Cai et al. find that Rspo1 cooperates with another hormonal mediator, Wnt4, to promote mammary stem cell (MaSC) self-renewal through Wnt/β-catenin signaling. Hormonal treatment that stimulates the expression of both Rspo1 and Wnt4 can completely substitute for exogenous Wnt proteins, potently expand MaSCs, and maintain their full development potential in transplantation. This study shows that hormones can induce a collaborative local niche environment for stem cells.
Keywords: mammary stem cell, niche, luminal cell, hormone, Rspo1, Wnt4
Abstract
Signals from the niche play pivotal roles in regulating adult stem cell self-renewal. Previous studies indicated that the steroid hormones can expand mammary stem cells (MaSCs) in vivo. However, the facilitating local niche factors that directly contribute to the MaSC expansion remain unclear. Here we identify R-spondin1 (Rspo1) as a novel hormonal mediator in the mammary gland. Pregnancy and hormonal treatment up-regulate Rspo1 expression. Rspo1 cooperates with another hormonal mediator, Wnt4, to promote MaSC self-renewal through Wnt/β-catenin signaling. Knockdown of Rspo1 and Wnt4 simultaneously abolishes the stem cell reconstitution ability. In culture, hormonal treatment that stimulates the expression of both Rspo1 and Wnt4 can completely substitute for exogenous Wnt proteins, potently expand MaSCs, and maintain their full development potential in transplantation. Our data unveil the intriguing concept that hormones induce a collaborative local niche environment for stem cells.
Adult stem cells reside in unique microenvironments known as niches. Signals from the niche play dominant roles in controlling stem cell self-renewal and maintaining the undifferentiated state. Mapping the niche composition and identifying its signals are important for understanding stem cell regulatory mechanisms.
Mammary gland development is governed by the concerted action of systemic hormones and local growth factors. The mammary gland is an epithelial organ, consisting of a basal layer of myoepithelial cells and an inner layer of luminal cells. Hormone receptor-positive cells are in the luminal layer of the epithelium. The basal population, harboring mammary stem cells (MaSCs), is able to generate new mammary glands in transplantation assays (Shackleton et al. 2006; Stingl et al. 2006; Zeng and Nusse 2010) and differentiate into all epithelial cell lineages (Rios et al. 2014). Despite the fact that MaSCs show a hormone receptor-negative phenotype (Asselin-Labat et al. 2006; Lim et al. 2009), steroid hormone signaling profoundly influences the behavior of MaSCs (Asselin-Labat et al. 2010; Joshi et al. 2010). Thus, the action of hormones on stem cells certainly involves other local factors. Previous studies have shown that hormonal treatment expands MaSCs in vivo and that elevated Wnt4 and Rankl levels are correlated with hormonal treatment (Asselin-Labat et al. 2010; Joshi et al. 2010). However, whether Wnt4 or Rankl controls MaSCs self-renewal and expansion has not been formally demonstrated. On the other hand, the output of Wnt4 in the mammary gland is controversial, and whether Wnt4 participates in canonical Wnt/β-catenin signaling is unclear (Bradbury et al. 1995; Brisken et al. 2000; Kim et al. 2009).
Self-renewal of MaSCs is dependent on canonical Wnt signals (Badders et al. 2009; Zeng and Nusse 2010; van Amerongen et al. 2012). Upon the binding of Wnt ligands to the receptor Frizzled (Fz) and lipoprotein receptor-related protein 5/6 (LRP5/6), signaling from Wnt receptors leads to nuclear translocation of β-catenin and its association with the LEF-1/TCF transcription factors for the activation of target genes (Clevers and Nusse 2012). Various natural inhibitors and agonists have been identified that regulate receptor assembly and activation (for review, see Clevers and Nusse 2012). One such secreted agonist is R-spondins (Rspos). Rspo proteins enhance Wnt signaling through interaction with their receptors, Lgr4/5/6, to potentiate the LRP phosphorylation (Carmon et al. 2011; de Lau et al. 2011; Glinka et al. 2011; Gong et al. 2012). Rspo1 has been implicated in many adult stem cell in vitro expansion systems, such as the intestine, stomach, and liver (Kim et al. 2005; Sato et al. 2009; Barker et al. 2010; Huch et al. 2013). However, it remains unclear in vivo which cells produce Rspo proteins in these organs.
In vitro, it has been very challenging to increase the number of adult stem cells and maintain their stem cell properties. Our previous study demonstrated that Wnt3A proteins can promote MaSC self-renewal and expansion in culture (Zeng and Nusse 2010). Despite its potent application in vitro, Wnt3A is not expressed in the mammary gland (Weber-Hall et al. 1994; Brisken et al. 2000). The identity of Wnt members participating in regulating MaSCs and which cells secrete Wnts also remain elusive. The niche is traditionally portrayed as the location where stem cells are kept in a self-renewal state (Morrison and Spradling 2008), and stromal fibroblasts are postulated to act as the MaSC niche cells (Malanchi et al. 2012; Weiland et al. 2012). In this study, we started by investigating secreted components of Wnt signaling in luminal cells. We found that luminal cells secrete Rspo1, providing synergistic self-renewal signals with Wnt4 for basal stem cells. Interestingly, despite the fact that Rspo1 is expressed in progesterone receptor (PR)-negative cells, steroid hormones indirectly induce Rspo1 expression. Finally, we developed a novel method to clonally expand MaSCs in culture by establishing a Rspo1 and Wnt4 synergistic niche environment by hormonal stimulation.
Results
Luminal cells produce Rspo1
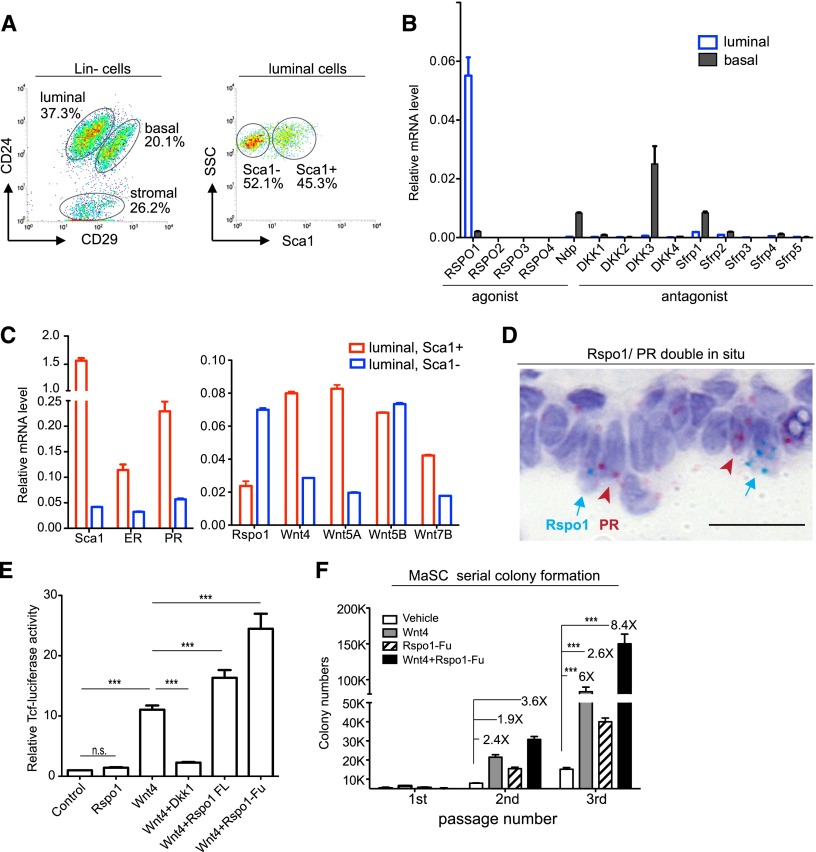
The luminal layer contains hormone-responsive cells in the mammary epithelium. To investigate which secreted components of Wnt signaling are expressed in luminal cells, the expression of different Wnt genes, natural agonists, and inhibitors was examined in FACS-isolated basal (Lin−, CD24+, CD29hi) and luminal (Lin−, CD24+, CD29lo) populations (Fig. 1A). Marker analysis by quantitative PCR (qPCR) confirmed the clear separation of luminal (K8), basal (K14), and stromal (vimentin) cells (Supplemental Fig. S1a). We found that among 10 Wnt candidates that had been reportedly expressed in the mammary gland (Weber-Hall et al. 1994; Chu et al. 2004; Veltmaat et al. 2004; Dwyer et al. 2010), Wnt4, Wnt5A, Wnt5B, and Wnt7B were detected in luminal cells by qPCR. Among them, Wnt4 and Wnt7B showed predominant expression in luminal cells (Supplemental Fig. S1b). In profiling the expression of various secreted Wnt agonists (Rspos and Ndp) and antagonists (Dkks and Sfrps), we found that Rspo1 is significantly higher in luminal cells compared with basal cells (Fig. 1B). We also observed that antagonists (e.g., Dkk3 and Sfrp1) were expressed prominently in basal cells (Fig. 1B).
Figure 1.
Rspo1 produced from PR-negative cells synergize with Wnt4 signaling. (A) Primary mammary cells were FACS-isolated from 8- to 10-wk-old female virgins into basal (Lin−, CD24+, CD29hi), luminal (Lin−, CD24+, CD29lo), and stromal (Lin−, CD24−) populations. Luminal cells were further isolated into Sca1+ and Sca1− populations. (B) qPCR analysis of Wnt agonists (Rspo1–4 and Norrin) and antagonists (Dkk1–4 and Sfrp1–5) in basal and luminal populations. Rspo1 was expressed significantly higher in luminal cells. mRNA was normalized to GAPDH. (C) qPCR analysis of Sca1, ER, PR, Rspo1, and luminal Wnt genes in Sca1+ and Sca1− luminal cell populations. mRNA was normalized to GAPDH. (D) In situ hybridization of Rspo1 (in cyan) and PR (in pink), indicating their neighboring position. Nuclei were counterstained with hematoxylin (in purple). Bar, 20 μm. (E) Wnt4 lentiviral infection activated the TCF-luciferase reporter activities in L cells, while addition of Dkk1 inhibited the activities. Both the Rspo1 full-length protein and the Rspo1 furin domain (Rspo1-Fu) were able to enhance Wnt4 activities in Tcf-luciferase assays in L cells, while Rspo1-Fu displayed stronger effects. Quantification was performed on four independent experiments. Data are presented as mean ± SD. (***) P < 0.01. (F) In basal stem cell serial colony formation assays, Wnt4 conditioned medium promoted the clonogenicity. Addition of Rspo1-Fu further increased the basal stem cell colony numbers in the second and third passages. (***) P < 0.01.
Hormone receptor-positive luminal cells can be further separated by their Sca1 expression (Regan et al. 2012; Shehata et al. 2012). We next investigate the expression of luminal Wnts and Rspo1 in the FACS-isolated Sca1+ and Sca1− subpopulation (Fig. 1A). Proper separation of the cells was confirmed by qPCR analysis of Sca1, estrogen receptor (ER), and PR expression (Fig. 1C). Interestingly, we observed that Rspo1 is expressed in the Sca1− luminal cells that are hormone receptor-negative, and Wnt4, Wnt5A, and Wnt7B are expressed in Sca1+ luminal cells, while Wnt5B has equal distribution in these two populations (Fig. 1C). Double-colored RNA in situ hybridization was performed to validate Rspo1 and Wnt genes in relation to PR expression. We found that, consistent with qPCR results, Wnt4 is expressed in PR+ cells (Supplemental Fig. S1c), whereas Rspo1 is expressed in PR− cells (Fig. 1D). Intriguingly, Rspo1-expressing cells are in close proximity to PR+ cells (Fig. 1D).
Rspo1 synergizes with Wnt4, not Wnt7B, in activating canonical Wnt signaling
Rspo1 has been implicated in promoting Wnt/β-catenin signaling activities instructed by Wnt3 in the intestine stem cells (Sato et al. 2009). To understand Rspo1’s role in the mammary gland, we first addressed whether any of the luminal Wnts activate canonical Wnt/β-catenin signaling and drive the stem cell self-renewal in the mammary gland. Given the inhibitory roles of Wnt5A and Wnt5B in the mammary gland (Roarty and Serra 2007; Kessenbrock et al. 2013), these Wnts are unlikely to promote MaSC self-renewal; hence, we focused our efforts on Wnt4 and Wnt7B. The output of Wnt4 in the mammary gland is controversial and not well established (see the Discussion for detail). We first investigated whether Wnt4 functions as a canonical Wnt by using a TCF reporter assay. L cells stably expressing TCF-luciferase reporter were infected with Wnt4-expressing lentivirus. We found that Wnt4 enhanced the luciferase activities, while addition of the canonical Wnt inhibitor Dkk1 suppressed the TCF reporter activation (Supplemental Fig. S1d). Furthermore, activation of canonical Wnt target gene Axin2 expression was also examined in the presence of Wnt4. Basal cells (Lin−, CD24+, CD29hi) from Axin2-lacZ mammary glands were isolated and cultured in three-dimensional (3D) Matrigel as previously described (Zeng and Nusse 2010), we found that Wnt4 conditioned medium activated Axin2-lacZ expression in the colonies (Supplemental Fig. S1e). Conversely, supplement of canonical Wnt inhibitor Dkk1 suppressed the Axin2 reporter activation (Supplemental Fig. S1f). Together, these data suggest that Wnt4 can activate canonical Wnt/β-catenin signaling in mammary cells.
To determine whether Rspo1 is able to synergize with Wnt4 activities, we carried out Tcf-luciferase assays by using Rspo1 full-length protein and a truncated form containing only the Rspo1 furin domain (Rspo1-Fu) that is critical for β-catenin/Tcf activation (Kim et al. 2008). We found that both Rspo1 full-length protein and Rspo1-Fu could significantly amplify Wnt4 activities (Fig. 1E). To investigate the impact of Rspo1 on MaSC self-renewal, we examined their serial colony formation capability in the presence of Wnt4 conditioned medium and Rspo1. Addition of Rspo1-Fu increased the clonogenicity of basal stem cells, as indicated by the continuously increasing colony numbers in serial colony formation assays (Fig. 1F). Together, these observations suggest that Wnt4 and Rspo1 can cooperate to promote self-renewal of basal stem cells.
We next investigated the role of Wnt7B in activating canonical Wnt signaling. In situ hybridization confirmed Wnt7B’s luminal expression pattern (Supplemental Fig. S2a). In contrast to Wnt4, we found that lentivirus expressing Wnt7B alone or in combination with Rspo1 cannot stimulate the TCF-luciferase activities in L cells (Supplemental Fig. S2b). In mammary basal cell 3D cultures, Wnt7B conditioned medium was not able to enhance Axin2 expression (Supplemental Fig. S2c). To further examine Wnt7B’s capability in activating the canonical Wnt activity in mammary cells, coculture experiments were carried out using primary mammary epithelial cells isolated from Axin2-lacZ reporter mice and an Eph4 mammary cell line. Eph4 cells were virally infected with control vector tagged with green fluorescence (named Eph4-GFP) or Wnt7B (named Eph4-Wnt7B-GFP). Eph4 cells expressing Wnt4 (named Eph4-Wnt4-GFP) were used as a positive control. We found that Axin2-lacZ expression could not be detected in either the control or Eph4-Wnt7B-GFP mixed culture (Supplemental Fig. S2d,e), while Eph4-Wnt4-GFP was able to turn on lacZ expression (Supplemental Fig. S2f). Conversely, addition of IWP-2 (a Porcupine inhibitor that blocks Wnt secretion) (Chen et al. 2009) inhibited the Axin2-lacZ expression in the mixed culture with Eph4-Wnt4-GFP (Supplemental Fig. S2g). These data suggest that Wnt4, not Wnt7B, activates canonical Wnt/β-catenin signaling in primary mammary cells.
Cooperation of Rspo1 and Wnt4 is critical for mammary gland reconstitution
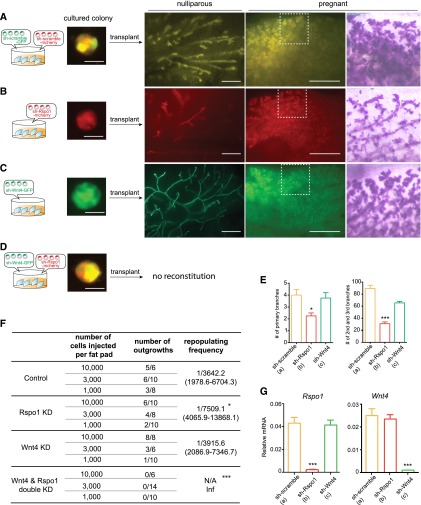
Increased MaSC serial colony formation by Rspo1 and Wnt4 suggests a synergy in MaSC self-renewal (Fig. 1F). We next investigated this cooperation in vivo by knockdown using shRNA and transplantation methods established in a previous study (Welm et al. 2008). Mammary cells were infected with lentivirus containing mCherry-tagged Rspo1 shRNA (named sh-Rspo1-mCherry), GFP-labeled Wnt4 shRNA (named sh-Wnt4-GFP), or both, and infected cells were isolated by their fluorescent tags and transplanted to cleared fat pads of recipients. To minimize potential off-target effects, two independent shRNAs were constructed for Wnt4 or Rspo1 knockdown. The knockdown efficiency of sh-Wnt4 and sh-Rspo1 was confirmed by Western blot analysis (Fig. 4A, below; data not shown). We observed that knockdown of Rspo1 resulted in fewer side branches in the virgin animal, as illustrated in whole-mount imaging and quantification of branches (Fig. 2B,E). In addition, knockdown of Rspo1 also led to alveologenesis defects during pregnancy (Fig. 2B). These observations are reminiscent of phenotypes seen in Rspo1 mutant outgrowths (Chadi et al. 2009). Moreover, we found that Rspo1 knockdown also led to a reduced number of stem cells, as evidenced by decreased repopulating frequency (Fig. 2F). We found that MaSCs with Wnt4 knockdown generated rather normal mammary outgrowth in virgins and during pregnancy (Fig. 2C), similar to the scramble control (Fig. 2A). These phenotypes are consistent with the observations of a previous report that used Wnt4-null transplants (Brisken et al. 2000). Cells from the mammary outgrowth were analyzed by qPCR, confirming the successful knockdown of both endogenous genes in vivo (Fig. 2G). Strikingly, MaSCs with Wnt4 and Rspo1 double knockdown failed to generate mammary outgrowths in transplantation assays (Fig. 2D,F). Together, these data suggest that the cooperation of Wnt4 and Rspo1 is critical for MaSC regenerative capacity.
Figure 4.
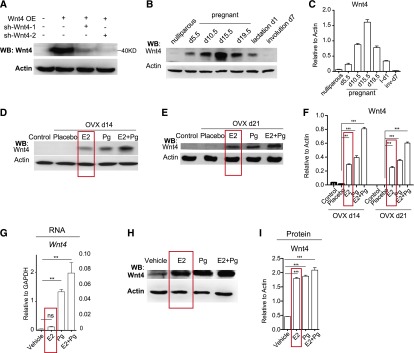
E2 up-regulates Wnt4 protein level. (A) Western blot analysis of Wnt4 overexpression (OE) to examine the Wnt4 antibody specificity and knockdown efficiency of two independent Wnt4 shRNA. (B,C) Western blot and quantification analysis of Wnt4 proteins during pregnancy, lactation, and involution. (***) P < 0.01. (D–F) Mammary glands of control and ovariectomized mice (OVX) were harvested and analyzed after 2 wk or 3 wk by Western blot and quantification. Either E2 or Pg was sufficient to enhance Wnt4 protein expression, while the augmentation was more pronounced in the presence of both E2 and Pg. The estrous cycle was not controlled in these experiments. (***) P < 0.01. (G) qPCR analysis of cultured luminal cells indicated that E2 had a subtle influence on the Wnt4 mRNA level, and Pg increased Wnt4 mRNA significantly. (H,I) Western blot analysis of cultured luminal cells indicated that the Wnt4 protein level significantly increased in the presence of E2, Pg, or both. (***) P < 0.01.
Figure 2.
Cooperation of Wnt4 and Rspo1 is critical for MaSC reconstitution in vivo. (A) Isolated mammary cells were infected with GFP and mCherry scramble control lentivirus and cultured in Matrigel for colony formation. The infected cells were isolated using fluorescence and transplanted into recipients. Outgrowths (in yellow) with normal morphology were detected in nulliparous virgins (10 wk post-transplantation). When harvesting late pregnant recipients, a dense ductal system ending in a cluster of alveoli was observed in live fluorescence as well as with carmine staining. (B) Mammary cells with sh-Rspo1 knockdown led to decreased side branch formation in virgins and alveologenesis defects during pregnancy. (C) Mammary cells with sh-Wnt4 knockdown generated normal mammary outgrowth in virgins and during pregnancy. (D) Mammary cells with sh-Wnt4 and sh-Rspo1 double knockdown were able to form a colony in vitro but failed to generate mammary outgrowths in vivo. (E) Quantification of primary branches and the numbers of second and third branches in reconstituted mammary glands. (***) P < 0.05. (F) Infected cells were transplanted by limiting dilution. Rspo1 knockdown led to a decreased number of stem cells. Wnt4 and Rspo1 double knockdown completely abolished stem cell regeneration in vivo. Results are combined from three independent experiments. Bars: in colonies, 20 μm; in the whole mount, 2 mm. (G) qPCR analysis of mammary outgrowth indicated the successful knockdown Rspo1 (in B) or Wnt4 (in C) in vivo. mRNA was normalized to GAPDH. (***) P < 0.01.
Overexpression of Wnt4 in luminal cells promotes basal stem cell self-renewal and expansion in a paracrine manner
Given the importance of Rspo1 and Wnt4 in self-renewal, we tested whether Rspo1 and Wnt4 secreted from luminal cells can serve as self-renewal factors by performing coculture experiments of luminal and basal cells. We found that endogenous Wnt4 and Rspo1 in luminal cells decrease rapidly in culture (Supplemental Fig. S3a).Endogenous Wnt5A expression also decreased noticeably, while Wnt5B and Wnt7B expression remained unaltered in culture (Supplemental Fig. S3a). In order to maintain Wnt4 expression, luminal cells were infected with Wnt4-expressing lentivirus and then used for coculture studies with basal cells. The Wnt4 mRNA level was indeed restored in cultured luminal cells and exceeded its original endogenous level prior to culture (Supplemental Fig. S3b); hypothetically, overexpression of Wnt4 induced more robust Wnt/β-catenin signaling activities, similar to the effect of Rspo1 and Wnt4 cooperation. As illustrated in Supplemental Figure S3c, we first FACS-isolated luminal cells from wild-type mammary glands and infected them with control-GFP or Wnt4-GFP lentivirus in suspension. DsRed-marked basal cells were isolated from mammary glands of Actin-DsRed mice and mixed with GFP-expressing or Wnt4-GFP-expressing luminal cells (Supplemental Fig. S3d, green) and then plated in Matrigel for culture in 3D (Supplemental Fig. S3d). After 1 wk of coculture, colonies formed by DsRed-marked basal cells were analyzed. We found that basal colony numbers were increased in serial passages in the presence of Wnt4 (Supplemental Fig. S3e), suggesting that the number of stem cells increases in each passage. In these assays, we also tested the requirement for Wnt4 secretion by addition of IWP2 and found that blocking Wnt secretion inhibits the expansion of basal stem cells (Supplemental Fig. S3e). These observations are reminiscent of basal stem cell cultures in the presence of purified Wnt3A proteins (Zeng and Nusse 2010) and indicate that overexpression of Wnt4 in luminal cells is sufficient for self-renewal and expansion of basal stem cells in vitro. We subsequently isolated and transplanted the DsRed-marked basal stem cell colonies in the third passage to the cleared fat pads of recipients. Consistent with our previous study (Zeng and Nusse 2010), MaSCs cultured in the third passage completely lost their reconstitution abilities. However MaSCs cocultured with Wnt4-secreting luminal cells readily regenerated normal mammary glands, while IWP-2 treatment markedly reduced their reconstitution capabilities (Supplemental Fig. S3f,g). We also investigated whether overexpression of Wnt7B can exert an effect similar to that of Wnt4. We found that overexpression of Wnt7B cannot promote basal cell serial colony formation or maintain their reconstitution abilities (Supplemental Fig. S3h,i). Taken together, these data suggest that Wnt4 is able to maintain MaSC self-renewal and promote their expansion in a paracrine manner.
Rspo1 expression is up-regulated during pregnancy and upon hormonal treatment
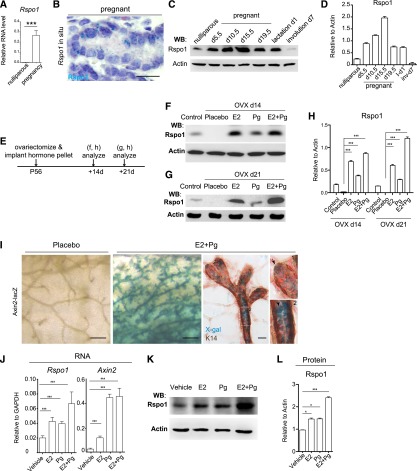
Previous studies indicated that Wnt4 expression is regulated by progesterone (Pg) (Brisken et al. 2000). We next investigated how Rspo1 expression is regulated. We found that Rspo1 levels are significantly increased in pregnant mammary glands, as measured by qPCR (Fig. 3A). In situ hybridization confirmed elevated Rspo1 mRNA expression in luminal cells of the pregnant gland (Fig. 3B). We next examined their protein expression in the mammary gland during early pregnancy (day 5.5), mid-pregnancy (day 10.5), late pregnancy (days 15.5 and 19.5), lactation day 1, and involution day 7. By Western blot analysis, we found that Rspo1 proteins are indeed up-regulated during pregnancy, becoming evident in early pregnancy, and continue to increase until the beginning of late pregnancy (day 15.5) (Fig. 3C,D). After that, Rspo1 level declined continuously and returned to the nulliparous level after involution (Fig. 3C,D). These observations are consistent with a previous report of Rspo1 mRNA (Chadi et al. 2009).
Figure 3.
Hormones induce the expression of Rspo1 in vivo and in vitro. (A) Rspo1 qPCR analysis indicated that Rspo1 expression was significantly increased in late pregnancy mammary epithelial cells. (***) P < 0.01. (B) In situ hybridization showed the robust Rspo1 level in pregnancy (in cyan). Nuclei were counterstained with hematoxylin (in purple). Bar, 20 μm. (C,D) Western blot and quantification indicate the Rspo1 expression during pregnancy, lactation, and involution. (***) P < 0.01. (E) Mice were ovariectomized and implanted with E2 or Pg pellets alone or in combination (E2 + Pg) for 14 or 21 d. (F–H) Mammary glands of control and ovariectomized mice (OVX) were harvested and analyzed by Western blot and quantification. Either E2 or Pg treatment was sufficient to enhance Rspo1 expression, while the augmentation was more pronounced in the presence of both E2 and Pg. The estrous cycle was not controlled in these experiments. (***) P < 0.01. (I) Axin2-lacZ mice were ovariectomized and implanted with E2 and Pg pellets for 21 d. The mammary glands were harvested and subjected to X-gal and K14 staining. Axin2 expression was induced in both basal cells (arrow in insert 1) and luminal cells (arrowhead in insert 2) by hormonal treatment. Bars: in the whole mount, 1 mm; in sections, 20 μm. (J) qPCR analysis of cultured luminal cells indicated that Rspo1 and Axin2 expression were significantly increased in all conditions of hormonal treatment. (***) P < 0.01. (K,L) Western blot analysis of cultured luminal cells indicated that the Rspo1 level significantly increased in the presence of hormones.(***) P < 0.01; (*) P < 0.05.
We next examined whether pregnancy hormones regulate the expression of Rspo1. We examined the effects of exogenous E2 and Pg in ovariectomized mice, which are depleted of the endogenous hormones. In ovariectomized mice, E2 or Pg pellets alone or in combination (E2 + Pg) were implanted for 14 d (Fig. 3E). We found that either E2 or Pg is sufficient to increase Rspo1 expression by Western blot analysis (Fig. 3F,H). Similar results were observed at 21 d post-implantation (Fig. 3G,H). Notably, the up-regulation is more pronounced in the presence of both E2 and Pg (Fig. 3G,H). Furthermore, we investigated whether the Wnt/β-catenin signaling is activated with exogenous hormones. We observed strong lacZ staining in the mammary gland of ovariectomized Axin2-lacZ mice implanted with E2 and Pg pellets (Fig. 3I). Axin2 activation was detected in basal cells as well as in luminal cells, suggesting that luminal cells can also respond to Wnt signaling upon elevated hormonal stimulation. In addition, we examined the impact of E2 and Pg on Rspo1 expression in vitro. Luminal cells were cultured in Matrigel in the presence or absence of hormones. Consistent with the findings in ovariectomized mice, we observed that E2 or Pg alone was sufficient to induce Rspo1 RNA (Fig. 3J) and protein expression in culture (Fig. 3K,L). Notably, Axin2 expression was also increased in hormonal treatment in vitro (Fig. 3J). Together, these data suggest that the expression Rspo1 is positively regulated by steroid hormone.
Wnt4 and Rspo1 accumulation timing is synchronized
Previous studies showed that Wnt4 mRNA is increased during pregnancy, peaking at days 5.5∼10.5 and declining during mid-pregnancy and late pregnancy (Gavin and McMahon 1992; Weber-Hall et al. 1994; Badders et al. 2009). Considering the Rspo1 level is continuously elevated during pregnancy, the proposed Wnt4 decline in the mid and late stages is not aligned with the synergistic feature of Rspo1 and Wnt4. To measure the Wnt4 protein level, we generated a Wnt4 antibody. By Western blot analysis, Wnt4 overexpression induced a specific 40-kDa band that would disappear in the presence of two independent Wnt4 shRNA, indicating that the antibody can accurately detect Wnt4 (Fig. 4A). We next examined Wnt4 protein expression at various stages of pregnant, lactating, and involuting mammary glands and found that Wnt4 protein continues to accumulate until pregnancy day 15.5 (Fig. 4B,C). We discovered that E2 might be the cause for Wnt4 protein accumulation at this stage. Previous reports showed that the Wnt4 mRNA level is induced by Pg but is unaffected by E2 (Brisken et al. 2000; Joshi et al. 2010). We found that either E2 or Pg (treatment for 14 d or 21 d) is sufficient to increase Wnt4 protein expression in ovariectomized mice by Western blot analysis (Fig. 4D–F). In luminal cell culture, we observed that Wnt4 mRNA is indeed unaffected by E2 (Fig. 4G), whereas its protein level is significantly increased (Fig. 4H,I). The increased Wnt4 protein levels by E2 and Pg were comparable (Fig. 4H,I). These observations suggest that E2 might preferentially promote Wnt4 protein expression.
To further investigate the timing of Rspo1 and Wnt4 levels influenced by hormones, we analyzed their expression in different stages of the estrous cycle. By Western blot analysis, we found that both Rspo1 and Wnt4 proteins peak at diestrus, in keeping with the high Pg level and robust MaSC activities at the stage (Supplemental Fig. S4a,b; Joshi et al. 2010). Interestingly, we also observed the second highest expression of both Rspo1 and Wnt4 at estrus, during which the E2 level surges, supporting the idea that E2 up-regulates Wnt4 protein (Supplemental Fig. S4a,b). Collectively, our results indicate the synchronized timing of Wnt4 and Rspo1 accumulation during estrous cycles and the course of pregnancy, in line with their synergistic function in MaSC regulation.
Hormones induce a collaborative niche for MaSC expansion in culture
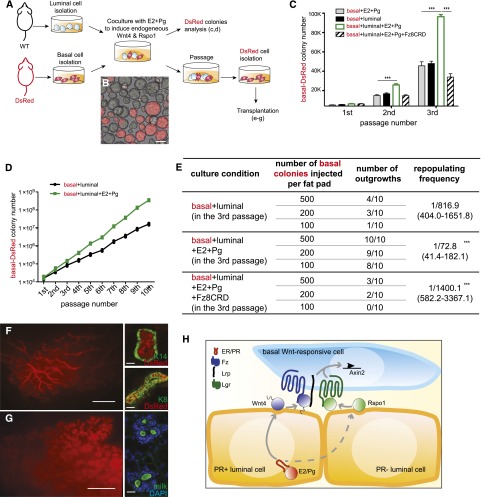
Knowing that hormones can maintain the expression of Rspo1 and Wnt4 in culture, we modified the luminal and basal cell coculture experiments described in Supplemental Figure S3 by replacing the exogenous Wnt4 with hormonal treatment. As illustrated in Figure 5A, DsRed-marked basal cells were isolated from mammary glands of DsRed mice, mixed with wild-type luminal cells, and then plated in Matrigel for culture in the presence of E2 and Pg. After 1 wk of coculture, colonies formed by DsRed-marked basal cells were analyzed (Fig. 5B). We found that basal colony numbers were significantly increased in serial passages in coculture with luminal cells in the presence of E2 and Pg (Fig. 5C), suggesting that the number of stem cells increases in each passage. In these assays, we also tested the requirement for Wnt proteins by addition of Fz8CRD, a soluble domain of the Wnt receptor that binds and sequesters Wnt proteins. Blocking Wnt signaling activation by Fz8CRD inhibited the expansion of basal stem cells (Fig. 5C). Blocking Wnt secretion by IWP2 displayed a similar inhibition effect (data not shown). We maintained the coculture for 10 passages (∼10 wk) and found that stem cells cultured with luminal cells and hormones continue to expand and produce increasing numbers of colonies (Fig. 5D). To examine the properties of these stem cells in vivo, DsRed-marked basal stem cell colonies in the third passage were isolated and transplanted to the cleared fat pads of immunocompromised recipients. We found that stem cells cocultured with luminal cells and hormones regenerated a normal mammary gland at a significantly higher frequency, while Fz8CRD treatment markedly reduced the reconstitution capabilities (Fig. 5E,F). When recipient mice were in the lactating stage, the mammary glands resulting from the transplanted colonies consisted of a dense ductal system with alveoli and abundant milk proteins (Fig. 5G). These results suggest that hormonal treatment creates a collaborative niche for MaSC expansion in culture and maintains their full development potential.
Figure 5.
Hormonal treatment induces a collaborative niche for MaSC expansion in culture. (A) Coculture experiments of basal and luminal cells were established as illustrated. (B) A representative picture of colonies formed in the coculture system. Bar, 20 μm. (C) Quantification of the numbers of basal DsRed-marked colonies that were maintained in the described culture conditions for three passages. The numbers of primary colonies were similar. The numbers of secondary and tertiary colonies were increased with hormonal treatment. The increasing colony numbers were inhibited by Fz8CRD in each passage. (***) P < 0.01. (D) Coculture was maintained for 10 passages. Colony numbers were continuously increased with hormonal treatment. One of three similar independent experiments is shown. (E–G) Tertiary basal DsRed-marked colonies maintained in the described culture conditions were transplanted into cleared fat pads by limiting dilution. Recipient fat pads were harvested at 8-wk post-transplantation. (E) DsRed outgrowth was detected in a higher percentage when cultured with hormones, whereas Fz8CRD inhibited the DsRed-marked basal reconstitution rate. Results are combined from three independent experiments. (F) Representative pictures in virgin recipients are shown in whole mount with live DsRed and keratin marker expression in tissue sections. (G) The reconstituted mammary gland displayed normal alveogenesis during pregnancy and produced abundant milk protein. Bars: in the whole mount, 2 mm; in the tissue sections, 20 μm. (H) Model of the differentiated luminal cells synergistically regulating the self-renewal of MaSCs. Hormone stimulates the expression of Wnt4 in PR+ luminal cells and Rspo1 in PR− luminal cells. Rspo1 and Wnt4 collaborate to induce basal cell Wnt response, enabling MaSC property maintenance or their expansion in number.
Discussion
Steroid hormone signaling profoundly influences the behavior of MaSCs (Asselin-Labat et al. 2010; Joshi et al. 2010). Hormones regulate many local growth factors, yet which ones are responsible for the action of hormones on stem cell self-renewal have not been determined. In this study, we found that Rspo1, as a novel hormone mediator, synergized with Wnt4 to promote MaSC self-renewal. Deprivation of Rspo1 and Wnt4 abolishes MaSCs’ regenerative capabilities. An in vitro coculture system consisting of luminal cells and hormones stimulates the expression of both Rspo1 and Wnt4, which recapitulates the in vivo collaborative environment, enabling MaSC property maintenance and their expansion in number. Our findings reveal a mechanism through which differentiated luminal cells synergistically regulate the self-renewal of MaSCs (Fig. 5H).
Rspo1 as a novel hormone mediator
Rspo1 has been implicated in multiple stem cell in vitro culture systems, such as intestine stem cells and liver stem cells (Sato et al. 2009; Huch et al. 2013), highlighting its critical role as a niche factor. However, it remains elusive which cells produce Rspo proteins in these organs. We provide evidence that Rspo1 is expressed in the PR-negative luminal cells of the mammary gland. Despite the fact that Rspo1-expressing cells lack the hormone receptor, E2 and Pg induce the expression of Rspo1 through unknown intermediate factors. In vivo, the Rspo1 level is elevated at estrus and diestrus phases in the nulliparous mammary gland and drastically increased during pregnancy and in ovariectomized mice with hormonal stimulation. Our research adds Rspo1 to a short list of hormone-mediated local factors for stem cells in the mammary gland. By analogy, such a mechanism could be extended to other organ systems.
Ovarian hormones control MaSC self-renewal through the synergy action of Rspo1 and Wnt4
Mammary gland development is governed by the concerted action of hormones and local growth factors. Previous work by Joshi et al. (2010) had found that hormonal treatment expands MaSCs in vivo and that elevated Wnt4 levels are correlated with hormonal treatment. However, these studies did not establish whether Wnt4 is the stem cell self-renewal signal and whether the action of Pg on MaSCs is through the regulation of Wnt4. In the present study, we do show that Wnt4 is the endogenous self-renewal niche factor for MaSCs. Coculture with Wnt4-expressing cells is sufficient to maintain the MaSCs’ properties and expand their numbers in culture. Blocking paracrine function of Wnt4 by IWP2 counteracts the hormonal impact on MaSCs.
Brisken et al. (2000) have shown that Wnt4 mRNA expression is induced by Pg and demonstrated a role for Wnt4 in alveologenesis during pregnancy. In the present study, we reveal that E2 takes part in Wnt4 protein regulation, allowing Wnt4 proteins continue to accumulate to the beginning of late pregnancy (day 15.5). The synchronized protein accumulation timing of Rspo1 and Wnt4 at diestrus phase and during early to mid-pregnancy is in line with their synergistic function in regulating MaSCs, mediating the Pg effect on MaSCs. Intriguingly, the elevated Rspo1 and Wnt4 also occurs during the estrus phase and late pregnancy when E2 surges, possibly suggesting (1) undocumented MaSC activities at these stages or (2) additional physiological roles of Rspo1 and Wnt4; e.g., regulation of luminal cell proliferation. Indeed, it has been proposed that Wnt4 can act on luminal cells (Hiremath et al. 2007; Joshi et al. 2010). Recent studies have demonstrated that Rspo1 and Wnt4 cooperate during early gonad proliferation in ovary differentiation (Chassot et al. 2008, 2012; Tomizuka et al. 2008). It will be interesting to understand whether such cooperation mediates the influence of hormones in these processes.
A previous study by Asselin-Labat et al. (2010) identified RANKL as the hormone-mediated local factor for MaSCs. Wnt4 and RANKL share many similar traits, including their expression in luminal cells, expression fluctuation during estrous cycle (Silberstein et al. 2006), up-regulation during pregnancy (Fata et al. 2000; Brisken et al. 2002), and essential functions in alveologenesis (Brisken et al. 2000). We attempted to maintain the MaSC properties in vitro using recombinant RANKL. However, we were not able to observe increasing numbers of colonies forming in serial passaging MaSC culture, and the cultured colonies have also lost their reconstitution abilities when transplanted (data not shown). The inability to maintain MaSC properties in the above condition could be due to a lack of RANK receptor in the culture MaSCs, reflecting the limitation of the in vitro system. In contrast, coculture with Wnt4-expressing cells potently expands MaSCs and retains their full developmental potential, underlining Wnt4 as a self-renewal niche factor for MaSCs. Nevertheless, the complexity of the niche adopts the likely redundancy of self-renewal factors. Notably, Wnt4-null transplants display transient defects in mid-pregnancy and are rescued in a later stage (Brisken et al. 2000). The redundant players may not be other luminal Wnts (Wnt5A, Wnt5B, and Wnt7B), as none of them stimulates canonical Wnt/β-catenin signaling in mammary cells. It is more possible that while Wnt4 is absent and Rspo1 is intact, other Wnt members generated from the basal layer (e.g., Wnt10A and Wnt11) contribute to the MaSC regulation mechanism, specifically synergizing with Rspo1. These possibilities need to be addressed in future studies.
Wnt4 activates canonical Wnt/β-catenin signaling
The output of Wnt4 in the mammary gland has been controversial, and whether Wnt4 participates in canonical Wnt/β-catenin signaling is not established (Bradbury et al. 1995; Brisken et al. 2000; Kim et al. 2009). Ectopic side branches were observed in transplantation assays upon Wnt4 overexpression (Bradbury et al. 1995). On the other hand, Brisken et al. (2000) showed transient side branching defects in Wnt4 mutant outgrowths. Although neither of the above-mentioned studies measured β-catenin/TCF activities, they both support a proproliferation function for Wnt4. Conversely, Kim et al. (2009) suggested that Wnt4 cannot activate TCF-dependent gene expression and is not sufficient to induce hyper side branching. In contrast, our study showed that Wnt4 activates the TCF-luciferase activities, while canonical Wnt inhibitor Dkk1 suppresses the TCF reporter activation, and agonist Rspo1 enhances the activation. More evidence of canonical Wnt/β-catenin signaling activation came from the facts that Wnt4 activates Axin2-lacZ, the canonical Wnt target gene expression in primary MaSC culture, which is suppressed by addition of Dkk1.
Apply ovarian hormones to expand MaSCs in vitro
It has been surprisingly difficult to increase the number of adult stem cells in culture and maintain their stem cell properties. Steered by the prominent role of Wnt signaling in stem cell maintenance, Sato et al. (2009; Barker et al. 2010) used Rspo1 to augment Wnt signaling and successfully expand Lgr5+ intestine stem cells in culture. Subsequently, expansion of Lgr5+ adult stem cells of stomach and liver tissue has also been accomplished by using Rspo1 (Barker et al. 2010; Huch et al. 2013). Our previous study revealed that MaSCs can be expanded in vitro under serum-free and feeder-free defined conditions when supplemented with Wnt3A (Zeng and Nusse 2010). However, the identity of the Wnt action molecules in vivo and the precise location of the physiological niche remain unknown. Our present study demonstrates that Wnt4, the endogenous Wnt in regulating MaSCs in vivo, can also expand MaSCs in culture. However, the purification of Wnt has proven difficult, and the resources for purified and biologically active Wnt proteins are limited. Given the rapid decrease of endogenous Wnt4 in mammary cell culture, Rspo1 is predicted to have limited application in creating a Wnt-augmented condition, as it does for intestine, stomach, and liver stem cell cultures. Knowing that both of the MaSC niche factors, Wnt4 and Rspo1, are under hormonal regulation, we engaged E2 and Pg to recapitulate Wnt-induced MaSC expansion in vitro. In our study, we cocultured MaSCs with luminal cells and applied hormones to stimulate the endogenous expression of Wnt4 and Rspo1 in the niche. We successfully expanded MaSCs in culture, notably maintaining the full development potential when examined in transplantation assays. This is a novel approach, nicely recapitulating the in vivo physiological situations in vitro. The easy accessibility of E2 and Pg will allow the MaSC expanding method to be widely implemented.
In conclusion, our study revealed Rspo1 as a novel hormone mediator and demonstrated that the cooperation of Rspo1 and Wnt4 is critical for the maintenance of MaSC self-renewal. Our results illustrate that the signaling events critical for self-renewal indeed take place between the stem cells and their own progeny cells (the proposed niche). This may be a general principle, in light of the findings that paneth cells also act as the niche for intestinal stem cells (Sato et al. 2011). Furthermore, our study reinforces the connection between hormone and MaSC regulation, providing an important mechanistic link between the physiological state of the organism and the growth response of an organ through circulating hormones and locally acting Wnt signals.
Materials and methods
Experimental animals and estrous staging
Axin2lacZ/+ (Lustig et al. 2002), Actin-DsRed (Jackson Laboratories), and Nude strains were used in this study. Virgin female BALB/c mice aged 8–12 wk were examined by vaginal smear cytology as described previously (Joshi et al. 2010; Byers et al. 2012). Experimental procedures were approved by the Animal Care and Use Committee of the Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences.
Antibodies
Rabbit polyclonal anti-Wnt4 antibody was raised against a synthetic peptide (NH2-PRNAQFKPHTDEDC-CONH2) and affinity-purified. Other antibodies used were rat anti K8 (1:250; Developmental Hybridoma Bank), rabbit anti-K14 (1:1000; Covance), rabbit anti-Flag (1:1000; Sigma), mouse anti-β-Actin (1:3000; Sigma), and rabbit anti-milk (1:500; Nordic Immunological Laboratories).
Luciferase assays
Mouse L cells stably expressing SuperTOPflash reporter (Mikels and Nusse 2006) were cultured in DMEM, 10% fetal bovine serum (FBS), and antibiotics. L cells were plated into a 24-well plate, allowed to recover for 5–8 h, and then treated in duplicate or triplicate with Wnt3A, Wnt4, Wnt7B, Rspo1, or Dkk1 proteins, conditioned medium, or lentivirus as described in the text. Luciferase assays were performed using the Dual-Light reporter gene assay system (Applied Biosystems). Relative luciferase units were measured and normalized against β-galactosidase activity at 48 h post-treatment.
Primary cell preparation
Mammary glands from 8- to 12-wk-old virgin or pregnant female mice were isolated. The minced tissue was placed in culture medium (RPMI 1640 with 25 mM HEPES, 5% FBS, 1% PSQ, 300U mL−1 Collagenase III [Worthington]) and digested for 2 h at 37°C. After lysis of the red blood cells in NH4Cl, a single-cell suspension was obtained by sequential incubation with 0.25% Trypsin-EDTA for 5 min at 37°C and 0.1 mg/mL DNase I (Sigma) for 5 min with gentle pipetting followed by filtration through 40-mm cell strainers.
Cell labeling and flow cytometry
The following antibodies in 1:200 dilutions were used: PE-conjugated CD31, CD45, and TER119 (BD PharMingen); and Sca1-PE, CD24-PE/cy7, and CD29-APC (Biolegend). Antibody incubation was performed on ice for 15 min in HBSS with 10% FBS. All sortings were performed using a FACSAria or FCASJazz (Becton Dickinson). The purity of sorted population was routinely checked and ensured to be >95%.
In vitro colony formation assay
FACS-sorted cells were resuspended at a density of 4 × 105 cells per milliliter in chilled 100% growth factor-reduced Matrigel (BD Bioscience), and the mixture was allowed to polymerize before covering with culture medium (DMEM/F12; ITS [1:100; Sigma]; 50 ng mL−1 EGF; plus either vehicle [1% CHAPS in PBS], 200 ng mL−1 Wnt3A [Willert et al. 2003], 200 ng mL−1 Dkk1, 1 μM E2, 2.5 μM Pg, or 2uM IWP2). Culture medium was changed every 24 h. Primary colony numbers were scored after 6–7 d in culture. The colonies were mostly spherical. In cases in which colonies were oval, the long axis was measured. For passaging colonies, the medium was aspirated, and Matrigel was digested by incubation in 500 μL of Matrigel recovery solution (BD Bioscience) for 1 h on ice. Colonies released from Matrigel were harvested after pelleting. Single cells were obtained through incubation with 0.25% Trypsin-EDTA for 5 min at 37°C followed by gentle pipetting. Single cells were then replated in Matrigel as described above.
Lentiviral vector and infection
A Wnt4 or Wnt7B ORF was subcloned into the pWPI-EGFP backbone to generate the Wnt4-EGFP or Wnt7B construct. Wnt4 shRNA and Rspo1 shRNA were synthesized and subcloned into plko backbone with EGFP and mCherry, respectively. Lentivirus was produced by transient transfection in 293T cells. Mammary cells were isolated from 8- to 12-wk-old virgin female glands as described above, followed by sorting into basal and luminal cells. The infection protocol was modified from methods described in Welm et al. (2008). The sorted cells were collected and cultured in a low adherent plate in 10% FBS-supplemented DMEM-F12 with virus. At 6 h after infection, cells were collected and resuspened in Matrigel for consequent culturing.
Immunohistochemistry and in situ hybridization
Frozen sections were prepared by air-drying and fixation for 1 h in cold MeOH. Tissue sections were incubated with primary antibodies overnight at 4°C followed by washes, incubation with secondary antibodies for 2 h at 25°C, and counterstaining with DAPI (Vector Laboratories). The following antibodies were used: rat anti-K8 (1:250; Developmental Hybridoma Bank) and rabbit anti-K14 (1:1000; Covance). In situ hybridization was performed using the RNAscope kit (Advanced Cell Diagnostics) following the manufacturer’s instructions. Wnt4, Wnt7B, Rspo1, and PR probes were ordered from Advanced Cell Diagnostics. For all of the immunofluorescence stainings, in situ and in immunoblots, at least three independent experiments were conducted. Representative images are shown in the figures.
Mammary fat pad transplantation and analysis
Sorted cells were resuspended in 50% Matrigel, PBS with 20% FBS, and 0.04% Trypan Blue (Sigma) and injected in 10-μL volumes into the cleared fat pads of 3-wk-old females. Reconstituted mammary glands were harvested after 6–10 wk post-surgery. All transplanted cells were labeled with GFP, DsRed, or mCherry. Outgrowths were detected under a fluorescence dissection microscope (Leica). Outgrowths with >10% of the host fat pad filled were scored as positive.
RT-qPCR
RNA from whole embryos or human adenoma tissues was isolated with Trizol (Invitrogen). The cDNA library was prepared with the SuperScript III kit (Invitrogen). RT–PCR was performed on a StepOne Plus (Applied Biosystems). RNA level was normalized to GAPDH. The primers used were Axin2-F, AGCCTAAAGGTCTTATGTGGCTA; Axin2-R, ACCTACGTGATAAGGATTGACT; Krt8-F, AGGATGAGATCAACAAGCGT; Krt8-R, CTTCATGGATCTGCCGGA; Krt14-F, TGACCATGCAGAACCTCAATGA; Krt14-R, ATTGGCATTGTCCACGG; Vim-F, GCTGCGAGAGAAATTGCAGGA; Vim-R, CCACTTTCCGTTCAAGGTCAAG; Wnt2-F, CAGTGACAATATTGACTACGG; Wnt2-R, CTTTACAGCCTTCCTTCCA; Wnt4-F, GCAATTGGCTGTACCTGG; Wnt4-R, GCACTGAGTCCATCACCT; Wnt5a-F,CGCTAGAGAAAGGGAACGAATC; Wnt5a-R, TTACAGGCTACATCTGCCAGGTT; Wnt5b-F, CACCAGTTTCGACAGAGGC; Wnt5b-R, GCAGTCTCTCGGCTACCTATCT; Wnt6-F, GCGGAGACGATGTGGACTTC; Wnt6-R, ATGCACGGATATCTCCACGG; Wnt7a-F, GGCTTCTCTTCGGTGGTAGC; Wnt7a-R, TGAAACTGACACTCGTCCAGG; Wnt7b-F, CTTCACCTATGCCATCACGG; Wnt7b-R, TGGTTGTAGTAGCCTTGCTTCT; Wnt10b-F, TTCTCTCGGGATTTCTTGGATTC; Wnt10b-R, TGCACTTCCGCTTCAGGTTTTC; Wnt10a-F, CATGCTCGAATGAGACTCCAC; Wnt10a-R, CCCTACTGTGCGGAACTCAG; Wnt11-F, GCTGGCACTGTCCAAGACTC; Wnt11-R, CTCCCGTGTACCTCTCTCCA; Rspo1-F, GCAACCCCGACATGAACAAAT; Rspo1-R, GGTGCTGTTAGCGGCTGTAG; Rspo2-F, CCAAGGCAACCGATGGAGAC; Rspo2-R, TCGGCTGCAACCATTGTCC; Rspo3-F, ATGCACTTGCGACTGATTTCT; Rspo3-R, GCAGCCTTGACTGACATTAGGAT; Rspo4-F, GCTGCAAAAGGCGGTTTCAC; Rspo4-R, TGGACTCAGATAACACTCGGC; Ndp-F1, TCAACGCTGCATGAGACA; Ndp-R1, ACAGGAGGAACGGAAAGG; DKK1-F, AGGAGTGCGGCTCTGACG; DKK1-R, CGAGGAAAATGGCTGTGG; DKK2-F, GAGCATCCTCACCCCACA; DKK2-R, CGAGCACAACAAAACCCA; DKK3-F, GGTGGGAAATAACACAGTCCAT; DKK3-R, CTCGGGTGCATAGCATCTGC; DKK4-F, TTCTGCTTAGCGTTTCAC; DKK4-R, TCCCTTACTGCTTTGTGA; Sfrp1-F, TCTAAGCCCCAAGGTACAACC; Sfrp1-R, GCTTGCACAGAGATGTTCAATG; Sfrp2-F, GCCAGCCCGACTTCTCCTA; Sfrp2-R, CCGCATGTTCTGGTACTCGAT; Sfrp3-F, GAGCGGATTTTCCTATGG; Sfrp3-R, GATTGACGGTGTCCCTTG; Sfrp4-F, TATGACCGTGGAGTTTGT; Sfrp4-R, TTTTGCTCAGGTATGTTGC; Sfrp5-F, GACCGAAAGTTGATTGGA; and Sfrp5-R, AGAGGGAACAGGGATAGG.
Statistical analysis
One-way ANOVA or Student’s t-test was performed, and the P-value was calculated in Prism on data represented by bar charts, which consisted of results from three independent experiments unless otherwise specified. For all experiments with error bars, the standard deviation (SD) was calculated to indicate the variation within each experiment. No statistical method was used to predetermine sample size. The experiments were not randomized. The investigators were not blinded to allocation during experiments and outcome assessment.
Supplementary Material
Acknowledgments
We are grateful to Chi-Chung Hui, Esther Verheyen, and Roel Nusse for critical reading of the manuscript. We thank Dangsheng Li for helpful discussion. This work is supported by grants from the Ministry of Science and Technology of China (2012CB945000 and 2014CB964800 to Y.A.Z.), National Natural Science Foundation of China (31171421 and 31371500 to Y.A.Z., and 31201098 to C.C.), the Chinese Academy of Sciences (XDA01010307 and 2010OHTP03 to Y.A.Z.), and Shanghai Municipal Science and Technology Commission (12PJ1410100 to Y.A.Z.).
Footnotes
Supplemental material is available for this article.
Article published online ahead of print. Article and publication date are online at http://www.genesdev.org/cgi/doi/10.1101/gad.245142.114.
References
- Asselin-Labat ML, Shackleton M, Stingl J, Vaillant F, Forrest NC, Eaves CJ, Visvader JE, Lindeman GJ. 2006. Steroid hormone receptor status of mouse mammary stem cells. J Natl Cancer Inst 98: 1011–1014 [DOI] [PubMed] [Google Scholar]
- Asselin-Labat ML, Vaillant F, Sheridan JM, Pal B, Wu D, Simpson ER, Yasuda H, Smyth GK, Martin TJ, Lindeman GJ, et al. 2010. Control of mammary stem cell function by steroid hormone signalling. Nature 465: 798–802 [DOI] [PubMed] [Google Scholar]
- Badders NM, Goel S, Clark RJ, Klos KS, Kim S, Bafico A, Lindvall C, Williams BO, Alexander CM. 2009. The Wnt receptor, Lrp5, is expressed by mouse mammary stem cells and is required to maintain the basal lineage. PLoS ONE 4: e6594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker N, Huch M, Kujala P, van de Wetering M, Snippert HJ, van Es JH, Sato T, Stange DE, Begthel H, van den Born M, et al. 2010. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell 6: 25–36 [DOI] [PubMed] [Google Scholar]
- Bradbury JM, Edwards PA, Niemeyer CC, Dale TC. 1995. Wnt-4 expression induces a pregnancy-like growth pattern in reconstituted mammary glands in virgin mice. Dev Biol 170: 553–563 [DOI] [PubMed] [Google Scholar]
- Brisken C, Heineman A, Chavarria T, Elenbaas B, Tan J, Dey SK, McMahon JA, McMahon AP, Weinberg RA. 2000. Essential function of Wnt-4 in mammary gland development downstream of progesterone signaling. Genes & Dev 14: 650–654 [PMC free article] [PubMed] [Google Scholar]
- Brisken C, Ayyannan A, Nguyen C, Heineman A, Reinhardt F, Tan J, Dey SK, Dotto GP, Weinberg RA. 2002. IGF-2 is a mediator of prolactin-induced morphogenesis in the breast. Dev Cell 3: 877–887 [DOI] [PubMed] [Google Scholar]
- Byers SL, Wiles MV, Dunn SL, Taft RA. 2012. Mouse estrous cycle identification tool and images. PLoS ONE 7: e35538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmon KS, Gong X, Lin Q, Thomas A, Liu Q. 2011. R-spondins function as ligands of the orphan receptors LGR4 and LGR5 to regulate Wnt/β-catenin signaling. Proc Natl Acad Sci 108: 11452–11457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadi S, Buscara L, Pechoux C, Costa J, Laubier J, Chaboissier MC, Pailhoux E, Vilotte JL, Chanat E, Le Provost F. 2009. R-spondin1 is required for normal epithelial morphogenesis during mammary gland development. Biochem Biophys Res Commun 390: 1040–1043 [DOI] [PubMed] [Google Scholar]
- Chassot AA, Gregoire EP, Magliano M, Lavery R, Chaboissier MC. 2008. Genetics of ovarian differentiation: Rspo1, a major player. Sex Dev 2: 219–227 [DOI] [PubMed] [Google Scholar]
- Chassot AA, Bradford ST, Auguste A, Gregoire EP, Pailhoux E, de Rooij DG, Schedl A, Chaboissier MC. 2012. WNT4 and RSPO1 together are required for cell proliferation in the early mouse gonad. Development 139: 4461–4472 [DOI] [PubMed] [Google Scholar]
- Chen B, Dodge ME, Tang W, Lu J, Ma Z, Fan CW, Wei S, Hao W, Kilgore J, Williams NS, et al. 2009. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat Chem Biol 5: 100–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu EY, Hens J, Andl T, Kairo A, Yamaguchi TP, Brisken C, Glick A, Wysolmerski JJ, Millar SE. 2004. Canonical WNT signaling promotes mammary placode development and is essential for initiation of mammary gland morphogenesis. Development 131: 4819–4829 [DOI] [PubMed] [Google Scholar]
- Clevers H, Nusse R. 2012. Wnt/β-catenin signaling and disease. Cell 149: 1192–1205 [DOI] [PubMed] [Google Scholar]
- de Lau W, Barker N, Low TY, Koo BK, Li VS, Teunissen H, Kujala P, Haegebarth A, Peters PJ, van de Wetering M, et al. 2011. Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling. Nature 476: 293–297 [DOI] [PubMed] [Google Scholar]
- Dwyer MA, Joseph JD, Wade HE, Eaton ML, Kunder RS, Kazmin D, Chang CY, McDonnell DP. 2010. WNT11 expression is induced by estrogen-related receptor α and β-catenin and acts in an autocrine manner to increase cancer cell migration. Cancer Res 70: 9298–9308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fata JE, Kong YY, Li J, Sasaki T, Irie-Sasaki J, Moorehead RA, Elliott R, Scully S, Voura EB, Lacey DL, et al. 2000. The osteoclast differentiation factor osteoprotegerin-ligand is essential for mammary gland development. Cell 103: 41–50 [DOI] [PubMed] [Google Scholar]
- Gavin BJ, McMahon AP. 1992. Differential regulation of the Wnt gene family during pregnancy and lactation suggests a role in postnatal development of the mammary gland. Mol Cell Biol 12: 2418–2423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glinka A, Dolde C, Kirsch N, Huang YL, Kazanskaya O, Ingelfinger D, Boutros M, Cruciat CM, Niehrs C. 2011. LGR4 and LGR5 are R-spondin receptors mediating Wnt/β-catenin and Wnt/PCP signalling. EMBO Rep 12: 1055–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong X, Carmon KS, Lin Q, Thomas A, Yi J, Liu Q. 2012. LGR6 is a high affinity receptor of R-spondins and potentially functions as a tumor suppressor. PLoS ONE 7: e37137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiremath M, Lydon JP, Cowin P. 2007. The pattern of β-catenin responsiveness within the mammary gland is regulated by progesterone receptor. Development 134: 3703–3712 [DOI] [PubMed] [Google Scholar]
- Huch M, Dorrell C, Boj SF, van Es JH, Li VS, van de Wetering M, Sato T, Hamer K, Sasaki N, Finegold MJet al. 2013. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature 494: 247–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi PA, Jackson HW, Beristain AG, Di Grappa MA, Mote PA, Clarke CL, Stingl J, Waterhouse PD, Khokha R. 2010. Progesterone induces adult mammary stem cell expansion. Nature 465: 803–807 [DOI] [PubMed] [Google Scholar]
- Kessenbrock K, Dijkgraaf GJ, Lawson DA, Littlepage LE, Shahi P, Pieper U, Werb Z. 2013. A role for matrix metalloproteinases in regulating mammary stem cell function via the Wnt signaling pathway. Cell Stem Cell 13: 300–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KA, Kakitani M, Zhao J, Oshima T, Tang T, Binnerts M, Liu Y, Boyle B, Park E, Emtage P, et al. 2005. Mitogenic influence of human R-spondin1 on the intestinal epithelium. Science 309: 1256–1259 [DOI] [PubMed] [Google Scholar]
- Kim KA, Wagle M, Tran K, Zhan X, Dixon MA, Liu S, Gros D, Korver W, Yonkovich S, Tomasevic N, et al. 2008. R-spondin family members regulate the Wnt pathway by a common mechanism. Mol Biol Cell 19: 2588–2596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YC, Clark RJ, Pelegri F, Alexander CM. 2009. Wnt4 is not sufficient to induce lobuloalveolar mammary development. BMC Dev Biol 9: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim E, Vaillant F, Wu D, Forrest NC, Pal B, Hart AH, Asselin-Labat ML, Gyorki DE, Ward T, Partanen A, et al. 2009. Aberrant luminal progenitors as the candidate target population for basal tumor development in BRCA1 mutation carriers. Nat Med 15: 907–913 [DOI] [PubMed] [Google Scholar]
- Lustig B, Jerchow B, Sachs M, Weiler S, Pietsch T, Karsten U, van de Wetering M, Clevers H, Schlag PM, Birchmeier W, et al. 2002. Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol Cell Biol 22: 1184–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malanchi I, Santamaria-Martínez A, Susanto E, Peng H, Lehr HA, Delaloye JF, Huelsken J. 2012. Interactions between cancer stem cells and their niche govern metastatic colonization. Nature 481: 85–89 [DOI] [PubMed] [Google Scholar]
- Mikels AJ, Nusse R. 2006. Purified Wnt5a protein activates or inhibits β-catenin-TCF signaling depending on receptor context. PLoS Biol 4: e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SJ, Spradling AC. 2008. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell 132: 598–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan JL, Kendrick H, Magnay FA, Vafaizadeh V, Groner B, Smalley MJ. 2012. c-Kit is required for growth and survival of the cells of origin of Brca1-mutation-associated breast cancer. Oncogene 31: 869–883 [DOI] [PubMed] [Google Scholar]
- Rios AC, Fu NY, Lindeman GJ, Visvader JE. 2014. In situ identification of bipotent stem cells in the mammary gland. Nature 506: 322–327 [DOI] [PubMed] [Google Scholar]
- Roarty K, Serra R. 2007. Wnt5a is required for proper mammary gland development and TGF-β-mediated inhibition of ductal growth. Development 134: 3929–3939 [DOI] [PubMed] [Google Scholar]
- Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, et al. 2009. Single Lgr5 stem cells build crypt–villus structures in vitro without a mesenchymal niche. Nature 459: 262–265 [DOI] [PubMed] [Google Scholar]
- Sato T, van Es JH, Snippert HJ, Stange DE, Vries RG, van den Born M, Barker N, Shroyer NF, van de Wetering M, Clevers H. 2011. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 469: 415–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackleton M, Vaillant F, Simpson KJ, Stingl J, Smyth GK, Asselin-Labat ML, Wu L, Lindeman GJ, Visvader JE. 2006. Generation of a functional mammary gland from a single stem cell. Nature 439: 84–88 [DOI] [PubMed] [Google Scholar]
- Shehata M, Teschendorff A, Sharp G, Novcic N, Russell A, Avril S, Prater M, Eirew P, Caldas C, Watson CJ, et al. 2012. Phenotypic and functional characterization of the luminal cell hierarchy of the mammary gland. Breast Cancer Res 14: R134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberstein GB, Van Horn K, Hrabeta-Robinson E, Compton J. 2006. Estrogen-triggered delays in mammary gland gene expression during the estrous cycle: evidence for a novel timing system. J Endocrinol 190: 225–239 [DOI] [PubMed] [Google Scholar]
- Stingl J, Eirew P, Ricketson I, Shackleton M, Vaillant F, Choi D, Li HI, Eaves CJ. 2006. Purification and unique properties of mammary epithelial stem cells. Nature 439: 993–997 [DOI] [PubMed] [Google Scholar]
- Tomizuka K, Horikoshi K, Kitada R, Sugawara Y, Iba Y, Kojima A, Yoshitome A, Yamawaki K, Amagai M, Inoue A, et al. 2008. R-spondin1 plays an essential role in ovarian development through positively regulating Wnt-4 signaling. Hum Mol Genet 17: 1278–1291 [DOI] [PubMed] [Google Scholar]
- van Amerongen R, Bowman AN, Nusse R. 2012. Developmental stage and time dictate the fate of Wnt/β-catenin-responsive stem cells in the mammary gland. Cell Stem Cell 11: 387–400 [DOI] [PubMed] [Google Scholar]
- Veltmaat JM, Van Veelen W, Thiery JP, Bellusci S. 2004. Identification of the mammary line in mouse by Wnt10b expression. Dev Dyn 229: 349–356 [DOI] [PubMed] [Google Scholar]
- Weber-Hall SJ, Phippard DJ, Niemeyer CC, Dale TC. 1994. Developmental and hormonal regulation of Wnt gene expression in the mouse mammary gland. Differentiation 57: 205–214 [DOI] [PubMed] [Google Scholar]
- Weiland A, Roswall P, Hatzihristidis TC, Pietras K, Ostman A, Strell C. 2012. Fibroblast-dependent regulation of the stem cell properties of cancer cells. Neoplasma 59: 719–727 [DOI] [PubMed] [Google Scholar]
- Welm BE, Dijkgraaf GJ, Bledau AS, Welm AL, Werb Z. 2008. Lentiviral transduction of mammary stem cells for analysis of gene function during development and cancer. Cell Stem Cell 2: 90–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willert K, Brown JD, Danenberg E, Duncan AW, Weissman IL, Reya T, Yates JR 3rd, Nusse R. 2003. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature 423: 448–452 [DOI] [PubMed] [Google Scholar]
- Zeng YA, Nusse R. 2010. Wnt proteins are self-renewal factors for mammary stem cells and promote their long-term expansion in culture. Cell Stem Cell 6: 568–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.