Abstract
Study Objective
To compare cumulus cell structure and timing of oocyte maturation of in vitro and in vivo matured nonhuman primate oocytes.
Design
In vivo (VVM) and in vitro (IVM) maturation of oocytes.
Setting
Animal cell culture laboratory.
Animal(s)
48 female rhesus macaques.
Interventions
15 females were administered FSH and aspirated oocytes were cultured in vitro for 0, 3, 6, 12 or 24 hours (IVM). 33 females were administered FSH and hCG and oocytes were collected 3, 6, 12, or 28–30 hours following hCG (VVM).
Main Outcome Measures
Nuclear maturation and microtubule scores of oocytes and actin and tubulin transzonal processes of cumulus cells. Embryo development was observed for VVM oocytes.
Results
The rate of nuclear maturation was faster for IVM oocytes compared to VVM oocytes. Actin transzonal processes decreased 0 to 12 hours post hCG for VVM oocytes. Tubulin transzonal processes of IVM and VVM oocytes decreased from 0 to 24 hours and 0 to 3 hours respectively. Embryo development improved as VVM time increased.
Conclusions
Nuclear maturation and remodeling of cumulus oocyte complex structural components associated with IVM do not parallel that of oocyte maturation in vivo, indicating that in vitro culture conditions continue to be sub-optimal.
Keywords: Rhesus monkey, tubulin, actin, zona pellucida, ovary, spindle
INTRODUCTION
In vitro maturation (IVM) of human and non-human primate oocytes has yielded suboptimal results compared to standard in vitro fertilization (IVF) technology (1–3). Interestingly, the term IVM is applied to several different procedures involving oocyte culture. In most infertility clinics, it is associated with the oocytes that are retrieved from ovaries that have received FSH and hCG, but are still not at metaphase II at 36 hours post hCG. At the other end of the spectrum, for some mammalian species, oocytes are obtained from non-stimulated ovaries and IVM is relatively successful (4,5). In humans and nonhuman primates, IVM of oocytes from unstimulated ovaries is not successful. Therefore, to study specific aspects of IVM, a rhesus monkey model has been developed in which ovaries are primed with FSH for several days, but hCG is not given, thus assuring that germinal vesicle (GV) oocytes are obtained for the study of final maturation in vitro (6,7). Because of the many similarities in reproductive biology, the nonhuman primate model for IVM will likely have direct application to improving human oocyte maturation.
The cumulus cells that surround the oocyte, forming the cumulus-oocyte complex (COC), are required for IVM of nonhuman primate oocytes (6,8). The structure and function of cumulus cells during the IVM process has not been well studied, especially in nonhuman primates. However, there is increasing interest in using cumulus cell morphology to predict the developmental competence of oocytes (9). Using cumulus cells to predict IVM oocyte quality in a clinically relevant way will require that cumulus cells be removed so that nuclear maturation status of the oocyte can be observed. Once removed, the cumulus cells cannot be replaced and if the oocyte has not matured, the process of IVM has been irrevocably disrupted. Therefore, the relationship of the COC structure to the timing of nuclear maturation is important for developing optimal protocols for IVM. Additionally, comparison of the COCs from IVM and conventional IVF cycles can determine if maturation rates for these two technologies are similar.
The timing of nuclear maturation of oocytes is also important to the emerging technology of somatic cell nuclear transfer (SCNT). Prior to transfer of the somatic cell nucleus, the nuclear DNA of the oocyte must be removed; this is most easily accomplished at the MII stage when the spindle is well developed and the appearance of the first polar body can be used as an indicator of maturation. The current inefficiency of SCNT reprogramming and embryo development may be influenced by the timing of spindle removal (10). Therefore, understanding the timing of nuclear maturation and spindle development is crucial for the efficient application of this technology.
This study compares the structural components of the cumulus oocyte complex and the nuclear maturation status of oocytes. These endpoints are compared in both in vitro and in vivo matured oocytes at various time points.
MATERIALS AND METHODS
Adult female rhesus macaques (Macaca mulatta) were housed and cared for at the California National Primate Research Center (CNPRC) as described previously (11). Only females with a history of normal menstrual cycles were selected for this study. All procedures for maintenance and handling of the animals were reviewed and approved in advance by the Institutional Animal Use and Care Administrative Advisory Committee at the University of California at Davis.
Females were observed daily for signs of vaginal bleeding and the first day of menses was assigned cycle day 1. Starting on cycle days 1–4 recombinant macaque FSH (r-mFSH; National Hormone and Peptide Program, UCLA) was administered (37.5 IU) twice daily, intramuscularly for 7 days total. Antide (Ares-Serono, Randolph, MA) was administered subcutaneously (0.5 mg/kg body wt.) once daily in the morning on days when r-mFSH was given to prevent an endogenous LH surge. Immature oocytes were collected for in vitro maturation experiments (IVM) on the morning following the last dose of r-mFSH. IVM oocytes were also collected for the 0 hour in vivo maturation time point. For collection of in vivo matured oocytes (VVM), females were given recombinant hCG (1000 IU Ovidrel; Serono, Rockland, MA) on treatment day 8 in addition to the FSH treatment outlined above. Follicular contents were aspirated at 3, 6, 12, or 28–30 hours following hCG. Cumulus oocytes complexes (COCs) were aspirated by ultrasound-guided oocyte collection as previously described (11–13). COCs were retrieved from aspirates as previously described (11). In vivo matured COCs were rinsed in Tyrodes lactate (TL)-Hepes medium (37°C) containing 0.1 mg/ml polyvinyl alcohol (PVA) (14) and 5 ng/ml recombinant human FSH (r-hFSH; Organon Inc., Roseland, NJ) and fixed for assessment of nuclear maturation and structural components. COCs were fixed in a microtubule stabilizing buffer (15) for 1 hour and then placed in blocking solution of phosphate buffered saline consisting of 2% powdered milk, 2% normal goat serum, 2% BSA, 0.1M glycine and 0.01% TritonX-100 overnight at 4°C (15).
In vitro maturation of oocytes
Retrieved immature COCs were randomly placed into treatment drops of 70 μl Connaught Medical Research Laboratories Medium- 1066 (37°C) containing 10 % bovine calf serum (Gemini Bioproducts, West Sacramento, CA) (14) and further modified with hFSH and hLH (0.03 IU/ml Pergonal; Ares-Serono, Randolph, MA) and 1 μg/ml androstenedione (Steraloids, Newport, RI)(M1A) and incubated in a humidified atmosphere of 5% CO2 in air for 0, 3, 6, 12 or 24 hours at 37°C. In vitro maturation culture periods (0, 3, 6, 12 and 24) correspond to the extent of vivo hCG exposure for VVM oocytes. Following incubation, COCs were fixed for assessment of nuclear maturation and structural components as described above.
Staining for nuclear status and cytoplasmic microtubules of oocytes and transzonal processes of cumulus cells
Nuclear status and cytoskeletal elements were assessed by immunostaining oocytes for tubulin, actin and nuclear material (15). COCs were transferred from blocking solution to 30 μl DPBS drops on ten well slides and stained as previously described (11). Nuclear maturation was assessed using a Delta Vision microscope (Applied Precision, Issaquah, WA) with an Olympus 60X (1.20NA) water immersion objective. Each oocyte was given a nuclear maturation score based on the arrangement of tubulin and chromatin. Oocytes with a germinal vesicle (GV) pocket were categorized as GV. In the absence of a GV pocket, the condensed chromatin and accompanying tubulin spindle structure were assessed to determine the post GV status of the oocyte. Early spindle formation was noted in eggs that displayed very tightly condensed chromatin and few to many unorganized microtubules. Metaphase I (MI) oocytes displayed the classical single spindle structure with the chromatin aligned at the central plane of the spindles. The transition from MI to Metaphase II (MII) is characterized by the separation of the chromatin bundles and intermediate spindle structures. MII oocytes were noted to have extruded a single polar body, which has no discernable spindle or chromatin organization, and a well defined spindle structure a distance away from the polar body with the chromatin aligned at the center plane of the spindle structure.
Cytoskeletal elements were examined on an inverted Zeiss Axiovert microscope (Carl Zeiss, Jena, Germany) fitted with Argon and Helium-Neon lasers for confocal microscopy (Zeiss Model LSM 510). Cumulus cell transzonal processes and oocyte cytoplasmic microtubules were imaged through a 63X (1.4 NA) oil immersion lens. Fluorescence of Alexa 488 was observed by use of 488 nm excitation and 505–530 nm bandpass filters, and that of Alexa 555 was observed by use of 543 nm excitation and 560 nm long pass filters. Z-sections of 30 – 40 0.5 μm were collected for each oocyte and a z-projection was created. The images were examined with Adobe Photoshop (Adobe Systems Inc., San Jose, CA). Cytoplasmic microtubules were evaluated for completeness of the network (Figure 1). Oocytes demonstrating a complete or nearly complete network were assigned a score of 4; 25–50% degradation =3; 75% degradation = 2 and complete or nearly complete degradation = 1. Transzonal processes were manually counted in the separated channels of each z-projection. Green channel processes were counted as f-actin and red as tubulin.
Figure 1.
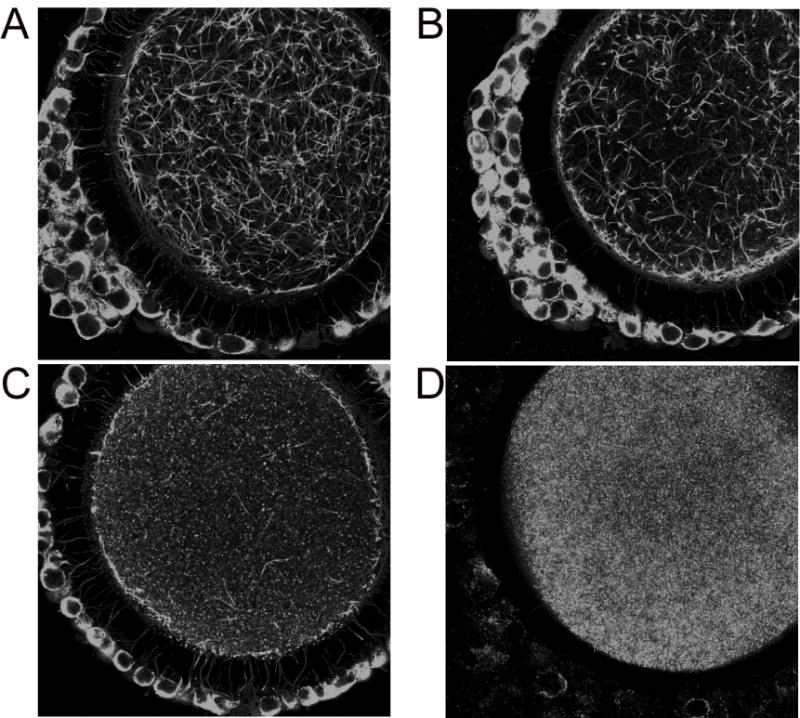
Oocyte cytoplasmic microtubules were evaluated for completeness of the network. Oocytes demonstrating a complete or nearly complete network were assigned a score of 4 (A), 25–50% degradation =3 (B), 75% degradation = 2 (C) and complete or nearly complete degradation = 1 (D).
In vivo maturation and embryo development
COCs collected for time points 0, 3, 6 and 12 hours post hCG injection were transferred into 70 μl drops of M1A medium (as described above) under oil (37°C) and incubated at 37°C in a humidified atmosphere of 5% CO2 in air. After experiments using M1A medium were complete we investigated whether COCs collected 12 hours post hCG injection would benefit from culture in chemically-defined, protein-free hamster embryo culture medium 9 (HECM-9) (16) as used for standard IVF. COCs from different females collected 12 hours post hCG injection were placed in HECM-9 drops under oil. COCs collected 28–30 hours post hCG were placed in drops of HECM-9 under oil (37°C) and incubated at 37°C in a humidified atmosphere of 5% CO2 in air. Regardless of when COCs were retrieved they were inseminated between 28–33 hours post hCG injection which is standard for IVF. All COCs were inseminated regardless of nuclear status. Only COCs from two females, aspirated 12 hours post hCG (HECM-9 medium), were inseminated 21–22 hours post hCG injection due to animal and IVF scheduling restraints. Immature COCs collected for time point 0 (no hCG administered) were inseminated approximately 32 hours after retrieval. It should be noted that insemination time refers to the time in which sperm and COCs begin colocalization. Fertilization may take place any time during their co-culture together. Briefly, COCs were placed in 10 mg/ml hyaluronidase (MP Biomedicals, Solon, OH) in TL-PVA that was pre-equilibrated to 37°C. Some of the cumulus cells were removed with gentle pipetting in the hyaluronidase solution. Oocytes were rinsed and transferred into TL-PVA medium (37°C) under oil and inseminated according to standard procedure for IVF of rhesus macaque oocytes (17). Semen was collected from male macaques that had been trained for this procedure as previously reported (18). Sperm were washed from seminal plasma and resuspended in TL-BSA medium (14). The next morning, oocytes were transferred into 70 μl drops of HECM-9 medium under oil (37°C) and incubated at 37°C in a humidified atmosphere of 5% CO2, 10% O2 and 85% N2 for 48 hours. Embryos were transferred into 70 μl drops of HECM-9 medium with 5% bovine calf serum (Gemini Bioproducts, West Sacramento, CA) under mineral oil and incubated as described above. Chemically defined embryo culture media for all stages of embryo development would be ideal, however at this time the addition of serum at the 8-cell stage is necessary to support the development of viable blastocysts (13,16,19,20). Embryos were transferred to fresh medium every other day until no further development was observed. When development ceased, embryos were classified as: < 8 cell, 9–16 cell, 17–32 cell, morula or blastocyst stage embryos.
Statistical Analysis
Nuclear maturation and microtubule scores were tested for a linear association with time using an extension of the Mantel-Haenszel Chi-Square statistic. When cell counts were too low for the asymptotic Mantel-Haenszel statistic to be valid, exact p-values were found using a Monte Carlo estimate of the Exact test. P < 0.05 suggests that the data have a linear association. Statistics were run with SAS version 9.1 (SAS Institute Inc., Cary, NC).
The time point data for actin and tubulin processes were each analyzed using a One-way Anova and the Tukey-Kramer post test when p < 0.05. Actin and tubulin processes are expressed as the mean ± SEM and N represents the number of oocytes used for each experiment. Actin and tubulin processes were compared between in vivo (time: 28–30 hrs) and in vitro matured oocytes (time: 24 hr) using an unpaired t-test. Statistics were run with Prism software (GraphPad Software, Inc., San Diego, CA).
Developmental data was first analyzed as percentage of embryos progressing to or beyond each developmental stage with a One-Way Anova blocked for female. No significance was found likely due to the low sample size after blocking for female. Embryos were also categorized as individuals into developmental stages and an extension of the Mantel Haenszel Chi-Square statistic was ran to test for a linear correlation with in vivo maturation time. An extension of the Mantel Haenszel Chi-Square statistic was also ran to test for a location shift in development between HECM-9 versus IVM maturation medium for COCs collected 12 hours post hCG injection.
RESULTS
Nuclear Maturation
A linear association was found between nuclear status of IVM oocytes and time in culture (Monte Carlo Estimate of Exact test, p < 0.0001). As time advanced from 0 to 24 hours the percentage of IVM oocytes at each time point progressed towards a more mature nuclear status (Table I). The first time point in which the nuclear status of IVM oocytes was observed to reach beyond germinal vesicle breakdown (GVBD) was at 12 hours. VVM oocytes did not mature beyond GVBD until 28–32 hours post hCG injection. As time advanced the percentage of VVM oocytes at each time point progressed towards a more mature nuclear status (Monte Carlo Estimate of Exact test, p < 0.001) (Table I). The nuclear status of IVM oocytes (time:24 hr) was more advanced than VVM (time: 28–30 hrs) oocytes (test for location shift, p < 0.0001).
Table I.
Contingency table of oocytes cross-categorized according to IVM or VVM time and nuclear status. Percentage of oocytes within each row are indicated.
| Time (hours) | # of females | Total oocytes | Nuclear Status | |||||
|---|---|---|---|---|---|---|---|---|
| GV | GVBD | MI | MI–MII | MII | ||||
| IVM | 0a | 3 | 23 | 100 | 0 | 0 | 0 | 0 |
| 3a | 3 | 20 | 95 | 5 | 0 | 0 | 0 | |
| 6a | 3 | 23 | 100 | 0 | 0 | 0 | 0 | |
| 12a | 3 | 23 | 43.5 | 52.2 | 4.4 | 0 | 0 | |
| 24a,c | 12 | 47 | 2.1 | 0 | 40.4 | 21.3 | 36.2 | |
|
| ||||||||
| VVM | 0b | 3 | 23 | 100 | 0 | 0 | 0 | 0 |
| 3b | 4 | 15 | 93.3 | 6.7 | 0 | 0 | 0 | |
| 6b | 4 | 13 | 92.3 | 7.7 | 0 | 0 | 0 | |
| 12b | 4 | 11 | 100 | 0 | 0 | 0 | 0 | |
| 28–30b,c | 5 | 15 | 60 | 26.7 | 6.7 | 6.7 | 0 | |
Indicates IVM data lines analyzed as a complete set for a linear association, p < 0.0001
Indicates VVM data lines analyzed as a complete set for a linear association, p < 0.001
Indicates a location shift between data lines of last maturation time points of IVM and VVM only, p < 0.0001
Microtubule scores
Oocytes were given microtubule scores based on completeness of the cytoplasmic microtubule network (Figure 1). A linear correlation was found between microtubule scores of IVM oocytes with time in culture (p < 0.0001). Increased time in culture was associated with decreased microtubule scores (Table II). Microtubule scores of VVM oocytes were associated with collection times post hCG administration (p < 0.01). The linear association found shows microtubule scores to decrease as time after hCG administration increases (Table II). When microtubule scores were compared between IVM and VVM at the 6 hr time point IVM oocytes were associated with higher microtubule scores than VVM oocytes (p < 0.0001). When microtubule scores were compared between IVM (time: 24 hr) and VVM oocytes (time: 28–30 hrs) no significant difference was found and the majority of oocytes in each group had a microtubule score of 0.
Table II.
Contingency table of oocytes cross-categorized according to IVM or VVM time and microtubule scores. Percentage of oocytes within each row are indicated.
| Time (hours) | # of females | Total oocytes | Microtubule Scores | ||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||||
| IVM | 0a | 3 | 23 | 4.4 | 13 | 73.9 | 8.7 |
| 3a | 3 | 21 | 9.5 | 4.7 | 76.2 | 9.5 | |
| 6a,c | 3 | 23 | 4.4 | 0 | 65.2 | 30.4 | |
| 12a | 3 | 23 | 26.1 | 30.4 | 34.8 | 8.7 | |
| 24a | 12 | 49 | 83.7 | 14.3 | 0 | 2 | |
|
| |||||||
| VVM | 0b | 3 | 23 | 4.4 | 13 | 73.9 | 8.7 |
| 3b | 4 | 19 | 52.6 | 21 | 26.3 | 0 | |
| 6b,c | 4 | 15 | 66.7 | 20 | 0 | 13.3 | |
| 12b | 4 | 9 | 88.9 | 0 | 11.1 | 0 | |
| 28–30b | 2 | 8 | 52.5 | 112.5 | 25 | 0 | |
Indicates IVM data lines analyzed as a complete set for a linear association, p < 0.0001
Indicates VVM data lines analyzed as a complete set for a linear association, p < 0.01
Indicates a location shift between data lines of IVM and VVM 6 hour time points only, p < 0.0001
Actin and Tubulin Processes
The number of cumulus cell actin transzonal processes of IVM COCs did not change with increased maturation time (Figure 2a). Actin transzonal processes from VVM COCs decreased from 0 to 12 hours post hCG administration (p < 0.05) (Figure 2b). No difference was found between the number of actin transzonal processes of IVM (time: 24 hr) and VVM oocytes (time: 28–30 hrs).
Figure 2.
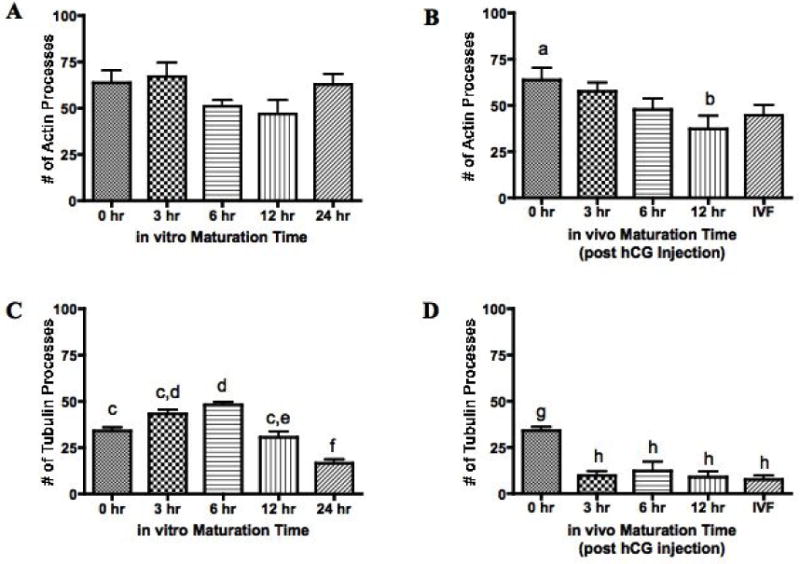
Cumulus cell actin and tubulin transzonal processes were counted and compared to in vitro or in vivo maturation time. Actin and tubulin processes are expressed as the mean ± SEM and n represents the number of oocytes used for each experiment (n=137 (A), n=83 (B), n=137 (C) and n=74 (D)). Values with different superscripts within each figure differ significantly; P < 0.05 (B), P < 0.01 (C), P < 0.001 (D).
The number of cumulus cell tubulin transzonal processes changed with in vitro maturation time (Figure 2c). An increase in tubulin processes was seen from 0 to 6 hours followed by a decrease in processes from 6 to 24 hours (p < 0.01). Overall there was a decrease in tubulin processes from 0 to 24 hours IVM with the lowest number of tubulin processes present at the 24 hour time point (p < 0.01). Tubulin processes decreased drastically from 0 to 3 hours for VVM COCs and remained low for the remainder of time points observed (p < 0.001) (Figure 2d). The number of tubulin transzonal processes was not different between IVM (time: 24 hr) and VVM COCs (time: 28–30 hrs).
In vivo maturation and development
A linear association was found between in vivo maturation time and developmental status using an extension of the Mantel Haenszel Chi-Square statistic (Table III). The development of embryos advanced as in vivo maturation time increased (p < 0.01). There was no significant difference in the percentage of embryos that reached the blastocyst stage between IVM (0 hrs post hCG) and VVM oocytes. However, embryos from all VVM time points reached the blastocyst stage one day before the IVM group embryos (data not shown). A location shift was found in development when HECM-9 and M1A groups were compared for COCs aspirated 12 hours post hCG injection, indicating a difference in developmental progression (p < 0.001) (Table III). The development of embryos from the M1A group was more advanced than the HECM-9 group.
Table III.
Contingency table of embyros cross-categorized according to in vivo maturation time and developmental status. Percentage of embryos within each row are indicated.
| Time (hours) | Culture Medium | # of females | Total embryos | Developmental Status | ||||
|---|---|---|---|---|---|---|---|---|
| < 8-cell | 9–16 cell | 17–32 cell | Morula | Blastocyst | ||||
| 0a | M1A | 5 | 47 | 21.3 | 25.5 | 4.3 | 14.9 | 34.0 |
| 3a | M1A | 4 | 45 | 31.1 | 26.7 | 8.9 | 6.7 | 26.7 |
| 6a | M1A | 5 | 47 | 23.4 | 12.8 | 25.5 | 19.2 | 19.2 |
| 12a,b | M1A | 2 | 32 | 18.8 | 15.6 | 28.1 | 0 | 37.5 |
| 12b | HECM-9 | 4 | 74 | 81.1 | 4.1 | 0 | 0 | 14.9 |
| 28–30a | HECM-9 | 3 | 46 | 15.2 | 10.9 | 8.7 | 10.9 | 54.4 |
Indicates data lines analyzed as a complete set for a linear association, p < 0.01
Indicates a location shift between data lines of 12 hour times points only, p < 0.001
DISCUSSION
This is the first comparison of IVM and VVM in the nonhuman primate that not only compares the timing of nuclear maturation but also the remodeling of cumulus cell transzonal projections.
The time required for VVM rhesus oocytes to reach GV breakdown and enter metaphase I is similar to that reported for humans. Human oocytes that were obtained from healthy women undergoing ovarian hyperstimulation at various times after hCG, underwent GV breakdown between 15 and 20 h post hCG and had entered metaphase I by 20 to 26 h post hCG (21). A separate study in the rhesus macaque found the majority of oocytes collected 12 hours post hCG injection to be at the GV stage while 36 hours post hCG injection yielded a majority of MII oocytes (22). The nuclear maturation of IVM oocytes by 24 hours in culture was presumed to be faster than VVM in a previous study (7), but this finding was not compared to VVM oocytes recovered at the earlier time after hCG. The timing to nuclear maturation during IVM found in this study was similar to that found by Schramm and Bavister [1999]. In contrast, a recent study in the cynomolgus monkey, oocytes retrieved prior to hCG did not mature to metaphase II even after 40 hours of culture (23). The culture media used in that study was different than the basic medium used in the current study, as further described below, and probably explains the dramatic differences in the results of the two studies.
A high incidence of spindle disruption, especially for IVM COCs has been reported previously in the cynomolgus monkey (23). In the current study of rhesus IVM oocytes, the incidence of spindle disruption was very low (less than 5%) in the MII oocytes. The results of Yin et al. [2006] may be due to exposure of COCs to hypoxanthine contained in the commercial Medium 199 that was used for COC culture or to room temperature during oocyte collection. In contrast to some other species such as the mouse, rhesus monkey oocytes are extremely sensitive to chilling injury (24) and must be maintained at about 37°C to assure viability (25). It is possible that since the cynomolgus and rhesus macaque are reproductively similar that these factors also influenced the outcome of the experiments of Yin et al. [2006]. Hypoxanthine has been shown to maintain meiotic arrest of rhesus monkey oocytes (26) and perhaps could be a problematic factor in the Yin et al. study where IVM was desired. A recent study of human IVM oocytes found abnormalities of the spindle and chromosome structure similar to that found by Yin et al., [2006] and the human oocytes were also cultured in Medium 199 (aka TCM-199) during in vitro maturation (27). Because oocytes were donated from women with polycystic ovarian syndrome, the abnormalities could also be due to poor oocyte quality, however, the similarities with the study of Yin et al. would also suggest that a medium that did not contain hypoxanthine might be indicated for IVM of primate oocytes. The lack of spindle disruption in our results supports this hypothesis.
The association of cumulus cell tubulin transzonal processes remodeling and eventual decline in the actin transzonal processes with the meiotic maturation of oocytes was first reported in the bovine model (15). In rhesus COCs there was a significant increase and then decrease in tubulin processes in the IVM group, evidence of the remodeling that has been reported in the bovine model. In contrast, in the VVM group there was an abrupt decline in tubulin process number at 3 hr after hCG that continued in all time points observed. The number of actin processes in the VVM group decreased with time, becoming significant at the 12 hr time point, however, there was no significant decrease in the IVM group. The bovine study reported a decline in actin processes and an increase in tubulin processes as meiotic maturation progressed which seems to be most similar to the rhesus IVM COCs. It is interesting to note that the bovine COCs were obtained from non-stimulated ovaries and all oocytes in that study were matured in vitro. In the rhesus VVM COCs, the number of actin and tubulin processes consistently decreased with no evidence of processes remodeling that was observed in the IVM group and the bovine COCs. The lack of remodeling in the VVM group may be further evidence of the limitation of in vitro culture conditions.
While our results are consistent with the bovine model in which depolymerization of the cytoplasmic microtubule network was found to be associated with in vitro oocyte maturation, we further show that there are fundamental differences between IVM and VVM oocytes (15). At the 6 hr time point IVM oocytes were associated with higher microtubule scores and therefore more complete networks than VVM oocytes. Neither IVM nor VVM oocytes had progressed beyond the GVBD stage at 6 hrs indicating that differences in remodeling were likely a result of IVM or VVM conditions.
The driving force behind remodeling of cumulus cell transzonal processes and the cytoplasmic microtubule network in IVM oocytes is unknown. IVM oocytes are matured in medium that contains serum, exposing the oocyte to a wide array of growth factors and nutrients. These results support the view that to better understand the mechanisms involved in the IVM process, defined, serum-free media must be developed (28). Although there has been one report of serum-free IVM culture media for rhesus oocytes, the success of that media has not been confirmed by other laboratories (29). The remodeling seen in the current study leads to speculation that serum added to maturation medium either provides a catalyst that initiates microtubule remodeling or lacks sufficient components for IVM to parallel VVM. The amount of cellular resources spent in the remodeling process is unknown, however, a large expenditure of resources could partially explain why IVM oocytes are compromised compared to VVM oocytes.
While there was no significant difference in the percentage of embryos that reached the blastocyst stage of development between IVM (0 hrs post hCG) and VVM, embryos from all VVM time points reached the blastocyst stage one day before the IVM group embryos. This lag in development of rhesus IVM embryos is consistent with previous reports and is attributed to sub-optimal culture conditions during IVM and before in vitro fertilization (3).
This comparison of IVM and VVM oocytes reveals that the cumulus cell remodeling that is associated with IVM in rhesus oocytes may be similar to that seen for IVM of oocytes from non-stimulated ovaries in some species, but does not parallel the process of oocyte maturation in vivo. The significant differences in nuclear maturation rates and structural components of the COC indicate that in vitro culture conditions continue to be suboptimal.
Acknowledgments
This research was supported by RR13439 (CAV), RR00169 (CNPRC), NIH HD043358 (CLC)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cha KY, Chian RC. Maturation in vitro of immature human oocytes for clinical use. Hum Reprod Update. 1998;4(2):103–20. doi: 10.1093/humupd/4.2.103. [DOI] [PubMed] [Google Scholar]
- 2.Picton HM. Oocyte maturation in vitro. Curr Opin Obstet Gynecol. 2002;14(3):295–302. doi: 10.1097/00001703-200206000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Schramm RD, Paprocki AM, VandeVoort CA. Causes of developmental failure of in-vitro matured rhesus monkey oocytes: impairments in embryonic genome activation. Hum Reprod. 2003;18(4):826–33. doi: 10.1093/humrep/deg144. [DOI] [PubMed] [Google Scholar]
- 4.Krisher RL. The effect of oocyte quality on development. J Anim Sci. 2004;82(E-Suppl):E14–23. doi: 10.2527/2004.8213_supplE14x. [DOI] [PubMed] [Google Scholar]
- 5.Lonergan P. State-of-the-art embryo technologies in cattle. Soc Reprod Fertil Suppl. 2007;64:315–25. doi: 10.5661/rdr-vi-315. [DOI] [PubMed] [Google Scholar]
- 6.Schramm RD, Bavister BD. Effects of granulosa cells and gonadotrophins on meiotic and developmental competence of oocytes in vitro in non-stimulated rhesus monkeys. Hum Reprod. 1995;10:887–95. doi: 10.1093/oxfordjournals.humrep.a136056. [DOI] [PubMed] [Google Scholar]
- 7.Schramm RD, Bavister BD. A macaque model for studying mechanisms controlling oocyte development and maturation in human and non-human primates. Hum Reprod. 1999;14(10):2544–55. doi: 10.1093/humrep/14.10.2544. [DOI] [PubMed] [Google Scholar]
- 8.Schramm RD, Bavister BD. Granulosa cells from follicle stimulating hormone-primed monkeys enhance the development competence of in-vitro-matured oocytes from non-stimulated rhesus monkeys. Hum Reprod. 1996;11:1698–702. doi: 10.1093/oxfordjournals.humrep.a019472. [DOI] [PubMed] [Google Scholar]
- 9.Sato C, Shimada M, Mori T, Kumasako Y, Otsu E, Watanabe H, et al. Assessment of human oocyte developmental competence by cumulus cell morphology and circulating hormone profile. Reprod Biomed Online. 2007;14:49–56. doi: 10.1016/s1472-6483(10)60763-8. [DOI] [PubMed] [Google Scholar]
- 10.Simerly CR, Navara CS. Nuclear transfer in the rhesus monkey: opportunities and challenges. Cloning Stem Cells. 2003;5:319–31. doi: 10.1089/153623003772032826. [DOI] [PubMed] [Google Scholar]
- 11.Nyholt de Prada JK, VandeVoort CA. Growth hormone and in vitro maturation of rhesus macaque oocytes and subsequent embryo development. J Assist Reprod Genet. 2008 doi: 10.1007/s10815-008-9208-3. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vandevoort CA, Baughman WL, Stouffer RL. Comparison of different regimens of human gonadotropins for superovulation of rhesus monkeys: ovulatory response and subsequent luteal function. J In Vitro Fert Embryo Transf. 1989;6:85–91. doi: 10.1007/BF01130732. [DOI] [PubMed] [Google Scholar]
- 13.VandeVoort CA, Leibo SP, Tarantal AF. Improved collection and developmental competence of immature macaque oocytes. Theriogenology. 2003;59:699–707. doi: 10.1016/s0093-691x(02)01129-9. [DOI] [PubMed] [Google Scholar]
- 14.Boatman DE. In vitro growth of non-human primate pre- and peri-implantation embryos. In: Bavister BD, editor. The Mammalian Preimplantation Embryo: regulation of growth and differentiation in vitro. New York: Plenum Press; 1987. pp. 273–308. [Google Scholar]
- 15.Allworth AE, Albertini DF. Meiotic maturation in cultured bovine oocytes is accompanied by remodeling of the cumulus cell cytoskeleton. Dev Biol. 1993;158:101–12. doi: 10.1006/dbio.1993.1171. [DOI] [PubMed] [Google Scholar]
- 16.McKiernan SH, Bavister BD. Culture of one-cell hamster embryos with water soluble vitamins: pantothenate stimulates blastocyst production. Hum Reprod. 2000;15:157–64. doi: 10.1093/humrep/15.1.157. [DOI] [PubMed] [Google Scholar]
- 17.Schramm RD, Bavister BD. Follicle-stimulating hormone priming of rhesus monkeys enhances meiotic and developmental competence of oocytes matured in vitro. Biol Reprod. 1994;51:904–12. doi: 10.1095/biolreprod51.5.904. [DOI] [PubMed] [Google Scholar]
- 18.Sarason RL, VandeVoort CA, Mader DR, Overstreet JW. The use of nonmetal electrodes in electroejaculation of restrained but unanesthetized macaques. J Med Primatol. 1991;20:122–5. [PubMed] [Google Scholar]
- 19.Gardner DK, Lane M. Culture and selection of viable blastocysts: a feasible proposition for human IVF? Hum Reprod Update. 1997;3(4):367–82. doi: 10.1093/humupd/3.4.367. [DOI] [PubMed] [Google Scholar]
- 20.Pinyopummintr T, Bavister BD. In vitro-matured/in vitro-fertilized bovine oocytes can develop into morulae/blastocysts in chemically defined, protein-fee culture media. Biol Reprod. 1991;45:736–42. doi: 10.1095/biolreprod45.5.736. [DOI] [PubMed] [Google Scholar]
- 21.Bomsel-Helmreich O, Huyen LV, Durand-Gasselin I, Salat-Baroux J, Antoine JM. Timing of nuclear maturation and cumulus dissociation in human oocytes stimulated with clomiphene citrate, human menopausal gonadotropin, and human chorionic gonadotropin. Fert Steril. 1987;48(4):586–95. doi: 10.1016/s0015-0282(16)59469-2. [DOI] [PubMed] [Google Scholar]
- 22.Borman SM, Chaffin CL, Schwinof KM, Stouffer RL, Zelinski-Wooten MB. Progesterone promotes oocyte maturation, but not ovulation, in nonhuman primate follicles without a gonadotropin surge. Biol Reprod. 2004;71(1):366–73. doi: 10.1095/biolreprod.103.023390. [DOI] [PubMed] [Google Scholar]
- 23.Yin H, Duffy DM, Gosden RG. Comparative maturation of cynomolgus monkey oocytes in vivo and in vitro. Reprod Biol Endocrinol. 2006;4:14. doi: 10.1186/1477-7827-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Songsasen N, Yu IJ, Ratterree MS, VandeVoort CA, Leibo SP. Effect of chilling on the organization of tubulin and chromosomes in rhesus monkey oocytes. Fert and Steril. 2002;77:818–25. doi: 10.1016/s0015-0282(01)03240-x. [DOI] [PubMed] [Google Scholar]
- 25.Wolf DP, Vandevoort CA, Meyer-Haas GR, Zelinski-Wooten MB, Hess DL, Baughman WL, et al. In vitro fertilization and embryo transfer in the rhesus monkey. Biol Reprod. 1989;41:335–46. doi: 10.1095/biolreprod41.2.335. [DOI] [PubMed] [Google Scholar]
- 26.Warikoo PK, Bavister BD. Hypoxanthine and cyclic adenosine 5′-monophosphate maintain meiotic arrest of rhesus monkey oocytes in vitro. Fertil Steril. 1989;51:886–9. [PubMed] [Google Scholar]
- 27.Liu L, Oldenbourg R, Trimarchi JR, Keefe DL. A reliable, noninvasive technique for spindle imaging and enucleation of mammalian oocytes. Nat Biotechnol. 2000;18:223–5. doi: 10.1038/72692. [DOI] [PubMed] [Google Scholar]
- 28.Bavister BD. Culture of preimplantation embryos: facts and artifacts. Hum Reprod Update. 1995;1(2):91–148. doi: 10.1093/humupd/1.2.91. [DOI] [PubMed] [Google Scholar]
- 29.Zheng P, Wang H, Bavister BD, Ji W. Maturation of rhesus monkey oocytes in chemically defined culture media and their functional assessment by IVF and embryo development. Hum Reprod. 2001;16(2):300–5. doi: 10.1093/humrep/16.2.300. [DOI] [PubMed] [Google Scholar]


