Abstract
OBJECTIVES
Cystatin C, a measure of kidney function, has been linked to cognitive impairment, but the association between cognition and levels of cystatin C in persons with chronic kidney disease (CKD) is unknown.
DESIGN/SETTING/PARTICPANTS
We studied 821 participants from the Chronic Renal Insufficiency Cohort Cognitive Study with a baseline cognitive assessment completed at the same visit as serum cystatin C measurement.
MEASUREMENTS
Levels of serum cystatin C were categorized into tertiles; cognitive function was assessed with six neuropsychological tests. We compared scores on these tests across tertiles of cystatin C using linear regression and logistic regression to examine the association between cystatin C level and poor cognitive performance (1 SD difference from the mean).
RESULTS
The mean participant age was 64.9 years, 50.6% were male, and 48.6% were white. After multivariable adjustment for age, race, education, and medical comorbidities in linear models, higher levels of cystatin C were associated with worse cognition on the 3MS, Buschke Delayed Recall, Trails A, Trails B, and Boston Naming (p<0.05 for all). This association remained statistically significant for Buschke Delayed Recall (p=0.01) and Trails A (p=0.03) after additional adjustment for eGFR. Compared to the lowest tertile of cystatin C, the highest tertile was associated with increased likelihood of poor cognitive performance on Trails A (OR=2.17; 95% CI: 1.16-4.06), Trails B (OR=1.89; 95% CI: 1.09-3.27), and Boston Naming (OR=1.85; 95% CI: 1.07-3.19) after multivariate adjustment in logistic models.
CONCLUSION
Among patients with CKD, higher levels of serum cystatin C were associated with worse cognition and increased likelihood of poor cognitive performance on attention, executive function, and naming. Cystatin C is a marker of cognitive impairment and may be associated with cognition independent of eGFR levels.
Keywords: cystatin C, cognition, chronic kidney disease
INTRODUCTION
Cystatin C, a protease inhibitor, has been studied as a measure of kidney function and as a biomarker of cognitive impairment.1-3 In a number of community-based cohort studies, high cystatin C levels were associated with an increased risk for cognitive impairment,4,5 while other observational studies have reported an association between low cystatin C levels (both in serum and cerebrospinal fluid (CSF)) and an increased risk of dementia.6,7 Cystatin C has also been linked to risk of cognitive impairment through genetic and neuropathological studies. A common polymorphism of the cystatin C gene is associated with increased risk of AD,8-10 and cystatin C colocalizes with β-amyloid in the brain, especially in areas involved in AD pathology.11,12
As a measure of kidney function, cystatin C is much less affected by age, sex, and muscle mass compared to serum creatinine.13,14 Studies also suggest that cystatin C is a prognostic indicator of adverse health outcomes, although it is unclear if these associations are independent of cystatin C’s role as a marker of glomerular filtration rate.15 Among older adults, higher levels of cystatin C are associated with increased risk of mortality, cardiovascular events, and poor physical function,4,16-18 and in patients with chronic kidney disease (CKD), cystatin C may better predict kidney decline and progression to ESRD as well as identify those at higher risk for heart failure and mortality.14,19 Although individuals with CKD are also at high risk for cognitive decline and dementia,20-22 the potential of cystatin C as a marker of poor cognitive performance in this population has not been investigated.
In a prospective observational study, the Chronic Renal Insufficiency Cohort (CRIC) Study, we sought to determine the association between cystatin C levels and cognitive function on six cognitive tests among older adults with CKD and to evaluate whether this association was independent of estimated glomerular filtration rate (eGFR) levels. We hypothesized that higher levels of serum cystatin C would be associated with worse cognitive performance and that these associations would remain significant even after adjusting for eGFR.
METHODS
Setting
The Chronic Renal Insufficiency Cohort Cognitive (CRIC COG) Study is an ancillary study of the CRIC Study.
Parent Study
Participants enrolled in the main CRIC Study from seven clinical centers across the United States (July 1, 2003-December 31, 2006) were between 21 and 74 years old and had an eGFR of 20-70 ml/min/1.73 m2 if aged 21-44 years, 20-60 ml/min/1.73 m2 if aged 45-64 years, and 20-50 ml/min/1.73 m2 if aged 65-74. Participants were considered ineligible if they were institutionalized, did not provide informed consent, had previously undergone dialysis for longer than one month, were diagnosed with polycystic kidney disease, had received a prior organ/bone marrow transplant, were taking immunosuppressive drugs for kidney in the past 6 months, or were currently participating in a clinical trial or an ongoing cohort study at some of the centers, the African American Study of Kidney Disease and Hypertension. Other exclusion criteria included New York Heart Association Class III/IV heart failure, cirrhosis, HIV infection or AIDS, chemotherapy for cancer within 2 years, multiple myeloma, or renal cell carcinoma. Additional details of the parent study have been previously published.23
CRIC COG Study
CRIC COG studied cognitive function in elders (eligibility criteria: adults ≥55 years old enrolled in the main CRIC study) with CKD.24 Participants were recruited from 4 of the 7 clinical CRIC centers located in Philadelphia, PA; Cleveland, OH; Oakland, CA; and Chicago, IL. Of the 825 CRIC COG participants (83.9 % of those invited to participate, all were English speaking), 821 participants completed a cognitive assessment and had cystatin C measurement during the same in-clinic visit of the CRIC parent study. The institutional review boards at each of the participating sites and at the University of California San Francisco approved the study. All participants gave written informed consent.
Predictor Variable
Blood samples were collected during the main CRIC clinic visit. Samples were stored at −80° C, and cystatin C was measured at the CRIC central laboratory with a coefficient of variation of 4.9% using the Siemens (Dade/Behring) BN II ProSpec nephelometer (Siemens Healthcare Diagnostics, Deerfield, IL) which used a particle-enhanced immunonepholometric assay (N Latex Cystatin C, Dade Behring, Inc.). 25 To correct for drift over time when using different calibrator and reagent lots manufactured by Siemens, an internal standardization for CRIC cystatin C was implemented. 26 Cystatin C values for the 821 participants ranged from 0.55 mg/L to 6.59 mg/L. We categorized cystatin C into tertiles of low (≤1.230 mg/L), middle (1.231-1.710 mg/L), and high levels (>1.711 mg/L).
Cognitive Function Measures
Baseline cognitive testing consisted of 6 interviewer-administered tests in English including the Modified Mini-Mental State Examination (3MS, global cognitive function), Trails A (attention) and B (executive function), Category Fluency (verbal fluency), Buschke Selective Reminding Test – Delayed Recall (delayed memory), and the Boston Naming Test (naming). The 3MS is a general cognitive battery assessing orientation, concentration, language, praxis, and immediate and delayed memory. Scores on the 3MS range from 0-100 with higher scores (points) indicating better function.27 Visuospatial scanning, motor speed, executive function and attention are all measured with Trails A and B, but Trails A is largely a test of attention where participants draw a line between randomly placed numbered circles in sequence and Trails B, in which the participant draws a line between alternating patterns of circled letters and numbers in sequence, is primarily a test of executive function. Lower scores (seconds) indicate higher function, as they are based on the time to complete a particular task. Scores range from 0 to 300.28 Category (Verbal) Fluency evaluates verbal production, semantic memory, and language. The test measures the number of animals named in a one-minute period. Higher scores indicate better function.29 The Buschke Selective Reminding Test measures verbal memory with delayed components. Participants are read a list of 12 words and asked to recall as many as possible after 1 trial (immediate recall) and after a 20-30 minute delay (delayed recall) higher scores indicate better performance.30 The Boston Naming Test assesses language function by asking participants to name 15 objects presented in pictures with higher scores indicating higher function.29
For each cognitive test, test-specific clinically significant poor cognitive performance was defined as a score one standard deviation (SD) or more below the mean for the 3MS, Verbal Fluency, Buschke Delayed Recall, and the Boston Naming tests. For Trails A and B, lower scores indicate better function, and poor cognitive performance on each test was defined as a score one SD or greater above the mean.
Covariates
All covariates were measured at the same visit for the parent study as was the baseline cognitive assessment. Demographic variables included age, sex, race, Hispanic ethnicity, and education. Comorbid conditions were identified through patient self-report, laboratory test values, and use of medications for diabetes mellitus, stroke, hypertension, and coronary heart disease. Serum creatinine was calibrated to isotope dilution mass spectrometry (IDMS), and used to eGFR with the Modification of Diet in Renal Disease (MDRD) estimating equation.31
Statistical Analyses
To assess whether there was a linear trend in patient characteristics across cystatin C tertiles, ANOVAs were used for continuous variables along with an orthogonal polynomial contrast for test of linear trend; and the Cochran-Armitage trend test was used for categorical variables. We examined the relationship between eGFR and cystatin C with a Pearson correlation. To test for a linear trend in the association between cystatin C tertile and cognitive function score, we again used an ANOVA model along with an orthogonal polynomial contrast. Models were also adjusted for age, race, education, and medical comorbidities that were significantly associated with cystatin C in bivariate analyses (p<0.10). Finally, models were also adjusted for eGFR to investigate whether the association between cystatin C and cognition was independent of eGFR levels. In addition, we tested for an interaction between cystatin C level and age group (< 65, 65-74, >75).
To examine the association between cystatin C level and clinically significant poor cognitive performance, odds ratios (OR) and 95% confidence intervals (CI) were measured in unadjusted logistic regression models. Participants in the lowest tertile of cystatin C (≤1.230 mg/L) served as the reference group. Models were then adjusted for age, race, education, and comorbidities. All analyses were completed using SAS version 9.1, and significance testing was 2-sided with a Type I error rate of 5%.
RESULTS
The 821 participants had a mean age of 64.9 years, 50.6% were male, and 48.6% were white. Participants with higher cystatin C tended to be older, non-white, and have less education (p<0.001 for all). The prevalence of diabetes, hypertension, coronary heart disease, and stroke increased (p<0.05 for each comparison) while eGFR decreased (p<0.001) with increasing level of cystatin C (Table 1). The Pearson correlation between eGFR and cystatin-C was -0.82 (p<0.001).
Table 1. Baseline Characteristics of 821 Participants by Cystatin C Tertile.
| Characteristic N (%) or Mean (SD) | Low (0-1.230 mg/L) N=273 |
Middle (1.231-1.710 mg/L) N=273 |
High (>1.711 mg/L) N=275 |
p-valuea |
|---|---|---|---|---|
| Age, years | 63.6 (5.0) | 65.8 (5.9) | 65.2 (5.7) | <0.001 |
| Age Group | ||||
| <65 years | 168 (61.5) | 123 (45.1) | 129 (46.9) | <0.001 |
| 65-74 years | 100 (36.6) | 128 (46.9) | 131 (47.6) | |
| >75 years | 5 (1.8) | 22 (8.1) | 15 (5.5) | |
| Male (%) | 142 (52.0) | 135 (49.5) | 138 (50.2) | 0.67 |
| White (%) | 151 (55.3) | 136 (49.8) | 112 (40.7) | <0.001 |
| Education, >High School (%) | 201 (73.6) | 172 (63.0) | 156 (56.7) | <0.001 |
| Hispanic ethnicity (%) | 6 (2.2) | 9 (3.3) | 7 (2.6) | 0.80 |
| Diabetes (%) | 93 (34.1) | 155 (56.8) | 176 (64.0) | <0.001 |
| Hypertension (%) | 223 (81.7) | 258 (94.5) | 267 (97.1) | <0.001 |
| Coronary Heart Disease (%) | 55 (20.2) | 78 (28.6) | 104 (37.8) | <0.001 |
| Stroke (%) | 25 (9.2) | 41 (15.0) | 41 (14.9) | 0.046 |
| eGFR, mL/min per 1.73m2 | 54.8 (8.8) | 41.8 (7.7) | 27.5 (8.2) | <0.001 |
p-value is for test of trend across tertiles of cystatin C
Participants with higher cystatin C level had worse cognitive performance on all cognitive tests compared to those with intermediate and lower cystatin C levels in unadjusted linear regression models (Table 2). After multivariable adjustment for age, race, education, and medical comorbidities including diabetes, hypertension, coronary heart disease, and stroke, higher cystatin C levels were associated with lower cognitive performance on 3MS: High tertile (Mean (SE)) = 91.2 (0.6) vs. Middle tertile = 92.5 (0.6) and Low tertile =93.2 (0.6); Buschke Delayed Recall: 6.8 (0.3) vs. 7.2 (0.3) and 7.3 (0.3); Trails A: 70.5 (3.4) vs. 61.9 (3.3) and 55.3 (3.4); Trails B: 176.5 (6.9) vs. 161.3 (6.7) and 152.7 (6.8), and Boston Naming: 13.4 (0.2) vs. 13.8 (0.2) and 13.9 (0.2) (p<0.05 for each comparison). The association with Verbal Fluency was no longer significant (Verbal Fluency: 17.3 (0.5) vs. 16.8 (0.5) and 17.3 (0.5), p=0.90). In order to determine whether the association between cystatin C and cognition was independent of another measure of kidney function, we also adjusted for eGFR in the full multivariable-adjusted model. In these models, Trails A and the Buschke Delayed Recall were still significantly associated with cystatin C (p<0.05 for each). There were no significant interactions between age group and cystatin C level (p>0.05 for all).
Table 2. Unadjusted and Adjusteda Mean and Standard Error (SE) of Cognitive Test Scores by Cystatin C Tertile.
| Low (0-1.230mg/L) N=273 |
Mid (1.231-1.710mg/L) N=273 |
High (>1.711mg/L) N=275 |
p-valueb | |
|---|---|---|---|---|
| 3MS | ||||
| Unadjusted | 94.9(0.4) | 93.0(0.4) | 91.1(0.4) | <0.001 |
| Adjusted | 93.2(0.6) | 92.5(0.6) | 91.2(0.6) | <0.001 |
| BuschkeDelayedRecall | ||||
| Unadjusted | 7.9(0.2) | 7.4(0.2) | 6.9(0.2) | <0.001 |
| Adjusted | 7.3(0.3) | 7.2(0.3) | 6.8(0.3) | 0.02 |
| TrailsA | ||||
| Unadjusted | 43.9(2.2) | 55.4(2.2) | 66.8(2.2) | <0.001 |
| Adjusted | 55.3(3.4) | 61.9(3.3) | 70.5(3.4) | <0.001 |
| TrailsB | ||||
| Unadjusted | 116.9(4.7) | 140.7(4.7) | 163.1(4.8) | <0.001 |
| Adjusted | 152.7(6.8) | 161.3(6.7) | 176.5(6.9) | <0.001 |
| BostonNaming | ||||
| Unadjusted | 14.2(0.1) | 13.9(0.1) | 13.4(0.1) | <0.001 |
| Adjusted | 13.9(0.2) | 13.8(0.2) | 13.4(0.2) | 0.001 |
| VerbalFluency | ||||
| Unadjusted | 18.7(0.3) | 17.5(0.3) | 17.6(0.3) | 0.01 |
| Adjusted | 17.3(0.5) | 16.8(0.5) | 17.3(0.5) | 0.97 |
adjusted for age, race, education, diabetes, hypertension, coronary heart disease, and stroke
p-value is for test of linear trend
When compared to those within the lowest tertile of cystatin C in unadjusted models, participants within the highest tertile had an increased risk for poor cognitive performance based on the 3MS (OR = 2.73; 95% CI: 1.60, 4,66), Buschke Delayed Recall (OR = 1.99; 95% CI: 1.25, 3.16), Trails A (OR = 3.58; 95% CI: 2.03, 6.31), Trails B (OR = 3.00; 95% CI: 1.85, 4.85), and Boston Naming (OR = 2.54; 95% CI: 1.57, 4.11). In models adjusted for age, race, education, and comorbidities, these associations remained statistically significant for Trails A (OR = 2.17; 95% CI: 1.16, 4.06) and B (OR = 1.89; 95% CI: 1.07, 3.27), and Boston Naming (OR = 1.85; 95% CI: 1.07, 3.19) (Figure),
Figure. Adjusteda Odds Ratios and 95% Confidence Intervals for Poor Cognitive Performance across Cystatin C Tertile (low as the reference).
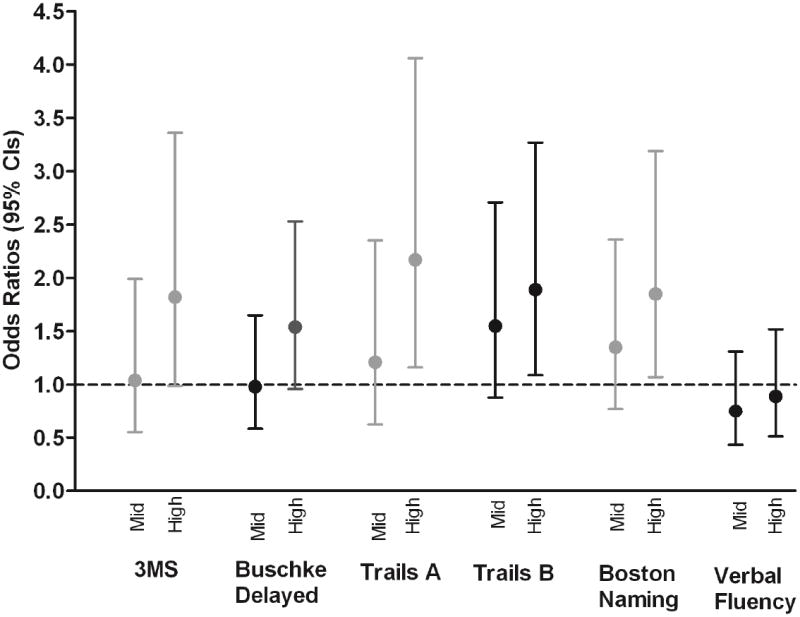
a Models are adjusted for age, race, education, diabetes, hypertension, coronary heart disease, and stroke
CONCLUSION
Among a subgroup of older adults with CKD, enrolled in a prospective cohort study, we found a significant association between higher levels of cystatin C and worse cognitive function for most cognitive domains including global cognition, delayed memory, attention, executive function, and naming. Participants in the highest tertile of cystatin C were almost two times more likely to have significant poor cognitive performance on attention, executive function, and naming compared to the lowest tertile. Cystatin C was associated with attention and delayed memory after adjusting for eGFR levels. In addition, there was no difference in the relationship between cystatin C level and cognitive function by age group.
Several studies have demonstrated that elderly adults with impaired kidney function have an increased risk of cognitive impairment and dementia based on measures of serum creatinine.32-34 Our findings of an association between cystatin C and cognitive function are consistent with these previous studies and provide additional evidence of a risk relationship between kidney function and cognitive impairment. The reported associations also parallel our previous findings from the CRIC COG study which described associations between eGFR by MDRD and worse performance on multiple cognitive domains including global cognition, executive function, naming, attention, and delayed memory.24
The results of this study are also consistent with longitudinal cohort studies of community dwelling older adults which demonstrate an association between high cystatin C levels and incident cognitive decline as well as cognitive and physical disability.4,5 High levels of cystatin C have also been associated with increased presence of subclinical brain infarcts,35,36 and in a recent cohort study of older adults, markers of kidney function, including elevated cystatin C levels, were associated with poor global cognition and increased white matter deficits in the anterior limb of the internal capsulem37 a region associated with impairment on executive function and episodic memory.38 However, our results are in contrast to other studies which also support cystatin C’s relationship with cognitive function but suggest that lower cystatin C levels correspond with increased risk for poor cognitive outcomes including incident AD in a cohort of older men, conversion to AD in patients with mild cognitive impairment, and dementia in patients with Lewy body disease.6,7,39 These conflicting reports may be due to differences in study populations, study design, outcome definitions, or methods used to measure cystatin C.
The cross sectional association between cystatin C and cognitive function in this study could be related to vascular pathology and share pathways with cardiovascular outcomes also linked to cystatin C including hypertension, obesity, and coronary heart disease.3,40-42 Our results also suggest a significant relationship between cystatin-C and attention as well as delayed memory even after adjusting for eGFR which suggests that cystatin-C could affect the pathological changes found in AD through pathways that are not associated with kidney function. Several lines of evidence also support a role for cystatin C in neurodegenerative pathways involving amyloid plaque formation as demonstrated by its co-localization with β-amyloid in brain tissue,11 and a high correlation with β-amyloid and tau levels in CSF.43 In animal models, cystatin-C knockout mice have reduced levels of soluble β-amyloid, reduced plaque formation, and lower cognitive deficits,44 suggesting higher levels of cystatin-C may increase AD pathology. However, some in vitro and in vivo studies indicate that cystatin-C is neuroprotective and binds directly to β-amyloid to prevent plaque formation and preserve cognition.1,45,46
A number of studies indicate that cystatin C is a reliable marker for risk stratification in elderly adults with and without chronic kidney disease which may be attributable to its role as a sensitive measure of kidney function or to its relationships with non-GFR determinants such as inflammation.14-17,47,48 Our results suggest a possible relationship between cystatin C and worse cognition independent of eGFR levels. In earlier investigations of the association between cystatin C and cognitive function, studies that accounted for eGFR, either by stratification or adjustment, reported that cystatin C remained significantly associated with cognitive impairment; however, these cohorts focused on healthy older adults.5,6 The findings from this study suggest that among those with chronic kidney disease, cystatin C is complementary to creatinine-based eGFR in its association with cognitive function, but it may also provide additional prognostic value. The observed association between cystatin C and specific cognitive domains could be related to non-GFR determinants of cystatin C.
Our study had a number of strengths including participant recruitment from the ongoing, multi-center CRIC study which is comprised of a large cohort of diverse, patients. As a result, we were also able to evaluate cognitive performance on a variety of cognitive tests. In addition, CRIC participants are well characterized with complete data on many patient characteristics including sociodemographic variables and cardiovascular comorbidities allowing for adjustment of several potential covariates and confounders as well as level of kidney function.
These results should be interpreted in light of the study limitations. This was a cross-sectional analysis; therefore, causality cannot be inferred. In addition, we did not have data on structural brain imaging or pathological results to determine the etiology of clinically significant poor cognitive performance. Finally, these findings may not be generalizable to younger populations as the CRIC COG cohort was composed of older adults.
In this study, we found that high cystatin C levels were associated with increased likelihood of poor cognitive performance among older adults with CKD, and for some domains this association was independent of eGFR levels. Although the effect sizes were small and the findings are preliminary, our findings suggest a need for further investigation to determine the relationship between cystatin C level and cognitive outcomes in both healthy and CKD populations as well as for additional longitudinal studies to determine the potential role for cystatin C as a biomarker for cognitive decline.
Acknowledgments
Study Funding:
Funding for the CRIC Study was obtained under a cooperative agreement from National Institute of Diabetes and Digestive and Kidney Diseases (U01DK060990, U01DK060984, U01DK061022, U01DK061021, U01DK061028, U01DK060980, U01DK060963, and U01DK060902). In addition, this work was supported in part by: the Perelman School of Medicine at the University of Pennsylvania Clinical and Translational Science Award NIH/NCATS UL1TR000003, Johns Hopkins University UL1 TR-000424, University of Maryland GCRC M01 RR-16500, Clinical and Translational Science Collaborative of Cleveland, UL1TR000439 from the National Center for Advancing Translational Sciences (NCATS) component of the National Institutes of Health and NIH roadmap for Medical Research, Michigan Institute for Clinical and Health Research (MICHR) UL1TR000433, University of Illinois at Chicago CTSA UL1RR029879, Tulane University Translational Research in Hypertension and Renal Biology P30GM103337, Kaiser NIH/NCRR UCSF-CTSI UL1 RR-024131. The CRIC COG ancillary study is supported by a National Institute of Diabetes and Digestive and Kidney Diseases grant, R01DK069406.
Footnotes
Author Contributions:
Study concept and design: Kristine Yaffe
Acquisition of subjects and/or data: Kristine Yaffe, Manjpula Kurella-Tamura, Mark Duckworth, Alan S. Go, John W. Kusek, James P. Lash, Akinlolu Ojo, Nancy Robinson, Raymond R. Townsend
Analysis and interpretation of data: Kristine Yaffe, Lynn Ackerson
Preparation of manuscript: Kristine Yaffe, Manjula Kurella-Tamura, Lynn Ackerson, Tina D. Hoang, Amanda H. Anderson, Mark Duckworth, Alan S. Go, Marie Krousel-Wood, John W. Kusek, James P. Lash, Akinlolu Ojo, Nancy Robinson, Ashwini R. Sehgal, James H. Sondheimer, Susan Steigerwalt, Raymond R. Townsend
Sponsor’s Role:
The study sponsor’s did not participate in the design, methods, subject recruitment, data collections, analysis and preparation of the paper.
Conflict of Interest:
Dr. Yaffe is a consultant for Novartis and serves on data safety monitoring boards for Takeda, Inc and a study sponsored by the NIH and on the Beeson Scientific Advisory Board.
References
- 1.Kaeser SA, Herzig MC, Coomaraswamy J, et al. Cystatin C modulates cerebral [beta]-amyloidosis. Nat Genet. 2007;39:1437–1439. doi: 10.1038/ng.2007.23. [DOI] [PubMed] [Google Scholar]
- 2.Kaur G, Levy E. Cystatin C in Alzheimer’s disease. Frontiers Mol Neurosci. 2012;5:79. doi: 10.3389/fnmol.2012.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bugnicourt J-M, Godefroy O, Chillon J-M, et al. Cognitive disorders and dementia in CKD: The neglected kidney-brain axis. J Am Soc Nephrol. 2013;24:353–363. doi: 10.1681/ASN.2012050536. [DOI] [PubMed] [Google Scholar]
- 4.Sarnak MJ, Katz R, Fried LF, et al. Cystatin C and Aging Success. Arch Intern Med. 2008;168:147–153. doi: 10.1001/archinternmed.2007.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yaffe K, Lindquist K, Shlipak MG, et al. Cystatin-C as a marker of cognitive function in elders: Findings from the Health ABC study. Ann Neurol. 2008;63:798–802. doi: 10.1002/ana.21383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sundelof J, Arnlov J, Ingelsson E, et al. Serum cystatin C and the risk of Alzheimer disease in elderly men. Neurology. 2008;71:1072–1079. doi: 10.1212/01.wnl.0000326894.40353.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maetzler W, Schmid B, Synofzik M, et al. The CST3 BB genotype and low cystatin C cerebrospinal fluid levels are associated with dementia in Lewy body disease. J Alzheimers Dis. 2010;19:937–942. doi: 10.3233/JAD-2010-1289. [DOI] [PubMed] [Google Scholar]
- 8.Cathcart HM, Huang R, Lanham IS, et al. Cystatin C as a risk factor for Alzheimer disease. Neurology. 2005;64:755–757. doi: 10.1212/01.WNL.0000151980.42337.44. [DOI] [PubMed] [Google Scholar]
- 9.Finckh U, von der Kammer H, Velden J, et al. Genetic association of a cystatin C gene polymorphism with late-onset Alzheimer disease. Arch Neurol. 2000;57:1579–1583. doi: 10.1001/archneur.57.11.1579. [DOI] [PubMed] [Google Scholar]
- 10.Hua Y, Zhao H, Lu X, et al. Meta-Analysis of the Cystatin C(CST3) Gene G73A Polymorphism and Susceptibility to Alzheimer’s Disease. Int J Neurosci. 2012;122:431–438. doi: 10.3109/00207454.2012.672502. [DOI] [PubMed] [Google Scholar]
- 11.Deng A, Irizarry MC, Nitsch RM, et al. Elevation of Cystatin C in susceptible neurons in Alzheimer’s disease. Am J Pathol. 2001;159:1061–68. doi: 10.1016/S0002-9440(10)61781-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levy EP, Sastre MP, Kumar AP, et al. Codeposition of cystatin C with amyloid-[beta] protein in the brain of alzheimer disease patients. J Neuropathol Exp Neurol. 2001;60:94–104. doi: 10.1093/jnen/60.1.94. [DOI] [PubMed] [Google Scholar]
- 13.Coll E, Botey A, Alvarez L, et al. Serum cystatin C as a new marker for noninvasive estimation of glomerular filtration rate and as a marker for early renal impairment. Am J Kidney Dis. 2000;36:29–34. doi: 10.1053/ajkd.2000.8237. [DOI] [PubMed] [Google Scholar]
- 14.Menon V, Shlipak MG, Wang X, et al. Cystatin C as a risk factor for outcomes in chronic kidney disease. Ann Intern Med. 2007;147:19–27. doi: 10.7326/0003-4819-147-1-200707030-00004. [DOI] [PubMed] [Google Scholar]
- 15.Tangri N, Inker LA, Tighiouart H, et al. Filtration markers may have prognostic value independent of glomerular filtration rate. J Am Soc Nephrol. 2012;23:351–359. doi: 10.1681/ASN.2011070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shlipak MG, Katz R, Sarnak MJ, et al. Cystatin C and prognosis for cardiovascular and kidney outcomes in elderly persons without chronic kidney disease. Ann Intern Med. 2006;145:237–246. doi: 10.7326/0003-4819-145-4-200608150-00003. [DOI] [PubMed] [Google Scholar]
- 17.Shlipak MG, Sarnak MJ, Katz R, et al. Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med. 2005;352:2049–2060. doi: 10.1056/NEJMoa043161. [DOI] [PubMed] [Google Scholar]
- 18.Peralta CA, Shlipak MG, Judd S, et al. Detection of chronic kidney disease with creatinine, cystatin c, and urine albumin-to-creatinine ratio and association with progression to end-stage renal disease and mortality. JAMA. 2011;305:1545–1552. doi: 10.1001/jama.2011.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhavsar NA, Appel LJ, Kusek JW, et al. Comparison of Measured GFR, Serum Creatinine, Cystatin C, and Beta-Trace Protein to Predict ESRD in African Americans With Hypertensive CKD. Am J Kidney Dis. 2011;58:886–893. doi: 10.1053/j.ajkd.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kurella M, Chertow GM, Fried LF, et al. Chronic kidney disease and cognitive impairment in the elderly: The health, aging, and body composition study. J Am Soc Nephrol. 2005;16:2127–2133. doi: 10.1681/ASN.2005010005. [DOI] [PubMed] [Google Scholar]
- 21.Kurella M, Chertow GM, Luan J, et al. Cognitive impairment in chronic kidney disease. J Am Geriatr Soc. 2004;52:1863–1869. doi: 10.1111/j.1532-5415.2004.52508.x. [DOI] [PubMed] [Google Scholar]
- 22.Kurella M, Yaffe K, Shlipak MG, et al. Chronic kidney disease and cognitive impairment in menopausal women. Am J Kidney Dis. 2005;45:66–76. doi: 10.1053/j.ajkd.2004.08.044. [DOI] [PubMed] [Google Scholar]
- 23.Feldman HI, Appel LJ, Chertow GM, et al. The Chronic Renal Insufficiency Cohort (CRIC) Study: Design and Methods. J Am Soc Nephrol. 2003;14:S148–153. doi: 10.1097/01.asn.0000070149.78399.ce. [DOI] [PubMed] [Google Scholar]
- 24.Yaffe K, Ackerson L, Tamura MK, et al. Chronic kidney disease and cognitive function in older adults: Findings from the chronic renal insufficiency cohort cognitive study. J Am Geriatr Soc. 2010;58:338–345. doi: 10.1111/j.1532-5415.2009.02670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Erlandsen EJ, Randers E, Kristensen JH. Evaluation of the Dade Behring N Latex Cystatin C assay on the Dade Behring Nephelometer II System. Scand J Clin Lab Invest. 1999;59:1–8. doi: 10.1080/00365519950185940. [DOI] [PubMed] [Google Scholar]
- 26.Anderson A, Xie D, Tao K, et al. J Am Soc Nephrol. Vol. 21. ASN abstract 287; 2010. Internal standardization of cystatin C measurements in the Chronic Renal Insufficiency Cohort (CRIC) Study; p. 176A. [Google Scholar]
- 27.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 28.Reitan RM. Validity of the Trail Making Test as an indicator of organic brain damage. Percept Mot Skills. 1958;8:271–276. [Google Scholar]
- 29.Welsh KA, Butters N, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) . Part V. A normative study of the neuropsychological battery. Neurology. 1994;44:609–614. doi: 10.1212/wnl.44.4.609. [DOI] [PubMed] [Google Scholar]
- 30.Buschke H, Fuld PA. Evaluating storage, retention, and retrieval in disordered memory and learning. Neurology. 1974;24:1019–1025. doi: 10.1212/wnl.24.11.1019. [DOI] [PubMed] [Google Scholar]
- 31.Coresh J, Astor BC, McQuillan G, et al. Calibration and random variation of the serum creatinine assay as critical elements of using equations to estimate glomerular filtration rate. Am J Kidney Dis. 2002;39:920–929. doi: 10.1053/ajkd.2002.32765. [DOI] [PubMed] [Google Scholar]
- 32.Seliger SL, Siscovick DS, Stehman-Breen CO, et al. Moderate renal impairment and risk of dementia among older adults: The Cardiovascular Health Cognition Study. J Am Soc Nephrol. 2004;15:1904–1911. doi: 10.1097/01.asn.0000131529.60019.fa. [DOI] [PubMed] [Google Scholar]
- 33.Slinin Y, Paudel ML, Ishani A, et al. Kidney function and cognitive performance and decline in older men. J Am Geriatr Soc. 2008;56:2082–2088. doi: 10.1111/j.1532-5415.2008.01936.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tamura MK, Wadley V, Yaffe K, et al. Kidney function and cognitive impairment in US adults: The Reasons for Geographic and Racial Differences in Stroke (REGARDS) study. Am J Kidney Dis. 2008;52:227–234. doi: 10.1053/j.ajkd.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seliger SL, Longstreth WT, Jr, Katz R, et al. Cystatin C and subclinical brain infarction. J Am Soc Nephrol. 2005;16:3721–3727. doi: 10.1681/ASN.2005010006. [DOI] [PubMed] [Google Scholar]
- 36.Wada M, Nagasawa H, Kawanami T, et al. Cystatin C as an index of cerebral small vessel disease:Results of a cross-sectional study in community-based Japanese elderly. Eur J Neurol. 2010;17:383–390. doi: 10.1111/j.1468-1331.2009.02809.x. [DOI] [PubMed] [Google Scholar]
- 37.Rajagopalan P, Refsum H, Hua X, et al. Mapping creatinine- and cystatin C-related white matter brain deficits in the elderly. Neurobiol Aging. 2013;34:1221–1230. doi: 10.1016/j.neurobiolaging.2012.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith EE, Salat DH, Jeng J, et al. Correlations between MRI white matter lesion location and executive function and episodic memory. Neurology. 2011;76:1492–1499. doi: 10.1212/WNL.0b013e318217e7c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ghidoni R, Benussi L, Glionna M, et al. Plasma Cystatin C and Risk of Developing Alzheimer’s Disease in Subjects with Mild Cognitive Impairment. J Alzheimers Dis. 2010;22:985–991. doi: 10.3233/JAD-2010-101095. [DOI] [PubMed] [Google Scholar]
- 40.Kestenbaum B, Rudser KD, de Boer IH, et al. Differences in kidney function and incident hypertension: The multi-ethnic study of atherosclerosis. Ann Intern Med. 2008;148:501–508. doi: 10.7326/0003-4819-148-7-200804010-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muntner P, Winston J, Uribarri J, et al. Overweight, obesity, and elevated serum cystatin C levels in adults in the United States. Am J Med. 2008;121:341–348. doi: 10.1016/j.amjmed.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luc Gr, Bard J-M, Lesueur Cl, et al. Plasma cystatin-C and development of coronary heart disease: The PRIME Study. Atherosclerosis. 2006;185:375–380. doi: 10.1016/j.atherosclerosis.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 43.Sundelof J, Sundstrom J, Hansson O, et al. Cystatin C Levels are Positively Correlated with both A-Beta 42 and Tau Levels in Cerebrospinal Fluid in Persons with Alzheimer’s Disease, Mild Cognitive Impairment, and Healthy Controls. J Alzheimers Dis. 2010;21:471–478. doi: 10.3233/JAD-2010-091594. [DOI] [PubMed] [Google Scholar]
- 44.Sun B, Zhou Y, Halabisky B, et al. Cystatin C-Cathepsin B Axis Regulates Amyloid Beta Levels and Associated Neuronal Deficits in an Animal Model of Alzheimer’s Disease. Neuron. 2008;60:247–257. doi: 10.1016/j.neuron.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tizon B, Ribe EM, Mi W, et al. Cystatin C protects neuronal cells from amyloid-beta-induced toxicity. J Alzheimers Dis. 2010;19:885–894. doi: 10.3233/JAD-2010-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sastre M, Calero M, Pawlik M, et al. Binding of cystatin C to Alzheimer’s amyloid β inhibits in vitro amyloid fibril formation. Neurobiol Aging. 2004;25:1033–1043. doi: 10.1016/j.neurobiolaging.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 47.Shlipak MG, Wassel Fyr CL, Chertow GM, et al. Cystatin C and mortality risk in the elderly: The health, aging, and body composition study. J Am Soc Nephrol. 2006;17:254–261. doi: 10.1681/ASN.2005050545. [DOI] [PubMed] [Google Scholar]
- 48.Peralta CA, Katz R, Sarnak MJ, et al. Cystatin C identifies chronic kidney disease patients at higher risk for complications. J Am Soc Nephrol. 2011;22:147–155. doi: 10.1681/ASN.2010050483. [DOI] [PMC free article] [PubMed] [Google Scholar]


