Abstract
Background: Continuous glucose monitoring (CGM) is a tool widely used in the treatment of patients with type 1 diabetes. The purpose of the current study was to evaluate whether accuracy and patient treatment satisfaction differ between the Enlite™ (Medtronic MiniMed, Inc., Northridge, CA) and Dexcom® (San Diego, CA) G4 PLATINUM CGM sensors.
Subjects and Methods: Thirty-eight ambulatory patients with type 1 diabetes used the Dexcom G4 and Enlite sensors simultaneously for a minimum of 4 and maximum of 6 days. Patients measured capillary glucose levels with a HemoCue® (Ängelholm, Sweden) system six to 10 times a day. In addition, two inpatient studies were performed between Days 1–3 and 4–6.
Results: The mean absolute relative difference (MARD) in blood glucose for the Dexcom G4 was significantly lower (13.9%) than for the Enlite sensor (17.8%) (P<0.0001). The corresponding MARDs for Days 1–3 were 15.0% versus 19.4% (P=0.0027) and 13.6% versus 15.9% (P=0.026) for Days 4–6. For glucose levels in the hypoglycemic range (<4.0 mmol/L), the MARD for the Dexcom G4 was 20.0% compared with 34.7% for the Enlite (P=0.0041). On a visual analog scale (VAS) (0–100), patients rated the Dexcom G4 more favorably than the Enlite in 12 out of the 13 user experience questions. For example, more patients rated their experience with the Dexcom G4 as positive (VAS, 79.7 vs. 46.6; P<0.0001) and preferred to use it in their daily lives (VAS, 79.1 vs. 42.1; P<0.0001).
Conclusions: The Dexcom G4 sensor was associated with greater overall accuracy than the Enlite sensor during initial (Days 1–3) and later (Days 4–6) use and for glucose levels in the hypoglycemic range. Patients reported a significantly more positive experience using the Dexcom G4 than the Enlite.
Introduction
Good glycemic control is essential to prevent complications in patients with type 1 diabetes.1–3 However, few patients reach hemoglobin A1c (HbA1c) targets,4,5 and a large proportion still have very poor glycemic control.4,5 In younger patients, a large proportion of deaths are due to acute complications of hypo- or hyperglycemic coma.6–8
Continuous glucose monitoring (CGM) is a tool used in optimizing glycemic control in patients with type 1 diabetes.9 A continuous glucose monitor is a subcutaneous tissue sensor that provides an interstitial fluid glucose measurement every 1–5 min. During the last decade, CGM has been approved in many countries and is an increasingly common method to manage type 1 diabetes. The Dexcom® G4 PLATINUM (Dexcom, Inc., San Diego, CA), and the Enlite™ (Medtronic MiniMed, Inc., Northridge, CA) sensors are widely used in clinical practice and can be used with separate CGM systems or in connection with insulin pumps.
On the basis of available evidence, CGM is recommended for patients with certain clinical conditions such as unawareness or frequent episodes of hypoglycemia.10 In certain countries, patients with very poor glycemic control are also reimbursed for CGM use.11 Although several benefits make use of CGM a highly attractive method for diabetes management, significant limitations remain.
Numerous clinical trials have shown different findings on the accuracy of CGM,12–15 which may depend on factors such as the use of relatively small datasets, short study duration, use of different generations of sensors, and various types of reference methods. No official recommendations currently exist for selecting a particular CGM system, and individual diabetes centers generally base the selection on local experience and preferences. To our knowledge, there are no clinical trials comparing patients' subjective experiences with different CGM sustems, and the accuracy of the Enlite and Dexcom G4 sensors has not been compared in ambulatory patients, within the hypoglycemic range, or for a duration of longer than 48 h.13
The aim of this study was to compare the accuracy and treatment experiences of the Dexcom G4 and Enlite sensors in patients with type 1 diabetes.
Subjects and Methods
This study was performed at the NU-Hospital Organization, consisting of five hospitals in the western part of Sweden. A non-randomized, non-blinded, 6-day study was performed on type 1 diabetes patients to compare the accuracy of the Dexcom G4 and Enlite sensors. The study protocol was approved by the internal review board of the NU-Hospital Organization.
Study procedures
All enrolled patients gave written informed consent before any study-related procedures were performed or patients were admitted to the clinical research unit (CRU). Inclusion and exclusion criteria are shown in Table 1. In brief, type 1 diabetes patients who were 18 years or older and younger than 75 years were included. Exclusion criteria consisted of current pregnancy, cognitive dysfunction or other disease making CGM use difficult, continuous use of paracetamol, or current use of a CGM sensor.
Table 1.
Inclusion and Exclusion Criteria
| Criteria | |
|---|---|
| Inclusion | 1. Type 1 diabetes 2. Age 18 years or older and <75 years 3. Written informed consent |
| Exclusion | 1. Pregnancy 2. Severe cognitive dysfunction or other disease making CGM use difficult 3. Continuous use of paracetamol. Paracetamol use was not allowed in the week preceding the study or throughout the study because it disturbs the interpretation of blood glucose levels estimated by the Dexcom G4. Use of other pain-relieving medications was allowed. 4. Current CGM use 5. History of allergic reaction to any CGMS materials or adhesives making contact with the skin 6. History of allergic reaction to chlorhexidine or alcohol antiseptic solution 7. Abnormal skin at the anticipated glucose sensor attachment sites (excessive hair, burn, inflammation, infection, rash, and/or tattoo) |
CGM, continuous glucose monitor; CGMS, continuous glucose monitoring system.
Each participant made three visits. At the first visit, demographic and baseline characteristics, medical history, and concomitant medication use (according to the anatomical therapeutic chemical classification system) were collected. Trained study personnel inserted sensors subcutaneously in the abdominal area at least 10 cm from the umbilicus or insulin pump infusion site. Tattoos, stretch marks, bumps, or other skin deformities were not allowed around the insertion area.
Patients were educated on how to use the sensors, and sensors were calibrated according to the manufacturer's instructions. All participants used both CGM systems unmasked simultaneously for a minimum of 4 and maximum of 6 days. All participants were educated on use of the HemoCue® (Ängelholm, Sweden) analyzer and cuvettes and were advised to measure capillary glucose values six to 10 times per day. Participants recorded HemoCue capillary glucose values, meal, and activity data in a written diary. Patients were informed that finger-stick and sensor glucose values do not always match and thus were instructed to perform capillary testing when dosing insulin or performing other treatment-related procedures to correct blood glucose levels (e.g., with respect to diet or physical activity).
At visits 2 and 3, each patient was admitted to the CRU to obtain seven venous blood samples on Days 1–3 and 4–6, with an interval of at least 15 min. An intravenous catheter was inserted to facilitate blood acquisition. Three capillary finger-stick blood glucose samples were obtained simultaneous with venous samples on each occasion. The HemoCue analyzer was used to measure both venous and capillary blood samples. Data from CGM systems were downloaded during the scheduled patient visit at the CRU. Capillary values and time points were recorded by participants and compared by study personnel with those recorded by the meter.
After the CGM was removed, sensor insertion sites were observed for bleeding, inflammation, or infection and photographed in the event of an abnormal finding. Patient experience was evaluated using 13 predefined questions and a visual analogue scale (VAS), with the lowest value (0) indicating “not true at all” and the highest value (100) indicating “completely true.”
Predefined end points
All end points were predefined and registered on ClinicalTrials.gov with other trial information. The primary end point was the difference in mean absolute relative difference (MARD) between the sensors, using capillary glucose values as the reference. In all secondary predefined end points, capillary glucose values were also used as the reference. Secondary end points were accuracy of both sensors during Days 1–3 and 4–6, as well as during hypoglycemia (<4 mmol/L), euglycemia (4–10 mmol/L), and hyperglycemia (>14 mmol/L). End points were analyzed by first investigating MARD and then the mean absolute difference, absolute correlation coefficient, median absolute relative difference, and median absolute difference.
In the questionnaire, 13 concepts were measured as independent variables. Questions were subjectively phrased evaluative statements that allowed participants to agree or disagree according to the VAS. In brief, statements pertained to experience in setting up the system, interpreting the user screen, feelings of safety with respect to the system, ease of use, sensor discomfort, problems with sensor contacts, system calibrations, disturbances from alarms, and willingness to use the system in daily life. In addition, patients estimated their total number of problems with sensor contact. The questionaire also allowed patients to provide free-form text comments on their experience.
Other predefined analyses
The correlation between capillary and venous blood samples was estimated. A sensitivity analysis was performed by excluding outliers of the CGM values versus HemoCue capillary glucose values.
Statistical analysis
All statistical analyses of predefined end points were described in the statistical analysis plan (SAP) before the database was locked. For descriptive purposes, mean with SD, median with minimum and maximum values, and within-individual SDs are presented for continuous variables, and number with percentages are presented for categorical variables. All enrolled subjects with at least 10 time points (within a 5-min time frame) with evaluable values from both CGM systems and the reference capillary value during the whole study period were included in the intention-to-treat population. According to the power calculation, 36 patients were required to detect a 5 percentage unit greater accuracy of one CGM system over the other, assuming an SD of 8 percentage units.16 The primary end point and other continuous variables were tested using the Wilcoxon signed-rank test. Categorical differences between the two CGM systems were compared using the sign test. The relation between two continuous variables was expressed by Spearman correlation coefficient (rs). All tests were two-tailed and conducted at the 0.05 significance level. Analyses were performed using SAS® version 9.3 (SAS, Inc., Cary, NC).
Results
Characteristics of the study cohort
Among 46 subjects enrolled, 38 met the criteria for the predefined intention-to-treat analysis (i.e., at least 10 capillary glucose values matched at a maximum deviation of 5 min from the CGM values). Among the reasons for the eight other subjects not completing the study were difficulties complying with the study procedure and disturbances by sensor alarms, such as low sensor signal at night. Baseline characteristics of the cohort are shown in Table 2. The mean age was 50.0 years, 66% were men, and mean diabetes duration was 22.9 years. The mean HbA1c level was 58.9 mmol/mol (7.5%). Ten patients used continuous subcutaneous insulin infusion for insulin delivery, and 28 used multiple daily injection therapy.
Table 2.
Demographic Characteristics
| Intention-to-treat population (n=38) | |
|---|---|
| Age (years) | 50.0 (14.3) |
| 50.1 (20.8; 73.6) | |
| n=38 | |
| Sex | |
| Male | 25 (65.8%) |
| Female | 13 (34.2%) |
| Body mass index (kg/m2) | 24.6 (3.6) |
| 25.1 (18.9; 31.5) | |
| n=32 | |
| Waist circumference (cm) | 90.0 (10.3) |
| 91.0 (71.0; 108.0) | |
| n=34 | |
| Smoking | |
| Never smoked | 29 (76.3%) |
| Previous smoker | 8 (21.1%) |
| Current smoker | 1 (2.6%) |
| HbA1c (IFCC, mmol/mol) | 58.9 (10.5) |
| 59.0 (34.0; 77.0) | |
| n=37 | |
| Diabetes duration (years) | 22.9 (16.0) |
| 18.0 (1.0; 57.0) | |
| n=38 | |
| Insulin delivery | |
| CSII | 10 (26.3%) |
| MDI | 28 (73.7%) |
| Total insulin dose per day | 41.4 (16.7) |
| 40.0 (10.0; 81.0) | |
| n=37 | |
| Albumin/creatinine ratio (μg/mg) | 1.87 (6.23) |
| 0.70 (0.20; 37.50) | |
| n=35 | |
| Blood pressure (mm Hg) (left arm) | |
| Systolic | 120.9 (12.2) |
| 120.0 (99.0; 149.0) | |
| n=35 | |
| Diastolic | 71.0 (12.2) |
| 73.0 (48.0; 90.0) | |
| n=35 | |
| Myocardial infarction | 1 (2.6%) |
| Percutaneous coronary intervention | 2 (5.3%) |
| Bypass surgery | 1 (2.6%) |
| Stroke | 2 (5.3%) |
| Retinopathy | |
| None | 19 (50.0%) |
| Simplex | 10 (26.3%) |
| Nonproliferative | 6 (15.8%) |
| Proliferative | 3 (7.9%) |
| Neuropathy | 5 (13.2%) |
For continuous variables data are mean (SD)/median (minimum; maximum)/n. For categorical variables data are n (%).
CSII, continuous subcutaneous insulin infusion; HbA1c, hemoglobin A1c; IFCC, International Federation of Clinical Chemistry; MDI, multiple daily injections.
Predefined end points on CGM system accuracy
Results of all predefined endpoints on sensor accuracy are shown in Table 3. The primary end point, difference in MARD between the two systems over the whole study period, showed that the MARD for the Dexcom G4 was significantly lower (13.9%) than for the Enlite sensor (17.8%) (P<0.0001), among a total of 1,012 sets of measurements. The corresponding MARDs for Days 1–3 (n=545 sets of measurements) were 15.0% versus 19.4%, respectively (P=0.0027); MARDs for Days 4–6 were 13.6% versus 15.9%, respectively (P=0.026) (n=467 sets of measurements). For glucose levels <4.0 mmol/L (n=80 sets of measurements) the MARD was 20.0% for the Dexcom G4 and 34.7% for the Enlite (P=0.0041). For glucose values of 4–10 mmol/L (n=668 sets of measurements) the corresponding MARDs were 14.1% versus 17.3% (P=0.0008). No significant difference in MARD was apparent for glucose levels >14 mmol/L (n=70 sets of measurements): 12.1% for Dexcom G4 and 13.9% for Enlite (P=0.24). Using MAD and the absolute correlation coefficient as measures for accuracy showed a similar pattern, with generally greater accuracy for the Dexcom G4 (Table 3).
Table 3.
Primary and Secondary End Points of Accuracy Evaluations (in the Intention-to-Treat Population)
| Dexcom G4 | Enlite | Difference (Dexcom G4 – Enlite) | P value | |
|---|---|---|---|---|
| All data | ||||
| MARD | 13.87 (5.24) | 17.85 (5.65) | −3.98 (5.87) | <0.0001 |
| 12.4 (6.9; 29.1) | 16.8 (9.3; 30.5) | −2.7 (−22.7; 5.5) | ||
| n=38 | n=38 | n=38 | ||
| IISD=12.83 | IISD=17.42 | IISD=16.83 | ||
| MAD | 1.09 (0.45) | 1.35 (0.44) | −0.26 (0.40) | 0.0002 |
| 1.0 (0.4; 2.4) | 1.3 (0.6; 2.6) | −0.2 (−1.3; 0.6) | ||
| n=38 | n=38 | n=38 | ||
| IISD=1.00 | IISD=1.29 | IISD=1.28 | ||
| Absolute Pearson correlation coefficient | 0.88 (0.11) | 0.82 (0.12) | 0.06 (0.11) | 0.0009 |
| 0.9 (0.5; 1.0) | 0.9 (0.5; 1.0) | 0.0 (−0.1; 0.4) | ||
| n=38 | n=38 | n=38 | ||
| MedARD | 11.22 (4.26) | 13.47 (4.81) | −2.26 (4.55) | |
| 10.7 (3.5; 24.1) | 12.9 (5.7; 25.7) | −1.9 (−14.0; 5.9) | ||
| n=38 | n=38 | n=38 | ||
| MedAD | 0.88 (0.49) | 1.05 (0.42) | −0.16 (0.33) | |
| 0.7 (0.2; 2.5) | 1.0 (0.4; 2.5) | −0.1 (−0.9; 0.5) | ||
| n=38 | n=38 | n=38 | ||
| Capillary glucose levels <4.0 mmol/L | ||||
| MARD | 20.04 (15.13) | 34.69 (21.43) | −14.65 (26.33) | 0.0041 |
| 17.0 (2.6; 61.7) | 30.4 (2.8; 97.1) | −7.2 (−81.3; 31.7) | ||
| n=28 | n=28 | n=28 | ||
| IISD=16.55 | IISD=22.30 | IISD=20.41 | ||
| MAD | 0.67 (0.49) | 1.15 (0.71) | −0.48 (0.90) | 0.0040 |
| 0.6 (0.1; 2.2) | 1.0 (0.1; 3.3) | −0.2 (−3.2; 1.1) | ||
| n=28 | n=28 | n=28 | ||
| IISD=0.49 | IISD=0.57 | IISD=0.56 | ||
| Absolute Pearson correlation coefficient | 0.54 (0.26) | 0.42 (0.24) | 0.13 (0.45) | 0.43 |
| 0.5 (0.2; 0.9) | 0.4 (0.1; 0.8) | 0.2 (−0.6; 0.8) | ||
| n=10 | n=10 | n=10 | ||
| MedARD | 19.07 (14.84) | 33.53 (21.89) | −14.60 (25.95) | |
| 16.4 (2.6; 61.7) | 29.2 (2.8; 97.1) | −10.8 (−81.3; 31.7) | ||
| n=28 | n=28 | n=28 | ||
| MedAD | 0.64 (0.48) | 1.14 (0.73) | −0.49 (0.90) | |
| 0.5 (0.1; 2.2) | 1.0 (0.1; 3.3) | −0.4 (−3.2; 1.1) | ||
| n=28 | n=28 | n=28 | ||
| Capillary glucose levels >14.0 mmol/L | ||||
| MARD | 12.13 (7.96) | 13.94 (7.75) | −1.81 (11.19) | 0.24 |
| 11.6 (0.3; 31.5) | 11.8 (2.4; 31.1) | −1.5 (−25.0; 28.5) | ||
| n=25 | n=25 | n=25 | ||
| IISD=7.11 | IISD=11.95 | IISD=13.02 | ||
| MAD | 1.93 (1.22) | 2.27 (1.36) | −0.33 (1.79) | 0.31 |
| 2.1 (0.0; 4.7) | 1.8 (0.3; 6.0) | −0.3 (−3.8; 4.3) | ||
| n=25 | n=25 | n=25 | ||
| IISD=1.20 | IISD=2.19 | IISD=2.31 | ||
| Absolute Pearson correlation coefficient | 0.72 (0.28) | 0.67 (0.31) | 0.05 (0.28) | 0.49 |
| 0.8 (0.2; 1.0) | 0.8 (0.1; 1.0) | 0.1 (−0.5; 0.5) | ||
| n=15 | n=15 | n=15 | ||
| MedARD | 11.41 (8.38) | 13.91 (8.09) | −1.47 (11.08) | |
| 8.5 (0.3; 31.5) | 13.4 (2.4; 33.0) | −1.2 (−25.0; 28.5) | ||
| n=25 | n=25 | n=25 | ||
| MedAD | 1.79 (1.28) | 2.18 (1.34) | −0.25 (1.73) | |
| 1.7 (0.0; 4.7) | 1.9 (0.3; 6.0) | −0.2 (−3.8; 4.3) | ||
| n=25 | n=25 | n=25 | ||
| Capillary glucose levels 4.0–10.0 mmol/L | ||||
| MARD | 14.09 (5.91) | 17.33 (6.29) | −3.25 (5.42) | 0.0008 |
| 12.9 (5.6; 32.1) | 16.4 (7.8; 36.3) | −2.3 (−15.7; 6.5) | ||
| n=38 | n=38 | n=38 | ||
| IISD=12.86 | IISD=16.86 | IISD=15.93 | ||
| MAD | 0.99 (0.43) | 1.19 (0.43) | −0.21 (0.35) | 0.0004 |
| 0.9 (0.4; 2.5) | 1.1 (0.6; 2.4) | −0.2 (−1.0; 0.6) | ||
| n=38 | n=38 | n=38 | ||
| IISD=0.90 | IISD=1.12 | IISD=1.07 | ||
| Absolute Pearson correlation coefficient | 0.79 (0.15) | 0.69 (0.19) | 0.10 (0.17) | 0.0003 |
| 0.8 (0.4; 1.0) | 0.7 (0.2; 0.9) | 0.1 (−0.2; 0.5) | ||
| n=38 | n=38 | n=38 | ||
| MedARD | 12.04 (6.58) | 13.66 (6.40) | −1.68 (4.71) | |
| 10.1 (2.6; 37.1) | 12.8 (5.6; 37.9) | −1.9 (−11.8; 8.8) | ||
| n=38 | n=38 | n=38 | ||
| MedAD | 0.81 (0.42) | 0.96 (0.41) | −0.11 (0.34) | |
| 0.7 (0.2; 2.2) | 0.9 (0.5; 2.5) | −0.1 (−0.9; 0.7) | ||
| n=38 | n=38 | n=38 | ||
| Days 1–3 | ||||
| MARD | 15.01 (6.78) | 19.41 (7.76) | −4.41 (7.88) | 0.0027 |
| 12.9 (5.7; 36.7) | 17.6 (7.0; 38.2) | −3.2 (−30.6; 7.2) | ||
| n=37 | n=37 | n=37 | ||
| IISD=13.44 | IISD=17.76 | IISD=18.49 | ||
| MAD | 1.17 (0.51) | 1.45 (0.59) | −0.28 (0.52) | 0.0022 |
| 1.2 (0.4; 2.4) | 1.3 (0.6; 2.9) | −0.3 (−1.7; 0.8) | ||
| n=37 | n=37 | n=37 | ||
| IISD=1.03 | IISD=1.30 | IISD=1.41 | ||
| Absolute Pearson correlation coefficient | 0.87 (0.15) | 0.82 (0.14) | 0.05 (0.12) | 0.0012 |
| 0.9 (0.2; 1.0) | 0.9 (0.4; 1.0) | 0.0 (−0.3; 0.3) | ||
| n=37 | n=37 | n=37 | ||
| MedARD | 12.46 (6.13) | 15.42 (6.92) | −2.58 (5.94) | |
| 10.6 (3.3; 35.8) | 13.4 (4.7; 32.0) | −1.4 (−16.5; 11.3) | ||
| n=37 | n=37 | n=37 | ||
| MedAD | 0.94 (0.54) | 1.18 (0.61) | −0.17 (0.44) | |
| 0.8 (0.2; 2.5) | 1.0 (0.3; 3.2) | −0.1 (−1.2; 0.8) | ||
| n=37 | n=37 | n=37 | ||
| Days 4–6 | ||||
| MARD | 13.57 (6.70) | 15.88 (5.22) | −2.30 (7.29) | 0.026 |
| 12.8 (6.8; 41.1) | 15.8 (6.4; 28.5) | −3.2 (−14.6; 23.8) | ||
| n=37 | n=37 | n=37 | ||
| IISD=12.53 | IISD=15.69 | IISD=13.80 | ||
| MAD | 1.07 (0.48) | 1.27 (0.56) | −0.20 (0.57) | 0.046 |
| 1.0 (0.4; 2.3) | 1.0 (0.6; 2.7) | −0.2 (−1.3; 1.1) | ||
| n=37 | n=37 | n=37 | ||
| IISD=1.03 | IISD=1.34 | IISD=1.16 | ||
| Absolute Pearson correlation coefficient | 0.89 (0.17) | 0.84 (0.15) | 0.05 (0.13) | 0.0055 |
| 0.9 (0.0; 1.0) | 0.9 (0.3; 1.0) | 0.0 (−0.3; 0.4) | ||
| n=36 | n=36 | n=36 | ||
| MedARD | 11.05 (6.11) | 12.40 (5.21) | −1.85 (6.71) | |
| 11.0 (4.3; 41.1) | 10.8 (5.5; 25.7) | −1.7 (−16.8; 23.8) | ||
| n=37 | n=37 | n=37 | ||
| MedAD | 0.87 (0.46) | 1.00 (0.44) | −0.16 (0.49) | |
| 0.8 (0.2; 2.2) | 0.9 (0.4; 2.4) | −0.2 (−1.5; 1.1) | ||
| n=37 | n=37 | n=37 | ||
| Day 1 2–8 h post-sensor insertion | ||||
| Absolute Pearson correlation coefficient | 0.88 (0.20) | 0.74 (0.32) | 0.14 (0.26) | 0.13 |
| 1.0 (0.3; 1.0) | 0.9 (0.2; 1.0) | 0.0 (-0.1; 0.7) | ||
| n=10 | n=10 | n=10 | ||
For continuous variables data are mean (SD)/median (minimum; maximum)/n/intra-individual SD (IISD) (as appropriate). The statistical difference between CGM systems was tested by using the Wilcoxon signed-rank test.
MAD, mean absolute difference; MARD, mean absolute relative difference; MedAD, median absolute difference; MedARD, median absolute relative difference.
Predefined end points of treatment experience
Patients rated the Dexcom G4 significantly more favorably than the Enlite system in 12 out of 13 user experience questions, including general treatment satisfaction, sensor problem, ease of use, feelings of safety and freedom using the system, disturbances from alarms, and sensor discomfort (Table 4). For example, more patients rated their experience with the Dexcom G4 as positive (VAS, 79.7 vs. 46.6; P<0.0001), and more patients preferred to use the Dexcom G4 in their daily lives (VAS, 79.1 vs. 42.1; P<0.0001). No significant differences existed on ease of system set-up.
Table 4.
End Points of Patient Treatment Experience with the Dexcom G4 and Enlite Sensors (in the Intention-to-Treat Population)
| Dexcom G4 | Enlite | Difference (Dexcom G4 – Enlite) | P value | |
|---|---|---|---|---|
| My experience of the system was very positive. | 79.7 (24.0) | 46.6 (28.1) | 33.1 (29.4) | <0.0001 |
| 86 (10; 100) | 43 (7; 100) | 37 (−36; 82) | ||
| n=36 | n=36 | n=36 | ||
| The set-up of the system was very easy and problem-free. | 85.9 (18.4) | 80.3 (28.4) | 5.6 (23.6) | 0.34 |
| 92 (10; 100) | 93 (5; 100) | −1 (−17; 80) | ||
| n=36 | n=36 | n=36 | ||
| I felt safe and free when using the system. | 75.2 (23.3) | 54.4 (32.6) | 20.8 (24.0) | <0.0001 |
| 79 (10; 100) | 57 (5; 100) | 9 (−1; 71) | ||
| n=36 | n=36 | n=36 | ||
| It was easy to use the system. | 86.1 (16.0) | 56.7 (26.4) | 29.3 (28.0) | <0.0001 |
| 90 (10; 100) | 55 (7; 100) | 30 (−35; 84) | ||
| n=36 | n=35 | n=35 | ||
| It was easy to interpret the display of the system. | 90.0 (15.0) | 62.5 (28.1) | 27.5 (29.0) | <0.0001 |
| 94 (10; 100) | 64 (9; 100) | 26 (−54; 73) | ||
| n=36 | n=36 | n=36 | ||
| I did not experience pain/discomfort when using the sensor/system. | 87.8 (19.9) | 86.1 (20.2) | 1.7 (3.6) | 0.0015 |
| 96 (10; 100) | 94 (10; 100) | 1 (−2; 17) | ||
| n=36 | n=36 | n=36 | ||
| I had no problem with contact of the sensors of the system. | 85.5 (22.1) | 43.0 (30.1) | 42.6 (31.1) | <0.0001 |
| 93 (0; 100) | 43 (0; 100) | 45 (−25; 90) | ||
| n=36 | n=36 | n=36 | ||
| Total estimated number of problems with contact of the sensors of the system | 0.7 (1.5) | 4.1 (3.8) | −3.4 (3.1) | <0.0001 |
| 0 (0; 6) | 3 (0; 20) | −3 (−14; 1) | ||
| n=35 | n=34 | n=33 | ||
| It was easy to calibrate the system. | 89.9 (15.8) | 80.1 (20.1) | 9.7 (16.7) | <0.0001 |
| 94 (8; 100) | 87 (27; 100) | 2 (−19; 57) | ||
| n=36 | n=36 | n=36 | ||
| The system's alarm did not disturb me. | 73.8 (26.9) | 47.4 (31.6) | 26.5 (33.7) | <0.0001 |
| 84 (10; 100) | 43 (4; 100) | 24 (−50; 75) | ||
| n=36 | n=36 | n=36 | ||
| The system's sensor was comfortable to wear. | 72.5 (28.4) | 68.6 (28.6) | 3.9 (11.8) | 0.022 |
| 83 (3; 100) | 78 (4; 100) | 0 (−16; 48) | ||
| n=36 | n=36 | n=36 | ||
| The system's sensor did not disturb me. | 72.8 (29.9) | 70.1 (30.5) | 2.7 (9.3) | 0.0069 |
| 85 (1; 100) | 83 (1; 100) | 1 (−2; 53) | ||
| n=36 | n=36 | n=36 | ||
| I would like to use the system in my daily life. | 79.1 (23.6) | 42.1 (32.9) | 39.3 (33.3) | <0.0001 |
| 89 (1; 100) | 39 (0; 97) | 34 (−1; 100) | ||
| n=36 | n=35 | n=35 | ||
| Secretion of blood and other fluids at removala | No: 32 (94.1%) | No: 32 | D=E: 32 | 1.00 |
| Yes: 2 (5.9%) | (97.0%) | (97.0%) | ||
| Yes: 1 (3.0%) | D<E: 1 (3.0%) | |||
| Visible skin reaction after removala | No: 34 (100.0%) | No: 32 | D>E: 1 (3.0%) | 1.00 |
| (97.0%) | D=E: 32 | |||
| Yes: 1 (3.0%) | (97.0%) |
For continuous variables data are mean (SD)/median (minimum; maximum)/n. For categorical variables data are n (%). Statistical differences between continuous glucose monitoring systems were tested by using the Wilcoxon signed-rank test for continuous variables and the sign test for categorical variables.
D>G, Dexcom better than Enlite; D=G, Dexcom equal to Enlite; D<G, Dexcom worse than Enlite.
Other predefined end points
Average rates of patient-reported signal disturbances were significantly lower for the Dexcom G4 than the Enlite: 0.7 and 4.1, respectively (P<0.0001) (Table 4). Skin reactions and blood or fluid secretion when the sensors were removed were rare for both and did not differ significantly (Table 4).
Results of open questions
Some patients noted sensor problems (e.g., low signal) as extremely important. One patient commented that even if sensor accuracy increased, she would not feel safe using it if a low sensor signal became a frequent problem.
Predefined analyses of time of CGM data lost
Results from reliability analysis for the Dexcom G4 and the Enlite sensor are shown in Supplementary Data (available online at www.liebertonline.com/dia). The mean number of minutes per day not displaying data over 6 days was 13.0 min for the Dexcom G4 and 98.2 min for the Enlite sensor, resulting in a mean of all failure minutes per patient in the study of 70.9 min versus 546 min, respectively (Supplementary Table S1). Expressed in percentages, the mean failure time was 1.3% for the Dexcom G4 compared with 7.5% of time for the Enlite sensor. The time of CGM data loss was also numerically lower for the Dexcom G4 when each day was studied separately for Days 1–6 (Supplementary Table S2).
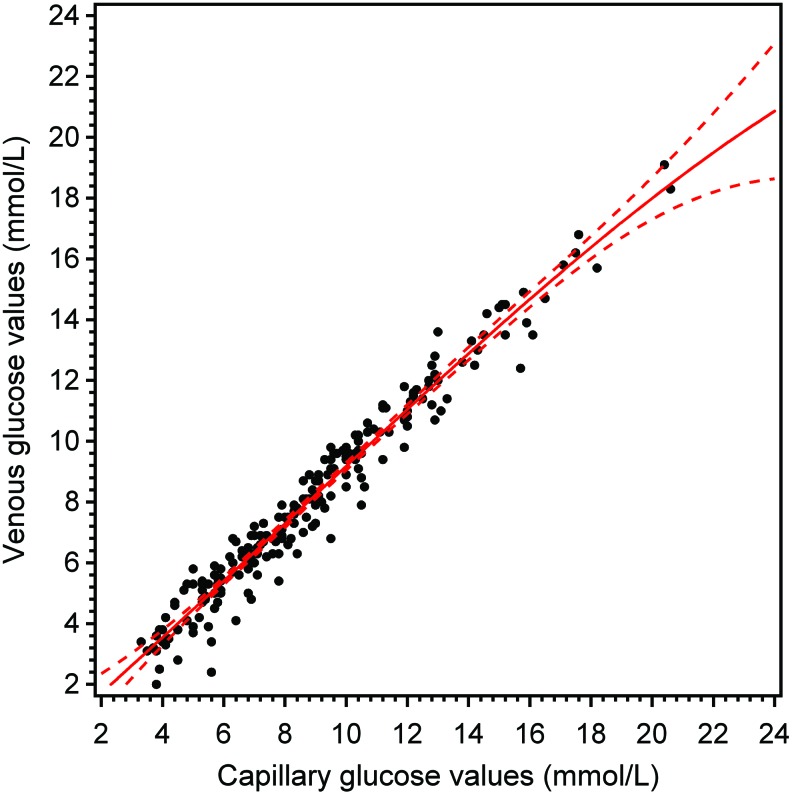
Association between capillary and venous glucose values
The correlation coefficient between capillary and venous glucose values was rs=0.98, and the mean difference between venous and capillary values was −0.78 (SD=0.72). The relation between capillary and venous glucose values is shown in Figure 1.
FIG. 1.
Venous glucose values versus capillary glucose values (in the intention-to-treat population). Solid curve is estimated by cubic regression analysis and dashed curves represent 95% confidence limits. Color images available online at www.liebertonline.com/dia
Direction of the deviation in CGM values
The mean difference when the Dexcom G4 values were subtracted from capillary values was 0.12 (SD=0.46) mmol/L, and the corresponding mean difference for the Enlite sensor was 0.10 (SD=0.65) mmol/L. For glucose values <4 mmol/L, the corresponding mean differences were −0.23 (SD=0.73) and −1.08 (SD=0.77) mmol/L, respectively. For glucose levels >14.0 mmol/L, mean differences were 0.74 (SD=1.98) and 1.86 (SD=1.77) mmol/L, respectively. For glucose levels between 4.0 and 10.0 mmol/L, the mean difference between capillary and Dexcom G4 values was 0.01 (SD=0.63), and that between capillary and Enlite values was −0.15 (SD 0.66) mmol/L.
Sensitivity analysis
In the sensitivity analysis, the scatterplot of capillary and CGM values for both sensors revealed only one clear outlier, which did not affect the results.
Post hoc analysis of MARD as a continuous function of capillary glucose levels
In Supplementary Figure S1, MARD is shown as a continuous function for both CGM sensors in relation to capillary glucose levels.17
Discussion
The Dexcom G4 sensor showed greater accuracy than the Enlite sensor over the entire study period and in separate analyses of early (Days 1–3) and late (Days 4–6) use. The Dexcom G4 also showed greater accuracy in the hypoglycemic and euglycemic ranges, whereas no significant difference was found at very high glucose levels (>14 mmol/L). Patients rated treatment experience to be more positive with the Dexcom G4 in 12 out of 13 user-related questions, including interpretation of the user screen, feelings of safety using the system, ease of use, pain or discomfort, problems with sensor contact, system calibration, disturbances from alarms, and willingness to use the system in daily life. No differences existed in the ease of system set-up. When capillary and venous glucose samples were measured simultaneously at the CRU, the relationship was very strong (correlation coefficient=0.98), but venous glucose levels were 0.78 mmol/L lower than capillary values.
Similar to the present study, a recent clinical trial by Damiano et al.13 that examined 24 patients with type 1 diabetes found greater accuracy associated with the Dexcom G4 than the Enlite sensor. However, the design of the previous study was different from ours in several ways. Venous samples were used as a reference and for calibrating the CGM systems in a closed-loop design. An advantage of using venous samples may be greater accuracy than capillary values, but on the other hand, as Damiano et al.13 noted, patients perform calibrations in daily life with capillary values, which is essential in evaluating sensors. Moreover, in our study the HemoCue system was used to estimate capillary glucose values, which has been shown to have a high accuracy also in comparison with venous samples in previous studies18 and was also used in the A1C-Derived Average Glucose (ADAG) trial to relate HbA1c to mean glucose levels.19 Other differences between the present study and the previous trial13 are that we included more patients and evaluated the sensors over a longer period of time. Therefore, we were also able to evaluate accuracy in the hypoglycemic range and sensor use up to 6 days instead of 48 h. Our results indicate that the Enlite sensor showed systematically higher glucose values in the hypoglycemic range, implying a risk that hypoglycemia may be missed rather than an excess number of false alarms. Hence, we believe that our study and the trial of Damiano et al.13 are complementary in many aspects, in that both show greater accuracy for the Dexcom G4 sensor.
In addition to the fact that this study showed greater accuracy of the Dexcom G4 sensor, the size of the difference for the various treatment-related questions is of interest. Although significant, the difference may not be clinically meaningful. However, both relative and absolute differences were surprisingly large for many reasons. For example, the VAS rating was 71–95% higher for the Dexcom G4 regarding problems with sensor contact, having generally positive experiences, and willingness to use the system in daily life. For feelings of safety and freedom using the systems, interpreting the display, ease of use, and alarm disturbances, the VAS was 39–56% higher in favor of the Dexcom G4. Because the amount of time using a CGM system has been strongly associated with beneficial treatment effects on HbA1c,20,21 it is possible that the Dexcom G4 may lead to a greater reduction in HbA1c level for many patients, perhaps because of better compliance from the more positive treatment experience patients reported. Our study underscores the need for future clinical trials to likewise study the CGM treatment experience, especially because it would be a relatively straightforward addition to studies on the accuracy of CGM systems. The result may be more rapid improvement in systems, possibly leading to better treatment effects and satisfaction. Moreover, the fact that venous samples in our study showed regularly lower glucose levels than capillary values implies that the same type of glucose measurement should be used as the reference and for system calibration in future accuracy studies.
A strength of the present study is that it was performed independently from Dexcom, the manufacturer of the Dexcom G4 system, and Medtronic, the manufacturer of the Guardian® REAL-Time system. CGM systems, sensors, and all other costs including salaries for research staff were paid for by independent funders (see Acknowledgments). Other strengths are that all end points were predefined and that the exact statistical procedures used were declared in a signed SAP before the database was locked. A limitation to the present study is the short duration (4–6 days) used to measure the treatment experience, which may differ over a longer period. However, the large differences found here may indicate that these differences will persist also over longer time periods. Moreover, it is important for patients to have positive experiences when starting CGM therapy, to reduce the likelihood they will quit using it later.
In conclusion, the Dexcom G4 CGM system showed greater accuracy than the Enlite system, and patients had a significantly more positive treatment experience. Hence, our findings indicate that the Dexcom G4 is more optimal when dosing insulin, when taking other treatment actions, and in avoiding hypoglycemia. The results also indicate that the Dexcom G4 may make it possible for more patients to wear a CGM device for a longer period of time. These findings should be considered by treatment providers when recommending suitable CGM systems and evaluating ongoing therapies.
Supplementary Material
Acknowledgments
We would like to thank Agneta Ruderfeldt, diabetes educator and research nurse for being involved in examining patients, and HemoCue AB for partial support with cuvettes and HemoCue meters. We also want to thank Josh Murphy who assisted in editing the manuscript. This study was performed independently from Dexcom, the manufacturer of the Dexcom G4 system, and Medtronic, the manufacturer of the Guardian REAL-Time system. This study was supported by grants from the following: the Swedish state, under the agreement between the Swedish government and the county councils concerning economic support of research and education of doctors (ALF-agreement); the Swedish Society for Physicians; and the Health & Medical Care Committee of the Regional Executive Board, Region Västra Götaland, Sweden.
Author Disclosure Statement
The laboratory of J.I.J. has received funds for consulting and clinical trial research from Medtronic. I.B.H. has received research grants from Sanofi-US and Halozyme. D.K. is a consultant for Google, Lifecare, Roche, and Sanofi. M.L. has received honoraria and/or Consulting fees from Astra Zeneca, Medtronic, Novo Nordisk, and Pfizer and research grants from Abbott Scandinavia, Astra Zeneca, Dexcom, Novo Nordisk, and Pfizer and has participated on advisory boards for Novo Nordisk. V.M., M.A., S.A. A.P., S.D. and B.H. declare no conflicts of interests.
References
- 1.Diabetes Control and Complications Trial Study Group: The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 2.Nathan DM, Cleary PA, Backlund JY, et al. : Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lind M, Bounias I, Olsson M, et al. : Glycaemic control and incidence of heart failure in 20,985 patients with type 1 diabetes: an observational study. Lancet 2011;378:140–146 [DOI] [PubMed] [Google Scholar]
- 4.Swedish National Diabetes Register. Annual Report 2012. www.ndr.nu/pdf/Annual_Report_NDR_2012.pdf (accessed January9, 2014)
- 5.Livingstone SJ, Looker HC, Hothersall EJ, et al. : Risk of cardiovascular disease and total mortality in adults with type 1 diabetes: Scottish registry linkage study. PLoS Med 2012;9:e1001321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laing SP, Swerdlow AJ, Slater SD, et al. : The British Diabetic Association Cohort Study, I: all-cause mortality in patients with insulin-treated diabetes mellitus. Diabet Med 1999;1:459–465 [DOI] [PubMed] [Google Scholar]
- 7.Skrivarhaug T, Bangstad HJ, Stene LC, et al. : Long-term mortality in a nationwide cohort of childhood-onset type 1 diabetic patients in Norway. Diabetologia 2006;49:298–305 [DOI] [PubMed] [Google Scholar]
- 8.Secrest AM, Becker DJ, Kelsey SF, et al. : Cause-specific mortality trends in a large population-based cohort with long-standing childhood-onset type 1 diabetes. Diabetes 2010;59:3216–3222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American Diabetes Association: Position statement: standards of medical care in diabetes—2014. Diabetes Care 2014;37(Suppl 1):S14–S80 [DOI] [PubMed] [Google Scholar]
- 10.Blevins TC, Bode BW, Garg SK, et al. : Statement by the American Association of Clinical Endocrinologists Consensus Panel on Continuous Glucose Monitoring. Endocr Pract 2010;16:730–745 [DOI] [PubMed] [Google Scholar]
- 11.Heinemann L, Franc S, Phillip M, et al. : Reimbursement for continuous glucose monitoring: a European view. J Diabetes Sci Technol 2012;6:1498–1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kovatchev B, Anderson S, Heinemann L, et al. : Comparison of the numerical and clinical accuracy of four continuous glucose monitors. Diabetes Care 2008;31:1160–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Damiano ER, McKeon K, El-Khatib FH, et al. : A comparative effectiveness analysis of three continuous glucose monitors: the Navigator, G4 Platinum and Enlite. J Diabetes Sci Technol 2014;8:699–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keenan DB, Mastrototaro JJ, Zisser H, et al. : Accuracy of the Enlite 6-day glucose sensor with Guardian and Veo calibration algorithms. Diabetes Technol Ther 2012;14:225–231 [DOI] [PubMed] [Google Scholar]
- 15.Calhoun P, Lum J, Beck RW, et al. : Performance comparison of the Medtronic Sof-Sensor and Enlite glucose sensors in inpatient studies of individuals with type 1 diabetes. Diabetes Technol Ther 2013;15:758–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Damiano ER, El-Khatib FH, Magyar KL, et al. : A comparative analysis of three continuous glucose monitors: not all are created equal [abstract]. Diabetes 2012;61(Suppl 1):A2 [Google Scholar]
- 17.Rodbard D: Characterizing accuracy and precision of glucose sensors and meters. J Diabetes Sci Technol 2014;8:980–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hannestad U1, Lundblad A: Accurate and precise isotope dilution mass spectrometry method for determining glucose in whole blood. Clin Chem 1997;43:794–800 [PubMed] [Google Scholar]
- 19.Borg R, Kuenen JC, Carstensen B, et al. : Associations between features of glucose exposure and A1C: the A1C-Derived Average Glucose (ADAG) study. Diabetes 2010;59:1585–1590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group, Beck RW, Buckingham B, et al. : Factors predictive of use and of benefit from continuous glucose monitoring in type 1 diabetes. Diabetes Care 2009;32:1947–1953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bergenstal RM, Tamborlane WV, Ahmann A, et al. : Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med 2010;363:311–320 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.