Abstract
Objective:
This study aimed to 1) determine the sensitivity of the electrically evoked auditory change complex (eACC) to changes in stimulating electrode position; and 2) investigate the association between results of eACC measures and behavioral electrode discrimination and their association with speech-perception performance in pediatric cochlear implant (CI) users who have auditory neuropathy spectrum disorder (ANSD).
Design:
Fifteen children with ANSD ranging in age between 5.4 and 18.6 yrs participated in this study. All subjects used Cochlear Nucleus devices. For each subject, open-set speech perception ability was assessed using the Phonetically Balanced Kindergarten (PBK) word lists presented at 60 dB SPL using monitored live voice in a sound booth. Behavioral and objective measures of electrode discrimination were made in a non-clinical test environment. The stimuli used to elicit these measures were 800-ms biphasic pulse trains delivered by a direct interface to the cochlear implant. Data were collected from two basic stimulation conditions. In the standard condition, the entire pulse train was delivered to a mid-array electrode (electrode 11 or 12) at the maximum comfortable level (C level). In the change condition, the stimulus was split into two 400-ms pulse train segments presented sequentially on two different electrodes. The stimulation level of the second 400-ms pulse train was loudness balanced to the C level of the mid-array electrode used in the standard condition. The separation between the pair of stimulating electrodes was systematically varied. For behavioral electrode discrimination measures, each subject was required to determine whether they heard one or two sounds for stimuli presented in different stimulation conditions. For the eACC measures, two replicates of 100 artifact-free sweeps were recorded for each stimulation condition.
Results:
The eACC in response to changes in stimulating electrode position was recorded from all subjects with ANSD using direct electrical stimulation. Electrode discrimination thresholds determined with the eACC and behavioral measures were consistent. Children with ANSD using cochlear implants who showed poorer speech performance also required larger separations between the stimulating electrode pair to reliably elicit the eACC than subjects with better speech-perception performance. There was a robust correlation between electrode discrimination capacities and speech-perception performances in subjects tested in this study. The effect of electrode separation on eACC amplitudes was not monotonic.
Conclusions:
These results demonstrate the feasibility of using the eACC to evaluate electrode discrimination capacities in children with ANSD. These results suggest that the eACC elicited by changes in stimulating electrode position holds great promise as an objective tool for evaluating spectral pattern detection in this population, which may be predictive of their potential speech-perception performance.
Keywords: electrical stimulation, auditory neuropathy spectrum disorders, auditory event-related response, speech perception, cochlear implant
INTRODUCTION
Auditory neuropathy spectrum disorder (ANSD) is a form of hearing impairment that is characterized by evidence of cochlear function in conjunction with an aberrant auditory neural system. The prevalence of ANSD is about 10-15% of children with newly identified hearing loss (Berlin et al., 2010; Rance et al., 1999). Unlike sensorineural hearing loss, the effect of ANSD on auditory capability cannot be predicted based on the severity of hearing loss as measured by pure tone audiometry, cochlear microphonic threshold, presence/absence of otoacoustic emissions, or steady state evoked potentials (Rance et al. 1999). Cochlear implantation has been used to manage hearing deficits for patients with ANSD who do not benefit from hearing aids. Substantial across-patient variations in speech-perception skills have been reported for implanted children with ANSD (Breneman et al., 2012; Buss et al., 2002; Gibson and Sanli, 2007; Madden et al., 2002; Mason et al., 2003; Miyamoto et al., 1999; Rance et al., 1999; Rance and Baker, 2008, 2009; Shallop et al., 2001; Teagle et al., 2010; Zeng and Liu, 2006). Whereas many children with ANSD who receive cochlear implants (CIs) exhibit substantial benefit (Teagle et al., 2010; Buss et al., 2002; Shallop et al., 2001; Madden et al., 2002; Mason et al., 2003), a sub-group of children fail to show significant improvement in speech-perception performance (Teagle et al., 2010; Gibson and Sanli, 2007; Miyamoto et al., 1999; Rance et al., 1999). The mechanisms responsible for these variations are not yet well understood.
Accurate speech recognition depends partly on the ability of the auditory system to detect ongoing changes in the spectral patterns of incoming signals. CI users are known to have impaired spectral pattern detection (e.g. Friesen et al., 2001; Fu et al., 1998; Henry and Turner, 2003; Loizou and Poroy, 2001). Performance on tasks evaluating spectral pattern detection correlates with vowel and consonant recognition in quiet (Henry and Turner, 2003; Henry et al., 2005; Litvak et al., 2007), speech perception in noise (Friesen et al., 2001; Fu et al., 1998; Fu and Nogaki, 2004; Won et al., 2007), music perception (Won et al., 2010), and identification of environmental sounds (Shafiro, 2008) in CI users. One factor shown to partially account for the impaired spectral pattern detection in implanted listeners is the number of “effective spectral channels” (Friesen et al., 2001; Jones et al., 2013). The electrode array of a CI consists of 12 or more electrodes placed along the longitudinal axis of the cochlea. For devices that do not use current steering technology, the electrical current delivered by each electrode creates an electric field that stimulates the surrounding neural tissue. The electrical fields created by different electrodes typically overlap with each other resulting in channel interactions wherein the same neural population is excited by more than one stimulating electrode. The number of “effective spectral channels” of a multichannel CI will be reduced due to the lack of across-fiber independence. Psychophysical measures of electrode discrimination have been used to assess whether individual electrodes provide perceptually distinct spectral information in adult and child listeners with CIs (Dawson et al., 2000; Friesen et al., 2001; Henry et al., 2000; Throckmorton and Collins, 1999). Results of these studies showed that electrode discrimination and speech perception abilities were correlated, and speech understanding could be improved if the number of “effective spectral channels” increased. While this behavioral measure can be used in adult and older child listeners, doing so in young prelingually-deafened children can be a challenge. In addition, many children with ANSD have multiple disabilities or medical conditions that limit their ability to provide reliable behavioral responses despite advanced age. Therefore, it will be critical to identify some objective measures to evaluate electrode discrimination capacities for these listeners so that decisions about communication mode can be made in a timely manner. If it could be determined that a child is unable to resolve spectral cues in speech, then it may be appropriate to consider a visual supplement to communication should be recommended.
The auditory event-related potentials (ERPs), including the onset response and the auditory change complex (ACC), are cortically generated potentials that can be recorded from surface electrodes placed on the scalp. The onset ERP response is typically evoked by a brief stimulus and the ACC is elicited by stimulus change(s) that occur within an ongoing, long-duration stimulation. The onset ERP and the ACC signal the neural encoding of sound onset and sound change(s) at the level of the auditory cortex, respectively. Results of several studies suggest that the ACC provides important insight into potential auditory discrimination capability of the brain (e.g. Het et al., 2012; Martin et al., 2008; Martin et al., 2010). Previous studies have shown that the ACC response can be used to evaluate spectral discrimination abilities in listeners with normal hearing (He et al., 2012; Martin et al., 2010) and listeners with ANSD (Dimitrijevic et al., 2011). The ACC response increases in amplitude as the magnitude of spectral change increases (He et al., 2012; Martin et al., 2010). Martin (2007) described the electrically evoked ACC (eACC) recorded from one adult listener with ANSD using acoustic stimuli presented in the sound field. The stimulus set tested in this study included spectral changes in the second formant ranging from 0 to 1200 Hz. Results showed that stimuli that could not be perceptually distinguished did not elicit the eACC. However, this conclusion is based on results recorded from only one adult subject with ANSD. It is unknown whether this finding would generalize to implanted pediatric listeners with ANSD.
Brown et al. (2008) described the eACC in response to changes in stimulating electrode position recorded from implanted adult listeners using direct-in stimulation. In this study, the speech processor microphone was bypassed and the electrical biphasic pulse train delivered directly to a single electrode using proprietary software. This technique allows better stimulus control, which simplifies interpretation of results. In addition, previous studies (Brown et al., 2008; He et al., 2013) have shown that the amount of contamination caused by electric stimulus artifact on eACC responses recorded using this technique is negligible. Two basic stimulation conditions were used in Brown et al. (2008) to elicit electrically-evoked auditory event-related responses (eERPs). In the “no change” condition, the entire pulse train was delivered uninterrupted to a single electrode, and the onset eERP was measured relative to the onset of the stimulus. In the “change” condition, the eACC responses were evoked by presenting two pulse train bursts sequentially to two stimulating electrodes, each pulse train being half the duration of that used in the “no change” condition. The separation between these two stimulating electrodes was systematically varied. Their results showed that the eACC was recorded from all subjects and the amplitude of the eACC increased as the magnitude of separation between two stimulating electrodes increased. Overall, results of these studies suggest that the eACC might be a promising tool for evaluating electrode discrimination in CI users. However, it remains unknown whether the eACC can be elicited by changes in stimulating electrode position in children with ANSD who use cochlear implants. In addition, the relationship between measures of the eACC and behavioral electrode discrimination and their association with speech-perception performance has not been systematically investigated.
The purpose of this study was twofold: 1) to determine the sensitivity of the eACC in response to changes in stimulating electrode position, and 2) to evaluate the association between measures of the eACC and behavioral electrode discrimination and their association with speech-perception performance in children with ANSD. It was hypothesized that 1) eACC recordings could be used to objectively evaluate electrode discrimination capabilities, and 2) variations in electrode discrimination capabilities could, at least partially, account for differences in speech perception performance in implanted children with ANSD.
METHODS
Subjects
Fifteen implanted children with ANSD ranging in age between 5.4 and 18.6 yrs (mean: 9.6 yrs, SD: 3.7 yrs) participated in this study. All subjects were diagnosed with ANSD based on the presence of a CM (+/− OAEs) with absent ABRs. None of these subjects had any known cognitive or neurological conditions that might affect central auditory processing. All subjects had been implanted with a Cochlear Corporation device (Englewood, CO) with full electrode insertions in the test ear, and the duration of use was at least 12 months prior to testing. All subjects received audiological management at the University of North Carolina at Chapel Hill. Five subjects were implanted unilaterally (S4, S5, S10, S11, and S15); all others received sequential bilateral cochlear implants. Two bilaterally implanted subjects (S6 and S7) received Advanced Bionics devices (Valencia, CA) in their first ear of implant and, at a later date, received a Cochlear Nucleus Freedom device in their second ear. Only the ears implanted with Cochlear Corporation devices were tested in this study. It should be noted that with their Advanced Bionics device only, S6 obtained a score of 20% correct and S7 obtained a score of 48% correct on PBK words presented at 60 dB SPL live voice after more than 12 years of device use for both subjects. For all other bilaterally implanted subjects, one ear was chosen randomly for testing. For 13 of the subjects with ANSD, English is the only language used in their families. Two subjects (S2 and S11) were learning English as their primary language in school and used a combination of English and Spanish at home. Detailed demographic information for subjects is shown in Table 1.
Table 1.
Demographic information of all subjects who participated in this study.
| Subject number |
Gender | Risk Factor |
Ear tested |
Age at testing |
Age at implantation |
CI type | Processing strategy and rate |
|---|---|---|---|---|---|---|---|
| S1 | M | Unknown | R | 6.5 | 2.3 | Nucleus 24R (CA) | ACE 1200 |
| S2 | M | Premature | L | 7.8 | 1.9 | Nucleus 24R (CA) | ACE 1200 |
| S3 | F | Unknown | L | 5.5 | 3.6 | Nucleus N5 | ACE 900 |
| S4 | M | Jaundiced | R | 11.3 | 1.6 | Nucleus 24R (CA) | ACE 900 |
| S5 | M | Premature | L | 13.4 | 2.5 | Nucleus N5 | ACE 900 |
| S6 | F | Unknown | L | 17.1 | 15.6 | Nucleus 24RE | ACE 900 |
| S7 | F | Unknown | L | 14.2 | 12.7 | Nucleus N5 | ACE 720 |
| S8 | F | Unknown | R | 6.9 | 2.5 | Nucleus 24R (CA) | ACE 900 |
| S9 | M | Unknown | R | 5.4 | 2.8 | Nucleus 24R (CA) | ACE 900 |
| S10 | F | Unknown | R | 9.5 | 3.0 | Nucleus 24R (CA) | ACE 900 |
| S11 | M | Unknown | R | 10.4 | 4.3 | Nucleus 24RE | ACE 900 |
| S12 | F | Premature | R | 8.5 | 3.2 | Nucleus 24RE | ACE 900 |
| S13 | F | Unknown | R | 7.6 | 5.9 | Nucleus N5 | ACE 900 |
| S14 | M | Unknown | L | 5.7 | 1.8 | Nucleus 24RE (CA) | ACE 900 |
| S15 | F | Unknown | R | 14.1 | 2.5 | Nucleus 24R (CS) | ACE 900 |
CI: cochlear implant
All subjects were recruited from the Carolina Children’s Communicative Disorders Program (CCCDP) within the Department of Otolaryngology-Head and Neck Surgery at the University of North Carolina at Chapel Hill (UNC). The institutional review board (IRB) at UNC approved this study. Written informed consents (using UNC IRB-approved forms) were obtained before testing. All subjects received payment for their participation.
General Procedures
Each subject participated in open-set speech perception testing, behavioral measures of dynamic ranges and electrode discrimination, and electrophysiological measures of the eACC in this study. These tests were undertaken in different sessions scheduled on the same day; test sessions lasted for up to 3.5 hours. In addition to spoken language, Sign language and Cued Speech were used when necessary to facilitate subject instructions.
For behavioral and electrophysiological measures, the speech processor microphone was bypassed and electrical stimulation created with custom-designed software incorporating Nucleus Implant Communicator (NIC) programming routines was delivered directly to individual electrodes. The software also generated a trigger pulse coinciding with the onset of the stimulus pulse train that was used to synchronize the stimulating and the recording system. This technique has been previously described by Brown et al. (2008) and He et al. (2013). The stimulus was a train of biphasic current pulses presented in a monopolar stimulation mode (MP1). The pulse width, duration of interphase gap and pulse rate was adjusted for individual subjects based on their preferred programming map. Two stimulation conditions were used for both measures in this study: the “standard” condition and the “change” condition. In the “standard” condition, the 800-ms pulse train was delivered to a single mid-array electrode (electrode 12 for 14 subjects; electrode 11 for subject S5 due to an open circuit of electrode 12). In the “change” condition, the stimulus consisted of two constant amplitude pulse trains, each 400 ms in duration. The leading pulse train segment was presented on the same mid-array electrode as selected in the “standard” condition, and the trailing pulse train segment was presented on one of the following nine electrodes: 13, 14, 15, 16, 17, 18, 19, 20, and 22. For subject S5, electrodes 13 and 22 were not tested because they were disabled due to short circuits; instead, electrode 21 was tested. For subject S9, only electrodes 12-16 were tested due to time constraints. For subject S14, electrodes 20 and 22 were not tested due to short circuits.
Speech Perception Tests
For each subject, the open-set speech perception ability was evaluated using PBK word lists presented at 60 dB SPL using monitored live-voice through a loudspeaker placed at 0° azimuth in a single-walled sound attenuating booth. Responses were transcribed by experienced audiologists and scored for percent correct of words repeated. The stimuli (25 monosyllabic words) were presented in an auditory-only condition using preferred CI settings. For bilateral implant subjects, each ear was tested separately. Only scores for the experimental ear were included in this study.
Behavioral Measures
Behavioral detection thresholds and maximum comfortable levels were measured for each electrode tested using an ascending method of adjustment. The stimulus was a 400-ms biphasic pulse train. The initial stimulation level was set to be inaudible and the subject was instructed to notify the experimenter when she/he first heard the stimulus. Once threshold (T level) was determined the stimulation level was slowly increased and the subject was asked to indicate when the stimulation level was judged to be “loud but comfortable” (C level).
After the T and C levels were obtained, two pulse trains were presented sequentially to the mid-array electrode (electrode 11 for S5 and electrode 12 for all other subjects) and one of the other experimental electrodes. Each pulse train was 400 ms in duration and there was no silent interval between the two pulse trains. The stimulation levels were initially set at the C level measured individually for each respective stimulating electrode. Subjects were instructed to compare the loudness of the two stimuli and inform the experimenter whether the second stimulus was louder, softer or the same as the first stimulus. In cases where the two stimuli differed in loudness, the level of the second stimulating electrode was adjusted and the procedure was repeated until these two stimuli were perceived as equally loud. This loudness balancing procedure was repeated three times for each electrode pair tested in the “change” condition. The loudness-balanced stimulation level was defined as the average of three estimations obtained for each electrode pair. The variations in three estimations ranged from 1 to 8 CU with a mean of 4.7 CU. Younger children tended to show more variation across three estimations than older children.
In the behavioral electrode discrimination task, two bursts of pulse trains were presented sequentially to each electrode pair tested in the “change” condition at the loudness-balanced stimulation level. Each pulse train was 400 ms in duration and there was no silent interval between the two pulse trains. As reference, the discrimination task also included the condition where the two 400-ms pulse trains were presented sequentially to the same mid-array electrode (equivalent to an uninterrupted train of 800 ms). Before data collection, three practice runs were completed in order to familiarize the listener with the task and response requirements. In one practice run, the stimuli were presented to an electrode pair that was widely spaced and, therefore, easy to discriminate (e.g., electrode 12 vs. electrode 22). The other two runs included stimuli presented to electrode pairs that were more closely spaced and therefore presumed to be more difficult to discriminate. Subjects were instructed to listen carefully to the stimuli and inform the experimenter whether they heard one or two sounds. For each electrode pair, this procedure was repeated six times and the order of these tests was randomized across electrode pairs. As a result, there were six responses for each electrode pair. The criterion level of 4 out of 6 responses was required for electrode pairs to be considered discriminable (i.e. reported as “two sounds”). Response time was subject driven and no feedback was given. The subject was able to listen to the stimulus pair again prior to responding if they so desired. The smallest electrode separation between two stimulating electrodes that could be discriminated was defined as the behavioral electrode discrimination threshold. Our pilot data collection showed that using this approach rather than a fine-tuned psychophysical method for behavioral electrode discrimination measure was necessary in this study in order to obtain results with reasonable accuracy in a timely fashion.
Electrophysiological Measures
Stimulation Conditions
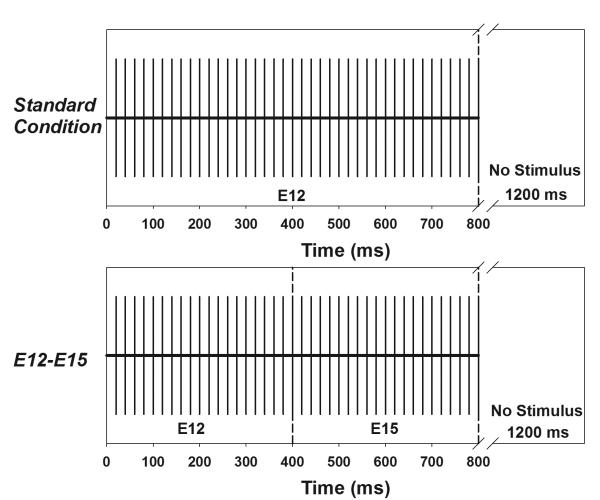
The stimulus was a train of biphasic current pulses 800 ms in duration with an inter-stimulation interval of 1200 ms. In the “standard” condition, the 800-ms pulse train was presented at the C level measured for electrode 12 (electrode 11 for subject S5). The “standard” condition was used to elicit the onset eERP response. In the “change” condition, the two 400-ms pulse trains were presented at the loudness-balanced stimulation level to each electrode pair as described above and eERPs were recorded for each stimulation condition. The sequence of recordings was pseudo-randomized on a trial-by-trial basis across stimulation conditions. Figure 1 shows a schematic of the “standard” condition (upper panel) and one “change” condition (lower panel). In the “change” condition, the stimulating electrode changed from electrode 12 to electrode 15 after 400-ms of stimulation.
Figure 1.
Schematic illustrations of the stimulation paradigm used in the “standard” condition (upper panel) where the entire pulse train is delivered to electrode 12 and the “change” condition (lower panel) where the stimulating electrode shifts from electrode 12 to electrode 15 after 400-ms of stimulation.
Electrophysiological Recordings
Subjects were tested in a single-walled sound booth. They were seated in a reclining chair and watched a silent movie with captions. Subjects were instructed to ignore the stimulus and to relax but not fall asleep during the recording session. Breaks were provided as necessary to ensure that they were able to comply with these instructions. Each recording session took approximately two to three hours to complete.
Electroencephalographic (EEG) activity was recorded using a Neuroscan SCAN system (version 4.4, Compumedics, Charlotte, NC) and a SynAmpRT amplifier. Disposable, sterile Ag-AgCl surface recording electrodes were used to record the EEG. Responses were recorded differentially between electrodes positioned at Fz (positive) and contralateral mastoid (reference). A ground electrode was placed on the low forehead (Fpz). Eye-blink activity was monitored using two recording electrodes placed superior and inferior to the eye that was contralateral to the stimulating ear. Ocular artifacts exceeding 100 μV were rejected from averaging. Electrode impedances were maintained below 5000 Ohms with an inter-electrode impedance difference of less than 2000 Ohms. The recording window consisted of a 100-ms pre-stimulus baseline and a 1900-ms peri/post-stimulus time. Responses were amplified (X10 gain) and analog filtered online between 0.1 and 100 Hz (12 dB/octave roll-off) prior to averaging. In all conditions, the raw EEG signal was sampled at a rate of 1000 Hz. After eye-blink rejection, the remaining (at least 100) artifact-free sweeps were averaged and two averaged responses were recorded for each stimulation condition and each subject. These replicates were averaged together, digitally filtered between 1-30 Hz (12 dB/octave) offline using custom-designed MATLAB software, and smoothed using a 40-msec wide boxcar filter before response identifications and amplitude measurements.
Data Analysis
The eERP responses were independently assessed by two experienced researchers who were blind to subject identification and stimulation conditions. Grand mean averages were computed and used to determine the time windows in which the onset eERP response and the eACC occurred. The windows for the onset and the eACC response were from 20 to 250 ms and from 430 to 680 ms, relative to the stimulus onset, respectively. For each subject, all replicates measured for each stimulation condition as well as the averaged responses of these replicates were overlapped to show the repeatability. Intra-class correlation tests with a two-way random model evaluating the consistency were used to assess the test-retest reliability of the eACC replicates recorded for the same stimulating condition. In this model, both replicates for each subject/condition were considered random effects. Therefore, results of these tests can be generalized to other subjects, and they are not affected by the sequence of eACC recordings for each stimulation condition. The time window over which the correlations was assessed was from 430 to 680 ms. Visual identification of the onset eERP response and the eACC was based on peak latency, waveform morphology, and the replicable property of neural responses. Root mean squared (RMS) amplitudes were measured for both the onset and the eACC within these time windows. The RMS amplitude of a baseline period (1800-1900 ms) was also computed in order to estimate the noise floor for these recording traces. The presence of the eACC response was determined based on two criteria: 1) a visually detectable eACC response in the recording trace based on mutual agreement between the two researchers; and 2) an RMS amplitude during the eACC response window that was at least 50% larger than that of the noise floor. The objective electrode discrimination threshold was defined as the smallest electrode separation that could reliably evoke the eACC response. The correlation between electrode discrimination threshold and the PBK word score was evaluated using a Goodman-Kruskal's gamma test.
RESULTS
The PBK word scores measured for all subjects with ANSD ranged from 0% to 96% with a median of 76% correct. Results measured from individual subjects are listed in Table 2. Subjects were classified as good or poor performers depending on whether their PBK word scores were higher or lower than 70% correct, respectively. This criterion was used in our previous study (He et al., 2013). It was adopted in the present study in order to be consistent.
Table 2.
PBK word scores and behavioral electrode discrimination thresholds for all subjects.
| Subject number |
PBK word score (%) |
Behavioral electrode discrimination threshold |
|---|---|---|
| S1 | 96 | 1 |
| S2 | 88 | 1 |
| S3 | 88 | 1 |
| S4 | 76 | 1 |
| S5 | 76 | 2 |
| S6 | 16 | 2 |
| S7 | 44 | 2 |
| S8 | 88 | 1 |
| S9 | 82 | 1 |
| S10 | 60 | 2 |
| S11 | 72 | 1 |
| S12 | 32 | 2 |
| S13 | 88 | 1 |
| S14 | 36 | 2 |
| S15 | 0 | 2 |
|
| ||
| Median | 76 | 1 |
PBK: Phonetically Balanced Kindergarten Words test
The behavioral electrode discrimination threshold was one electrode separation for eight subjects. At least two electrode separations were required for the other seven subjects to reliably distinguish two sounds. Results measured for individual subjects are listed in Table 2; they suggest that subjects who had larger behavioral electrode discrimination thresholds also exhibited poorer speech-perception performance. This observation is confirmed by results of a Mann-Whitney U test (p<0.01).
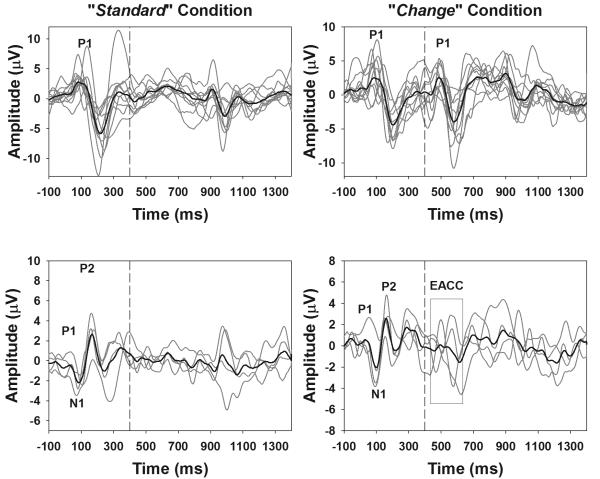
The onset eERPs and the eACCs in response to changes in stimulating electrode position were successfully recorded from all subjects tested in this study. Figure 2 shows a collection of eERP responses recorded from these subjects. Left panels show responses recorded in the “standard” condition where the entire 800-ms pulse train was delivered to electrode 12 (electrode 11 for S5). Right panels show waveforms recorded in one “change” condition where the stimulation electrode shifted from electrode 12 (electrode 11 for S5) to electrode 22 (electrode 21 for S5, electrode 16 for S9, electrode 19 for S14) after 400 ms of stimulation. Display window for all graphs includes only the 100-ms pre-stimulus baseline and 1400 ms after stimulus onset for the sake of clarity. Responses recorded from individual subjects and the group average waveforms are shown with grey and black lines, respectively. The vertical dashed line indicates the time when the first 400 ms of stimulation ended. The onset eERP and the eACC response recorded from 11 subjects consist of a P1 peak and followed by a N2 peak occurring approximately 100 ms later. These responses are shown in the top panels. The P1 peaks of the onset and the eACC are labeled for these responses. The onset eERP responses recorded from four subjects (i.e. S6, S7, S10, and S11) consist of a typical P1-N1-P2 complex occurring within a time window of 20 to 250 ms after the stimulus onset. The eACC recorded from three subjects (i.e. S6, S7 and S10) also consists of a P1-N1-P2 complex occurring within a time window of 430 to 680 ms. In contrast, the eACC response recorded from subject S11 is dominated by a P1 peak followed by a N2 peak, which is less mature in waveform morphology than the onset response recorded from the same subject. These waveforms are shown in the lower panels of the Figure 2. The P1, N1 and P2 peaks are labeled for the onset eERP responses and eACC responses recorded from these subjects are indicated using a black rectangle and labeled as the eACC. Individual peaks of the eACC were not labeled due to variations in response morphology.
Figure 2.
eERP responses recorded from all subjects for the “standard” condition (the left panels) and one “change” condition where there was the largest separation in electrode position tested for each subject (the right panels). Responses recorded from 11 subjects only showed P1 and N2 peaks. These responses are shown in the top panels. P1 peaks are labeled for onset and eACC responses in graphs shown in the top panels. Graphs in the bottom panels show eERPs recorded from four subjects whose onset responses showed the P1-N1-P2 complex. P1, N1 and P2 peaks are labeled for onset responses in these graphs. The eACC recorded from the “change” condition is indicated by a rectangle
Peak latencies and RMS amplitudes of the onset and the eACC responses measured from individual subjects are listed in Table 3. For 11 subjects whose onset responses consist of P1 and N2 peaks, P1 latencies of the onset response ranged from 67 to 144 ms with a mean of 99.1 ms (SD: 18.6 ms). N2 latencies ranged from 148 to 265 ms with a mean of 201.7 ms (SD: 26.9 ms). RMS amplitudes of the onset responses measured from these subjects ranged from 0.8 to 6.2 μV with a mean of 3.1 μV (SD: 1.1 μV). For four subjects whose onset responses consist of the P1-N1-P2 complex, P1 latencies of the onset response ranged from 27 to 76 ms with a mean of 43.9 ms (SD: 14.0 ms). N1 latencies ranged from 74 to 116 ms with a mean of 89.7 ms (SD: 13.2 ms). P2 latencies ranged from 132 to 191 ms with a mean of 156.4 ms (SD: 14.2 ms). RMS amplitude of these onset responses ranged from 0.6 to 3.4 μV with a mean of 1.5 μV (SD: 0.5 μV). The eACC recorded from 12 subjects was dominated by a P1 peak followed by a N2 peak. P1 latencies of the eACC measured from these subjects ranged from 464 to 588 ms with a mean of 500.6 ms (SD: 24.1 ms). N2 latencies of the eACC ranged from 525 to 712 ms with a mean of 593.4 ms (SD: 37.6 ms). The RMS amplitude of the eACC measured from these subjects ranged from 0.4 to 4.8 μV with a mean of 2.1 μV (SD: 1.1 μV). The eACC recorded from S6, S7 and S10 consists of a typical P1-N1-P2 complex. The P1 latencies of the eACC measured from these three subjects ranged from 417 to 505 ms with a mean of 443.6 ms (SD: 21.4 ms). The N1 latencies ranged from 466 to 584 ms with a mean of 492.8 ms (SD: 25.6 ms). The P2 latencies ranged from 535 to 694 ms with a mean of 580.1 ms (SD: 16.6 ms). The RMS amplitude of the eACC measured from these two subjects ranged from 0.6 to 2.4 μV with a mean of 1.4 μV (SD: 0.5 μV). Results of a two-tailed, dependent samples t-test indicate that the onset responses show significantly larger RMS amplitudes than the eACC responses (t=4.3, p<0.01). Results of independent samples Mann-Whitney U tests show that RMS amplitudes of the onset response measured from good performers are significantly larger than those measured from poor performers (p<0.01). Peak latencies of onset responses were not compared for these two groups due to variation in response morphologies.
Table 3.
Means and standard deviations (parentheses) for latency and amplitude of eERP components.
| Onset Response |
eACC Response |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Subject Number |
Latency (ms ± SD) |
RMS Amplitude (μV ± SD) |
Latency (ms ± SD) |
RMS Amplitude (μV ± SD) |
||||||
| P1 | N1 | P2 | N2 | P1 | N1 | P2 | N2 | |||
| S1 | 98.5 (8.9) |
188.4 (9.4) |
3.5 (0.5) |
490.1 (7.0) |
570.6 (16.2) |
2.4 (1.1) |
||||
| S2 | 87.3 (9.2) |
164.1 (15.8) |
3.0 (0.6) |
495 (8.4) |
578.9 (14.5) |
3.7 (1.1) |
||||
| S3 | 104.6 (20.6) |
208.3 (12.1) |
2.8 (0.9) |
487.8 (12.3) |
591.6 (15.9) |
3.5 (0.8) |
||||
| S4 | 103.9 (5.6) |
244.3 (13.3) |
3.3 (0.5) |
518 (8.7) |
642 (13.7) |
2.8 (0.7) |
||||
| S5 | 82.4 (15.7) |
205.1 (17.4) |
1.4 (0.7) |
484.5 (7.5) |
556.2 (16.4) |
1.1 (0.4) |
||||
| S6 | 26.2 (15.5) |
86.9 (15.7) |
151.2 (8.7) |
1.5 (0.6) |
435 (10.6) |
486.9 (18.7) |
555.6 (12.5) |
1.2 (0.4) |
||
| S7 | 27.4 (14.1) |
80.8 (14.9) |
161.8 (14.4) |
1.6 (0.4) |
447.9 (27.9) |
495.1 (31.2) |
563.1 (19.6) |
1.6 (0.6) |
||
| S8 | 106.1 (15.8) |
203.7 (7.9) |
3.8 (0.6) |
488.3 (12.0) |
575.9 (18.4) |
2.0 (0.8) |
||||
| S9 | 75.2 (6.5) |
189.4 (9.7) |
5.8 (0.4) |
495.8 (9.7) |
563.3 (5.3) |
3.0 (0.6) |
||||
| S10 | 42.7 (19.6) |
98 (15.6) |
165.4 (17.4) |
1.5 (0.8) |
477 (10.4) |
564 (19.1) |
682.6 (16.0) |
1.5 (0.6) |
||
| S11 | 48.1 (24.1) |
82.1 (17.5) |
148.6 (9.6) |
1.5 (0.4) |
490.6 (13.1) |
568.6 (17.8) |
1.8 (0.6) |
|||
| S12 | 88.3 (10.7) |
174.5 (13.9) |
1.8 (0.4) |
526.6 (20.0) |
587.4 (18.7) |
1.5 (0.3) |
||||
| S13 | 89.6 (10.4) |
192.2 (7.6) |
3.5 (0.7) |
480.7 (8.0) |
568.2 (13.9) |
2.5 (0.7) |
||||
| S14 | 108.3 (15.3) |
208.9 (17.3) |
3.2 (0.8) |
493.1 (23.5) |
593.7 (17.8) |
2.1 (0.7) |
||||
| S15 | 132.1 (7.1) |
236 (14.2) |
3.6 (1.3) |
539.3 (27.7) |
669.5 (35.3) |
0.7 (0.2) |
||||
eERP: electrically evoked event-related potential
eACC: electrically evoked auditory change complex
RMS: root mean squared
SD: standard deviation
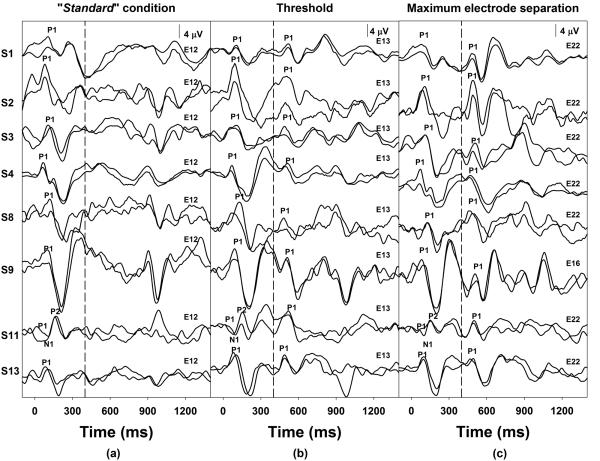
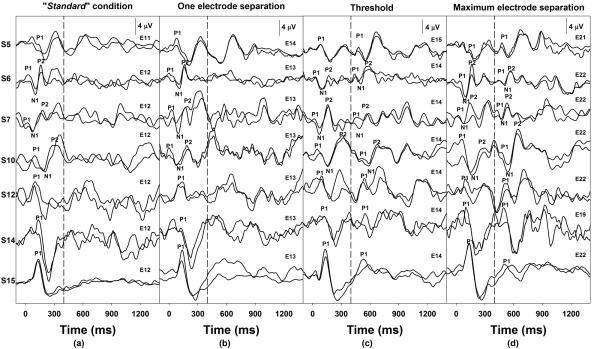
The objective electrode discrimination threshold was one electrode separation for eight of nine subjects who were classified as good performers based on PBK word scores. In other words, the eACC could be reliably elicited when the stimulating electrode changed to an adjacent-apical electrode in these subjects. The remaining good performer subject, S5, deviated from this finding with a PBK score of 76% and an objective electrode discrimination threshold of two electrodes separation. For those six subjects who were classified as poor performers, a spatial separation of two stimulating electrodes was required for the eACC measures. Figure 3 and Figure 4 show individual waveforms recorded for subjects who required one-electrode or two-electrode separation for the eACC measures, respectively. In each figure, dashed lines indicate the time when the first 400-ms of stimulation ended. Each trace represents an averaged response of 100 artifact-free sweeps. Two replicates recorded for each stimulation condition were overlapped to show the repeatability. The stimulating electrode used for the second 400-ms of stimulation is labeled for traces recorded for each stimulation condition. Response peaks are labeled for these waveforms, and subject numbers are listed on the left in each figure. Panel (a) of Figure 3 shows responses recorded for the “standard” condition, panel (b) shows responses measured for the eACC condition associated with their objective electrode discrimination threshold (i.e. one electrode separation), and panel (c) shows eACC responses elicited by the largest electrode separation tested in these subjects. Responses recorded from seven subjects are shown in Figure 4. Panel (a) shows responses recorded for the “standard” condition, panel (b) shows responses recorded when the stimulating electrode moved to the adjacent-apical electrodes, panel (c) shows responses measured for the eACC condition associated with their objective electrode discrimination threshold (i.e. two electrode separation), and panel (d) shows responses elicited by the largest electrode separation in these subjects. It should be pointed out that a small response to the offset of stimulation was also observed at a latency of 900 to 1150 ms. The general morphologic characteristics of the offset response were similar to those of the onset response recorded for the same subject. In addition, this offset response was recorded for all subjects who required one-electrode separation for the eACC measures but was only recorded for a subgroup of three subjects who required two-electrode separation for the eACC measures in the “standard” condition (left panels of Figure 3 and 4). However, a careful inspection of responses recorded from individual subjects revealed that the offset response was not reliably recorded for all stimulating condition. Further inspection of results shown in these two figures and those listed in Table 2 suggests that results of objective and behavioral electrode discrimination thresholds were consistent. Changes in stimulating electrode position that could not be reliably distinguished on the behavioral task did not elicit an eACC and vice versa.
Figure 3.
eERP responses recorded for three stimulation conditions in eight subjects with ANSD who show an objective electrode discrimination threshold of one electrode. The first dashed line indicates the time when the first 400 ms segment of stimulation ended.
Figure 4.
eERP responses recorded for four stimulation conditions in seven subjects with ANSD who show an objective electrode discrimination threshold of two electrodes. The dashed line indicates the time when the first 400 ms of stimulation ended.
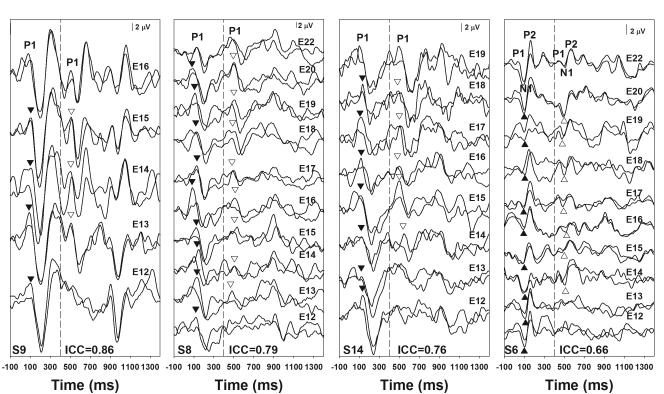
In general, the two replicate eACC responses recorded for any given stimulating conditions showed good replicability. For each subject, the test-retest reliability of these replicates was evaluated using intra-class correlation tests with a two-way random model evaluating the consistency. The mean intra-class correlation coefficients (ICCs) range from 0.66 to 0.86 for these subjects. Figure 5 shows eERP responses of four subjects recorded for all stimulation conditions tested in these subjects. These responses show various degrees of test-retest reliabilities as indicated by the mean of ICCs calculated for all stimulation conditions listed in each panel. Each trace represents an averaged response of 100 artifact-free sweeps. Two replicates recorded for the same stimulation condition were overlapped together. Vertical dashed lines indicate the time point when the first 400-ms of stimulation ended. Subject numbers are indicated in the low left corner of each panel. For subject S8, S9 and S14, the onset and the eACC responses are dominated by a P1 peak and followed by a N2 peak. These peaks are labeled for the top two traces. Filled and open triangles indicate P1 peaks of the onset and the eACC responses for other traces, respectively. For subject S6, eERP responses consist of a P1-N1-P2 complex. These peaks are labeled for the top two traces. Filled and open triangles indicate N1 peaks of the onset and the eACC responses for other traces, respectively. The objective electrode discrimination threshold was one electrode for subjects S8 and S9 and was two electrodes for subjects S6 and S14.
Figure 5.
eERP responses recorded from four subjects with ANSD whose response showed various degrees of test-retest reliability of the eACC response as indicated by the intra-correlation coefficients (ICCs). Traces recorded for the same stimulation condition were overlapped to show the repeatability of these responses. Subject number and the mean ICC of the eACC are shown in each panel.
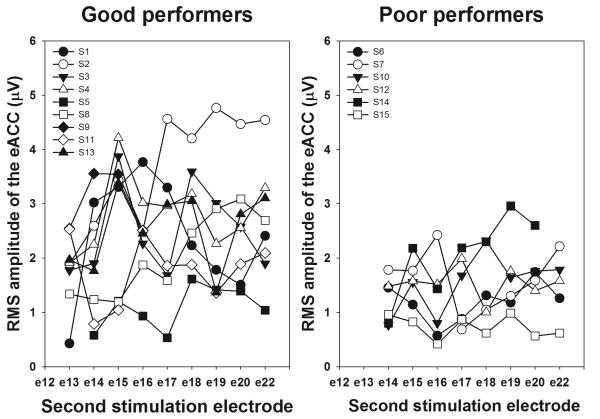
Figure 6 shows RMS amplitudes of the eACC response plotted as a function of the second stimulating electrode number. Each type of symbol indicates results measured from one subject. The left panel shows results from good performers and the right panel shows results from poor performers. RMS amplitudes exhibit marked inter-subject variability. While some subjects show large RMS amplitudes (e.g. S2), others show small amplitudes (e.g. S15). For all subjects, the dependency of RMS amplitude on degree of electrode separation is non-monotonic. Again, there is variability across subjects. While increasing separation between stimulating electrodes generally causes the RMS amplitude to increase for some subjects (e.g. S2), it shows little or no effect on other subjects (e.g. S5 and S15). Results of an independent sample Mann-Whitney U test show that RMS amplitudes of the eACC measured from good performers are significantly larger than those measured from poor performers (p<0.05).
Figure 6.
The RMS amplitude of the eACC responses plotted as a function of the second stimulating electrode number. The left panel and right panel show results measured for good and poor performers, respectively.
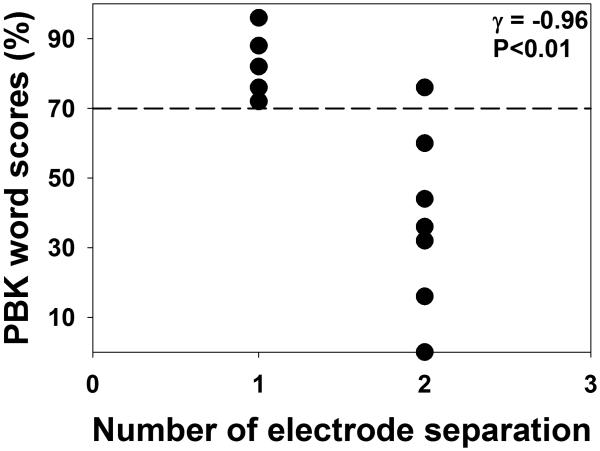
Figure 7 shows PBK word scores plotted as a function of electrode discrimination threshold for all subjects. Results of a Goodman-Kruskal's gamma test are shown at the upper right corner. This test was chosen because data of electrode discrimination thresholds were ordinal with many ties. These results showed that electrode discrimination threshold was significantly correlated with PBK word score (γ= −0.96; p<0.01), which suggests that the larger the electrode discrimination threshold, the poorer the speech-perception performance in these subjects.
Figure 7.
The relationship between electrode discrimination threshold and PBK word scores for all subjects tested in this study. Each dot represents result measured from one subject. The horizontal dashed line indicates a score of 70% correct on PBK word lists. Results of a Goodman-Kruskal's gamma test are shown in the upper right corner.
DISCUSSION
The first aim of this study was to investigate the feasibility of recording the eACC in response to changes in stimulating electrode position through the cochlear implants of children with ANSD using direct electrical stimulation. Our results indicated that eERPs, including the onset and the eACC responses, could reliably be recorded from children with ANSD using direct electrical stimulation, which is consistent with results of our previous study (He et al., 2013). The onset response recorded in this study showed characteristics that were generally similar to responses recorded from normal hearing children (Martin et al., 2010) as well as from children with sensorineural hearing loss (SNHL) who are CI users (e.g. Sharma et al., 2005). However, significant variations across subjects in peak latencies and RMS amplitudes were observed for these onset responses.
The eACC in response to changes in stimulating electrode position has been described for adult CI users with acquired SNHL (Brown et al., 2008). In the present study, the eACC was recorded from all subjects with ANSD using a similar experimental paradigm and testing technique. Morphologic characteristics of the eACC were similar to those of the onset response for 12 subjects. However, for three subjects, the eACC response showed less mature morphologic characteristics than the respective onset response recorded from the same subject. RMS amplitudes of the eACC response were significantly smaller than those of the onset response, which is consistent with published literature (Brown et al., 2008). The test-retest reliability of the eACC was assessed using intra-class correlation tests. The mean ICCs ranged from 0.66 to 0.86, which is generally consistent with results of previous studies (Friesen and Tremblay, 2006; He et al., 2013). Unlike results reported by Brown et al. (2008), the effect of increasing separation between the two stimulating electrodes on RMS amplitudes was non-monotonic for all of the ANSD subjects tested in this study. Whereas Brown et al. (2008) showed that eACC amplitudes generally increased as the distance between the two stimulating electrodes increased for the majority of their subjects, there was no consistent pattern of RMS amplitude dependency across subjects in the present study. This discrepancy could be due to differences in subject populations and methodologies across the two studies. Brown et al. (2008) measured the eACC from adult CI users with SNHL. In contrast, pediatric CI users with ANSD were tested in this study. One fundamental attribute of ANSD is reduced neural synchrony, which by definition can cause aberrant neural response patterns in far-field recordings. It is unknown precisely how much neural synchrony is restored by electrical stimulation in subjects with ANSD, and it is possible that some degree of neural dys-synchrony remains in these subjects even with electrical stimulation. We speculate that this difference in functionality of the neural substrate may partially account for the difference between our results and those reported for adult CI users by Brown et al. (2008). Moreover, as Brown et al. themselves pointed out, a possible limitation of their study was a potential confound between electrode differences and loudness differences due to the stimulation levels used. It has been shown that the eACC can be evoked by changes in stimulation levels in CI users using direct electrical stimulation (Kim et al., 2009), and that the eACC evoked by changes in stimulation level cannot be distinguished from responses evoked by changing stimulating electrode position based on general characteristics of the change potential (Kim et al., 2009). Martin and Boothroyd (2000) have shown that the acoustic ACC evoked by a concurrent change in spectrum and stimulation level is larger in amplitude than the ACC evoked by either change alone. Therefore, the discrepancy between our study and Brown et al. (2008) could partially be caused by differences in stimuli used in these two studies.
The second aim of this study was to assess the association(s) between results of eACC recordings, behavioral electrode discrimination measures, and speech-perception performance in implanted children with ANSD. In the present study, results of eACC recordings were consistent with performance on behavioral electrode discrimination tasks. The eACC could be reliably elicited by a change in stimulating electrode position that could also be behaviorally distinguished. Conversely, the eACC could not be recorded in stimulation conditions where the subject could not behaviorally detect changes in stimulating electrode positions. Elicitation of the eACC response from the poor performers required a larger minimum electrode separation between the two stimulating electrodes than for the good performers. For the six poor performers, a minimum of two electrode separations was required for the eACC measures. Electrode discrimination capacities were correlated with speech-perception performance for subjects tested in this study. However, these results need to be interpreted with caution since subject S5 required two electrode separations for the eACC recordings even though he achieved 76% correct on PBK Word score.
Results revealed clear differences between the groups with different speech perception performance, with the onset and eACC responses appearing more robust in the good performers. While this observation is consistent with those reported previously for non-implanted ANSD subjects (Narne and Vanaja, 2008; Sharma et al., 2011), the underlying mechanism(s) responsible for these differences remains to be elucidated. One possible explanation is that these poor performers have less neural synchrony and/or less neural activation than the good performers despite the use of electrical stimulation. Studies using positron emission tomography (PET) have shown that CI users with good speech performance have higher levels of cortical activity than those with poor speech performance (Herzog, et al., 1991; Fujiki et al., 1999, 2000). Compared with poor performers, good performers showed greater neural activity in the auditory association cortices but not in the primary auditory cortex (Herzog, et al., 1991; Fujiki et al., 1999, 2000). These observations indicate that differing neurophysiological states exist within the auditory system of these two groups of children that have similar ANSD profiles. Further work is clearly needed in this area. While varying degrees of neural dys-sychrony and/or neural activity is highly probable among children with ANSD, age differences might account for some of the findings in the present study. Specifically, the adult-like P1, N1 and P2 peaks observed in the two poor performing children could partially be explained by their older age at implantation and testing (Table 1). Previous work in non-ANSD subjects suggests that the RMS amplitude of the onset response recorded from Fz decreases from age 9 years onward due to development changes in cortical auditory event-related potentials (Ponton et al., 2000). Further studies testing larger groups of ANSD subjects and incorporating more complicated recording paradigms, such as high density EEG recording, are needed to better delineate this issue.
Another interesting finding of this study is that eERPs recorded from the second implanted ear of two poor performers (i.e., the test ear in this study) showed general characteristics that are age appropriate for these two subjects (Ponton et al., 2000). Both subjects received their second implant after a substantial time of unilateral CI use. Specifically, subject S6 received her first implant at age 2.1 years and her second implant at 15.6 years of age. Subject S7 received her first and second implants at ages 5.7 and 12.7 years, respectively. (Recall that the first implants in these two subjects were Advanced Bionics devices and therefore were not testable using our procedure. Only the second implants – both Cochlear Nucleus devices – were tested.) Our results (Figures 4 and 5) showed that responses from these two older subjects did not exhibit prolonged latencies or negative peaks associated with hearing deprivation as described by Sharma et al. (2005; 2009). Differences between our results and those of Sharma et al. (2005; 2009) could be due to differences in methodologies used across studies. In the Sharma et al. studies, a synthesized speech syllable /ba/ was presented in the sound field, and this stimulus was transduced by the subject’s speech processor to evoke the eERPs. Therefore, the entire electrode array was stimulated during the recording. In contrast, here the eERP was evoked by a biphasic pulse train that was directly presented to a single electrode. It is possible that abnormal responses might have been recorded if complex stimuli such as speech had been used in this study. A second difference across studies is that the two bilateral CI users reported in Sharma et al. (2005) were followed only up to 9 months after they received their second CIs. It is possible that eERPs could have shown age-appropriate characteristics if these two subjects had been followed for a longer period of time. In congenitally deaf cats, O’Neil et al. (2011) showed that synapses in the central auditory cortex could be preserved by contralateral electrical stimulation via an indirect pathway at early age. Therefore, unilateral CI use, in theory, could preserve the contralateral auditory pathway to a certain degree. Therefore, our results suggested that it might be premature to make clinical recommendations and/or conclusions based only on morphological characteristics of the electrically-evoked onset response since all six poor performers showed normal morphology of onset response.
Our previous study (He et al., 2013) showed a robust correlation between temporal resolution abilities as revealed by measures of the eACC in response to temporal gaps and speech-perception abilities in children with ANSD. Results of the present study demonstrated a strong correlation between spectral pattern detection and speech-perception abilities in children with ANSD. It should be pointed out that seven subjects participated in both studies. Subject number assigned to these participants, their PBK word scores and results of eACC measures recorded in these two studies are shown in the Appendix Table. PBK word scores recorded in different test sessions showed small variations. However, these variations didn’t change their subject classification (i.e. good vs. poor performers). Among these seven subjects, three children had less than 70% correct on PBK word scores measured in both testing sessions. These three subjects required at least two-electrode separation and a minimum of 20 ms of temporal gap for eACC measures, which suggested that both temporal resolution and spectral pattern detection deficits might account for their poor speech-perception performance. All good performers except for subject S5 (S6 in He et al., 2013) required only one-electrode separation and a temporal gap of 10 ms or less for eACC measures. S5 achieved a PBK word score of 76% in both testing sessions. His gap detection threshold was 5 ms, which was comparable with all other good performers. However, the fact that his electrode discrimination threshold was two electrodes away suggested a deficit in spectral pattern detection, which might account for his less than ideal PBK word score.
CONCLUSION
The eACC in response to changes in stimulating electrode position can be reliably recorded from ANSD subjects with cochlear implants using direct electrical stimulation. These responses show variations within and across ANSD subjects. Electrode discrimination capacities measured using eACC recordings were consistent with those measured with behavioral testing procedures. Compared with good performers, the six poor performers tested in this study required a larger separation in stimulating electrode position to evoke the eACC response. Electrode discrimination capacities were correlated with speech perception performance for subjects tested in this study. Increasing separation between two stimulating electrodes did not show consistent effects on eACC amplitude. These results suggest that the eACC holds great promise as an objective tool for evaluating spectral pattern detections in implanted children with ANSD, which may provide predictive information about their speech-perception performance.
ACKNOWLEDGMENTS
This work was supported by grants from the NIH/NIDCD (1R21DC011383) and Deafness Research Foundation. Portions of this paper were presented at the Objective Measures in Auditory Implants - 7th International Symposium, Amsterdam, The Netherlands in 2012. The authors thank Lisa R. Park, Jennifer Woodard, and Debora R. Hatch for their assistances with subject recruitment, and all ANSD subjects and their parents for participating in this study. We also want to express our great appreciation to Professors Carolyn J. Brown and Paul J. Abbas from the University of Iowa for generously providing the stimulating software for the eACC recording.
Source of Funding: This work was supported in part by grants from NIH/NIDCD (1R21 DC011383) and The Deafness Research Foundation.
Footnotes
Conflicts of Interest: Dr. Craig A. Buchman is a member of Cochlear Corp. Surgeon’s Advisory Board and Dr. Holly F.B. Teagle is a member of a Cochlear Corp. Audiology Advisory Board. For the remaining authors none were declared.
REFERENCES
- Berlin CI, Hood LJ, Morlet T, et al. Multi-site diagnosis and management of 260 patients with auditory neuropathy/dys-synchrony (auditory neuropathy spectrum disorder) Int J Audiol. 2010;49:30–43. doi: 10.3109/14992020903160892. [DOI] [PubMed] [Google Scholar]
- Breneman AI, Gifford RH, DeJong MD. Cochlear implantation in children with auditory neuropathy spectrum disorder: long-term outcomes. J Am Acad Audiol. 2012;23:5–17. doi: 10.3766/jaaa.23.1.2. [DOI] [PubMed] [Google Scholar]
- Brown CJ, Etler C, He S, et al. The electrically evoked auditory change complex: preliminary results from nucleus cochlear implant users. Ear Hear. 2008;29:704–717. doi: 10.1097/AUD.0b013e31817a98af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss E, Labadie RF, Brown CJ, et al. Outcome of cochlear implantation in pediatric auditory neuropathy. Otol Neurotol. 2002;23:328–332. doi: 10.1097/00129492-200205000-00017. [DOI] [PubMed] [Google Scholar]
- Dawson PW, McKay CM, Busby PA, et al. Electrode discrimination and speech perception in young children using cochlear implants. Ear Hear. 2000;21:597–607. doi: 10.1097/00003446-200012000-00007. [DOI] [PubMed] [Google Scholar]
- Dmitrijevic A, Starr A, Bhatt S, et al. Auditory cortical N100 in pre- and post-synaptic auditory neuropathy to frequency or intensity changes of continuous tones. Clin Neurophysiol. 2011;122:594–604. doi: 10.1016/j.clinph.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friesen LM, Shannon RV, Baskent D, et al. Speech recognition in noise as a function of the number of spectral channels: comparison of acoustic hearing and cochlear implants. J Acoust Soc Am. 2001;110:1150–1163. doi: 10.1121/1.1381538. [DOI] [PubMed] [Google Scholar]
- Friesen LM, Tremblay KL. Acoustic change complex recorded in adult cochlear implant listeners. Ear Hear. 2006;27:678–685. doi: 10.1097/01.aud.0000240620.63453.c3. [DOI] [PubMed] [Google Scholar]
- Fu QJ, Nogaki G. Noise susceptibility of cochlear implant users: the role of spectral resolution and smearing. J Assoc Res Otolaryngol. 2004;6:19–27. doi: 10.1007/s10162-004-5024-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu QJ, Shannon RV, Wang XS. Effects of noise and spectral resolution on vowel and consonant recognition: acoustic and electric hearing. J Acoust Soc Am. 1998;104:3586–3596. doi: 10.1121/1.423941. [DOI] [PubMed] [Google Scholar]
- Fujiki N, Naito Y, Hirano S, et al. Cortical activity and seech perception performance in cochlear implant users. Adv Otorhinolaryngol. 2000;57:32–35. doi: 10.1159/000059146. [DOI] [PubMed] [Google Scholar]
- Fujiki N, Naito Y, Hirano S, et al. Correlation between rCBF and speech perception in cochlear implant users. Auris Nasus Larynx. 1999;26:229–236. doi: 10.1016/s0385-8146(99)00009-7. [DOI] [PubMed] [Google Scholar]
- Gibson WPR, Sanli H. Auditory neuropathy: an update. Ear Hear. 2007;28(Suppl):102S–106S. doi: 10.1097/AUD.0b013e3180315392. [DOI] [PubMed] [Google Scholar]
- Herzog H, Lamprecht A, Kuhn A, et al. Cortical activation in profoundly deaf patients during cochlear implant stimulation demonstrated by H2(15)O PET. J Comput Assist Tomogr. 1991;15:369–375. doi: 10.1097/00004728-199105000-00005. [DOI] [PubMed] [Google Scholar]
- He S, Grose J, Teagle HFB, et al. Gap detection measured with electrically-evoked auditory event-related potentials and speech perception abilities in children with auditory neuropathy spectrum disorder. Ear Hear. 2013 doi: 10.1097/AUD.0b013e3182944bb5. (Accepted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S, Grose JH, Buchman CA. Auditory discrimination: the relationship between psychophysical and electrophysiological measures. Int. J. Audiol. 2012;51:771–782. doi: 10.3109/14992027.2012.699198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry BA, McKay CM, McDermott HJ, et al. The relationship between speech perception and electrode discrimination in cochlear implantee. J Acoust Soc Am. 2000;108:1269–1280. doi: 10.1121/1.1287711. [DOI] [PubMed] [Google Scholar]
- Henry BA, Turner CW, Behrens A. Spectral peak resoltuion and speech recognition in quiet: normal hearing, hearing impaired, and cochlear implant users. J Acoust Soc Am. 2005;118:1111–1121. doi: 10.1121/1.1944567. [DOI] [PubMed] [Google Scholar]
- Henry BA, Turner CW. The resolution of complex spectral patterns by cochlear implant and normal-hearing listeners. J Acoust Soc Am. 2003;113:2861–2873. doi: 10.1121/1.1561900. [DOI] [PubMed] [Google Scholar]
- Jones GL, Won JH, Drennan WR, et al. Relationship between channel interaction and spectral-ripple discrimination in cochlear implant users. J Acoust Soc Am. 2013;133:425–433. doi: 10.1121/1.4768881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JR, Brown CJ, Abbas PJ, et al. The effect of changes in stimulus level on electrically evoked cortical auditory potentials. Ear Hear. 2009;30:320–329. doi: 10.1097/AUD.0b013e31819c42b7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loizou PC, Poroy O. Minimum spectral constrast needed for vowel identification by normal hearing and cochlear implant listeners. J Acoust Soc Am. 2001;110:1619–1627. doi: 10.1121/1.1388004. [DOI] [PubMed] [Google Scholar]
- Litvak LM, Spahr AJ, Saoji AA, et al. Relationship between perception of spectral ripple and speech recognition in cochlear implant and vocoder listeners. J Acoust Soc Am. 2007;122:982–991. doi: 10.1121/1.2749413. [DOI] [PubMed] [Google Scholar]
- Madden C, Hilbert L, Rutter M, et al. Pediatric cochlear implantation in auditory neuropathy. Otol Neurotol. 2002;23:163–168. doi: 10.1097/00129492-200203000-00011. [DOI] [PubMed] [Google Scholar]
- Martin BA. Can the acoustic change complex be recorded in an individual with a cochlear implant? Separating neural responses from cochlear implant artifact. J Am Acad Audiol. 2007;18:126–140. doi: 10.3766/jaaa.18.2.5. [DOI] [PubMed] [Google Scholar]
- Martin BA, Tremblay KL, Korczak P. Speech evoked potentials: From the laboratory to the clinic. Ear Hear. 2008;29:285–313. doi: 10.1097/AUD.0b013e3181662c0e. [DOI] [PubMed] [Google Scholar]
- Martin BA, Boothroyd A, Ali D, et al. Stimulus Presentation Strategies for Eliciting the Acoustic Change Complex: Increasing Efficiency. Ear Hear. 2010;31:356–366. doi: 10.1097/AUD.0b013e3181ce6355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin BA, Boothroyd A. Cortical, auditory, evoked potentials in response to changes of spectrum and amplitude. J Acoust Soc Am. 2000;107:2155–2161. doi: 10.1121/1.428556. [DOI] [PubMed] [Google Scholar]
- Mason JC, De Michelle A, Stevens C. Cochlear implantation in auditory neuropathy of varied etiologies. Laryngoscope. 2003;109:181–185. doi: 10.1097/00005537-200301000-00009. [DOI] [PubMed] [Google Scholar]
- Miyamoto RT, Kirk KI, Renshaw J, et al. Cochlear implantation in auditory neuropathy. Laryngoscope. 1999;109:181–185. doi: 10.1097/00005537-199902000-00002. [DOI] [PubMed] [Google Scholar]
- Narne VK, Vanaja CS. Speech identification and cortical potentials in individuals with auditory neuropathy. Behavioral Brain Funct. 2008;4:15–23. doi: 10.1186/1744-9081-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neil JN, Limb CJ, Baker CA, et al. Bilateral effects of unilteral cochlear implantation in congenitally deaf cats. J Comparative Neurol. 2010;518:2382–2404. doi: 10.1002/cne.22339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponton CW, Eggermont JJ, Kwong B, et al. Maturation of human central auditory sytem activity:evidence from multi-channel evoked potentials. Clin Neurophysiol. 2000;111:220–236. doi: 10.1016/s1388-2457(99)00236-9. [DOI] [PubMed] [Google Scholar]
- Rance G, Barker EJ. Speech and language outcomes in children with auditory neuropathy/dys-synchrony managed with either cochlear implants or hearing aids. Int J Audiol. 2009;48:313–320. doi: 10.1080/14992020802665959. [DOI] [PubMed] [Google Scholar]
- Rance G, Barker EJ. Speech perception in children with auditory neuropathy/dyssynchrony managed with either hearing AIDS or cochlear implants. Otol Neurotol. 2008;29:179–182. doi: 10.1097/mao.0b013e31815e92fd. [DOI] [PubMed] [Google Scholar]
- Rance G, Beer DE, Cone-Wesson B, et al. Clinical findings for a group of infants and young children with auditory neuropathy. Ear Hear. 1999;20:238–252. doi: 10.1097/00003446-199906000-00006. [DOI] [PubMed] [Google Scholar]
- Sharma A, Cardon G, Henion K, et al. Cortical maturation and behavioral outcomes in children with auditory neuropathy spectrum disorder. Int J Audiol. 2011;50:98–106. doi: 10.3109/14992027.2010.542492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Dorman MF, Spahr AJ. The influence of a sensitive period on central auditory development in children with unilateral and bilateral cochlear implants. Hear Res. 2005;203:134–143. doi: 10.1016/j.heares.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Sharma A, Nash AA, Droman M. Cortical development, plasticity and re-organization in chidren with cochlear implants. J Commun Disord. 2009;42:272–279. doi: 10.1016/j.jcomdis.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafiro V. Identification of environmental sounds with varying spectral resolution. Ear Hear. 2008;29:401–420. doi: 10.1097/AUD.0b013e31816a0cf1. [DOI] [PubMed] [Google Scholar]
- Shallop JK, Peterso A, Facer GW, et al. Cochlear implants in 5 cases of auditory neuropathy: postoperative findings and progress. Laryngoscope. 2001;111:555–562. doi: 10.1097/00005537-200104000-00001. [DOI] [PubMed] [Google Scholar]
- Teagle HFB, Roush PA, Woodard JS, et al. Cochlear implantation in children with auditory neuropathy spectrum disorder. Ear Hear. 2010;31:325–3. doi: 10.1097/AUD.0b013e3181ce693b. [DOI] [PubMed] [Google Scholar]
- Throckmorton CS, Collins LM. Investigation of the effects of temporal and spatial interactions on speech-recognition skills in cochlear-implant subjects. J Acoust Soc Am. 1999;105:861–873. doi: 10.1121/1.426275. [DOI] [PubMed] [Google Scholar]
- Won JH, Drennan WR, Kang RS, et al. Psychoacoustic abilities associated with music perception in cochlear implant users. Ear Hear. 2010;31:796–805. doi: 10.1097/AUD.0b013e3181e8b7bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won JH, Drennan WR, Rubinstein JT. spectral-ripple resolution correlates with speech reception in noise in cochlear implant users. J Assoc Res Otolaryngol. 2007;8:384–392. doi: 10.1007/s10162-007-0085-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng FG, Liu S. Speech perception in individuals with auditory neuropathy. J Speech Lang Hear Res. 2006;49:367–380. doi: 10.1044/1092-4388(2006/029). [DOI] [PubMed] [Google Scholar]