Abstract
A series of unnatural amino acids functionalized with sterically shielded pyrroline nitroxides were synthesized. Their reduction by ascorbate/glutathione indicates that l-cysteine functionalized with gem-diethylpyrroline nitroxide is reduced at the slowest rate and is comparable to that measured for the most resistant to reduction pyrroline and pyrrolidine nitroxides.
The spin labeling of proteins by incorporating unnatural amino acids functionalized with nitroxides has many potential advantages over the established, site-directed spin labeling (SDSL) methods.1,2 Unnatural amino acid spin labels offer access to novel approaches to spin labeling of proteins via nonsense codon suppression of an introduced stop codon: (1) via a chemically aminoacylated tRNA3,4 or flexizyme-prepared aminoacylated tRNA5,6 and (2) via genetic encoding of unnatural, spin-labeled amino acids in live cells.7 However, despite the tremendous promise for genetic encoding strategies, a major challenge remains in the survival of such probes in the reducing conditions during the ribosome-mediated protein synthesis, resulting in partially irreversible chemical reduction of the nitroxide to the corresponding diamagnetic hydroxylamine.8 Thus, chemical modifications of the spin labels are required to render them resistant to such reduction before they can be applied for the structure–function study of expressed proteins in biological environments.
To this end, we9,10 and others11,12 recently prepared sterically shielded pyrrolidine nitroxides that are by far the most resistant to ascorbate and ascorbate/glutathione mediated reduction9 and, therefore, would be valuable as modified unnatural amino acid spin labels. Note that functionalization of l-amino acids with racemic pyrrolidine nitroxides would give two possible stereoisomeric products, but in the case of pyrroline nitroxides, without stereocenters, only one enantiomer for the product is expected.
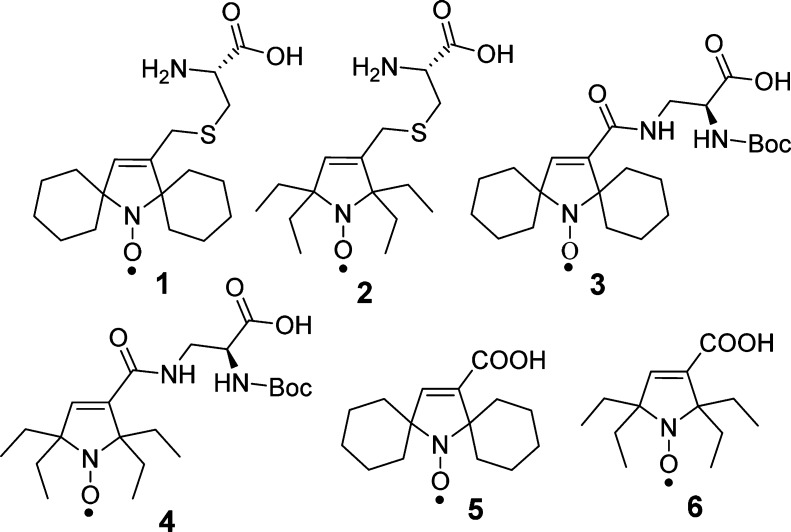
Here, we report the synthesis of amino acids 1–4, functionalized with sterically shielded pyrroline nitroxides (Figure 1), and their reduction kinetics by ascorbate/glutathione. For reference, we study reduction kinetics of pyrroline nitroxides 5 and 6 (Figure 1).
Figure 1.
Amino acids 1–4 and pyrroline nitroxides 5 and 6.
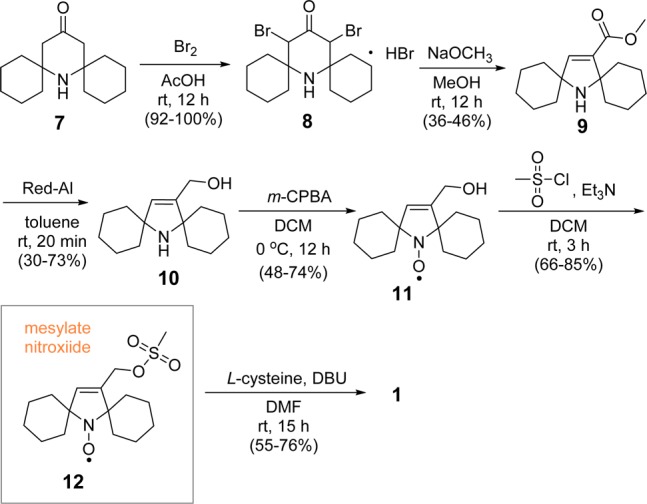
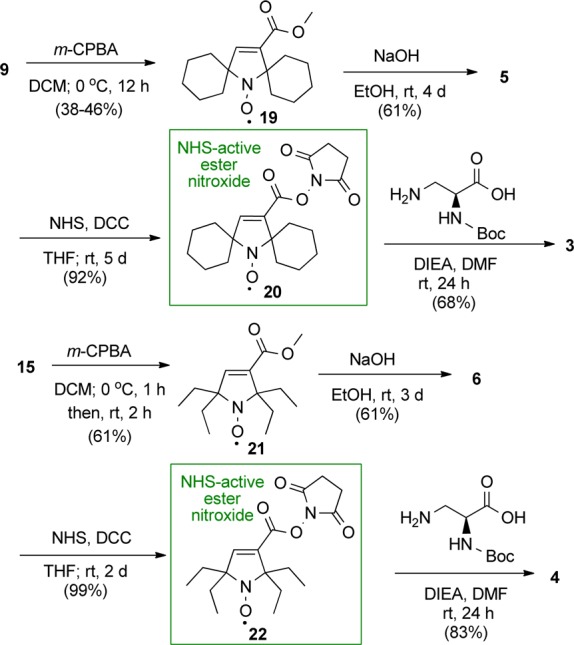
Synthesis of amino acid 1 is outlined in Scheme 1. Dibromination of 7,13,14 according to the previously published procedure, provided 8.11 Subsequent Favorskii rearrangement of 8 gave 5-membered ring amine 9. Selective reduction of 9 using Red-Al provided allylic alcohol amine 10, which was then selectively oxidized with m-CPBA (1.6 equiv) to yield pyrroline nitroxide 11.15 Note that the conversion of 8 to 10 was accomplished by modifications of reaction conditions for the analogous gem-dimethylamines.17,18 Reaction of the allylic alcohol nitroxide 11 with MsCl gave mesylate nitroxide 12.15 Nucleophilic substitution of 12 with the sulfhydryl group of l-cysteine provided the target amino acid 1.
Scheme 1. Synthesis of Amino Acid 1.
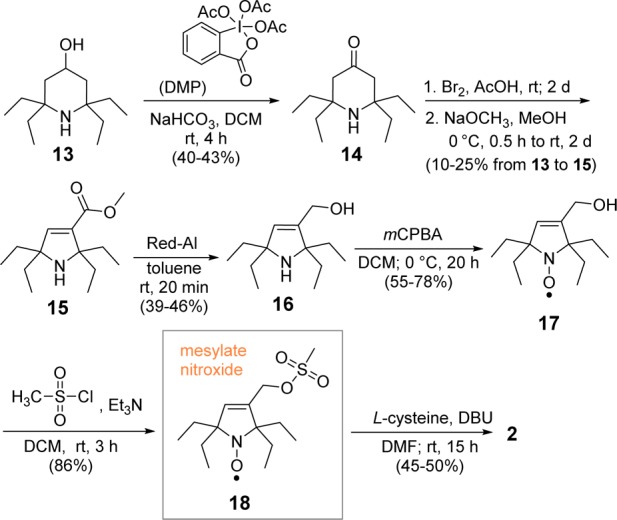
The synthesis of amino acid 2 is outlined in Scheme 2. In the first step, alcohol amine 13(9,15,16) is selectively oxidized to ketone amine 14(15) using Dess–Martin reagent (DMP).19,20 Subsequent steps leading to mesylate nitroxide 18 are implemented in an analogous way to that for spirocyclohexyl nitroxide 12. Given the increased steric hindrance of gem-diethyl groups, we were surprised to attain selective oxidation of amine 16 with m-CPBA (1.8 equiv) to provide pyrroline nitroxide 17 in 78% yield (100% spin purity) and negligible amounts of alkene epoxidation byproduct. Finally, reaction of 18 with l-cysteine gave the target amino acid 2.
Scheme 2. Synthesis of Amino Acid 2.
The synthesis of N-Boc-protected amino acids 3 and 4 as well as pyrroline nitroxides 5(15) and 6 is outlined in Scheme 3. In the initial steps, the Favorskii rearrangement products 9 and 15 (Schemes 1 and 2) are subjected to oxidation with m-CPBA to provide corresponding nitroxides 19 and 21. Ester hydrolysis of 19 and 21 gives the pyrroline nitroxides 5 and 6, respectively. The carboxylic acids in nitroxides 5 and 6 are converted to N-hydroxysuccinimidyl (NHS) esters by standard methods, using NHS and DCC, to give the corresponding NHS-active esters 20(15) and 22, which are then coupled with N-Boc-dab to yield target N-Boc-protected amino acids 3 and 4, respectively.
Scheme 3. Synthesis of N-Boc-Protected Amino Acids 3 and 4.
The high purity of the bulk samples of amino acid nitroxides 1–4, as well as pyrroline nitroxides 5 and 6, used for kinetic studies, is determined by paramagnetic 1H NMR spectroscopy (Table S2, Supporting Information) and EPR spin concentrations. Pyrroline nitroxide 3 and 4 are reduced, using a large excess of ascorbate and reduced glutathione (GSH), to the corresponding diamagnetic hydroxylamines, and their structures are confirmed by 1H NMR spectroscopy.21
EPR spectra of amino acid nitroxides 1–4 in chloroform show triplet patterns due to 14N hyperfine splitting, aN ≈ 14–16 G, and g values of about 2.006, similar to those for the reference pyrroline nitroxides 5 and 6.
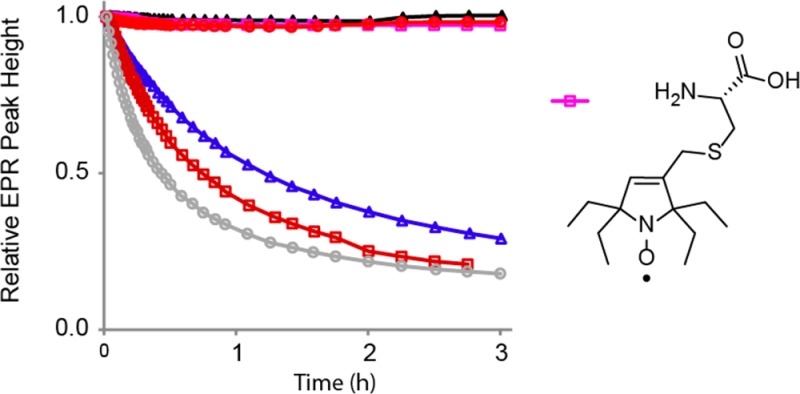
Rates of reduction for pyrroline nitroxides 1–6 are studied under pseudo-first-order conditions using a 20-fold excess ascorbate and 25-fold exccess of GSH in pH 7.4 PBS buffer. Addition of GSH leads to higher conversion of nitroxide to hydroxylamine and provides only slightly increased initial rates of the reduction of nitroxides, compared to that in the absence of GSH (ascorbate only).9,11 Second-order rate constants, k, are obtained by monitoring the decay of the low-field EPR peak height of nitroxides at 295 K (Figure 2 and Table 1). Single integrated peak heights are examined and found to produce similar values of k for most nitroxides (Table S1, Supporting Information).9 For comparison, the rate of reduction of the gem-dimethylpyrrolidine nitroxide (3-carboxy-PROXYL),9,11,22 is measured under identical conditions (Table 1).
Figure 2.
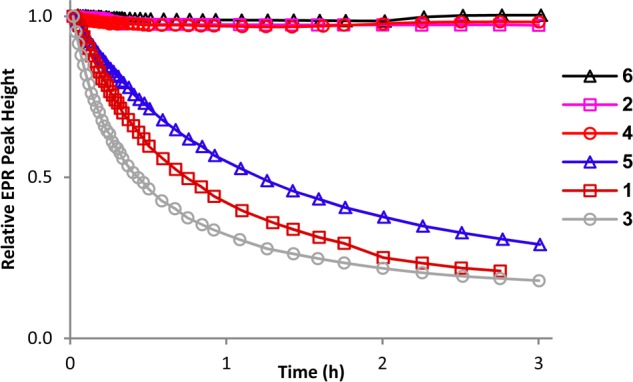
Reduction profiles of 0.2 mM nitroxides with 4 mM ascorbate and 5 mM GSH in 25 mM PBS pH 7.4 at 295 K.
Table 1. Second-Order Rate Constants, k (M–1 s–1), for Initial Rates of Reduction of Pyrroline Nitroxides (0.2 or 1 mM) with 20-fold Excess Ascorbate and 25-fold Excess of GSH in PBS (25 or 125 mM) pH 7.4 at 295 Ka,b.
| 0.2 mM spirocylohexyl
nitroxides |
1.0 mM gem-diethyl
nitroxides |
||
|---|---|---|---|
| k | k | ||
| 1 | 0.0845 ± 0.0018 | 2 | 0.0014 ± 0.0005 |
| 3 | 0.1370 ± 0.0023 | 4 | 0.0025 ± 0.0003 |
| 5c | 0.0509 ± 0.0039 | 6 | 0.0012 ± 0.0000 |
Mean ± two standard deviations from three measurements; see Table S1 in the Supporting Information.
k = 0.0603 ± 0.0025 M–1 s–1 was measured for 3-carboxy-PROXYL under identical conditions.
k = 0.07 M–1 s–1 was reported for 5, based on imprecise temperature control, which could vary 4–5° (ref (11)).
gem-Diethylpyrroline nitroxides 2 (k ≈ 0.001 M–1 s–1), 4 (k ≈ 0.003 M–1 s–1), and 6 (k ≈ 0.001 M–1 s–1) are reduced at significantly slower rates than the corresponding spirocyclohexyl nitroxides 1 (k ≈ 0.08 M–1 s–1), 3 (k ≈ 0.14 M–1 s–1), and 5 (k ≈ 0.05 M–1 s–1). More importantly, all three gem-diethylpyrroline nitroxides, which require increased concentration (from 0.2 to 1 mM) to observe measurable initial decay, show a very small decrease in concentration, leaving 97+ % of 0.2 mM nitroxide intact after 3 h (Figure 2). These results imply that amino acids functionalized with gem-diethylpyrroline nitroxides have great potential for spin labeling via the ribosome-mediated protein synthesis.
Acknowledgments
This work was primarily supported by a National Institutes of Health consortium grant (U54GM087519). Additional support was provided by the National Science Foundation (Grant Nos. CHE-1012578 and CHE-1362454), in particular for the research of undergraduate students (K.B. and E.R.). E.R. was supported in part by the Research Experiences for Undergraduates (REU) Program of the National Science Foundation (CHE-1156560). We thank Robert Nickel, an undergraduate at UNL, who was supported by the UCARE program, for his contribution to the early stages in the synthesis of amino acids. We thank Yao Liu, an undergraduate at UNL, for her contribution to the synthesis of 7 and 15. We thank Christopher Richardson (Hamilton College), who was supported by the above referenced REU Program, for his synthesis of nitroxide 19.
Supporting Information Available
Additional experimental details. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Hubbell W. L.; Lopez C. J.; Altenbach C.; Yang Z. Curr. Opin. Struct. Biol. 2013, 23, 725–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minnihan E. C.; Yokoyama K.; JoAnne Stubbe J. A. F1000 Biol. Rep. 2009, 1, 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish V. W.; Benson D. R.; Altenbach C. A.; Hideg K.; Hubbell W. L.; Schultz P. G. Proc. Natl. Acad. Sci. U.S.A. 1994, 91, 2910–2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer A. M.; Kalai T.; Liu S. Q. B.; Hideg K.; Voss J. C. Biochemistry 2004, 43, 8470–8482. [DOI] [PubMed] [Google Scholar]
- Goto Y.; Katoh T.; Suga H. Nat. Protoc. 2011, 6, 779–790. [DOI] [PubMed] [Google Scholar]
- Goto Y.; Suga H. Methods Mol. Biol. 2012, 848, 465–478. [DOI] [PubMed] [Google Scholar]
- Schmidt M. J.; Borbas J.; Drescher M.; Summerer D. J. Am. Chem. Soc. 2014, 136, 1238–1241. [DOI] [PubMed] [Google Scholar]
- Belkin S.; Mehlhorn R. J.; Hideg K.; Hankovsky O.; Packer L. Arch. Biochem. Biophys. 1987, 256, 232–243. [DOI] [PubMed] [Google Scholar]
- Paletta J. T.; Pink M.; Foley B.; Rajca S.; Rajca A. Org. Lett. 2012, 14, 5322–5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Rajca A.; Wang Y.; Boska M.; Paletta J. T.; Olankitwanit A.; Swanson M. A.; Mitchell D. G.; Eaton S. S.; Eaton G. R.; Rajca S. J. Am. Chem. Soc. 2012, 134, 15724–15727. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Correction:Rajca A.; Wang Y.; Boska M.; Paletta J. T.; Olankitwanit A.; Swanson M. A.; Mitchell D. G.; Eaton S. S.; Eaton G. R.; Rajca S. J. Am. Chem. Soc. 2014, 136, 3318–3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirilyuk I. A.; Polienko Y. F.; Krumkacheva O. A.; Strizhakov R. K.; Gatilov Y. V.; Grigor’ev I. A.; Bagryanskaya E. G. J. Org. Chem. 2012, 77, 8016–8027. [DOI] [PubMed] [Google Scholar]
- Morozov D. A.; Kirilyuk I. A.; Komarov D. A.; Goti A.; Bagryanskaya I. Y.; Kuratieva N. V.; Grigor’ev I. A. J. Org. Chem. 2012, 77, 10688–10698. [DOI] [PubMed] [Google Scholar]
- Sakai K.; Yamada K.; Yamasaki T.; Kinoshita Y.; Mito F.; Utsumi H. Tetrahedron 2010, 66, 2311–2315. [Google Scholar]
- Rajca A.; Kathirvelu V.; Roy S. K.; Pink M.; Rajca S.; Sarkar S.; Eaton S. S.; Eaton G. R. Chem.—Eur. J. 2010, 16, 5778–5782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spirocyclohexyl nitroxides 5, 11, 12, and 20 and gem-diethylamines 13 and 14 were prepared using different synthetic routes in refs (11) and (16), respectively.
- Wetter C.; Gierlich J.; Knoop C. A.; Müller C.; Schulte T.; Studer A. Chem.—Eur. J. 2004, 10, 1156–1166. [DOI] [PubMed] [Google Scholar]
- Hatano B.; Araya H.; Yoshimura Y.; Sato H.; Ito T.; Ogata T.; Kijima T. Heterocycles 2010, 81, 349–356. [Google Scholar]
- Krishna M. C.; DeGraff W.; Hankovszky O. H.; Sár C. P.; Kálai T.; Jekő J.; Russo A.; Mitchell J. B.; Hideg K. J. Med. Chem. 1998, 41, 3477–3492. [DOI] [PubMed] [Google Scholar]
- Meyer S. D.; Schreiber S. L. J. Org. Chem. 1994, 59, 7549–7552. [Google Scholar]
- Boeckman R. K. Jr.; Shao P.; Mullins J. J. Org. Synth. 2000, 77, 141–152. [Google Scholar]
- Compound 4 (∼5 mM) could be only partially reduced to hydroxylamine with ∼70% of its gem-diethyl nitroxide remaining. For ∼3 mM 3 reduced under similar conditions, only <1% of its spirocyclohexyl nitroxide remained (Supporting Information).
- Vianello F.; Momo F.; Scarpa M.; Rigo A. Magn. Reson. Imaging 1995, 13, 219–226. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.