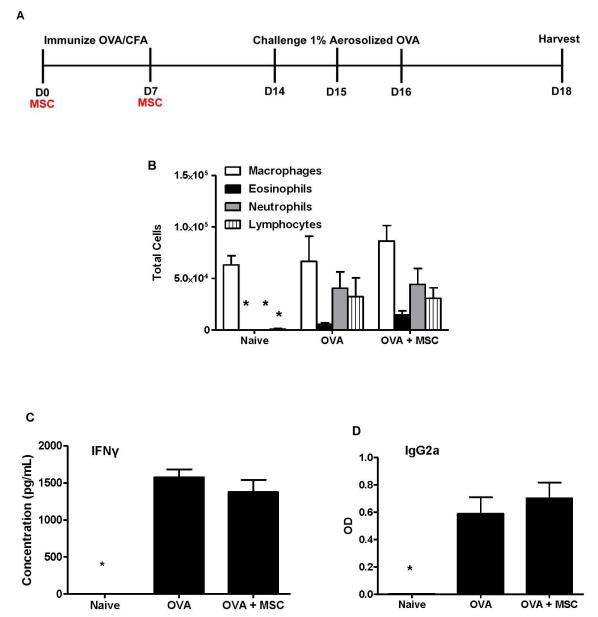
Abstract
Bone marrow-derived mesenchymal stromal cells (BMSCs) mitigate inflammation in mouse models of acute lung injury. However, specific mechanisms of BMSC actions on CD4 T lymphocyte-mediated inflammation in vivo remain poorly understood. Limited data suggests promotion of Th2 phenotype in models of Th1-mediated diseases. However whether this might alleviate or worsen Th2-mediated diseases such as allergic asthma is unknown. To ascertain the effects of systemic administration of BMSCs in a mouse model of Th2-mediated allergic airways inflammation, ovalbumin-induced allergic airways inflammation was induced in wild type C57BL/6 and BALB/c mice as well as in IFNγ receptor null mice. Effects of systemic administration during antigen sensitization of either syngeneic or allogeneic BMSC on airways hyper-reactivity, lung inflammation, antigen-specific CD4 T lymphocytes, and serum immunoglobulins were assessed. Both syngeneic and allogeneic BMSCs inhibited airways hyper-reactivity and lung inflammation through a mechanism partly dependent on IFNγ. However, contrary to existing data, BMSCs did not affect antigen-specific CD4 T lymphocyte proliferation but rather promoted Th1 phenotype in vivo as assessed by both ova-specific CD4 T lymphocyte cytokine production and ova-specific circulating immunoglobulins. BMSCs treated to prevent release of soluble mediators and a control cell population of primary dermal skin fibroblasts only partly mimicked the BMSC effects and in some cases worsened inflammation. In conclusion, BMSCs inhibit Th2-mediated allergic airways inflammation by influencing antigen-specific CD4 T lymphocyte differentiation. Promotion of a Th1 phenotype in antigen-specific CD4 T lymphocytes by BMSCs is sufficient to inhibit Th2-mediated allergic airways inflammation through an IFNγ-dependent process.
Keywords: mesenchymal stromal cell, allergic airways disease, CD4 lymphocyte
Introduction
Mesenchymal stromal cells (MSCs) are adult, multipotent progenitor cells that were first identified in the bone marrow and have the ability to differentiate into bone, fat and cartilage (1). MSCs have subsequently been isolated from virtually all post-natal tissues as well as umbilical cord blood, placenta, and amniotic fluid and can be induced in vitro to differentiate into a variety of cell types (2). While MSCs isolated from different sources share key identifying characteristics, differences in gene expression and their secretome have been observed. MSCs derived from bone marrow (BMSCs) have been best characterized and have been found to have significant immunomodulatory and non-immunogenic properties, allowing administration of allogeneic BMSCs without eliciting an immunogenic response within the host (3-5). BMSCs inhibit the proliferation and function of a broad range of immune cells in vitro, including T cells, B cells, natural killer (NK) cells and dendritic cells (DCs) (6-20). Notably, BMSCs can suppress CD4 T lymphocyte responses in vitro by inhibiting T cell proliferation induced by mitogens or specific antigens (21-32). These effects likely occur through a paracrine effect by the release of soluble mediators by the BMSCs although cell-cell contact may also be involved (23, 24, 27-29, 31-34). However, whether the mechanisms by which BMSCs suppress immune cells in vitro are similar to those in vivo remains unclear.
Published reports evaluating BMSC effects on CD4 T lymphocyte differentiation in in vitro model systems generally demonstrate that MSCs promote a Th2 phenotype in CD4 lymphocytes. In vivo, systemic administration of human BMSCs decreased numbers of Th1 and Th17 CD4 T lymphocytes, with corresponding decreases in IFNγ and IL-17 production, and increased numbers of IL-4 producing Th2 lymphocytes when administered in a mouse model of experimental autoimmune encephalitis (35). These findings suggest that administration of BMSCs would be either ineffective or could potentially exacerbate the immune response in diseases characterized by Th2-mediated inflammation, such as allergic asthma. Since little is known about the in vivo effects of BMSC administration in Th2 models of inflammation, it was of particular interest to investigate the effects of BMSCs on the generation of antigen-specific CD4 T cells in allergic airways inflammation, a mouse model of allergic asthma. Sensitization to ovalbumin with the Th2-promoting adjuvant aluminum hydroxide, followed by challenge with aerosolized ovalbumin is a well established model of inducing Th2-mediated eosinophilic allergic airways inflammation in mice (36). Initial clonal expansion and differentiation of antigen-specific CD4 T cells occurs during the sensitization phase of the ova model. Given this, we investigated whether administration of either syngeneic or allogeneic bone marrow derived BMSCs during antigen sensitization would effect the generation of allergic airways inflammation, including clonal proliferation and differentiation of antigen-specific CD4 Th2 lymphocytes.
Materials and Methods
Mice
Female C57BL/6, BALB/c and IFNγRKO (IFNγ−/−) mice (6-8wk) from the Jackson Laboratory were maintained in accordance with institutional and American Association for Accreditation of Laboratory Animal Care standards, with studies subject to Institutional Animal Care and Use Committee review at the University of Vermont (Burlington, VT).
Bone Marrow Mesenchymal stem cells (BMSCs)
MSCs derived from bone marrow of adult male C57BL/6 mice were obtained from the Tulane University Center for Gene Therapy (Sekiya et al., 2002). BMSCs were cultured in Iscoves Modified Dulbecco Medium with 2mM L-glutamine, 100 U/ml penicillin, 100μg/ml streptomycin, 10% fetal bovine serum and 10% horse serum (HS) (GIBCO, Invitrogen). Cells were used at passage 6 or lower and maintained in culture at confluency no greater than 70%. Purity was determined by expression of Sca-1, CD106, CD29, absent expression of CD11b, CD11c, CD34, CD45, and the ability to differentiate into chondrocytes and adipocytes in vitro.
OVA Sensitization
Mice were immunized by intraperitoneal (IP) injection with 20μg of ovalbumin (ova, grade V; Sigma Aldrich) or PBS with 2.25 mg Aluminum hydroxide (Alum, ImjectAlum; Pierce), or Complete (day 0) or Incomplete (day 7) Freud’s adjuvant on days 0 and 7. Immediately prior to each immunization, experimental mice received tail vein injection of 2 × 106 MSCs, while control mice received the same number of MSCs treated with the cross-linker 1-ethyl-3-[3-dimethylaminopropyl] carbodiimide hydrochloride (EDCI)(37), primary dermal C57BL/6 fibroblasts, viable MSCs with PBS/alum-immunization, or PBS control. Mice were challenged with aerosolized 1% ova in saline for 30 minutes on days 14, 15, and 16.
Respiratory mechanics
Pulmonary function was analyzed on day 18 using the forced oscillation technique (flexiVent; SCIREQ Scientific Respiratory Equipment) as previously described (38). The peak responses for airway resistance (RN), tissue resistance (G), or tissue elastance (H) were determined in response to sequential inhalation of nebulized saline, 3.125 mg/mL and 12.5 mg/mL of methacholine.
Bronchoalveolar lavage, total and differential cell counts, and cytokine assessment
Bronchoalveolar lavage (BAL) fluid was collected by instilling and recovering 1 mL of saline into the airways. Cells were counted using an Advia cell counter (Bayer), cytospun onto glass slides and stained with Hema-3 (Biochemical Sciences). Two hundred cells per slide were scored by characteristic morphology and staining. BAL cytokine levels were measured using a mouse 23-plex kit on the Bio-Plex suspension array system (Bio-rad).
Lung histology and inflammation scoring
Lungs were inflated, gravity fixed (20 cm) with 4% paraformaldehyde, paraffin embedded, cut into 5-μm sections, and stained with hematoxylin and eosin. Inflammation was scored on a scale from 0-3, in blinded fashion by a collaborating veterinary pathologist (Dr. Ziats) based on the presence and intensity of peri-bronchial cell infiltrates compared to known positive and negative controls using an established scoring system. 4 airways per animal were analyzed and 6-8 animals were analyzed per condition.
Serum collection and immunoglobulin analyses
Serum samples were diluted 1:50 for IgG1, 1:20 for IgG2a and 1:15 for IgE and analyzed as previously described (39).
CD4 T lymphocyte isolation, proliferation, and cytokine production
Splenic CD4 T lymphocytes were positively selected (MACS CD4 T lymphocyte separation system, Miltenyi); purity was above 98% by flow cytometry with antibodies against CD4 and TCR. The irradiated (2000 Rads) CD4-negative fraction of splenocytes from naïve C57BL/6 mice provided antigen presenting cells (APCs). Antigen specific CD4 T lymphocyte proliferation was assessed by 3[H]-labeled thymidine (NEN #NET027A) incorporation as previously described (40) using serial ovalbumin dilutions. IFNγ and IL-4 were measured by ELISA (R&D Systems; DuoSet ELISA Development Systems).
Statistical analyses
Mean values were compared by Students’ T test or ANOVA (Zar, 1974). For analysis of inflammation scores, a non-parametric, Kruskal-Wallis rank sum test was performed.
Results
Systemic administration of either syngeneic or allogeneic BMSCs during antigen sensitization inhibits methacholine-induced airways hyper-reactivity and eosinophilic lung inflammation
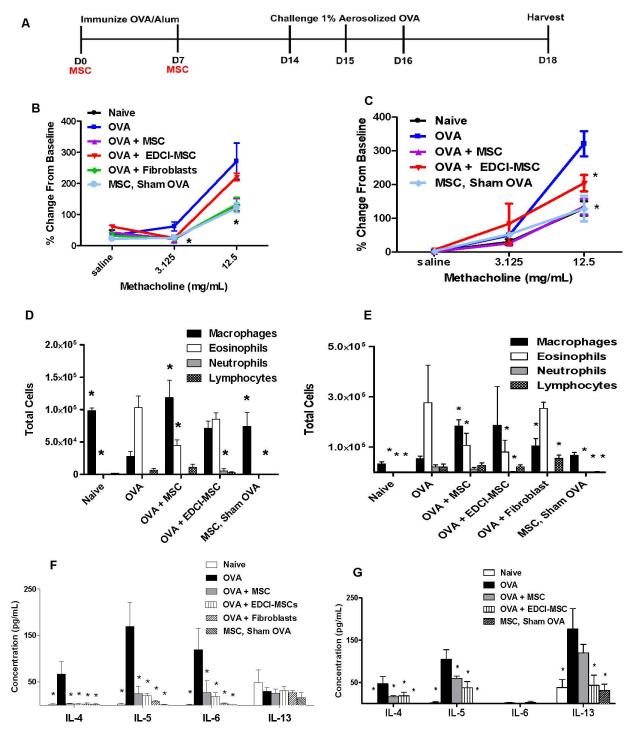
To determine if systemic BMSCs administration during antigen sensitization inhibited the ova-stimulated increase in airways hyper-reactivity of the large conducting airways, the primary physiologic outcome, adult mice were immunized by intraperitoneal injection of ovalbumin (ova) in the presence of the Th2 promoting adjuvant aluminum hydroxide (alum) on days 0 and 7 (Figure 1A). BMSCs isolated from the bone marrow of adult C57BL/6 mice (Tulane Mesenchymal Stem Cell Core Facility) or vehicle control (phosphate-buffered saline, PBS) were administered by tail vein injection immediately prior to immunization on days 0 and 7 to either adult C57BL/6 (syngeneic) or adult BALB/c (allogeneic) mice. Following aerosol challenge with ova on days 14-16, airways hyper-reactivity was assessed on day 18. C57BL/6 mice receiving syngeneic BMSCs during immunization demonstrated significant inhibition of the ova-stimulated increase in methacholine-induced airways hyper-reactivity in the large conducting airways (Rn) (Figure 1B). BMSC administration had no effects on the other physiologic parameters measured, lung tissue elastance (H) or tissue resistance (G) (Schuessler and Bates, 1995) (Figures E1A, E1B). Adult BALB/c mice receiving allogeneic BMSCs during antigen sensitization comparably demonstrated a significant decrease in airways hyper-reactivity (Rn) in response to the higher methacholine dose (Figure 1C). Allogeneic BMSC administration to the BALB/c mice also decreased tissue elastance (H) but no effects were observed on tissue resistance (G) (Figures E1C, E1D). Administration of BMSCs to mice receiving control (PBS/alum) immunizations (ie, “MSCs, Sham OVA”) followed by ova challenge did not affect airways hyper-reactivity in either the C57BL/6 or BALB/c mice (Figures 1A, B).
Figure 1. Systemic administration of syngeneic and allogeneic BMSCs during ova/alum sensitization inhibits airways hyper-responsiveness and allergic airways inflammation.
A) Schematic of experimental studies. B) Airways hyper-responsiveness in syngeneic or C) Allogeneic BMSC, EDCI-MSC, fibroblast or PBS-treated ova immunized mice as determined by peak airways resistance responses at each methacholine dose as a function of % change from resting baseline. D) BAL fluid cell differentials in syngeneic or E) Allogeneic BMSC, EDCI-MSC, fibroblast or PBS-treated ova immunized mice models. Data represents percent of total cells counted. F) Syngeneic and G) allogeneic BAL fluid cytokine levels as measured using luminex multi-bead technology (Bioplex, Biorad, Hercules, CA). Data depicts means and ± SE from one of 3 similar experiments w/ 6-8 mice per condition for each experiment. * p<0.05 compared to ova.
We next evaluated whether BMSC administration during antigen sensitization would also inhibit the Th2-mediated eosinophilic lung inflammation induced by ova/alum sensitization and subsequent challenge. In ova-treated C57BL/6 mice receiving syngeneic BMSCs during ova/alum sensitization, the total numbers of eosinophils in bronchoalveolar lavage (BAL) fluid was significantly decreased two days after final aerosol challenge compared to the characteristic increase provoked by ova/alum sensitization and ova challenge (Figure 1D). While BMSC administration had no effects on the small increase in numbers of BAL fluid lymphocytes and neutrophils observed in ova-treated mice, administration did result in significant increases in BAL fluid macrophages compared to ova treated mice. Similar effects were observed in BALB/c mice receiving allogeneic BMSCs whereby there was a significant decrease in the number of eosinophils with a concordant increase in numbers of macrophages and no effect on lymphocyte or neutrophil counts (Figure 1E). BMSCs administered in both the syngeneic and allogeneic sham ova models resulted in similar cellular profiles as to what was observed in naïve mice (Figures 1D, E). The Th2 cytokines IL-4 and IL-5 are known promoters of eosinophil recruitment, and immunoglobulin class switching, and are elevated in BAL fluid on day 18 following aerosolized ova challenge in both C57BL/6 and BALB/c ova-treated mice. Administration of BMSCs in either the syngeneic or allogeneic models resulted in a significant decrease in levels of IL-4 and IL-5 in the BAL fluid (Figures 1F,G). Levels of another prominent Th2 cytokine, IL-13, which characteristically peak earlier after ova challenge, were not elevated in BAL fluid of C57BL/6 ova-treated mice on day 18 and were otherwise not affected by syngeneic BMSC administration (Figure 1F). Although IL-13 levels were still detectable on Day 18 in the BALB/c mice, BMSC administration did not result in a significant decrease compared to ova controls. (Figure 1G). No detectable levels of IFNγ or IL-17, indicative of a Th1 or Th17 response respectively, were measured in BAL fluid for any of the groups (data not shown). IL-6 is rapidly produced by stimulated antigen presenting cells and promotes a Th2 phenotype in CD4 cells. Syngeneic BMSC administration significantly decreased IL-6 levels in the BAL fluid compared to OVA treated mice (Figure 1F) No measurable levels of BAL fluid IL-6 was detected in any of the BALB/c treated mice (Figure 1G).
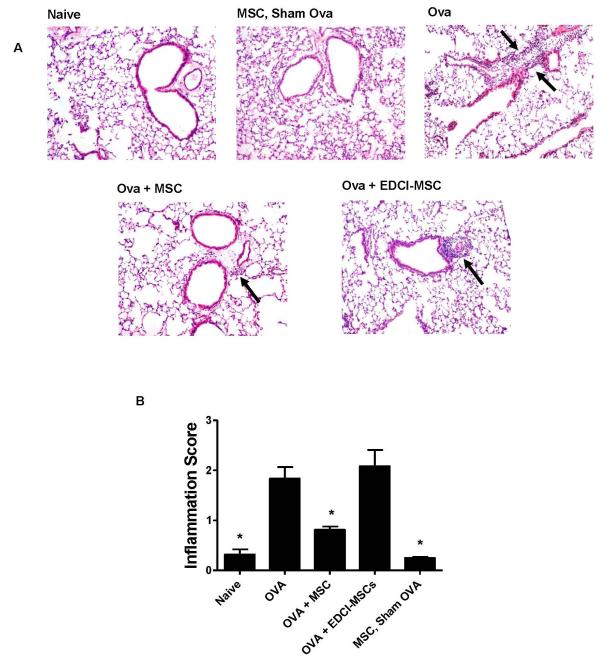
Qualitative and quantitative assessment of histologic lung inflammation demonstrated that administration of allogeneic BMSCs to BALB/c mice decreased peribronchial inflammatory cell infiltrates characteristic of this model (Figures 2A,B). Similarly, administration of syngeneic BMSCs to C57BL/6 mice also resulted in a significant decrease in lung inflammation as assessed by the number of inflammatory cells surrounding the airways (Figures E2A, E2B).
Figure 2. Systemic administration of allogeneic BMSCs during ova/alum sensitization decreases peribronchial inflammation.
A) Representative photomicrographs, stained with hematoxylin and eosin, depicting histologic inflammation on day 18 for allogeneic models are depicted for each experimental condition. Arrows designate areas of peribronchial inflammatory cell infiltration. Original magnification 100X. B) Airways inflammation scoring. Data depicts means and ± SE from one of 3 similar experiments w/ 6-8 mice per condition for each experiment. * p<0.05 compared to ova.
To determine whether soluble mediators secreted by BMSCs were required for the observed inhibition of airways hyper-reactivity and Th2-mediated, eosinophil lung inflammation, administration of BMSCs treated with 1-ethyl-3-[3-dimethylaminopropyl]carbodiimide hydrochloride (EDCI) to prevent release of soluble mediators (“EDCI-MSCs”) was assessed (37). Unlike viable MSCs, administration of the EDCI-MSCs did not inhibit all the end points assessed. EDCI-MSCs suppressed AHR only at the lower 3.125 mg/mL dose in the syngeneic model as opposed to the allogeneic model whereby EDCI-MSCs were only effective at suppressing AHR at the higher 12.5 mg/mL dose (Figures 1A, B). While administration of EDCI-MSCs decreased levels of IL-4 and IL-5 in both the syngeneic and allogeneic models, they were only able to decrease BAL fluid eosinophils within the syngeneic model (Figures 1B,C,D,E). The effects of EDCI-MSCs were also variable with respect to their effects on airways hyper-reactivity. Furthermore, administration of EDCI-MSCs did not reduce histologic inflammation in either the syngeneic or allogeneic models as assessed by the number of inflammatory cells found surrounding the airways (Figure 2, Figures E2A, E2B). These results suggest that while cell surface structures contribute to the BMSC effects, soluble mediators are required in order for BMSCs to exhibit their full suppressive capabilities in this model.
To further examine whether the effects observed were specific to the administration of BMSCs and not a result of introducing a foreign cell type into circulation, we assessed the effects of a control cell type, primary mouse dermal fibroblasts obtained from skin biopsies of C57BL/6 mice. Notably, syngeneic administration of C57BL/6 mouse primary dermal fibroblasts significantly inhibited airways hyper-reactivity (Rn) in C57BL/6 mice (Figure 1B) and decreased levels of Th2-associated cytokines in the BAL fluid (Figure 1F). However, administration of syngeneic primary dermal fibroblasts did not decrease the numbers of BAL fluid eosinophils and, importantly, significantly increased histologic airways inflammation compared to the control ova-treated mice (Figure 1D and Figures E2A, E2B).
The above results demonstrate that both syngeneic and allogeneic administration of BMSCs during sensitization to ovalbumin decreases airways hyper-responsiveness and eosinophilic Th2-mediated lung inflammation following subsequent challenge with aerosolized ovalbumin. Furthermore, administration of the control cell types, EDCI-MSCs and primary dermal fibroblasts only partially mimicked the suppressive effects observed compared to the administration of viable BMSCs and in some cases increased cellular infiltration.
Systemic administration of syngeneic or allogeneic BMSCs during antigen sensitization does not inhibit in vivo antigen-specific CD4 T lymphocyte proliferation but alters antigen-specific CD4 T lymphocyte differentiation
As BMSCs have been demonstrated to inhibit CD4 T lymphocyte proliferation in in vitro mixed lymphocyte reactions (21, 24, 41), we reasoned that inhibition of antigen (ie., ovalbumin)-specific CD4 T lymphocyte generation might be a mechanism by which the BMSCs inhibit allergic airways inflammation. To assess this, C57BL/6 or BALB/c mice were immunized with ova/alum on days 0 and 7 and splenic CD4 T lymphocytes were harvested on day 14 (Figure 4A). C57BL/6 BMSCs or PBS control was administered by tail vein injection 15 minutes prior to each immunization. Notably, BMSC administration during ova immunization had no effect on ovalbumin-specific CD4 T lymphocyte proliferation following either syngeneic administration to C57BL/6 mice or allogeneic administration to BALB/c mice (Figures 3B and Figures E3A). While the in vitro re-stimulation of CD4 lymphocytes occurred in the absence of BMSCs, we conclude that ova-specific T cell proliferation was unaffected by in vivo exposure to BMSCs due to the fact that any differences in the generation of ova-specific T lymphocytes during clonal expansion would be reflected by in vitro antigen-specific stimulation of proliferation.
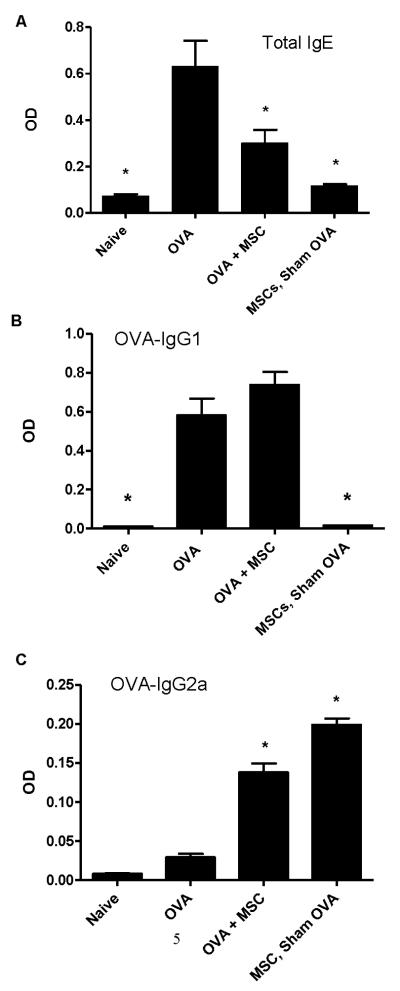
Figure 4. Systemic administration of allogeneic BMSCs increases circulating antigen-specific IgG2a but has no effect on IgGE or IgG1.
A) Total IgE from the allogeneic model. B) Ova-specific IgG1 from the allogeneic model. C) Ova-specific IgG2a from the allogeneic model. Data depicts means and ± SE from one of 3 similar experiments w/ 6-8 mice per condition for each experiment. * p<0.05 compared to ova.
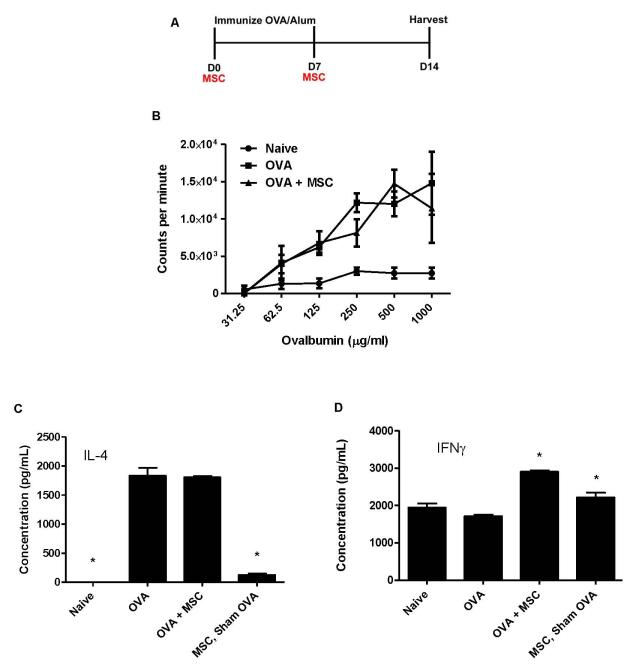
Figure 3. Systemic administration of allogeneic BMSCs does not affect antigen-specific CD4 T lymphocyte proliferation but promotes CD4 Th1 differentiation.
A) Schematic of experimental protocol. B) Allogeneic antigen specific CD4 T lymphocyte proliferation as assessed by 3[H]-thymidine incorporation. C) IL-4 content in conditioned media from splenic CD4 T lymphocytes isolated from allogeneic model. D) IFNγ content in conditioned media from splenic CD4 T lymphocytes isolated from the allogeneic model. Data depicts means and ± SE from one of 3 similar experiments w/ 6-8 mice per condition for each experiment. * p<0.05 compared to ova.
We next assessed whether systemic BMSC administration during antigen sensitization would affect antigen-specific CD4 T lymphocyte differentiation. Use of the adjuvant alum during ova immunization results in generation of Th2 antigen-specific CD4 T lymphocytes. Notably, administration of allogeneic BMSCs during ova sensitization had no effect on IL-4 release from antigen-specific splenic CD4 T lymphocytes cultured ex vivo in the presence of ova and irradiated splenic antigen presenting cells (Figure 3C)) and had either no effect or increased levels of IL-4 as a result of syngeneic BMSC administration (Figure E3B). However, administration of either syngeneic or allogeneic BMSCs, resulted in significant increases in the production of IFNγ by antigen-specific splenic CD4 T lymphocytes (Figure 3D and Figure E3C). No significant change in either IL-4 or IFNγ release by antigen-specific splenic CD4 T lymphocytes was observed in sham-ova treated mice that received allogeneic BMSCs compared to naïve mice (Figures 3C,D).
Systemic administration of sygeneic or allogeneic MSCs during antigen sensitization alters levels of circulating antigen-specific immunoglobulins
As BMSC administration during ova sensitization promoted Th1 differentiation of antigen-specific CD4 T lymphocytes, we assessed whether MSC administration affected levels of circulating antigen-specific antibodies provoked by ova sensitization and challenge. Systemic administration of allogeneic BMSCs to the BALB/c mice significantly mitigated the increases in ovalbumin-specific IgE and IgG1 typically provoked by ova/alum sensitization and ova challenge (Figure 4A,B). However, levels of circulating antigen-specific IgG2a, indicative of a Th1 phenotype, were significantly increased in mice receiving BMSCs (Figure 4C). In contrast, administration of syngeneic BMSCs to C57BL/6 mice during ova sensitization had no effect on the characteristic increase in antigen specific IgE or IgG1, however, did resulted in a significant increase in the Th1-associated ova-IgG2a (Figures E4A, E4B, E4C). No significant change in levels of circulating ovalbumin-specific IgE or IgG1 were observed in sham-ova treated mice that had received either syngeneic or allogeneic BMSCs compared to naïve mice (Figures 4A-C and Figures E4A, E4B, E4C). Increases in ova-specific IgG2a were observed in sham-ova treated mice receiving allogeneic BMSCs whereby, ova-specific responses were generated during the challenge phase of the experimental protocol.
These results demonstrate that while neither syngeneic nor allogeneic BMSC administration alters genesis and proliferation of antigen-specific CD4 lymphocytes in vivo, differentiation of these cells is skewed from a Th2 phenotype towards a Th1 as measured both by release of IL-4 and IFNγ as well as by patterns of circulating antigen-specific immunoglobulins. The promotion of a Th1 phenotype in CD4 lymphocytes was observed in both the Th1 biased C57BL/6 mouse strain and notably in the Th2 biased BALB/c strain. Differences between syngeneic and allogeneic BMSC administration on levels of circulating IgE and IgG1 were observed, however, it is unclear whether this is due to strain differences in production or a result of the different types of transplants performed.
Systemic administration of syngeneic BMSCs during antigen sensitization has no effect on Th1-mediated allergic airways inflammation
As MSC administration promotes differentiation of antigen-specific Th1 CD4 T lymphocytes in this Th2 model of allergic airways inflammation, we questioned what the BMSCs might do in a model of Th1-mediated allergic airways inflammation in the Th1-biased C57BL/6 mice. To assess this, we utilized complete Freund’s adjuvant (CFA) rather than alum to create a model of ova-induced Th1-mediated lung inflammation (Figure 5A). Administration of BMSCs during ova sensitization had no effect on the characteristic Th1-mediated increase in BAL fluid neutrophils and lymphocytes (Figure 5B). Notably, BMSC administration did not increase but rather slightly decreased IFNγ release from antigen-specific splenic CD4 T lymphocytes (Figure 5C). IL-4 release by antigen-specific CD4 T lymphocytes was not detected under any experimental condition (data not shown). In parallel, BMSCs did not alter levels of the circulating antigen-specific Th1 immunoglobulin IgG2a (Figure 5D). These studies demonstrate that while BMSCs promote a Th1 phenotype in the setting of an in vivo antigen-stimulated Th2 response in both Th1 and Th2-biased mouse strains, MSCs do not either augment the Th1-mediated inflammation or promote a Th2 phenotype in the setting of an antigen-stimulated allergic airways Th1 response in a Th1-biased mouse.
Figure 5. Systemic administration of syngeneic BMSCs does not augment Th1-mediated allergic airways inflammation.
A) Schematic of experimental studies. B) BAL fluid cell differentials. Data represent percent of total cells counted. C) Antigen-specific CD4 T lymphocyte IFNγ production 72 hours after ova stimulation. D) Circulating ova-specific IgG2a levels. Data depict means and ± SE of 1 experiment w/ 4 mice per condition * p<0.05 compared to ova.
BMSC inhibition of allergic airways inflammation is partly dependent on IFNγ
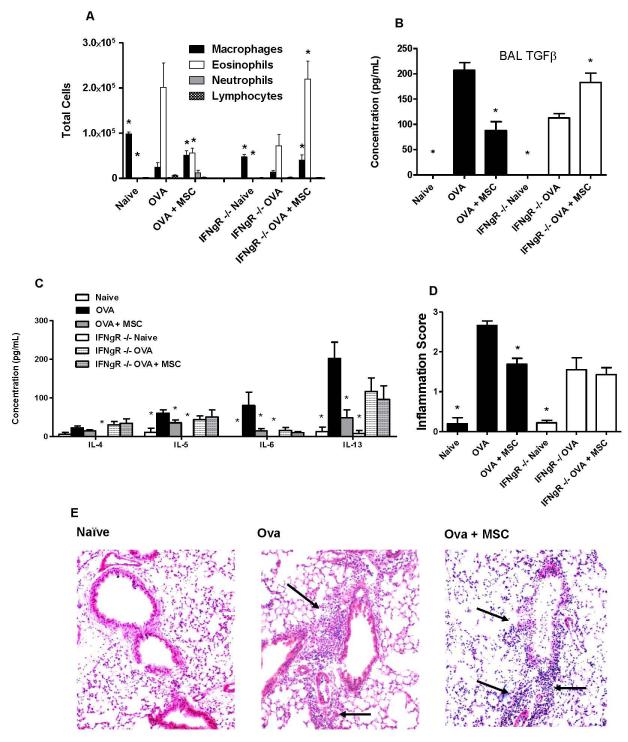
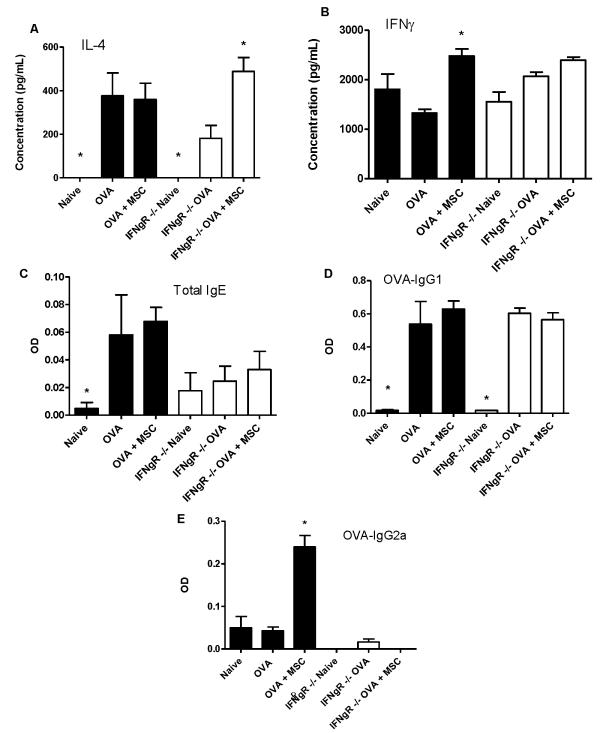
Promotion of a Th1 phenotype and production of IFNγ by antigen-specific CD4 T lymphocytes has been demonstrated to be sufficient for ameliorating Th2-mediated allergic airways inflammation (26, 42). To determine if amelioration of allergic airways inflammation by BMSCs was comparably dependent on IFNγ, studies were repeated in IFNγ receptor null mice (IFNγR−/−), which develop ova/alum-stimulated allergic airways inflammation comparable in many respects to wild type mice (43, 44). In contrast to strain control C57BL/6 mice, systemic administration of syngeneic BMSCs during antigen sensitization of IFNγR−/− mice did not inhibit the ova-stimulated increase in BAL fluid eosinophils or BAL fluid IL-4, IL-5, or IL-13 content (Figure 6A, C). Another cytokine, TGFβ1, which has been implicated in generation of allergic airways hyper-responsiveness in the ovalbumin model (45), was significantly increased by ova/alum treatment in BAL fluid of both strain control C57BL/6 and IFNγR−/− mice compared to respective naive controls (Figure 6B). However, while BMSC administration during antigen sensitization decreased the ova/alum-stimulated increase in BAL fluid TGFβ levels in the C57BL/6 mice, BMSC administration significantly increased BAL fluid TGFβ in the IFNγR−/− mice (Figure 6B). While BMSC administration inhibited the increase in histologic inflammation provoked by ova/alum sensitization and ova challenge in the strain control C57BL/6 mice, histologic inflammation was not inhibited following BMSC administration to the IFNγR−/− mice (Figures 6D,E). In contrast to strain control C57BL/6 mice, BMSC administration to the IFNγR−/− mice during ovalbumin sensitization increased ova-stimulated IL-4 release from ovalbumin-specific CD4 T lymphocytes (Figure 7A). However, IFNγ release from ova-specific CD4 T lymphocytes was not significantly increased in the IFNγR−/− mice, compared to the increase observed in the C57Bl/6 control mice (Figure 7B). Circulating ova-specific IgE levels were blunted in the IFNγR−/− mice compared to strain control mice, however BMSCs had no effect in either cohort (Figure 7C). MSC administration had no effect on circulating ova-specific IgG1 in either wildtype or IFNγR−/− mice (Figure 7D), but in contrast to strain control mice in which ova-specific IgG2a is increased in MSC–treated mice, circulating ova-specific IgG2a was nearly undetectable under all experimental conditions in the IFNγR−/− mice (Figure 7E). Therefore, the mechanism by which BMSCs suppress Th2-mediated allergic airways inflammation in this model is thus partly through development of a Th1 response and the ability of the recipient to respond to IFNγ.
Figure 6. Administration of syngeneic BMSCs does not abrogate allergic airways inflammation in IFNγR−/− mice.
A) BAL cell differentials. Data represent percent of total cells counted. B) BAL fluid cytokine content. C) BAL fluid TGFβ levels as measured by ELISA. D) Semiquantitative scoring of histologic peribronchial inflammation. E) Representative histologic inflammation on Day 18. Arrows designate areas of peribronchial inflammatory cell infiltration. Lungs sections were stained with hematoxylin and eosin. Original magnification 100X. Values represent means ± SE of one of 3 similar separate experiments with 5-8 mice studied for each experimental condition. * p<0.05 compared to ova.
Figure 7. BMSCs do not promote Th1 phenotype in IFNR−/− mice.
A) IL-4 and B) IFNγ content in conditioned media from splenic CD4 T lymphocytes. C) Total IgE. D) Ova-specific IgG1. E) Ova-Specific Ig2a measured by ELISA from serum. Values represent means ± SE of one of 3 similar separate experiments with 5-8 mice studied for each experimental condition. * p<0.05 compared to ova.
Discussion
Mesenchymal stromal cells are increasingly being found to have potent anti-inflammatory effects in a wide range of inflammatory and immune-mediated disease models (3-5) . However, the mechanisms of MSC actions in vivo, particularly those affecting CD4 T lymphocyte-mediated inflammation, remain poorly understood. Although MSCs can inhibit CD4 T lymphocyte proliferation in in vitro assays, whether this occurs in vivo remains unclear. Further, the few published reports evaluating MSC effects on CD4 T lymphocyte differentiation generally demonstrate that MSCs tend to shift CD4 T lymphocytes towards a Th2 phenotype both in vitro and in vivo (35, 46). These findings suggest that administration of MSCs might be either ineffective or could potentially exacerbate immune responses in a model of Th2 mediated inflammation, such as allergic asthma. As such, it was of particular interest to investigate the effects of MSCs on the generation and differentiation of antigen-specific CD4 T cells in a model of Th2-mediated inflammation. Utilizing a mouse model of ovalbumin-induced Th2-mediated eosinophilic allergic airways inflammation, we found that systemic administration of either syngeneic or allogeneic BMSCs during antigen sensitization inhibited both airways hyper-reactivity and lung inflammation through a mechanism partly dependent on IFNγ. BMSCs promoted a Th1 phenotype in vivo as assessed by both IFNγ production following antigen-specific CD4 T lymphocyte stimulation and antigen-specific circulating immunoglobulins, in both the Th1-biased C57BL/6 and Th2-biased BALB/c mouse strains. As promotion of a Th1 CD4 lymphocyte and subsequent, IFNγ production can inhibit Th2-mediated allergic airways inflammation (26, 42), BMSCs therefore inhibit Th2-mediated allergic airways inflammation by influencing antigen-specific CD4 T lymphocyte differentiation and promoting IFNγ production. Further, the BMSC effects on allergic airways inflammation are dependent on the ability of the mouse to respond to IFNγ.
To address whether soluble mediators released by the BMSCs played a role in amelioration of allergic airways inflammation, we utilized BMSCs treated with EDCI to inhibit secretion of soluble mediators yet preserve cell surface epitopes. Administration of either syngeneic or allogeneic EDCI-treated BMSCs did not inhibit airways hyper-reactivity or significantly decrease ova-stimulated histologic inflammation. However, BAL fluid eosinophilia and levels of Th2 cytokines were reduced similarly to that observed in the mice receiving untreated BMSCs. This suggests that cell surface molecules play a role in the BMSC actions in vivo but that soluble mediators are required for MSCs to exhibit their full suppressive potential in this model.
MSCs were first identified as an adherent, fibroblast-like population of cells in the bone marrow and initially identified as colony-forming fibroblasts. Several recent publications have argued that their immunosuppressive properties are not unique to MSCs, but rather a fundamental property shared among stromal cells and fibroblasts (47, 48). For example, fibroblasts derived from a variety of sources including skin, are capable of inhibiting T lymphocyte proliferation and TNFα and IFNγ production in response to polyclonal stimulation in vitro (48). To investigate whether fibroblasts could mimic the inhibitory affects of BMSCs in allergic airways inflammation, we included primary dermal fibroblasts obtained from skin biopsies from C57BL/6 mice as a cell control. While systemic administration of these fibroblasts during ova/alum sensitization decreased airways hyper-responsiveness and levels of Th2 cytokines in the BAL fluid, syngeneic fibroblast administration did not decrease the number of eosinophils in the airways and conversely increased the number of peribronchial inflammatory cell infiltrates compared to ova-treated mice. While we are uncertain as to why fibroblast administration resulted in beneficial effects for some of the end points investigated, syngeneic fibroblast administration did not result in comparable overall anti-inflammatory actions and in some cases promoted inflammation and antigen-specific immune responses. These data correspond to findings in endotoxin-induced injury in mouse lungs where intratracheal administration of 3T3 fibroblasts did not improve survival or severity of disease as did MSC administration (49).
Precisely how BMSCs induce a Th1 response in vivo in this model remains unclear. Notably, induction of a Th1 CD4 phenotype was observed in two strains of mice, including BALB/c mice which are known to exhibit an inherent Th2 bias in response to inflammatory injuries (50). Administration of either syngeneic or allogeneic BMSCs to sham (ie., PBS)-treated mice was well tolerated and did not affect any of the measured endpoints of airways hyper-reactivity, lung inflammation, antigen-specific CD4 T lymphocyte differentiation, or levels of antigen-specific circulating immunoglobulins, as compared to naïve control mice. This is an important observation which highlights a specific immunomodulatory role of BMSCs only under conditions of immune stimulation or disruption. Further, BMSCs administration to mice with ova/CFA-mediated Th1-mediated allergic airways inflammation did not worsen the inflammation suggesting that MSCs do not further contribute to a comparable Th1-mediated allergic airways injury. These data contrast with data in other experimental models of Th1-mediated inflammatory or immune injury in which MSC administration decreases injury by promoting Th2 CD4 T lymphocyte differentiation and demonstrates that MSC effects are dependent on the model utilized. This suggests that the specific local inflammatory environments both in vitro and in vivo affect MSC actions as has been recently found in other model systems (51, 52).
It has recently been demonstrated by Nemeth and colleagues that systemic administration of either syngeneic or allogeneic BMSCs in a ragweed induced model of allergic airways disease also led to a decrease in airways inflammation as assessed by BAL cytokine levels, circulating antibodies and histology (53). In contrast to our study where MSCs were administered during immunization, MSCs were delivered during challenge, a time when antigen specific CD4 T cells previously generated during sensitization are re-stimulated. In this model, no increase in Th1-specific immunoglobulins or levels of IFNγ in BAL fluid was observed. Rather release of TGFβ from the BMSCs stimulated an increase in T regulatory cells in the lung with subsequent suppression of allergic airways inflammation. Thus BMSC administration can decrease airways inflammation when given during either immunization or challenge, however the mechanism by which they do so differs. This highlights a growing appreciation of MSCs to “sense” their microenvironment and respond in different ways to suppress inflammatory and immune responses. Further, administration of dermal fibroblasts to the ragweed-sensitized mice also partly mimicked the BMSC responses reinforcing the growing appreciation that stromal cells such as fibroblasts may share some of the immunosuppressive effects of BMSC.
Syngeneic adipose-derived MSCs (ASCs) have also been described to inhibit both ovalbumin-induced allergic rhinitis and allergic airways inflammation when administered during or prior to antigen challenge in previously sensitized mice (54,55). Interestingly, the mechanisms appear similar to our findings as the ASCs appear to promote a Th1 environment as measured by levels of BAL fluid IFNγ levels and by polyclonally activated CD4 T lymphocytes recovered from BAL and spleen which stained positively for intracellular IFNγ. Most recently xenogeneic administration of human bone marrow-derived MSCs (hBMSCs) during challenge in a mouse model of ova-induced chronic airway inflammation decreased airways inflammation (56) Although the mechanisms for the hBMSC effects are not clear, hBMSC administration increased serum IFNγ when in mice challenged with ova but not saline, indicating that the ability of MSCs to induce IFNγ release only occurred in the context of an on going inflammatory response.
To further assess the importance of IFNγ in the ability of BMSCs to suppress inflammation in our model, we utilized IFNγR deficient mice that are known to have an impaired ability to generate a Th1 inflammatory response. We found that not only do BMSCs induce a Th1 phenotype in CD4 cells which are capable of secreting IFNγ upon antigen stimulation, but that the ability of the host to respond to IFNγ is necessary to achieve the suppressive effects exerted by the BMSCs. We are currently working to address the mechanisms by which BMSCs are inducing Th1 phenotype in CD4 cells as well as which are the critical cell types which need to be able to respond to IFNγ.
In conclusion, the current study demonstrates that systemic administration of syngeneic or allogeneic BMSCs during antigen sensitization suppresses allergic airways inflammation in mice, in part by promotion of Th1 differentiation of antigen-specific CD4 T lymphocytes. Elucidation of the mechanisms by which MSCs induce a Th1 inflammatory response and other mechanisms by which the MSCs may be acting in this model is the subject of ongoing investigations. Due to the timing of administration of the BMSCs during antigen sensitization, our studies were not designed to serve as a model for BMSC treatment as a possible therapy for pre-established asthma but rather to investigate the effects of BMSCs on CD4 T lymphocyte differentiation in a model of Th2-mediated inflammation. Collectively, our study along with previously published reports whereby BMSCs were administered either before or during antigen challenge provide a better overall understanding of how MSCs can suppress the inflammatory response generated in asthma, beginning with the initial development of antigen specific T cells, to their suppressive effects on effector functions. Collectively, these data will be necessary should MSCs be one day used as a therapy for asthma. Allergic asthma is an immune-mediated inflammatory disease of the lung in which Th2 CD4 T lymphocytes are central to the pathogenesis. While most patients can be effectively managed with a combination of medications, commonly β-agonists and inhaled corticosteroids, approximately 5% of patients have severe refractory disease that is difficult to treat (54-57). In addition to higher morbidity and mortality, these patients also account for a disproportionate use of health care resources with estimates of up to 50% of health care costs associated with asthma being utilized by the 5% of patients with severe refractory disease. Currently available treatments for severe refractory asthma are either poorly effective or have significant toxicities and new treatment approaches are thus urgently needed. (58, 59).
Supplementary Material
Acknowledgements
The authors are grateful to the staff of the University of Vermont Office of Animal Care Management and Dr. Jeffrey Spees and Charla Poole of the University of Vermont Stem Cell Core for technical assistance, Taka Ashikaga of the University of Vermont Statistics Department, Drs. Jason Bates, Lennart Lundblad, Charles Irvin of the Vermont Lung Center, and Dr. Alan Howe of the University of Vermont College of Medicine for helpful discussions and advice.
Funding: The work performed in this manuscript was supported by HL087274 (DJW), HL081289 (DJW), a research grant from the Cystic Fibrosis Foundation (DJW), NCRR COBRE P20 RR-155557 (Charles Irvin PI), and a T32 HL76122 NIH/NHBLI Multidisciplinary Training Grant in Lung Biology (Vermont Lung Center, Charles Irvin, PI).
Footnotes
There are no conflicts of interest for any of the authors.
Author Contribution Summary: Meagan Goodwin: concept and design, collection and/or assembly of data, data interpretation, manuscript writing, final approval of manuscript Viranuj Sueblinvong: data analysis and interpretation Philip Eisenhauer: data analysis and interpretation Nicholas Ziats: data analysis and interpretation Laurie LeClair: concept and design Matthew Poynter: concept and design Chad Steele: data analysis and interpretation Mercedes Rincon: concept and design Daniel Weiss: concept and design, manuscript writing, final approval of manuscript
References
- 1.Friedenstein AJ, Chailakhyan RK, Latsinik NV, Panasyuk AFK-B IV. Stromal Cells Responsible for Transferring the Microenvironment of the Hemopoietic Tissues. Cloning in Vitro and Retransplantation in Vivo. Transplantation. 1974;17:331–340. doi: 10.1097/00007890-197404000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Weiss DJ, Kolls JK, Ortiz LA, Panoskaltsis-Mortari A, Prockop DJ. Stem Cells and Cell Therapies in Lung Biology and Lung Diseases. Proceedings of the American Thoracic Society. 2008;5(5):637–667. doi: 10.1513/pats.200804-037DW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keating A. Mesenchymal Stromal Cells. Current Opinion in Hematology. 2006;13(6):419–425. doi: 10.1097/01.moh.0000245697.54887.6f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rasmusson I. Immune Modulation by Mesenchymal Stem Cells. Experimental Cell Research. 2006;312(12):2169–2179. doi: 10.1016/j.yexcr.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 5.Nauta AJ, Fibbe WE. Immunomodulatory Properties of Mesenchymal Stromal Cells. Blood. 2007;110(10):3499–3506. doi: 10.1182/blood-2007-02-069716. [DOI] [PubMed] [Google Scholar]
- 6.Sotiropoulou PA, Perez SA, Gritzapis AD, Baxevanis CN, Papamichail M. Interactions between Human Mesenchymal Stem Cells and Natural Killer Cells. Stem Cells (Dayton, Ohio) 2006;24(1):74–85. doi: 10.1634/stemcells.2004-0359. [DOI] [PubMed] [Google Scholar]
- 7.Tabera S, Perez-Simon JA, Diez-Campelo M, Sanchez-Abarca LI, Blanco B, Lopez A, Benito A, Ocio E, Sanchez-Guijo FM, Canizo C, et al. The Effect of Mesenchymal Stem Cells on the Viability, Proliferation and Differentiation of B-Lymphocytes. Haematologica. 2008;93(9):1301–1309. doi: 10.3324/haematol.12857. [DOI] [PubMed] [Google Scholar]
- 8.Ivanova-Todorova E, Bochev I, Mourdjeva M, Dimitrov R, Bukarev D, Kyurkchiev S, Tivchev P, Altunkova I, Kyurkchiev D. Adipose Tissue-Derived Mesenchymal Stem Cells Are More Potent Suppressors of Dendritic Cells Differentiation Compared to Bone Marrow-Derived Mesenchymal Stem Cells. Immunology Letters. 2009 doi: 10.1016/j.imlet.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 9.Spaggiari GM, Abdelrazik H, Becchetti F, Moretta L. Mscs Inhibit Monocyte-Derived Dc Maturation and Function by Selectively Interfering with the Generation of Immature Dcs: Central Role of Msc-Derived Prostaglandin E2. Blood. 2009;113(26):6576–6583. doi: 10.1182/blood-2009-02-203943. [DOI] [PubMed] [Google Scholar]
- 10.Li YP, Paczesny S, Lauret E, Poirault S, Bordigoni P, Mekhloufi F, Hequet O, Bertrand Y, Ou-Yang JP, Stoltz JF, et al. Human Mesenchymal Stem Cells License Adult Cd34+ Hemopoietic Progenitor Cells to Differentiate into Regulatory Dendritic Cells through Activation of the Notch Pathway. J Immunol. 2008;180(3):1598–1608. doi: 10.4049/jimmunol.180.3.1598. [DOI] [PubMed] [Google Scholar]
- 11.English K, Barry FP, Mahon BP. Murine Mesenchymal Stem Cells Suppress Dendritic Cell Migration, Maturation and Antigen Presentation. Immunology Letters. 2008;115(1):50–58. doi: 10.1016/j.imlet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 12.Jung YJ, Ju SY, Yoo ES, Cho SJ, Cho KA, Woo SY, Seoh JY, Park JW, Han HS, Ryu KH. Msc-Dc Interactions: Msc Inhibit Maturation and Migration of Bm-Derived Dc. Cytotherapy. 2007;9(5):451–458. doi: 10.1080/14653240701452057. [DOI] [PubMed] [Google Scholar]
- 13.Djouad F, Charbonnier LM, Bouffi C, Louis-Plence P, Bony C, Apparailly F, Cantos C, Jorgensen C, Noel D. Mesenchymal Stem Cells Inhibit the Differentiation of Dendritic Cells through an Interleukin-6-Dependent Mechanism. Stem Cells (Dayton, Ohio) 2007;25(8):2025–2032. doi: 10.1634/stemcells.2006-0548. [DOI] [PubMed] [Google Scholar]
- 14.Ramasamy R, Fazekasova H, Lam EW, Soeiro I, Lombardi G, Dazzi F. Mesenchymal Stem Cells Inhibit Dendritic Cell Differentiation and Function by Preventing Entry into the Cell Cycle. Transplantation. 2007;83(1):71–76. doi: 10.1097/01.tp.0000244572.24780.54. [DOI] [PubMed] [Google Scholar]
- 15.Beyth S, Borovsky Z, Mevorach D, Liebergall M, Gazit Z, Aslan H, Galun E, Rachmilewitz J. Human Mesenchymal Stem Cells Alter Antigen-Presenting Cell Maturation and Induce T-Cell Unresponsiveness. Blood. 2005;105(5):2214–2219. doi: 10.1182/blood-2004-07-2921. [DOI] [PubMed] [Google Scholar]
- 16.Zhang W, Ge W, Li C, You S, Liao L, Han Q, Deng W, Zhao RC. Effects of Mesenchymal Stem Cells on Differentiation, Maturation, and Function of Human Monocyte-Derived Dendritic Cells. Stem Cells and Development. 2004;13(3):263–271. doi: 10.1089/154732804323099190. [DOI] [PubMed] [Google Scholar]
- 17.Nauta AJ, Kruisselbrink AB, Lurvink E, Willemze R, Fibbe WE. Mesenchymal Stem Cells Inhibit Generation and Function of Both Cd34+-Derived and Monocyte-Derived Dendritic Cells. J Immunol. 2006;177(4):2080–2087. doi: 10.4049/jimmunol.177.4.2080. [DOI] [PubMed] [Google Scholar]
- 18.Jiang XX, Zhang Y, Liu B, Zhang SX, Wu Y, Yu XD, Mao N. Human Mesenchymal Stem Cells Inhibit Differentiation and Function of Monocyte-Derived Dendritic Cells. Blood. 2005;105(10):4120–4126. doi: 10.1182/blood-2004-02-0586. [DOI] [PubMed] [Google Scholar]
- 19.Chen L, Zhang W, Yue H, Han Q, Chen B, Shi M, Li J, Li B, You S, Shi Y, et al. Effects of Human Mesenchymal Stem Cells on the Differentiation of Dendritic Cells from Cd34+ Cells. Stem Cells and Development. 2007;16(5):719–731. doi: 10.1089/scd.2007.0065. [DOI] [PubMed] [Google Scholar]
- 20.Ramasamy RFH, Lam EW, Soeiro I, Lombardi G, Dazzi F. Mesenchymal Stem Cells Inhibit Dendritic Cell Differentiation and Function by Preventing Entry into the Cell Cycle. Transplantation. 2007;83(1):71–76. doi: 10.1097/01.tp.0000244572.24780.54. [DOI] [PubMed] [Google Scholar]
- 21.Le Blanc K, Tammik L, Sundberg B, Haynesworth SE, Ringden O. Mesenchymal Stem Cells Inhibit and Stimulate Mixed Lymphocyte Cultures and Mitogenic Responses Independently of the Major Histocompatibility Complex. Scandinavian Journal of Immunology. 2003;57(1):11–20. doi: 10.1046/j.1365-3083.2003.01176.x. [DOI] [PubMed] [Google Scholar]
- 22.Ramasamy R, Tong CK, Seow HF, Vidyadaran S, Dazzi F. The Immunosuppressive Effects of Human Bone Marrow-Derived Mesenchymal Stem Cells Target T Cell Proliferation but Not Its Effector Function. Cellular Immunology. 2008;251(2):131–136. doi: 10.1016/j.cellimm.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 23.Nasef A, Chapel A, Mazurier C, Bouchet S, Lopez M, Mathieu N, Sensebe L, Zhang Y, Gorin NC, Thierry D, et al. Identification of Il-10 and Tgf-Beta Transcripts Involved in the Inhibition of T-Lymphocyte Proliferation During Cell Contact with Human Mesenchymal Stem Cells. Gene Expression. 2007;13(4-5):217–226. doi: 10.3727/000000006780666957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rasmusson I, Ringden O, Sundberg B, Le Blanc K. Mesenchymal Stem Cells Inhibit Lymphocyte Proliferation by Mitogens and Alloantigens by Different Mechanisms. Experimental Cell Research. 2005;305(1):33–41. doi: 10.1016/j.yexcr.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 25.Fang L, Lange C, Engel M, Zander AR, Fehse B. Sensitive Balance of Suppressing and Activating Effects of Mesenchymal Stem Cells on T-Cell Proliferation. Transplantation. 2006;82(10):1370–1373. doi: 10.1097/01.tp.0000232450.62408.f9. [DOI] [PubMed] [Google Scholar]
- 26.Gajewski TF, Fitch FW. Anti-Proliferative Effect of Ifn-Gamma in Immune Regulation. I. Ifn-Gamma Inhibits the Proliferation of Th2 but Not Th1 Murine Helper T Lymphocyte Clones. J Immunol. 1988;140(12):4245–4252. [PubMed] [Google Scholar]
- 27.Augello A, Tasso R, Negrini SM, Amateis A, Indiveri F, Cancedda R, Pennesi G. Bone Marrow Mesenchymal Progenitor Cells Inhibit Lymphocyte Proliferation by Activation of the Programmed Death 1 Pathway. European Journal of Immunology. 2005;35(5):1482–1490. doi: 10.1002/eji.200425405. [DOI] [PubMed] [Google Scholar]
- 28.Meisel R, Zibert A, Laryea M, Gobel U, Daubener W, Dilloo D. Human Bone Marrow Stromal Cells Inhibit Allogeneic T-Cell Responses by Indoleamine 2,3-Dioxygenase-Mediated Tryptophan Degradation. Blood. 2004;103(12):4619–4621. doi: 10.1182/blood-2003-11-3909. [DOI] [PubMed] [Google Scholar]
- 29.Yang SH, Park MJ, Yoon IH, Kim SY, Hong SH, Shin JY, Nam HY, Kim YH, Kim B, Park CG. Soluble Mediators from Mesenchymal Stem Cells Suppress T Cell Proliferation by Inducing Il-10. Experimental & Molecular Medicine. 2009;41(5):315–324. doi: 10.3858/emm.2009.41.5.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM. Human Bone Marrow Stromal Cells Suppress T-Lymphocyte Proliferation Induced by Cellular or Nonspecific Mitogenic Stimuli. Blood. 2002;99(10):3838–3843. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- 31.Sato K, Ozaki K, Oh I, Meguro A, Hatanaka K, Nagai T, Muroi K, Ozawa K. Nitric Oxide Plays a Critical Role in Suppression of T-Cell Proliferation by Mesenchymal Stem Cells. Blood. 2007;109(1):228–234. doi: 10.1182/blood-2006-02-002246. [DOI] [PubMed] [Google Scholar]
- 32.Cui L, Yin S, Liu W, Li N, Zhang W, Cao Y. Expanded Adipose-Derived Stem Cells Suppress Mixed Lymphocyte Reaction by Secretion of Prostaglandin E2. Tissue Engineering. 2007;13(6):1185–1195. doi: 10.1089/ten.2006.0315. [DOI] [PubMed] [Google Scholar]
- 33.Ortiz LA, Dutreil M, Fattman C, Pandey AC, Torres G, Go K, Phinney DG. Interleukin 1 Receptor Antagonist Mediates the Antiinflammatory and Antifibrotic Effect of Mesenchymal Stem Cells During Lung Injury. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(26):11002–11007. doi: 10.1073/pnas.0704421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oh I, Ozaki K, Sato K, Meguro A, Tatara R, Hatanaka K, Nagai T, Muroi K, Ozawa K. Interferon-Gamma and Nf-Kappab Mediate Nitric Oxide Production by Mesenchymal Stromal Cells. Biochemical and Biophysical Research Communications. 2007;355(4):956–962. doi: 10.1016/j.bbrc.2007.02.054. [DOI] [PubMed] [Google Scholar]
- 35.Bai L, Lennon DP, Eaton V, Maier K, Caplan AI, Miller SD, Miller RH. Human Bone Marrow-Derived Mesenchymal Stem Cells Induce Th2-Polarized Immune Response and Promote Endogenous Repair in Animal Models of Multiple Sclerosis. Glia. 2009;57(11):1192–1203. doi: 10.1002/glia.20841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wagers S, Lundblad LK, Ekman M, Irvin CG, Bates JH. The Allergic Mouse Model of Asthma: Normal Smooth Muscle in an Abnormal Lung? J Appl Physiol. 2004;96(6):2019–2027. doi: 10.1152/japplphysiol.00924.2003. [DOI] [PubMed] [Google Scholar]
- 37.Jenkins MK, Schwartz RH. Antigen Presentation by Chemically Modified Splenocytes Induces Antigen-Specific T Cell Unresponsiveness in Vitro and in Vivo. The Journal of Experimental Medicine. 1987;165(2):302–319. doi: 10.1084/jem.165.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schuessler TF, Bates JH. A Computer-Controlled Research Ventilator for Small Animals: Design and Evaluation. IEEE Transactions on Bio-medical Engineering. 1995;42(9):860–866. doi: 10.1109/10.412653. [DOI] [PubMed] [Google Scholar]
- 39.Paveglio SA, Allard J, Mayette J, Whittaker LA, Juncadella I, Anguita J, Poynter ME. The Tick Salivary Protein, Salp15, Inhibits the Development of Experimental Asthma. J Immunol. 2007;178(11):7064–7071. doi: 10.4049/jimmunol.178.11.7064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Allard JB, Poynter ME, Marr KA, Cohn L, Rincon M, Whittaker LA. Aspergillus Fumigatus Generates an Enhanced Th2-Biased Immune Response in Mice with Defective Cystic Fibrosis Transmembrane Conductance Regulator. J Immunol. 2006;177(8):5186–5194. doi: 10.4049/jimmunol.177.8.5186. [DOI] [PubMed] [Google Scholar]
- 41.Crop MJ, Baan CC, Korevaar SS, Ijzermans JN, Alwayn IP, Weimar W, Hoogduijn MJ. Donor-Derived Mesenchymal Stem Cells Suppress Alloreactivity of Kidney Transplant Patients. Transplantation. 2009;87(6):896–906. doi: 10.1097/TP.0b013e31819b3d72. [DOI] [PubMed] [Google Scholar]
- 42.Parronchi P, De Carli M, Manetti R, Simonelli C, Sampognaro S, Piccinni MP, Macchia D, Maggi E, Del Prete G, Romagnani S. Il-4 and Ifn (Alpha and Gamma) Exert Opposite Regulatory Effects on the Development of Cytolytic Potential by Th1 or Th2 Human T Cell Clones. J Immunol. 1992;149(9):2977–2983. [PubMed] [Google Scholar]
- 43.Coyle AJ, Tsuyuki S, Bertrand C, Huang S, Aguet M, Alkan SS, Anderson GP. Mice Lacking the Ifn-Gamma Receptor Have Impaired Ability to Resolve a Lung Eosinophilic Inflammatory Response Associated with a Prolonged Capacity of T Cells to Exhibit a Th2 Cytokine Profile. J Immunol. 1996;156(8):2680–2685. [PubMed] [Google Scholar]
- 44.Zhao LL, Lotvall J, Linden A, Tomaki M, Sjostrand M, Bossios A. Prolonged Eosinophil Production after Allergen Exposure in Ifn-Gammar Ko Mice Is Il-5 Dependent. Scandinavian Journal of Immunology. 2008;67(5):480–488. doi: 10.1111/j.1365-3083.2008.02098.x. [DOI] [PubMed] [Google Scholar]
- 45.Alcorn JF, Rinaldi LM, Jaffe EF, van Loon M, Bates JH, Janssen-Heininger YM, Irvin CG. Transforming Growth Factor-Beta1 Suppresses Airway Hyperresponsiveness in Allergic Airway Disease. American Journal of Respiratory and Critical Care Medicine. 2007;176(10):974–982. doi: 10.1164/rccm.200702-334OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aggarwal S, Pittenger MF. Human Mesenchymal Stem Cells Modulate Allogeneic Immune Cell Responses. Blood. 2005;105(4):1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 47.Haniffa MA, Wang XN, Holtick U, Rae M, Isaacs JD, Dickinson AM, Hilkens CM, Collin MP. Adult Human Fibroblasts Are Potent Immunoregulatory Cells and Functionally Equivalent to Mesenchymal Stem Cells. J Immunol. 2007;179(3):1595–1604. doi: 10.4049/jimmunol.179.3.1595. [DOI] [PubMed] [Google Scholar]
- 48.Jones SHN, Cope A, Dazzi F. The Antiproliferative Effect of Mesenchymal Stem Cells Is a Fundamental Property Shared by All Stromal Cells. J Immunol. 2007;179(5):2824–2831. doi: 10.4049/jimmunol.179.5.2824. [DOI] [PubMed] [Google Scholar]
- 49.Gupta N, Su X, Popov B, Lee JW, Serikov V, Matthay MA. Intrapulmonary Delivery of Bone Marrow-Derived Mesenchymal Stem Cells Improves Survival and Attenuates Endotoxin-Induced Acute Lung Injury in Mice. J Immunol. 2007;179(3):1855–1863. doi: 10.4049/jimmunol.179.3.1855. [DOI] [PubMed] [Google Scholar]
- 50.Gueders MM, Paulissen G, Crahay C, Quesada-Calvo F, Hacha J, Van Hove C, Tournoy K, Louis R, Foidart JM, Noel A, et al. Mouse Models of Asthma: A Comparison between C57bl/6 and Balb/C Strains Regarding Bronchial Responsiveness, Inflammation, and Cytokine Production. Inflamm Res. 2009;58(12):845–854. doi: 10.1007/s00011-009-0054-2. [DOI] [PubMed] [Google Scholar]
- 51.Ryan JM, Barry F, Murphy JM, Mahon BP. Interferon-Gamma Does Not Break, but Promotes the Immunosuppressive Capacity of Adult Human Mesenchymal Stem Cells. Clinical and Experimental Immunology. 2007;149(2):353–363. doi: 10.1111/j.1365-2249.2007.03422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Waterman RS, Tomchuck SL, Henkle SL, Betancourt AM. A New Mesenchymal Stem Cell (Msc) Paradigm: Polarization into a Pro-Inflammatory Msc1 or an Immunosuppressive Msc2 Phenotype. PloS one. 5(4):e10088. doi: 10.1371/journal.pone.0010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nemeth K, Keane-Myers A, Brown JM, Metcalfe DD, Gorham JD, Bundoc VG, Hodges MG, Jelinek I, Madala S, Karpati S, et al. Bone Marrow Stromal Cells Use Tgf-Beta to Suppress Allergic Responses in a Mouse Model of Ragweed-Induced Asthma. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(12):5652–5657. doi: 10.1073/pnas.0910720107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Proceedings of the ATS Workshop on Refractory Asthma: Current Understanding, Recommendations, and Unanswered Questions. American Thoracic Society. American Journal of Respiratory and Critical Care Medicine. 2000;162(6):2341–2351. doi: 10.1164/ajrccm.162.6.ats9-00. [DOI] [PubMed] [Google Scholar]
- 55.The Enfumosa Cross-Sectional European Multicentre Study of the Clinical Phenotype of Chronic Severe Asthma. European Network for Understanding Mechanisms of Severe Asthma. Eur Respir J. 2003;22(3):470–477. doi: 10.1183/09031936.03.00261903. [DOI] [PubMed] [Google Scholar]
- 56.Adcock IM, Ito K. Steroid Resistance in Asthma: A Major Problem Requiring Novel Solutions or a Non-Issue? Current Opinion in Pharmacology. 2004;4(3):257–262. doi: 10.1016/j.coph.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 57.Chanez P, Wenzel SE, Anderson GP, Anto JM, Bel EH, Boulet LP, Brightling CE, Busse WW, Castro M, Dahlen B, et al. Severe Asthma in Adults: What Are the Important Questions? The Journal of Allergy and Clinical Immunology. 2007;119(6):1337–1348. doi: 10.1016/j.jaci.2006.11.702. [DOI] [PubMed] [Google Scholar]
- 58.Polosa R, Morjaria J. Immunomodulatory and Biologic Therapies for Severe Refractory Asthma. Respiratory Medicine. 2008;102(11):1499–1510. doi: 10.1016/j.rmed.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 59.Morjaria JB, Chauhan AJ, Babu KS, Polosa R, Davies DE, Holgate ST. The Role of a Soluble Tnfalpha Receptor Fusion Protein (Etanercept) in Corticosteroid Refractory Asthma: A Double Blind, Randomised, Placebo Controlled Trial. Thorax. 2008;63(7):584–591. doi: 10.1136/thx.2007.086314. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.