Abstract
Thousands of odors are sensed and discriminated by G protein-coupled odorant receptors (ORs) expressed in olfactory sensory neurons (OSNs). G protein-coupled receptor kinases (GRKs) may have a role in desensitization of ORs. However, whether ORs are susceptible to agonist-dependent desensitization and whether GRKs affect odorant responsiveness of OSNs are currently unknown. Here we show that GRK3 attenuated the agonist responsiveness of a specific mouse odorant receptor for eugenol (mOR-EG) upon agonist pretreatment in HEK293 cells, but GRK3 did not affect the response amplitude or the recovery kinetics upon repeated agonist stimulation. We performed electrophysiological recordings of single OSNs which expressed mOR-EG and green fluorescent protein (GFP) in the presence or absence of GRK3. The kinetics and amplitude of agonist responsiveness of individual GFP-labeled mOR-EG neurons were not significantly affected by the absence of GRK3. These results indicate that the role of GRK3 in attenuating ORs responsiveness in OSNs may have been overestimated.
Key words: calcium imaging, cAMP assay, electrophysiology, GRK3, odorant receptor
Introduction
The vertebrate olfactory system can recognize and discriminate among thousands of structurally diverse odorants. A molecular basis for the detection of and discrimination among natural odorants was provided by the discovery of the odorant receptor (OR) gene superfamily, which comprises thousands of genes encoding rhodopsin-like G protein-coupled receptors (GPCRs) (Buck and Axel 1991). The first step in olfactory signal transduction is the activation of G protein Gαolf by odorant-bound activated ORs. The activated Gαolf in turn stimulates adenylyl cyclase III (ACIII), resulting in an increase in intracellular cAMP, which then interacts with and opens a cyclic nucleotide-gated (CNG) channel; the resulting cation influx leads to depolarization of the olfactory sensory neuron (OSN) (Mombaerts 2004; Kleene 2008; Touhara and Vosshall 2009).
Most non-OR GPCRs rapidly lose responsiveness in the continued (or recurring) presence of an agonist or stimulus (Ferguson 2001). This process, termed desensitization, is the consequence of a combination of multiple mechanisms. These mechanisms include 1) the uncoupling of the receptor from heterotrimeric G protein following receptor phosphorylation (Ohguro et al. 1995; Lee et al. 2000; Liggett 2002), 2) the internalization of cell surface receptors into cytosolic vesicles (Tsuga et al. 1998; Matharu et al. 2001), and 3) the down-regulation of the total receptor amount due to reduced receptor mRNA and protein synthesis and to increased degradation of pre-existing receptors in lysosomes and the plasma membrane (Hausdorff et al. 1992; Tsuga et al. 1998). The uncoupling of GPCRs from G proteins results mainly from phosphorylation of receptors by intracellular kinases. Second messenger-dependent protein kinases—including cAMP-dependent protein kinase (PKA) and Ca2+-dependent protein kinase (PKC)—and GPCR kinases (GRKs) phosphorylate serine and threonine residues within the intracellular loops and C-terminal region of GPCRs (Ferguson 2001; Reiter and Lefkowitz 2006; Shukla et al. 2011). GRKs selectively phosphorylate agonist-activated receptors, thereby promoting the binding of cytosolic cofactor proteins called arrestins which then sterically uncouple the receptor from the G protein (Shukla et al. 2011).
Although there have been multiple studies on the roles of GRKs and arrestins in the regulation of some GPCRs such as adrenergic receptor, little is known about the precise mechanisms responsible for desensitization of ORs. Reportedly, G protein coupled receptor kinase 3 (GRK3), also known as β-adrenergic receptor kinase 2, and β-arrestin 2 are highly enriched in OSNs, and preincubation with antibodies to GRK3 and β-arrestin 2 resulted in an elevation of cAMP response in the presence of odorant (Dawson et al. 1993). Moreover, GRK3 is translocated from the cytosol to the cell membrane upon odorant stimulation (Boekhoff et al. 1994). Cilia preparations derived from GRK3-deficient mice lack the rapid agonist-induced desensitization normally observed following odorant stimulation (Peppel et al. 1997). These findings indicate that phosphorylation of ORs by GRK3 might be involved in the mechanisms responsible for desensitization of ORs. In addition, human OR17-4 and OR2AG1 are targeted for phosphorylation by PKA and β-arrestin-mediated internalization (Mashukova et al. 2006; Neuhaus et al. 2006).
Here, we aimed to elucidate the mechanisms underlying agonist-dependent desensitization of a specific mouse OR and its contribution to response amplitude and kinetics. We used a mouse OR whose cognate ligand is a spicy odor, eugenol (EG), as a model; we chose this particular OR, mOR-EG, because it has been well characterized both in vitro and in vivo (Kajiya et al. 2001; Katada et al. 2004; Oka et al. 2004; Katada et al. 2005; Oka et al. 2006). Using mammalian cultured cells, we examined whether mOR-EG exhibited agonist-dependent desensitization. In addition, we used GRK3 knock-out mice in which mOR-EG-expressing neurons were tagged with green fluorescent protein (GFP) to assess the role of GRK3 in mOR-EG-expressing neurons.
Materials and methods
Materials and reagents
Odorants and isoproterenol hydrochloride (Iso) were purchased from Nacalai Tesque or Tokyo Chemical Industry. Olfr73, Olfr16, Olfr510, and Olfr151 genes each encode a mouse OR—mOR-EG (Kajiya et al. 2001), MOR23, MOR204-34, and M71, respectively.
Cell culture
HEK293 cells were grown in Dulbecco’s modified Eagle’s medium (Nacalai Tesque) supplemented with 10% fetal bovine serum (JRH Bioscience). Cells were cultured at 37°C under a humidified atmosphere containing 5% CO2. Cell cultures were split every 2 days before becoming confluent. Cells were discarded after 2 months of continuous growth and splitting; new cell cultures were started from a frozen stock every 2 months.
cAMP assay
Transfection of HEK293 cells and the cAMP assay in these cells were conducted as described earlier (Katada et al. 2003). In brief, HEK293 cultures which were 60–70% confluent in wells of 24-well plates were transfected with 0.7 μg of receptor cDNA using 1.75 μL of Lipofectamine 2000 (Invitrogen). Transfected cells were then cultured for 24h and further incubated for 30min with 1mM 3-isobutyl-1-methylxanthine (IBMX, Sigma). These cells were then exposed to 1mM EG solution containing 1mM IBMX for 15min. The cAMP levels were determined using a cAMP-ScreenTM system (Applied Biosystems) according to the manufacturer’s instructions.
For pretreatment assays with EG (the mOR-EG agonist) or Iso (the β2AR agonist), HEK293 cells transfected with mOR-EG were incubated with 50 μM EG, 50 μM allyl anisole (AA), or 50nM Iso for 30min. For pretreatment assays with protein kinase inhibitors, HEK293 cells transfected with mOR-EG were incubated with 1mM Staurosporine (Sigma), and then exposed to 1mM EG solution containing 1mM IBMX for 15min.
Flow cytometry
HEK293 cultures which were 60–70% confluent in a 10-cm dish were transfected with 8 μg of the receptor cDNA using 2.5 μL Lipofectamine 2000/μg DNA (Invitrogen). Transfected cells were cultured for 24h. Cells were then washed twice with PBS and stripped off each dish with PBS containing 5mM EDTA. Cells were collected and filtered through a nylon mesh. Filtered cells were then labeled with anti-Flag IgG and Alexa Fluor-488 antimouse IgG and applied to a FACS Calibur instrument (Becton–Dickinson). CELLQUEST software (Becton–Dickinson) was used to generate each histogram.
Calcium imaging
HEK293 cultures which were 50–60% confluent in a 35-mm glass-bottomed dish precoated with 100 μg/mL of poly-D-lysine were transfected with 2.5 μg of pME18S-Flag-Rho-mOR-EG and 1.8 μg of pME18S-Gα15 together with or without 0.5 μg of pME18S-GRK3 using lipofectamine 2000 (2.5 μL/μg DNA); pME18S was cotransfected to adjust the total amount of transfected DNA in each culture to 4.8 μg. Cells were cultured for 24h after transfection, and then loaded with 2.5 μM fura-2 AM for 30min at 37°C. EG solutions were repeatedly applied to cultures for 30 s separated by 2-min intervals; all solutions moved via a peristaltic pump at a flow rate of 1.5mL/min. For pretreatment experiments, cells were incubated with 50 μM EG or 50 μM AA for 30min prior to stimulation with 1mM EG. AQUA COSMOS (Hamamatsu Photonics, Shizuoka, Japan) was used to measure Fura-2/AM fluorescence at 510nm following excitation with 340 or 380nm; this fluorescence was a measure of intracellular Ca2+ levels.
Generation of mOR-EG-ires-GFP/GRK3–/– mice
GRK3–/– mice (Peppel et al. 1997) were kindly provided by Dr Robert J. Lefkowitz (Duke University). mOR-EG-ires-GFP, GRK3-/- mice were generated by crossing GRK3-/- mice with mOR-EG-ires-GFP described earlier (Oka et al. 2006). Mice were genotyped using the following two primer sets for GRK3 alleles. One primer set were 5′-TAAGGTTTTCAGAGTAGAGGGACAGT-3′, and 5′-A GAGGTAGGAGCATATCTCTGAGTCT-3′. This primer set amplified 850bp containing a part of exon b sequence in the GRK3 gene which was removed in the knockout allele. Another primer set were 5′-TCCTGAGT CACAGGTACAGGGGTTG-3′, and 5′-CACACGCTC AGCAGGATTCCTACAC-3′. These primers are located outside the 2.4kb BamHI fragment (Peppel et al. 1997) which has been replaced with the neomycin cassette (1.9 kbp). The 2.6-kbp product was amplified from intact GRK3 allele, whereas 2.1 kbp was amplified from KO mice. The presence of neomycin cassette was checked using the following primer set: 5′-AGGATCTCGTCGTGACCCATGG-3′ and 5′-GAAGAACTCGTCAAGAAGGCG-3′.
Electrophysiology of OSNs
All electrophysiological studies involving mice were performed according to protocols approved by the Monell Chemical Senses Center Institutional Animal Care and Use Committee. Single-cell suction recordings (Lowe and Gold 1991) were performed as described (Ponissery Saidu et al. 2012). The cell body of an isolated GFP-positive OSN was sucked into the tip of a recording pipette, leaving the cilia and the dendritic knob accessible for solution changes. The recorded signals were filtered at DC-50 Hz and sampled at 10kHz using a Cambridge Electronic Design (CED) acquisition board and Signal software (CED). Experiments were performed at 37°C. Rapid solution exchanges were achieved by transferring the tip of the recording pipette across the interface of neighboring streams of solution using the Perfusion Fast-Step SF-77B solution changer (Warner Instruments).
Results
Agonist-dependent desensitization of mOR-EG in HEK293 cells
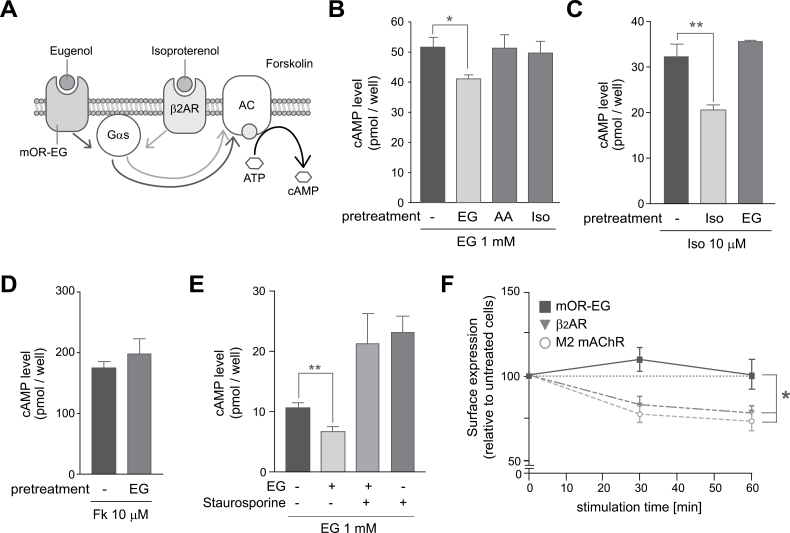
To examine whether signaling via a mouse OR is susceptible to the agonist-dependent desensitization, we used HEK293 cells to assess the effect of agonist pretreatment on agonist-induced intracellular cAMP accumulation which is mediated by Gαs and adenylyl cyclase (Figure 1A). Pretreatment of mOR-EG-expressing HEK293 cells with 50 μM EG for 30min resulted in 20±2.8% reduction of the cAMP response to EG, but pretreatment with a nonligand, 4- AA (50 μM), or a β2AR agonist, isoproterenol (Iso) (50nM) did not attenuate the response to EG (Figure 1B). Pretreatment of HEK293 cells with Iso resulted in 37±3.5% reduction of the cAMP responsiveness of β2AR to Iso, whereas pretreatment with EG did not attenuate this response (Figure 1C). Pretreatment of mOR-EG-expressing HEK293 cells with EG did not affect the forskolin-induced cAMP increase, suggesting that adenylyl cyclase was not desensitized by EG pretreatment (Figure 1D). When mOR-EG-expressing HEK293 cells were exposed to staurosporine (a potent inhibitor of multiple kinases) before EG pretreatment, EG-dependent mOR-EG desensitization was diminished (Figure 1E). These results indicated that mOR-EG was desensitized by some endogenous kinase(s) in an agonist-dependent manner in HEK293 cells.
Figure 1.
Agonist-dependent desensitization of mOR-EG. (A) Gαs-cAMP pathway in HEK293 cells. EG and isoproterenol activate the cAMP-pathway via mOR-EG and β2-adrenergic receptor (AR), respectively. Forskolin (Fk) is an adenylyl cyclase stimulant. (B) Agonist-dependent desensitization of mOR-EG. mOR-EG-expressing HEK293 cells pretreated with 50 μM EG, 50 μM 4- AA, or 50nM isoproterenol (Iso) for 30min were stimulated with 1mM EG for 15min, and were then subjected to the cAMP assay (±SEM, n = 3, Student’s t-test, *P < 0.05). (C) Agonist-dependent desensitization of β2AR. HEK293 cells pretreated with 50nM of Iso or 50 μM of EG for 30min were stimulated with 10 μM Iso for 15min and were then subjected to the cAMP assay (±SEM, n = 3–4, Student’s t-test, **P < 0.01). (D) Fk-induced cAMP increases after pretreatment of EG. mOR-EG-expressing HEK293 cells pretreated with 50 μM EG for 30min were stimulated with 10 μM Fk for 15min (±SEM, n = 3). (E) Effect of the kinase inhibitor staurosporine on agonist-dependent desensitization of mOR-EG. mOR-EG-expressing HEK293 cells pretreated with 50 μM EG for 30min were stimulated with 10 μM Fk for 15min and were subjected to the cAMP assay (±SEM, n = 3, Student’s t-test, **P < 0.01). (F) Quantification of the amount of receptors on the cell surface after agonist stimulation by flow cytometric analysis. HEK293 cells expressing a Flag-tagged OR—mOR-EG, M2 mAChR, or β2AR —were stimulated with EG (50 μM), carbachol (1mM), or Iso (1mM), respectively, for 30min or 60min. Then, cells were labeled with anti-Flag monoclonal antibodies and analyzed by FACS. The cells with high level of fluorescence were counted, and fluorescence of these cells was normalized relative to fluorescence of untreated cells (±SEM, n = 3–7, Student’s t-test, *P < 0.05).
mOR-EG is not internalized into HEK293 cells following agonist treatment
To determine whether the attenuation of mOR-EG signaling was due to internalization of mOR-EG, we used a flow-cytometric analysis to measure the amount of cell-surface mOR-EG. A 60-min agonist treatment of HEK293 cells expressing M2-type muscarinic acetylcholine receptor (M2 mAChR) or β2AR caused a 73±5.5% or 78±3.8%, respectively, reduction in the amount of the respective receptor on the cell surface, suggesting that the agonist treatment cause receptor internalization. In contrast, after a 30- or 60-min agonist treatment, the amount of mOR-EG on the cell surface appeared to be unaltered (Figure 1F). These results indicated that the decrease in the responsiveness of mOR-EG after agonist treatment was not associated with receptor internalization in HEK293 cells.
Effect of GRK3 overexpression on desensitization of mOR-EG in HEK293 cells
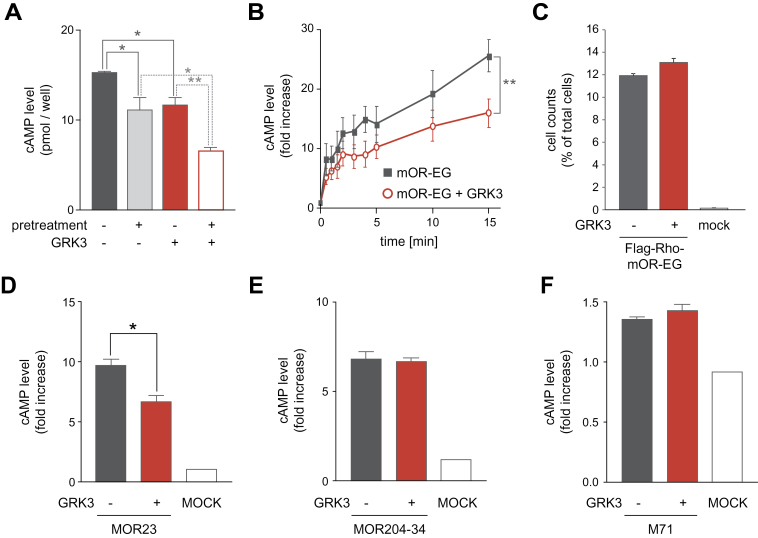
GRK3, a member of the GRK family, is reportedly highly enriched in OSNs (Dawson et al., 1993), and GRK3 is translocated from cytosol to the cell membrane upon odorant stimulation (Boekhoff et al. 1994). Furthermore, olfactory neural cilia preparations derived from GRK3–/– mice lack rapid odorant-induced desensitization, suggesting that GRK3 may play a role in OR desensitization (Peppel et al. 1997). Therefore, we examined the effect of overexpression of GRK3 on EG-responsiveness of mOR-EG in HEK293 cells. Coexpression of GRK3 with mOR-EG resulted in a 24±12% reduction of cAMP response to EG (Figure 2A). Time course analysis revealed that the effect of GRK3 overexpression was significant after 15min EG exposure (Figure 2B). Furthermore, the GRK3-overexpressing HEK293 cells showed an enhanced reduction in EG-responsiveness of mOR-EG following EG pretreatment (Figure 2A). The amount of mOR-EG on the cell surface did not differ significantly between cells transfected with and cells non-transfected with GRK3 (Figure 2C). These results indicated that GRK3 desensitized mOR-EG in an agonist-dependent manner in HEK293 cells without causing mOR-EG to undergo receptor internalization.
Figure 2.
Effect of GRK3 overexpression on desensitization of ORs. (A) Agonist-dependent desensitization of mOR-EG in the presence or absence of transfected GRK3. HEK293 cells expressing mOR-EG alone (black) or mOR-EG with GRK3 (red) were pretreated with 50 μM EG for 30min and then stimulated with 1mM EG for 15min and were subjected to the cAMP assay (±SEM, n = 3–4, Student’s t-test, *P < 0.05, **P < 0.01). (B) Time course of cAMP production in HEK293 cells expressing mOR-EG alone (black) or mOR-EG with GRK3 (red). The cells were stimulated with 1mM EG for 0, 0.5, 1.0, 1.5, 2.0, 3.0, 4.0, 5.0, 10, or 15min (±SEM, n = 3, Paired t-test, **P < 0.01). (C) Quantification of the amount of mOR-EG on the cell surface by flow cytometric analysis. HEK293 cells expressing Flag-tagged mOR-EG alone (black) or Flag-mOR-EG with GRK3 (red) were labeled with anti-Flag monoclonal antibodies and analyzed by FACS. The cells with high level of fluorescence were counted (±SEM, n = 3). (D-F) Effects of GRK3 on agonist-dependent cAMP responses of 3 different ORs. HEK293 cells transfected with MOR23 (D), MOR204-34 (E), or M71 (F) alone (black) or with GRK3 (red) were stimulated with 1mM lyral, methyl isoeugenol or acetophenone, respectively, for 15min (±SEM, n = 3, Student’s t-test, *P < 0.05).
Some ORs do not exhibit GRK3-mediated desensitization
We next assessed the effect of GRK3 on agonist-induced signaling of three other ORs. MOR23, MOR204-34, and M71 receptors were transiently expressed with GRK3 in HEK293 cells, and cell responsiveness to the respective odorants was analyzed. Lyral-induced cAMP levels were 31±6.2% lower in cells expressing both GRK3 and MOR23 than in those expressing only MOR23 (Figure 2D), but agonist-induced cAMP levels did not differ between cells expressing GRK3 and MOR204-34 or M71 and those expressing only MOR204-34 or M71, respectively (Figure 2E,F). These results indicated that the molecular mechanisms underlying OR desensitization differ among ORs.
Desensitization of mOR-EG in a Ca2+ response assay in HEK293 cells
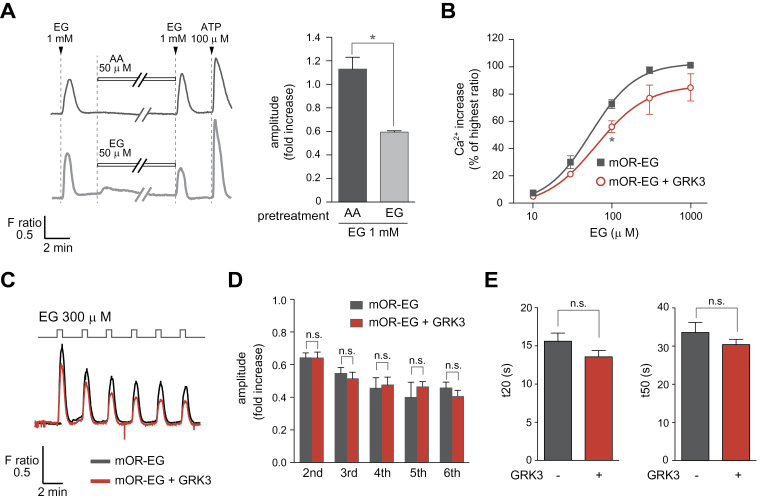
We next used Ca2+ imaging to examine desensitization of mOR-EG following EG pretreatment. In the presence of Gα15, a promiscuous G protein, a transient Ca2+ increase was observed upon EG stimulation of mOR-EG-expressing HEK293 cells. A 30-min pretreatment of mOR-EG/Gα15-expressing HEK293 cells with EG resulted in 54±5.3% decrease in the calcium response amplitude to EG compared with that observed in the cells pretreated with AA, a nonagonist for mOR-EG (Figure 3A).
Figure 3.
Desensitization of mOR-EG assessed via the Ca2+ response. (A) Agonist-dependent decreases in mOR-EG-mediated Ca2+ response in HEK293 cells. HEK293 cells were stimulated with 1mM EG for 30 s before and after treatment with 50 μM 4- AA (left top) or EG (left bottom) for 30min, followed by stimulation with 100 μM of ATP. The average ratio increase in the Ca2+ response of the second EG stimulation was normalized with that of the first agonist stimulation (right) (±SEM, n = 3, Student’s t-test, *P < 0.05). (B) Dose-dependent Ca2+ responses of HEK293 cells expressing mOR-EG alone (black) or mOR-EG with GRK3 (red). The dose-response curve is shown; the response is represented as the percentage of the highest ratio induced by 1mM EG (±SEM, n = 4, Student’s t-test, *P < 0.05). (C–D) Effects of GRK3 on repeated agonist stimulation. HEK293 cells expressing mOR-EG alone (black) or mOR-EG with GRK3 (red) were stimulated with 300 μM EG for 30 s; a 300-μM 30 s EG stimulations were repeated 5 more times at intervals of 2min (C). The average ratio increases of the 2nd to 6th response were normalized to the 1st response (±SEM, n = 3–4) (D). (E) Effects of GRK3 on recovery time after agonist stimulation. HEK293 cells expressing mOR-EG alone (black) or mOR-EG with GRK3 (red) were stimulated with 300 μM EG for 2min and 2 recovery times, t20 and t50 (the time it takes for the Ca2+ response to fall to 20% or 50% of the peak value, respectively), were determined for the profile of averaged response (±SEM, n = 18, Student’s t-test, P = 0.138 for t20, P = 0.298 for t50).
We also examined the effect of GRK3-overexpression on Ca2+ responses. Based on the dose–response curves, the Ca2+ increase was lower in cells expressing GRK3 and mOR-EG than in those expressing only mOR-EG (Figure 3B). We then used the Ca2+ assay to determine whether the desensitization was observed when repeated EG stimulation was applied to mOR-EG in the presence of GRK3. It turns out that we did not see any enhanced reduction of the Ca2+ increases in GRK3-overexpressing cells when the cells were stimulated with EG for 6 times with a 2-min interval (Figure 3C,D). To determine whether the recovery kinetics were affected by GRK3 overexpression, we measured the time required for a 20% or a 50% decrease from the maximal response (t20 and t50, respectively); t20 and t50 values for cells expressing mOR-EG or for cells expressing both GRK3 and mOR-EG were not significantly different (Figure 3E).
Together, a modest decrease in the amplitude of mOR-EG response was observed in GRK3-expressing HEK293 cells, but GRK3 overexpression did not have significant effects on recovery time or response kinetics.
Response properties of mOR-EG-expressing OSNs that lack GRK3
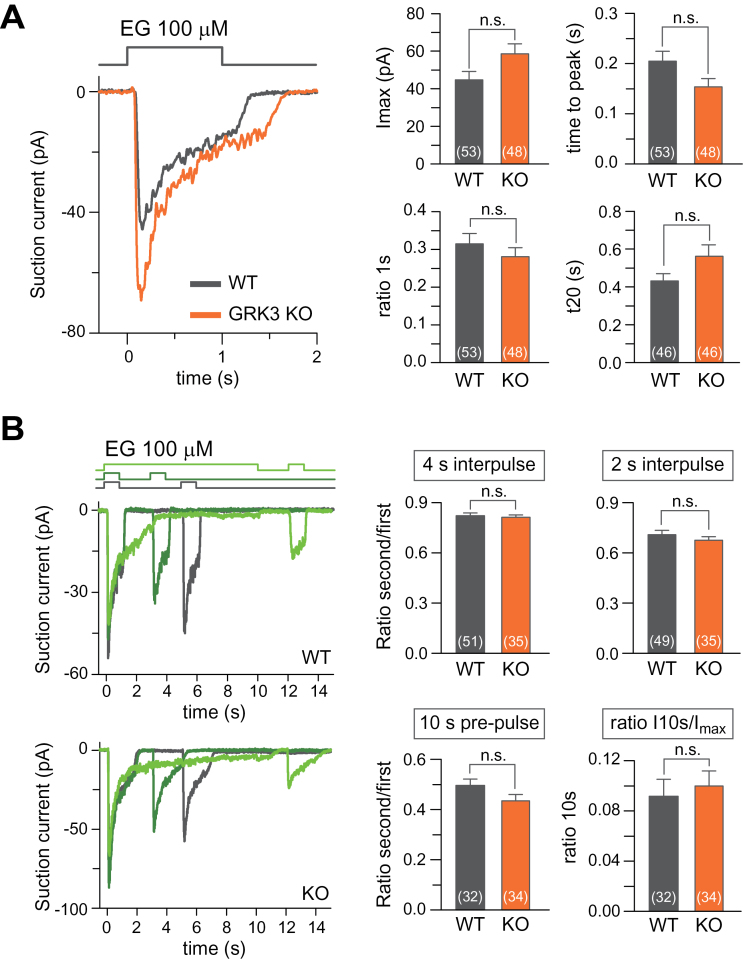
To examine a role of GRK3 in mOR-EG signaling in OSNs, we generated mice in which mOR-EG-expressing neurons were tagged by GFP and lacked GRK3 by crossing GRK3–/– mice with mOR-EG-ires-GFP transgenic mice (Oka et al. 2006). Mice were genotyped by PCR using two primer sets for GRK3 alleles (Supplementary Figure 1). We used the suction pipette technique to record electrophysiological responses from single GFP-positive neurons. OSNs from wild-type and GRK3–/– mice that expressed mOR-EG-GFP were exposed to 100 µM EG for 1 s (Figure 4A). Regardless of genotype, each OSN showed a fast increase in current with the response settling to a lower level following the peak current during the 1 s stimulation. The averaged maximally evoked current was slightly larger in the GRK3–/– OSNs than in wild-type OSNs, but this difference was not significant (P = 0.051, t-test). A possible manifestation of GRK3 could be a faster decline of the current after reaching its peak due to increased mOR-EG desensitization in wild-type compared with GRK3–/– OSN. No such difference, however, was evident. The ratio between the maximal current and the current after 1 s (Figure 4A ratio 1 s) did not differ significantly between wild-type and GRK3–/– OSNs (see also the following section for results during a 10-s odorant exposure). The response kinetics for wild-type and GRK3–/– OSNs remained similar for the responses to reach their maxima (time to peak), as well as the time required for the response to decline to 20% of its value measured at the end of 1 s stimulation (t20).
Figure 4.
Response properties of mOR-EG-expressing OSNs which lack GRK3. Odorant responses were recorded from GFP-positive OSNs using the suction pipette technique. (A) Left panel: Response of wild-type (black) or GRK3–/– (orange) OSNs to 100 µM EG. Right panels plot the averages of the maximal current (I max), the ratio of the current at 1s normalized to the peak current (ratio 1s), the time required of the maximal current to peak from the onset of stimulation (time to peak) and the time it takes for the current to fall to 20% of its value measured at the end of stimulation at 1 s (t20). (B) Repeated stimulation of wild-type and GRK3–/– OSNs. OSNs were exposed twice to 100 µM EG as indicated by the solution monitors at the top. Right panels: The response of the second exposure was normalized to the first for each OSN for the 4 s and 2 s interpulse periods and also when the first odorant exposure lasted 10 s (10 s prepulse). “Ratio I10s/ I max” is a measure of the decline of the response during the 10-s prepulse. Plotted are mean ± SEM, numbers in parenthesis are OSNs recorded from, “n.s.” not significant.
We used a double pulse protocol to examine whether GRK3-mediated OR desensitization led to an adaptive change in odorant-induced responses during repeated stimulation. OSNs were exposed to EG for 1 s and re-exposed after an interpulse period of either 2 s or 4 s (Figure 4B, dark green and black traces). Responses to the second stimulation were smaller than the responses to the first stimulation, but wild-type and GRK3–/– OSNs did not differ significantly in the level of adaptation with either interpulse period. The wild-type and GRK3–/– levels of adaptation during the response to the second stimulation are represented in Figure 4B (right panels); specifically, the maximal current response of the second response was normalized to the first response for each genotype. To increase the amount of time allowed for agonist-induced GRK3-mediated phosphorylation to occur, we pre-exposed OSNs to EG for 10 s before stimulating them again with a pulse of EG after an interpulse period of 2 s (Figure 4B, light green). OSN responses declined to similar levels during the 10 s EG exposures (see Figure 4B right panel, “ratio I10s/I max”) regardless of genotype. In addition, the responses to the second exposure did not differ significantly between wild-type and GRK3–/– OSNs.
Discussion
It has been proposed that GRK3 is involved in agonist-dependent phosphorylation and desensitization of ORs in OSNs (Peppel et al. 1997). However, the evidence supporting this hypothesis derives mainly from biochemical measurement of second messengers in olfactory cilia preparations in vitro; therefore, the role of GRK3 in OR desensitization in vivo remained unclear. Here, we describe the first study that successfully examined GRK3-mediated OR desensitization in a mammalian cell line and the in vivo role of GRK3 in OR desensitization by focusing on mOR-EG in individual OSNs. We demonstrated a decrease in the response amplitude of a particular OR in HEK293 cells upon agonist pretreatment or upon overexpression of GRK3, although interestingly these effects were not applicable to all ORs. However, repeated agonist-stimulation did not result in clear OR desensitization; moreover, GRK3 overexpression did not affect recovery kinetics, suggesting that GRK3 may not be involved in a rapid desensitization of mOR-EG or other ORs. Consistently, the electrical recording showed no significant difference between receptor-targeted OSNs with GRK3 and those without GRK3; more specifically, neither OR response kinetics nor current amplitudes differed significantly between OSN genotypes. Therefore, these finding indicated that the in vivo contribution of GRK3 in attenuating OR responsiveness in OSNs might have been overestimated based on in vitro approaches.
Generally, two specific physiological phenomena are measured to determine whether receptor desensitization is occurring. One is the kinetic time course of recovery to basal levels after a maximal response, and the other is the maximal amplitude of a response to an agonist as an agonist is applied continuously. Treatment of olfactory cilia preparations with anti-GRK3 antibodies or GRK3 peptide inhibitor causes a loss of odorant-induced rapid decrease in cAMP levels (Dawson et al. 1993; Boekhoff et al. 1994). These findings are further supported by genetic GRK3 knock-out studies in which olfactory cilia preparations from GRK3-null mutant mice did not exhibit the rapid decrease in the cAMP response normally observed in preparations from wild-type mice (Peppel et al. 1997). These results led to the proposal that GRK3 affects response kinetics of ORs in OSNs. Here, we used response assays to measure the kinetics of OR responses to a cognate ligand in HEK293 cells and in single intact OSNs from GRK3–/– or wild-type mice. Our results showed recovery time (t20) tended to be shorter in GRK3-overexpressing HEK293 cells than in control cells and that recovery time (t20) for neural response to agonists tended to be longer in GRK3–/– mice than in wild-type mice, but neither effect was as dramatic as was expected from earlier studies. This discrepancy between our findings and those of previous studies may result from the difference in experimental strategies; specifically, we recorded Ca2+ or electrophysiological responses, but second messenger levels were measured in earlier studies. Nevertheless, GRK3 may not significantly contribute to a rapid desensitization and response termination after a maximal response.
Findings from earlier studies of response amplitude are somewhat contradictory. Boekhoff et al. (1994) found that the maximal cAMP response is not altered in the presence or absence of GRK3 peptide inhibitor; in contrast, Dawson et al. (1993) found an increase in the maximal response in the presence of anti-GRK3 antibodies. Notably, Peppel et al. (1997) found that the maximal rise in cAMP level generated in isolated cilia preparations upon odorant stimulation is markedly lower in preparations from GRK3–/– mice than in those from wild-type mice. Thus, the effects of GRK3 on OR-mediated response amplitudes are unclear. Using two assays (cAMP and Ca2+ assays), we found that GRK3 did affect response amplitude, but no desensitization was observed upon repeated agonist-mediated stimulation. In OSNs, the amplitude (I max) of agonists-induced responses tended to be larger in GRK3–/– mice than in wild-type mice; this finding was reasonable if we assumed that GRK3 was involved in agonist-dependent phosphorylation and desensitization of ORs. However, the differences between genotypes were not significant. Overall, these findings indicated that the effect of GRK3 on receptor efficacy may have been overestimated or that other factors may function in parallel to GRK3 in OR desensitization in OSNs; alternatively, GRK3 may not function in OR desensitization in OSNs.
Some groups have suggested that PKA, like GRK3, may be involved in desensitization of ORs (Mashukova et al. 2006). These researchers found that two human ORs, OR17-4 and OR2AG1, are targets of PKA-catalyzed phosphorylation. Here, we found that some ORs including mOR-EG and MOR23 were desensitized by GRK3 in the heterologous HEK293 cells, but that other ORs such as MOR204-3 and M71 were not. Therefore, mechanisms of desensitization may differ among ORs. In addition, Mashukova et al. (2006) showed that β-arrestin mediates internalization of ORs; notably, other GPCRs also undergo ligand-dependent, arrestin-mediated internalization that results in a decrease in the amount of receptor on the cell surface. However, the flow cytometric analysis described here revealed that the decrease in mOR-EG-mediated responsiveness of HEK293 cells was not associated with mOR-EG internalization. Ligand-dependent OR internalization may be unsuited for the olfactory system because olfactory responses must adapt quickly to detect constant changes in the odor environment. Taken together, current and earlier findings indicate that several different kinases may be responsible for phosphorylation-dependent OR desensitization and that particular ORs (e.g., mOR-EG) may require a specific kinase and have particular subcellular dynamics upon ligand-dependent desensitization. This hypothesis could partially explain our observation that there are some odorants which undergoes quick desensitization and other odorants which take more time for adaptation (Stuck et al. 2014).
OSNs have some mechanisms for negative feedback regulation of responses to odorants. The CNG channel-dependent rise in Ca2+ concentration mediates inactivation of phophodiesterases and CNG channels (Yan et al. 1995; Zufall and Leinders-Zufall 2000; Kato and Touhara 2009). Ca2+ and the calcium-binding protein, calmodulin, bind to and close CNG channels, which is thought to affect response termination (Song et al. 2008). ACIII may also be a target of negative feedback regulation. However, Ca2+-induced phosphorylation of ACIII reportedly does not cause neural adaptation (Cygnar et al. 2012). Because ORs are the beginning of signal transduction in OSNs, phosphorylation and desensitization of ORs has been thought to play a role in the termination of odorant responses. Our present data, however, indicated that the role of GRK3 is not as significant for odorant responsiveness in OSNs as has been imagined, at least not for all ORs. Our observations are somewhat consistent with earlier findings that GRK3 knock-out mice did not have any deficit in their responses to odorants (Peppel et al. 1997).
Arguably, the best investigated example of GPCR desensitization to sensory transduction is desensitization of mouse rod photoreceptors. Rhodopsin kinase (GRK1) phosphorylates light-activated rhodopsin, and this phosphorylation allows for a reliably controlled inactivation of activated rhodopsin. This inactivation is in contrast to the relatively modest GRK3-mediated changes that we observe in OSNs. Therefore, why do olfaction and photoreception, which superficially have quite similar second messenger transduction cascades, have such hugely different roles for the respective GRKs? A plausible reason might be the different ways in which the respective receptors are activated. Rhodopsin is kept in an inactive configuration by covalently bound chromophore 11-cis-retinal until the latter is converted to 11-trans-retinal by capture of a photon. Without further modifications, this active state might only decay with a time course as slow as 40 s (Xu et al. 1997). However, its lifetime is curtailed by phosphorylation, followed by arrestin binding to in the order of 100ms (Burns and Pugh 2009; Doan et al. 2009). In contrast, an OR is activated by binding of its cognate odorant and inactivated upon dissociation of the odorant–OR complex. The dwell-time and, therefore, the lifetime of the activated odorant–OR complex has been estimated to be 1ms (Bhandawat et al. 2005), orders of magnitudes shorter than the lifetime of activated rhodopsin. Thus, phosphorylation might not be the most suitable mechanism for shortening the lifetime of the active configuration of an OR by rapidly terminating or controlling the activity of ORs and therefore determining the time course of physiological responses to odorants. This hypothesis does not exclude the possibility that GRK3 or other kinases may mediate effects on a longer odorant-exposure timescale.
In summary, we demonstrated that OR signaling was attenuated upon ligand stimulation on a 15–30-min scale in GRK3-expressing HEK293 cells in an OR-specific manner; however, rapid OR desensitization was not observed upon repeated stimulation. In OSNs, the effect of GRK3 on the response amplitude and recovery kinetics of ORs was marginal, suggesting that GRK3 may not substantively contribute to the rapid desensitization of ORs. Although our results cannot be applied to all ORs, our findings provide insights into the long-term examination of GRK3-mediated OR desensitization. Obviously, it is desirable to determine the phosphorylation state of ORs, but the detection of OR phosphorylation has been technically quite difficult; therefore, the role or roles of OR phosphorylation in OSNs, if any exist, remain to be elucidated. The fundamental question which still remains is what molecular mechanism drives the conformational switch between the active and inactive states of ORs upon ligand entering and leaving the OR binding sites.
Supplementary material
Supplementary material can be found at http://www.chemse.oxfordjournals.org/
Funding
This work was supported in part by Grant-in-Aid for Scientific Research on Priority Areas from JSPS Japan [grant number 18077001] and ERATO Touhara Chemosensory Signal Project from JST Japan to K.T., and by National Institutes of Health [grant DC009613] to J.R. Genotyping was in part performed at the Monell Genotyping and DNA/RNA Analysis Core, which is supported, in part, by funding from the NIH-NIDCD Core grant 1P30DC011735-01.
Supplementary Material
Acknowledgements
The authors thank Dr Robert J. Lefkowitz (Duke University) for providing GRK3 knock-out mice. The authors also thank Touhara’s lab members for their generous support and Dr V. Kefalov for insightful discussions.
References
- Bhandawat V, Reisert J, Yau KW. 2005. Elementary response of olfactory receptor neurons to odorants. Science. 308:1931–1934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boekhoff I, Inglese J, Schleicher S, Koch WJ, Lefkowitz RJ, Breer H. 1994. Olfactory desensitization requires membrane targeting of receptor kinase mediated by beta gamma-subunits of heterotrimeric G proteins. J Biol Chem. 269:37–40 [PubMed] [Google Scholar]
- Buck L, Axel R. 1991. A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell. 65:175–187 [DOI] [PubMed] [Google Scholar]
- Burns ME, Pugh EN, Jr. 2009. RGS9 concentration matters in rod phototransduction. Biophys J. 97:1538–1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cygnar KD, Collins SE, Ferguson CH, Bodkin-Clarke C, Zhao H. 2012. Phosphorylation of adenylyl cyclase III at serine1076 does not attenuate olfactory response in mice. J Neurosci. 32:14557–14562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson TM, Arriza JL, Jaworsky DE, Borisy FF, Attramadal H, Lefkowitz RJ, Ronnett GV. 1993. Beta-adrenergic receptor kinase-2 and beta-arrestin-2 as mediators of odorant-induced desensitization. Science. 259:825–829 [DOI] [PubMed] [Google Scholar]
- Doan T, Azevedo AW, Hurley JB, Rieke F. 2009. Arrestin competition influences the kinetics and variability of the single-photon responses of mammalian rod photoreceptors. J Neurosci. 29:11867–11879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SS. 2001. Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling. Pharmacol Rev. 53:1–24 [PubMed] [Google Scholar]
- Hausdorff WP, Sung J, Caron MG, Lefkowitz RJ. 1992. Recent molecular analyses of β-adrenergic receptor phosphorylation, sequestration and down regulation. Asia Pac J Pharmacol. 7: 149–158 [Google Scholar]
- Kajiya K, Inaki K, Tanaka M, Haga T, Kataoka H, Touhara K. 2001. Molecular bases of odor discrimination: Reconstitution of olfactory receptors that recognize overlapping sets of odorants. J Neurosci. 21:6018–6025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katada S, Hirokawa T, Oka Y, Suwa M, Touhara K. 2005. Structural basis for a broad but selective ligand spectrum of a mouse olfactory receptor: mapping the odorant-binding site. J Neurosci. 25:1806–1815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katada S, Nakagawa T, Kataoka H, Touhara K. 2003. Odorant response assays for a heterologously expressed olfactory receptor. Biochem Biophys Res Commun. 305:964–969 [DOI] [PubMed] [Google Scholar]
- Katada S, Tanaka M, Touhara K. 2004. Structural determinants for membrane trafficking and G protein selectivity of a mouse olfactory receptor. J Neurochem. 90:1453–1463 [DOI] [PubMed] [Google Scholar]
- Kato A, Touhara K. 2009. Mammalian olfactory receptors: pharmacology, G protein coupling and desensitization. Cell Mol Life Sci. 66:3743–3753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleene SJ. 2008. The electrochemical basis of odor transduction in vertebrate olfactory cilia. Chem Senses. 33:839–859 [DOI] [PubMed] [Google Scholar]
- Lee KB, Ptasienski JA, Bunemann M, Hosey MM. 2000. Acidic amino acids flanking phosphorylation sites in the M2 muscarinic receptor regulate receptor phosphorylation, internalization, and interaction with arrestins. J Biol Chem. 275:35767–35777 [DOI] [PubMed] [Google Scholar]
- Liggett SB. 2002. Update on current concepts of the molecular basis of beta2-adrenergic receptor signaling. J Allergy Clin Immunol. 110(6 Suppl):S223–S228 [DOI] [PubMed] [Google Scholar]
- Lowe G, Gold GH. 1991. The spatial distributions of odorant sensitivity and odorant-induced currents in salamander olfactory receptor cells. J Physiol. 442:147–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashukova A, Spehr M, Hatt H, Neuhaus EM. 2006. Beta-arrestin2-mediated internalization of mammalian odorant receptors. J Neurosci. 26:9902–9912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matharu AL, Mundell SJ, Benovic JL, Kelly E. 2001. Rapid agonist-induced desensitization and internalization of the A(2B) adenosine receptor is mediated by a serine residue close to the COOH terminus. J Biol Chem. 276:30199–30207 [DOI] [PubMed] [Google Scholar]
- Mombaerts P. 2004. Genes and ligands for odorant, vomeronasal and taste receptors. Nat Rev Neurosci. 5:263–278 [DOI] [PubMed] [Google Scholar]
- Neuhaus EM, Mashukova A, Barbour J, Wolters D, Hatt H. 2006. Novel function of beta-arrestin2 in the nucleus of mature spermatozoa. J Cell Sci. 119(Pt 15):3047–3056 [DOI] [PubMed] [Google Scholar]
- Ohguro H, Van Hooser JP, Milam AH, Palczewski K. 1995. Rhodopsin phosphorylation and dephosphorylation in vivo. J Biol Chem. 270:14259–14262 [DOI] [PubMed] [Google Scholar]
- Oka Y, Katada S, Omura M, Suwa M, Yoshihara Y, Touhara K. 2006. Odorant receptor map in the mouse olfactory bulb: in vivo sensitivity and specificity of receptor-defined glomeruli. Neuron. 52:857–869 [DOI] [PubMed] [Google Scholar]
- Oka Y, Omura M, Kataoka H, Touhara K. 2004. Olfactory receptor antagonism between odorants. EMBO J. 23:120–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peppel K, Boekhoff I, McDonald P, Breer H, Caron MG, Lefkowitz RJ. 1997. G protein-coupled receptor kinase 3 (GRK3) gene disruption leads to loss of odorant receptor desensitization. J Biol Chem. 272:25425–25428 [DOI] [PubMed] [Google Scholar]
- Ponissery Saidu S, Dibattista M, Matthews HR, Reisert J. 2012. Odorant-induced responses recorded from olfactory receptor neurons using the suction pipette technique. J Vis Exp. e3862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter E, Lefkowitz RJ. 2006. GRKs and beta-arrestins: roles in receptor silencing, trafficking and signaling. Trends Endocrinol Metab. 17:159–165 [DOI] [PubMed] [Google Scholar]
- Shukla AK, Xiao K, Lefkowitz RJ. 2011. Emerging paradigms of β-arrestin-dependent seven transmembrane receptor signaling. Trends Biochem Sci. 36:457–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Cygnar KD, Sagdullaev B, Valley M, Hirsh S, Stephan A, Reisert J, Zhao H. 2008. Olfactory CNG channel desensitization by Ca2+/CaM via the B1b subunit affects response termination but not sensitivity to recurring stimulation. Neuron. 58:374–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuck BA, Fadel V, Hummel T, Sommer JU. 2014. Subjective olfactory desensitization and recovery in humans. Chem Senses. 39:151–157 [DOI] [PubMed] [Google Scholar]
- Touhara K, Vosshall LB. 2009. Sensing odorants and pheromones with chemosensory receptors. Annu Rev Physiol. 71:307–332 [DOI] [PubMed] [Google Scholar]
- Tsuga H, Kameyama K, Haga T, Honma T, Lameh J, Sadée W. 1998. Internalization and down-regulation of human muscarinic acetylcholine receptor m2 subtypes. Role of third intracellular m2 loop and G protein-coupled receptor kinase 2. J Biol Chem. 273:5323–5330 [DOI] [PubMed] [Google Scholar]
- Xu J, Dodd RL, Makino CL, Simon MI, Baylor DA, Chen J. 1997. Prolonged photoresponses in transgenic mouse rods lacking arrestin. Nature. 389:505–509 [DOI] [PubMed] [Google Scholar]
- Yan C, Zhao AZ, Bentley JK, Loughney K, Ferguson K, Beavo JA. 1995. Molecular cloning and characterization of a calmodulin-dependent phosphodiesterase enriched in olfactory sensory neurons. Proc Natl Acad Sci USA. 92:9677–9681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zufall F, Leinders-Zufall T. 2000. The cellular and molecular basis of odor adaptation. Chem Senses. 25:473–481 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.