Abstract
Cell-based therapies with embryonic or adult stem cells, including induced pluripotent stem cells, have emerged as potential novel approaches for several devastating and otherwise incurable lung diseases, including emphysema, pulmonary fibrosis, pulmonary hypertension, and the acute respiratory distress syndrome. Although initial studies suggested engraftment of exogenously administered stem cells in lung, this is now generally felt to be a rare occurrence of uncertain physiologic significance. However, more recent studies have demonstrated paracrine effects of administered cells, including stimulation of angiogenesis and modulation of local inflammatory and immune responses in mouse lung disease models. Based on these studies and on safety and initial efficacy data from trials of adult stem cells in other diseases, groundbreaking clinical trials of cell-based therapy have been initiated for pulmonary hypertension and for chronic obstructive pulmonary disease. In parallel, the identity and role of endogenous lung progenitor cells in development and in repair from injury and potential contribution as lung cancer stem cells continue to be elucidated. Most recently, novel bioengineering approaches have been applied to develop functional lung tissue ex vivo. Advances in each of these areas will be described in this review with particular reference to animal models.
Abbreviations: AEC, alveolar epithelial cell; ALI, acute lung injury; ARDS, acute respiratory distress syndrome; BASC, bronchioalveolar stem cell; CCSP, Clara cell secretory protein; CF, cystic fibrosis; CFTR, cystic fibrosis transmembrane conductance regulator; CLP, cecal ligation and puncture; COPD, chronic obstructive pulmonary disease; eNOS, endothelial nitric oxide synthetase; EPC, endothelial progenitor cell; ESC, embryonic stem cell; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; GFP, green fluorescent protein; HSC, hematopoietic stem cell; IPF, idiopathic pulmonary fibrosis; KGF, keratinocyte growth factor; LPS, lipopolysaccharide; MCT, monocrotaline; MHC, major histocompatibility complex; MSC, mesenchymal stromal (stem) cell; PH, pulmonary hypertension; pro-SPC, pro-surfactant protein C; Sca-1, stem cell antigen-1
Many lung diseases remain incurable and continue to have substantial morbidity and mortality. This includes chronic obstructive pulmonary disease (COPD), notably emphysema, the only major disease category increasing in prevalence and one of the leading causes of death worldwide, as well as less common but equally devastating diseases such as pulmonary fibrosis, cystic fibrosis (CF), pulmonary hypertension (PH), and the acute respiratory distress syndrome (ARDS). Although symptomatic care for each of these conditions has improved, lung transplantation remains the only option for many patients. However, there is a severe shortage of donor lungs, and thus, many patients on waiting lists die prior to transplantation. Furthermore, lung transplantation is often a poor option with a 5-year mortality of approximately 50% and many problems associated with the required lifelong immunosuppression. New options are thus desperately needed.
Recent findings that embryonic stem cells and stem cells derived from adult tissues might be used by surgeons in repair and regeneration of injured or diseased tissues has stimulated extensive investigations into whether these approaches could be used for lung diseases. In parallel, there is increased understanding of the identity and roles of endogenous progenitor cells in the lungs, including possible roles as lung cancer stem cells. These are exciting and rapidly moving fields that hold promise for improved understanding of lung biology and as a potential therapeutic approach for lung diseases.
This review will highlight recent advances in understanding of endogenous lung progenitor cells followed by respective consideration of embryonic and adult-derived stem cells, with particular focus on advances in cell therapy approaches for specific lung diseases with adult stem cells (summarized in Table I 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21).
Table I.
Cell therapy with MSCs in experimental lung disease models
| Animal model | Outcome | Potential mechanism | Reference |
|---|---|---|---|
| Bleomycin IT | Reduce fibrosis Reduce inflammation |
IL-1RA Suppression of NO metabolite, proinflammatory and angiogenic cytokines Increase of MMP2 activity and reduction of Timp2 |
1, 2, 3, 4, 5, 6 |
| E coli LPS IP | Reduce inflammation Improve mortality |
Direct cell–cell contact and release of cytokines | 7, 8 |
| LPS IT | Reduce inflammation | Angiopoietin-1 | 9, 10 |
| Hyperoxia-induced lung injury | Prevent arrested alveolar and vascular growth | Paracrine-mediated mechanism | 11, 12, 13 |
| Cecal ligation and puncture-induced sepsis and acute lung injury | Improve mortality | Prostaglandin E(2)-induced IL-10 production | 15 |
| Papain-induced emphysema | Amelioration of emphysema | Reduce apoptosis by upregulation of Bcl-2 and Bax gene | 14 |
| Ovalbumin-induced asthma | Reduced airway hyperresponsiveness to methacholine Reduced airway eosinophilia |
Inhibit Th2 phenotype development through paracrine effect | 16 |
| CFTR-KO mice with Naphthalene-induced injury | Detection of CFTR by PCR Restored CFTR function by patch clamp |
Engraftment of airway epithelial cells | 17 |
| 2% polidocanol-induced airway injury | Airway epithelium | Early engraftment Paracrine effect |
18 |
| MCT-induced PAH (Rat) | Improved RV pressure, RVH and RV function | ECM remodeling Secretion of VEGF |
19, 20, 21 |
Abbreviations: IT, intratracheal; IP, intraperitoneal; LPS, lipopolysaccharide; ECM, extracellular matrix; RV, right ventricle; RVH, right ventricle hypertrophy; PAH, pulmonary artery hypertension; IL, interleukin; IL-1RA, interleukin-1 receptor antagonist; NO, nitric oxide; MMP2, matrix metalloproteinase 2; Timp2, tissue inhibitor of metalloproteinases 2.
Endogenous Lung Stem and Progenitor Cells
Undifferentiated multipotent endogenous tissue stem cells have been identified in nearly all tissues and are thought to contribute to tissue maintenance and repair. These are rare unspecialized cells that are often localized to specialized niches within each tissue and usually cycle infrequently. Importantly, they exhibit self-renewal capacity and can give rise to daughter progenitor or transit amplifying cells. Both stem and progenitor cells may give rise to the differentiated cells of the organ.22, 23
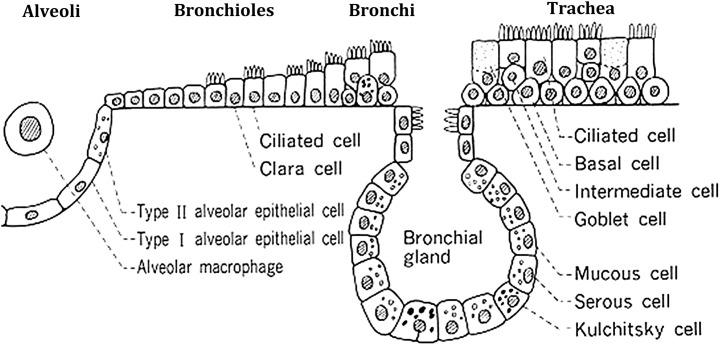
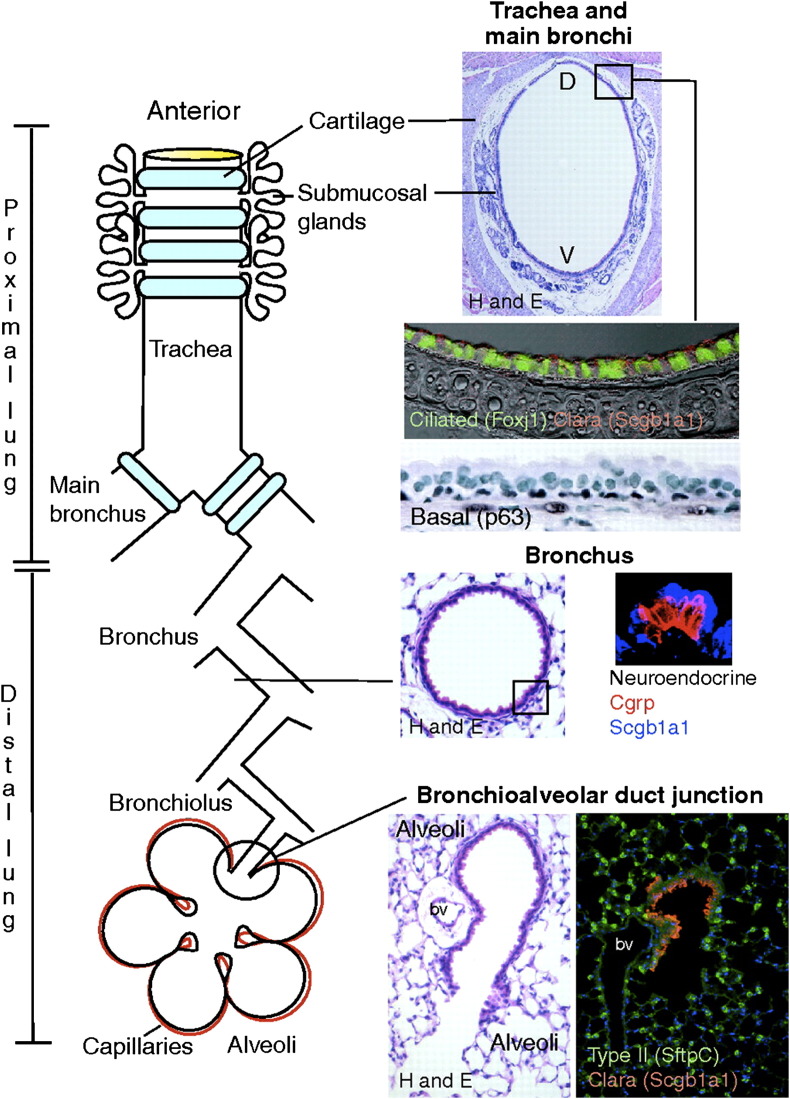
As the lung is a complex organ composed of more than 40 different differentiated cell types (Fig 1 ), identification of endogenous progenitor cells has been challenging, and it is now clear that different progenitor cell population are localized in different anatomic regions of the lung. In the alveoli, surfactant producing type 2 alveolar epithelial cells (AECs) are well recognized as progenitors for the type 1 AEC.23 In the airways, different putative airway progenitor cell populations and hierarchies have been identified along the tracheobronchial tree in mouse models after selective ablation of epithelial cells by exposure to toxic chemicals or through cell-type-specific expression of cell-toxic genes in transgenic mice (Fig 2 ).22, 23, 24, 25, 26 In the trachea, large airways, and large airway submucosal glands, a subpopulation of basal epithelial cells that express p63 and cytokeratins 5 and 14 have been implicated. In mice, the predominant epithelial cell of the smaller airways is the nonciliated Clara cell that exhibits characteristics of transit-amplifying cells after injury to ciliated airway epithelial cells. However, unlike transit-amplifying cells in tissues with higher rates of epithelial turnover, such as intestine, Clara cells exhibit a low proliferative frequency in the steady state, are broadly distributed throughout the bronchiolar epithelium, and contribute to the specialized tissue function. In more distal airways, cells resistant to the epithelial toxin naphthalene, which poisons the cytochrome P450 isozyme found in Clara cells and results in desquamation of the differentiated airway epithelial lining, termed toxin-resistant variant Clara cells have been identified as having progenitor functions and have been termed as bronchiolar stem cells (reviewed in Reference 25). Naphthalene-resistant cells are also located within discrete microenvironments within bronchioles that include the neuroepithelial body and bronchoalveolar duct junction (reviewed in 22, 25).
Fig 1.
Schematic showing different cell types in the lung. Figure courtesy of Barry Stripp, PhD, Duke University, North Carolina, USA.
Fig 2.
Schematic showing various types of lung endogenous progenitor cells located in different anatomic regions of the lung. CGRP = calcitonin gene-related peptide, Scgb1a1 = secretoglobulin, family 1A (also known as uteroglobulin), SftpC = surfactant protein C, D = dorsal, and V = ventral. Figure reproduced with permission from Rawlins and Hogan.24 (Color version of figure is available online.)
At the bronchoalveolar duct junction in mouse lungs, a population of naphthalene-resistant cells that stain for the Clara cell marker, Clara cell secretory protein (CCSP), and type 2 AEC marker pro-surfactant protein C (pro-SPC) have been described.27 Although rare, these cells proliferate in response to naphthalene injury and during compensatory lung growth after unilateral pneumonectomy in mice.28 Furthermore, when pro-SPC/CCSP dual-positive cells were isolated using methods developed for enrichment of type 2 AEC, some dual-labeled cells exhibited a unique cell-surface phenotype, Sca1pos/CD34pos/CD45neg/CD31neg. These cells were found to self-renew in culture and give rise to progeny expressing CCSP, pro-SPC, or the type 1 AEC marker aquaporin 5. As such, these cells have been termed bronchioalveolar stem cells (BASCs).27 However, depending on the techniques used, there is some ambiguity in published results and the toxin-resistant Clara cells, BASCs, and other cells may represent different interpretations of the same cell population(s).22 This highlights the need for specific markers of the different cell populations and rigorous methods of lineage tracing as well as further underscoring the importance of the in vivo microenvironment on cell behavior.29 For example, expression of stem cell antigen-1 (Sca-1), once thought to be a feature of murine hematopoietic cells, has now been described as a marker for both putative bronchiolar progenitor cells as well as for fibroblastic progenitor cells in the lung.30, 31, 32 Furthermore, recent data using chimeric wild-type–green fluorescent protein (GFP) chimeric mice, generated by implanting 8–16 cell GFP mouse embryo aggregates into pseudopregnant wild-type females, demonstrates that the bronchiolar progenitor cells do not seem to play a role in normal airway epithelial homeostasis and turnover but do participate in the setting of lung injury.33
Less information is available concerning potential progenitor cell populations contributing to other cells populations such as interstitial, smooth muscle, or endothelial cells in the lung.31, 34 Recently, several groups have identified what seem to be resident mesenchymal stromal (stem) cells in mouse lung as well as in both neonatal and adult human lungs.35, 36, 37 At present, it is unclear whether these cells may participate in structural repair of lung tissue or may play a role in immune surveillance and immunomodulation. In addition to the role of endogenous lung stem and progenitor cells in repair from lung injury, increasing information suggests that mature differentiated lung cells may transdifferentiate and change phenotype. Best described for epithelial–mesenchymal transition, recent investigations describe a wider range of reversible phenotypes in epithelial and mucus cells.38, 39, 40, 41, 42, 43 These mechanisms may also play significant roles in injury or repair from injury. Thus, overall, although progress is being made in clarifying the identity and role of airway progenitor cells in mice, the role(s) of these cell populations remains unclear. Moreover, little corresponding data as yet exist in other animal models or in human lungs.
Endogenous lung progenitor cells in human lung diseases
Although it is attractive to speculate that failure of normal endogenous airway stem cell reparative function may contribute to chronic acquired lung diseases such as emphysema, evidence for this remains sketchy at present. More suggestive information is available for the genetic lung disease, cystic fibrosis (CF). Airway epithelium in CF patients contains primitive cuboidal cells that express primitive cell markers, including thyroid transcription factor and cytokeratin 7.44 Neuroepithelial cells also express the cystic fibrosis transmembrane conductance regulator protein (CFTR), the defective protein in patients with CF that seems to play a role in neuropeptide secretion.45, 46 CFTR –/– mice also contain fewer pulmonary neuroendocrine cells during embryonic development but increased numbers of these cells postnatally.47 This suggests that endogenous airway progenitor cell pathways in CF lungs may be altered, but this has not been extensively investigated. Recently, another population of putative airway progenitor cells expressing CCSP, SCA-1, stage-specific embryonic antigen 1, and the embryonic stem cell marker Oct-4 have been identified in neonatal mice.48, 49 These cells were able to form epithelial colonies and differentiate into both type 1 and type 2 alveolar epithelial cells. Interestingly, these cells were susceptible to infection with the severe acute respiratory syndrome virus raising the possibility that endogenous lung progenitor cells may be specific disease targets. Comparably, the basal epithelial cells of the trachea and upper airways seem more susceptible to infection with the common cold rhinovirus.50 Endogenous progenitor cells may also be attractive candidates for targeting with gene transfer vectors that provide sustained expression. Variant Clara cells seem to be specifically targeted by recombinant adeno-associated vectors in adult mice.51 The possibility remains that other endogenous stem or progenitor populations exist, and there is much room for additional information on regulatory mechanisms and pathways as have been elucidated in other epithelial progenitor cell populations.52
Lung cancer stem cells
There is intense current interest in the connections between endogenous stem or progenitor cells and cancer stem cells. Cancer stem cells have been defined in transplantation assays as the critical cells from tumors that are capable of propagating disease and are hypothesized to be the cells that maintain tumor progression and disease resistance.53, 54 Best described in breast and other solid cancer, increasing evidence suggests there may be multiple lung cancer stem cells.55, 56, 57 Given the heterogeneity of lung cancers, this may not be surprising. BASCs have been suggested to have a role in development of lung cancer in mice, but their role in tumor maintenance has not been established, nor has the human correlation been demonstrated. Side population CD45-Hoechst-effluxing cells have been identified in several human lung cancer cell lines and exhibit tumorigenic properties when subcutaneously implanted into NOD SCID mice.58 Side population cells have also been identified in clinical lung cancer specimens.58 Several recent reports implicate CD133+ cells as both conferring resistance to chemotherapy and having tumor initiating properties for lung cancers.59, 60, 61, 62 Recent studies have begun elucidating cell signaling and gene expression pathways including Wnt, hedgehog, c-kit, Akt, and others that may play roles in transformation of endogenous progenitor cells into lung cancer cells.60, 61, 63, 64, 65, 66, 67, 68 Despite growing data, additional work is needed to clarify the connections between endogenous lung progenitor cells and their potential roles as lung cancer stem cells and to determine their potential role as therapeutic targets.
Embryonic Stem Cells (ESCs) and Lung Repair
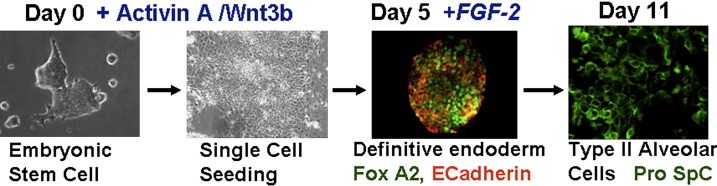
Progress using ESCs for lung regeneration or repair has been slower to develop. In studies during the past approximate 5 years, several laboratories have demonstrated that both mouse and human ESCs can be induced in culture to acquire phenotypic markers of type 2 AEC, including expression of surfactant proteins and lamellar bodies, and even form pseudoglandular structures.69, 70, 71, 72 In general, this occurred at a low level unless the ESCs were genetically engineered to select for surfactant protein-expressing cells.73 More recent protocols and manipulation of cell signaling pathways guiding embryologic lung development and development of definitive endoderm have yielded more robust in vitro derivation of cells with phenotypic characteristics of type 2 alveolar epithelial cells from murine ESCs (Fig 3 ).74 Derivation of airway epithelial cells from ESCs has proven even more elusive although development of cells with phenotypic markers of airway epithelial cells has been demonstrated after culture of the ESCs under air–liquid interface conditions.75, 76 Moreover, there are still only limited available studies of effects of ESC administration to lung in vivo. Endotracheal administration of mESC-derived type 2 AEC to fetal mice resulted in survival of the cells in the lungs and maintenance of pro-SPC expression during a 24-hour period.74 Whether the cells truly engrafted or had functional significance was not determined. Most recently, transduction of one of the approved human ESC lines developed at the University of Wisconsin (line H9.2) with an SPC-promoter-driven neomycin expression cassette was used to develop a >99% pure population of ESC-derived cells with phenotypic characteristics of type 2 AEC, including surfactant expression and presence of lamellar bodies (hES-ATII).77 Intratracheal administration of the hES-ATII cells 1 or 2 days after induction of acute lung injury following intratracheal bleomycin administration to immunocompromised SCID mice resulted in a substantial number of hES-ATII cells seeming to have engrafted in lung with persistent SPC expression up to 9 days later. Up to 20% of the total SPC-expressing cells seemed to be of hES-ATII origin. A small number of hES-ATII cells also seemed to have differentiated into type 1 AEC in vivo. No apparent engraftment was observed if hES-ATII cells were administered to naïve uninjured mice or if a control monocyte population was administered. In parallel, bleomycin-induced lung injury was significantly reduced in mice receiving hES-ATII cells but not monocytes or vehicle control. Reduced injury was measured by both qualitative and quantitative measures, including functional measurements of tidal volumes and arterial oxygen saturations. Whether the observed amelioration of lung injury resulted from structural engraftment of the administered cells or reflected a previously unsuspected paracrine effect of the hES-derived cells is not yet clear. Nonetheless, these results have several ramifications, including the study and potential use of ESCs in genetic lung diseases as human deltaF508 embryonic stem cell lines have been established in England and Belgium.78, 79 These cells exhibit normal morphology and protein expression compared with other hES cell lines but have not been studied in detail. With the new loosening of restrictions on study of human ESCs in the United States, it is anticipated that there will be further rapid advances in study of ESCs for lung injury and repair.
Fig 3.
ESCs can be effectively manipulated in vitro to differentiate into type 2 alveolar epithelial cells using the lung development cell signaling pathway to guide ESCs differentiation. FGF-2 = fibroblast growth factor-2 and pro-Spc = pro-surfactant protein C. Figure courtesy of Christine Finck, MD, University of Connecticut. (Color version of figure is available online.)
Adult Stem Cells and Lung Repair
Structural engraftment of lung tissues by circulating or exogenously administered stem or progenitor cells
It was initially speculated that adult bone-marrow-derived cells including hematopoietic stem cells (HSCs), mesenchymal stromal (stem) cells (MSCs), endothelial progenitor cells (EPCs), circulating fibrocytes, and other populations could structurally engraft as mature differentiated airway and alveolar epithelial cells or as vascular endothelial or interstitial lung cells. As such, a sizeable literature has developed over the past approximate 10 years predominantly based on studies in mice using techniques that evaluated histologic demonstration of tagged donor-derived marrow cells in recipient lungs after systemic or more intratracheal administration of tagged donor marrow cells. Experimental approaches have included use of donor marrow cells from transgenic GFP expressing mice or use of sex-mismatched transplantation (ie, male donor cells into female recipients) and subsequent identification of donor cells using fluorescent in situ hybridization to detect the Y chromosome (reviewed in Reference 80). Lethal irradiation and myeloablation of the recipient mouse bone marrow prior to cell administration was frequently but not always used. Notably, prior lung injury, including radiation-induced lung injury resulting from myeloablative total body irradiation, was usually required to observe engraftment although lung injury did not always result in apparent increase of engraftment.81, 82, 83
However, it has become apparent that engraftment of exogenously administered adult stem cells as airway and alveolar epithelial cells is generally a rare occurrence of uncertain physiologic significance.84, 85 Several technical issues contributed to misinterpretation of results in the earlier reports including inadequate microscopic techniques in which donor-derived cells superimposed on resident airway or alveolar epithelial cells were not effectively discriminated. Furthermore, a variety of leukocytes, notably airway and alveolar macrophages, reside in the lung. Many of the early reports did not use antibodies directed against CD45 or other leukocyte markers to exclude the possibility that cells of donor origin detected in airway or alveolar epithelium were donor-derived leukocytes rather than epithelial cells. Other tools, such as detection of GFP by direct fluorescence as a marker of donor-derived marrow cells, can be subject to error in the presence of autofluorescent cells resident in the lung, notably alveolar macrophages.86 Furthermore, fusion of marrow-derived cells with resident organ cells, rather than phenotypic conversion of the marrow cells, has been demonstrated in several organs, notably liver and skeletal muscle, but also in lung.87, 88, 89 The extent of fusion in lung after sex-mismatched transplantation may be underestimated as the Y chromosome may be lost from the heterokaryon cells resulting from fusion of donor-derived marrow cells with type 2 alveolar epithelial cells in mouse lungs.89 A smaller body of literature in human bone marrow and lung transplantation that had also demonstrated apparent chimerism in lungs of the transplant recipients has also been called into question for the same reasons (reviewed in Reference 80).90, 91, 92, 93, 94, 95, 96, 97
Nonetheless, recent studies with more rigorous techniques continue to demonstrate that engraftment of airway and alveolar epithelium, as well as of pulmonary vascular endothelium and of lung interstitium, with adult bone marrow or cord blood-derived stem cells, although rare, can occur under certain conditions, usually after previous perturbation through induction of lung injury.17, 98, 99, 100, 101, 102, 103, 104, 105, 106 Interestingly, several recent reports suggest that chronic or progressive lung injury may result in more substantial engraftment of type 2 AECs and of interstitial and pulmonary vascular cells with donor-derived cells in mouse or rat models.101 However, not all chronic lung injury models resulted in substantial epithelial engraftment.107 Notably, engraftment of pulmonary vascular endothelium and stimulation of no-angiogenesis by exogenously administered EPCs has fostered recent clinical trials for treatment of pulmonary hypertension (as will be discussed further).108, 109, 110 However, there are many variables still left to be explored that may increase epithelial, interstitial, or pulmonary vascular engraftment with circulating or donor-derived cells. For example, the types of marrow-derived, cord-blood-derived, or fully differentiated nonpulmonary cells that might engraft as lung epithelium, interstitium, or pulmonary vasculature remain to be fully explored. In addition to existing studies of HSCs, MSCs (of bone marrow and cord blood origin), EPCs, and fibrocytes, the possibility remains that there may be other cell populations that could be recruited to the lung or localized to the lung after systemic or other routes of administration.111, 112 A population of circulating bone-marrow-derived CD45+/CXCR4+/cytokeratin+ cells has been described to participate in re-epitheliazation of denuded tracheal xenografts.113 Most recently, a population of CCSP-expressing marrow cells has been suggested to engraft as airway epithelium.114 Other sources of stem or progenitor cells, such as umbilical cord blood or adipose tissues, also have not yet been extensively characterized for ability to engraft as lung tissue 115, 116, 117 The effect of age of either donor cells or of recipients is also less well explored, although one report demonstrated that transplantation of whole marrow into 1-day-old mouse pups, using a variety of conditioning regimens, did not increase the number of bone-marrow-derived cells over that observed after total marrow administration to adult mice.105 Furthermore, the route of administration of donor-derived cells is less well characterized as most studies have investigated engraftment after systemic administration of donor cells. Direct intratracheal administration of MSCs may enhance retention of donor-derived cells in the lung and possible epithelial engraftment.8, 114 However, this area is controversial and remains under-explored.
Furthermore, the ability to structurally engraft in adult lung may not solely be a property of stem or progenitor cells. For example, intratracheal administration of isolated type 2 alveolar epithelial cells results in rare engraftment in areas of injured lung after bleomycin administration to rats.118 Notably, bleomycin-injured rats that received the type 2 AEC had less histologic injury and decreased hydroxyproline content. Comparably, intratracheal administration of neonatal mouse lung fibroblasts resulted in apparent alveolar and interstitial engraftment and engraftment was higher in areas of elastase-induced lung injury.119 Systemically administered skin fibroblasts, transduced ex vivo to express angiopoietin-1, protected against lung injury produced by intratracheal endotoxin administration in rats.120 These results suggest that lung injuries might be amenable to cell therapy approaches using a variety of cell types other than stem or progenitor cells.
The mechanisms by which circulating or systemically administered stem or progenitor cells might be recruited to lung remain poorly understood. After systemic (ie, venous) administration, many cells initially localize in lungs as the first major capillary bed encountered. Lung injury can result in increased localization and/or retention of marrow-derived cells in lung.87, 88, 89 The range and identity of chemotactic soluble mediators released by injured lung cells and the role of upregulation of adhesion molecules with which circulating cells might interact remains poorly understood, but several recognized pathways including the SDF-1/CXCR4 axis and CD44 seem to be involved (reviewed in Reference 80).121, 122, 123 Recipient immune responses also play significant yet poorly characterized roles in retention of cells in lung.89 Commonly used approaches of sex-mismatched transplantation or cell administration may also result in clearance of cells.89 The timing of cell administration after lung injury can also influence recruitment and phenotypic conversion. Systemic administration of MSCs 4 hours after lung irradiation resulted in apparent engraftment of cells as epithelial and vascular endothelial cells.124 However, MSCs administered at later time points seemed to engraft as interstitial cells and to participate in the development of fibrosis.124, 125 As with engraftment, several factors including age of donor or recipient, type of cell administered, and route of administration might affect recruitment to lung.
Once lodged in or recruited to the lung, the mechanisms by which stem or progenitor cells might be induced to acquire phenotype of lung epithelial, interstitial, or vascular endothelial cells remain poorly understood. In vitro studies continue to demonstrate that soluble factors released from lung epithelial cells or from injured lung homogenates can induce expression of lung epithelial markers in several types of marrow-derived cells, possibly through activation of β-catenin signaling pathways.7, 126, 127 One novel mechanism of inducing phenotypic change might involve release of membrane-derived microvesicles, a recently appreciated means of intercellular communication that involves horizontal transfer of mRNA and proteins between cells.128, 129
Immunomodulation of lung diseases by mesenchymal stem cells
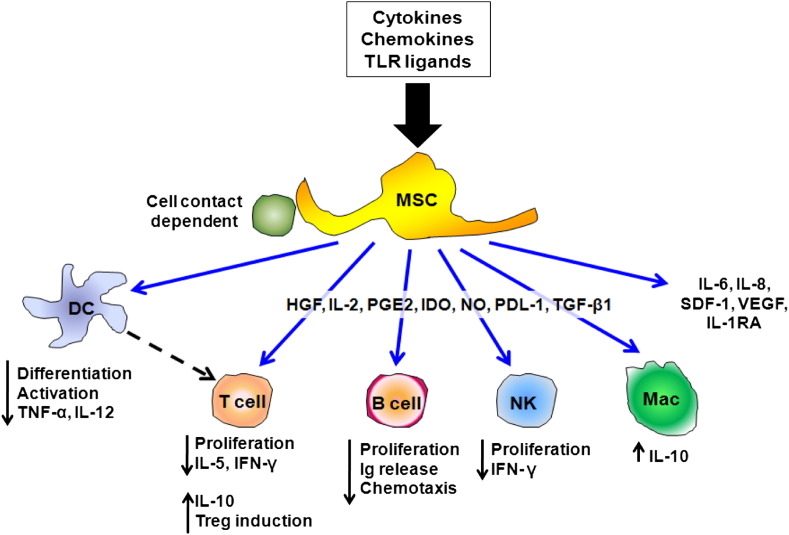
MSCs were first described in 1968 as an adherent, clonogenic, nonphagocytic, and fibroblastic-like population of bone marrow cells.130 Since then they have been isolated from many other tissues including adipose tissues, cord blood, placenta, and amniotic fluid.59 MSCs are multipotent and can differentiate into cells of mesodermal, endodermal, and ectodermal lineages.80 Furthermore, these cells also have the ability to regulate hematopoietic cells and to secrete multiple regulatory molecules such as growth factors and anti-inflammatory cytokines that can modulate immune responses.131 The mechanisms of MSC actions on inflammatory and immune cells are not well understood but likely involve both secretion of soluble mediators as well as cell–cell contact (Fig 4 ). Although the frequency of MSCs in the adult bone marrow is low (less than 0.1%); once isolated, MSCs can be expanded ex vivo, which makes it possible to manufacturer these cells for potential therapeutic purposes. Furthermore, MSCs can easily be transduced or genetically manipulated to deliver or to secrete selected disease-modifying molecules. Moreover, MSCs also seem to be relatively immunoprivileged because of their low constitutive expression of major histocompatibility complex (MHC) type 1 and lack of constitutive expression of MHC type 2 and the costimulatory molecules CD80, CD86, and CD40.132 This allows administration of allogeneic MSCs without significant host responses. Overall, these properties of MSCs make them an attractive therapeutic tool as a vector for treatment delivery or as immunomodulatory agents. As such, MSCs have now been used in clinical trials for inflammatory and autoimmune diseases such as graft versus host and Crohn’s diseases as well as in acute myocardial infarction.133 As will be discussed, immunomodulation and amelioration of lung injury has now been demonstrated in a variety of mouse models and suggests the most direct translational route toward clinical trials of cell therapies in lung diseases. As such, a trial of allogeneic MSCs in patients with moderate–severe chronic obstructive pulmonary disease has been started in the United States.
Fig 4.
Schematic illustrating the range of in vitro immune-modulating effects described for MSCs. DC = dendritic cell; HGF = hepatocyte growth factor; IDO = indoleamine 2,3-dioxygenase; IFN-γ = interferon γ; Ig = immunoglobulin; IL = interleukin; IL-1RA = interleukin-1 receptor antagonist; Mac = macrophage; NK = natural killer; PGE2 = prostaglandin E-2; SDF-1 = stem-cell-derived factor 1; TNF-α = tumor necrosis factor-α; TGF-β1 = transforming growth factor- β1; TLR = toll-like receptor; and VEGF = vascular endothelial growth factor. (Color version of figure is available online.)
Stem Cell and Cell Therapy Approaches for Specific Lung Diseases
Acute lung injury (ALI)
A growing number of studies have demonstrated compelling data on the beneficial effects of MSCs in mouse models of ALI including acute bacterial pneumonia, administration of endotoxin or bleomyicn, hyperoxia exposure, and cecal ligation and puncture (CLP)-induced sepsis.7, 8, 9, 10, 11, 12, 13, 15, 134, 135 Other available animal models of ALI have been used to evaluate for pathogenesis and pathophysiology of lung injury, such as acid-induced acute lung injury135, 136 and ventilator-induced lung injury.137 However, none of these has yet been tested for therapeutic efficacy of MSC.
In the endotoxin injury model, mice receive Escherichia coli lipopolysaccharide (LPS) either via intraperitoneally or intratracheally followed by MSC administration systemically or intratracheally at 1 hour or 4 hours after injury, respectively. The LPS administration results in acute lung inflammation that begins several hours peaks between 24 and 48 hours and resolves after that.134 Both systemic and intrapulmonary administration of bone-marrow-derived MSCs have been demonstrated to result in improved mortality, improvement of alveolar fluid clearance, and attenuation of inflammation and lung injury despite minimal, if any, engraftment of MSCs in the lung. Several paracrine mechanisms have been proposed for the MSC effects in this model including release of anti-inflammatory mediators such as IL-10, angiopoetin-1, and keratinocyte growth factor (KGF). Moreover, MSCs engineered to further express angiopoietin-1 potentiated the protective effect of MSCs.116 The MSCs themselves may be influenced by the specific inflammatory environment in the injured lung. For example, endotoxin can act on toll-like receptor 4 (TLR-4) expressed by the MSCs resulting in increased production of cyclooxygenase-2 and subsequent increased production of the anti-inflammatory agent prostaglandin E2 by the MSCs.15 Similar anti-inflammatory effects of MSCs have been found in endotoxin-treated isolated perfused human lungs.138 Another interesting study performed by Zhao et al showed that the protection of lung injury prevented endothelial leakage by strengthening of the endothelial adherent junction barrier through activation of RhoGTPase Cdc42.139 Altogether, these studies demonstrate the potent immunomodulatory effect of MSCs in mouse and isolated human lung models of acute lung injury and provide important preclinical data for potential clinical trials of allogeneic MSCs in ALI and ARDS.
Fibrotic lung diseases
Idiopathic pulmonary fibrosis (IPF) is a devastating chronic progressive pulmonary disease with high morbidity and mortality, and no effective treatment for this disease has been identified.140 IPF is now considered to be caused by pro-inflammatory and noninflammatory pathways that lead to chronic epithelial injury. This triggers cellular apoptosis and a dysregulated immune response characterized by, among other things, elicitation of Th2 cytokines,141, 142, 143 which promotes the expression of pro-fibrotic factors like transforming growth factor beta.144 Other fibrotic lung diseases are a consequence of underlying auto_underimmune and inflammatory conditions through as yet unclear mechanisms. Several animal models of fibrotic lung diseases have been developed to study the pathophysiology and pathogenesis of human fibrotic lung diseases.145 The best characterized model is the bleomycin model of pulmonary fibrosis. The drug was initially used as an anti-neoplastic agent; however, its use was limited by pulmonary toxicity and development of pulmonary fibrosis.145 It can be administered via intraperitoneal, intravenous, subcutaneous, or intratracheal routes.145 Other fibrotic lung disease models that have been characterized are fluorescein-isothiocyanate-induced fibrosis,146 irradiation-induced fibrosis,147 silica,148 asbestos-induced fibrosis,149 various transgenic mice including T-bet defcient mice,150 and viral models of chronic fibrosis.151
The murine model that has been most extensively evaluated for the protective or therapeutic effects of MSCs in fibrotic lung disease is intratracheal administration of bleomycin. The advantage of this model is that it requires only a single dose to result in injury and fibrosis, with fibrosis starting around day 14 and maximal responses around days 21–28.145 However, resolution of fibrosis can occur in mice after day 21.145 Systemic administration of MSCs 4 hours after bleomycin administration was demonstrated to reduce inflammation, fibrosis formation, and mortality at 14 days after injury, in part mediated by the IL-1α receptor antagonist released by the MSCs.1, 2 However, the protective effects of MSCs were abrogated when MSCs were given at day 7 after injury.1 In another study using asbestos-induced pulmonary fibrosis in mice, after total body irradiation and bone marrow transplantation, the chimeric mice were protected from development of lung injury and fibrosis as compared with nonchimeric mice.107 However, the protective effect of transplantation is most likely from changes occurred after irradiation and bone marrow transplantation. Another study demonstrated a reduction in collagen deposition in bleomycin-induced lung fibrosis in mice after administration of MSC or lineage-negative HSCs engineered to overexpress KGF.152
In contrast, another bone-marrow-derived stem cell population, circulating fibrocytes, have been implicated in the pathogenesis of several lung diseases in both mouse and clinical models including pulmonary fibrosis, pulmonary hypertension, and the subepithelial fibrosis that can develop in severe asthma, as well as in clinical bronchiolitis obliterans in lung and bone marrow transplant patients.153, 154, 155, 156 Further levels of circulating fibrocytes may be an indicator of worse prognosis in both asthma and IPF.157, 158 As such, based on current available data in the mouse models, cell-based therapy approaches may be less effective in established fibrotic lung diseases. However, studies using cell therapy as preventive modality for development of fibrosis (ie, after irradiation-induced lung injury) are needed. For example, administering marrow-derived cells early after radiation-induced lung injury in mice was protective, whereas administering the cells later resulted in the cells contributing to the radiation-induced damage.124 One potential therapeutic avenue for fibrotic lung diseases is to inhibit the fibrocytes. Alternatively, these cells might be excellent vectors to deliver therapeutic agents directly at the site of fibrosis (ie, fibroblastic foci).
Chronic obstructive pulmonary disease (COPD)
COPD is currently the fourth leading cause of death in worldwide.159 Approximately 20% of patients with COPD present with emphysema that is characterized by destruction of terminal bronchioles and alveolar walls causing enlargement of air spaces. Patients generally die from progressive respiratory failure, and despite advances in the symptomatic treatment of emphysema, no cure remains.
Available experimental mouse models of emphysema include transgenic and knockout models and exposure to environmental agents such as tobacco smoke or intratracheal administration of degradative enzymes such as elastase.160, 161 Several studies have evaluated the role of stem cells on remodeling of the lung in mouse emphysema models using elastase-treated and transgenic (Tight-skin or Tsk) mice.161, 162, 163 These studies used transplantation of wild-type mouse bone marrow cells into emphysematous mice and demonstrated that there were decreased emphysematous structural changes in chimeric mice. In particular, a study in Tsk mice that had already developed emphysematous changes at the time of transplantation demonstrated a reversal of these changes such that the lungs resembled those of age-controlled normal mice with nonsignificant engraftment of donor-derived cells in chimeric mice lungs.87 Another study in rats was done to specifically evaluate the role of bone marrow stromal cells in irradiation and papain-induced emphysema.14 In this study, plastic adherent bone marrow cells (bone-marrow-derived stromal cells) were systemically administered immediately after irradiation (7.5 Gy total body irradiation) and intratracheal instillation of 8% papain (0.05 mL/100 g body weight). Lungs were harvested after 28 days and demonstrated a reduction in air-space enlargement, as quantified by mean linear intercept, and apoptotic cells in the bone-marrow-derived stromal cells treated group as compared with the controlled irradiated, papain-treated group.14 Minimal engraftment by donor-derived cells was observed. These data suggest that bone-marrow-derived stromal cells can protect against progression of emphysema. The potential mechanism is likely through paracrine effects resulting in decreased alveolar apoptosis after injury. However, there is much yet to be learned about anti-inflammatory effects of bone-marrow-derived and other stem cell populations in models of COPD.
In a phase I, double-blind, placebo-controlled trial of PROCHYMAL™ (ex vivo cultured adult human mesenchymal stem cells) conducted by Osiris Therapeutics Inc. (Columbia, Md) in patients with acute myocardial infarction, an improvement in both forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC) was noted in treated patients.164 Although the mechanisms of improvement in pulmonary function in this patient population are not yet well understood, these observations have stimulated a multicenter, double-blind, placebo-controlled phase II trial of PROCHYMAL™ (Osiris Therapeutics) for patients with moderate–severe COPD (FEV1/FVC < 0.70, 30% ≤ FEV1 ≤ 70%), which was initiated in May 2008. The primary goal of the trial is to determine the safety of MSC infusions in patients with lung disease. The secondary goal is initial estimation of the potential efficacy of MSCs for decreasing the chronic inflammation associated with COPD, thus improving both pulmonary function and quality of life. The trial has recruited 62 patients in 6 participating U.S. sites. In the 6-month interim data analysis, no infusional toxicities or significant adverse events were reported. Notably, a significant decrease in the circulating inflammatory marker C-reactive protein, which is commonly increased in COPD patients, was noted in treated patients as was a trend towards improvement in quality of life indicators.165 The trial ends in the fall of 2010, and further results will be forthcoming.
Cystic fibrosis
CF is an autosomal recessive disease caused by mutations in the gene encoding for the CF transmembrane CFTR resulting in abnormal ion transport across the respiratory epithelium leading to CF lung disease. The initial cell therapy approach for CF in mouse models was based on the concept of replacing defective airway epithelial cells with wild-type donor cells containing the correct CFTR gene. However, only rare engraftment (<0.025% of total airway epithelial cells) of airway and alveolar epithelium with adult bone-marrow- or cord-blood-derived stem cells with corresponding CFTR protein expression has been found (Fig 5 ).17, 80, 104, 105 This was despite using naphthalene to denude the airway epithelium and conceivably enhance donor cell engraftment. In one study, intestinal engraftment was also assessed, and despite being rare, functional CFTR activity was detected by potential difference measurements in the rectal mucosa of recipient mice. This observation remains unexplained, as it is difficult to reconcile the rare engrafted cell observed with a physiologic change in potential difference. Transplant of whole marrow into 1-day-old CFTR knockout mice also did not increase the number of donor origin cells engrafted as either respiratory or intestinal epithelium.104 These studies suggest that systemic administration of adult bone-marrow- or cord-blood-derived progenitor cells do not result in significant structural engraftment as CFTR-expressing cells as either airway or intestinal epithelium in mouse models. Although not yet investigated in CF mouse models, several recent studies have also found only low levels of airway epithelial engraftment after direct airway administration of donor marrow-derived cells in mice.8, 103 Overall these observations suggest that, unless significant advances are made in understanding the mechanisms by which cells are recruited to airway epithelium and induced to undergo phenotypic conversion to functional airway epithelial cells, correction of CF lung disease by structural engraftment of adult marrow- or cord-blood-derived stem cells is not likely at present. In contrast, as CF lung disease is characterized by chronic neutrophilic airway inflammation and high expression of multiple proinflammatory cytokines,166 a potential therapeutic role of MSCs to abate inflammation in CF lung disease is suggested. However, there is no available information concerning the immunomodulatory effects of MSC in CF lungs. This would seem a promising area for study.
Fig 5.
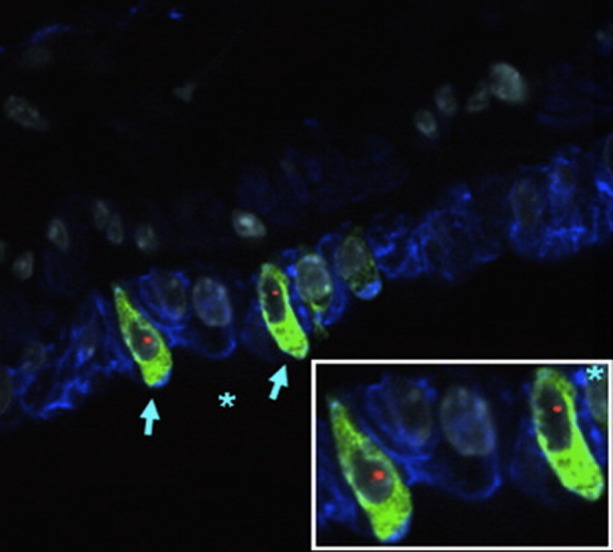
CFTR expressed cells can be detected in female CFTR knockout mouse lungs after transplantation with male GFP stromal marrow cells. Donor-derived (Y chromosome, red), CFTR-positive (green), and cytokeratin-positive (blue) cells are indicated by light blue arrows in airway walls of lungs assessed 1 week after transplantation. Inset is a higher power view of the area marked by asterisk. Original magnification: ×1000. Figure reproduced with permission from Loi et al.17 (Color version of figure is available online.)
Asthma
Asthma is characterized by reversible airflow obstruction, hyper-responsiveness of the airway to smooth muscle agonists, and airway inflammation. In most cases of asthma, the patients remain symptomatically controlled by available medications including corticosteroids and bronchodilators. However, approximately 5% of asthmatics are refractory to conventional treatment and suffer substantial morbidity and mortality. The ability of MSCs to modulate immune systems has led to increasing interest in using MSCs as a potential therapeutic modality for severe refractory asthma. Several laboratories have now demonstrated that MSCs can ameliorate allergic airways inflammation in mice.16, 167, 168, 169 However the optimal dose, route of administration, timing of administration, and longevity of MSCs effects are not yet known.
Pulmonary vascular diseases
Pulmonary hypertension (PH) is a disease characterized by pulmonary vascular remodeling resulting in pulmonary artery obstruction that, in turn, leads to elevation of pulmonary arterial pressure, right-heart dysfunction, and ultimately, progressive respiratory failure. MSCs-based therapy had been explored as a potential therapeutic modality in this devastating disease.
Several rodent models of PH are available, but the most commonly used involve administration of monocrotaline (MCT) or exposure to chronic hypoxia. Systematic or intratracheal MSC administration can attenuate MCT-induced increase in pulmonary artery pressure and improve pulmonary vascular resistance.19, 20 The protective effects of MSCs in this model occurred even when cells were administered systemically 7 days after MCT administration.21 MSCs express several growth factors including vascular endothelial growth factors that promote neovascularization, and this might be one of the mechanisms by which MSCs protect against development of PH. Furthermore, EPCs can be transduced to express pro-angiogenic factors such as endothelial nitric oxide synthetase (eNOS) or inhibitors of smooth muscle cell proliferation such as calcitonin gene related peptide, which further improve right ventricle function.21 Several studies in mice and dogs have demonstrated a role for exogenously administered EPCs in vasculogenesis and vascular repair in experimental models of pulmonary hypertension.108, 170, 171, 172 EPCs can also preferentially localize to areas of injured lung after systemic administration and may also have paracrine effects to decrease inflammation.173 As such, two pilot trials of autologous EPC administration for primary pulmonary hypertension conducted at Zhejiang University, Hangzhou, China, in both adult and pediatric patients demonstrated an increased 6-minute walk capacity and improved hemodynamic variables, including mean pulmonary artery pressure, pulmonary vascular resistance, and cardiac output, 12 weeks after systemic administration of autologous EPCs with conventional therapy compared with patients receiving conventional therapy alone.110, 174 Importantly, no adverse effects of EPC administration were noted, although long-term follow-up is pending. A therapeutic trial of administration of autologous EPCs transduced to express eNOS for patients with pulmonary hypertension, the Pulmonary Hypertension and Cell Therapy (PHaCeT) trial has been initiated at St. Michael’s Hospital in Toronto, Ontario, Canada, and the Jewish General Hospital in Montreal, Quebec, Canada. As of March 2010, the PHACeT trial has completed enrollment of the first 2 dose-panels with 3 patients receiving a total of 7 million early growth EPCs transfected to overexpress human eNOS in panel 1 and three more patients receiving 23 million cells in panel 2 (information courtesy of Duncan Stewart, MD, FRCPC). The cell delivery procedure was well tolerated, and there were no safety concerns. Notably, the first 6 patients showed a remarkable reduction in total pulmonary vascular resistance during the course of the 3-day delivery period, which might represent the effect of increased nitric oxide (NO) release by the engineered EPCs within the pulmonary microcirculation. The trial’s Data Safety Monitoring Board has approved moving to panel 3, which calls for a total of 50 million cells, again in 3 divided doses over 3 days. Completion of the third dose panel will be followed by enrollment of an additional 3 patients at the highest tolerated cell dose, which should provide sufficient support to move forward with the design of a randomized controlled trial that can assess potential efficacy of this cell therapy approach in pulmonary artery hypertension.
Lung cancer
In addition to the possibility of endogenous lung progenitor cells functioning as cancer stem cells, bone-marrow-derived or circulating MSCs, EPCs, and fibrocytes may each contribute to development of primary and metastatic lung and other malignancies in mice, in part by providing a supportive stroma for the cancers and/or by participating in tumor vascularization.175, 176, 177, 178, 179 In contrast, MSCs and EPCs have been demonstrated to home to areas of tumor development and EPCs and MSCs, as well as HSCs, engineered to release anti-tumor or pro-apoptotic agents, have been used to suppress tumor growth in mouse tumor models.180, 181, 182, 183, 184, 185, 186 Cell-based therapies may thus be powerful new tools for lung cancer. In parallel, increased numbers of circulating EPCs also portended worse survival among those with non–small-cell lung cancer. Thus, circulating stem or progenitor cells may serve as useful biomarkers for lung cancer response to therapies.
Lung tissue bioengineering
Most recently, focus has been on exploration of 3-dimensional culture systems and bioengineering approaches to generate functional lung tissue ex vivo and in vivo.187, 188 These approaches have been increasingly successfully used in regeneration of other tissues including skin, vasculature, cartilage, and bone. Notably, MSCs isolated from amniotic fluid, umbilical cord blood, adipose tissues, or bone marrow can be seeded on biodegradable polyglycolic acid or other biosynthetic scaffolds and can generate tracheal cartilage for use in repair of congenital tracheal defects, as well as tendon tissue for use in congenital diaphragmatic defects.189, 190 Studies in animal models and a recent clinical investigation suggest safety and efficacy, and clinical trials in neonates with congenital tracheal or diaphragmatic defects are planned.189, 190, 191 Most recently these approaches have resulted in successful clinical use of a bioengineered airway.192
The complex 3-dimensional architecture of the lung makes engineering functional lung parenchyma ex vivo a more difficult task. Studies both in vitro and in vivo using mixed fetal lung cells cultured in a 3-dimensional glycosaminoglycan or other type of scaffolds resulted in formation of alveolar-like structures in the scaffold.188, 193 Furthermore, stimulation of murine fetal lung cells in polymer scaffolds with different isoforms of fibroblast growth factor resulted in different patterns of development demonstrating the power of 3-dimensional culture systems to evaluate lung development and repair.194 Fetal rat lung cells cultured in a biodegradable gelatin sponge, and subsequently injected into normal rat lungs, induced formation of branching, sacculated epithelial structures reminiscent of lung parenchymal architecture.193 Mixed fetal murine epithelial cells admixed with Matrigel and injected subcutaneously into the abdominal wall of adult mice demonstrated cells that expressed pro-surfactant protein C (pro-SPC) after 1 week.195 These studies demonstrate the potential of in vivo lung tissue generation using mixed populations of fetal lung cells. However, using fetal lung cells is not a practical approach, and lung tissue engineering with stem or progenitor cells is a more practical option.
However, there are few studies as yet evaluating whether stem or progenitor cells isolated from adult bone marrow, cord blood, or other sources can also comparably form airway or alveolar-like structures when cultivated in a 3-dimensional matrix or other scaffolding material and whether stem or progenitor cells cultured in such fashion can be used for functional lung regeneration in vivo. A population of cells described as adult lung somatic progenitor cells isolated from adult sheep lungs cultured in synthetic polymer constructs resulted in expression of airway and alveolar epithelial markers by the cells.196 Structures resembling lung airways and parenchyma developed when impregnated constructs were implanted subcutaneously in nude mice or inserted into the wound cavity after wedge lung resection in sheep. Adipose-derived MSCs, cultured ex vivo in sheets of polyglycolic acid and then applied to wound edges after lung volume reduction surgery in rats, accelerated alveolar and vascular regeneration.197 Successful engineering of a portion of a bronchus and subsequent transplantion into a patient with bronchial stenosis has also been reported.198 In this particular study, autologous bone-marrow-derived MSCs were cultured and differentiated into chrondroblasts prior to seeding onto a de-cellularized allograft section of bronchus along with the patient’s epithelial cells. By applying appropriate growth factors and physical force, a functional bronchus resulted and was used in transplantation. These studies demonstrate that MSCs and other stem cell populations can be used in ex vivo tissue bioengineering and herald a potential new era for lung transplantation.
Conclusion
A continuing rapid accumulation of data in both animal models and in clinical trials suggests that cell-based therapies using adult tissue-derived stem/progenitor cells may be potential therapeutic strategies for lung repair and remodeling after injury and as a source of cells for ex vivo lung tissue engineering. In parallel, further understanding of the role of endogenous lung progenitor cells will provide insight into mechanisms of lung development and repair after injury and may provide novel therapeutic strategies. It is hoped new research programs will provide further understanding of mechanisms of repair of lung injury and provide a sound scientific basis for therapeutic use of stem and cell therapies in lung diseases.
Footnotes
Supported by NHLBIHL087274 and HL081289 research grants from the Cystic Fibrosis Foundation and the American Lung Association and NCRR COBREP20 RR-155557.
References
- 1.Ortiz L.A., Gambelli F., McBride C. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci U S A. 2003;100:8407–8411. doi: 10.1073/pnas.1432929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rojas M., Xu J., Woods C.R. Bone marrow-derived mesenchymal stem cells in repair of the injured lung. Am J Respir Cell Mol Biol. 2005;33:145–152. doi: 10.1165/rcmb.2004-0330OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ortiz L.A., Dutreil M., Fattman C. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc Natl Acad Sci U S A. 2007;104:11002–11007. doi: 10.1073/pnas.0704421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee S.H., Jang A.S., Kim Y.E. Modulation of cytokine and nitric oxide by mesenchymal stem cell transfer in lung injury/fibrosis. Respir Res. 2010;11:16. doi: 10.1186/1465-9921-11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moodley Y., Atienza D., Manuelpillai U. Human umbilical cord mesenchymal stem cells reduce fibrosis of bleomycin-induced lung injury. Am J Pathol. 2009;175:303–313. doi: 10.2353/ajpath.2009.080629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumamoto M., Nishiwaki T., Matsuo N., Kimura H., Matsushima K. Minimally cultured bone marrow mesenchymal stem cells ameliorate fibrotic lung injury. Eur Respir J. 2009;34:740–748. doi: 10.1183/09031936.00128508. [DOI] [PubMed] [Google Scholar]
- 7.Xu J., Woods C.R., Mora A.L. Prevention of endotoxin-induced systemic response by bone marrow-derived mesenchymal stem cells in mice. Am J Physiol Lung Cell Mol Physiol. 2007;293:L131–L141. doi: 10.1152/ajplung.00431.2006. [DOI] [PubMed] [Google Scholar]
- 8.Gupta N., Su X., Popov B., Lee J.W., Serikov V., Matthay M.A. Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J Immunol. 2007;179:1855–1863. doi: 10.4049/jimmunol.179.3.1855. [DOI] [PubMed] [Google Scholar]
- 9.Mei S.H., McCarter S.D., Deng Y., Parker C.H., Liles W.C., Stewart D.J. Prevention of LPS-induced acute lung injury in mice by mesenchymal stem cells overexpressing angiopoietin 1. PLoS Med. 2007;4:e269. doi: 10.1371/journal.pmed.0040269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu J., Qu J., Cao L. Mesenchymal stem cell-based angiopoietin-1 gene therapy for acute lung injury induced by lipopolysaccharide in mice. J Pathol. 2008;214:472–481. doi: 10.1002/path.2302. [DOI] [PubMed] [Google Scholar]
- 11.van Haaften T., Byrne R., Bonnet S. Airway delivery of mesenchymal stem cells prevents arrested alveolar growth in neonatal lung injury in rats. Am J Respir Crit Care Med. 2009;180:1131–1142. doi: 10.1164/rccm.200902-0179OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aslam M., Baveja R., Liang O.D. Bone marrow stromal cells attenuate lung injury in a murine model of neonatal chronic lung disease. Am J Respir Crit Care Med. 2009;180:1122–1130. doi: 10.1164/rccm.200902-0242OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang Y.S., Oh W., Choi S.J. Human umbilical cord blood-derived mesenchymal stem cells attenuate hyperoxia-induced lung injury in neonatal rats. Cell Transplant. 2009;18:869–886. doi: 10.3727/096368909X471189. [DOI] [PubMed] [Google Scholar]
- 14.Zhen G., Liu H., Gu N., Zhang H., Xu Y., Zhang Z. Mesenchymal stem cells transplantation protects against rat pulmonary emphysema. Front Biosci. 2008;13:3415–3422. doi: 10.2741/2936. [DOI] [PubMed] [Google Scholar]
- 15.Nemeth K., Leelahavanichkul A., Yuen P.S. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15:42–49. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goodwin M, Eisenhauer PL, Sueblinvong V, Weiss DJ. Systemic administration of mesenchymal stem cells abrogates allergic airways inflammation by inhibiting CD4 T cell Th2 phenotype development. ATS 2009. San Diego, CA.
- 17.Loi R., Beckett T., Goncz K.K., Suratt B.T., Weiss D.J. Limited restoration of cystic fibrosis lung epithelium in vivo with adult bone marrow-derived cells. Am J Respir Crit Care Med. 2006;173:171–179. doi: 10.1164/rccm.200502-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leblond A.L., Naud P., Forest V. Developing cell therapy techniques for respiratory disease: intratracheal delivery of genetically engineered stem cells in a murine model of airway injury. Hum Gene Ther. 2009;20:1329–1343. doi: 10.1089/hum.2009.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Umar S., de Visser Y.P., Steendijk P. Allogenic stem cell therapy improves right ventricular function by improving lung pathology in rats with pulmonary hypertension. Am J Physiol Heart Circ Physiol. 2009;297:H1606–H1616. doi: 10.1152/ajpheart.00590.2009. [DOI] [PubMed] [Google Scholar]
- 20.Baber S.R., Deng W., Master R.G. Intratracheal mesenchymal stem cell administration attenuates monocrotaline-induced pulmonary hypertension and endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2007;292:H1120–H1128. doi: 10.1152/ajpheart.00173.2006. [DOI] [PubMed] [Google Scholar]
- 21.Kanki-Horimoto S., Horimoto H., Mieno S. Implantation of mesenchymal stem cells overexpressing endothelial nitric oxide synthase improves right ventricular impairments caused by pulmonary hypertension. Circulation. 2006;114:I181–I185. doi: 10.1161/CIRCULATIONAHA.105.001487. [DOI] [PubMed] [Google Scholar]
- 22.Snyder J.C., Teisanu R.M., Stripp B.R. Endogenous lung stem cells and contribution to disease. J Pathol. 2009;217:254–264. doi: 10.1002/path.2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rawlins E.L. Lung epithelial progenitor cells: lessons from development. Proc Am Thorac Soc. 2008;5:675–681. doi: 10.1513/pats.200801-006AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rawlins E.L., Hogan B.L. Epithelial stem cells of the lung: privileged few or opportunities for many? Development. 2006;133:2455–2465. doi: 10.1242/dev.02407. [DOI] [PubMed] [Google Scholar]
- 25.Chen H., Matsumoto K., Stripp B.R. Bronchiolar progenitor cells. Proc Am Thorac Soc. 2009;6:602–606. doi: 10.1513/pats.200907-078RM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu X., Engelhardt J.F. The glandular stem/progenitor cell niche in airway development and repair. Proc Am Thorac Soc. 2008;5:682–688. doi: 10.1513/pats.200801-003AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim C.F., Jackson E.L., Woolfenden A.E. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121:823–835. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 28.Nolen-Walston R.D., Kim C.F., Mazan M.R. Cellular kinetics and modeling of bronchioalveolar stem cell response during lung regeneration. Am J Physiol Lung Cell Mol Physiol. 2008;294:L1158–L1165. doi: 10.1152/ajplung.00298.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zemke A.C., Snyder J.C., Brockway B.L. Molecular staging of epithelial maturation using secretory cell-specific genes as markers. Am J Respir Cell Mol Biol. 2009;40:340–348. doi: 10.1165/rcmb.2007-0380OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Teisanu R.M., Lagasse E., Whitesides J.F., Stripp B.R. Prospective isolation of bronchiolar stem cells based upon immunophenotypic and autofluorescence characteristics. Stem Cells. 2009;27:612–622. doi: 10.1634/stemcells.2008-0838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McQualter J.L., Brouard N., Williams B. Endogenous fibroblastic progenitor cells in the adult mouse lung are highly enriched in the Sca-1 positive cell fraction. Stem Cells. 2009;27:623–633. doi: 10.1634/stemcells.2008-0866. [DOI] [PubMed] [Google Scholar]
- 32.McQualter J.L., Yuen K., Williams B., Bertoncello I. Evidence of an epithelial stem/progenitor cell hierarchy in the adult mouse lung. Proc Natl Acad Sci U S A. 2010;107:1414–1419. doi: 10.1073/pnas.0909207107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Giangreco A., Arwert E.N., Rosewell I.R., Snyder J., Watt F.M., Stripp B.R. Stem cells are dispensable for lung homeostasis but restore airways after injury. Proc Natl Acad Sci U S A. 2009;106:9286–9291. doi: 10.1073/pnas.0900668106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murphy J., Summer R., Fine A. Stem cells in airway smooth muscle: state of the art. Proc Am Thorac Soc. 2008;5:11–14. doi: 10.1513/pats.200704-052VS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hennrick K.T., Keeton A.G., Nanua S. Lung cells from neonates show a mesenchymal stem cell phenotype. Am J Respir Crit Care Med. 2007:1751158–1751164. doi: 10.1164/rccm.200607-941OC. [DOI] [PubMed] [Google Scholar]
- 36.Lama V.N., Smith L., Badri L. Evidence for tissue-resident mesenchymal stem cells in human adult lung from studies of transplanted allografts. J Clin Invest. 2007;117:989–996. doi: 10.1172/JCI29713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karoubi G., Cortes-Dericks L., Breyer I., Schmid R.A., Dutly A.E. Identification of mesenchymal stromal cells in human lung parenchyma capable of differentiating into aquaporin 5-expressing cells. Lab Invest. 2009;89:1100–1114. doi: 10.1038/labinvest.2009.73. [DOI] [PubMed] [Google Scholar]
- 38.Willis B.C., Borok Z. TGF-beta-induced EMT: mechanisms and implications for fibrotic lung disease. Am J Physiol Lung Cell Mol Physiol. 2007;293:L525–L534. doi: 10.1152/ajplung.00163.2007. [DOI] [PubMed] [Google Scholar]
- 39.Willis B.C., duBois R.M., Borok Z. Epithelial origin of myofibroblasts during fibrosis in the lung. Proc Am Thorac Soc. 2006;3:377–382. doi: 10.1513/pats.200601-004TK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Demayo F., Minoo P., Plopper C.G., Schuger L., Shannon J., Torday J.S. Mesenchymal-epithelial interactions in lung development and repair: are modeling and remodeling the same process? Am J Physiol Lung Cell Mol Physiol. 2002;283:L510–L517. doi: 10.1152/ajplung.00144.2002. [DOI] [PubMed] [Google Scholar]
- 41.Park K.S., Wells J.M., Zorn A.M. Transdifferentiation of ciliated cells during repair of the respiratory epithelium. Am J Respir Cell Mol Biol. 2006;34:151–157. doi: 10.1165/rcmb.2005-0332OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu R., Harper R., Kao C.Y. New insights into airway mucous cell differentiation. J Organ Dysfunct. 2006;2:30–36. [Google Scholar]
- 43.Wang J., Edeen K., Manzer R. Differentiated human alveolar epithelial cells and reversibility of their phenotype in vitro. Am J Respir Cell Mol Biol. 2007;36:661–668. doi: 10.1165/rcmb.2006-0410OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hollande E., Cantet S., Ratovo G., Daste G., Brémont F., Fanjul M. Growth of putative progenitors of type II pneumocytes in culture of human cystic fibrosis alveoli. Biol Cell. 2004;96:429–441. doi: 10.1016/j.biolcel.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 45.Pan J.C., Bear C., Farragher S., Cutz E., Yeger H. Cystic fibrosis transmembrane conductance regulator modulates neurosecretory function in pulmonary neuroendocrine cell-related tumor cell line models. Am J Respir Cell Mol Biol. 2002;27:553–560. doi: 10.1165/rcmb.4843. [DOI] [PubMed] [Google Scholar]
- 46.Yeger H., Pan J., Fu X.W., Bear C., Cutz E. Expression of CFTR and Cl(−) conductances in cells of pulmonary neuroepithelial bodies. Am J Physiol Lung Cell Mol Physiol. 2001;281:L713–L721. doi: 10.1152/ajplung.2001.281.3.L713. [DOI] [PubMed] [Google Scholar]
- 47.Pan J., Luk C., Kent G., Cutz E., Yeger H. Pulmonary neuroendocrine cells, airway innervation, and smooth muscle are altered in CFTR null mice. Am J Respir Cell Mol Biol. 2006;35:320–326. doi: 10.1165/rcmb.2005-0468OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ling T.Y., Kuo M.D., Li C.L. Identification of pulmonary Oct-4+ stem/progenitor cells and demonstration of their susceptibility to SARS coronavirus (SARS-CoV) infection in vitro. Proc Natl Acad Sci U S A. 2006;103:9530–9535. doi: 10.1073/pnas.0510232103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ratajczak M.Z., Machalinski B., Wojakowski W., Ratajczak J., Kucia M. A hypothesis for an embryonic origin of pluripotent Oct-4(+) stem cells in adult bone marrow and other tissues. Leukemia. 2007;21:860–867. doi: 10.1038/sj.leu.2404630. [DOI] [PubMed] [Google Scholar]
- 50.Jakiela B., Brockman-Schneider R., Amineva S., Lee W.M., Gern J.E. Basal cells of differentiated bronchial epithelium are more susceptible to rhinovirus infection. Am J Respir Cell Mol Biol. 2008;38:517–523. doi: 10.1165/rcmb.2007-0050OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu Z.J., Zhuge Y., Velazquez O.C. Trafficking and differentiation of mesenchymal stem cells. J Cell Biochem. 2009;106:984–991. doi: 10.1002/jcb.22091. [DOI] [PubMed] [Google Scholar]
- 52.Blanpain C., Horsley V., Fuchs E. Epithelial stem cells: turning over new leaves. Cell. 2007;128:445–458. doi: 10.1016/j.cell.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morrison S.J. Cancer stem cells. Clin Adv Hematol Oncol. 2005;3:171–172. [PubMed] [Google Scholar]
- 54.Reya T., Morrison S.J., Clarke M.F., Weissman I.L. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 55.Giangreco A., Groot K.R., Janes S.M. Lung cancer and lung stem cells: strange bedfellows? Am J Respir Crit Care Med. 2007;75:547–553. doi: 10.1164/rccm.200607-984PP. [DOI] [PubMed] [Google Scholar]
- 56.Park C.Y., Tseng D., Weissman I.L. Cancer stem cell-directed therapies: recent data from the laboratory and clinic. Mol Ther. 2009;17:219–230. doi: 10.1038/mt.2008.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alison M.R., Lebrenne A.C., Islam S. Stem cells and lung cancer: future therapeutic targets? Expert Opin Biol Ther. 2009;9:1127–1141. doi: 10.1517/14712590903103803. [DOI] [PubMed] [Google Scholar]
- 58.Ho M.M., Ng A.V., Lam S., Hung J.Y. Side population in human lung cancer cell lines and tumors is enriched with stem-like cancer cells. Cancer Res. 2007;67:4827–4833. doi: 10.1158/0008-5472.CAN-06-3557. [DOI] [PubMed] [Google Scholar]
- 59.Bertolini G., Roz L., Perego P. Highly tumorigenic lung cancer CD133+ cells display stem-like features and are spared by cisplatin treatment. Proc Natl Acad Sci U S A. 2009;106:16281–16286. doi: 10.1073/pnas.0905653106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sabisz M., Skladanowski A. Cancer stem cells and escape from drug-induced premature senescence in human lung tumor cells: implications for drug resistance and in vitro drug screening models. Cell Cycle. 2009;8:3208–3217. doi: 10.4161/cc.8.19.9758. [DOI] [PubMed] [Google Scholar]
- 61.Levina V., Marrangoni A., Wang T. Elimination of human lung cancer stem cells through targeting of the stem cell factor-c-kit autocrine signaling loop. Cancer Res. 2010;70:338–346. doi: 10.1158/0008-5472.CAN-09-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Salnikov A.V., Gladkich J., Moldenhauer G., Volm M., Mattern J., Herr I. CD133 is indicative for a resistance phenotype but does not represent a prognostic marker for survival of non-small cell lung cancer patients. Int J Cancer. 2010;126:950–958. doi: 10.1002/ijc.24822. [DOI] [PubMed] [Google Scholar]
- 63.Tian J., Mahmood R., Hnasko R., Locker J. Loss of Nkx2.8 deregulates progenitor cells in the large airways and leads to dysplasia. Cancer Res. 2006;66:10399–10407. doi: 10.1158/0008-5472.CAN-06-1564. [DOI] [PubMed] [Google Scholar]
- 64.Sicinski P., Zacharek S., Kim C. Duality of p27Kip1 function in tumorigenesis. Genes Dev. 2007;21:1703–1706. doi: 10.1101/gad.1583207. [DOI] [PubMed] [Google Scholar]
- 65.Watkins D.N., Berman D.M., Burkholder S.G., Wang B., Beachy P.A., Baylin S.B. Hedgehog signalling within airway epithelial progenitors and in small-cell lung cancer. Nature. 2003;422:313–317. doi: 10.1038/nature01493. [DOI] [PubMed] [Google Scholar]
- 66.Pei X.H., Bai F., Smith M.D., Xiong Y. p18Ink4c collaborates with Men1 to constrain lung stem cell expansion and suppress non-small-cell lung cancers. Cancer Res. 2007;67:3162–3170. doi: 10.1158/0008-5472.CAN-06-4517. [DOI] [PubMed] [Google Scholar]
- 67.Seo D.C., Sung J.M., Cho H.J. Gene expression profiling of cancer stem cell in human lung adenocarcinoma A549 cells. Mol Cancer. 2007;6:75. doi: 10.1186/1476-4598-6-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Regala R.P., Davis R.K., Kunz A., Khoor A., Leitges M., Fields A.P. Atypical protein kinase C{iota} is required for bronchioalveolar stem cell expansion and lung tumorigenesis. Cancer Res. 2009;69:7603–7611. doi: 10.1158/0008-5472.CAN-09-2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Samadikuchaksaraei A., Bishop A.E. Derivation and characterization of alveolar epithelial cells from murine embryonic stem cells in vitro. Methods Mol Biol. 2006;330:233–248. doi: 10.1385/1-59745-036-7:233. [DOI] [PubMed] [Google Scholar]
- 70.Rippon H.J., Polak J.M., Qin M., Bishop A.E. Derivation of distal lung epithelial progenitors from murine embryonic stem cells using a novel three-step differentiation protocol. Stem Cells. 2006;24:1389–1398. doi: 10.1634/stemcells.2005-0465. [DOI] [PubMed] [Google Scholar]
- 71.Denham M., Cole T.J., Mollard R. Embryonic stem cells form glandular structures and express surfactant protein C following culture with dissociated fetal respiratory tissue. Am J Physiol Lung Cell Mol Physiol. 2006;290:L1210–L1215. doi: 10.1152/ajplung.00427.2005. [DOI] [PubMed] [Google Scholar]
- 72.Samadikuchaksaraei A., Cohen S., Isaac K. Derivation of distal airway epithelium from human embryonic stem cells. Tissue Eng. 2006;12:867–875. doi: 10.1089/ten.2006.12.867. [DOI] [PubMed] [Google Scholar]
- 73.Wang D., Haviland D.L., Burns A.R., Zsigmond E., Wetsel R.A. A pure population of lung alveolar epithelial type II cells derived from human embryonic stem cells. Proc Natl Acad Sci U S A. 2007;104:4449–4454. doi: 10.1073/pnas.0700052104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Roszell B., Mondrinos M.J., Seaton A. Efficient derivation of alveolar type II cells from embryonic stem cells for in vivo application. Tissue Eng Part A. 2009;15:3351–3365. doi: 10.1089/ten.tea.2008.0664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Coraux C., Nawrocki-Raby B., Hinnrasky J. Embryonic stem cells generate airway epithelial tissue. Am J Respir Cell Mol Biol. 2005;32:87–92. doi: 10.1165/rcmb.2004-0079RC. [DOI] [PubMed] [Google Scholar]
- 76.Van Haute L., De Block G., Liebaers I., Sermon K., De Rycke M. Generation of lung epithelial-like tissue from human embryonic stem cells. Respir Res. 2009;10:105. doi: 10.1186/1465-9921-10-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang D., Morales J.E., Calame D.G., Alcorn J.L., Wetsel R.A. Transplantation of human embryonic stem cell-derived alveolar epithelial type II cells abrogates acute lung injury in mice. Mol Ther. 2010;18:625–634. doi: 10.1038/mt.2009.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pickering S.J., Minger S.L., Patel M. Generation of a human embryonic stem cell line encoding the cystic fibrosis mutation deltaF508, using preimplantation genetic diagnosis. Reprod Biomed Online. 2005;10:390–397. doi: 10.1016/s1472-6483(10)61801-9. [DOI] [PubMed] [Google Scholar]
- 79.Mateizel I., De Temmerman N., Ullmann U. Derivation of human embryonic stem cell lines from embryos obtained after IVF and after PGD for monogenic disorders. Hum Reprod. 2006;21:503–511. doi: 10.1093/humrep/dei345. [DOI] [PubMed] [Google Scholar]
- 80.Weiss D.J., Kolls J.K., Ortiz L.A., Panoskaltsis-Mortari A., Prockop D.J. Stem cells and cell therapies in lung biology and lung diseases. Proc Am Thorac Soc. 2008;5:637–667. doi: 10.1513/pats.200804-037DW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Beckett T., Loi R., Prenovitz R., Goncz K.K., Suratt B.T., Weiss D.J. Acute lung injury with endotoxin or NO2 does not enhance development of airway epithelium from bone marrow. Mol Ther. 2005;12:680–686. doi: 10.1016/j.ymthe.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 82.Theise N.D., Henegariu O., Grove J. Radiation pneumonitis in mice: a severe injury model for pneumocyte engraftment from bone marrow. Exp Hematol. 2002;30:1333–1338. doi: 10.1016/s0301-472x(02)00931-1. [DOI] [PubMed] [Google Scholar]
- 83.Herzog E.L., Van Arnam J., Hu B., Krause D.S. Threshold of lung injury required for the appearance of marrow-derived lung epithelia. Stem Cells. 2006;24:1986–1992. doi: 10.1634/stemcells.2005-0579. [DOI] [PubMed] [Google Scholar]
- 84.Kotton D.N., Fabian A.J., Mulligan R.C. Failure of bone marrow to reconstitute lung epithelium. Am J Respir Cell Mol Biol. 2005;33:328–334. doi: 10.1165/rcmb.2005-0175RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chang J.C., Summer R., Sun X., Fitzsimmons K., Fine A. Evidence that bone marrow cells do not contribute to the alveolar epithelium. Am J Respir Cell Mol Biol. 2005;33:335–342. doi: 10.1165/rcmb.2005-0129OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Swenson E.S., Price J.G., Brazelton T., Krause D.S. Limitations of green fluorescent protein as a cell lineage marker. Stem Cells. 2007;25:2593–2600. doi: 10.1634/stemcells.2007-0241. [DOI] [PubMed] [Google Scholar]
- 87.Spees J.L., Olson S.D., Ylostalo J. Differentiation, cell fusion, and nuclear fusion during ex vivo repair of epithelium by human adult stem cells from bone marrow stroma. Proc Natl Acad Sci U S A. 2003;100:2397–2402. doi: 10.1073/pnas.0437997100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Harris R.G., Herzog E.L., Bruscia E.M., Grove J.E., Van Arnam J.S., Krause D.S. Lack of a fusion requirement for development of bone marrow-derived epithelia. Science. 2004;305:90–93. doi: 10.1126/science.1098925. [DOI] [PubMed] [Google Scholar]
- 89.Herzog E.L., Van Arnam J., Hu B. Lung-specific nuclear reprogramming is accompanied by heterokaryon formation and Y chromosome loss following bone marrow transplantation and secondary inflammation. FASEB J. 2007;21:2592–2601. doi: 10.1096/fj.06-7861com. [DOI] [PubMed] [Google Scholar]
- 90.Suratt B.T., Cool C.D., Serls A.E. Human pulmonary chimerism after hematopoietic stem cell transplantation. Am J Respir Crit Care Med. 2003;168:318–322. doi: 10.1164/rccm.200301-145OC. [DOI] [PubMed] [Google Scholar]
- 91.Mattsson J., Jansson M., Wernerson A., Hassan M. Lung epithelial cells and type II pneumocytes of donor origin after allogeneic hematopoietic stem cell transplantation. Transplantation. 2004;78:154–157. doi: 10.1097/01.tp.0000132326.08628.74. [DOI] [PubMed] [Google Scholar]
- 92.Kleeberger W., Versmold A., Rothamel T. Increased chimerism of bronchial and alveolar epithelium in human lung allografts undergoing chronic injury. Am J Pathol. 2003;162:1487–1494. doi: 10.1016/S0002-9440(10)64281-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Spencer H., Rampling D., Aurora P., Bonnet D., Hart S.L., Jaffé A. Transbronchial biopsies provide longitudinal evidence for epithelial chimerism in children following sex mismatched lung transplantation. Thorax. 2005;60:60–62. doi: 10.1136/thx.2004.029678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bittmann I., Dose T., Baretton G.B. Cellular chimerism of the lung after transplantation. An interphase cytogenetic study. Am J Clin Pathol. 2001;115:525–533. doi: 10.1309/GAFN-5MPA-LY8E-DTPQ. [DOI] [PubMed] [Google Scholar]
- 95.Davies J.C., Potter M., Bush A., Rosenthal M., Geddes D.M., Alton E.W. Bone marrow stem cells do not repopulate the healthy upper respiratory tract. Pediatr Pulmonol. 2002;34:251–256. doi: 10.1002/ppul.10163. [DOI] [PubMed] [Google Scholar]
- 96.Zander D.S., Baz M.A., Cogle C.R., Visner G.A., Theise N.D., Crawford J.M. Bone marrow-derived stem-cell repopulation contributes minimally to the type II pneumocyte pool in transplanted human lungs. Transplantation. 2005;80:206–212. doi: 10.1097/01.tp.0000165095.39320.50. [DOI] [PubMed] [Google Scholar]
- 97.Albera C., Polak J.M., Janes S. Repopulation of human pulmonary epithelium by bone marrow cells: a potential means to promote repair. Tissue Eng. 2005;11:1115–1121. doi: 10.1089/ten.2005.11.1115. [DOI] [PubMed] [Google Scholar]
- 98.Sueblinvong V., Loi R., Eisenhauer P.L. Derivation of lung epithelium from human cord blood-derived mesenchymal stem cells. Am J Respir Crit Care Med. 2008;177:701–711. doi: 10.1164/rccm.200706-859OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Macpherson H., Keir P., Webb S. Bone marrow-derived SP cells can contribute to the respiratory tract of mice in vivo. J Cell Sci. 2005;118:2441–2450. doi: 10.1242/jcs.02375. [DOI] [PubMed] [Google Scholar]
- 100.MacPherson H., Keir P.A., Edwards C.J., Webb S., Dorin J.R. Following damage, the majority of bone marrow-derived airway cells express an epithelial marker. Respir Res. 2006;7:145. doi: 10.1186/1465-9921-7-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yan C., Lian X., Dai Y. Gene delivery by the hSP-B promoter to lung alveolar type II epithelial cells in LAL-knockout mice through bone marrow mesenchymal stem cells. Gene Ther. 2007;14:1461–1470. doi: 10.1038/sj.gt.3303006. [DOI] [PubMed] [Google Scholar]
- 102.Serikov V.B., Popov B., Mikhailov V.M., Gupta N., Matthay M.A. Evidence of temporary airway epithelial repopulation and rare clonal formation by BM-derived cells following naphthalene injury in mice. Anat Rec (Hoboken) 2007;290:1033–1045. doi: 10.1002/ar.20574. [DOI] [PubMed] [Google Scholar]
- 103.Wong A.P., Dutly A.E., Sacher A. Targeted cell replacement with bone marrow cells for airway epithelial regeneration. Am J Physiol Lung Cell Mol Physiol. 2007;293:L740–L752. doi: 10.1152/ajplung.00050.2007. [DOI] [PubMed] [Google Scholar]
- 104.Bruscia E.M., Price J.E., Cheng E.C. Assessment of cystic fibrosis transmembrane conductance regulator (CFTR) activity in CFTR-null mice after bone marrow transplantation. Proc Natl Acad Sci U S A. 2006;103:2965–2970. doi: 10.1073/pnas.0510758103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bruscia E.M., Ziegler E.C., Price J.E., Weiner S., Egan M.E., Krause D.S. Engraftment of donor-derived epithelial cells in multiple organs following bone marrow transplantation into newborn mice. Stem Cells. 2006;24:2299–2308. doi: 10.1634/stemcells.2006-0166. [DOI] [PubMed] [Google Scholar]
- 106.Spees J.L., Pociask D.A., Sullivan D.E. Engraftment of bone marrow progenitor cells in a rat model of asbestos-induced pulmonary fibrosis. Am J Respir Crit Care Med. 2007;176:385–394. doi: 10.1164/rccm.200607-1004OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Levis J., Loi R., Butnor K.J. Decreased asbestos-induced lung inflammation and fibrosis after radiation and bone marrow transplant. Am J Respir Cell Mol Biol. 2008;38:16–25. doi: 10.1165/rcmb.2007-0249OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhao Y.D., Courtman D.W., Deng Y., Kugathasan L., Zhang Q., Stewart D.J. Rescue of monocrotaline-induced pulmonary arterial hypertension using bone marrow-derived endothelial-like progenitor cells: efficacy of combined cell and eNOS gene therapy in established disease. Circ Res. 2005;96:442–450. doi: 10.1161/01.RES.0000157672.70560.7b. [DOI] [PubMed] [Google Scholar]
- 109.Raoul W., Wagner-Ballon O., Saber G. Effects of bone marrow-derived cells on monocrotaline- and hypoxia-induced pulmonary hypertension in mice. Respir Res. 2007;8:8. doi: 10.1186/1465-9921-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhu J.H., Wang X.X., Zhang F.R. Safety and efficacy of autologous endothelial progenitor cells transplantation in children with idiopathic pulmonary arterial hypertension: open-label pilot study. Pediatr Transplant. 2008;12:650–655. doi: 10.1111/j.1399-3046.2007.00863.x. [DOI] [PubMed] [Google Scholar]
- 111.Kucia M., Wysoczynski M., Ratajczak J., Ratajczak M.Z. Identification of very small embryonic like (VSEL) stem cells in bone marrow. Cell Tissue Res. 2008;331:125–134. doi: 10.1007/s00441-007-0485-4. [DOI] [PubMed] [Google Scholar]
- 112.Kucia M., Zuba-Surma E.K., Wysoczynski M. Adult marrow-derived very small embryonic-like stem cells and tissue engineering. Expert Opin Biol Ther. 2007;7:1499–1514. doi: 10.1517/14712598.7.10.1499. [DOI] [PubMed] [Google Scholar]
- 113.Gomperts B.N., Belperio J.A., Rao P.N. Circulating progenitor epithelial cells traffic via CXCR4/CXCL12 in response to airway injury. J Immunol. 2006;176:1916–1927. doi: 10.4049/jimmunol.176.3.1916. [DOI] [PubMed] [Google Scholar]
- 114.Wong A.P., Keating A., Lu W.Y. Identification of a bone marrow-derived epithelial-like population capable of repopulating injured mouse airway epithelium. J Clin Invest. 2009;119:336–348. doi: 10.1172/JCI36882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Berger M.J., Adams S.D., Tigges B.M. Differentiation of umbilical cord blood-derived multilineage progenitor cells into respiratory epithelial cells. Cytotherapy. 2006;8:480–487. doi: 10.1080/14653240600941549. [DOI] [PubMed] [Google Scholar]
- 116.Miao Z., Jin J., Chen L. Isolation of mesenchymal stem cells from human placenta: comparison with human bone marrow mesenchymal stem cells. Cell Biol Int. 2006;30:681–687. doi: 10.1016/j.cellbi.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 117.Traktuev D.O., Merfeld-Clauss S., Li J. A population of multipotent CD34-positive adipose stromal cells share pericyte and mesenchymal surface markers, reside in a periendothelial location, and stabilize endothelial networks. Circ Res. 2008;102:77–85. doi: 10.1161/CIRCRESAHA.107.159475. [DOI] [PubMed] [Google Scholar]
- 118.Serrano-Mollar A., Nacher M., Gay-Jordi G. Intratracheal transplantation of alveolar type II cells reverses bleomycin-induced lung fibrosis. Am J Respir Crit Care Med. 2007;176:1261–1268. doi: 10.1164/rccm.200610-1491OC. [DOI] [PubMed] [Google Scholar]
- 119.Kuang P.P., Lucey E., Rishikof D.C., Humphries D.E., Bronsnick D., Goldstein R.H. Engraftment of neonatal lung fibroblasts into the normal and elastase-injured lung. Am J Respir Cell Mol Biol. 2005;33:371–377. doi: 10.1165/rcmb.2004-0319OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.McCarter S.D., Mei S.H., Lai P.F. Cell-based angiopoietin-1 gene therapy for acute lung injury. Am J Respir Crit Care Med. 2007;175:1014–1026. doi: 10.1164/rccm.200609-1370OC. [DOI] [PubMed] [Google Scholar]
- 121.Viswanathan A., Painter R.G., Lanson N.A., Jr., Wang G. Functional expression of N-formyl peptide receptors in human bone marrow-derived mesenchymal stem cells. Stem Cells. 2007;25:1263–1269. doi: 10.1634/stemcells.2006-0522. [DOI] [PubMed] [Google Scholar]
- 122.Satoh K., Kagaya Y., Nakano M. Important role of endogenous erythropoietin system in recruitment of endothelial progenitor cells in hypoxia-induced pulmonary hypertension in mice. Circulation. 2006;113:1442–1450. doi: 10.1161/CIRCULATIONAHA.105.583732. [DOI] [PubMed] [Google Scholar]
- 123.Son B.R., Marquez-Curtis L.A., Kucia M. Migration of bone marrow and cord blood mesenchymal stem cells in vitro is regulated by stromal-derived factor-1-CXCR4 and hepatocyte growth factor-c-met axes and involves matrix metalloproteinases. Stem Cells. 2006;24:1254–1264. doi: 10.1634/stemcells.2005-0271. [DOI] [PubMed] [Google Scholar]
- 124.Epperly M.W., Guo H., Gretton J.E., Greenberger J.S. Bone marrow origin of myofibroblasts in irradiation pulmonary fibrosis. Am J Respir Cell Mol Biol. 2003;29:213–224. doi: 10.1165/rcmb.2002-0069OC. [DOI] [PubMed] [Google Scholar]
- 125.Yan X., Liu Y., Han Q. Injured microenvironment directly guides the differentiation of engrafted Flk-1(+) mesenchymal stem cell in lung. Exp Hematol. 2007;35:1466–1475. doi: 10.1016/j.exphem.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 126.Popov B.V., Serikov V.B., Petrov N.S., Izusova T.V., Gupta N., Matthay M.A. Lung epithelial cells induce endodermal differentiation in mouse mesenchymal bone marrow stem cells by paracrine mechanism. Tissue Eng. 2007;13:2441–2450. doi: 10.1089/ten.2007.0001. [DOI] [PubMed] [Google Scholar]
- 127.Field-Corbett C., O’Dea S. Soluble signals from mechanically disrupted lung tissue induce lung-related gene expression in bone marrow-derived cells in vitro. Stem Cells Dev. 2007;16:231–242. doi: 10.1089/scd.2006.0069. [DOI] [PubMed] [Google Scholar]
- 128.Ratajczak J., Miekus K., Kucia M. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia. 2006;20:847–856. doi: 10.1038/sj.leu.2404132. [DOI] [PubMed] [Google Scholar]
- 129.Aliotta J.M., Sanchez-Guijo F.M., Dooner G.J. Alteration of marrow cell gene expression, protein production, and engraftment into lung by lung-derived microvesicles: a novel mechanism for phenotype modulation. Stem Cells. 2007;25:2245–2256. doi: 10.1634/stemcells.2007-0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Friedenstein A.J., Petrakova K.V., Kurolesova A.I., Frolova G.P. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968;6:230–247. [PubMed] [Google Scholar]
- 131.Newman R.E., Yoo D., LeRoux M.A., Danilkovitch-Miagkova A. Treatment of inflammatory diseases with mesenchymal stem cells. Inflamm Allergy Drug Targets. 2009;8:110–123. doi: 10.2174/187152809788462635. [DOI] [PubMed] [Google Scholar]
- 132.Patel S.A., Sherman L., Munoz J., Rameshwar P. Immunological properties of mesenchymal stem cells and clinical implications. Arch Immunol Ther Exp (Warsz) 2008;56:1–8. doi: 10.1007/s00005-008-0001-x. [DOI] [PubMed] [Google Scholar]
- 133.Bernardo M.E., Locatelli F., Fibbe W.E. Mesenchymal stromal cells. Ann NY Acad Sci. 2009;1176:101–117. doi: 10.1111/j.1749-6632.2009.04607.x. [DOI] [PubMed] [Google Scholar]
- 134.Rojas M., Woods C.R., Mora A.L., Xu J., Brigham K.L. Endotoxin-induced lung injury in mice: structural, functional, and biochemical responses. Am J Physiol Lung Cell Mol Physiol. 2005;288:L333–L341. doi: 10.1152/ajplung.00334.2004. [DOI] [PubMed] [Google Scholar]
- 135.Su X., Lee J.W., Matthay Z.A. Activation of the alpha7 nAChR reduces acid-induced acute lung injury in mice and rats. Am J Respir Cell Mol Biol. 2007;37:186–192. doi: 10.1165/rcmb.2006-0240OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Allen G.B., Leclair T., Cloutier M., Thompson-Figueroa J., Bates J.H. The response to recruitment worsens with progression of lung injury and fibrin accumulation in a mouse model of acid aspiration. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1580–L1589. doi: 10.1152/ajplung.00483.2006. [DOI] [PubMed] [Google Scholar]
- 137.Allen G.B., Suratt B.T., Rinaldi L., Petty J.M., Bates J.H. Choosing the frequency of deep inflation in mice: balancing recruitment against ventilator-induced lung injury. Am J Physiol Lung Cell Mol Physiol. 2006;291:L710–L717. doi: 10.1152/ajplung.00532.2005. [DOI] [PubMed] [Google Scholar]
- 138.Lee J.W., Fang X., Gupta N., Serikov V., Matthay M.A. Allogeneic human mesenchymal stem cells for treatment of E. coli endotoxin-induced acute lung injury in the ex vivo perfused human lung. Proc Natl Acad Sci U S A. 2009;106:16357–16362. doi: 10.1073/pnas.0907996106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhao Y.D., Ohkawara H., Vogel S.M., Malik A.B., Zhao Y.Y. Bone marrow-derived progenitor cells prevent thrombin-induced increase in lung vascular permeability. Am J Physiol Lung Cell Mol Physiol. 2010;298:L36–L44. doi: 10.1152/ajplung.00064.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Noth I., Martinez F.J. Recent advances in idiopathic pulmonary fibrosis. Chest. 2007;132:637–650. doi: 10.1378/chest.06-1927. [DOI] [PubMed] [Google Scholar]
- 141.Marshall B.G., Shaw R.J. T cells and fibrosis. Chem Immunol. 2000;78:148–158. doi: 10.1159/000058824. [DOI] [PubMed] [Google Scholar]
- 142.Thannickal V.J., Lee D.Y., White E.S. Myofibroblast differentiation by transforming growth factor-beta1 is dependent on cell adhesion and integrin signaling via focal adhesion kinase. J Biol Chem. 2003;278:12384–12389. doi: 10.1074/jbc.M208544200. [DOI] [PubMed] [Google Scholar]
- 143.White E.S., Lazar M.H., Thannickal V.J. Pathogenetic mechanisms in usual interstitial pneumonia/idiopathic pulmonary fibrosis. J Pathol. 2003;201:343–354. doi: 10.1002/path.1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Willis B.C., Liebler J.M., Luby-Phelps K. Induction of epithelial-mesenchymal transition in alveolar epithelial cells by transforming growth factor-beta1: potential role in idiopathic pulmonary fibrosis. Am J Pathol. 2005;166:1321–1332. doi: 10.1016/s0002-9440(10)62351-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Moore B.B., Hogaboam C.M. Murine models of pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2008;294:L152–L160. doi: 10.1152/ajplung.00313.2007. [DOI] [PubMed] [Google Scholar]
- 146.Roberts S.N., Howie S.E., Wallace W.A. A novel model for human interstitial lung disease: hapten-driven lung fibrosis in rodents. J Pathol. 1995;176:309–318. doi: 10.1002/path.1711760313. [DOI] [PubMed] [Google Scholar]
- 147.Haston C.K., Travis E.L. Murine susceptibility to radiation-induced pulmonary fibrosis is influenced by a genetic factor implicated in susceptibility to bleomycin-induced pulmonary fibrosis. Cancer Res. 1997;57:5286–5291. [PubMed] [Google Scholar]
- 148.Davis G.S., Leslie K.O., Hemenway D.R. Silicosis in mice: effects of dose, time, and genetic strain. J Environ Pathol Toxicol Oncol. 1998;17:81–97. [PubMed] [Google Scholar]
- 149.Bozelka B.E., Sestini P., Gaumer H.R., Hammad Y., Heather C.J., Salvaggio J.E. A murine model of asbestosis. Am J Pathol. 1983;112:326–337. [PMC free article] [PubMed] [Google Scholar]
- 150.Xu J., Mora A.L., LaVoy J., Brigham K.L., Rojas M. Increased bleomycin-induced lung injury in mice deficient in the transcription factor T-bet. Am J Physiol Lung Cell Mol Physiol. 2006;291:L658–L667. doi: 10.1152/ajplung.00006.2006. [DOI] [PubMed] [Google Scholar]
- 151.Mora A.L., Woods C.R., Garcia A. Lung infection with gamma-herpesvirus induces progressive pulmonary fibrosis in Th2-biased mice. Am J Physiol Lung Cell Mol Physiol. 2005;289:L711–L721. doi: 10.1152/ajplung.00007.2005. [DOI] [PubMed] [Google Scholar]
- 152.Aguilar S., Scotton C.J., McNulty K. Bone marrow stem cells expressing keratinocyte growth factor via an inducible lentivirus protects against bleomycin-induced pulmonary fibrosis. PLoS ONE. 2009;4:e8013. doi: 10.1371/journal.pone.0008013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Andersson-Sjoland A., de Alba C.G., Nihlberg K. Fibrocytes are a potential source of lung fibroblasts in idiopathic pulmonary fibrosis. Int J Biochem Cell Biol. 2008;40:2129–2140. doi: 10.1016/j.biocel.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 154.Schmidt M., Sun G., Stacey M.A., Mori L., Mattoli S. Identification of circulating fibrocytes as precursors of bronchial myofibroblasts in asthma. J Immunol. 2003;171:380–389. doi: 10.4049/jimmunol.171.1.380. [DOI] [PubMed] [Google Scholar]
- 155.Brocker V., Langer F., Fellous T.G. Fibroblasts of recipient origin contribute to bronchiolitis obliterans in human lung transplants. Am J Respir Crit Care Med. 2006;173:1276–1282. doi: 10.1164/rccm.200509-1381OC. [DOI] [PubMed] [Google Scholar]
- 156.Nihlberg K., Larsen K., Hultgardh-Nilsson A., Malmström A., Bjermer L., Westergren-Thorsson G. Tissue fibrocytes in patients with mild asthma: a possible link to thickness of reticular basement membrane? Respir Res. 2006;7:50. doi: 10.1186/1465-9921-7-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Mehrad B., Burdick M.D., Zisman D.A., Keane M.P., Belperio J.A., Strieter R.M. Circulating peripheral blood fibrocytes in human fibrotic interstitial lung disease. Biochem Biophys Res Commun. 2007;353:104–108. doi: 10.1016/j.bbrc.2006.11.149. [DOI] [PubMed] [Google Scholar]
- 158.Wang C.H., Huang C.D., Lin H.C. Increased circulating fibrocytes in asthma with chronic airflow obstruction. Am J Respir Crit Care Med. 2008;178:583–591. doi: 10.1164/rccm.200710-1557OC. [DOI] [PubMed] [Google Scholar]
- 159.Chapman K.R., Mannino D.M., Soriano J.B. Epidemiology and costs of chronic obstructive pulmonary disease. Eur Respir J. 2006;27:188–207. doi: 10.1183/09031936.06.00024505. [DOI] [PubMed] [Google Scholar]
- 160.Mahadeva R., Shapiro S.D. Animal models of pulmonary emphysema. Curr Drug Targets Inflamm Allergy. 2005;4:665–673. doi: 10.2174/156801005774912860. [DOI] [PubMed] [Google Scholar]
- 161.Yuhgetsu H., Ohno Y., Funaguchi N. Beneficial effects of autologous bone marrow mononuclear cell transplantation against elastase-induced emphysema in rabbits. Exp Lung Res. 2006;32:413–426. doi: 10.1080/01902140601047633. [DOI] [PubMed] [Google Scholar]
- 162.Adachi Y., Oyaizu H., Taketani S. Treatment and transfer of emphysema by a new bone marrow transplantation method from normal mice to Tsk mice and vice versa. Stem Cells. 2006;24:2071–2077. doi: 10.1634/stemcells.2005-0575. [DOI] [PubMed] [Google Scholar]
- 163.Ishizawa K., Kubo H., Yamada M. Bone marrow-derived cells contribute to lung regeneration after elastase-induced pulmonary emphysema. FEBS Lett. 2004;556:249–252. doi: 10.1016/s0014-5793(03)01399-1. [DOI] [PubMed] [Google Scholar]
- 164.Hare J.M., Traverse J.H., Henry T.D. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54:2277–2286. doi: 10.1016/j.jacc.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Osiris Therapeutics, Inc. Osiris therapeutis reports interim data for COPD stem cell study. Available at http://investor.osiris.com/releasedetail.cfm?ReleaseID=391580.
- 166.Koehler D.R., Downey G.P., Sweezey N.B., Tanswell A.K., Hu J. Lung inflammation as a therapeutic target in cystic fibrosis. Am J Respir Cell Mol Biol. 2004;31:377–381. doi: 10.1165/rcmb.2004-0124TR. [DOI] [PubMed] [Google Scholar]
- 167.Nemeth K., Keane-Myers A., Brown J.M. Bone marrow stromal cells use TGF-beta to suppress allergic responses in a mouse model of ragweed-induced asthma. Proc Natl Acad Sci U S A. 2010;107:5652–5657. doi: 10.1073/pnas.0910720107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Cho K.S., Park H.K., Park H.Y. IFATS collection: Immunomodulatory effects of adipose tissue-derived stem cells in an allergic rhinitis mouse model. Stem Cells. 2009;27:259–265. doi: 10.1634/stemcells.2008-0283. [DOI] [PubMed] [Google Scholar]
- 169.Park H.K., Cho K.S., Park H.Y. Adipose-derived stromal cells inhibit allergic airway inflammation in mice. Stem Cells Dev. 2010 doi: 10.1089/scd.2009.0513. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 170.Nagaya N., Kangawa K., Kanda M. Hybrid cell-gene therapy for pulmonary hypertension based on phagocytosing action of endothelial progenitor cells. Circulation. 2003;108:889–895. doi: 10.1161/01.CIR.0000079161.56080.22. [DOI] [PubMed] [Google Scholar]
- 171.Takahashi M., Nakamura T., Toba T., Kajiwara N., Kato H., Shimizu Y. Transplantation of endothelial progenitor cells into the lung to alleviate pulmonary hypertension in dogs. Tissue Eng. 2004;10:771–779. doi: 10.1089/1076327041348563. [DOI] [PubMed] [Google Scholar]
- 172.Zhao Q., Liu Z., Wang Z., Yang C., Liu J., Lu J. Effect of prepro-calcitonin gene-related peptide-expressing endothelial progenitor cells on pulmonary hypertension. Ann Thorac Surg. 2007;84:544–552. doi: 10.1016/j.athoracsur.2007.03.067. [DOI] [PubMed] [Google Scholar]
- 173.Kahler C.M., Wechselberger J., Hilbe W. Peripheral infusion of rat bone marrow derived endothelial progenitor cells leads to homing in acute lung injury. Respir Res. 2007;8:50. doi: 10.1186/1465-9921-8-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wang X.X., Zhang F.R., Shang Y.P. Transplantation of autologous endothelial progenitor cells may be beneficial in patients with idiopathic pulmonary arterial hypertension: a pilot randomized controlled trial. J Am Coll Cardiol. 2007;49:1566–1571. doi: 10.1016/j.jacc.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 175.Aghi M., Chiocca E.A. Contribution of bone marrow-derived cells to blood vessels in ischemic tissues and tumors. Mol Ther. 2005;12:994–1005. doi: 10.1016/j.ymthe.2005.07.693. [DOI] [PubMed] [Google Scholar]
- 176.Aghi M., Cohen K.S., Klein R.J. Tumor stromal-derived factor-1 recruits vascular progenitors to mitotic neovasculature, where microenvironment influences their differentiated phenotypes. Cancer Res. 2006;66:9054–9064. doi: 10.1158/0008-5472.CAN-05-3759. [DOI] [PubMed] [Google Scholar]
- 177.Oh H.K., Ha J.M., O E. Tumor angiogenesis promoted by ex vivo differentiated endothelial progenitor cells is effectively inhibited by an angiogenesis inhibitor, TK1-2. Cancer Res. 2007;67:4851–4859. doi: 10.1158/0008-5472.CAN-06-2979. [DOI] [PubMed] [Google Scholar]
- 178.Nolan D.J., Ciarrocchi A., Mellick A.S. Bone marrow-derived endothelial progenitor cells are a major determinant of nascent tumor neovascularization. Genes Dev. 2007;21:1546–1558. doi: 10.1101/gad.436307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Gao D., Nolan D.J., Mellick A.S., Bambino K., McDonnell K., Mittal V. Endothelial progenitor cells control the angiogenic switch in mouse lung metastasis. Science. 2008;319:195–198. doi: 10.1126/science.1150224. [DOI] [PubMed] [Google Scholar]
- 180.Studeny M., Marini F.C., Dembinski J.L. Mesenchymal stem cells: potential precursors for tumor stroma and targeted-delivery vehicles for anticancer agents. J Natl Cancer Inst. 2004;96:1593–1603. doi: 10.1093/jnci/djh299. [DOI] [PubMed] [Google Scholar]
- 181.Hall B., Dembinski J., Sasser A.K., Studeny M., Andreeff M., Marini F. Mesenchymal stem cells in cancer: tumor-associated fibroblasts and cell-based delivery vehicles. Int J Hematol. 2007;86:8–16. doi: 10.1532/IJH97.06230. [DOI] [PubMed] [Google Scholar]
- 182.Stoff-Khalili M.A., Rivera A.A., Mathis J.M. Mesenchymal stem cells as a vehicle for targeted delivery of CRAds to lung metastases of breast carcinoma. Breast Cancer Res Treat. 2007;105:157–167. doi: 10.1007/s10549-006-9449-8. [DOI] [PubMed] [Google Scholar]
- 183.Miletic H., Fischer Y., Litwak S. Bystander killing of malignant glioma by bone marrow-derived tumor-infiltrating progenitor cells expressing a suicide gene. Mol Ther. 2007;15:1373–1381. doi: 10.1038/sj.mt.6300155. [DOI] [PubMed] [Google Scholar]
- 184.Xin H., Kanehira M., Mizuguchi H. Targeted delivery of CX3CL1 to multiple lung tumors by mesenchymal stem cells. Stem Cells. 2007;25:1618–1626. doi: 10.1634/stemcells.2006-0461. [DOI] [PubMed] [Google Scholar]
- 185.Rachakatla R.S., Marini F., Weiss M.L., Tamura M., Troyer D. Development of human umbilical cord matrix stem cell-based gene therapy for experimental lung tumors. Cancer Gene Ther. 2007;14:828–835. doi: 10.1038/sj.cgt.7701077. [DOI] [PubMed] [Google Scholar]
- 186.Kanehira M., Xin H., Hoshino K. Targeted delivery of NK4 to multiple lung tumors by bone marrow-derived mesenchymal stem cells. Cancer Gene Ther. 2007;14:894–903. doi: 10.1038/sj.cgt.7701079. [DOI] [PubMed] [Google Scholar]
- 187.Nichols J.E., Cortiella J. Engineering of a complex organ: progress toward development of a tissue-engineered lung. Proc Am Thorac Soc. 2008;5:723–730. doi: 10.1513/pats.200802-022AW. [DOI] [PubMed] [Google Scholar]
- 188.Tomei A.A., Boschetti F., Gervaso F. 3D collagen cultures under well-defined dynamic strain: A novel strain device with a porous elastomeric support. Biotechnol Bioeng. 2009;103:217–225. doi: 10.1002/bit.22236. [DOI] [PubMed] [Google Scholar]
- 189.Omori K., Nakamura T., Kanemaru S., Magrufov A., Yamashita M., Shimizu Y. In situ tissue engineering of the cricoid and trachea in a canine model. Ann Otol Rhinol Laryngol. 2008;117:609–613. doi: 10.1177/000348940811700811. [DOI] [PubMed] [Google Scholar]
- 190.Urita Y., Komuro H., Chen G., Shinya M., Saihara R., Kaneko M. Evaluation of diaphragmatic hernia repair using PLGA mesh-collagen sponge hybrid scaffold: an experimental study in a rat model. Pediatr Surg Int. 2008;24:1041–1045. doi: 10.1007/s00383-008-2212-y. [DOI] [PubMed] [Google Scholar]
- 191.Omori K., Tada Y., Suzuki T. Clinical application of in situ tissue engineering using a scaffolding technique for reconstruction of the larynx and trachea. Ann Otol Rhinol Laryngol. 2008;117:673–678. doi: 10.1177/000348940811700908. [DOI] [PubMed] [Google Scholar]
- 192.A pipe dream becomes reality. Nat Med. 2009;15:4. doi: 10.1038/nm0109-4b. [DOI] [PubMed] [Google Scholar]
- 193.Andrade C.F., Wong A.P., Waddell T.K., Keshavjee S., Liu M. Cell-based tissue engineering for lung regeneration. Am J Physiol Lung Cell Mol Physiol. 2007;292:L510–L518. doi: 10.1152/ajplung.00175.2006. [DOI] [PubMed] [Google Scholar]
- 194.Mondrinos M.J., Koutzaki S., Lelkes P.I., Finck C.M. A tissue-engineered model of fetal distal lung tissue. Am J Physiol Lung Cell Mol Physiol. 2007;293:L639–L650. doi: 10.1152/ajplung.00403.2006. [DOI] [PubMed] [Google Scholar]
- 195.Mondrinos M.J., Koutzaki S.H., Poblete H.M., Crisanti M.C., Lelkes P.I., Finck C.M. In vivo pulmonary tissue engineering: contribution of donor-derived endothelial cells to construct vascularization. Tissue Eng Part A. 2008;14:361–368. doi: 10.1089/tea.2007.0041. [DOI] [PubMed] [Google Scholar]
- 196.Cortiella J., Nichols J.E., Kojima K. Tissue-engineered lung: an in vivo and in vitro comparison of polyglycolic acid and pluronic F-127 hydrogel/somatic lung progenitor cell constructs to support tissue growth. Tissue Eng. 2006;12:1213–1225. doi: 10.1089/ten.2006.12.1213. [DOI] [PubMed] [Google Scholar]
- 197.Shigemura N., Okumura M., Mizuno S. Lung tissue engineering technique with adipose stromal cells improves surgical outcome for pulmonary emphysema. Am J Respir Crit Care Med. 2006;174:1199–1205. doi: 10.1164/rccm.200603-406OC. [DOI] [PubMed] [Google Scholar]
- 198.Macchiarini P., Jungebluth P., Go T. Clinical transplantation of a tissue-engineered airway. Lancet. 2008;372:2023–2030. doi: 10.1016/S0140-6736(08)61598-6. [DOI] [PubMed] [Google Scholar]