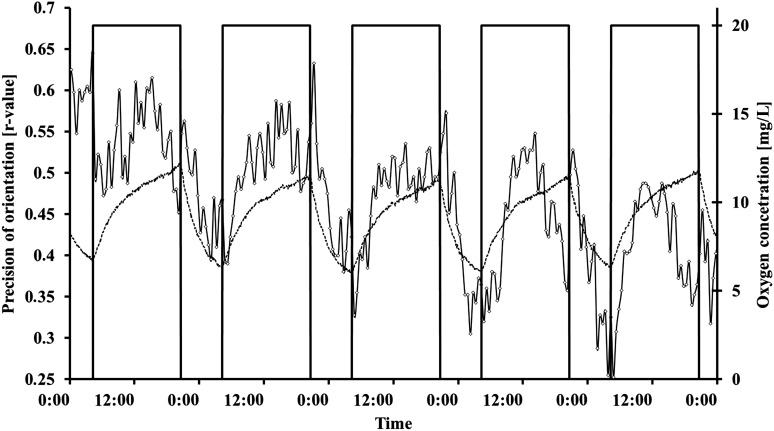
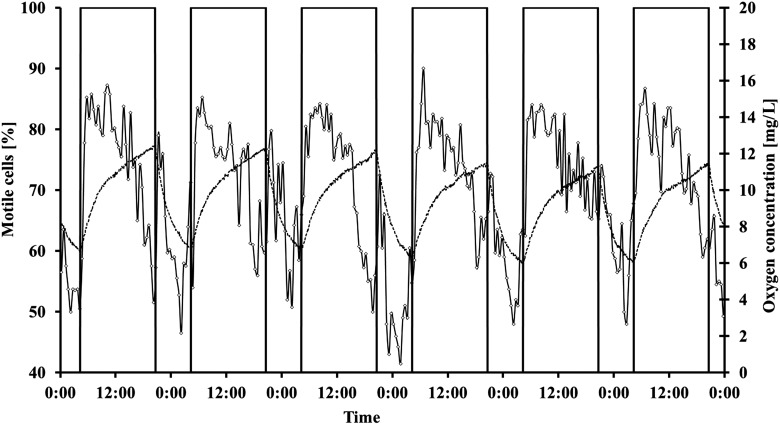
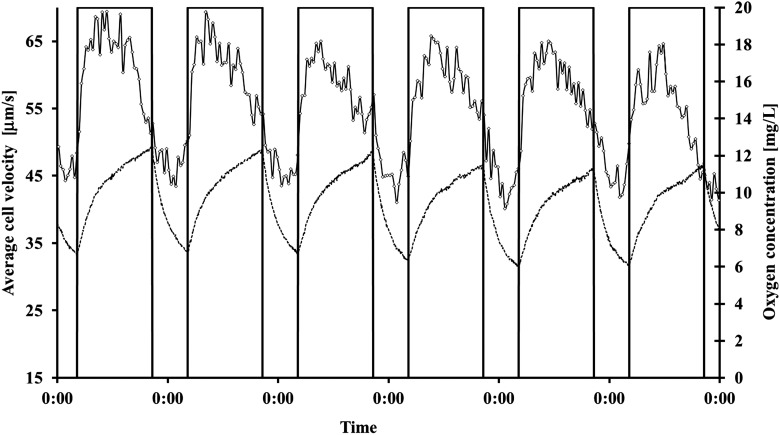
During a dark-light cycle of 8 h–16 h, no significant changes in graviorientation were measured (Table 5). The average of the r values was 0.5 in darkness and 0.49 in light; 73.14% of the cells swam upward in the darkness, and 73.49% swam upward in light (Figs. 6 and 7). By looking at the kinetics of the interaction, it can be seen that Euglena gracilis shows a pronounced response upon transition from dark to light and vice versa. After transition from dark to light, graviorientation initially decreased but increased after about 4 h to at least the values obtained in the dark period. After about 12 h illumination, gravitactic orientation often decreased again. Upon transition to the dark period, the precision of orientation first increased considerably but in most cases dropped to relatively low values. Average motility was 49.54% in darkness and 63.31% in light. After transition from light into the dark period, the motility increased immediately about 2 h, after which motility began to decrease again. After several cycles (about seven or eight), motility recovered about 1 h before the light switched on again (Fig. 6 shows representative cycles). After transition from dark into light, motility showed a pronounced increase that reached a maximum after about 2 h, and subsequently motility decreased again. After about 14 h of light, there was a pronounced drop in motility that lasted until the transition to darkness, when motility rose rapidly. Swimming speed is much faster in light (54.44 μm/s) compared to darkness (44.42 μm/s). The kinetic of the swimming velocity was pronounced. In darkness velocity was almost constant, while in light velocity initially increased very quickly. After about 12 h, velocity decreased almost to the dark values (Fig. 8). Oxygen evolution followed the course of illumination. In the dark period, the oxygen level did not reach values below 5 mg/L.
Table 5.
A Bioreactor with Euglena gracilis Cells under Alternating Illumination (8 h Dark–16 h Light)
| Light profile | r Value | Theta | Up (%) | Motile (%) | Velocity (μm/s) | Counts |
|---|---|---|---|---|---|---|
| DP | 0.51 | 280.73 | 73.14 | 49.54 | 44.43 | 297.76 |
| DP stdev | 0.15 | 87.03 | 12.39 | 20.58 | 5.82 | 146.38 |
| LP | 0.49 | 270.45 | 73.50 | 63.32a | 54.54a | 350.79a |
| LP stdev | 0.14 | 96.69 | 11.48 | 24.82 | 7.47 | 188.86 |
Mean values of movement parameters at different illumination phases. The table shows average and standard deviation (stdev) of all recorded movement parameters of the dark and light cycles. For detailed information, see the caption to Table 1. Effects of constant light were not determined.
FIG. 6.
Influence of 8 h–16 h dark-light cycles on gravitactic orientation of Euglena gracilis in a closed bioreactor. Cells were measured every 30 min. The bars indicate the light period. The figure shows representative dark-light cycles with subsequent constant illumination. The r value is a parameter of the precision of the movement direction (0: no orientation, 1: all cells swim in the same direction). The dashed line shows the oxygen concentration in the reactor (mg/L). For details, see the text.
FIG. 7.
Influence of 8 h–16 h dark-light cycles on motility of Euglena gracilis cells in a closed bioreactor. Cells were measured every 30 min. The bars indicate the light period. The figure shows representative dark-light cycles with subsequent constant illumination. Due to limitations in the modified tracking software, the fraction of motile cells is underestimated. But the deviation is the same in all measurements (for details, see the text). The dashed line shows the oxygen concentration in the reactor (mg/L).
FIG. 8.
Influence of 8 h–16 h dark-light cycles on swimming velocity of Euglena gracilis (μm/s). Cells were measured every 30 min. The bars indicate the light period. The figure shows representative dark-light cycles with subsequent constant illumination. Due to limitations in the modified tracking software, the fraction of motile cells is underestimated. But the deviation is the same in all measurements (for details, see the text). The dashed line shows the oxygen concentration in the reactor (mg/L).
4. Discussion
4.1. Discussion of motility data
Under microgravity, buoyancy-driven convection is absent, so that particles are moved solely by the far slower process of diffusion. As a consequence, the environment around immotile cells is soon depleted of nutrients and begins to accumulate metabolic waste products, including oxygen in the case of photosynthetic algae and carbon dioxide in the case of respiring cells. Motile cells are less affected because their constant movement inhibits the formation of a depleted zone around the cell. Observations of cell motility provide information about the actual physiological conditions and can serve as a measure of the degree of mixing of nutrients and metabolic substances (the more motile the cells are, the better mixed the environment). Our measurements reveal that at least a considerable portion of the cells was motile in all the various light-dark treatments. A high degree of motility has been observed in Euglena gracilis under constant red illumination during previous space missions (Häder et al., 1996; Strauch, 2009).
Pronounced motility, photosynthetic efficiency, and low demands in environmental factors make Euglena gracilis a promising candidate for oxygen production in future space experiments. Another advantage of Euglena gracilis is that the cells can use ammonia and reduced amino groups as their sole nitrogen source (Buetow, 1966; Oda et al., 1982). However, they are unable to use nitrate or nitrite. Therefore, they can effectively reduce the concentration of toxic ammonia and contribute to system stability of biological life-support systems without being nitrate competitors for co-cultivated higher plants.
In addition, the experiments yielded interesting results concerning general effects of light-dark cycles on the movement behavior, most of all gravitaxis. The data provided by the present study show a clear correlation between illumination and movement behavior of Euglena gracilis cells in a closed reactor. At all short-term light cycles (light-dark: 1 h–1 h, 2 h–2 h, 4 h–4 h, 6 h–6 h), cells responded with a significant increase in negative gravitactic orientation in darkness and an increase of velocity in the light phase. The observed increase in swimming speed in light is possibly due to photokinesis, a light-induced increase in swimming speed. Photokinesis has been described for Euglena mutabilis (Melkonian et al., 1986).
Cells responded immediately to a change of light condition. In most cases, a pronounced change in movement parameters (e.g., precision of orientation, swimming speed) between the last time point of a dark phase and the first time point in the subsequent light phase (and vice versa) was observed. Euglena gracilis is an intensively investigated model organism in chronobiology (Anderson et al., 1985; Edmunds et al., 1992; Edmunds, 2005). Euglena gracilis displays a well-known endogenous rhythmicity in its cellular functions. These cycles are synchronized by periodic external factors (e.g., light). This nearly temperature-independent synchronization is termed entrainment, and the external periodic synchronizing signals are termed pacemaker or Zeitgeber (Harmer et al., 2001). However, no analysis of signal-transduction change of circadian rhythm components was performed in this study. Thus it is not clear whether the observed periodicities of the motility parameters are due to entrained internal rhythms or are direct responses to light. However, the gravitactic measurement points in the direction of entrained ultradian rhythms because cells show a periodicity in gravitaxis even in the presence of constant light after light and dark cycles (2h-2h light-dark).
4.2. Discussion of oxygen data
It was found that Euglena gracilis in closed reactors produced oxygen under all applied illumination intervals without reaching oversaturation or depletion (range between 4 and 10 mg/L). Permanent light, in contrast, resulted in excessive concentrations around 20 mg/L. Accordingly, a 4 h–4 h light-dark cycle was employed in subsequent experiments. Under these conditions, oxygen concentration remained above 8 mg/L; the system ran at least 50 days without any disturbance (Lebert et al., unpublished results). This demonstrates that short-term light cycles do not impair the viability of a Euglena gracilis culture. The ability of Euglena gracilis to adapt even to very short light-dark cycles is of practical importance as an oxygen-producing element in a life-support system for space applications. The main purpose of this system is production of oxygen for zoological partners in a life-support system. Additionally, the algae utilize carbon dioxide and excretion products of the animals. During a long-term (16 d) space experiment (Aquacells on Foton M2), no negative effects were observed in Euglena gracilis cells (Häder et al., 2006). In a later experiment (OMEGAHAB on Foton M3), where a reactor with Euglena gracilis cells was coupled with a fish tank, it was shown that the cells can produce enough oxygen to ensure survival of fish larvae (Strauch, 2009). During these experiments, Euglena gracilis cells were constantly irradiated with red light. To save electrical power, it would be beneficial to drive a light-dark cycle. Long dark periods always bear a risk of oxygen depletion, with lethal consequences for animals in a coupled compartment. A possible strategy of flexible light-dark cycles is to avoid oxygen depletion as well as oversaturation.
5. Conclusion
Euglena gracilis has become a model organism in gravitational research and can be subjected to physiological and molecular biological studies.
The data of this report show that Euglena gracilis can tolerate different light profiles, which enables precise dosing of oxygen suitable for organisms with different needs in oxygen. In addition, the cells only consume ammonia and therefore do not compete with higher plants for nitrate. Above all, Euglena gracilis is an approved space-compliant organism that is already in use in a variety of short- and long-term space experiments.
Acknowledgments
This work was funded by the DLR on behalf of the BMBF (grant 50WB1128). The authors thank the anonymous reviewers for critical comments and fruitful suggestions.
Disclosure Statement
No competing financial interests exist.
References
- Ahmad A.L., Yasin N.M., Derek C., and Lim J.K. (2011) Microalgae as a sustainable energy source for biodiesel production: a review. Renewable and Sustainable Energy Reviews 15:584–593 [Google Scholar]
- Anderson R.W., Laval-Martin D.L., and Edmunds L.N., Jr. (1985) Cell cycle oscillators. Temperature compensation of the circadian rhythm of cell division in Euglena. Exp Cell Res 157:144–158 [DOI] [PubMed] [Google Scholar]
- Batschelet E. (1981) Circular Statistics in Biology, Mathematics in Biology, Academic Press, London [Google Scholar]
- Benedetti P.A., Bianchini G., Checcucci A., Ferrara R., and Grassi S. (1976) Spectroscopic properties and related functions of the stigma measured in living cells of Euglena gracilis. Arch Microbiol 111:73–76 [DOI] [PubMed] [Google Scholar]
- Blüm V. (2003) Aquatic modules for bioregenerative life support systems: developmental aspects based on the space flight results of the C.E.B.A.S. MINI-MODULE. Adv Space Res 31:1683–1691 [DOI] [PubMed] [Google Scholar]
- Blüm V., Andriske M., Kreuzberg K., and Schreibman M.P. (1995) Animal protein production modules in biological life support systems: novel combined aquaculture techniques based on the Closed Equilibrated Biological Aquatic System (C.E.B.A.S.). Acta Astronaut 36:615–623 [DOI] [PubMed] [Google Scholar]
- Buetow D.E. (1966) Amino acids as nitrogen sources for the growth of Euglena gracilis and Astasia longa. The Journal of Protozoology 13:585–587 [Google Scholar]
- Carre I.A. and Edmunds L.N. (1992) cAMP-dependent kinases in the algal flagellate Euglena gracilis. J Biol Chem 267:2135–2137 [PubMed] [Google Scholar]
- Carvalho A.P., Meireles L.A., and Malcata F.X. (2006) Microalgal reactors: a review of enclosed system designs and performances. Biotechnol Prog 22:1490–1506 [DOI] [PubMed] [Google Scholar]
- Checcucci A. (1976) Molecular sensory physiology of Euglena. Naturwissenschaften 63:412–417 [DOI] [PubMed] [Google Scholar]
- Committee on the Sustainable Development of Algal Biofuels (2012) Sustainable Development of Algal Biofuels, National Academies Press, Washington, DC [Google Scholar]
- Douskova I., Doucha J., Livansky K., Machat J., Novak P., Umysova D., Zachleder V., and Vitova M. (2009) Simultaneous flue gas bioremediation and reduction of microalgal biomass production costs. Appl Microbiol Biotechnol 82:179–185 [DOI] [PubMed] [Google Scholar]
- Edmunds L.N. (2005) Circadian organization in the algal flagellate Euglena. In Madame Curie Bioscience Database [Internet], Landes Bioscience, Austin, TX [Google Scholar]
- Edmunds L.N., Jr., Carre I.A., Tamponnet C., and Tong J. (1992) The role of ions and second messengers in circadian clock function. Chronobiol Int 9:180–200 [DOI] [PubMed] [Google Scholar]
- Gerber S. and Häder D.-P. (1995) Effects of artificial UV-B and simulated solar radiation on the flagellate Euglena gracilis: physiological, spectroscopical and biochemical investigations. Acta Protozool 34:13–20 [Google Scholar]
- Gòdia F., Albiol J., Pérez J., Creus N., Cabello F., Montràs A., Masot A., and Lasseur C. (2004) The MELiSSA pilot plant facility as an integration test-bed for advanced life support systems. Adv Space Res 34:1483–1493 [DOI] [PubMed] [Google Scholar]
- Häder D.-P. (1987) Polarotaxis, gravitaxis and vertical phototaxis in the green flagellate, Euglena gracilis. Arch Microbiol 147:179–183 [DOI] [PubMed] [Google Scholar]
- Häder D.-P. (1996) NIZEMI—experiments on the slow rotating centrifuge microscope during the IML-2 mission. J Biotechnol 47:223–224 [DOI] [PubMed] [Google Scholar]
- Häder D.-P. and Vogel K. (1991) Interactive image analysis system to determine the motility and velocity of cyanobacterial filaments. J Biochem Biophys Meth 22:289–300 [DOI] [PubMed] [Google Scholar]
- Häder D.-P., Reinecke E., Vogel K., and Kreuzberg K. (1991) Responses of the photosynthetic flagellate, Euglena gracilis, to hypergravity. Eur Biophys J 20:101–107 [DOI] [PubMed] [Google Scholar]
- Häder D.-P., Rosum A., Schäfer J., and Hemmersbach R. (1996) Graviperception in the flagellate Euglena gracilis during a shuttle space flight. J Biotechnol 47:261–269 [DOI] [PubMed] [Google Scholar]
- Häder D.-P., Hemmersbach R., and Lebert M. (2005) Gravity and the Behavior of Unicellular Organisms, Cambridge University Press, Cambridge, UK [Google Scholar]
- Häder D.-P., Richter P.R., Strauch S.M., and Schuster M. (2006) Aquacells—flagellates under long-term microgravity and potential usage for life support systems. Microgravity Sci Technol 18:210–214 [Google Scholar]
- Harmer S.L., Panda S., and Kay S.A. (2001) Molecular bases of circadian rhythms. Annu Rev Cell Dev Biol 17:215–253 [DOI] [PubMed] [Google Scholar]
- Lebert M., Porst M., and Häder D.-P. (1999) Circadian rhythm of gravitaxis in Euglena gracilis. J Plant Physiol 155:344–349 [DOI] [PubMed] [Google Scholar]
- Mata T.M., Martins A.A., and Caetano N.S. (2010) Microalgae for biodiesel production and other applications: a review. Renewable and Sustainable Energy Reviews 14:217–232 [Google Scholar]
- Melkonian M., Meinicke-Liebelt M., and Häder D.-P. (1986) Photokinesis and photophobic responses in the gliding flagellate, Euglena mutabilis. Plant Cell Physiol 27:505–513 [Google Scholar]
- Mohabir G. and Edmunds L.N., Jr. (1999) Circadian clock regulation of the bimodal rhythm of cyclic AMP in wild-type Euglena. Cell Signal 11:143–147 [DOI] [PubMed] [Google Scholar]
- Nasir A., Strauch S.M., Becker I., Sperling A., Schuster M., Richter P.R., Weißkopf M., Ntefidou M., Daiker V., An Y.A., Li X.Y., Liu Y.D., Lebert M., and Legué V. (2014) The influence of microgravity on Euglena gracilis as studied on Shenzhou 8. Plant Biol (Stuttg) 16(Supplement 1):113–119 [DOI] [PubMed] [Google Scholar]
- Oda Y., Nakano Y., and Kitaoka S. (1982) Utilization and toxicity of exogenous amino acids in Euglena gracilis. J Gen Microbiol 128:853–858 [Google Scholar]
- Porterfield D.M. (1997) Orientation of motile unicellular algae to oxygen: oxytaxis in Euglena. Biol Bull 193:229–230 [DOI] [PubMed] [Google Scholar]
- Sanderson J.E., Wise D.L., and Augenstein D.C. (1978) Organic chemicals and liquid fuels from algal biomass. Biotechnol Bioeng Symp 8:131–151 [Google Scholar]
- Schlösser U.G. (1994) SAG-Sammlung von Algenkulturen at the University of Göttingen. Bot Acta 107:113–186 [Google Scholar]
- Starr R.C. (1964) The culture collection of algae at Indiana University. Am J Bot 51:1013–1044 [Google Scholar]
- Strauch S.M. (2009) Euglena gracilis als Sauerstoffproduzent eines bioregenerativen Lebenserhaltungssystems und ihre physiologische Reaktion auf Änderungen der Schwerkraft. Thesis, Friedrich-Alexander-University Erlangen-Nuremberg, Erlangen, Germany [Google Scholar]
- Vogel K. and Häder D.-P. (1990) Simultaneous tracking of flagellates in real time by image analysis. In Proceedings of the Fourth European Symposium on Life Sciences Research in Space, ESA SP-307, European Space Agency, Paris, pp 541–545 [Google Scholar]