Abstract
The polarization of sterol- and sphingolipid-enriched domains (lipid rafts) has been linked to morphogenesis and cell movement in diverse cell types. In the yeast Saccharomyces cerevisiae, a dramatic polarization of sterol-rich domains to the shmoo tip was observed in pheromone-induced cells (M. Bagnat and K. Simons, Proc. Natl. Acad. Sci. USA 99:14183-14188, 2002). We therefore examined whether plasma membrane lipid polarization contributes to the ability of the fungal pathogen Candida albicans to grow in a highly polarized manner to form hyphae. Interestingly, staining with filipin revealed that membrane sterols were highly polarized to the leading edge of growth during all stages of hyphal growth. Budding and pseudohyphal cells did not display polarized staining. Filipin staining was also enriched at septation sites in hyphae, where colocalization with septin proteins was observed, suggesting a role for the septins in forming a boundary domain. Actin appeared to play a role in sterol polarization and hyphal morphogenesis in that both were disrupted by low concentrations of latrunculin A that did not prevent budding. Furthermore, blocking either sphingolipid biosynthesis with myriocin or sterol biosynthesis with ketoconazole resulted in a loss of ergosterol polarization and caused abnormal hyphal morphogenesis, suggesting that lipid rafts are involved. Since hyphal growth is required for the full virulence of C. albicans, these results suggest that membrane polarization may contribute to the pathogenesis of this organism.
Candida albicans is an opportunistic human fungal pathogen that can grow in a variety of morphologies, including round buds, elongated pseudohyphae, and filamentous hyphae (7). The ability to switch between budding and hyphal growth is important for the virulence of this organism (10, 31, 32). Hyphae are thought to play an important role in pathogenesis because their filamentous growth pattern can facilitate invasive growth. In addition to their unique morphology, hyphae are further distinguished from budding cells and pseudohyphae by the production of hypha-specific virulence factors (36, 50). For example, hyphae produce adhesin proteins that facilitate attachment to host cells (46) and also secrete proteases that contribute to invasion into tissues (13, 40). Investigations of the molecular mechanisms underlying hyphal growth have identified many factors that are involved in hyphal morphogenesis (7, 31, 50). For example, studies have identified signal transduction components that stimulate hyphal growth and transcription factors that induce hypha-specific genes.
The filamentous growth of hyphae occurs by localized morphogenesis at the hyphal tip (20). As in other eukaryotic organisms, this polarized growth is mediated by the actin cytoskeleton. Actin and other morphogenesis proteins are highly enriched at the leading edge of hyphal growth. Interestingly, recent studies of other organisms have indicated that a polarized distribution of membrane lipids can also contribute to morphogenesis. In particular, these studies have focused on membrane microdomains known as lipid rafts that have been observed in organisms from yeast to humans (2, 8). Lipid rafts have a distinct membrane composition in that they are highly enriched in sterols and sphingolipids. An asymmetric distribution of lipid rafts in the plasma membrane has been implicated in promoting the cell polarity that mediates cell migration in a wide range of cells, including leukocytes, carcinomas, and epithelial cells (33). A remarkable example of membrane lipid asymmetry identified in a fungus is the dramatic polarization of sterol-rich membrane domains at the tips of pheromone-induced mating projections in Saccharomyces cerevisiae (4). Lipid polarization was not detected during budding growth and is thought to contribute to the highly polarized morphogenesis that occurs to create the mating projection. In view of the observation of lipid raft polarization in a wide variety of cell types, it seems likely that polar distribution of raft domains may be a common underlying feature of cell polarity and morphogenesis.
Lipid rafts are thought to contribute to morphogenesis in part because their distinct lipid composition allows for the partitioning of specific proteins into these domains. This results in the formation of specialized zones that are enriched in proteins involved in signal transduction pathways, cell adhesion, and other cell polarity processes. Interestingly, one subset of proteins that are enriched in raft domains are glycosylphosphatidylinositol (GPI)-anchored proteins (8, 9). This is significant in regard to hyphal morphogenesis in that several proteins involved in cell wall biogenesis are thought to contain GPI anchors (28). In addition, the adhesin proteins (e.g., Hwp1 and Als1p) are GPI anchored (46). Therefore, in the present study we examined whether polarized membrane domains are found in C. albicans. The results indicate that a highly polarized ergosterol-rich domain is present specifically during hyphal growth. This suggests that lipid rafts contribute to hyphal morphogenesis in C. albicans and are likely to also contribute to the presentation of virulence factors by this pathogen.
MATERIALS AND METHODS
Media and growth conditions.
Cells were propagated on YPD (1% yeast extract, 2% peptone, 2% dextrose) medium supplemented with 80 mg of uridine/liter (YPD+uri). Pseudohyphal growth was promoted by diluting cells from an overnight culture into fresh YPD+uri and incubating them at 37°C for 4 h. Hyphal growth was carried out at 37°C in YPD+uri containing bovine calf serum at a final concentration of 10%. For filipin staining, cells were induced to form hyphae on the surfaces of 12-mm-diameter coverslips (Fisher) placed in the wells of flat-bottomed tissue culture dishes (Corning). For rhodamine-phalloidin staining, cells were grown in liquid medium inside culture tubes. Farnesol (Sigma) was dissolved in methanol and added to serum-containing cultures at a final concentration of 1 mM. Latrunculin A was dissolved in dimethyl sulfoxide to 20 mM and added to serum-containing cultures at a final concentration of 200 or 400 μM. Myriocin was dissolved in methanol and added to budding cultures at a final concentration of 1 μM and to serum-containing cultures at a final concentration of 100 μM. Factors in serum apparently affected the delivery of myriocin into cells, necessitating the use of higher concentrations of the drug. However, similar abnormalities in hyphal morphology were obtained in Lee's medium supplemented with 1 μM myriocin (not shown). Phytosphingosine was dissolved in ethanol and added to serum-containing cultures at a final concentration of 30 μM. Ketoconazole was dissolved in methanol and added to cultures at a final concentration of 100 or 300 μM. In all drug studies, an equal concentration of solvent alone was added to untreated control samples, and the solvent concentration never exceeded 2% in the final culture volume.
Fluorescence microscopy.
Cells were stained with 10 μg of filipin/ml for 10 min and then analyzed by fluorescence microscopy. For colocalization studies, C. albicans strain yAW2, expressing CDC10-GFP under the control of the ADH promoter, was stained with filipin and analyzed by fluorescence microscopy. Rhodamine-phalloidin staining of actin was performed as described previously (1). Samples were photographed under an Olympus BH2 microscope by using a Zeiss Axio Cam camera run by Openlab 3.0.8 software from Improvision.
DRM isolation and Western blot analysis.
The isolation of detergent-resistant membranes (DRMs) was performed essentially as previously described (3). Briefly, cell pellets were lysed by agitation with glass beads in 100 μl of lysis buffer (50 mM Tris-HCl [pH 7.4], 150 mM NaCl, 5 mM EDTA) supplemented with a protease inhibitor mixture (1 μg of pepstatin A/ml, 1 mM benzamidine, and 0.5 mM phenylmethylsulfonyl fluoride). The lysates were centrifuged at a low speed to remove glass beads and unbroken cells. Cleared lysates were incubated in the presence of cold 1% Triton X-100 for 30 min at 4°C. After the detergent treatment, lysates were subjected to density gradient centrifugation using a step gradient of different concentrations of Optiprep (Sigma). Fractions of equal volumes were removed from the tops of gradients and analyzed by Western blotting using a polyclonal antibody raised against Pma1p from Neurospora crassa (kindly provided by Amy Chang), a polyclonal anti-Cdc11p antibody (Santa Cruz Biotechnology, Santa Cruz, Calif.), or concanavalin A conjugated to horseradish peroxidase (Sigma). The antibodies were diluted in a solution of 1% powdered milk in TBS (10 mM Tris [pH 7.5], 150 mM NaCl), and concanavalin A was diluted in a solution of 0.3% bovine serum albumin in TBS. Control concanavalin A blots were preincubated with 2 mM methyl-α-d-mannopyranoside. Blots probed with antibodies were incubated with horseradish peroxidase-conjugated secondary antibodies, and then the cross-reacting proteins were detected by chemiluminescence by use of a SuperSignal West Dura kit as specified by the manufacturer (Pierce Inc., Rockford, Ill.). Concanavalin A blots were washed and incubated with the SuperSignal West Dura substrate to visualize glycosylated proteins. The protein concentrations of the cell lysates were assayed by using a BCA protein assay kit (Pierce Inc.).
RESULTS
Membrane polarization during hyphal growth.
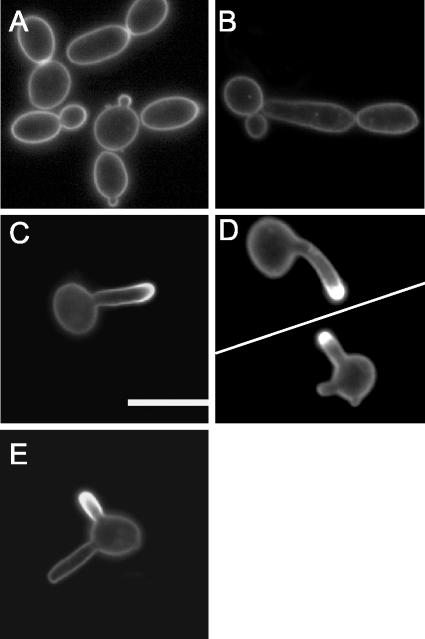
To determine whether membrane lipids are polarized during different types of morphogenesis in C. albicans, we grew cells as buds, pseudohyphae, or true hyphae and stained them with filipin to detect the major membrane sterol, ergosterol (Fig. 1). Filipin is a polyene antibiotic that is similar in structure to amphotericin B in that it binds sterols. It has been used to visualize membrane sterols in a wide range of cell types because it has fluorescent properties that can be observed by microscopy (16). During budding, growth is polarized to form round daughter cells that display uniform filipin staining throughout the mother and daughter membranes (Fig. 1A). Likewise, for pseudohyphal cells, in which growth is more highly polarized to form chains of elongated buds, uniform filipin staining was detected throughout the membrane (Fig. 1B). In contrast, cells grown in the presence of serum to induce the formation of hyphae showed intense filipin staining at the leading edge of morphogenesis (Fig. 1C). Hyphae are distinguishable from pseudohyphae by their parallel walls, lack of constrictions at septation sites, and production of hypha-specific virulence factors (7). Similar results were obtained when hyphal growth was stimulated by growth in synthetic medium (Lee's medium), demonstrating that this was not a serum-specific phenomenon (Fig. 1D). Thus, membrane lipid polarization is a unique feature of true hyphae, which suggests that membrane domains may play a special role in hyphal morphogenesis.
FIG. 1.
Polarization of membrane ergosterol during hyphal growth of C. albicans. The images show filipin staining of strain BWP17 grown at 30°C in YPD to promote bud formation (A), at 37°C to promote pseudohyphal growth (B), at 37°C in medium containing 10% bovine calf serum (BCS) to promote hyphal growth (C and E), or at 37°C in Lee's medium to promote hyphal growth (D). The cells were stained with 10 μg of filipin/ml for 10 min and then analyzed by fluorescence microscopy. Bar, 10 μm.
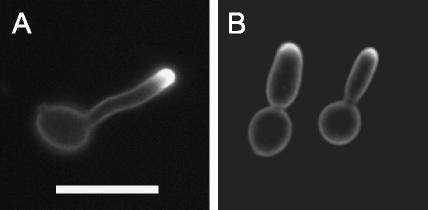
To test whether the polarization of ergosterol-rich domains resulted from the presence of a unique morphological structure at the hyphal tip or whether it occurred in response to specific hypha-promoting signals, we induced cells with serum in the presence of farnesol. Farnesol is a quorum-sensing molecule produced by C. albicans that prevents the formation of hyphae (23). The addition of farnesol to serum-containing cultures resulted in most cells forming pseudohyphae. Interestingly, these cells still displayed strong filipin staining at their growing tips (85.5%, n = 113) (Fig. 2B). These results also indicate that membrane polarization occurs in response to the hyphal morphogenesis program and is not due solely to the unique geometry of the hyphal tip. This result is also interesting in that it indicates that farnesol does not appear to act by disrupting lipid polarization.
FIG. 2.
Filipin staining of farnesol-treated cells. (A) Hyphal growth was induced in the presence of 10% BCS at 37°C. (B) Farnesol was added to serum-containing cultures at a final concentration of 1 mM to prevent the formation of hyphae. The cells were stained with 10 μg of filipin/ml for 10 min and then analyzed by fluorescence microscopy. Bar, 10 μm.
An analysis of cells that had formed two small hyphal outgrowths (germ tubes) showed that filipin staining was only detected at the tip of one outgrowth (Fig. 1E). These cells arise by a phenomenon known as germ tube switching that has been described previously for C. albicans and other fungi (22, 49) in which cells abort the formation of an initial hyphal outgrowth and switch to a new site of morphogenesis to form another hypha. These results demonstrate that the observed staining pattern is not simply due to the membrane curvature at the hyphal tip. The fact that these outgrowths are formed in a sequential manner indicates that lipid polarization is only directed toward sites of active hyphal morphogenesis. In addition, hyphae that were stained with a second membrane dye (FM-464) displayed uniform staining across the entire plasma membrane, indicating that the membrane polarization observed with filipin is not due to differences in permeability at the hyphal tip (data not shown).
Analysis of DRMs in C. albicans.
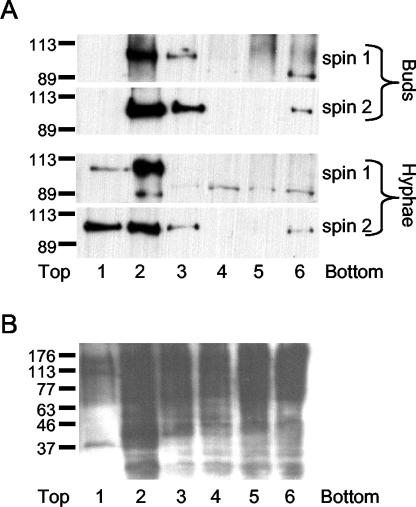
Since lipid rafts are enriched in ergosterol, we examined whether C. albicans contains lipid rafts by taking advantage of the detergent-resistant properties of the raft domains (8, 9). For this analysis, cell lysates were extracted with cold 1% Triton X-100 and then the DRMs were isolated by density gradient centrifugation. Fractions from the gradient were then analyzed in a Western blot probed with antibodies against Pma1p, a plasma membrane ATPase that has been shown in S. cerevisiae to be present in DRMs (2). Pma1p was detected in the DRM fraction of both budding and hyphal cells, indicating that lipid rafts also exist in C. albicans (Fig. 3). As a control for DRM isolation, fractions were analyzed by Western blotting with a concanavalin A probe to detect glycosylated membrane proteins. As shown in Fig. 3B, many glycosylated proteins were detected in fractions at the bottom of the gradient, indicating that they were efficiently solubilized by 1% Triton X-100. This is consistent with the previously reported ability of 1% Triton X-100 to discriminate between detergent-resistant and nonresistant fractions (43).
FIG. 3.
Pma1p is present in the DRM fraction of budding and hyphal cells. DRMs were isolated from budding and hyphal cells by density gradient centrifugation after membrane extraction with cold 1% Triton X-100, as described in Materials and Methods. Fractions of equal volumes were removed from the top of the gradient and analyzed by Western blotting using a polyclonal antibody raised against Pma1p (A) or concanavalin A (B). In panel A, fraction 2 from the first density gradient (spin 1), which consistently contained most of the Pma1p, was separated through a second density gradient (spin 2). The relative positions of protein standards, with masses indicated in kilodaltons, are shown to the left.
The marker protein Pma1p fractionated with DRMs during both the budding and hyphal phases of growth. This indicates that the polarization of ergosterol-enriched domains is apparently due to their localization at the hyphal tip and not to de novo formation of DRMs in hyphae.
Time course analysis of membrane polarization during hyphal growth.
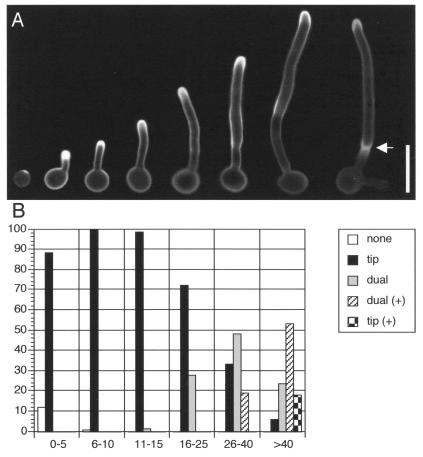
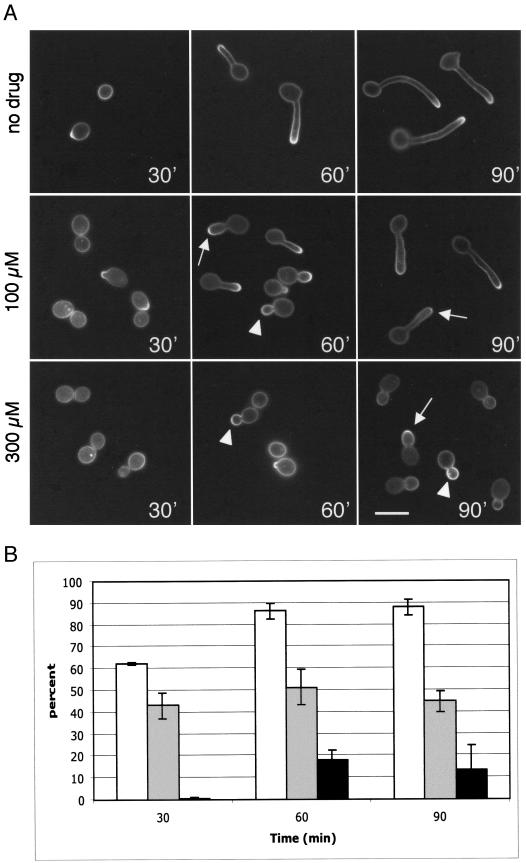
The pattern of membrane polarization during hyphal growth was examined by staining cells with filipin at various time points after induction with serum (Fig. 4). At early time points, cells lacking a hyphal outgrowth showed intense staining at the presumptive site of germ tube formation. During the subsequent initiation of hyphal growth, staining was detected at the leading edge of growth.
FIG. 4.
Polarization of membrane ergosterol at different stages of hyphal growth. (A) Strain BWP17 was induced to form hyphae in medium containing 10% BCS, and then samples were collected for filipin staining at various time points up to 3 h. Representative cells from different times of induction are shown. The arrow indicates a cell that has completed septum formation. (B) Patterns of filipin staining quantified by different lengths of hyphae observed during the time course. Legend: none, uniform membrane staining; tip, staining only at hyphal tips; dual, staining at hyphal tips and presumptive septation sites; dual (+), dual staining after septum formation; tip (+), tip staining only after septum formation. At least 300 cells were counted for each hyphal length from 0 to 10 μm, at least 100 cells were counted for each hyphal length from 11 to 40 μm, and 67 cells were counted for hyphae of >40 μm. Bar, 10 μm.
At later stages of hyphal growth, a second site of filipin staining appeared distal from the tip at the presumptive site of septum formation. This second site of localization occurred in two distinct phases. Before a mature septum could be discerned by light microscopy, filipin staining was diffusely localized. After septum formation, filipin staining was more restricted, and the membrane separating the two newly formed cell compartments was also intensely stained (Fig. 4A). As hyphal growth continued, filipin staining at the first septum faded and staining was observed at the sites where the second septum was forming, further demonstrating that membrane polarization is restricted to actively growing sites (Fig. 4A, arrow). Strong tip staining persisted throughout all phases of hyphal growth.
Septins colocalize with ergosterol-rich membranes during hyphal growth.
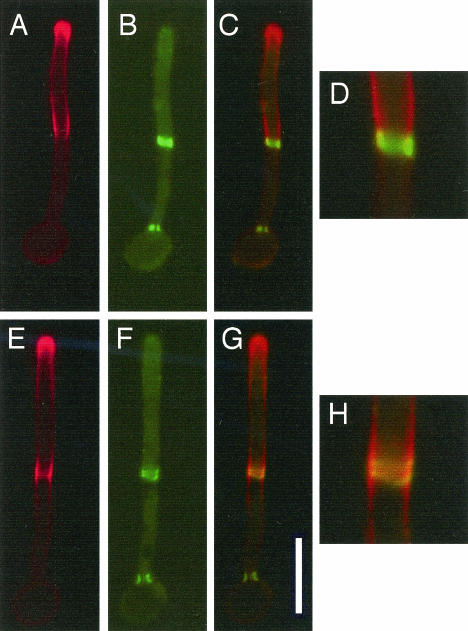
The pattern of filipin staining described above was reminiscent of that of septin proteins, which also localize to the hyphal tip and to sites of septation (45, 49). For a comparison of the localization of septins and ergosterol-rich membranes, CDC10-GFP cells, which produce the Cdc10 septin fused to green fluorescent protein, were grown in serum for various time points and analyzed by fluorescence microscopy. Septins colocalized with ergosterol-rich membranes at the septation site in two distinct phases (Fig. 5). At early stages, septins formed an immature single ring at the incipient septation site. Immature ring formation occurred independent of ergosterol-rich membranes, as no colocalization with filipin staining was observed at this stage (data not shown). At somewhat later stages, septins were localized adjacent to ergosterol-rich membranes at the boundary between the growing hyphal tip and the mother cell. Filipin staining extended back from the hyphal tip until it reached the septin ring and no further (Fig. 5A to D). Finally, when septum formation was under way, the septins separated into characteristic double rings and overlapped directly with filipin staining. The filipin staining extended away from the septin double ring in both directions for a short distance before becoming uniform with the rest of the membrane (Fig. 5E to H). These data suggest a role for septins in forming a boundary for lipid domains during hyphal growth, consistent with reports that septins act as a boundary domain for membrane proteins and actin during the budding growth of S. cerevisiae (6, 47). Septins also localized to the leading edge of growth, similar to ergosterol, but this is not obvious in Fig. 5 because septin localization at the tip is more diffuse and requires longer exposure times.
FIG. 5.
Septin colocalization with ergosterol-rich domains during hyphal growth. Strain yAW2, expressing the septin CDC10-GFP fusion construct under the control of the ADH promoter (49), was grown in 10% BCS at 37°C to promote hyphal formation and then was stained with filipin. Filipin staining is indicated in red, while CDC10-GFP staining is depicted in green. Images were merged with Openlab 3.0.8 software from Improvision. (A to D) Hyphal cells prior to division of the septin ring; (E to H) hyphal cells after division of the septin ring. (A and E) Filipin stain; (B and F) Cdc10-GFP stain; (C and G) merged images; (D and H) close-ups of merged images. Bar, 10 μm.
The colocalization between septins and sterol-rich membranes suggested that septins may directly interact with these membrane microdomains. However, a significant proportion of septins did not consistently fractionate with DRMs by density gradient centrifugation (data not shown). Two possible explanations can account for this discrepancy. First, it is possible that only a minor fraction of the total cellular pool of septins associates directly with plasma membrane domains, making analysis of this subfraction difficult. Second, septins may associate with membrane domains indirectly through another protein, and this protein-protein interaction may not be resistant to detergent extraction. One candidate that could mediate this interaction is a homologue of the S. cerevisiae protein Rvs161p, which colocalizes with septins and is also found in lipid rafts (5, 18).
Actin is involved in membrane polarization.
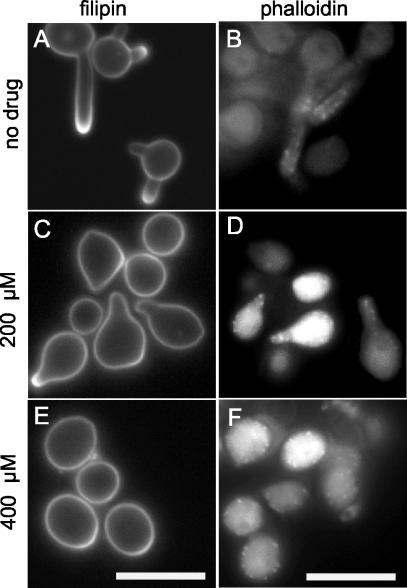
The actin cytoskeleton is involved in polar growth in many cell types, including hyphal growth in C. albicans (21, 39). To test whether the actin cytoskeleton is also involved in lipid raft polarization, we induced cells with serum containing either a high dose of latrunculin A (400 μM), which blocks budding and hyphal growth, or a low dose (200 μM) that was previously reported to prevent hyphal growth, but not budding (21). After 1 h of growth in the presence of the drug, ergosterol polarization was detected in only 53% ± 4.2% of cells treated with 200 μM latrunculin A, in contrast to the case for untreated controls (94.5% ± 5%) (Fig. 6A and C). Furthermore, rhodamine-phalloidin staining revealed reduced polarization of actin patches to sites of morphogenesis in cells treated with 200 μM latrunculin A compared to untreated controls (Fig. 6B and D). With 400 μM latrunculin A, polarized morphogenesis was not observed and ergosterol polarization was detected in only a very small minority of cells (Fig. 6E and F). These results suggest a role for actin in the polarization of plasma membrane lipids during C. albicans hyphal growth.
FIG. 6.
Actin is required for polarization of membrane lipids. Strain BWP17 was grown overnight in YPD medium and then diluted into medium containing 10% BCS and grown at 37°C to promote hypha formation. Latrunculin A was added to the cultures at a concentration of 200 or 400 μM. The cells were then grown for 1 h in the presence of the drug and stained with filipin to detect membrane sterols (A, C, and E) or rhodamine-phalloidin to detect actin (B, D, and F). Bar, 10 μm.
Sterol and sphingolipid biosynthesis is required for membrane polarization and hyphal growth in C. albicans.
To determine whether sterol biosynthesis is required for hyphal growth, we grew C. albicans cells in the presence of ketoconazole, a specific inhibitor of sterol biosynthesis. Ketoconazole belongs to the azole family of antifungal agents and blocks 14-α-demethylation of lanosterol in the ergosterol biosynthesis pathway (16, 37). When cells were treated with a low dose of ketoconazole (100 μM), hyphae grew more slowly than in untreated controls and often appeared swollen at their tips (Fig. 7). In addition, sterol polarization was detected in only 50% of cells, even after 1.5 h of growth in serum (Fig. 7B). When staining was observed, it was frequently localized to a more restricted region of the hyphal tip relative to the case for untreated controls (Fig. 7A, arrows). At a higher dose of ketoconazole (300 μM), hyphal growth was completely blocked and sterol polarization to bud tips was observed in only a small minority of cells. When staining was present, it was sometimes localized to a more restricted region (Fig. 7A, arrows) or uniformly present across the entire surface of the growing bud (Fig. 7A, arrowheads). These results indicate a role for sterol biosynthesis in hyphal growth and membrane lipid polarization in C. albicans.
FIG. 7.
Sterol biosynthesis is required for membrane polarization and hyphal growth. Strain BWP17 was diluted from an overnight culture into medium containing 10% BCS and grown for 1.5 h at 37°C. The cells were harvested at the indicated times. Ketoconazole was added at a final concentration of 100 or 300 μM. (A) Filipin staining of ketoconazole-treated cells and untreated controls. Arrows indicate cells displaying filipin staining at a more restricted region of the hyphal tip, and arrowheads indicate cells with uniform filipin staining across the entire surface of the growing bud. Cells were seeded onto coverslips at a low density to prevent clumping of the hyphae, and representative cells were moved closer together by using Adobe Photoshop. Bar, 10 μm. (B) Percent cells displaying filipin staining at sites of polarized morphogenesis in untreated control cells (white bar) or cells treated with 100 μM (gray bar) or 300 μM (black bar) ketoconazole. Error bars represent standard deviations for three experiments.
To determine whether sphingolipids, a major component of lipid rafts, are also required for hyphal growth, we incubated C. albicans cells in the presence of myriocin. Myriocin is a specific inhibitor of serine palmitoyltransferase, which catalyzes the first committed step of sphingolipid biosynthesis (34). In S. cerevisiae, the two subunits of serine palmitoyltransferase are encoded by the genes LCB1 and LCB2, both of which are required for lipid raft formation and protein delivery to the cell surface (3, 35). Myriocin has also been shown to disrupt polar growth in the fungal pathogen Aspergillus nidulans, and studies of other organisms have demonstrated that depleting membranes of sphingolipids results in a loss of lipid rafts and DRMs (9, 11, 25). Cells that were treated with myriocin for 6 h at 30°C exhibited a cell separation defect and grew as connected chains of buds (data not shown).
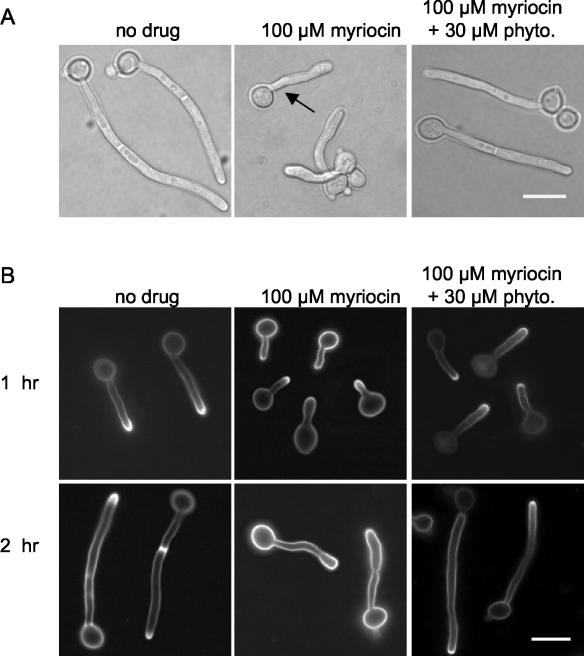
The addition of myriocin at a concentration (100 μM) that partially inhibited the growth of serum-stimulated cells disrupted hyphal morphogenesis (Fig. 8A). Hyphae appeared to emerge normally from mother cells (Fig. 8A, arrow), but hyphal tips subsequently became swollen and the morphology appeared less regular. The relatively normal morphology during the initial stages of hyphal growth in myriocin-treated samples suggested that cells from the overnight culture had a pool of sphingolipids that was used during the early phases of hyphal growth. Time course experiments were therefore performed to understand how hyphal morphology and sterol polarization change over time in the presence of myriocin (Fig. 8B). After 1 h of growth in the presence of myriocin, the percentage of cells displaying sterol polarization was slightly reduced relative to that for untreated controls (Table 1). By 2 h, the majority of cells exhibited abnormal hyphal morphologies and no longer stained for filipin (Fig. 8; Table 1). An analysis of the DRMs from the myriocin-treated cells also demonstrated that the level of Pma1p was diminished after 3 h (data not shown). This was consistent with the behavior of Pma1p in S. cerevisiae, which is targeted for vacuolar degradation when it is unable to enter DRMs in the secretory pathway (2, 19).
FIG. 8.
Sphingolipid biosynthesis is required for membrane polarization and hyphal morphogenesis. Strain BWP17 was diluted from an overnight culture into medium containing 10% BCS and grown for 2 h at 37°C. Myriocin was added at a final concentration of 100 μM to serum-stimulated cells, and phytosphingosine was added to rescued cultures at a final concentration of 30 μM. Representative morphologies of cells observed by light microscopy after 2 h of growth under the indicated conditions (A) or of cells from each sample stained with filipin after 1 h and 2 h of growth (B) are shown. Cells were seeded onto coverslips at a low density to prevent clumping of the hyphae, and representative cells were moved closer together by using Adobe Photoshop. Bar, 10 μm.
TABLE 1.
Sphingolipid biosynthesis is necessary for sterol polarization to hyphal tips
| Timea (h) | Drug treatment (concn [μM]) | % Polarized filipin stain ± SD |
|---|---|---|
| 1 | None | 94.5 ± 6.4 |
| Myriocin (100) | 51 ± 4.2 | |
| Myriocin (100) plus phytosphingosine (30) | 92 ± 7.1 | |
| 2 | None | 90.5 ± 6.4 |
| Myriocin (100) | 10.5 ± 3.5 | |
| Myriocin (100) plus phytosphingosine (30) | 84 ± 11.3 |
Time after BWP17 cells were treated with 10% BCS and the indicated drug(s).
To confirm that the effects of myriocin were due to an inhibition of sphingolipid biosynthesis, we examined the ability of phytosphingosine to rescue the effects of myriocin treatment. Phytosphingosine is an intermediate in sphingolipid synthesis that is downstream of serine palmitoyltransferase. When added to myriocin-treated cultures, phytosphingosine largely rescued the hyphal morphology and sterol polarization phenotypes produced by the drug treatment (Fig. 8; Table 1). Taken together, the results from the ketoconazole and myriocin experiments suggest that lipid raft polarization plays an important role in hyphal morphogenesis in C. albicans.
DISCUSSION
Biological membranes are composed of many types of lipids, and it has been proposed that the aggregation of distinct subsets of lipids can result in the formation of distinct membrane domains known as lipid rafts. These raft domains are highly enriched in sphingolipids and sterols, and their polar distribution within the plasma membrane has been implicated in polarized morphogenesis and motility in a wide range of cell types (4, 8, 33). For example, a polar localization of sterol-enriched membrane domains was detected at the tips of pheromone-induced mating projections in S. cerevisiae. The significance of this observation is underscored by the discovery that several lipid raft proteins that can be isolated from DRMs are also localized to the tips of mating projections (4). In the present study, we demonstrated sterol polarization at the growing tip of hyphal cells at all stages of hyphal growth and at sites of septum formation in mature hyphae in C. albicans. Since sterol polarization was dependent on both sterol and sphingolipid biosynthesis, it seems likely that these sterol-rich domains represent lipid rafts. Furthermore, the presence of DRMs in both budding and hyphal cells indicates that lipid raft polarization in hyphae does not result from de novo formation of these domains (Fig. 3), but is brought about by another process. Consistent with a role in polarized morphogenesis, the disruption of sterol or sphingolipid biosynthesis coincided with the production of aberrant hyphal morphologies. These results indicate that lipid raft polarization has a special role in hyphal morphogenesis in C. albicans.
Sterol polarization is specific to hyphae, as budding cells and pseudohyphae did not display sterol polarization at sites of active morphogenesis. When hyphal growth was prevented by a treatment with the quorum-sensing molecule farnesol, sterol polarization was still observed at the tips of growing buds. However, although farnesol-treated cells resemble pseudohyphae, they are distinct from pseudohyphae in that they express a hypha-specific reporter gene, HWP1-GFP (data not shown), suggesting that these cells are actively engaged in a hyphal differentiation program at the transcriptional level. This result further indicates that sterol polarization is specific to cells engaged in the hyphal morphogenesis program and is not a result of the unique geometry of the hyphal tip versus that of budding cells. The mechanism by which farnesol disrupts hyphal growth in C. albicans is not understood. However, a possible connection was suggested by the large increase in farnesol production that was observed when ergosterol biosynthesis was prevented downstream of farnesyl pyrophosphate (24). This suggests a link between farnesol and ergosterol production that could influence lipid raft functions and hyphal morphogenesis.
One interesting question that arose from the present study concerns the mechanism that maintains sterol polarization at the hyphal tip. Studies of several mammalian cell types have suggested that lipid raft aggregation at specific locations within the plasma membrane is enhanced by interactions between proteins found within these lipid domains. For example, T-cell-receptor interactions are thought to promote raft clustering and consequently the formation of multimeric signaling complexes at sites of T-cell aggregation (26, 27). It is possible that lipid raft polarization at the hyphal tips in C. albicans is brought about in a similar manner. Also, a role for the septin family of proteins in this process was suggested by the colocalization of septins with sterol-rich domains at hyphal tips and septation sites (Fig. 5) (45, 49). Septins establish domain boundaries between mother cells and buds in S. cerevisiae for a variety of proteins, including a transmembrane protein, Ist2p (6, 47). It is tempting to speculate a more general role for septins in forming domain boundaries for distinct lipid domains. However, our failure to detect significant amounts of septin proteins in DRM fractions suggests that other proteins are also involved.
The significance of lipid polarization during polarized morphogenesis in S. cerevisiae has recently been called into question (48). Valdez-Taubas and Pelham (48) argued that the sterol polarization observed in mating projections of S. cerevisiae results from endocytic recycling and the slow diffusion of membranes at the tip of mating projections. However, they failed to consider a role for septins in establishing a boundary domain at the base of mating projections. Septins localize at the base of mating projections and help to sequester proteins involved in mating to the shmoo tips (12, 14, 17). It is possible that they similarly act to maintain the polarization of lipid domains to this site. In addition, the polarization of sterol-rich domains to the tips of farnesol-treated cells suggests that membrane recycling and diffusion alone are not adequate for membrane polarization. Farnesol-treated cells grow with budding or pseudohyphal morphologies but are nevertheless able to activate some aspects of the hyphal differentiation program (Fig. 2; also data not shown). Thus, factors other than unique geometry and tip dynamics function to promote membrane polarization during hyphal growth.
Relationship between lipid rafts and C. albicans virulence.
The detection of polarized lipid domains in C. albicans has many interesting implications for the ability of this organism to act as a pathogen. One aspect of this is the ability of proteins, including acylated proteins, pleckstrin homology (PH) domain-containing proteins, and GPI-anchored proteins, to partition into lipid raft domains. It is particularly interesting that C. albicans produces many GPI-anchored proteins that are specific to the hyphal phase of growth. The proteins thought to contain GPI anchors include members of the adhesin protein family (e.g., Hwp1p and Als1p) that mediate adhesion to host cells and members of the secreted aspartyl protease family (i.e., Sap9p and Sap10p) (28, 42, 46). These virulence factors are upregulated during hyphal growth, and their production is required for the invasive growth and virulence of the organism. Bioinformatic approaches have also predicted other likely GPI-anchored proteins whose functions are not yet known (29). Because many GPI-anchored proteins require sequestration into raft domains for delivery to the plasma membrane in other organisms, including S. cerevisiae, the present studies suggest that the polarization of membrane domains to the hyphal tip may directly contribute to the virulence of C. albicans.
Other GPI-anchored proteins, such as Eap1p, Dfg5p, and Phr1p, are known to be involved in epithelial adhesion, virulence, and proper hyphal growth (30, 41, 44). Phr1p is the closest C. albicans homolog of Gas1p from S. cerevisiae. Gas1p is involved in cell wall biogenesis and is a known lipid raft protein that fails to reach the cell surface when lipid rafts are disrupted (3). Interestingly, the hyphal phenotypes conferred by myriocin were similar to those observed in phr1Δ/phr1Δ strains of C. albicans grown at a neutral pH (41). Thus, the hyphal growth defects observed upon treatment with myriocin might be accounted for, in part, by a loss of Phr1p trafficking to the cell surface. Alternatively, Phr1p could have a more direct role in promoting membrane polarization. In some cases, clusters of GPI-anchored proteins are thought to promote the formation of lipid rafts (8). To examine this possibility, we used S. cerevisiae to test whether the overproduction of GPI-anchored proteins could promote ectopic lipid raft polarization in budding cells. However, GAL1 promoter overexpression in budding cells either GAS1 or SAG1, the gene encoding α-agglutinin, did not promote detectable membrane polarization (data not shown), suggesting that other proteins are required for membrane lipid polarization in yeast.
The presence of ergosterol-rich membrane domains at the tips of hyphae is also significant in that amphotericin, a drug that is widely used to treat C. albicans infections, acts by binding ergosterol and forming pores in membranes (15, 38). These observations suggest the interesting possibility that amphotericin analogs may be useful for targeting novel therapeutic agents that neutralize other virulence functions to hyphal tips.
Acknowledgments
We thank Deborah Brown for invaluable technical advice, helpful discussions, and critical reviews of the manuscript and Amy Chang for providing antibodies.
This work was supported by grant RO1 AI47837 from the National Institutes of Health to J.B.K.
REFERENCES
- 1.Adams, A. E., and J. R. Pringle. 1991. Staining of actin with fluorochrome-conjugated phalloidin. Methods Enzymol. 194:729-731. [DOI] [PubMed] [Google Scholar]
- 2.Bagnat, M., A. Chang, and K. Simons. 2001. Plasma membrane proton ATPase Pma1p requires raft association for surface delivery in yeast. Mol. Biol. Cell 12:4129-4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bagnat, M., S. Keranen, A. Shevchenko, and K. Simons. 2000. Lipid rafts function in biosynthetic delivery of proteins to the cell surface in yeast. Proc. Natl. Acad. Sci. USA 97:3254-3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bagnat, M., and K. Simons. 2002. Cell surface polarization during yeast mating. Proc. Natl. Acad. Sci. USA 99:14183-14188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balguerie, A., M. Bagnat, M. Bonneu, M. Aigle, and A. M. Breton. 2002. Rvs161p and sphingolipids are required for actin repolarization following salt stress. Eukaryot. Cell 1:1021-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barral, Y., V. Mermall, M. S. Mooseker, and M. Snyder. 2000. Compartmentalization of the cell cortex by septins is required for maintenance of cell polarity in yeast. Mol. Cell 5:841-851. [DOI] [PubMed] [Google Scholar]
- 7.Berman, J., and P. E. Sudbery. 2002. Candida albicans: a molecular revolution built on lessons from budding yeast. Nat. Rev. Genet. 3:918-932. [DOI] [PubMed] [Google Scholar]
- 8.Brown, D. A., and E. London. 1998. Functions of lipid rafts in biological membranes. Annu. Rev. Cell Dev. Biol. 14:111-136. [DOI] [PubMed] [Google Scholar]
- 9.Brown, D. A., and J. K. Rose. 1992. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell 68:533-544. [DOI] [PubMed] [Google Scholar]
- 10.Calderone, R. A., and W. A. Fonzi. 2001. Virulence factors of Candida albicans. Trends Microbiol. 9:327-335. [DOI] [PubMed] [Google Scholar]
- 11.Cheng, J., T. S. Park, A. S. Fischl, and X. S. Ye. 2001. Cell cycle progression and cell polarity require sphingolipid biosynthesis in Aspergillus nidulans. Mol. Cell. Biol. 21:6198-6209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Faty, M., M. Fink, and Y. Barral. 2002. Septins: a ring to part mother and daughter. Curr. Genet. 41:123-131. [DOI] [PubMed] [Google Scholar]
- 13.Felk, A., M. Kretschmar, A. Albrecht, M. Schaller, S. Beinhauer, T. Nichterlein, D. Sanglard, H. C. Korting, W. Schafer, and B. Hube. 2002. Candida albicans hyphal formation and the expression of the Efg1-regulated proteinases Sap4 to Sap6 are required for the invasion of parenchymal organs. Infect. Immun. 70:3689-3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ford, S. K., and J. R. Pringle. 1991. Cellular morphogenesis in the Saccharomyces cerevisiae cell cycle: localization of the CDC11 gene product and the timing of events at the budding site. Dev. Genet. 12:281-292. [DOI] [PubMed] [Google Scholar]
- 15.Fujii, G., J. E. Chang, T. Coley, and B. Steere. 1997. The formation of amphotericin B ion channels in lipid bilayers. Biochemistry 36:4959-4968. [DOI] [PubMed] [Google Scholar]
- 16.Ghannoum, M. A., and L. B. Rice. 1999. Antifungal agents: mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin. Microbiol. Rev. 12:501-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giot, L., and J. B. Konopka. 1997. Functional analysis of the interaction between Afr1p and the Cdc12p septin, two proteins involved in pheromone-induced morphogenesis. Mol. Biol. Cell 8:987-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gladfelter, A. S., J. R. Pringle, and D. J. Lew. 2001. The septin cortex at the yeast mother-bud neck. Curr. Opin. Microbiol. 4:681-689. [DOI] [PubMed] [Google Scholar]
- 19.Gong, X., and A. Chang. 2001. A mutant plasma membrane ATPase, Pma1-10, is defective in stability at the yeast cell surface. Proc. Natl. Acad. Sci. USA 98:9104-9109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gow, N. A. R. 2002. Cell biology and cell cycle of Candida, p. 145-158. In R. A. Calderone (ed.), Candida and candidiasis. ASM Press, Washington, D.C.
- 21.Hazan, I., and H. Liu. 2002. Hyphal tip-associated localization of Cdc42 is F-actin dependent in Candida albicans. Eukaryot. Cell 1:856-864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heitefuss, R. 2001. Defence reactions of plants to fungal pathogens: principles and perspectives, using powdery mildew on cereals as an example. Naturwissenschaften 88:273-283. [DOI] [PubMed] [Google Scholar]
- 23.Hornby, J. M., E. C. Jensen, A. D. Lisec, J. J. Tasto, B. Jahnke, R. Shoemaker, P. Dussault, and K. W. Nickerson. 2001. Quorum sensing in the dimorphic fungus Candida albicans is mediated by farnesol. Appl. Environ. Microbiol. 67:2982-2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hornby, J. M., B. W. Kebaara, and K. W. Nickerson. 2003. Farnesol biosynthesis in Candida albicans: cellular response to sterol inhibition by zaragozic acid B. Antimicrob. Agents Chemother. 47:2366-2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horvath, A., C. Sutterlin, U. Manning-Krieg, N. R. Movva, and H. Riezman. 1994. Ceramide synthesis enhances transport of GPI-anchored proteins to the Golgi apparatus in yeast. EMBO J. 13:3687-3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Janes, P. W., S. C. Ley, and A. I. Magee. 1999. Aggregation of lipid rafts accompanies signaling via the T cell antigen receptor. J. Cell Biol. 147:447-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Janes, P. W., S. C. Ley, A. I. Magee, and P. S. Kabouridis. 2000. The role of lipid rafts in T cell antigen receptor (TCR) signalling. Semin. Immunol. 12:23-34. [DOI] [PubMed] [Google Scholar]
- 28.Kapteyn, J. C., H. Van Den Ende, and F. M. Klis. 1999. The contribution of cell wall proteins to the organization of the yeast cell wall. Biochim. Biophys. Acta 1426:373-383. [DOI] [PubMed] [Google Scholar]
- 29.Lee, S. A., S. Wormsley, S. Kamoun, A. F. Lee, K. Joiner, and B. Wong. 2003. An analysis of the Candida albicans genome database for soluble secreted proteins using computer-based prediction algorithms. Yeast 20:595-610. [DOI] [PubMed] [Google Scholar]
- 30.Li, F., and S. P. Palecek. 2003. EAP1, a Candida albicans gene involved in binding human epithelial cells. Eukaryot. Cell. 2:1266-1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu, H. 2001. Transcriptional control of dimorphism in Candida albicans. Curr. Opin. Microbiol. 4:728-735. [DOI] [PubMed] [Google Scholar]
- 32.Lo, H. J., J. R. Kohler, B. DiDomenico, D. Loebenberg, A. Cacciapuoti, and G. R. Fink. 1997. Nonfilamentous C. albicans mutants are avirulent. Cell 90:939-949. [DOI] [PubMed] [Google Scholar]
- 33.Manes, S., R. Ana Lacalle, C. Gomez-Mouton, and A. C. Martinez. 2003. From rafts to crafts: membrane asymmetry in moving cells. Trends Immunol. 24:320-326. [DOI] [PubMed] [Google Scholar]
- 34.Miyake, Y., Y. Kozutsumi, S. Nakamura, T. Fujita, and T. Kawasaki. 1995. Serine palmitoyltransferase is the primary target of a sphingosine-like immunosuppressant, ISP-1/myriocin. Biochem. Biophys. Res. Commun. 211:396-403. [DOI] [PubMed] [Google Scholar]
- 35.Nagiec, M. M., J. A. Baltisberger, G. B. Wells, R. L. Lester, and R. C. Dickson. 1994. The LCB2 gene of Saccharomyces and the related LCB1 gene encode subunits of serine palmitoyltransferase, the initial enzyme in sphingolipid synthesis. Proc. Natl. Acad. Sci. USA 91:7899-7902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nantel, A., D. Dignard, C. Bachewich, D. Harcus, A. Marcil, A. P. Bouin, C. W. Sensen, H. Hogues, M. van het Hoog, P. Gordon, T. Rigby, F. Benoit, D. C. Tessier, D. Y. Thomas, and M. Whiteway. 2002. Transcription profiling of Candida albicans cells undergoing the yeast-to-hyphal transition. Mol. Biol. Cell 13:3452-3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Odds, F. C., A. J. Brown, and N. A. Gow. 2003. Antifungal agents: mechanisms of action. Trends Microbiol. 11:272-279. [DOI] [PubMed] [Google Scholar]
- 38.Paquet, M. J., I. Fournier, J. Barwicz, P. Tancrede, and M. Auger. 2002. The effects of amphotericin B on pure and ergosterol- or cholesterol-containing dipalmitoylphosphatidylcholine bilayers as viewed by 2H NMR. Chem. Phys. Lipids 119:1-11. [DOI] [PubMed] [Google Scholar]
- 39.Pruyne, D., and A. Bretscher. 2000. Polarization of cell growth in yeast. J. Cell Sci. 113:571-585. [DOI] [PubMed] [Google Scholar]
- 40.Sanglard, D., B. Hube, M. Monod, F. C. Odds, and N. A. Gow. 1997. A triple deletion of the secreted aspartyl proteinase genes SAP4, SAP5, and SAP6 of Candida albicans causes attenuated virulence. Infect. Immun. 65:3539-3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saporito-Irwin, S. M., C. E. Birse, P. S. Sypherd, and W. A. Fonzi. 1995. PHR1, a pH-regulated gene of Candida albicans, is required for morphogenesis. Mol. Cell. Biol. 15:601-613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schaller, M., M. Bein, H. C. Korting, S. Baur, G. Hamm, M. Monod, S. Beinhauer, and B. Hube. 2003. The secreted aspartyl proteinases Sap1 and Sap2 cause tissue damage in an in vitro model of vaginal candidiasis based on reconstituted human vaginal epithelium. Infect. Immun. 71:3227-3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schuck, S., M. Honsho, K. Ekroos, A. Shevchenko, and K. Simons. 2003. Resistance of cell membranes to different detergents. Proc. Natl. Acad. Sci. USA 100:5795-5800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spreghini, E., D. A. Davis, R. Subaran, M. Kim, and A. P. Mitchell. 2003. Roles of Candida albicans Dfg5p and Dcw1p cell surface proteins in growth and hypha formation. Eukaryot. Cell 2:746-755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sudbery, P. E. 2001. The germ tubes of Candida albicans hyphae and pseudohyphae show different patterns of septin ring localization. Mol. Microbiol. 41:19-31. [DOI] [PubMed] [Google Scholar]
- 46.Sundstrom, P. 2002. Adhesion in Candida spp. Cell. Microbiol. 4:461-469. [DOI] [PubMed] [Google Scholar]
- 47.Takizawa, P. A., J. L. DeRisi, J. E. Wilhelm, and R. D. Vale. 2000. Plasma membrane compartmentalization in yeast by messenger RNA transport and a septin diffusion barrier. Science 290:341-344. [DOI] [PubMed] [Google Scholar]
- 48.Valdez-Taubas, J., and H. R. Pelham. 2003. Slow diffusion of proteins in the yeast plasma membrane allows polarity to be maintained by endocytic cycling. Curr. Biol. 13:1636-1640. [DOI] [PubMed] [Google Scholar]
- 49.Warenda, A. J., and J. B. Konopka. 2002. Septin function in Candida albicans morphogenesis. Mol. Biol. Cell 13:2732-2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Whiteway, M. 2000. Transcriptional control of cell type and morphogenesis in Candida albicans. Curr. Opin. Microbiol. 3:582-588. [DOI] [PubMed] [Google Scholar]