Abstract
Mesenchymal stromal cells (MSCs) are multipotent cells that possess broad immunomodulatory properties; the mechanisms underlying these properties have not been completely clarified. Aim of this study was to compare in vitro immunomodulatory effects of MSCs with those of microvesicles (MVs) released in supernatants from the same MSCs. MSCs were generated from bone marrow of 12 healthy donors (HDs) and MVs were isolated from their supernatant by serial ultracentrifugation according to two different procedures. Both MSCs and MVs were characterized by flow cytometry and incubated in vitro with peripheral blood mononuclear cells (PBMCs) of 12 HDs after stimulation with PHA and CpG. Growth factors and cytokines were quantified by ELISA. MVs were identified as 0.1–1 μm particles positive for CMFDA, CD107, and CD13. MSCs were significantly more capable to inhibit in vitro PHA-induced T-cell proliferation as compared with the corresponding MVs (P<0.01 and P<0.05 for MSC:PBMC ratio 1:2 and 1:10, respectively). While MVs displayed similar inhibitory activity on B-cell proliferation (P=0.43 as compared with PBMCs/CpG/MSCs; MSC:PBMC ratio 1:10) they induced lower inhibitory effect on plasmacell differentiation and antibody secretion (P<0.05 as compared with PBMCs/CpG/MSCs). For both T and B cells, MSC co-colture induced a statistically significant increase in IL-10 and TGFβ and decrease of GM-CSF and IFNγ, as compared with MV incubation. Our data indicate a lower in vitro immunomodulatory effect of MVs on T-cell proliferation and antibody formation, as compared with their cellular counterpart. The relative clinical benefit of either MSCs or MVs needs to be compared in proper prospective studies.
Introduction
Human mesenchymal stromal cells (MSCs) are multipotent cells, able to differentiate into mesenchymal lineages, that can be isolated from bone marrow (BM) and adipose tissue, or from cord blood and fetal tissues [1]. Among their extensively characterized in vitro properties [2,3], MSCs have been reported to display a strong antiproliferative and anti-inflammatory effect on immune responses [4,5]. In several in vitro studies, MSCs have been shown to inhibit T-cell proliferation in response to allo-antigens and mitogens, preventing the development of cytotoxic T-cells and inducing functional regulatory T cells [6–8]. In most studies it has been demonstrated that MSCs can inhibit in vitro B-cell proliferation and differentiation [9,10], whereas other researchers have reported a stimulatory effect of MSCs on B cells [11].
Based on these in vitro data, MSCs have been tested in phase I/II clinical trials aimed at treating steroid-resistant acute graft-versus-host disease (aGvHD) and favoring hematopoietic engraftment/recovery after hematopoietic stem cell transplantation [12–14], and in strategies of Regenerative Medicine [15,16]. The mechanisms by which MSCs exert their immunomodulatory and reparative effects in vitro and in vivo are still under investigation: although some authors consider MSCs-mediated immunosuppressive effects as mostly dependent on the secretion of antiproliferative soluble factors, such as TGFβ, prostaglandin E2 (PGE2), indoleamine-2,3-dioxygenase (IDO), hepatocyte growth factor (HGF), and human leucocyte antigen G, cell-to-cell contact mechanisms may also play a role4,5. Moreover, recent reports have indicated that MSCs are not per se constitutively inhibitory, but need to be activated by an inflammatory environment in the host to deliver their immunomodulatory signals [17,18].
Recent studies have suggested that, besides growth factors and cytokines, most cell types, including MSCs, secrete large amounts of microvesicles (MVs), either constitutively or upon activation. MVs have been shown to play a role in a variety of cellular events, such as cardiovascular and kidney repair and modulation of the immune response [19–24]. There are various types of secreted membrane vesicles that show distinct structural and biochemical properties, depending on their intracellular site of origin, consequently exerting different effects on target cells [19,20,23,24]. Most in vitro studies focused on two main and distinct types of signaling vesicles: exosomes and small shedding vesicles [21,22,24]. Exosomes derive from the endosomal membrane cell compartment and are released into the extracellular space after fusion of multivesicular bodies with the plasma membrane. They are more homogenous in size (30–120 nm) and contain large amounts of annexins and heat-shock proteins. Shedding vesicles or microparticles originate from direct budding of small cytoplasmic protrusions from the plasma membrane and are more heterogeneous in size (100 nm–1 μm). They expose high amounts of phosphatidylserine and are rich in cholesterol and ceramide [21,22,24]. Given the heterogeneity of vesicles released by cells in response of any kind of stimuli, mixed vesicles populations, containing both shedding vesicles and exosomes, are commonly referred to as MVs. MVs may influence the behavior of target cells by different mechanisms: they may act as signaling complexes by direct stimulation of target cells, they may fuse with target cell membranes and transfer receptors and/or bioactive factors, and they may mediate a horizontal transfer of genetic material, such as mRNA and microRNAs.
Aim of this study was to assess in vitro the immunomodulatory effect of MVs generated from BM-derived MSCs on T and B cells and to compare it with that of their cellular counterpart, to draw some useful information for implementing cellular therapy approaches based on the use of MVs as MSC substitutes.
Materials and Methods
Isolation, ex vivo culture, and characterization of BM-derived MSCs
Isolation of mononuclear cells, ex vivo expansion and characterization of MSCs were performed from healthy donors (HDs) who donated BM for transplantation at the Bambino Gesù Children's Hospital, as previously described [25]. HDs gave their written informed consent and the study was conducted according to the Declaration of Helsinki.
Briefly, mononuclear cells were obtained from BM aspirates (10 mL) of 12 HDs by density gradient centrifugation (Ficoll 1.077 g/mL; Lympholyte, Cedarlane Laboratories Ltd.) and plated in noncoated 75–175 cm2 tissue culture flasks (BD Falcon) at a density of 160,000/cm2 in complete culture medium: DMEM (Eurocloney) supplemented with 10% fetal bovine serum (FBS; Gibco, Life Technologies Ltd.), penicillin 50 U/mL, 50 mg/mL streptomycin, and 2 mM L-glutamine (Euroclone). Cultures were maintained at 37°C in a humidified atmosphere, containing 5% CO2. After 48-h adhesion, nonadherent cells were removed and culture medium was replaced twice a week. MSCs were harvested, after reaching ≥80% confluence, using Trypsin (Euroclone), and they were propagated at 4,000 cells/cm2.
MSCs were phenotypically characterized by flow cytometry at passage (P) 2–3. Fluorescein isothiocyanate- or phycoerythrin (PE)-conjugated monoclonal antibodies specific for CD13, CD14, CD34, CD45, CD73, CD80, CD90, class I-HLA and HLA-DR, CD73, CD105 (BD PharMingen) were used. Analysis of cell populations was performed by means of direct immunofluorescence with a FACSCanto flow cytometer (BD PharMingen) and data were calculated using the FACSDiva software (Tree Star, Inc.).
The osteogenic and adipogenic differentiation capacity of MSCs was assessed at P2–3 according to previously reported methods [25]. To detect osteogenic differentiation, cells were stained for calcium deposition with Alizarin Red (Sigma-Aldrich), whereas adipogenic differentiation was evaluated through the morphological appearance of fat droplets stained with Oil Red O (Sigma-Aldrich).
Isolation and characterization of MVs
On the basis of the published literature, two of the most commonly employed protocols for the isolation of MVs were employed [26,27]. Aim of this double approach was to possibly identify and select the best MV isolation method. To remove endogenous MVs, FBS was ultracentrifuged at 100,000 g by Optima XL-100K Ultracentrifuge (Beckman Coulter) before use. For both MV isolation protocols, supernatant was collected after 4-day MSC culture at P2–3 from 2×106 MSCs, when ∼90% cell confluence was reached. In the first procedure (MVs-1), to obtain a more purified final product, the medium was centrifuged as follows: 800 g for 10 min, 2,500 g for 15 min, and 10,000 g for 30 min.26 This was followed by centrifugation of the supernatant at 100,000 g for 1 h to isolate MVs, which were then washed at 100,000 g for 1 h in phosphate-buffered saline (PBS; Euroclone). In the second procedure (MVs-2), the MSC medium was first centrifuged at 1,000 g for 20 min to remove cell debris and then concentrated by centrifugation for 20 min at 2,000 g in sterile hydrated 30 kDa MWCO Amicon Ultra Centrifugal filter (Millipore) up to a volume of 200 μL [27]. Afterward, this intermediate product was diluted in 12 mL of PBS in polyallomer tubes (Beckman Coulter) and washed at 100,000 g at 4°C for 1 h. At the end of the ultracentrifugation, the suspension was once again concentrated by centrifuging for 20 min at 2,000 g in a sterile 30 kDa MWCO Amicon Ultra Centrifugal filter (Millipore) up to a volume of 400 μL.
Once isolated, both MV preparations were labeled with 5-chloromethylfluorescein diacetate at the final concentration of 0.1 mg/mL (CellTracker CMFDA; Molecular Probes) and phenotypically characterized by flow cytometry with PE-conjugated monoclonal antibody specific for CD13 and allophycocyanin-conjugated monoclonal antibody specific for CD107a (BD PharMingen). Calibration beads were employed to gate MVs by dimension parameters. Analysis of cell populations isolated from seven different HDs was performed by means of direct immunofluorescence with a FACSCanto flow cytometer (BD PharMingen) and data were calculated using the FACSDiva software (Tree Star).
Protein content of both MV preparations was measured via Bradford protein assay (Bio-Rad) following manufacturer's instructions; results were expressed as mean and range.
Peripheral blood mononuclear cell isolation
Peripheral blood mononuclear cells (PBMCs) were obtained by conventional Ficoll separation from heparinized peripheral blood samples from 12 HDs (different from those who donated BM), who gave informed consent for this study. Cells were employed on the same day of collection.
In vitro T-cell proliferation assay with phytohemagglutinin
PBMC proliferation in response to phytohemagglutinin (PHA-P; Sigma-Aldrich) was evaluated in triplicate in flat-bottom 96-well tissue culture plates (BD Falcon) in RPMI 1640 medium (Gibco, Life Technologies Ltd.) supplemented with 10% FBS in the presence or absence of MSCs, MVs-1, or MVs-2. Briefly, MSCs were seeded at MSCs:PBMCs ratios of 1:2 and 1:10 and allowed to adhere overnight before adding 105 PBMCs per well with or without PHA (5 μg/mL). MVs-1 or MVs-2, generated from 2×106 MSCs, were added (100 μL) directly to PHA-stimulated PBMCs by diluting them 1:2 in co-culture final volume. After 3-day incubation at 37°C in a humidified 5% CO2 atmosphere, cultures with both MSCs and MVs were pulsed with 3H-thymidine (1 μCi/well, specific activity 6.7 Ci/mmole; Perkin Elmer) and harvested after 18 h. 3H-thymidine incorporation was measured by standard procedure with Microbeta Trilux 1450 instrument (Perkin Elmer). Results, obtained from 12 experiments employing 12 different MSC/MV donors, were expressed as mean percentage (±SD) of PBMC proliferation. All experiments were performed in triplicate, in an allogeneic setting (HD-PBMCs/HD-MSCs; HD-PBMCs/MVs-1; HD-PBMCs/MVs-2).
With the aim to investigate a potential dose- and time-dependent effect of MVs, MVs generated from increasing numbers of MSCs (2×106, 5×106 and 10×106) or added at different time points of the culture (t0, t+0 and t+24 h; t+0, t+24 and t+48 h) were evaluated in the PHA assay.
In vitro B-cell proliferation and differentiation assay with CpG
Total PBMCs were labeled with 5-chloromethylfluorescein diacetate at the final concentration of 0.1 mg/mL (CellTracker CMFDA; Molecular Probes). Then, 2×105 PBMCs were seeded on 96 well-plates in the presence or absence of MSCs (MSCs:PBMCs ratio 1:10) or MVs-1 or MVs-2 and then stimulated with RPMI 1640, 10% FBS, 2% L-glutamine, 5×10−5M 2-βmercaptoethanol (Sigma-Aldrich), and 20 mg/mL gentamycin (Gibco), supplemented or not with 2.5 μg/mL CpG-ODN (Hycult Biotechnology). After 7-day culture, B cells were collected and stained with an appropriate combination of fluorescent-labeled antibodies: monoclonal clone HIB19 (anti-CD19), clone M-T271 (anti-CD27) and clone HIT2 (anti-CD38; all from BD Biosciences), and anti-IgM Fc5 μ fragment specific (Jackson Immuno Research Laboratories). Dead cells were excluded from analysis by side/forward scatter gating. Analysis was performed on a FACSCanto (BD PharMingen) interfaced to FACSDiva software. One hundred thousand events per sample were analyzed.
Measurement of growth factors and cytokines by ELISA
The concentration of IL-2, IL-6, IL-10, TGFβ, GM-CSF, and IFNγ in supernatants of both MSCs/PBMCs and MVs/PBMCs cultures was quantified by means of commercially available ELISA kits obtained from Mabtech after 72 h of incubation. Galectin-1, HGF, and PGE2 content was evaluated using ELISA kits obtained from R&D System, following the manufacturer's instructions. Plates were read either at 405 nm (for ALP-conjugated antibodies) or at 450 nm (for HRP-conjugated antibodies) through Envision Multilabel Reader (Perkin Elmer). The concentration of cytokines and growth factors was also measured in MVs-1 and MVs-2 preparations.
Immunoglobulin production
Quantitative analysis of IgA, IgG, and IgM concentrations in culture supernatants was performed by ELISA. Briefly, 96-well plates were coated overnight with purified goat anti-human IgA, IgG, or IgM antibodies (Jackson Immuno Research Laboratories). After washing with PBS/0.05% Tween20 and blocking with PBS/1% gelatine, plates were incubated for 1 h with the supernatants of the cultured cells. After washing, plates were incubated for 1 h with peroxidase-conjugated fragment of goat anti-human IgA, IgG or IgM antibodies (Jackson Immuno Research Laboratories). The assay was developed with o-phenylendiamine (Sigma-Aldrich) as chromogenic substrate. Optical density was measured on a microtiter plate reader at 450 nm and Ig concentrations were calculated by interpolation with the standard curve done using IgM, IgG, and whole IgA (Jackson Immuno Research Laboratories).
Statistical analysis
Statistical analysis was performed using the GraphPad Prism software package and determined with the Student's t-test, assuming paired data. A P value lower than 0.05 was considered to be statistically significant.
Results
Characterization of MSCs and of MV phenotype
BM-derived MSCs were characterized as adherent spindle-shaped cells expressing the typical pattern of surface markers reported in the literature [2]. In particular, MSCs highly expressed the surface markers CD13, CD73, CD90, and CD105, whereas hematopoietic markers such as CD14, CD34, CD45, CD80, and HLA-DR were no longer detectable by P2. MSCs were able to differentiate into osteoblasts, as demonstrated by the histologic detection of calcium depositions positive for Alzarin Red, and into adipocytes, as revealed by the formation of lipid droplets stained with Oil Red O (data not shown).
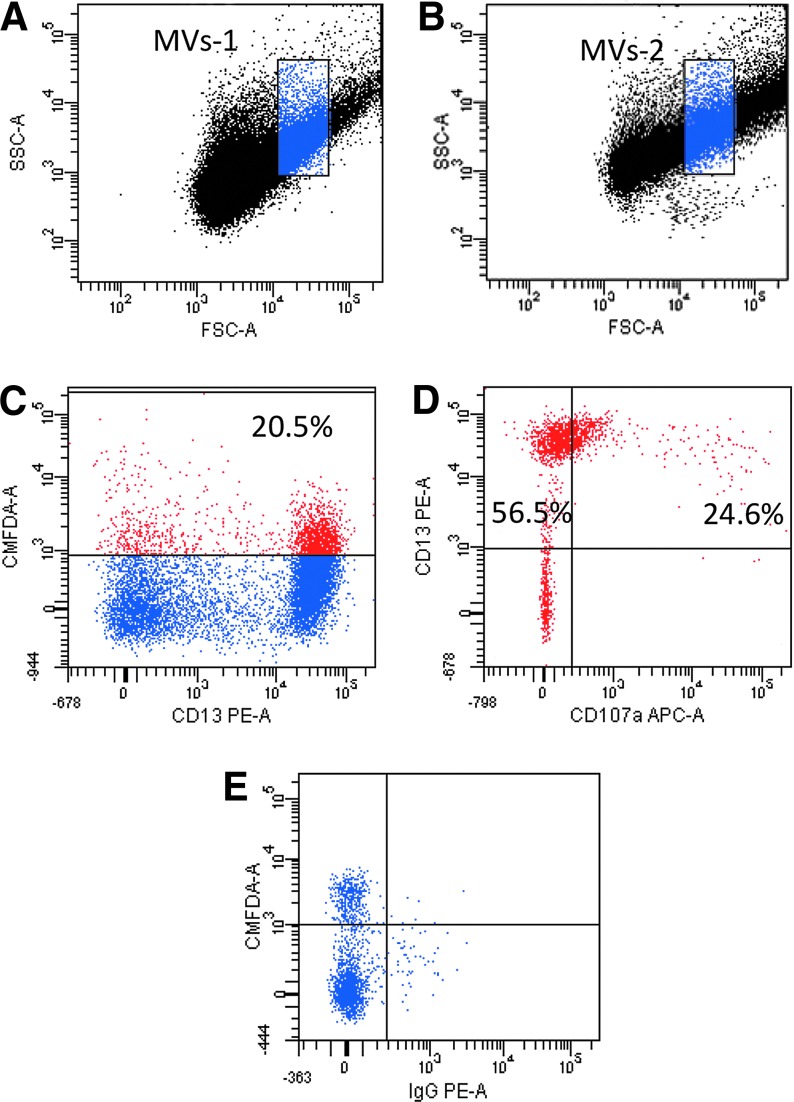
Both MVs-1 and MVs-2, after being isolated from MSCs by means of ultracentrifugation procedures, were phenotypically characterized by flow cytometry and, to precisely gate MVs by morphological parameters, calibration beads of 1 μm dimension were used. As shown in Fig. 1A and B, no differences in dimensions between the two preparations (MV-1 and MV-2) were revealed. Once gated, both MV preparations were shown to be CMFDA-positive (Fig. 1C), thus indicating that membrane-delimited fragments and not only free cytoplasm portions had been isolated. Moreover, CMFDA-positive MVs-1 and -2 were both shown to be positive for CD13 (Fig. 1C), a well-known surface marker expressed on MSC surface, and CD107a (Fig. 1D), a widely expressed intracellular protein. CD107a is normally located on the lysosomal/endosomal membrane and found on plasma membrane after extrusion of lysosomes/endosomes, suggesting that the gated population of MVs were derived from MSCs through a plasma membrane extrusion mechanism.
FIG. 1.
Characterization of microvesicles (MVs) isolated from mesenchymal stromal cell (MSC) supernatant by flow cytometry. (A, B) Representative example of dot-plot analysis of MVs-1 (A) and MVs-2 (B). The two preparations show similar dimension. (C–E) Representative example of the surface marker analysis performed on one sample of MVs-1. (C) After MVs were gated by dimension through calibration beads of 1 μm, the percentage of CMDFA+ MVs resulted to be 20.5% of total gated MVs. (D) Among CMFDA+ MVs, CD13+ MVs were 56.5% and CD13+ CD107a+ MVs were 24.6% (E). No signal was revealed when isotype control for CD13 was included. Dot plots analysis of MVs-2 was comparable.
Protein content was measured for both MV preparations: it was 0.64 μg/μL (range 0.35–0.87 μg/μL) and 5.04 μg/μL (range 4.50–5.67 μg/μL) for MVs-1 and MVs-2, respectively.
Effect of MSCs and MVs on PHA-induced T-cell proliferation
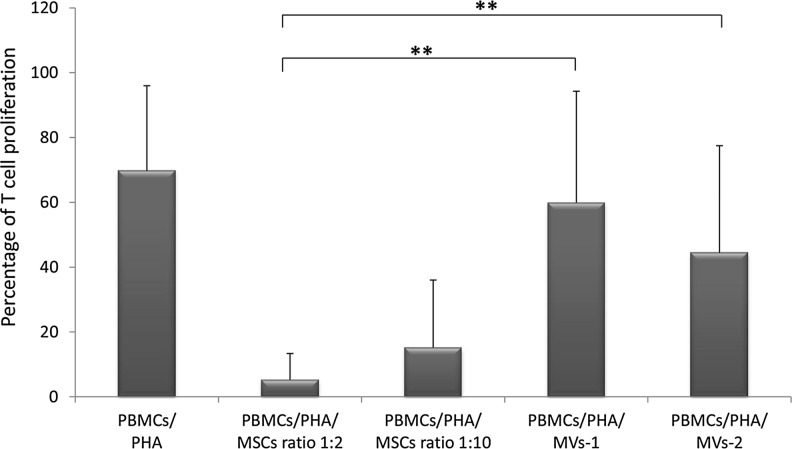
To evaluate the in vitro immunomodulatory capacity of ex vivo expanded MSCs and MSC-derived MVs, T-cell proliferation induced by PHA was measured in the presence or in the absence of either MSCs or MVs-1 or MVs-2 in an allogeneic setting. Twelve samples of MSC/MV obtained from HDs were employed and plated with PBMCs isolated from 12 different volunteers. In agreement with previously reported studies [6,7], MSCs proved to exert a strong in vitro inhibitory effect on PHA-induced T-cell proliferation with a median percentage of proliferation in the presence of MSCs of 5.19% (SD±8.16) and 15.14% (SD±20.86) at MSCs:PBMCs ratios 1:2 and 1:10, respectively. T-cell proliferation in the absence of MSCs was 69.70% (SD±26,28). When MVs-1 were incubated with PBMCs, T-cell proliferation was 59.90% (SD±34.37; P<0.01 and P<0.05 as compared with the condition PBMCs/PHA/MSCs ratios 1:2 and 1:10, respectively), whereas it was 44.41% (SD±39.32; P<0.01 and P<0.05 as compared with the condition PBMCs/PHA/MSCs ratios 1:2 and 1:10, respectively) when MVs-2 were added to PBMC cultures (Fig. 2). As compared with MVs-1, MVs-2 preparations showed a trend for a greater inhibitory activity, suggesting that the concentration step employed after the ultracentrifugation in the preparation procedure of MVs-2 may allow for saving more immune-active factors/cytokines in the final product. This is confirmed by the measurement of the concentration of cytokines and growth factors in both MV preparations; indeed, the concentration of the four detectable factors (TGFβ, Galectin-1, HGF, and PGE2) was higher in MVs-2 than in MVs-1 (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/scd).
FIG. 2.
In vitro immunomodulatory effect of MSCs and MSC-derived MVs on T-cell proliferation. The graph shows the proliferation of healthy donor peripheral blood mononuclear cells (PBMCs) stimulated with phytohemagglutinin (PHA), in the presence or in the absence of either MSCs (with two different MSCs:PBMCs ratios 1:2 and 1:10) or MVs, isolated from MSCs with two different procedures (MVs-1 and MVs-2). Each bar represents the percentage of proliferation of 105 PBMCs, calculated by measuring 3H-thymidine incorporation after 3-day co-culture. The counts per minute (cpm) values at each cell concentration were normalized to the cpm of PBMCs without MSCs or MVs in each experiment. Each bar represents the median±SD of 12 experiments (each point being in triplicate). P values lower than 0.01 (**) were considered highly statistically significant.
The addition of MVs generated from increasing numbers of MSCs (2×106, 5×106 and 10×106; Supplementary Fig. S2A) or added at different time points of the culture (t0, t+0 and t+24 h; t+0, t+24 and t+48 h; Supplementary Fig. S2B) did not influence their inhibitory effect.
The addition of supernatants collected at intermediate steps during the ultracentrifugation procedure was associated with an inhibition of PHA-induced T-cell proliferation, although inferior to that of MSCs and MVs (Supplementary Fig. S3).
Altogether, these in vitro findings suggest that, although MVs are able to display an antiproliferative effect on T cells, the corresponding MSCs possess a significantly superior ability of inhibition.
Effect of MSCs and MVs on CpG-induced B-cell proliferation and differentiation
As reported in previous studies [28], CpG was used to induce B-cell proliferation and plasmacell differentiation, in the presence or in the absence of either MSCs or MVs. As for T-cell analysis, the assays were performed in an allogeneic setting and in triplicate, by using PBMCs from the 12 different HDs. B-cell proliferation and differentiation were evaluated by flow cytometry and results were expressed as percentage of total B cells (CD19+), proliferating B cells (CD19+ CMFDA+), and plasmacells (CD19+ CD27+). As shown in Fig. 3, when PBMCs were stimulated with CpG, the percentage of CD19+ cells was 24.90% (SD±10.81); this was reduced to 10.70% (SD±14.11) in the presence of MSCs (MSCs:PBMCs ratio 1:10), to 13.60% (SD±18.15; P=0.70 as compared with PBMCs/CpG/MSCs) in the presence of MVs-1, and 14.54% (SD±16.34; P=0.66 as compared with PBMCs/CpG/MSCs) in the presence of MVs-2. As far as proliferating B cells were concerned, while the percentage of CD19+ CMFDA+ cells in the presence of CpG was 21.83% (SD±8.73), the addition of MSCs reduced it to 6.90% (SD±2.82). When MVs-1 and MVs-2 were co-coltured with PBMCs+CpG, the median percentage of proliferating B cells was 8.31% (SD±4.92; P=0.43 as compared with PBMCs/CpG/MSCs) and 9.00% (SD±9.95; P=0.50 as compared with PBMCs/CpG/MSCs), respectively. When CD19+CD27+ plasmacells were evaluated, their median percentage of proliferation after CpG stimulation was 59.30% (SD±5.16); this value dropped to 29.30% (SD±10.59) in the presence of MSCs, to 45.70% (SD±13.70; P=0.02 as compared with PBMCs/CpG/MSCs) in the presence of MVs-1, and 48.40% (SD±13.53; P=0.01 as compared with PBMCs/CpG/MSCs) in the presence of MVs-2.
FIG. 3.
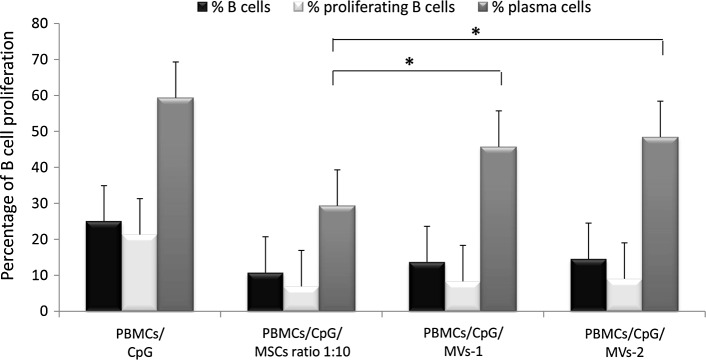
In vitro immunomodulatory effect of MSCs and MVs on CpG-stimulated PBMC proliferation. The graph shows the proliferation of healthy donor PBMCs stimulated with CpG in the presence or in the absence of MSCs (MSCs:PBMCs ratio 1:10), MVs-1 or MVs-2 (both diluted 1:2 in co-culture final volume). Black bars represent the mean percentage of proliferation of total B cells (CD19+), whereas white and gray bars represent the mean percentages of proliferating B cells (CD19+ CMFDA+; % prolif B) and plasmacells (CD19+CD27+; % PC), respectively. Each bar represents the median±SD of 12 experiments (each point being in triplicate). P values lower than 0.05 (*) were considered statistically significant.
These experimental findings indicate that, when considering the proliferation of B cells, MSCs and both MV preparations display a comparable ability of inhibition in vitro. As far as plasmacells are concerned, although MVs are able to induce an inhibitory effect on their differentiation, the immunomodulatory effect exerted by the corresponding MSCs on this cell subset is significantly superior.
Analysis of cytokines, growth factors, and immunoglobulin secretion in cell culture supernatants
Given the role attributed by many studies to soluble factors in mediating MSC immunomodulatory effects [29–31], cytokine and growth factor content in co-cultures of PHA- and CpG-stimulated PBMCs with MSCs and MVs were measured.
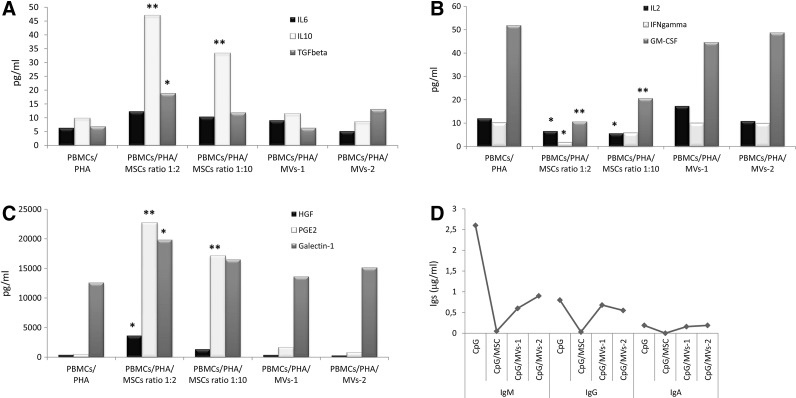
As shown in Fig. 4A, a highly significant increase in IL10 secretion was detected in co-cultures of PHA-stimulated PBMCs and MSCs, as compared with the conditions PBMCs/PHA/MVs-1 and -2 (P<0.01 for both MSCs:PBMCs ratios). A lower, but still statistically significant, increase in TGFβ levels was observed in co-cultures of PHA-stimulated PBMCs and MSCs as compared with the corresponding co-colture with MVs-1 and -2 (P<0.05 only at MSCs:PBMCs ratio 1:2). Not statistically significant difference in IL6 secretion profile was detected between co-cultures in the presence of MSCs or MVs.
FIG. 4.
Concentrations of cytokines and growth factors were quantified by ELISA in supernatants of co-culture of PHA-stimulated PBMCs with either MSCs or MVs. One representative experiment is shown. Results are expressed as pg/mL in case of cytokines and growth factors and μg/mL in case of immunoglobulins (Ig). (A) Measurement of the inhibitory/anti-inflammatory cytokines IL6 and IL10 and growth factor TGFβ. (B) Measurement of the stimulatory/pro-inflammatory cytokines IL2 and IFNγ and growth factor GM-CSF. (C) Measurement of paracrine factors involved in MSC-mediated immunemodulation: HGF, PGE2, and Galectin-1. (D) Immunoglobulin concentrations measured in co-cultures of CpG-stimulated PBMCs and MSCs or MVs. P values lower than 0.05 (*) were considered statistically significant, whereas P values lower than 0.01 (**) were considered highly statistically significant. HGF, hepatocyte growth factor; PGE2, prostaglandin E2.
As far as pro-inflammatory cytokines are concerned, a highly significant decrease in GM-CSF levels was measured in supernatants collected from co-cultures of PHA-stimulated PBMCs and MSCs (P<0.01 for both MSCs:PBMCs ratios), as compared with the conditions PBMCs/PHA/MVs-1 and -2. Furthermore, a significantly lower concentration of IL2 and IFNγ was measured in supernatants collected from co-cultures of PHA-stimulated PBMCs and MSCs (P<0.05 for both MSCs:PBMCs ratios in case of IL2 and only for MSCs:PBMCs ratio 1:2 in case of IFNγ), as compared with the condition PBMCs/PHA/MVs (Fig. 4B).
The concentration of some of the soluble factors known to be involved in the immunomodulatory effect displayed by MSCs, namely HGF, PGE2, and Galectin-1, was also measured in supernatants collected from co-cultures of PHA-stimulated PBMCs and MSCs/MVs. In the presence of MSCs, PGE2 levels significantly increased (P<0.05 for both MSCs:PBMCs ratios) as compared with the condition PBMCs/PHA/MVs-1 and -2. A significant increase was also measured in HGF and Galectin-1 levels (P<0.05 for both, but only for MSCs:PBMCs ratio 1:2).
As far as co-cultures of CpG-stimulated PBMCs and MSCs or MVs are concerned, similar cytokine and growth factors profiles, in terms of differential secretion pattern, were observed for IL6, TGFβ, GM-CSF, IFNγ, HGF, PGE2, and Galectin-1 as compared to those observed in co-cultures of PHA-stimulated PBMCs. Statistically significant higher levels of IL2 were measured in supernatants collected from co-cultures of CpG-stimulated PBMCs and MSCs as compared with the condition PBMCs/PHA/MVs (P<0.05); whereas, although a consistent production of IL10 was present after CpG stimulation, no significant difference was revealed in its levels in co-cultures with MSCs or MVs (data not shown).
Finally, the superior ability of MSCs to impair B-cell proliferation and plasmacell differentiation was confirmed by the significant reduction in IgM and IgG levels detected in supernatants collected from CpG-stimulated PBMC and MSC cultures (P<0.05 for both Igs), as compared with the condition PBMCs/PHA/MVs-1 and -2. On the contrary, a not statistically significant difference in IgA production was found between MSC and MV co-cultures (P>0.05 for both MVs preparations; Fig. 4D).
Discussion
In the present study, we isolated BM-derived MSCs and their corresponding MVs with the aim to phenotypically characterize them and to evaluate their respective immune regulatory function in vitro.
Previous studies have demonstrated the presence of MV in intercellular microenvironments and their role in cell-to-cell communication; however, many different procedures to obtain them have been described in the literature, thus generating uncertainty about their definition and biological effects according to the different preparations [19–24,32]. Since a well-defined and widely accepted procedure to isolate MVs is lacking, we focused on two previously described procedures [26,27]: the first one consists of serial ultracentrifugation steps, used to purify the preparation that we called microvesicles-1 (MVs-1), whereas the second adds a concentration step before serial ultracentrifugation, to obtain a more purified and soluble factor-enriched preparation (MVs-2). These two different procedures of MV preparation results into a greater mean protein content of MVs-2 in comparison to MVs-1.
Two main vesicle release processes have been described in the literature: MVs may derive from the endosomal membrane compartment and then be extruded from the cell surface of activated cells, after fusion with the plasma membrane; alternatively they may originate by direct budding from the cell plasma membrane [21,22,24]. To possibly identify an optimal method for the isolation of MSC-derived MVs, we characterized MVs by flow cytometry and found that the two preparations (MVs-1 and -2) were morphologically and phenotypically similar and both contained a heterogeneous population of 0.1–1 μm particles, expressing a characteristic MSC surface marker (CD13), thus indicating that they may be secreted by MSCs. Moreover, 25.8% (mean percentage calculated among 7 different HDs; range 19.3–30.4) of CD13+ MVs were found to be positive for CD107a, also known as lysosome-associated membrane protein 1 (LAMP-1), an intracellular protein normally expressed on the membrane of intracellular vesicles like endosomes or lysosomes, that can be found on the cell membrane after vesicle extrusion. The positivity for CD13 and CD107a suggests that MVs, independently from the mechanism of secretion, may represent intracellular vesicles that can be constitutively released from MSCs through membrane budding, thus maintaining MSC characteristic surface markers. Consistent with previous studies, a fraction of MVs may derive from the endosomal membrane and be extruded after fusion with the plasma membrane [22,24,32].
MSC immunoregulatory capacity is exerted through an array of different mechanisms, among which the release of soluble factors plays an important role [4,29–31]. A lot of attention has been recently paid to the release of membrane vesicles of any cell origin, which have been reported to affect the modulation of various compartments of the immune response [27]. Secreted MVs have been shown to induce activation of T cells [33], but also to inhibit natural killer [34] and B cells [35], and to impair monocyte differentiation into dendritic cells [36]. Moreover, it has been demonstrated that exosomes display the ability to promote regulatory T-cell generation [37].
MSC-derived MVs have been reported to display the capacity to reverse acute and chronic kidney injury in experimental models of ischemic renal damage and lethal toxic kidney injury [24,38–41]. Other authors have reported that MSC-derived MV infusion ameliorates reperfusion injury and reduces infarct size in a mouse model of myocardial ischemia/reperfusion injury [23,42]. MSC-derived MVs have been also shown to inhibit in vitro cell growth and survival of different tumor cell lines and to block in vivo the progression of established tumors [43]. The mechanisms by which MVs may influence the behavior of target cells are diverse: they may directly stimulate cells through surface-expressed receptors; they may transfer receptors or proteins from the cell of origin to the target cell; they may convey genetic information by horizontal transfer of mRNA and microRNA inducing functional changes in the target cell [21,22,24].
Our in vitro findings in the setting of mitogen-induced T-cell proliferation indicate that, although MVs display a sizeable antiproliferative effect on T cells, they are inferior in terms of inhibition ability compared with their corresponding MSCs. When considering CpG-induced B-cell proliferation, MSCs and MVs display in vitro a comparable ability of inhibition; by contrast, plasmacell differentiation and antibody production are significantly more impaired in the presence of MSCs, as compared with MVs. The secretion profile of pro- and anti-inflammatory cytokines measured in culture supernatants is in line with these results, indicating higher concentrations of inhibitory factors in MSC cultures as compared with MV cultures (Fig. 4A–C). This is, to the best of our knowledge, the first systematic comparison of the in vitro immunomodulatory effect of MSCs and their corresponding MVs on adaptive immunity. In the interpretation of these results, a note of caution derives from the difficulty in defining a precise comparison between widely accepted MSC/target cell ratios and MV/target cell ratios. Moreover, this study relates to a specific population of vesicles that sediments at 100,000 g, whereas the effect of other fractions of vesicles should be investigated in dedicated experiments.
According to some authors, MVs could represent a potential therapeutic tool in strategies of tissue repair, where they could be employed as a replacement for MSCs [23,24,41,44]. For example, Bruno et al. [39] demonstrated that recovery from acute kidney injury (AKI) after MSC administration may be mediated by the MVs released from MSCs that can mimic the original cells. MVs derived from human MSCs were shown to enhance animal survival in a cisplatin-induced lethal model of AKI in SCID mice, where multiple infusions of MVs are allowed to obtain better results in terms of mortality and tissue histology, as compared with a single MV infusion [38]. If MVs would display the same biological properties of the cell of origin, the development of therapeutic strategies that avoid the administration of MSCs could be foreseen, circumventing part of the safety issues related to the use of living cells, such as the risk of transformation of the cells [25,45,46]. Moreover, repeated administration of allogeneic MVs may not elicit immune responses, as they do not seem to express histocompatibility antigens [24]. It is also to be emphasized that the use of MVs could raise less regulatory issue since they are not considered ATMPs.
Despite these potential advantages, according to our findings obtained in a defined in vitro system, the use of MVs in the context of immune-mediated disorders where T cells play a major role in inducing tissue damage (ie, aGvHD occurring after allogeneic hematopoietic stem cell transplantation) could be less efficacious. MSC-derived MVs might not be able to elicit a sufficient antiproliferative effect on activated T cells and, therefore, may not induce meaningful clinical response in the majority of GvHD patients, as demonstrated with ex vivo expanded MSCs in phase I-II clinical studies [12,13]. Also, in the setting of disorders involving humoral immunity, infusion of MVs may not be the ideal substitute for MSC administration when the therapeutic aim is that of reducing the production of antibodies mediating tissue damage. Thus, in conclusion, whether the advantages of safety and ease of production compensate the less potent immunomodulatory effect of MVs in terms of clinical benefit for the patients remain to be demonstrated in proper clinical studies.
Supplementary Material
Acknowledgments
This work has been partly supported by grants from Associazione Italiana per la Ricerca sul Cancro (AIRC) IG9062 and Bando Giovani Ricercatori 2008 to MEB and by the special grant “5×1000” from AIRC to FL.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, et al. (2002). Pluripotency of mesenchymal stem cells derived from adult marrow. Nature 418:41–49 [DOI] [PubMed] [Google Scholar]
- 2.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S. and Marshak DR. (1999). Multilineage potential of adult human mesenchymal stem cells. Science 284:143–147 [DOI] [PubMed] [Google Scholar]
- 3.Deans RJ. and Moseley AB. (2000). Mesenchymal stem cells: biology and potential clinical use. Exp Hematol 28:875–884 [DOI] [PubMed] [Google Scholar]
- 4.Nauta AJ. and Fibbe WE. (2007). Immunomodulatory properties of mesenchymal stromal cells. Blood 110:3499–3506 [DOI] [PubMed] [Google Scholar]
- 5.Le Blanc K. and Mougiakakos D. (2012). Multipotent mesenchymal stromal cells and the innate immune system. Nat Rev Immunol 12:383–396 [DOI] [PubMed] [Google Scholar]
- 6.Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S. and Gianni AM. (2002). Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 99:3838–3843 [DOI] [PubMed] [Google Scholar]
- 7.Rasmusson I, Ringdén O, Sundberg B. and Le Blanc K. (2003). Mesenchymal stem cells inhibit the formation of cytotoxic T lymphocytes, but not activated cytotoxic T lymphocytes or natural killer cells. Transplantation 76:1208–1213 [DOI] [PubMed] [Google Scholar]
- 8.Maccario R, Podestà M, Moretta A, Cometa A, Comoli P, Montagna D, Daudt L, Ibatici A, Piaggio G, et al. (2005). Interaction of human mesenchymal stem cells with cells involved in alloantigen-specific immune response favours the differentiation of CD4+ T-cell subsets ex pressing regulatory/suppressive phenotype. Haematologica 90:516–525 [PubMed] [Google Scholar]
- 9.Corcione A, Benvenuto F, Ferretti E, Giunti D, Cappiello V, Cazzanti F, Risso M, Gualandi F, Mancardi GL, Pistoia V. and Uccelli A. (2006). Human mesenchymal stem cells modulate B-cell functions. Blood 107:367–372 [DOI] [PubMed] [Google Scholar]
- 10.Krampera M, Cosmi L, Angeli R, Pasini A, Liotta F, Andreini A, Santarlasci V, Mazzinghi B, Pizzolo G, et al. (2006). Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells 24:386–398 [DOI] [PubMed] [Google Scholar]
- 11.Traggiai E, Volpi S, Schena F, Gattorno M, Ferlito F, Moretta L. and Martini A. (2008). Bone marrow-derived mesenchymal stem cells induce both polyclonal expansion and differentiation of B cells isolated from healthy donors and systemic lupus erythematosus patients. Stem Cells 26:562–569 [DOI] [PubMed] [Google Scholar]
- 12.Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, Lanino E, Sundberg B, Bernardo ME, et al. (2008). Mesenchymal stem cells for treatment of steroid-resistant severe, acute graft-versus-host disease: a phase II study. Lancet 371:1579–1586 [DOI] [PubMed] [Google Scholar]
- 13.Ball LM, Bernardo ME, Roelofs H, van Tol MJ, Contoli B, Zwaginga JJ, Avanzini MA, Conforti A, Bertaina A, et al. (2013). Multiple infusions of mesenchymal stromal cells induce sustained remission in children with steroid-refractory, grade III-IV acute graft-versus-host disease. Br J Haematol 163:501–509 [DOI] [PubMed] [Google Scholar]
- 14.Ball LM, Bernardo ME, Roelofs H, Lankester A, Cometa A, Egeler RM, Locatelli F. and Fibbe WE. (2007). Cotransplantation of ex vivo expanded mesenchymal stem cells accelerates lymphocyte recovery and may reduce the risk of graft failure in haploidentical hematopoietic stem-cell transplantation. Blood 110:2764–2767 [DOI] [PubMed] [Google Scholar]
- 15.Bernardo ME, Pagliara D. and Locatelli. F. (2012). Mesenchymal stromal cell therapy: a revolution in regenerative medicine?. Bone Marrow Transplant 47:164–171 [DOI] [PubMed] [Google Scholar]
- 16.Frenette PS, Pinho S, Lucas D. and Scheiermann C. (2013). Mesenchymal stem cell: keystone of the hematopoietic stem cell niche and a stepping-stone for regenerative medicine. Annu Rev Immunol 31:285–316 [DOI] [PubMed] [Google Scholar]
- 17.Krampera M. (2011). Mesenchymal stromal cell ‘licensing’: a multistep process. Leukemia 25:1408–1414 [DOI] [PubMed] [Google Scholar]
- 18.Waterman RS, Tomchuck SL, Henkle SL. and Betancourt AM. (2010). A new mesenchymal stem cell (MSC) paradigm: polarization into a pro-inflammatory MSC1 or an immunosuppressive MSC2 phenotype. PLoS One 5:e10088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muralidharan-Chari V, Clancy JW, Sedgwick A. and D'Souza-Schorey C. (2010). Microvesicles: mediators of extracellular communication during cancer progression. J Cell Sci 123:1603–1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Théry C, Ostrowski M. and Segura E. (2009). Membrane vesicles as conveyors of immune responses. Nat Rev Immunol 9:581–593 [DOI] [PubMed] [Google Scholar]
- 21.György B, Szabó TG, Pásztói M, Pál Z, Misják P, Aradi B, László V, Pállinger E, Pap E, et al. (2011). Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell Mol Life Sci 68:2667–2688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cocucci E, Racchetti G. and Meldolesi J. (2008). Shedding microvesicles: artefacts no more. Trends Cell Biol 19:43–51 [DOI] [PubMed] [Google Scholar]
- 23.Lai RC, Chen TS. and Lim SK. (2011). Mesenchymal stem cell exosome: a novel stem cell-based therapy for cardiovascular disease. Regen Med 6:481–492 [DOI] [PubMed] [Google Scholar]
- 24.Biancone L, Bruno S, Deregibus MC, Tetta C. and Camussi G. (2012). Therapeutic potential of mesenchymal stem cell-derived microvesicles. Nephrol Dial Transplant 27:3037–3042 [DOI] [PubMed] [Google Scholar]
- 25.Bernardo ME, Zaffaroni N, Novara F, Cometa AM, Avanzini MA, Moretta A, Montagna D, Maccario R, Villa R, et al. (2007). Human bone marrow-derived mesenchymal stem cells do not undergo transformation after long-term in vitro culture and do not exhibit telomere maintenance mechanisms. Cancer Res 67:9142–9149 [DOI] [PubMed] [Google Scholar]
- 26.Muralidharan-Chari V, Clancy J, Plou C, Romao M, Chavrier P, Raposo G. and D'Souza-Schorey C. (2009). ARF6-regulated shedding of tumor cell-derived plasma membrane microvesicles. Curr Biol 19:1875–1885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Théry C, Amigorena S, Raposo G. and Clayton A. (2006). Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol Chapter 3:Unit 3.22 [DOI] [PubMed] [Google Scholar]
- 28.Capolunghi F, Cascioli S, Giorda E, Rosado MM, Plebani A, Auriti C, Seganti G, Zuntini R, Ferrari S, et al. (2008). CpG drives human transitional B cells to terminal differentiation and production of natural antibodies. J Immunol 180:800–808 [DOI] [PubMed] [Google Scholar]
- 29.Neuss S, Becher E, Woltje M, Tietze L. and Jahnen-Dechent W. (2004). Functional expression of HGF and HGF receptor/c-met in adult human mesenchymal stem cells suggest a role in cell mobilization, tissue repair, and wound healing. Stem Cells 22:405–414 [DOI] [PubMed] [Google Scholar]
- 30.Solchaga LA. and Zale EA. (2012). Prostaglandin E2: a putative potency indicator of the immunosuppressive activity of human mesenchymal stem cells. Am J Stem Cells 1:138–145 [PMC free article] [PubMed] [Google Scholar]
- 31.Gieseke F, Böhringer J, Bussolari R, Dominici M, Handgretinger R. and Müller I. (2010). Human multipotent mesenchymal stromal cells use galectin-1 to inhibit immune effector cells. Blood 116:3770–3779 [DOI] [PubMed] [Google Scholar]
- 32.Camussi G, Deregibus MC, Bruno S, Cantaluppi V. and Biancone L. (2010). Exosomes/microvesicles as a mechanism of cell-to-cell communication. Kidney Int 78:838–848 [DOI] [PubMed] [Google Scholar]
- 33.Théry C, Duban L, Segura E, Véron P, Lantz O. and Amigorena S. (2002). Indirect activation of naïve CD4+ T cells by dendritic cell-derived exosomes. Nat Immunol 3:1156–1162 [DOI] [PubMed] [Google Scholar]
- 34.Liu C, Yu S, Zinn K, Wang J, Zhang L, Jia Y, Kappes JC, Barnes S, Kimberly RP, Grizzle WE. and Zhang HG. (2006). Murine mammary carcinoma exosomes promote tumor growth by suppression of NK cell function. J Immunol 176:1375–1385 [DOI] [PubMed] [Google Scholar]
- 35.Koppler B, Cohen C, Schlöndorff D. and Mack M. (2006). Differential mechanisms of microparticle transfer to B cells and monocytes: anti-inflammatory properties of microparticles. Eur J Immunol 36:648–660 [DOI] [PubMed] [Google Scholar]
- 36.Yu s, Liu C, Su K, Wang J, Liu Y, Zhang L, Li C, Cong Y, Kimberly R, et al. (2007). Tumor exosomes inhibit differentiation of bone marrow dendritic cells. J Immunol 178:6867–6875 [DOI] [PubMed] [Google Scholar]
- 37.Wang GJ, Liu Y, Qin A, Shah SV, Deng ZB, Xiang X, Cheng Z, Liu C, Wang J, et al. Thymus exosomes-like particles induce regulatory T cells. J Immunol 181:5242–5248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bruno S, Grange C, Collino F, Deregibus MC, Cantaluppi V, Biancone L, Tetta C. and Camussi G. (2012). Microvesicles derived from mesenchymal stem cells enhance survival in a lethal model of acute kidney injury. PLoS One 7:e33115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bruno S, Grange C, Deregibus MC, Calogero RA, Saviozzi S, Collino F, Morando L, Busca A, Falda M, et al. (2009). Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J Am Soc Nephrol 20:1053–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gatti S, Bruno S, Deregibus MC, Sordi A, Cantaluppi V, Tetta C. and Camussi G. (2011). Microvesicles derived from human adult mesenchymal stem cells protect against ischaemia-reperfusion-induced acute and chronic kidney injury. Nephrol Dial Transplant 26:1474–1483 [DOI] [PubMed] [Google Scholar]
- 41.Morigi M. and Benigni A. (2013). Mesenchymal stem cells and kidney repair. Nephrol Dial Transplant 28:788–793 [DOI] [PubMed] [Google Scholar]
- 42.Lai RC, Yeo RW, Tan KH. and Lim SK. (2013). Mesenchymal stem cell exosome ameliorates reperfusion injury through proteomic complementation. Regen Med 8:197–209 [DOI] [PubMed] [Google Scholar]
- 43.Bruno S, Collino F, Deregibus MC, Grange C, Tetta C. and Camussi G. (2013). Microvesicles derived from human bone marrow mesenchymal stem cells inhibit tumor growth. Stem Cells Dev 22:758–771 [DOI] [PubMed] [Google Scholar]
- 44.Bruno S. and Camussi G. (2013). Role of mesenchymal stem cell-derived microvesicles in tissue repair. Pediatr Nephrol 28:2249–2254 [DOI] [PubMed] [Google Scholar]
- 45.Tarte K, Gaillard J, Lataillade JJ, Fouillard L, Becker M, Mossafa H, Tchirkov A, Rouard H, Henry C, et al. (2010). Clinical-grade production of human mesenchymal stromal cells: occurrence of aneuploidy without transformation. Blood 115:1549–1553 [DOI] [PubMed] [Google Scholar]
- 46.Ben-David U, Mayshar Y. and Benvenisty N. (2011). Large-scale analysis reveals acquisition of lineage-specific chromosomal aberrations in human adult stem cells. Cell Stem Cell 9:97–102 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.