Abstract
Purpose.
We investigated the progressive nature of neurodegenerative structural changes following injury to retinal ganglion cell (RGC) axons using quantifiable and noninvasive in vivo imaging techniques.
Methods.
To track degenerative RGC progression in retinas following optic nerve crush (ONC) injury, spectral-domain optical coherence tomography (SD-OCT) was used to quantitate the RGC nerve fiber layer (NFL) density. The RGC soma cell density (RCD) was measured by confocal scanning laser ophthalmoscopy (CSLO). The RCD counts were performed using blood vessels as landmarks to anatomically track defined progressive changes in enhanced yellow fluorescent fusion protein (EYFP)-labeled RGCs.
Results.
Following ONC injury, 68% of the observed decrease in RCD measured by CSLO and 54% of the NFL thickness obtained by SD-OCT imaging (N = 4 retinas) occurred within the first week. Between days 7 and 14, an additional 22% decrease in RCD was concurrent with a 31% decrease in overall NFL thickness. Finally, between days 14 and 21, an additional 10% decrease in RCD measured in vivo by CSLO and 15% decrease in NFL thickness by SD-OCT was observed.
Conclusions.
Our data suggest that in vivo CSLO imaging of EYFP-RGC expression and SD-OCT measured NFL thickness are fast and reliable methods that longitudinally track neurodegenerative progression following ONC injury. Neurodegenerative changes in NFL thickness measured by SD-OCT imaging have the same overall trajectory as those observed by CSLO for RCD; however, changes in NFL thickness initially lag behind in vivo RGC soma counts with a slower decline in overall measurable change.
Keywords: glaucoma, scanning laser ophthalmoscopy, optical coherence tomography, retinal ganglion cells, neurodegeneration
Neurodegenerative sequelae of optic nerve crush can be detected in vivo by CSLO and SD-OCT imaging modalities. Both RGC density and NFL thickness decrease as a function of time after injury; however, RGC density by CSLO imaging demonstrates a steeper initial decline than changes to the NFL by SD-OCT.
Introduction
Trauma or disease can lead to irreversible cell death, especially for the adult central nervous system (CNS). Glaucomatous pathophysiology exemplifies the CNS's vulnerability to axonal damage. Glaucoma consists of a family of neurodegenerative diseases wherein structural damage to the retinal ganglion cell (RGC), ultimately is associated with cell death,1,2 resulting in a major cause of irreversible blindness worldwide.3,4
In animal models of RGC neurodegeneration, increased IOP and optic nerve damage have grave impact on RGC survival. Previous work has focused on the commonly used DBA/2J mouse glaucoma model5–7 as well as mechanical injury to RGC axons, such as optic nerve crush (ONC).8 The RGC density and gene expression traditionally have been assessed using histologic and biochemical methods at static time points after injury.9 Molecular assessment of RGC-specific markers, such as the glycoprotein Thy-1,10,11 exhibit early decreases in gene expression and commitment to apoptotic cell death.9,11–15
Recent in vivo imaging advances take advantage of the clear optical media in the eye for direct noninvasive visualization of the retina's neurons, thereby allowing for longitudinal assessment of neurodegenerative processes.16–20 Combining the use of confocal scanning laser ophthalmoscopy (CSLO) and Thy-1 promoter–driven enhanced yellow fluorescent fusion protein (EYFP) expression within RGCs, we can longitudinally track RGCs and neurodegeneration in vivo subsequent to ONC injury. Concurrent to direct RGC visualization, we also track nerve fiber layer (NFL) structural thickness in vivo through segmentation of spectral domain-optical coherence tomography (SD-OCT) retinal imaging, as has been described previously by our laboratory.21 Therefore, we can correlate structural thinning of the ganglion cell layer to progressively decreasing RGC density in vivo.
Our results showed significant decreases in RGC density and NFL thickness following injury in individual animals tracked longitudinally in vivo. More importantly, we found that the density of RGC somas decreased kinetically at a more rapid rate than NFL thinning in the same eyes compared in tandem with different in vivo imaging modalities. Parallel comparison of the results of these in vivo noninvasive technologies in a single animal eye over time allowed us to assess the effectiveness of current clinically applicable tools to track RGC-associated diseases and will ultimately yield more sensitive measures with which to follow therapeutic windows of opportunity in animal models of glaucoma in vivo.
Materials and Methods
Animals
All animals used in this study were treated in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. All animal procedures were approved by the University of Miami Institutional Animal Care and Use Committee. The Thy1-COP4/EYFP line 9 mice were obtained from The Jackson Laboratory (Bar Harbor, ME, USA) and expressed EYFP homogeneously in RGCs distributed throughout the retina.22,23 We used 2-month-old transgenic Thy1-COP4/EYFP line 9 mice for this work. The animals were maintained in a temperature-regulated environment with a 12-hour light and 12-hour dark cycle. All mice were fed ad libitum.
ONC Injury
Mice were anesthetized with an intraperitoneal injection of a ketamine/xylazine mixture. A limbal conjunctival peritomy was performed in the superior region and a conjunctival flap was reflected posterior to allow access to the posterior quadrants of the globe. The optic nerve then was exposed gently through a small window created between the surrounding muscle bundles and fatty tissue with careful blunt dissection with Westcott scissors. Care was taken not to damage muscles or the vortex veins. At a site 1 mm posterior to the globe, the optic nerve was clamped with a pair of Dumont No. 5 self-closing tweezers (Ted Pella, Inc., Redding, CA, USA) for 5 seconds. All animals used in this study were unilaterally injured with the ONC, while the contralateral eye only had an optic nerve exposure (i.e., no crush injury). After this procedure, topical antibiotic drops were applied to the surgical site and mice were observed for recovery. In the postoperative period, the mice exhibited normal eating and drinking behavior, and were given buprenorphine (0.1 mg/kg) subcutaneously, twice daily for two days following procedure for pain control.
In Vivo CSLO Imaging
A modified commercially available CSLO (HRA2; Heidelberg Engineering GmbH, Dossenheim, Germany) with a 30° wide field mounted to the camera with fluorescence filters was used for RGC imaging. The scan rate of the CSLO was 12 frames per second. Eye-tracking was activated during imaging, enabling retinal recognition technology to account for slight eye movements. Four mice (N = 4) were anesthetized with an intraperitoneal injection of a ketamine/xylazine mixture and then placed on a positioning stage. The eyes were dilated with topical tropicamide and the corneas were moistened during imaging with regular application of artificial tears (Systane, Alcon, TX, USA). Averaged images (50 images at the same retinal location are averaged automatically by built-in software to augment the signal-to-noise ratio) were captured and simultaneously displayed. Images were taken serially walking across the retina. Each retinal frame represents approximately 40% of total retinal area at an optical resolution of 5 to 11 μm, as per the manufacture's specifications. The resolution factor for the CSLO imaging using the Spectralis HRA machine were obtained from the manufacturer's website publication (available in the public domain at http://www.epirusvisioncenter.gr/hra2.pdf). Other laboratories also have experimentally described HRA-II image resolution.24 Care was taken to keep the corneal surface moist throughout imaging with artificial tears. Relative counts over a specific area selected in each animal allowed us to focus on the relative change in cell density over time over an anatomically defined area in each eye. The data represented change in cell density counted over an area with a mean of 25.8 square pixels (SD = 4.0). An area with clearly visible and anatomically defined landmarks, for all of the time points in each eye, was selected by a masked observer and subsequently counted by a different laboratory member who was masked to sample and time point. The CSLO images were exported to image analysis software (FIJI/ImageJ; National Institutes of Health [NIH], Bethesda, MD, USA) and cells were counted manually. Investigators responsible for counts were masked and did not participate in initial image acquisition, and were not aware of animal group designations. Cell density was followed for each animal over the same area using retinal vasculature as guideposts. Images for cell density quantification were obtained 1 to 2 mm above the optic nerve head. For each animal in the study, we obtain longitudinal cell density measures from the exact same location to track progressive degenerative changes within that specific retinal region over time. The location (temporal, nasal) selected for each animal had to be far enough (1–2 mm) from the optic nerve head to yield flat 2D images for quantification. Change in soma density measured by CSLO was calculated as a proportion of the preinjury baseline value as well as discrete changes at each week (or time point). The CSLO data were statistically analyzed using a repeated measures ANOVA model, which found that the amount of variation between time points was statistically significant relative to the amount of variation within time points using the F-test (F[df] = 286[3,9], P < 0.0001). Paired t-tests were used to assess differences between weekly measurements. All statistical analyses were performed using SAS (SAS Institute, Inc., Chicago, IL, USA).
Immunofluorescence and Microscopy of Retina Whole Mounts
Animal globes were placed in 10% neutral phosphate buffered formalin fixative (NBF-4-G; Azer Scientific, Morgantown, PA, USA) overnight, retinas were dissected, rinsed in PBS (127 mM NaCl, 2.7 mM KCl, 10 mM phosphate) at 25°C, immunostained and then flat-mounted on a glass slide with DAPI Vectashield (Vector, Burlingame, CA, USA) and a coverslip. Goat-anti-green fluorescent protein (GFP) primary antibody (#600-101-215; Rockland, Gilbertsville, PA, USA) was diluted at 1:250 in PBS containing 0.5% Triton X-100 (IB07100; Shelton Scientific, Shelton, CT, USA). Donkey-anti-goat Alexa 488 (1:100 dilution, # A11055; Molecular Probes, Eugene, OR, USA) secondary antibody was diluted in PBS. As per the manufacturer's protocol, the anti-GFP antibody used in this study is designed to detect GFP and its variants (as do many other anti-GFP antibodies), such as yellow fluorescent protein (YFP), enhanced GFP (eGFP), and EYFP, which are all genetic mutants of GFP (available in the public domain at http://www.abcam.com/gfp-antibody-chip-grade-ab290.html and http://rockland-inc.com/store/Antibodies-to-GFP-and-Antibodies-to-RFP-600-101-215-O4L_24115.aspx). Retina whole mounts were blocked for nonspecific antibody binding with Rodent Block M (RBM96; BioCare Medical, Concord, CA, USA) for 30 minutes, incubated for 72 hours at 4°C with primary antibody, washed with PBS, incubated for 72 hours at 4°C with secondary antibody, washed with PBS, and then mounted onto glass coverslips using 4′6-diamidino-2-phenylindole (DAPI, Vectashield; Vector). Images were acquired with an inverted microscope (Axiovert 200M; Carl Zeiss Meditec, Oberkochen, Germany) running Zeiss AxioVision 4.7.2 software (Zeiss, Inc., Thornwood, NY, USA). Confocal microscopy images were obtained with a Leica TCP SP5 spectral confocal microscope (×63, water-immersion, 1.2 NA objective; Leica, Exton, PA, USA).
For immunofluorescence of sectioned retinas, animal globes were placed in 10% neutral phosphate buffered formalin fixative (NBF-4-G; Azer Scientific) overnight. After fixation, globes were embedded in paraffin, sectioned into 8-μm slices, and mounted on glass slides. Sectioned slides were deparaffinized and immunostained with goat-anti-GFP (1:250 dilution, #600-101-215; Rockland) and mouse-anti-Brn3a (1:100 dilution, #SC-8429; Santa Cruz Biotechnology, Santa Cruz, CA, USA) antibodies. Secondary antibodies were: donkey-anti-goat Alexa 488 (1:100 dilution, #A11055; Molecular Probes) and donkey-anti-mouse Alexa 546 (1:100 dilution, #A11030; Molecular Probes).
Cross-sectioned retina mounted on slides presented in Figure 1 were deparaffinized in xylene and sequential alcohol rinses, then treated with 95°C Trilogy (CMX833-C; Cell Marque, Rocklin, CA, USA) antigen retrieval reagent. Tissue sections were subsequently blocked with Rodent Block M (RBM96; BioCare Medical, Concord, CA, USA) for 30 minutes to reduce nonspecific antibody binding. Slides were incubated overnight at 4°C in PBS containing primary antibodies, washed with PBS, incubated in antibody buffer containing secondary antibodies for 1 hour at room temperature, washed with PBS, and mounted onto glass coverslips using DAPI Vectashield (Vector). Confocal microscopy images were obtained in a Leica TCP SP5 spectral confocal microscope (×63, water-immersion, 1.2 NA objective; Leica). Cell counts were taken across the extent of each retinal section examined transecting the optic nerve head providing total RGC counts at the longest retinal axis. Anti-Brn3a localizes RGC nuclei while anti-GFP signal localizes to RGC cell membranes resulting in several cross-sectioned cells missing Brn3a signal, because the cell's nucleus was present in a subsequent section while part of the cell's much larger cytoplasmic area is seen in each 8-μm section obtained. Total anti-Brn3a cell counts and anti-GFP cell counts of optic nerve crushed retinas and uninjured fellow eye sham optic nerve exposure retinas were compared using a paired t-test; P < 0.05 was considered statistically significant. Cross-sections immunostained using the anti-Brn3 antibody were treated with an antigen retrieval protocol. This step does not inhibit anti-GFP antibody binding, and was necessary for anti-Brn3 antibody binding. Panels presented in Figure 1 were treated with the same protocol as is highlighted by the merged image containing overlapping specific antibody stained patterns on the same retinal section consistent with both antibodies detecting cells within the ganglion cell layer. Mice used for these illustrative figures were not the same animals used for our analytical measures of in vivo neurodegenerative progression.
Figure 1.
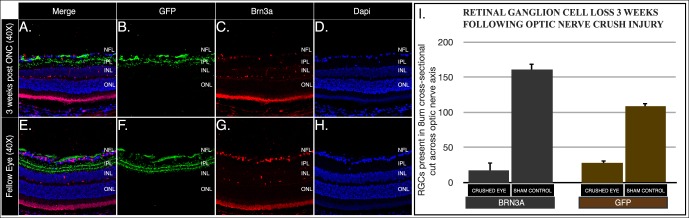
Histological analysis of RGC survival following ONC injury. Representative images from cross-sectioned globes 3 weeks following ONC (A–D) and uninjured sham nerve exposure (E–H). The RGC counts were obtained from sections across the optic nerve head for EYFP- and Brn3a-expressing cells (I). The average number of cells and standard deviation are shown. Sectioned retinas were immunostained with anti-GFP (green) and anti-Brn3a (red) antibodies. The EYFP fluorescence is enhanced using anti-GFP antibodies.
SD-OCT Mouse Imaging
An SD-OCT system designed for small animal imaging (Bioptigen, Research Triangle Park, NC, USA) was used for in vivo imaging of the murine retina. The axial resolution of the system is approximately 5 μm in retinal tissue per the manufacturer. Mice were anesthetized with an intraperitoneal injection of a ketamine/xylazine mixture then placed on a positioning stage. The head was fixated on a stabilizing bar and a heating blanket was placed under the anesthetized mouse. Eyes were dilated with topical tropicamide and corneas were moistened during imaging with regular application of artificial tears (Systane). Raster scans centered on the optic disc consisted of 1000 × 100 (horizontal × vertical) depth scans covering an area of 1 × 1 mm2 of the mouse retina. Alignment of the mouse occurred within less than a minute, and image acquisition required approximately 2 seconds per eye. No depth enhancement modifications have been made for imaging (similar to our previous published work using SD-OCT imaging). The images presented as representative examples, as well as the images used for the analyses, were of high quality amenable for manual segmentation.
The SD-OCT retinal layer manual segmentation was obtained for the OCT images acquired from control and injured mouse eyes, as per our established published protocols.21 Thickness maps resulting from segmentation of the RGC layer (RGCL) and inner plexiform layer (IPL) boundaries were calculated together with the average combined layer thickness and SD for each eye. The injury stage for each analyzed eye was masked for manual segmentation. Segmentation was repeated masked with similar results to validate the reliability of our manual segmentation results. The SD-OCT thickness data were fit using a repeated measures ANOVA model which found that the amount of variation between time points was statistically significant relative to the amount of variation within time points using the F-test (F[d] = 63.69 [3,9], P < 0.0001). Paired t-tests were used to assess differences between weekly measurements. All statistical analyses were performed using SAS.
Results
The RGC counts on sectioned retinas immunostained for anti-Brn3a (a well-recognized RGC marker, Figs. 1C, 1G) and anti-GFP (Figs. 1B, 1F) demonstrated a >80% reduction in RGC survival 3 weeks following ONC injury in comparison with uninjured optic nerve exposure sham controls retinas (Fig. 1). The Thy1-COP4/EYFP line 9 mouse expresses EYFP in the soma, dendrites, and axon of RGCs homogeneously distributed throughout the retina (Fig. 2A). Histological methods require that an animal be sacrificed for each specific time point analyzed. In contrast, clinical in vivo imaging modalities with modified optics for the mouse eye allow for direct and longitudinal visualization of retinal central nervous system (CNS) tissues of transgenic animals (Figs. 2B–D) and allow for tracking of the clinical course of optic nerve damage. An en face projection of SD-OCT B-scans illustrates the retinal vasculature exiting the optic nerve head (Fig. 2C). The CSLO imaging of transgenic mice expressing EYFP in RGC soma and axon illustrate RGCs as fluorescent dots with central projections of fluorescent axon bundles directed toward the optic nerve head (Fig. 2D).
Figure 2.
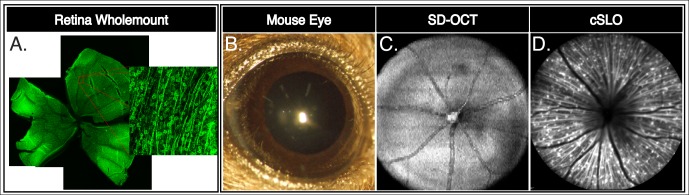
The RGCs expressing EYFP are homogeneously distributed throughout the retina of Thy1-COP4/EYFP transgenic mice. Representative retina whole mount montage showing RGCs evenly distributed throughout the retina of these transgenic mice (A). High magnification blow-out image illustrates RGC soma, dendrites, and axons contain EYFP fluorescence. The EYFP fluorescence is enhanced using anti-GFP antibodies. The mouse eye provides a window to visualize CNS structures in vivo by SD-OCT and CSLO (B–D). Depth-resolving SD-OCT highlights retinal microstructure, such as vessel architecture in the adult mouse retina in vivo (C). The CSLO imaging over the optic nerve head highlights vessels as areas devoid of fluorescence while Thy-1 driven EYFP expression show the RGC soma, dendrites, and axons (D).
Longitudinal RGC density following ONC injury visualized by CSLO imaging provides quantitative and topographic information across the retina of fluorescent transgenic retinas. A representative example of RGC density changes in single animal over a 3-week period is illustrated (Fig. 3). Figure 3 depicts the progressive decrease in RGCs over a similar area taking advantage of the unchanging retinal vasculature as landmarks for imaging the same region over time. In vivo imaging allows for direct longitudinal quantification of RGCs, as assessed by transgene EYFP expression, using a single animal at multiple time points. Preinjury baseline imaging shows a high confluence of fluorescent RGCs making individual RGC identification difficult. As RGCs die due to ONC injury, individual cells are more easily identified. At the 3-week postinjury time point, contrast of such few remaining RGCs allow for visualization of RGC dendritic arborization in the IPL of the retina.
Figure 3.
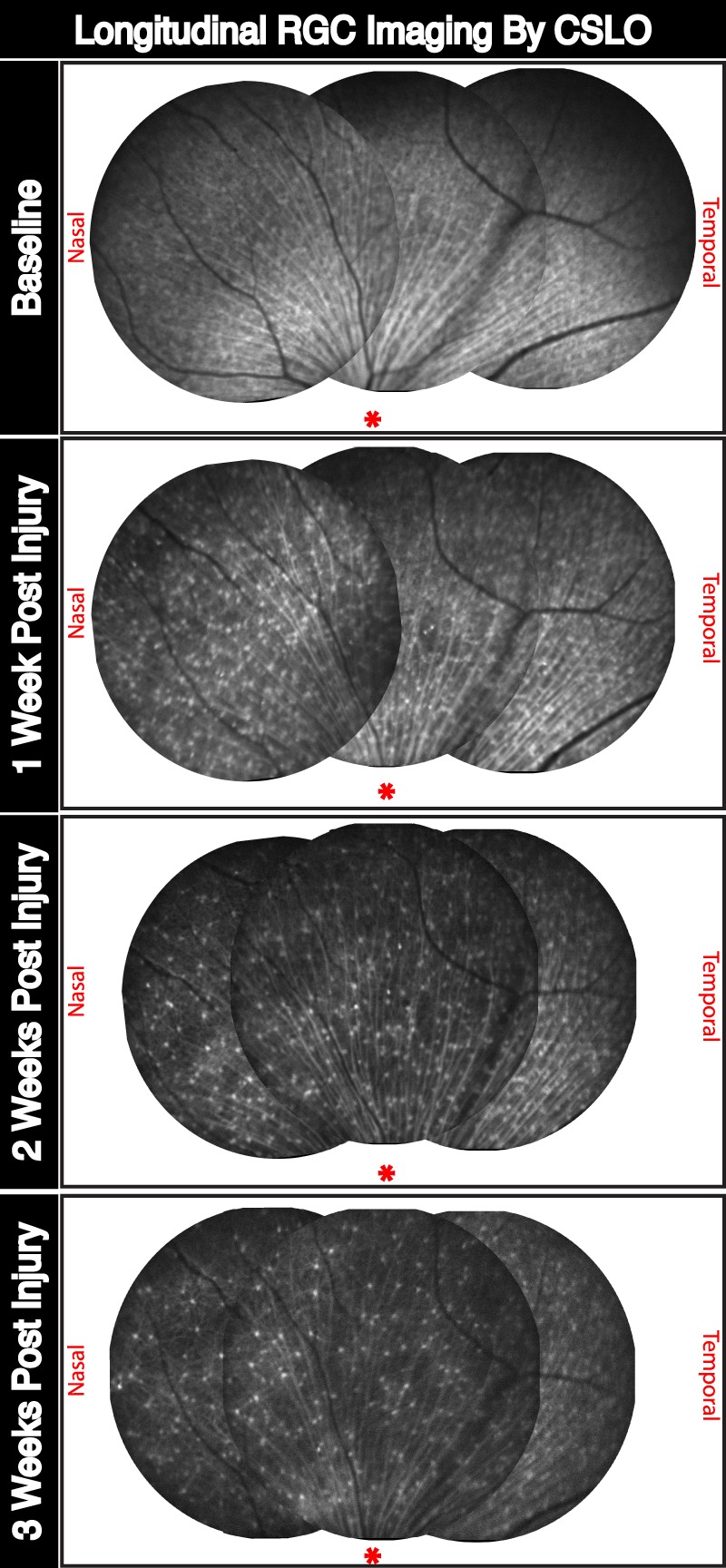
In vivo longitudinal and topographic assessment of neurodegenerative progression following ONC injury of adult RGC by CSLO imaging. Representative fundus images tracking longitudinal RGC density for a single mouse retina following ONC injury. Images were taken before, and 1, 2, and 3 weeks after injury. Tiled images represent a topographical view of the superior aspect of the retina followed longitudinally using vascular morphology as reference points. *Approximate location of the optic nerve head for reference. Nasal and temporal sides are labeled accordingly.
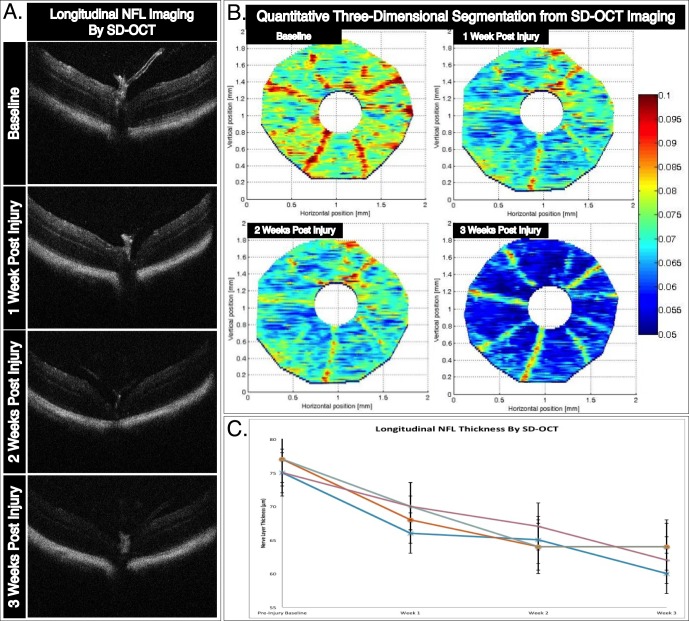
Longitudinal changes to retinal structure obtained by SD-OCT imaging immediately following CSLO imaging track the kinetics of progressive pathological changes to the NFL structure and RGC cell density. The SD-OCT images were centered at the posterior pole of each animal at every time point. Representative B-scans across the optic nerve head of each experimental retina are shown at multiple longitudinal time points (Fig. 4A), and show clearly distinct retinal layers. Manual segmentation of over 100 B-scans at each time point allow for construction of three-dimensional NFL thickness maps21 (Fig. 4B). The optic nerve, represented as a 245-μm radius circle centered on the elevated hyaloid remnant, was removed from our segmentation analysis so that the analysis measured the retina, as typically is performed for clinical assessment of human NFL. A color scale bar representing thickness measures ranging from 50 to 100 μm is shown. The average thickness of each manually segmented retina was calculated using a previously described segmentation algorithm modified for use with images obtained using the Bioptigen SD-OCT machine.21,25 These average thickness values and SD are plotted longitudinally for individual retinas (Fig. 4C). The average thickness across 4 retinas imaged longitudinally at preinjury baseline, and 1, 2, and 3 weeks after ONC injury were 76 (SD 1.1), 69 (SD 1.9), 65 (SD 1.4), and 63 (SD 1.9) μm, respectively.
Figure 4.
In vivo longitudinal and topographic assessment of NFL thickness as a surrogate marker for RGC survival. (A) Representative B-scan cross-sectional images tracking longitudinal thinning of the NFL layer following ONC injury. The B-scan images were taken directly over the optic nerve head at baseline, and 1, 2, and 3 weeks after injury. (B) Three-dimensional retinal thickness maps obtained through manual segmentation of SD-OCT imaging show progressive pathophysiological changes to the mouse retina following ONC injury. In this representative example, a single mouse retina is tracked over three weeks following injury. (C) Average NFL thickness for each manually segmented retina decreased in thickness following ONC. Topographic three-dimensional retina thickness maps were obtained by segmentation of NFL thickness of over 100 B-scans per retina. Error bars: standard deviation.
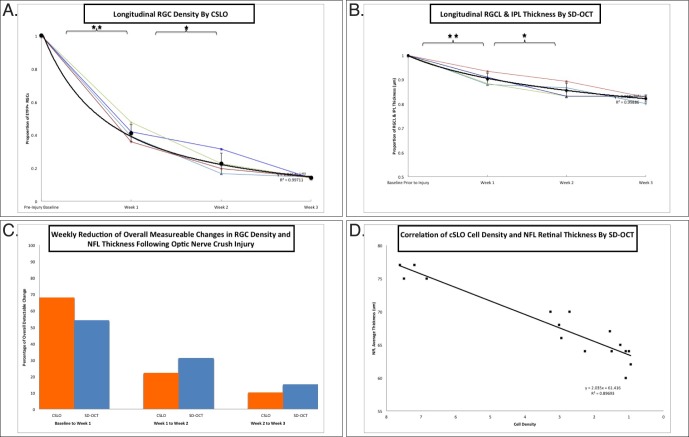
Weekly in vivo measurements of the proportion of EYFP+ RGCs observed by CSLO imaging relative to baseline cell counts were performed (Fig. 5A). Each line traces a single animal longitudinally from preinjury baseline to week 3 following ONC injury. Similarly, estimates of the proportion of segmented NFL thickness measurements also are shown (Fig. 5B). These results are summarized in the Table. Murine retinas demonstrate similar cell density changes and NFL thickness changes at each time point assessed. Individual and average EYFP+ RGC density is plotted (Fig. 5A) showing statistically significant reductions in cell density from baseline to week 1 (P < 0.01) and from week 1 to week 2 (P < 0.01). The average cell density obtained by CSLO in units of cells per pixel area at 1, 2, and 3 weeks following ONC injury were 7.3 (SD 0.3), 2.3 (SD 0.2), 1.6 (SD 0.4), and 1.0 (SD 0.1). At each week after ONC, a reduction in RGC density was detected. Approximately 68% of the total change in EYFP+ RGCs was observed in the first week following injury. Over the next 7 days, there was a 22% further reduction of the detected change, followed by a 10% decrease over the last 7 days (Fig. 5C). A best-fit power trend-line was fitted comparing each retina's longitudinal cell density dynamics (R2 = 0.99). Individual and average NFL thickness values relative to baseline are plotted (Fig. 5B). We found no significant changes in our sham optic nerve exposure when looking at baseline counts in animals of different ages to assess for any age-related changes over a 3-week period (data not shown).
Figure 5.
Quantification of longitudinal RGCs density by CSLO, SD-OCT. (A) Proportion of EYFP-positive RGCs surviving 3 weeks following ONC injury. Thin colored lines represent individual eye cell density followed longitudinally for 3 weeks. Black circles represent the mean value obtained from all eyes. A best-fit line was fitted as a power function (R2 = 0.99). Error bars: standard deviation. (B) Proportion of manually segmented NFL retina thickness. Thin colored lines represent individual retina average thickness followed longitudinally for 3 weeks. Black circles represent the mean value obtained from all eyes. A best-fit line was fitted as a power function (R2 = 0.99). Error bars: standard deviation. (C) Relative percentage reduction of total observable change is depicted for changes over first, second, and third weeks following ONC by CSLO (orange bars) and SD-OCT (blue bars). (D) Scatterplot using CSLO cell density counts and calculated retinal thickness as variables for all data points. A best-fit line was fitted as a linear function (R2 = 0.89). Significant difference with *P < 0.05 or **P < 0.01, respectively, by the paired t-test. Quantification of cell density by CSLO anatomically defined cell counts, and NFL thicknesses by SD-OCT manual segmentation of B-scans were performed by masked examiners. A total of four retinas (N = 4) was longitudinally analyzed for these experiments.
Table.
ONC Injury Induced Reduction in RGC Density by CSLO Imaging and RGCL Thickness by Manual Segmentation of SD-OCT B-Scans
|
Preinjury Baseline |
Wk 1 |
Wk 2 |
Wk 3 |
|
| CSLO imaging | ||||
| Eye 1 | 7.5 | 2.9 | 1.2 | 1.1 |
| Eye 2 | 7.2 | 3 | 2.3 | 1 |
| Eye 3 | 7.6 | 2.7 | 1.5 | 1.1 |
| Eye 4 | 6.8 | 3.3 | 1.5 | 0.9 |
| Relative overall decrease | 68% | 22% | 10% | |
| SD-OCT imaging | ||||
| Eye 1 | 75 | 66 | 65 | 60 |
| Eye 2 | 77 | 68 | 64 | 64 |
| Eye 3 | 77 | 70 | 64 | 64 |
| Eye 4 | 75 | 70 | 67 | 62 |
| Relative overall decrease | 54% | 31% | 15% |
Statistically significant reductions in SD-OCT measured NFL thickness are observed from baseline to week 1 (P < 0.05) and from weeks 1 to 3 (P < 0.01). Unlike retinal soma density changes observed with CSLO, structural changes to NFL thickness between weeks 1 and 2 were not statistically significant. Approximately 9.9% (SD 2.5) of the measured NFL thickness decreased in the first week following injury. Between weeks 1 and 2, there was a drop of 4.6% (SD 2.7) in structural thickness and another 3.3% (SD 3.8) drop between weeks 2 and 3 following injury (Fig. 5C). Structural integrity of NFL thickness relative to baseline was approximately 90% 1 week after injury, 86% 2 weeks after injury and 82% 3 weeks after injury. A best-fit power trend-line was fitted to the average longitudinal NFL thickness dynamics (R2 = 0.99). A comparison of SD-OCT NFL thickness and CSLO cell density is illustrated on a scatterplot (Fig. 5D). A correlation between the average NFL thickness and cell density was observed (R2 = 0.89).
Discussion
The CSLO and SD-OCT methods provide investigators with reliable and efficient means to longitudinally and topographically track progressive neurodegenerative changes in a single animal's eye in vivo and noninvasively. This study reliably assessed retinal neurodegenerative progression by comparing longitudinal changes to the NFL structure following RGC injury and longitudinal changes to RGC soma density using noninvasive in vivo imaging techniques. Neurodegenerative studies in the CNS have traditionally depended on histological methods for quantitative assessment of pathology. In such studies, using the retina as a model for CNS neurodegenerative diseases requires time-dependent enucleation of eyes, thereby providing a static measure of in vivo pathology progression.
Using SD-OCT and CSLO imaging allows investigators and clinicians to visualize the laminated retinal morphology noninvasively in vivo. Our laboratory has validated an effective approach using manual segmentation of SD-OCT images from living animals as a surrogate marker for RGC density in the murine retina.21 This experimental approach was designed to closely mimic the clinical assessment of RGC survival used for human glaucoma patients. Segmentation of SD-OCT images allowed assessment of quantitative and qualitative differences in RGC layer thickness between phenotypically wild-type and Brn3b−/− animals, which are born with approximately 30% of the normal number of RGCs.21,26–28 Our analysis of NFL thickness demonstrated that gross structural differences between transgenic animals born with large differences in RGC numbers correlate strongly with RGC counts obtained by histology. It remained unclear, however, whether our SD-OCT image analyses would acquire more subtle structural changes across a single retina tracked in vivo over time.
In this work we assessed the acute progression of pathological RGC death, such as those observed following ONC injury. We compared statistically significant NFL structural thinning to measure concomitant changes in RGC density by adding noninvasive in vivo CSLO imaging using Thy1-COP4/EYFP mice (Thy1-EYFP) with labeled RGCs. The data showed a similar general trend of RGC soma density and NFL thickness depicting a parallel progressive decline over the 3-week timeline following ONC injury. Based on our NFL thickness heat maps and CSLO imaging observations, we did not detect differential quadrants of NFL thinning or quadrant-specific changes in RGC density in response to ONC injury, as also has been reported by histology-based studies.29 Additionally, based on analysis of data 3 weeks after ONC, the percentage change observed in NFL thickness by SD-OCT initially lag those observed by CSLO for RGC soma in vivo imaging. This is not surprising, since SD-OCT measures the combination of space occupied by the RGC soma, axon, and shared dendritic structures as observed histologically, in contrast with RGC soma density measured by in vivo CSLO imaging. Our approach directly compares injury-induced changes in RGC-specific gene expression to structural changes in the retina as RGCs progressively die and the NFL degenerates. Despite having different geographical locations selected for cell-counts (temporal, nasal, superior, or inferior based upon consistent image quality for analyses in the same quadrants over time), the kinetics of density change over time over the same retinal region were remarkably reproducible and statistically significant (per data shown in Fig. 5). Thus, comparisons of longitudinal changes are valid and reproducible events for comparison relative to SD-OCT for the NFL and CSLO for retinal soma density.
The Thy1-EFYP mouse line takes advantage of the cell surface glycoprotein Thy1 promoter to drive a ChR2-YFP fusion protein.23 In the retina, Thy-1 has been used widely as an RGC marker, making the Thy1-COP4/EYFP animal an ideal tool for research assessing RGCs. Thy1-EYFP mice express EYFP in approximately 40% of homogeneously distributed RGCs (Fig. 2).22 Histological analysis of cross-sectioned retinas revealed EYFP expression limited to somas, dendrites, and axons of RGCs homogeneously distributed throughout the retina (Fig. 1). Thy-1 expression has been reported previously in a small subset of cells present in the IPL.16–18,30 Congruent with findings by Thyagarajan et al.,22 we found very few, if any, EYFP-positive cells outside of the RGC layer in Thy1-COP4/EYFP retinas, allowing us to compare NFL structural changes to RGC-specific EYFP signal density in vivo.16–18,31 To follow longitudinal changes in cell density by CSLO in vivo imaging, the authors make the necessary assumption that a loss of detectable EYFP signal represents a dead or dying cell. It is important to note, however, that decreased Thy1 expression may not necessarily represent cell death, and also may be a consequence of decrease mRNA and protein expression in injured cells.9,12
Our histology data demonstrated a >80% reduction in surviving RGCs 3 weeks following ONC injury. We observed comparable results when assessing changes in RGC density using the well-established RGC marker Brn3a (Fig. 1). In contrast with the easily discernible nucleus-localized Brn3a immunofluoresence signal (since Brna3a is a nuclear transcription factor), distinguishing neighboring EYFP signal spread throughout the soma and dendritic tree is far more challenging. This may explain the small difference in RGC counts we observed by histology. While using the in vivo CSLO imaging techniques, endogenous EYFP signal was used for quantification; however, for microscopic analyses we took advantage of ubiquitously used anti-GFP antibody targeted to our EYFP+ cells. The EYFP expression in the retina of these mice is limited to approximately 40% of RGCs, possibly resulting in a reduced total number of positive EYFP cells when compared to Brn3a-positive cells. These differences do not affect this study as EYFP-positive cells are distributed throughout the retina represented by many subtypes of RGCs, providing a suitable reference point for tracking injury-induced cell death throughout the retina and for the same region of cells observed over time in vivo. Even with a limited number of EYFP-positive RGCs in our transgenic model, baseline RGC counts from CSLO images proved to be a logistical challenge due to lack of contrast. Dendrites, axon, and soma of EYFP-positive RGCs significantly hindered rapid quantification of cells by investigator-driven counts. Automated cell counting using several software programs was unsuccessful until the 3-week time-point with fewer RGC somas for quantification (data not shown), whereas our manual segmentation was robust and provided statistically significant measurements. Using a nuclear localized fluorescent marker may provide a better target for future studies requiring high throughput automated quantification of CSLO RGC soma data.
Our in vivo longitudinal RGC density results from CSLO imaging showed that following ONC injury, approximately 59% of cells are no longer expressing EYFP signal by the first week, approximately 78% by the second week and approximately 86% by the third week after injury, relative to baseline (Fig. 5, Table). These kinetics closely resemble histopathological findings showing close to 60% reduction in RGCs 1 week after injury, with an initially fast onset over the first week followed by a slow continued reduction in cells thereafter.29,32–34 This also is corroborated by recent injury-induced CSLO RGC density kinetics by others.20 In work using an optic nerve transection injury model, Chauhan et al.20 elegantly describe longitudinal kinetics by CSLO in one group of animals and use another set of animals for SD-OCT imaging. This differs from our approach in a few ways. Our study followed each animal longitudinally using both imaging methods in tandem, allowing us to compare cell density changes directly to structural changes in the NFL. Furthermore, unlike the modest reduction in retinal thickness presented in their study, our manual segmentation approach allowed us to focus on the layers wherein RGC soma, axons, and dendrites reside, thereby yielding earlier distinguishable differences between time-points analyzed by our SD-OCT imaging. Remaining consistent with our previous work quantitating the NFL, the SD-OCT data presented are specific to manually segmented NFL thickness, defined as IPL + RGCL combined thickness. The reason for inclusion of the IPL and RGCL is related to current resolution parameters available in SD-OCT mouse imaging. Our approach has clinical relevance, as several NFL analysis programs used for human glaucoma analysis use not only a NFL analysis that includes the IPL, but also full thickness retina as surrogate markers for NFL change in the retina, including new work on the use of macular retinal thickness to correlate with areas of visual field loss.
Through week 1, we observed an approximately 54% decrease in NFL thickness, approximately 31% in week 2, and approximately 15% in week 3 following ONC injury (Fig. 5). Additionally, our manual segmentation of the NFL further allowed us to build three-dimensional thickness spatial models showing a progressive structural thinning that is symmetrical and nonlocalized (Fig. 4), in accordance with our observed findings by CSLO imaging of RGCs (Fig. 3). This 3D volumetric analysis is similar to what currently is performed in humans with a much larger eye and a greater number of RGCs with automated segmentation in contrast to our murine model, which requires manual segmentation because of the much smaller scale and lack of software sophisticated enough to match manual segmentation. This work demonstrates that decreasing RGC soma density proceeds more rapidly than NFL thinning, and that nerve fiber thinning depicts a slower degenerative process with ONC. It should be noted that our CSLO density kinetics appear to be somewhat slower than those reported by Leung et al.16 This difference may be due to different density measurement methodologies or expression profile in the mice studied, but in either case, both studies support a faster decrease in in vivo longitudinal Thy1 signal as compared to our observed NFL thinning kinetics. Several histologically-guided studies looking at RGC death in response to RGC insult suggest nerve degeneration as a biological event that precedes RGC soma loss.35–37 Using our in vivo approach, we obtained data tht depict disruption of RGC Thy1-derived EYFP signal expression as an early event, which is subsequently accompanied by NFL axonal thinning. Whether this finding is a limitation of these in vivo imaging modalities or suggests a more functional explanation into the pathophysiology of the RGC and axon injury process remains to be elucidated. We also must take into account the sample size in our observed findings. The sample size studied with our longitudinal imaging approach is limited by the need for near perfect imaging conditions at each time point assessed, and laborious manual nerve thickness segmentation and RGC density counts. Our comparison of both imaging technologies followed the same eyes over time and results are representative of each imaging modality with the same sample size (N = 4). The sample size also highlights our ability to take advantage of imaging technologies available to us to decrease the number of animals needed to track degenerative changes. We observed measurable degenerative progression from each eye and at every time point. Furthermore, our approach followed a specific area within each retina by CSLO imaging, which allowed us to obtain changes in cell density of the same dedicated area within the retina as a direct correlate to observed changes in NFL thickness measurements (which is very difficult and time-consuming to perform with histology without assurance that the exact same regions are compared with multiple different animals representing each region).
We provide statistical analysis of our data for CSLO and SD-OCT appropriate for small sample sizes using the paired t-test, which has been demonstrated in the literature as an adequate and appropriate measure for the sample size.38 Although baseline counts were very tediously obtained, which also explains the need for experienced image acquisition during baseline and subsequently acquisition at other longitudinal time points (a limiting factor for sample size) with clear media and other imaging quality factors, our RGC counts were consistent between masked analysts and statistically significant using various analyses. In addition to comparing our RGC density to the baseline results, we also compared our data at each time point to other relevant time points in the study. We believe this is a sound methodology and this is elaborated further by our statistical analyses, which show very close density measurement correlations observed at each given time point at each specific location for the murine retinas assessed.
This work takes advantage of tandem CSLO and SD-OCT clinical imaging modalities to assess and correlate structural and cell density changes to the RGC layer following RGC axonal injury. We found significant changes to the cellular density and structural thickness of injured RGCs undergoing similar kinetics over the course of 3 weeks following injury. Unlike other published reports assessing longitudinal neurodegenerative progression via similar methods, we compared our established imaging modalities on the same animal in tandem allowing us to directly correlate cell density and RGC layer thinning longitudinally and noninvasively in vivo. Furthermore, by using our optimized previously reported SD-OCT manual segmentation approach, we focus on 3-dimensional changes in structure limited to the RGC layer and IPLs where RGC somas, axons, and dendrites reside, providing a foundation for tracking early changes in retinal structure following acute optic nerve injury.
These data suggested potential windows of opportunity for the assessment of neuroprotective therapy effectiveness using efficient noninvasive in vivo imaging techniques in a prospective manner. The goal and scope for this study was to follow neurodegenerative progression using two clinically relevant technologies assessing two different structural retina components for which we have developed expertise in the human and mouse settings, and now take advantage of mouse genetics, which allow for further use of these techniques (fluorescently labeled RGCs), to investigate a longitudinal pathophysiological process (ONC). Using these techniques in a methodical fashion has allowed us to obtain two separate measures of neurodegenerative progression across the same exact animals and analyze the longitudinal progression of RGC loss using an increasingly powerful and experimentally applicable in vivo approach.
Acknowledgments
Supported by NIH National Eye Institute (NEI; Bethesda, MD, USA) Grants EY022872 (GCM) and EY016775 (RKL), the donors of the National Glaucoma Research program of the BrightFocus Foundation, an unrestricted research grant to the Bascom Palmer Eye Institute from Research to Prevent Blindness, and NIH Center Grant EY014801. The authors alone are responsible for the content and writing of the paper.
Disclosure: G.C. Munguba, None; S. Galeb, None; Y. Liu, None; D.C. Landy, None; D. Lam, None; A. Camp, None; S. Samad, None; M.L. Tapia, None; R.K. Lee, None
References
- 1. Quigley HA. Ganglion cell death in glaucoma: pathology recapitulates ontogeny. Aust N Z J Ophthalmol. 1995; 23: 85–91 [DOI] [PubMed] [Google Scholar]
- 2. Quigley HA, Nickells RW, Kerrigan LA, Pease ME, Thibault DJ, Zack DJ. Retinal ganglion cell death in experimental glaucoma and after axotomy occurs by apoptosis. Invest Ophthalmol Vis Sci. 1995; 36: 774–786 [PubMed] [Google Scholar]
- 3. Quigley HA. Number of people with glaucoma worldwide. Br J Ophthalmol. 1996; 80: 389–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Quigley HA. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006; 90: 262–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Anderson MG, Smith RS, Hawes NL, et al. Mutations in genes encoding melanosomal proteins cause pigmentary glaucoma in DBA/2J mice. Nat Genet. 2001; 30: 81–85 [DOI] [PubMed] [Google Scholar]
- 6. John SWM, Chang B, Smith RS, et al. Nat Genet. 1999; 21: 405–409 [DOI] [PubMed] [Google Scholar]
- 7. Munguba GC, Camp AS, Risco M, Tapia ML, Bhattacharya SK, Lee RK. Vesicular glutamate transporter 3 in age-dependent optic neuropathy. Mol Vis. 2011; 17: 413–419 [PMC free article] [PubMed] [Google Scholar]
- 8. Dowling JE. The Retina. Cambridge, MA: Belknap Press of Harvard University Press; 1987. [Google Scholar]
- 9. Schlamp CL, Johnson EC, Li Y, Morrison JC, Nickells RW. Changes in Thy1 gene expression associated with damaged retinal ganglion cells. Mol Vis. 2001; 7: 192–201 [PubMed] [Google Scholar]
- 10. Barnstable CJ, Dräger UC. Thy-1 antigen: a ganglion cell specific marker in rodent retina. Neuroscience. 1984; 11: 847–855 [DOI] [PubMed] [Google Scholar]
- 11. Perry VH, Morris RJ, Raisman G. Is Thy-1 expressed only by ganglion cells and their axons in the retina and optic nerve? J Neurocytol. 1984; 13: 809–824 [DOI] [PubMed] [Google Scholar]
- 12. Huang W, Fileta J, Guo Y, Grosskreutz CL. Downregulation of Thy1 in retinal ganglion cells in experimental glaucoma. Curr Eye Res. 2006; 31: 265–271 [DOI] [PubMed] [Google Scholar]
- 13. Nash MS, Osborne NN. Assessment of Thy-1 mRNA levels as an index of retinal ganglion cell damage. Invest Ophthalmol Vis Sci. 1999; 40: 1293–1298 [PubMed] [Google Scholar]
- 14. Osborne NN, Larsen AK. Antigens associated with specific retinal cells are affected by ischaemia caused by raised intraocular pressure: effect of glutamate antagonists. Neurochem Int. 1996; 29: 263–270 [DOI] [PubMed] [Google Scholar]
- 15. Li Y, Schlamp CL, Nickells RW. Experimental induction of retinal ganglion cell death in adult mice. Invest Ophthalmol Vis Sci. 1999; 40: 1004–1008 [PubMed] [Google Scholar]
- 16. Leung CK-S, Weinreb RN. Experimental detection of retinal ganglion cell damage in vivo. Exp Eye Res. 2009; 88: 831–836 [DOI] [PubMed] [Google Scholar]
- 17. Leung CKS, Lindsey JD, Crowston JG, et al. In vivo imaging of murine retinal ganglion cells. J Neurosci Methods. 2008; 168: 475–478 [DOI] [PubMed] [Google Scholar]
- 18. Leung CK, Lindsey JD, Crowston JG, Lijia C, Chiang S, Weinreb RN. Longitudinal profile of retinal ganglion cell damage after optic nerve crush with blue-light confocal scanning laser ophthalmoscopy. Invest Ophthalmol Vis Sci. 2008; 491: 4898–4902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Leung CK, Lindsey JD, Chen L, Liu Q, Weinreb RN. Longitudinal profile of retinal ganglion cell damage assessed with blue-light confocal scanning laser ophthalmoscopy after ischaemic reperfusion injury. Br J Ophthalmol. 2009; 93: 964–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chauhan BC, Stevens KT, Levesque JM, et al. Longitudinal in vivo imaging of retinal ganglion cells and retinal thickness changes following optic nerve injury in mice. PLoS One. 2012; 7: e40352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Camp AS, Ruggeri M, Munguba GC, et al. Structural correlation between the nerve fiber layer and retinal ganglion cell loss in mice with targeted disruption of the Brn3b gene. Invest Ophthalmol Vis Sci. 2011; 52: 5226–5232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Thyagarajan S, van Wyk M, Lehmann K, Löwel S, Feng G, Wässle H. Visual function in mice with photoreceptor degeneration and transgenic expression of channelrhodopsin 2 in ganglion cells. J Neurosci. 2010; 30: 8745–8758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang H, Peca J, Matsuzaki M, et al. High-speed mapping of synaptic connectivity using photostimulation in Channelrhodopsin-2 transgenic mice. Proc Natl Acad Sci U S A. 2007; 104: 8143–8148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Maass A, von Leithner PL, Luong V, et al. Assessment of rat and mouse RGC apoptosis imaging in vivo with different scanning laser ophthalmoscopes. Curr Eye Res. 2007; 32: 851–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Guy J, Qi X, Koilkonda RD, et al. Efficiency and safety of AAV-mediated gene delivery of the human ND4 complex I subunit in the mouse visual system. Invest Ophthalmol Vis Sci. 2009; 50: 4205–4214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gan L, Wang SW, Huang Z, Klein WH. POU domain factor Brn-3b is essential for retinal ganglion cell differentiation and survival but not for initial cell fate specification. Dev Biol. 1999; 210: 469–480 [DOI] [PubMed] [Google Scholar]
- 27. Xiang M. Requirement for Brn-3b in early differentiation of postmitotic retinal ganglion cell precursors. Dev Biol. 1998; 197: 155–169 [DOI] [PubMed] [Google Scholar]
- 28. Gan L, Xiang M, Zhou L, Wagner DS, Klein WH, Nathans J. POU domain factor Brn-3b is required for the development of a large set of retinal ganglion cells. Proc Natl Acad Sci U S A. 1996; 93: 3920–3925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Galindo-Romero C, Avilés-Trigueros M, Jiménez-López M, et al. Axotomy-induced retinal ganglion cell death in adult mice: quantitative and topographic time course analyses. Exp Eye Res. 2011; 92: 377–387 [DOI] [PubMed] [Google Scholar]
- 30. Feng G, Mellor RH, Bernstein M, et al. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000; 28: 41–51 [DOI] [PubMed] [Google Scholar]
- 31. Leung CK-S, Cheung CY-L, Lin D, Pang CP, Lam DSC, Weinreb RN. Longitudinal variability of optic disc and retinal nerve fiber layer measurements. Invest Ophthalmol Vis Sci. 2008; 49: 4886–4892 [DOI] [PubMed] [Google Scholar]
- 32. Levkovitch-Verbin H, Harris-Cerruti C, Groner Y, Wheeler LA, Schwartz M, Yoles E. RGC death in mice after optic nerve crush injury: oxidative stress and neuroprotection. Invest Ophthalmol Vis Sci. 2000; 41: 4169–4174 [PubMed] [Google Scholar]
- 33. Berkelaar M, Clarke DB, Wang YC, Bray GM, Aguayo AJ. Axotomy results in delayed death and apoptosis of retinal ganglion cells in adult rats. J Neurosci. 1994; 14: 4368–4374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Villegas-Perez MP, Vidal-Sanz M, Rasminsky M, Bray GM, Aguayo AJ. Rapid and protracted phases of retinal ganglion cell loss follow axotomy in the optic nerve of adult rats. J Neurobiol. 1993; 24: 23–36 [DOI] [PubMed] [Google Scholar]
- 35. Howell GR, Libby RT, Jakobs TC, et al. Axons of retinal ganglion cells are insulted in the optic nerve early in DBA/2J glaucoma. J Cell Biol. 2007; 179: 1523–1537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Buckingham BP, Inman DM, Lambert W, et al. Progressive ganglion cell degeneration precedes neuronal loss in a mouse model of glaucoma. J Neurosci. 2008; 28: 2735–2744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Soto I, Oglesby E, Buckingham BP, et al. Retinal ganglion cells downregulate gene expression and lose their axons within the optic nerve head in a mouse glaucoma model. J Neurosci. 2008; 28: 548–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. De Winter J. Using the Student's t-test with extremely small sample sizes. Pract Assess Res Eval. 2013; 18: 2 [Google Scholar]