Abstract
Not much is known about effects of gestational alcohol exposure on maternal and fetal cardiovascular adaptations. This study determined whether maternal binge alcohol exposure and L-glutamine supplementation could affect maternal-fetal hemodynamics and fetal regional brain blood flow during the brain growth spurt period. Pregnant sheep were randomly assigned to one of four groups: saline control, alcohol (1.75–2.5 g/kg body weight), glutamine (100 mg/kg body weight) or alcohol + glutamine. A chronic weekend binge drinking paradigm between gestational days (GD) 99 and 115 was utilized. Fetuses were surgically instrumented on GD 117 ± 1 and studied on GD 120 ± 1. Binge alcohol exposure caused maternal acidemia, hypercapnea, and hypoxemia. Fetuses were acidemic and hypercapnic, but not hypoxemic. Alcohol exposure increased fetal mean arterial pressure, whereas fetal heart rate was unaltered. Alcohol exposure resulted in ~40 % reduction in maternal uterine artery blood flow. Labeled microsphere analyses showed that alcohol induced >2-fold increases in fetal whole brain blood flow. The elevation in fetal brain blood flow was region-specific, particularly affecting the developing cerebellum, brain stem, and olfactory bulb. Maternal L-glutamine supplementation attenuated alcohol-induced maternal hypercapnea, fetal acidemia and increases in fetal brain blood flow. L-Glutamine supplementation did not affect uterine blood flow. Collectively, alcohol exposure alters maternal and fetal acid–base balance, decreases uterine blood flow, and alters fetal regional brain blood flow. Importantly, L-glutamine supplementation mitigates alcohol-induced acid–base imbalances and alterations in fetal regional brain blood flow. Further studies are warranted to elucidate mechanisms responsible for alcohol-induced programming of maternal uterine artery and fetal circulation adaptations in pregnancy.
Keywords: Endothelium, FASD, Alcohol, Uterine, Vascular, L-Glutamine
Introduction
Women who drink alcohol during pregnancy are at a high risk of giving birth to children with physical, behavioral or cognitive developmental problems that can persist as lifelong disabilities called fetal alcohol spectrum disorders (FASD) (Warren et al. 2001). Alcohol is a neurotoxic teratogen and many studies have shown that alcohol exposure has the potential to cause damage in different regions of the developing brain (Caetano et al. 2006; Sawant et al. 2013a). Although the deleterious effects of maternal alcohol exposure on fetal brain development and function are well documented, little is known about its effects on maternal and fetal cardiovascular adaptations (Ramadoss and Magness 2012c).
Alcohol is reported to produce dose-dependent changes in maternal and fetal systemic hemodynamics in animal models. For instance, in sheep, lower doses of alcohol (0.75 g/kg) do not result in any change in fetal mean arterial pressure (MAP) or heart rate (HR), whereas higher doses result in altered systemic hemodynamics (Cudd et al. 2001; Parnell et al. 2007). These alterations are accompanied by alcohol-induced maternal/fetal acidemia, hypercapnea, and hypoxemia (Parnell et al. 2007; Cudd et al. 2001; West et al. 2001; Sawant et al. 2013b). In the rat model, prenatal alcohol exposure (35 % alcohol-derived calories) during the final one-third of pregnancy had no effect on HR in the adult offspring (Handa et al. 2007). Ultrasound imaging studies have shown that binge-like alcohol (3 g/kg body weight) exposure in a murine second trimester equivalent model does not alter fetal HR (Bake et al. 2012).
Among the maternal cardiovascular changes during normal pregnancy, the most important are the vascular adaptations of the uterine artery that supplies nutrients and oxygen to the feto-placental compartment. Dramatic increases in uterine blood flow occur over the course of normal pregnancy to accommodate the increasing needs of nutrients and oxygen to the developing fetus and placenta (Ramadoss and Magness 2012d). These adaptations include a decrease in uterine vascular resistance as well as increases in angiogenesis and extracellular matrix remodeling, leading to a 53-fold increase in uterine blood flow as compared to the nonpregnant state (Rosenfeld 1977; Reynolds and Redmer 1995; Zygmunt et al. 2003; Osol and Mandala 2009). Therefore, a decrease in uterine perfusion is a cardinal feature of intrauterine growth restriction. Alterations in uterine blood flow can be closely associated with fetal cardiovascular adaptation and can predispose the fetus to cardiovascular diseases in the future by altering vascular reactivity (Alexander et al. 2005; Lang et al. 2003). Unfortunately, the impact of maternal alcohol exposure on uterine perfusion has received minimal attention and there is a paucity of information in the literature. An earlier study in sheep has shown that acute alcohol exposure leads to a significant reduction in uterine blood flow (Falconer 1990) and a series of in vitro studies have shown that binge-like alcohol exposure alters utero-placental vascular adaptations (Ramadoss et al. 2011, 2012; Ramadoss and Magness 2011, 2012a, b; Subramanian et al. 2014). However, to our knowledge, so far no study has been conducted to investigate the effect of repeated binge alcohol exposure during pregnancy on uterine blood flow. Therefore, the present study was designed to thoroughly investigate the effect of repeated maternal binge alcohol exposure on uterine artery blood flow.
Studies involving a sheep model have shown that gestational alcohol exposure alters fetal brain blood flow and vascular reactivity (Gleason et al. 1997; Mann et al. 1975; Mayock et al. 2007; Ngai et al. 2008; Parnell et al. 2007). Our laboratory (Parnell et al. 2007) and others (Mann et al. 1975) have shown that fetal brain blood flow increases in response to acute alcohol exposure in sheep. Cerebral vasodilatory response to hypoxemia was significantly attenuated in neonatal lambs exposed to alcohol during the first trimester-equivalent period of gestation (Gleason et al. 1997). Ultrasound imaging studies have shown that binge-like alcohol exposure in a murine second trimester equivalent model results in rapid and persistent decrease in blood flow from the umbilical artery to the fetal brain (Bake et al. 2012). Although these studies collectively imply that alcohol exposure leads to an alteration in fetal brain blood flow, no study has been done to investigate the effect of an intervention strategy to restore alcohol-induced alterations in brain blood flow.
In this study, the effect of L-glutamine was investigated as a nutritional intervention, based on the findings that repeated alcohol exposure or alcohol-induced acidemia results in a decrease in maternal and fetal L-glutamine bioavailability (Ramadoss et al. 2008; Washburn et al. 2013). Research has shown that L-glutamine plays a crucial role in pH homeostasis through its metabolism in the kidney (Heitmann and Bergman 1980; Welbourne 1987; Windus et al. 1984). Two diverse effects of L-glutamine on vascular reactivity have been observed. In vivo and in vitro studies have shown that L-glutamine influences endothelial-dependent relaxation by inhibiting availability of NADPH (Wu et al. 2001) and by inhibiting recycling of citrulline to arginine to limit nitric oxide release (Kawaguchi et al. 2005; Arnal et al. 1995; Meininger and Wu 1997; Okada et al. 2000), whereas other studies have postulated that a longer duration vasodilatory effect of L-glutamine is by its donation of nitrogen molecule for vasodilator synthesis (Xu and Pearl 1994). Thus, we hypothesized that repeated maternal alcohol exposure would lead to: (1) maternal and fetal hypercapnic acidemia; (2) alteration of systemic hemodynamics; (3) a decrease in uterine blood flow; and (4) subsequent changes in fetal regional brain blood flow. We further hypothesized that maternal L-glutamine supplementation would attenuate these negative effects of alcohol exposure during pregnancy.
Materials and methods
Animals
All aspects of the surgical and experimental protocols were approved by the Texas A&M University Institutional Animal Care and Use Committee. Suffolk ewes aged 2–5 years were obtained from a commercial supplier. Upon arrival at the animal facility, each ewe received an intramuscular injection of Covexin® 8 (Merck Animal Health, Summit, NJ) and an oral bolus of Valbazen® (Zoetis, Kalamazoo, MI). Ewes received progesterone impregnated vaginal implants (EAZI-BREED™, CIDR®, Zoetis, Kalamazoo, MI). Implants were removed 11 days after placement at which time prostaglandin F2α (20 mg; LUTALYSE®, Zoetis, Kalamazoo, MI) was intramuscularly administered. The following day, ewes were placed with a ram fitted with a marking harness for a period of 24 h. Marked ewes were presumed pregnant until confirmed pregnant ultrasonographically on gestation days (GD) 25 and 92 (Ramadoss et al. 2006).
Upon confirmation of pregnancy, ewes were housed individually where they were able to have visual contact with herd mates in adjacent pens at all times (Washburn et al. 2013). Conditions of constant temperature (22 °C) and fixed light/dark cycle (12 h:12 h) were maintained. During the entire pregnancy, ewes were fed a custom ration (Nutrena, Cargill Animal Nutrition, Minneapolis, MN) twice daily in the amount of 15 g of feed/kg body weight/day. Feed composition was the same as described by Lassala et al. (2011). Ewes were allowed free access to drinking water. Daily feed consumption was monitored and ewes consumed all of the food offered.
Treatment groups
Four treatment groups were used in this study: (1) a saline control group that received 0.9 % saline; (2) an alcohol group that received alcohol at a dosage of 1.75–2.5 g/kg body weight [40 % (w/v) diluted in 0.9 % saline]; (3) a glutamine group that received 0.9 % saline and 100 mg L-glutamine/kg body weight 3 times a day (i.e., 300 mg L-glutamine/kg body weight/day); and (4) an alcohol + glutamine group that received alcohol at a dosage of 1.75–2.5 g/kg body weight [40 % (w/v) diluted in 0.9 % saline] and 100 mg L-glutamine/kg body weight three times a day. 8–11 animals in all groups were used in this study. Losses of ewes from post-surgical complications resulted in unequal numbers of animals per treatment group for IV administration of saline, alcohol, L-glutamine, or alcohol plus L-glutamine. Detailed description of alcohol and L-glutamine dosing paradigm is given in the subsequent section.
Dosing paradigm
Dosing paradigm used in this study is depicted in Fig. 1. Saline or alcohol infusions were given intravenously (IV) through a jugular vein catheter over a period of 1 hour a day for 3 consecutive days per week from GD 99 to GD 115 to mimic a weekend binge drinking pattern. This period of gestation in sheep is associated with a steep rise in uterine blood flow (Rosenfeld 1977) and overlaps with the fetal brain growth spurt (Dobbing and Sands 1979). On GD 99, an intravenous catheter (16 gauge, 3.00 in Extended Use Catheter, Jorgensen, Loveland, CO) was placed percutaneously into the jugular vein.
Fig. 1.
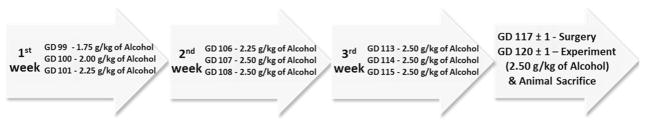
Model for chronic alcohol binging paradigm. Alcohol or saline infusions were given intravenously over a period of 1 h per day for 3 consecutive days per week between gestational days (GD) 99 and 115 to mimic a weekend binge drinking pattern exhibited by pregnant women. The first four doses of alcohol were 1.75, 2, 2.25 and 2.25 g/kg body weight on GD 99, 100, 101 and 106, respectively. Doses of alcohol were 2.5 g/kg body weight from GD 107. Some ewes received IV infusions of 100 mg L-glutamine/kg body weight three times a day (i.e., 300 mg L-glutamine/kg body weight/day) on three consecutive days per week. Fetuses were surgically instrumented on GD 117 ± 1 and studied on GD 120 ± 1. Full term in sheep is 147 days
On the day of infusions, ewes were connected to the infusion pump by 0830 h. Subsequently, saline or alcohol was infused continuously over 1 h into ewes. Infusion solutions were delivered intravenously by a peristaltic pump (VetFlo® 7701B IV Vet Infusion Pump, Grady Medical, Temecula, CA). The first four doses of alcohol were 1.75, 2, 2.25 and 2.25 g/kg body weight on GD 99, 100, 101 and 106, respectively, and thereafter were 2.5 g/kg body weight from GD 107 (Fig. 1). The alcohol dose of 2.5 g/kg body weight was selected to generate blood alcohol concentration (BAC) in the range of 300–370 mg/100 ml to mimic a binge alcohol exposure paradigm producing pathological fetal cerebellar Purkinje cell loss (Goodlett and Eilers 1997). This dose mimics clinically relevant patterns of drinking in women of child-bearing age and has been reported in clinical cases (Wells and Barnhill 1996; Urso et al. 1981; Church and Gerkin 1988; Hammond et al. 1973). The saline control and glutamine groups received a dose of saline (0.9 % NaCl) that was isovolumetric to the alcohol group. L-Glutamine powder (Sigma Aldrich, St. Louis, MO) was completely dissolved in sterile water at a concentration of 4.5 % (w/v) and passed through a 0.2-μm bacteriostatic filter. The solution was kept at room temperature and prepared 2 h prior to administration. Between GD 99 and 115, a 100 mg L-glutamine/kg body weight dose was administered intravenously as a 4.5 % (w/v) aqueous bolus three times a day (i.e., 300 mg L-glutamine/kg body weight/day) on three consecutive days per week (Fig. 1). In the alcohol plus L-glutamine group, L-glutamine was given as an IV bolus immediately before alcohol infusion. The IV administered L-glutamine per day amounted to 168 % of daily dietary L-glutamine intake by the gestating ewe (Table 1). No adverse effects or safety concerns of IV or oral glutamine supplementation were observed in newborns or in adult humans for L-glutamine doses in the range of 400–860 mg/kg body weight/day (Garlick 2001). In these studies, safety assessments were done by evaluating standard clinical chemistry, mental status, vital signs, temperature and clinical and subjective evidence of toxicity (Garlick 2001). Owing to the short half life of L-glutamine (Coster et al. 2004) and for the ease of administration, maternal L-glutamine supplementation was done 3 times per day. On GD 120 ± 1, animals received either saline, alcohol, L-glutamine, or alcohol plus L-glutamine infusion as described previously. Animals from the glutamine and the alcohol + glutamine groups received a single bolus of L-glutamine (100 mg/kg body weight) as a 4.5 % (w/v) solution just before the start of the final infusion.
Table 1.
Maternal L-glutamine intake from the enteral diet and L-glutamine supplementation to gestating ewes through intravenous administration
| Feed consumption by ewes (as-fed basis) | 15 g/kg body weight/day |
|---|---|
| L-Glutamine content in the enteral diet | 1.19 % (w/w; as-fed basis) |
| Dietary intake of L-glutamine | 1.19 % × 15 g/kg body weight/day = 178.5 mg/kg of body weight/day |
| IV bolus of L-glutamine | 100 mg/kg body weight × 3 times/day = 300 mg/kg of body weight/day |
L-Glutamine in the diet was determined using the enzymatic hydrolysis and high-performance liquid chromatography, as previously described (Dai et al. 2014; Li et al. 2011)
Procedure for surgical instrumentation
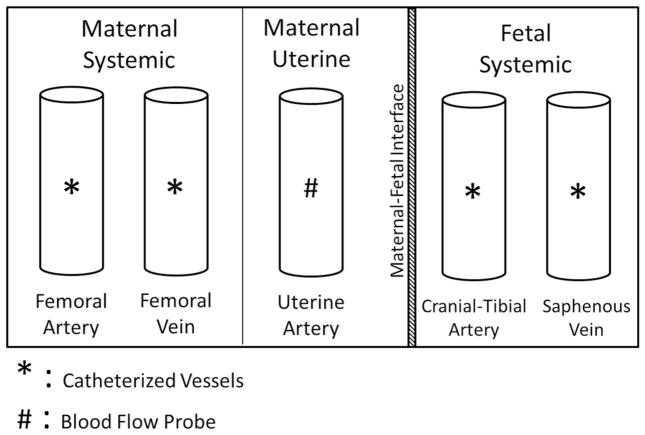
Surgical instrumentation and catheterization of the ewe and fetus were performed on GD 117 ± 1. Ewes were allowed free access to drinking water but underwent an 18 h fasting period before the surgical procedure. Surgery was conducted using sterile technique and all components were cold-gas sterilized using an AN-74i tabletop sterilizer (Andersen Products, Haw River, NC). Surgical anesthesia was induced with intravenous administration of 6.0 mg ketamine hydrochloride per kg body weight (Ketaved™, Vedco, St. Joseph, MO) and 0.3 mg diazepam per kg body weight (Abbott Labs, Abbott Park, IL) and the trachea was intubated. Surgical anesthesia was maintained with 2–3 % isoflurane (Fluriso, VetOne, Boise, ID) in oxygen delivered by a ventilation system (Matrx™ Model 3000, Midmark, Orchard Park, NY), and heart rate, ventilation rate, oxygen saturation, and expired carbon dioxide were monitored throughout the surgery using a Datascope Passport® 2 monitor (Mindray, Mahwah, NJ). Ewes were placed in dorsal recumbency throughout the surgery. Using standard surgical techniques (Cudd et al. 2001), catheters were inserted into the left and right maternal femoral artery and vein and advanced to the abdominal aorta and inferior vena cava (Fig. 2). Thereafter, laparotomy was performed via a ventral midline incision and the hind limb of the fetus was exteriorized. Both the left and right fetal cranial tibial arteries and saphenous veins were catheterized and advanced to the abdominal aorta and inferior vena cava, respectively, then the fetus was returned to the uterus (Fig. 2). A 6-mm transient-time ultrasonic perivascular flow probe (Transonic Systems Incorporated, Ithaca, NY) was secured around the primary uterine artery for recording uterine blood flow (UBF) (Fig. 2). As the incisions were closed, a catheter was installed inside the amniotic cavity. The catheters were filled with heparinized saline and sealed. All the catheters and flow probe leads were tunneled subcutaneously and then exteriorized through a small incision in the right flank of the ewe.
Fig. 2.
Illustration of surgical catheterization. Catheters were inserted into the maternal femoral artery and vein and they were advanced to the abdominal aorta and inferior vena cava, respectively. For the fetus, catheters were inserted into the cranial-tibial artery and saphenous vein and were advanced to the abdominal aorta and inferior vena cava, respectively. An ultrasonic perivascular flow probe was secured around the primary uterine artery
Upon completion of the surgery (1.5–2 h) and postoperative recovery (1 h), the ewe was returned to her pen. Buprenorphine hydrochloride (0.3 mg/ewe) (Buprenex®, Reckitt Benckiser, Berkshire, UK) was administered intramuscularly and flunixin meglumine (1.1 mg/kg body weight) (Banamine®, Merck Animal Health, Summit, NJ) was administered orally to the ewe every 12 h to control postoperative pain. In addition to penicillin G procaine (20,000 IU/kg) (PenOne Pro™, VetOne, Boise, ID), prophylactic antibiotic therapy included 3 doses of gentamicin sulfate (2.0 mg/kg body weight) (VetOne, Boise, ID) intramuscularly. All ewes were allowed to recover completely from instrumentation. During the recovery period (GD 117 and 119), ewes did not receive an infusion of saline, alcohol, or L-glutamine, had free access to drinking water, and received feed in the amount of 15 g/kg body weight/day. The final treatment with saline, alcohol, L-glutamine, or alcohol plus L-glutamine started on GD 120 ± 1, as described above. On this day, ewes had free access to drinking water but their morning feed was withheld before the start of the final infusion.
Measurement of systemic hemodynamic variables and uterine blood flow
On GD 120 ± 1, by 0830 h, one of the maternal arterial, fetal arterial and the amniotic catheters were connected to a strain-gauge pressure transducer and phasic blood pressure was measured at 1,000 Hz continuously throughout the experiment. Uterine artery blood flow was recorded using Transonic TS420 Perivascular Flowmeter and blood pressure was measured using a PowerLab® data acquisition system (PowerLab 8/30, model ML870). The data were analyzed using LabChart® software (ADInstruments, Inc., Colorado Springs, CO). Maternal and fetal arterial blood samples were collected at the baseline (0 min) and at the end of infusion (60 min) for the analysis of arterial pH, partial pressure of CO2 (PCO2), bicarbonate (HCO3) and partial pressure of O2 (PO2) using an i-Stat portable clinical analyzer (model 300A) (Abbott, Inc., Princeton, NJ). Maternal blood alcohol concentrations at the end of infusion (60 min) were measured using an enzymatic assay kit (Quantichrom® ethanol assay kit; BioAssay Systems, Hayward, CA).
Measurement of fetal regional brain blood flow
Fetal regional blood flow measurements were performed using the stable non-radioactive labeled microsphere technique (Reinhardt et al. 2001). Stable gold-labeled microspheres of 15 μm in diameter (BioPAL Inc., Worcester, MA), suspended in normal saline containing between 80 and 0.01 % thimerosal at a concentration of 2.5 × 106 spheres/ml were injected as a 1–1.5 mL bolus into the fetal inferior vena cava via the saphenous vein catheter at the baseline (0 min). The reference blood samples were withdrawn from the fetal abdominal aorta via the cranial-tibial artery catheter by a precision syringe pump (Harvard Apparatus, Holliston, MA) at a constant speed of 2.06 ml/min. A similar procedure was repeated at the end of the infusion (60 min) using samarium-labeled microspheres. It is important to note that because of the practicality of surgical procedures and experimentation, reference blood samples were drawn from the fetal abdominal aorta. Although the reference blood samples from the fetal abdominal aorta would not allow the measurement of actual fetal brain blood flow since distribution of blood between ascending and descending aortae is different (Rudolph and Heymann 1967), it will allow estimation of relative change in regional blood flow at the end of 60 min alcohol or saline infusion compared to their respective baseline. At the end of 60 min, ewes were euthanized using an IV injection of sodium pentobarbitone (75 mg/kg body weight). The uterus was exteriorized from the ewe and the fetus was removed. The brain was removed from the fetus and dissected into eight regions: frontal cortex, parietal cortex, temporal cortex, occipital cortex, cerebellum, brain stem, olfactory bulbs, and hippocampus. During sample dissection, careful consideration was given to maintain accurate and precise sectioning for each animal. The samples were weighed and placed into specialized vials. Tissue and reference blood samples were dried overnight at 70 °C and shipped to Bio Physics Assay Laboratory (BioPAL Inc., Worcester, MA), where they were exposed to a field of neutrons. The sample vials were stored for 48 h to allow short-lived activation products to decay. The results of the assay were reported as the number of disintegrations per min (DPM) measured for each labeled microsphere. Detailed description of the neutron activation procedure is explained elsewhere (Reinhardt et al. 2001). Absolute tissue blood flow at different times, measured by stable labeled microspheres was calculated using following formula:
where 2.06 ml/min is the reference blood withdrawal rate, [Microspheres in reference blood] is the DPM in reference blood, tissue weight was in grams, and [Microspheres in tissue] is the DPM in the tissue sample. Fold change in blood flow in any brain tissue sample was calculated by dividing blood flow at the end of infusion (60 min) by blood flow at the baseline (0 min).
Analysis of L-glutamine in plasma
Maternal and fetal plasma samples (50 μl) obtained at the baseline (0 min) and at 60 min after infusion were acidified with 50 μl of 1.5 mM HClO4 and then neutralized with 25 μL of 2 mM K2CO3 (Satterfield et al. 2012). HPLC-grade water (900 μl) was added to this solution and samples were centrifuged in a Microcentrifuge at 10,000 rpm for 5 min. The supernatant fluid was used for L-glutamine analysis by HPLC (Meininger and Wu 1997). Concentrations of L-glutamine in samples were calculated on the basis of authentic standard from Sigma Chemicals (St. Louis, MO) using the Waters Millenium-32 workstation (Waters Corporation, Milford, MA), as described earlier (Rezaei et al. 2013).
Statistical analysis
Two-way mixed ANOVA was performed on the maternal and fetal systemic hemodynamic parameters at the baseline (0 min) and at the end of infusion (60 min) on GD 120 ± 1 with treatment group and time point as factors. Differences among the treatment groups were analyzed using the Student–Newman–Keuls multiple comparison method. Mixed ANOVA was performed on the maternal uterine blood flow values for every 5 min time interval starting from the baseline (time 0) to the end of infusion (60 min) on GD 120 ± 1 using treatment group, and each time point as factors. Further pairwise comparisons were performed when appropriate using the Fisher’s protected least significant difference method. One-way ANOVA was performed among groups for the analysis of fold change in regional blood flow. A level of statistical significance was established at P < 0.05 and 0.05 < P < 0.1 were considered trends.
Results
Blood alcohol and plasma glutamine concentrations
The mean ± SEM maternal blood alcohol concentrations (BACs) at the end of alcohol infusion (60 min; the time point when values of BACs peaked) on GD 120 ± 1 were 304.2 ± 27.2 mg/dl and 325.4 ± 24.9 mg/dl in the alcohol and alcohol + glutamine groups, respectively. Data on concentrations of L-glutamine in maternal and fetal plasma at 0 and 60 min are summarized in Table 2. Alcohol administration reduced (P < 0.05) concentrations of L-glutamine in both maternal and fetal plasma. Notably, maternal L-glutamine supplementation increased (P < 0.05) concentrations of L-glutamine in both maternal and fetal plasma.
Table 2.
Concentration of L-glutamine in maternal and fetal plasma of gestating ewes with alcohol exposure
| Treatment | Number of ewes | Maternal plasma
|
Fetal plasma
|
||
|---|---|---|---|---|---|
| 0 min | 60 min | 0 min | 60 min | ||
| Saline control | 11 | 174 ± 5.3 | 142 ± 5.4c | 445 ± 24b | 425 ± 22b |
| Alcohol | 9 | 162 ± 9.3 | 110 ± 8.9d* | 222 ± 18d | 195 ± 15c* |
| L-Glutamine | 9 | 177 ± 5.6 | 340 ± 18a* | 704 ± 29a | 988 ± 47a* |
| Alcohol + L-Glutamine | 11 | 165 ± 6.4 | 232 ± 12b* | 324 ± 7.6c | 408 ± 14b* |
Values, expressed as nmol/ml, are mean ± SEM. See the text for experimental detail
0 min = baseline (before infusion)
60 min = the end of the 60-min infusion
P <0.05 vs the corresponding “0 min” value
Within a column, means sharing different superscript letters differ (P < 0.05)
Maternal systemic hemodynamic parameters
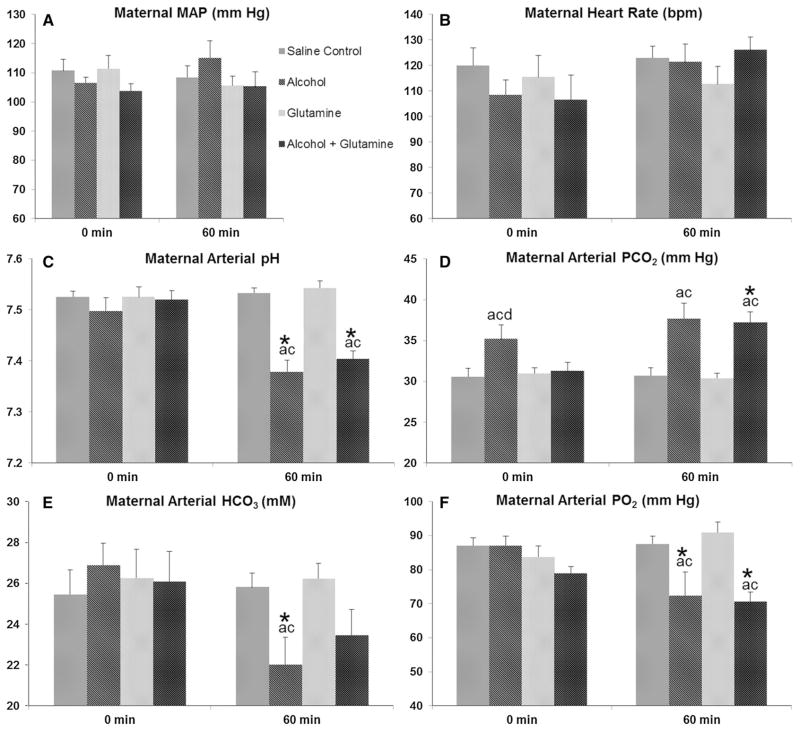
No changes were observed for maternal MAP and HR among groups at the baseline (0 min, i.e. the time when BAC was 0 mg/100 ml) or at the end of infusion (60 min) on GD 120 ± 1 (Fig. 3a, b). Baseline measurements on GD 120 ± 1 depict the chronic effect of repeated alcohol exposures and measurements done at the end of infusion on GD 120 ± 1 indicate the effect at the peak BAC. Baseline maternal arterial pH did not differ among groups, but at the end of infusion, maternal arterial pH was decreased in the alcohol and alcohol + glutamine groups, as compared to the saline control group or the glutamine group, and compared to their respective baseline (all P < 0.001) (Fig. 3c). Baseline maternal arterial PCO2 was higher in the alcohol group, as compared to the saline control (P = 0.008), glutamine (P = 0.021) and alcohol + glutamine (P = 0.022) groups (Fig. 3d). Maternal arterial PCO2 was elevated at the end of infusion in the alcohol and alcohol + glutamine groups, as compared to the saline control and glutamine groups (all P < 0.001). Levels of maternal arterial bicarbonate (HCO3) at the baseline did not differ among groups but were decreased at the end of infusion in the alcohol group, as compared to the saline control (P = 0.046) and glutamine (P = 0.031) groups (Fig. 3e). The level of maternal arterial bicarbonate (HCO3) at the end of infusion in the alcohol group was lower than its baseline (P = 0.019), whereas the level of maternal arterial bicarbonate (HCO3) at the end of infusion in the alcohol + glutamine group showed a decreasing trend, as compared to its baseline (P = 0.095). Baseline maternal arterial PO2 did not differ among groups, but at the end of infusion maternal arterial PO2 was decreased in the alcohol and alcohol + GLN groups, as compared to the saline control group (P = 0.003 and<0.001, respectively), and the glutamine group (all P < 0.001), as well as compared to their respective baselines (alcohol, P = 0.006 and alcohol + glutamine, P = 0.033) (Fig. 3f). These results indicate that maternal alcohol exposure results in maternal acidemia, hypercapnea and hypoxemia and maternal L-glutamine supplementation was able to compensate for these alcohol-induced acid–base imbalances.
Fig. 3.
Effects of alcohol and L-glutamine supplementation on maternal systemic hemodynamic parameters. Maternal systemic hemodynamic parameters and arterial blood gases were measured at the end of the chronic binge alcohol exposure paradigm on gestational day (GD) 120 ± 1. a, b Maternal mean arterial pressure (MAP) and heart rate (HR) did not differ among groups at the baseline (0 min) or at the end of alcohol infusion (60 min). c Maternal arterial pH was decreased at 60 min in the alcohol and alcohol + glutamine groups compared to the saline control and glutamine groups, as well as compared to their respective baselines. d Maternal arterial partial pressure of carbon dioxide (PCO2) was increased at 0 min in the alcohol group compared to all other groups and maternal arterial PCO2 was increased at 60 min in the alcohol and alcohol + glutamine groups compared to the saline control and glutamine groups. e Concentration of maternal arterial bicarbonate (HCO3) was decreased at 60 min in the alcohol group compared to the saline control and glutamine groups, as well as compared to the baseline. f Maternal arterial partial pressure of oxygen (PO2) was decreased at 60 min in the alcohol and alcohol + glutamine groups compared to the saline control and glutamine groups, and compared to their respective baselines. a, b, c and d indicate statistically significant differences (P < 0.05), compared to the saline control, alcohol, glutamine and alcohol + glutamine groups, respectively. Single asterisk indicate statistically significant differences (P < 0.05) in the alcohol and/or alcohol + glutamine group, compared to their respective baseline
Fetal systemic hemodynamic parameters
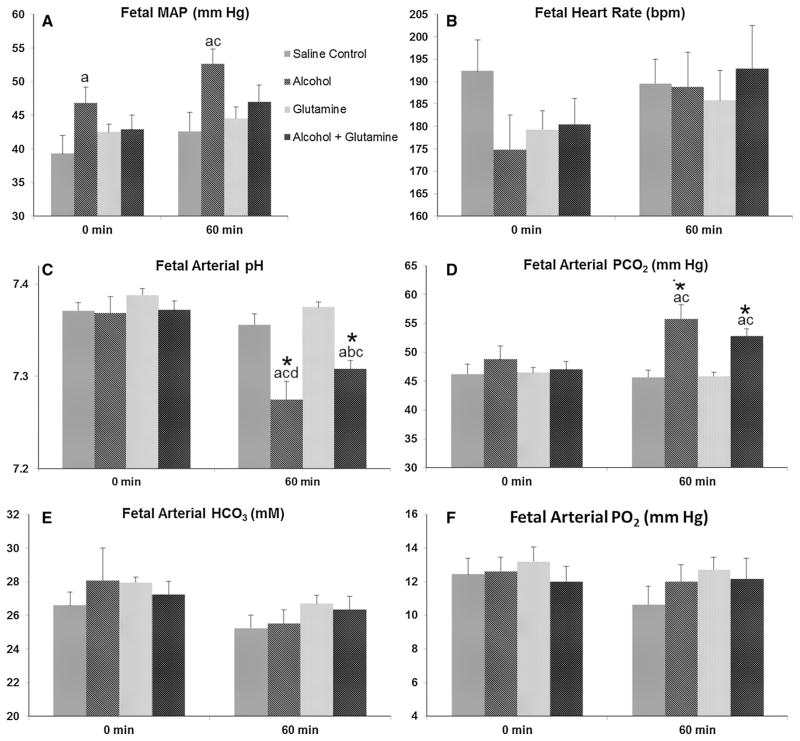
In the alcohol group, baseline (0 min) fetal MAP was higher, compared to the saline control group (P = 0.039) and fetal MAP at the end of infusion (60 min) was higher compared to the saline control (P = 0.006) and the glutamine (P = 0.025) groups on GD 120 ± 1 (Fig. 4a). No changes were observed for fetal HR among groups at the baseline or at the end of infusion on GD 120 ± 1 (Fig. 4b). Baseline fetal arterial pH did not differ among groups, but at the end of infusion fetal arterial pH was decreased in the alcohol and alcohol + GLN groups compared to the saline control group (P < 0.001 and =0.002, respectively), and the glutamine group (all P < 0.001), and as compared to their respective baselines (all P < 0.001) (Fig. 4c). Interestingly, fetal arterial pH at the end of infusion in the alcohol group was lower than the alcohol + glutamine group (P = 0.049). Baseline fetal arterial PCO2 did not differ among groups and fetal arterial PCO2 was increased at the end of infusion in the alcohol and alcohol + glutamine groups as compared to the saline control group (P < 0.001 and =0.001, respectively), and the glutamine group (all P < 0.001), and compared to their respective baselines (alcohol, P = 0.028 and alcohol + glutamine, P = 0.003) (Fig. 4d). No changes were observed for fetal arterial bicarbonate (HCO3) and PO2 among groups at the baseline (0 min) or at the end of infusion (60 min) on GD 120 ± 1 (Fig. 4e, f). These results indicate that the maternal alcohol exposure leads to an increase in fetal MAP, acidemia and hypercapnea and that maternal L-glutamine supplementation was able to mitigate these adverse effects of alcohol in the fetus.
Fig. 4.
Effects of alcohol and L-glutamine supplementation on fetal systemic hemodynamic parameters. Fetal systemic hemodynamic parameters and arterial blood gases were measured at the end of the chronic binge alcohol exposure paradigm on gestational day (GD) 120 ± 1. a Fetal mean arterial pressure (MAP) was elevated in the alcohol group at the baseline (0 min) compared to the saline control group and at the end of alcohol infusion (60 min), compared to the saline control and glutamine groups. b Fetal heart rate (HR) did not differ significantly among groups at 0 min and at 60 min. c Fetal arterial pH was decreased at 60 min in the alcohol and alcohol + glutamine groups, compared to the saline control group, glutamine group and compared to their respective baselines. Fetal arterial pH at 60 min in the alcohol group was significantly lower than the alcohol + glutamine group. d Fetal arterial partial pressure of carbon dioxide (PCO2) was increased at 60 min in the alcohol and alcohol + glutamine groups, compared to the saline control group, glutamine group and compared to their respective baseline. e, f Fetal arterial bicarbonate (HCO3) and partial pressure of oxygen (PO2) did not differ among groups at 0 or 60 min. a, b, c and d indicate statistically significant differences (P <0.05) compared to the saline control, alcohol, glutamine and alcohol + glutamine groups, respectively. Single asterisk indicate statistically significant differences (P < 0.05) in the alcohol and/or alcohol + glutamine group compared to their respective baseline
Maternal uterine artery blood flow
Uterine blood flow was measured on the last day of alcohol exposure. A significant main effect of treatment group (P <0.001) on uterine blood flow was present, but no significant effect of time or no significant interaction between treatment group and time was noted. Uterine artery blood flow was decreased in the alcohol and alcohol + glutamine groups compared to the saline control and glutamine groups (all P <0.001) (Fig. 5). No differences were observed between the saline control group and glutamine group, as well as between the alcohol group and alcohol + glutamine group. These results indicate that repeated maternal alcohol exposure decreased uterine artery blood flow and maternal L-glutamine supplementation did not affect uterine artery blood flow.
Fig. 5.
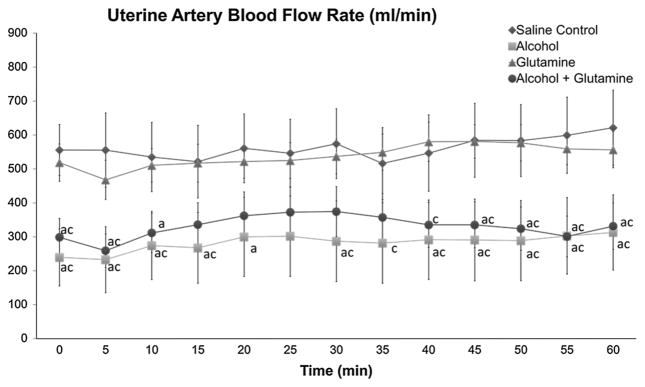
Effects of alcohol and L-glutamine supplementation on maternal uterine artery blood flow. Maternal uterine artery blood flow was measured at the end of the chronic binge alcohol exposure paradigm on gestational day (GD) 120 ± 1. Uterine artery blood flow (ml/min) was significantly decreased in the alcohol and alcohol + glutamine groups at the baseline (0 min) and during the period of the entire infusion (0–60 min). a and c indicate statistically significant differences (P < 0.05), compared to the saline control and glutamine groups, respectively
Fetal regional brain blood flow
A more than twofold increase was observed in the fetal cerebellar, brain stem and olfactory bulb blood flow at the end of 60 min from their respective baselines in the alcohol group (Fig. 6). The fold change in fetal cerebellar and brain stem blood flow in the alcohol group was higher than the saline control (cerebellum, P = 0.002 and brain stem, P = 0.001), glutamine (cerebellum, P = 0.011 and brain stem P = 0.009) and alcohol + glutamine (cerebellum, P = 0.025 and brain stem, P = 0.015) groups. Blood flow to the fetal olfactory bulb was higher in the alcohol group than in the saline control (P = 0.012) and glutamine (P = 0.025) groups. Further, the blood flow at the end of 60 min in the alcohol group exhibited a numerically increasing pattern in the hippocampus, frontal cortex, parietal cortex, temporal cortex and occipital cortex; however, the increases were not statistically significant. Estimated fetal whole-brain blood flow in the alcohol group was higher than in the saline control group (P = 0.013) (Fig. 6). These results indicate that maternal alcohol exposure results in a transient increase in fetal brain blood flow, especially to the cerebellum, brain stem and olfactory bulb. Maternal L-glutamine supplementation was able to prevent these alcohol-induced alterations in fetal regional brain blood flow.
Fig. 6.
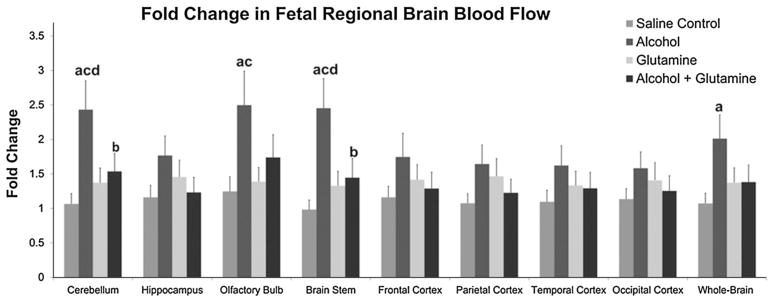
Effects of alcohol and glutamine supplementation on fetal regional brain blood flow. Fetal regional brain blood flows at the baseline (0 min) and at the end of alcohol infusion (60 min) were measured on the last day of alcohol treatment (GD 120 ± 1). The fold change in fetal cerebellar, olfactory bulb, brain stem and whole-brain blood flow was increased in the alcohol group at the end of 60 min from their respective baselines (0 min) compared to the saline control and glutamine groups. Maternal L-glutamine supplementation mitigated the alcohol-induced alteration in fetal regional brain blood flow
Discussion
Five major findings can be gleaned from this study. First, maternal alcohol exposure leads to maternal acidemia, hypercapnea, and hypoxemia. Second, maternal alcohol exposure results in an increase in fetal mean arterial pressure, acidemia and hypercapnea. A third and most important finding of this study is that maternal repeated binge alcohol exposure during the third trimester-equivalent period in the sheep model results in a more than 40 % reduction in uterine artery blood flow. Fourth, maternal alcohol exposure leads to a transient increase in fetal brain blood flow. The fifth and most innovative finding from this study is that maternal L-glutamine supplementation was able to diminish alcohol-induced maternal hypercapnea, fetal acidemia and alterations in fetal regional brain blood flow, hence limiting the brain-alcohol delivery during the periods of prenatal alcohol exposure.
Effect of alcohol on systemic hemodynamic parameters
The results from this study confirm the earlier findings (Cudd et al. 2001; Parnell et al. 2007; Ramadoss et al. 2007; West et al. 2001; Washburn et al. 2013) that alcohol exposure leads to maternal acidemia, hypercapnea and hypoxemia and fetal acidemia and hypercapnea but not fetal hypoxemia. Alcohol exposure in human clinical cases is known to result in mixed respiratory and metabolic acidosis (Lamminpaa and Vilska 1991; Sahn et al. 1975; Zehtabchi et al. 2005) and in animal models, acidemia has been described as a candidate mechanism for alcohol-induced developmental neuronal injury and altered amino acid homeostasis (Ramadoss et al. 2007, 2008; Cudd et al. 2001). In this study, our observation that alcohol exposure did not result in fetal hypoxemia is consistent with previous findings (Richardson et al. 1985; Cudd et al. 2001). Although the current report and results from our previous studies have shown that alcohol exposure results in maternal hypoxemia, the absence of alcohol-induced fetal hypoxemia may be due to fetal differences from adults in hemoglobin (Hb) concentration, a left-shifted O2-Hb saturation curve and higher cardiac output per unit body weight. Although there is no direct data from human studies on the effect of maternal alcohol consumption during pregnancy on systemic hemodynamic parameters, many clinical studies in men and non-pregnant women have shown that alcohol consumption directly or indirectly contributes to the development of hypertension (Fuchs et al. 2001; Saunders 1987; Witteman et al. 1990; Russell et al. 1991; van Leer et al. 1994).
The absence of alterations in alcohol-induced maternal HR and MAP demonstrates that alcohol-induced decreases in uterine blood flow are not due to a change in the uterine perfusion resulting from systemic hemodynamics, but rather is a local effect of alcohol on the gestational uterine artery adaptations. In rats, prenatal alcohol exposure (35 % alcohol derived calories) during the final one-third of pregnancy (from GD 14 to 20) had no effect on HR in the offspring as adults (Handa et al. 2007). Ultrasound imaging studies have shown that binge-like alcohol exposure (3 g/kg body weight) in a murine second trimester equivalent model did not alter fetal HR (Bake et al. 2012). Kenna and colleagues also reported that prenatal alcohol exposure (0.75 g/kg body weight) during the third trimester-equivalent period (from GD 95 to 133) in sheep did not affect fetal HR (Kenna et al. 2011). These reports support our findings that repeated maternal alcohol exposure had no significant effect on fetal HR. It is important to note that there are differences among these studies in the duration of exposure period and severity of alcohol exposure.
Alcohol exposure impairs uterine blood flow
We measured uterine blood flow on the last day of alcohol exposure, when we observed an approximately 40 % reduction in uterine artery blood flow. Our finding is strongly supported by results from an earlier acute alcohol exposure study in pregnant sheep, where intravenous infusion of 1 g alcohol/min over 1 h decreased uterine and placental blood flows, and the reductions were maintained for at least 2 h after the end of alcohol treatment; uterine blood flow was decreased by more than 20 %, whereas the umbilical blood flow was decreased by more than 30 % (Falconer 1990). In contrast, another acute study involving high BACs (up to 538 mg/100 ml) showed an increase in uterine blood flow possibly due to systemic hemodynamic effects and/or changes in perfusion pressure (Reynolds et al. 1996).
Studies have shown that enhanced production of nitric oxide (NO, a major vasodilator) by activation of endothelial NO synthase (eNOS) is a major local regulator of uterine blood flow during pregnancy (Magness et al. 2005; Rubanyi et al. 2002). A study in pregnant C57BL/6 J mice demonstrated that NO modulation of the systemic mesenteric artery vascular response was hampered due to alcohol exposure (Cook et al. 2001). In vitro studies have shown that binge-like alcohol exposure impairs eNOS activation and reduces mRNA levels for angiogenic genes and related proteome in endothelial cells of the ovine uterine artery, hence blunting the uterine vascular adaptations, including vasodilatory and angiogenic pathways (Ramadoss et al. 2010, 2011; Ramadoss and Magness 2011, 2012a, b, d). Finally, these findings have significant implications on fetal growth and development. These data lay the foundation for future studies aimed at investigating alcohol programming of uterine artery blood flow and fetal circulation, as well as underlying mechanistic perspectives, including programming of uterine artery endothelial function, and the endothelial vasodilatory NO system.
Alcohol exposure alters fetal cerebral blood flow
We herein hypothesized that repeated alcohol exposure would alter fetal brain blood flow and that maternal L-glutamine supplementation would ameliorate this effect. An acute alcohol exposure on GD 120 ± 1 resulted in a transient increase in fetal cerebellar, olfactory bulb, brain stem and overall whole-brain blood flow and maternal L-glutamine supplementation corrected this effect. Mann et al. (1975) have shown that fetal cerebral blood flow increases in response to acute alcohol exposure in sheep. Alcohol exposure on GD 132, which was preceded by chronic maternal alcohol exposure from GD 109 to 131, leads to increases in cerebral blood flow, especially in the ethanol-sensitive cerebellum (Parnell et al. 2007). Ultrasound imaging studies have revealed that binge alcohol (3 g/kg) exposure in a murine second trimester equivalent model results in rapid and persistent decrease in blood flow from the umbilical artery to the fetal brain on GD 12.5–14.5. In this study, blood flow was assessed by measuring fetal arterial blood acceleration and velocity time integral (VTI) data from pulse wave Doppler imaging experiments (Bake et al. 2012). Alcohol (1.5 g/kg body weight) exposure during the second trimester-equivalent period in sheep from GD 60–90 decreased and from GD 30–82 increased the subsequent cerebral vasodilatory responses to hypoxia and to vasodilatory hormones like vasoactive intestinal peptide, respectively (Mayock et al. 2007; Ngai et al. 2008). Gleason et al. (1997) demonstrated that the cerebral vasodilatory response to hypoxemia was substantially attenuated, showing that cerebral O2 delivery was not maintained in 1- to 4-day old neonatal lambs exposed to the alcohol (1 g/kg body weight) during the first trimester-equivalent period of gestation. In rats, moderate alcohol exposure (20–60 mmol/l) doesn’t alter the reactivity of the cerebral pial arteriole but exposure to higher doses of alcohol (80–100 mmol/l) impairs the dilatory response to agonists that trigger the synthesis of NO from the endothelium and neurons (Mayhan and Didion 1995). Human studies performed using the single photon emission computerized tomography technique revealed abnormalities and regional differences in cerebral blood flow in response to adult alcohol consumption and these differences were dependent on the degree of intoxication and age (Melgaard et al. 1990; Schwartz et al. 1993; Volkow et al. 1988). These clinical and preclinical investigations support our findings and emphasize that alcohol exposure alters cerebral vascular regulation.
Role of L-glutamine during development
Previously glutamine has been classified as a nutritionally nonessential amino acid, but due to its multifaceted roles in cell signaling, survival and growth, it is now classified as a conditionally essential dietary amino acid (Wu 2009; Xi et al. 2012). In gestating sheep, malnutrition results in reduced concentrations of L-glutamine in maternal and fetal plasma, as well as fetal growth restriction (Satterfield et al. 2013). Likewise, diminishing L-glutamine uptake and alterations in L-glutamine and L-glutamine-dependent metabolic processes leads to significant modifications of physiological and immunological functions (Muhling et al. 2007). L-Glutamine supplementation in postweaning pigs prevented jejunal atrophy, increased plasma amino acid levels, improved body weight gain, increased intestinal expression of genes related to cell growth and antioxidants, and suppressed expression of genes that promote oxidative stress and immune activation (Wu et al. 1996; Wang et al. 2008). Haynes et al. (2009) reported that L-glutamine administration in neonatal piglets enhanced growth performance and prevented endotoxin-induced enterocytes death. L-Glutamine supplementation in severely ill or extremely low birth weight infants has shown improvement in physical growth, neurodevelopmental outcomes, hepatic tolerance, plasma L-glutamine concentrations, and lowered infectious morbidity (Ehrenkranz et al. 2011; Wang et al. 2010; Poindexter et al. 2003; van den Berg et al. 2007). Thus, as a functional amino acid (Wu et al. 2013), humans and animals have requirements of dietary L-glutamine for maintenance, as well as optimal growth and development, particularly under such stress conditions as pregnancy, birth, lactation, and weaning (Lin et al. 2014; Wu et al. 2013).
Effect of L-glutamine on acid–base balance
Results from our study demonstrate that maternal L-glutamine supplementation was able to diminish the alcohol-induced maternal hypercapnea, transient fetal acidemia and alterations in fetal regional brain blood flow. Alcohol-induced acidemia stimulates glutamine uptake by renal mitochondria and its metabolism via the phosphate-dependent glutaminase gives stoichiometric amounts of glutamate and ammonia (Meister 1975; Nissim 1999). Ammonium ions are mainly excreted in the urine to facilitate the excretion of metabolic acids or anions, while conserving sodium and potassium ions, hence contributing to counteraction of the acidosis (Curthoys and Watford 1995). Mitigation of alcohol induced-acidemia in the fetal but not in the maternal compartment could be attributed to the fact that the fetus is capable of extracting L-glutamine from the placenta, and the extracted L-glutamine is delivered into the fetal circulation at a rate that is the highest among all other amino acids (Battaglia 2000; Bell et al. 1989; Lemons et al. 1976). Additionally, the placenta synthesizes a large amount of L-glutamine from branched-chain amino acids and α-ketoglutarate (Self et al. 2004). Also, the bioavailability of glutamine in ovine fetal plasma is 2–3 times greater than that of maternal plasma throughout gestation (Kwon et al. 2003). However, it should be noted that levels of maternal bicarbonate were decreased in the alcohol group but not in the alcohol + glutamine group at the end of 60 min on GD 120 ± 1. These findings indicate that maternal L-glutamine supplementation was able to correct alcohol-induced acid–base imbalances. We suggest that an increase in the dose and/or frequency of maternal L-glutamine supplementation will greatly improve the ability of L-glutamine to mitigate the negative effects of prenatal alcohol exposure.
Role of acid–base imbalance and glutamine on vascular function
One study in a rat model showed that cerebrovascular reactivity in response to alcohol exposure is mediated by hypercapnea (Hemmingsen and Barry 1979). Studies in non-pregnant monkey, dog and perinatal goat models have demonstrated that cerebral blood flow was increased in response to acute metabolic and respiratory acidosis, and this increase in cerebral flow was linearly related to the degree of hypercapnic acidemia (Harper and Glass 1965; Grubb et al. 1974; Bucciarelli and Eitzman 1979). Many studies have demonstrated the ability of L-glutamine to impair NO-mediated vasodilation by inhibiting citrulline uptake and arginine synthesis or by metabolism of L-glutamine to glucosamine which in turn inhibits availability of NADPH (an essential cofactor for NOS) (Lee et al. 1996; Arnal et al. 1995; Wu et al. 2001; Meininger and Wu 1997). Collectively, these findings support our results and imply that alcohol-induced acidemia-hypercapnea is possibly a key mechanism underlying alcohol-induced increases in fetal cerebral blood flow, and L-glutamine-induced decreases in NO release and the capability of L-glutamine to counteract acid–base imbalances through renal ammoniagenesis may be responsible for preventing alcohol-induced increases in brain blood flow. It should be noted that we and others have shown that L-glutamine exhibits a gluconeogenic effect (Washburn et al. 2013) via provision of carbon skeleton and activation of glucagon secretion (Greenfield et al. 2009) and glucagon is known to increase blood flow (Premen 1987; Shoemaker et al. 1959; Tibblin et al. 1970). Houdijk et al. (1994) have reported that a glutamine-enriched enteral diet increased splanchnic blood flow independent of activation of glucagon and NO. However, the current consensus is that L-glutamine does not directly enhance NO synthesis by constitutive NO synthases in endothelial cells or other cell types (Wu and Meininger 2002). In support of this view, we did not observe a significant effect of maternal L-glutamine supplementation on uterine blood flow. These findings indicate that the effect of hypercapnic acidemia and L-glutamine on blood flow differs temporally as well as regionally and contributes to the regional differences in alterations in blood flow in the fetus.
Although the cardiovascular adaptations of the mother during pregnancy and the offspring during perinatal, neonatal, juvenile, adolescent and adulthood periods are determined by a number of factors, altered vascular programming during gestation may influence development of cardiovascular disorders in the future. Although researchers in the field of FASD have focused their attention on maternal uterine vascular and fetal cardiovascular complications, effects of gestational alcohol exposure on the long-term cardiovascular health of offspring are unknown. Considering the devastating role of alcohol and the fact that the incidence of maternal drinking during pregnancy has not declined, with an estimated incidence of 2–5 % of the US population (Caetano et al. 2006; May et al. 2009), it is very important to conduct multi-institutional preclinical and clinical investigations to understand the long term effect of prenatal alcohol exposure on cardiovascular and metabolic adaptations of offspring. In addition to shedding light on the effects of gestational alcohol exposure on the maternal/fetal cardiovascular parameters, the results of the present study will also guide further scientific exploration of therapeutic interventions.
In summary, findings from this study indicate that repeated maternal alcohol exposure in gestating sheep results in maternal and fetal acidemia and hypercapnea, maternal, but not fetal hypoxemia, an elevation in fetal MAP, a decrease in uterine blood flow, and a transient increase in fetal brain blood flow. Maternal L-glutamine supplementation was able to mitigate alcohol-induced maternal hypercapnea, fetal acidemia and the transient increase in fetal brain blood flow. These results provide a strong foundation for future studies on assessing the effect of maternal L-glutamine supplementation to prevent the developmental damage in the fetal brain owing to prenatal alcohol exposure.
Acknowledgments
We would like to acknowledge the important role of the late Dr. Timothy Cudd in starting this study and making significant contributions. We would like to thank Raine Lunde, Brittney Kramer, Tracie Hohman, Kelsey Nation, Sarah Baker, Zoe Browning, Dillon Johnson, Lance Wheeler, Colt Sharpton, and Sudath Dahanayaka for their assistance in this study. This research was supported by NIAAA grant AA010940 (SW and GW) and AA18166-2 (SW), AA19446 (JR).
Footnotes
Conflict of interest The authors have no conflicts of interest to disclose.
Contributor Information
Onkar B. Sawant, Department of Veterinary Physiology and Pharmacology and Michael E. DeBakey Institute, College of Veterinary Medicine and Biomedical Sciences, 4466 Texas A&M University, College Station, TX 77843-4466, USA
Jayanth Ramadoss, Department of Obstetrics and Gynecology, University of Texas Medical Branch, Galveston, TX 77555, USA.
Gary D. Hankins, Department of Obstetrics and Gynecology, University of Texas Medical Branch, Galveston, TX 77555, USA
Guoyao Wu, Department of Animal Science, Texas A&M University, College Station, TX 77843, USA.
Shannon E. Washburn, Email: swashburn@cvm.tamu.edu, Department of Veterinary Physiology and Pharmacology and Michael E. DeBakey Institute, College of Veterinary Medicine and Biomedical Sciences, 4466 Texas A&M University, College Station, TX 77843-4466, USA
References
- Alexander BT, Hendon AE, Ferril G, Dwyer TM. Renal denervation abolishes hypertension in low-birth-weight offspring from pregnant rats with reduced uterine perfusion. Hypertension. 2005;45(4):754–758. doi: 10.1161/01.HYP.0000153319.20340.2a. [DOI] [PubMed] [Google Scholar]
- Arnal JF, Munzel T, Venema RC, James NL, Bai CL, Mitch WE, Harrison DG. Interactions between L-arginine and L-glutamine change endothelial NO production. An effect independent of NO synthase substrate availability. J Clin Invest. 1995;95(6):2565–2572. doi: 10.1172/JCI117957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bake S, Tingling JD, Miranda RC. Ethanol exposure during pregnancy persistently attenuates cranially directed blood flow in the developing fetus: evidence from ultrasound imaging in a murine second trimester equivalent model. Alcohol Clin Exp Res. 2012;36(5):748–758. doi: 10.1111/j.1530-0277.2011.01676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglia FC. Glutamine and glutamate exchange between the fetal liver and the placenta. J Nutr. 2000;130(4S Suppl):974S–977S. doi: 10.1093/jn/130.4.974S. [DOI] [PubMed] [Google Scholar]
- Bell AW, Kennaugh JM, Battaglia FC, Meschia G. Uptake of amino acids and ammonia at mid-gestation by the fetal lamb. Q J Exp Physiol. 1989;74(5):635–643. doi: 10.1113/expphysiol.1989.sp003316. [DOI] [PubMed] [Google Scholar]
- Bucciarelli RL, Eitzman DV. Cerebral blood flow during acute acidosis in perinatal goats. Pediatr Res. 1979;13(3):178–180. doi: 10.1203/00006450-197903000-00009. [DOI] [PubMed] [Google Scholar]
- Caetano R, Ramisetty-Mikler S, Floyd LR, McGrath C. The epidemiology of drinking among women of child-bearing age. Alcohol Clin Exp Res. 2006;30(6):1023–1030. doi: 10.1111/j.1530-0277.2006.00116.x. [DOI] [PubMed] [Google Scholar]
- Church MW, Gerkin KP. Hearing disorders in children with fetal alcohol syndrome: findings from case reports. Pediatrics. 1988;82(2):147–154. [PubMed] [Google Scholar]
- Cook JL, Zhang Y, Davidge ST. Vascular function in alcohol-treated pregnant and nonpregnant mice. Am J Physiol Regul Integr Comp Physiol. 2001;281(5):R1449–1455. doi: 10.1152/ajpregu.2001.281.5.R1449. [DOI] [PubMed] [Google Scholar]
- Coster J, McCauley R, Hall J. Glutamine: metabolism and application in nutrition support. Asia Pac J Clin Nutr. 2004;13(1):25–31. [PubMed] [Google Scholar]
- Cudd TA, Chen WJ, Parnell SE, West JR. Third trimester binge ethanol exposure results in fetal hypercapnea and acidemia but not hypoxemia in pregnant sheep. Alcohol Clin Exp Res. 2001;25(2):269–276. [PubMed] [Google Scholar]
- Curthoys NP, Watford M. Regulation of glutaminase activity and glutamine metabolism. Annu Rev Nutr. 1995;15:133–159. doi: 10.1146/annurev.nu.15.070195.001025. [DOI] [PubMed] [Google Scholar]
- Dai ZL, Wu ZL, Jia SC, Wu G. Analysis of amino acid composition in proteins of animal tissues and foods as pre-column o-phthaldialdehyde derivatives by HPLC with fluorescence detection. J Chromatogr B. 2014 doi: 10.1016/j.jchromb.2014.03.025. [DOI] [PubMed] [Google Scholar]
- Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev. 1979;3(1):79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- Ehrenkranz RA, Das A, Wrage LA, Poindexter BB, Higgins RD, Stoll BJ, Oh W. Early nutrition mediates the influence of severity of illness on extremely LBW infants. Pediatr Res. 2011;69(6):522–529. doi: 10.1203/PDR.0b013e318217f4f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falconer J. The effect of maternal ethanol infusion on placental blood flow and fetal glucose metabolism in sheep. Alcohol Alcohol. 1990;25(4):413–416. [PubMed] [Google Scholar]
- Fuchs FD, Chambless LE, Whelton PK, Nieto FJ, Heiss G. Alcohol consumption and the incidence of hypertension: the atherosclerosis risk in communities study. Hypertension. 2001;37(5):1242–1250. doi: 10.1161/01.hyp.37.5.1242. [DOI] [PubMed] [Google Scholar]
- Garlick PJ. Assessment of the safety of glutamine and other amino acids. J Nutr. 2001;131(9 Suppl):2556S–2561S. doi: 10.1093/jn/131.9.2556S. [DOI] [PubMed] [Google Scholar]
- Gleason CA, Iida H, Hotchkiss KJ, Northington FJ, Traystman RJ. Newborn cerebrovascular responses after first trimester moderate maternal ethanol exposure in sheep. Pediatr Res. 1997;42(1):39–45. doi: 10.1203/00006450-199707000-00007. [DOI] [PubMed] [Google Scholar]
- Goodlett CR, Eilers AT. Alcohol-induced Purkinje cell loss with a single binge exposure in neonatal rats: a stereological study of temporal windows of vulnerability. Alcohol Clin Exp Res. 1997;21(4):738–744. (pii:00000374-199706000-00028) [PubMed] [Google Scholar]
- Greenfield JR, Farooqi IS, Keogh JM, Henning E, Habib AM, Blackwood A, Reimann F, Holst JJ, Gribble FM. Oral glutamine increases circulating glucagon-like peptide 1, glucagon, and insulin concentrations in lean, obese, and type 2 diabetic subjects. Am J Clin Nutr. 2009;89(1):106–113. doi: 10.3945/ajcn.2008.26362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubb RL, Jr, Raichle ME, Eichling JO, Ter-Pogossian MM. The effects of changes in PaCO2 on cerebral blood volume, blood flow, and vascular mean transit time. Stroke. 1974;5(5):630–639. doi: 10.1161/01.str.5.5.630. [DOI] [PubMed] [Google Scholar]
- Hammond KB, Rumack BH, Rodgerson DO. Blood ethanol. A report of unusually high levels in a living patient. JAMA. 1973;226(1):63–64. doi: 10.1001/jama.226.1.63. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Zuloaga DG, McGivern RF. Prenatal ethanol exposure alters core body temperature and corticosterone rhythms in adult male rats. Alcohol. 2007;41(8):567–575. doi: 10.1016/j.alcohol.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper AM, Glass HI. Effect of alterations in the arterial carbon dioxide tension on the blood flow through the cerebral cortex at normal and low arterial blood pressures. J Neurol Neurosurg Psychiatry. 1965;28(5):449–452. doi: 10.1136/jnnp.28.5.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes TE, Li P, Li X, Shimotori K, Sato H, Flynn NE, Wang J, Knabe DA, Wu G. L-Glutamine or L-alanyl-L-glutamine prevents oxidant- or endotoxin-induced death of neonatal enterocytes. Amino Acids. 2009;37(1):131–142. doi: 10.1007/s00726-009-0243-x. [DOI] [PubMed] [Google Scholar]
- Heitmann RN, Bergman EN. Integration of amino acid metabolism in sheep: effects of fasting and acidosis. Am J Physiol. 1980;239(4):E248–E254. doi: 10.1152/ajpendo.1980.239.4.E248. [DOI] [PubMed] [Google Scholar]
- Hemmingsen R, Barry DI. Adaptive changes in cerebral blood flow and oxygen consumption during ethanol intoxication in the rat. Acta Physiol Scand. 1979;106(3):249–255. doi: 10.1111/j.1748-1716.1979.tb06395.x. [DOI] [PubMed] [Google Scholar]
- Houdijk AP, Van Leeuwen PA, Boermeester MA, Van Lambalgen T, Teerlink T, Flinkerbusch EL, Sauerwein HP, Wesdorp RI. Glutamine-enriched enteral diet increases splanchnic blood flow in the rat. Am J Physiol. 1994;267(6 Pt 1):G1035–G1040. doi: 10.1152/ajpgi.1994.267.6.G1035. [DOI] [PubMed] [Google Scholar]
- Kawaguchi T, Brusilow SW, Traystman RJ, Koehler RC. Glutamine-dependent inhibition of pial arteriolar dilation to acetylcholine with and without hyperammonemia in the rat. Am J Physiol Regul Integr Comp Physiol. 2005;288(6):R1612–1619. doi: 10.1152/ajpregu.00783.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenna K, De Matteo R, Hanita T, Rees S, Sozo F, Stokes V, Walker D, Bocking A, Brien J, Harding R. Daily ethanol exposure during late ovine pregnancy: physiological effects in the mother and fetus in the apparent absence of overt fetal cerebral dysmorphology. Am J Physiol Regul Integr Comp Physiol. 2011;301(4):R926–936. doi: 10.1152/ajpregu.00711.2010. [DOI] [PubMed] [Google Scholar]
- Kwon H, Spencer TE, Bazer FW, Wu G. Developmental changes of amino acids in ovine fetal fluids. Biol Reprod. 2003;68(5):1813–1820. doi: 10.1095/biolreprod.102.012971. [DOI] [PubMed] [Google Scholar]
- Lamminpaa A, Vilska J. Acid-base balance in alcohol users seen in an emergency room. Vet Hum Toxicol. 1991;33(5):482–485. [PubMed] [Google Scholar]
- Lang U, Baker RS, Braems G, Zygmunt M, Kunzel W, Clark KE. Uterine blood flow–a determinant of fetal growth. Eur J Obstet Gynecol Reprod Biol. 2003;110(Suppl 1):S55–61. doi: 10.1016/s0301-2115(03)00173-8. [DOI] [PubMed] [Google Scholar]
- Lassala A, Bazer FW, Cudd TA, Datta S, Keisler DH, Satterfield MC, Spencer TE, Wu G. Parenteral administration of L-arginine enhances fetal survival and growth in sheep carrying multiple fetuses. J Nutr. 2011;141(5):849–855. doi: 10.3945/jn.111.138172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TJ, Sarwinski S, Ishine T, Lai CC, Chen FY. Inhibition of cerebral neurogenic vasodilation by L-glutamine and nitric oxide synthase inhibitors and its reversal by L-citrulline. J Pharmacol Exp Ther. 1996;276(2):353–358. [PubMed] [Google Scholar]
- Lemons JA, Adcock EW, 3rd, Jones MD, Jr, Naughton MA, Meschia G, Battaglia FC. Umbilical uptake of amino acids in the unstressed fetal lamb. J Clin Invest. 1976;58(6):1428–1434. doi: 10.1172/JCI108598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XL, Rezaei R, Li P, Wu G. Composition of amino acids in feed ingredients for animal diets. Amino Acids. 2011;40:1159–1168. doi: 10.1007/s00726-010-0740-y. [DOI] [PubMed] [Google Scholar]
- Lin G, Wang X, Wu G, Feng C, Zhou H, Li D, Wang J. Improving amino acid nutrition to prevent intrauterine growth restriction in mammals. Amino Acids. 2014 doi: 10.1007/s00726-014-1725-z. [DOI] [PubMed] [Google Scholar]
- Magness RR, Phernetton TM, Gibson TC, Chen DB. Uterine blood flow responses to ICI 182 780 in ovariectomized oestradiol-17beta-treated, intact follicular and pregnant sheep. J Physiol. 2005;565(Pt 1):71–83. doi: 10.1113/jphysiol.2005.086439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann LI, Bhakthavathsalan A, Liu M, Makowski P. Effect of alcohol on fetal cerebral function and metabolism. Am J Obstet Gynecol. 1975;122(7):845–851. doi: 10.1016/0002-9378(75)90726-7. [DOI] [PubMed] [Google Scholar]
- May PA, Gossage JP, Kalberg WO, Robinson LK, Buckley D, Manning M, Hoyme HE. Prevalence and epidemiologic characteristics of FASD from various research methods with an emphasis on recent in-school studies. Dev Disabil Res Rev. 2009;15(3):176–192. doi: 10.1002/ddrr.68. [DOI] [PubMed] [Google Scholar]
- Mayhan WG, Didion SP. Acute effects of ethanol on responses of cerebral arterioles. Stroke. 1995;26(11):2097–2101. doi: 10.1161/01.str.26.11.2097. discussion 2102. [DOI] [PubMed] [Google Scholar]
- Mayock DE, Ness D, Mondares RL, Gleason CA. Binge alcohol exposure in the second trimester attenuates fetal cerebral blood flow response to hypoxia. J Appl Physiol. 2007;102(3):972–977. doi: 10.1152/japplphysiol.00956.2006. [DOI] [PubMed] [Google Scholar]
- Meininger CJ, Wu G. L-glutamine inhibits nitric oxide synthesis in bovine venular endothelial cells. J Pharmacol Exp Ther. 1997;281(1):448–453. [PubMed] [Google Scholar]
- Meister A. Function of glutathione in kidney via the gamma-glutamyl cycle. Med Clin North Am. 1975;59(3):649–666. doi: 10.1016/s0025-7125(16)32015-6. [DOI] [PubMed] [Google Scholar]
- Melgaard B, Henriksen L, Ahlgren P, Danielsen UT, Sorensen H, Paulson OB. Regional cerebral blood flow in chronic alcoholics measured by single photon emission computerized tomography. Acta Neurol Scand. 1990;82(2):87–93. doi: 10.1111/j.1600-0404.1990.tb01594.x. [DOI] [PubMed] [Google Scholar]
- Muhling J, Burchert D, Langefeld TW, Matejec R, Harbach H, Engel J, Wolff M, Welters ID, Fuchs M, Menges T, Krull M, Hempelmann G. Pathways involved in alanyL-glutamine-induced changes in neutrophil amino- and alpha-keto acid homeostasis or immunocompetence. Amino Acids. 2007;33(3):511–524. doi: 10.1007/s00726-006-0395-x. [DOI] [PubMed] [Google Scholar]
- Ngai AC, Mondares RL, Mayock DE, Gleason CA. Fetal alcohol exposure alters cerebrovascular reactivity to vasoactive intestinal peptide in adult sheep. Neonatology. 2008;93(1):45–51. doi: 10.1159/000105524. [DOI] [PubMed] [Google Scholar]
- Nissim I. Newer aspects of glutamine/glutamate metabolism: the role of acute pH changes. Am J Physiol. 1999;277(4 Pt 2):F493–497. doi: 10.1152/ajprenal.1999.277.4.F493. [DOI] [PubMed] [Google Scholar]
- Okada T, Watanabe Y, Brusilow SW, Traystman RJ, Koehler RC. Interaction of glutamine and arginine on cerebrovascular reactivity to hypercapnia. Am J Physiol Heart Circ Physiol. 2000;278(5):H1577–1584. doi: 10.1152/ajpheart.2000.278.5.H1577. [DOI] [PubMed] [Google Scholar]
- Osol G, Mandala M. Maternal uterine vascular remodeling during pregnancy. Physiology (Bethesda) 2009;24:58–71. doi: 10.1152/physiol.00033.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnell SE, Ramadoss J, Delp MD, Ramsey MW, Chen WJ, West JR, Cudd TA. Chronic ethanol increases fetal cerebral blood flow specific to the ethanol-sensitive cerebellum under normoxaemic, hypercapnic and acidaemic conditions: ovine model. Exp Physiol. 2007;92(5):933–943. doi: 10.1113/expphysiol.2007.038091. [DOI] [PubMed] [Google Scholar]
- Poindexter BB, Ehrenkranz RA, Stoll BJ, Koch MA, Wright LL, Oh W, Papile LA, Bauer CR, Carlo WA, Donovan EF, Fanaroff AA, Korones SB, Laptook AR, Shankaran S, Stevenson DK, Tyson JE, Lemons JA. Effect of parenteral glutamine supplementation on plasma amino acid concentrations in extremely low-birth-weight infants. Am J Clin Nutr. 2003;77(3):737–743. doi: 10.1093/ajcn/77.3.737. [DOI] [PubMed] [Google Scholar]
- Premen AJ. Splanchnic and renal hemodynamic responses to intraportal infusion of glucagon. Am J Physiol. 1987;253(6 Pt 2):F1105–1112. doi: 10.1152/ajprenal.1987.253.6.F1105. [DOI] [PubMed] [Google Scholar]
- Ramadoss J, Magness RR. 2-D DIGE uterine endothelial proteomic profile for maternal chronic binge-like alcohol exposure. J Proteomics. 2011;74(12):2986–2994. doi: 10.1016/j.jprot.2011.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadoss J, Magness RR. Alcohol-induced alterations in maternal uterine endothelial proteome: a quantitative iTRAQ mass spectrometric approach. Reprod Toxicol. 2012a;34(4):538–544. doi: 10.1016/j.reprotox.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadoss J, Magness RR. Multiplexed digital quantification of binge-like alcohol-mediated alterations in maternal uterine angiogenic mRNA transcriptome. Physiol Genomics. 2012b;44(11):622–628. doi: 10.1152/physiolgenomics.00009.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadoss J, Magness RR. Vascular effects of maternal alcohol consumption. Am J Physiol Heart Circ Physiol. 2012c;303(4):H414–421. doi: 10.1152/ajpheart.00127.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadoss J, Magness RR. Vascular effects of maternal alcohol consumption. Am J Physiol Heart Circ Physiol. 2012d doi: 10.1152/ajpheart.00127.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadoss J, Hogan HA, Given JC, West JR, Cudd TA. Binge alcohol exposure during all three trimesters alters bone strength and growth in fetal sheep. Alcohol. 2006;38(3):185–192. doi: 10.1016/j.alcohol.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Ramadoss J, Lunde ER, Pina KB, Chen WJ, Cudd TA. All three trimester binge alcohol exposure causes fetal cerebellar purkinje cell loss in the presence of maternal hypercapnea, acidemia, and normoxemia: ovine model. Alcohol Clin Exp Res. 2007;31(7):1252–1258. doi: 10.1111/j.1530-0277.2007.00422.x. [DOI] [PubMed] [Google Scholar]
- Ramadoss J, Wu G, Cudd TA. Chronic binge ethanol-mediated acidemia reduces availability of glutamine and related amino acids in maternal plasma of pregnant sheep. Alcohol. 2008;42(8):657–666. doi: 10.1016/j.alcohol.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadoss J, Liao WX, Chen DB, Magness RR. High-throughput caveolar proteomic signature profile for maternal binge alcohol consumption. Alcohol. 2010;44(7–8):691–697. doi: 10.1016/j.alcohol.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadoss J, Jobe SO, Magness RR. Alcohol and maternal uterine vascular adaptations during pregnancy-part I: effects of chronic in vitro binge-like alcohol on uterine endothelial nitric oxide system and function. Alcohol Clin Exp Res. 2011;35(9):1686–1693. doi: 10.1111/j.1530-0277.2011.01515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadoss J, Liao WX, Morschauser TJ, Lopez GE, Patankar MS, Chen DB, Magness RR. Endothelial caveolar hub regulation of adenosine triphosphate-induced endothelial nitric oxide synthase subcellular partitioning and domain-specific phosphorylation. Hypertension. 2012;59(5):1052–1059. doi: 10.1161/HYPERTENSIONAHA.111.189498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt CP, Dalhberg S, Tries MA, Marcel R, Leppo JA. Stable labeled microspheres to measure perfusion: validation of a neutron activation assay technique. Am J Physiol Heart Circ Physiol. 2001;280(1):H108–116. doi: 10.1152/ajpheart.2001.280.1.H108. [DOI] [PubMed] [Google Scholar]
- Reynolds LP, Redmer DA. Utero-placental vascular development and placental function. J Anim Sci. 1995;73(6):1839–1851. doi: 10.2527/1995.7361839x. [DOI] [PubMed] [Google Scholar]
- Reynolds JD, Penning DH, Dexter F, Atkins B, Hrdy J, Poduska D, Brien JF. Ethanol increases uterine blood flow and fetal arterial blood oxygen tension in the near-term pregnant ewe. Alcohol. 1996;13(3):251–256. doi: 10.1016/0741-8329(95)02051-9. [DOI] [PubMed] [Google Scholar]
- Rezaei R, Knabe DA, Tekwe CD, Dahanayaka S, Ficken MD, Fielder SE, Eide SJ, Lovering SL, Wu G. Dietary supplementation with monosodium glutamate is safe and improves growth performance in postweaning pigs. Amino Acids. 2013;44:911–923. doi: 10.1007/s00726-012-1420-x. [DOI] [PubMed] [Google Scholar]
- Richardson BS, Patrick JE, Bousquet J, Homan J, Brien JF. Cerebral metabolism in fetal lamb after maternal infusion of ethanol. Am J Physiol. 1985;249(5 Pt 2):R505–509. doi: 10.1152/ajpregu.1985.249.5.R505. [DOI] [PubMed] [Google Scholar]
- Rosenfeld CR. Distribution of cardiac output in ovine pregnancy. Am J Physiol. 1977;232(3):H231–235. doi: 10.1152/ajpheart.1977.232.3.H231. [DOI] [PubMed] [Google Scholar]
- Rubanyi GM, Johns A, Kauser K. Effect of estrogen on endothelial function and angiogenesis. Vascul Pharmacol. 2002;38(2):89–98. doi: 10.1016/s0306-3623(02)00131-3. [DOI] [PubMed] [Google Scholar]
- Rudolph AM, Heymann MA. The circulation of the fetus in utero. Methods for studying distribution of blood flow, cardiac output and organ blood flow. Circ Res. 1967;21(2):163–184. doi: 10.1161/01.res.21.2.163. [DOI] [PubMed] [Google Scholar]
- Russell M, Cooper ML, Frone MR, Welte JW. Alcohol drinking patterns and blood pressure. Am J Public Health. 1991;81(4):452–457. doi: 10.2105/ajph.81.4.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahn SA, Lakshminarayan S, Pierson DJ, Weil JV. Effect of ethanol on the ventilatory responses to oxygen and carbon dioxide in man. Clin Sci Mol Med. 1975;49(1):33–38. doi: 10.1042/cs0490033. [DOI] [PubMed] [Google Scholar]
- Satterfield CM, Dunlap KA, Keisler DH, Bazer FW, Wu G. Arginine nutrition and fetal brown adipose tissue development in diet-induced obese sheep. Amino Acids. 2012;43(4):1593–1603. doi: 10.1007/s00726-012-1235-9. [DOI] [PubMed] [Google Scholar]
- Satterfield MC, Dunlap KA, Keisler DH, Bazer FW, Wu G. Arginine nutrition and fetal brown adipose tissue development in nutrient-restricted sheep. Amino Acids. 2013;45(3):489–499. doi: 10.1007/s00726-011-1168-8. [DOI] [PubMed] [Google Scholar]
- Saunders JB. Alcohol: an important cause of hypertension. Br Med J (Clin Res Ed) 1987;294(6579):1045–1046. doi: 10.1136/bmj.294.6579.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawant OB, Lunde ER, Washburn SE, Chen WJ, Goodlett CR, Cudd TA. Different patterns of regional Purkinje cell loss in the cerebellar vermis as a function of the timing of prenatal ethanol exposure in an ovine model. Neurotoxicol Teratol. 2013a;35:7–13. doi: 10.1016/j.ntt.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawant OB, Ramadoss J, Hogan HA, Washburn SE. The role of acidemia in maternal binge alcohol-induced alterations in fetal bone functional properties. Alcohol Clin Exp Res. 2013b;37(9):1476–1482. doi: 10.1111/acer.12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz JA, Speed NM, Gross MD, Lucey MR, Bazakis AM, Hariharan M, Beresford TP. Acute effects of alcohol administration on regional cerebral blood flow: the role of acetate. Alcohol Clin Exp Res. 1993;17(6):1119–1123. doi: 10.1111/j.1530-0277.1993.tb05217.x. [DOI] [PubMed] [Google Scholar]
- Self JT, Spencer TE, Johnson GA, Hu J, Bazer FW, Wu G. Glutamine synthesis in the developing porcine placenta. Biol Reprod. 2004;70(5):1444–1451. doi: 10.1095/biolreprod.103.025486. [DOI] [PubMed] [Google Scholar]
- Shoemaker WC, Van Itallie TB, Walker WF. Measurement of hepatic glucose output and hepatic blood flow in response to glucagon. Am J Physiol. 1959;196(2):315–318. doi: 10.1152/ajplegacy.1959.196.2.315. [DOI] [PubMed] [Google Scholar]
- Subramanian K, Naik VD, Sathishkumar K, Sawant OB, Washburn SE, Wu G, Yallampalli C, Saade GR, Hankins GD, Ramadoss J. Interactive effects of in vitro binge-like alcohol and ATP on umbilical endothelial nitric oxide synthase post-translational modifications and redox modulation. Reprod Toxicol. 2014;43:94–101. doi: 10.1016/j.reprotox.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibblin S, Kock NG, Schenk WG., Jr Splanchnic hemodynamic responses to glucagon. Arch Surg. 1970;100(1):84–89. doi: 10.1001/archsurg.1970.01340190086020. [DOI] [PubMed] [Google Scholar]
- Urso T, Gavaler JS, Van Thiel DH. Blood ethanol levels in sober alcohol users seen in an emergency room. Life Sci. 1981;28(9):1053–1056. doi: 10.1016/0024-3205(81)90752-9. [DOI] [PubMed] [Google Scholar]
- van den Berg A, van Elburg RM, Westerbeek EA, van der Linde EG, Knol J, Twisk JW, Fetter WP. The effect of glutamine-enriched enteral nutrition on intestinal microflora in very low birth weight infants: a randomized controlled trial. Clin Nutr. 2007;26(4):430–439. doi: 10.1016/j.clnu.2007.03.002. [DOI] [PubMed] [Google Scholar]
- van Leer EM, Seidell JC, Kromhout D. Differences in the association between alcohol consumption and blood pressure by age, gender, and smoking. Epidemiology. 1994;5(6):576–582. doi: 10.1097/00001648-199411000-00004. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Mullani N, Gould L, Adler SS, Guynn RW, Overall JE, Dewey S. Effects of acute alcohol intoxication on cerebral blood flow measured with PET. Psychiatry Res. 1988;24(2):201–209. doi: 10.1016/0165-1781(88)90063-7. [DOI] [PubMed] [Google Scholar]
- Wang J, Chen L, Li P, Li X, Zhou H, Wang F, Li D, Yin Y, Wu G. Gene expression is altered in piglet small intestine by weaning and dietary glutamine supplementation. J Nutr. 2008;138(6):1025–1032. doi: 10.1093/jn/138.6.1025. [DOI] [PubMed] [Google Scholar]
- Wang Y, Tao YX, Cai W, Tang QY, Feng Y, Wu J. Protective effect of parenteral glutamine supplementation on hepatic function in very low birth weight infants. Clin Nutr. 2010;29(3):307–311. doi: 10.1016/j.clnu.2010.03.009. [DOI] [PubMed] [Google Scholar]
- Warren KR, Calhoun FJ, May PA, Viljoen DL, Li TK, Tanaka H, Marinicheva GS, Robinson LK, Mundle G. Fetal alcohol syndrome: an international perspective. Alcohol Clin Exp Res. 2001;25(5 Suppl ISBRA):202S–206S. doi: 10.1097/00000374-200105051-00033. [DOI] [PubMed] [Google Scholar]
- Washburn SE, Sawant OB, Lunde ER, Wu G, Cudd TA. Acute alcohol exposure, acidemia or glutamine administration impacts amino acid homeostasis in ovine maternal and fetal plasma. Amino Acids. 2013;45(3):543–554. doi: 10.1007/s00726-012-1453-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welbourne TC. Interorgan glutamine flow in metabolic acidosis. Am J Physiol. 1987;253(6 Pt 2):F1069–1076. doi: 10.1152/ajprenal.1987.253.6.F1069. [DOI] [PubMed] [Google Scholar]
- Wells DJ, Jr, Barnhill MT., Jr Unusually high ethanol levels in two emergency medicine patients. J Anal Toxicol. 1996;20(4):272. doi: 10.1093/jat/20.4.272. [DOI] [PubMed] [Google Scholar]
- West JR, Parnell SE, Chen WJ, Cudd TA. Alcohol-mediated Purkinje cell loss in the absence of hypoxemia during the third trimester in an ovine model system. Alcohol Clin Exp Res. 2001;25(7):1051–1057. [PubMed] [Google Scholar]
- Windus DW, Klahr S, Hammerman MR. Glutamine transport in basolateral vesicles from dogs with acute respiratory acidosis. Am J Physiol. 1984;247(3 Pt 2):F403–407. doi: 10.1152/ajprenal.1984.247.3.F403. [DOI] [PubMed] [Google Scholar]
- Witteman JC, Willett WC, Stampfer MJ, Colditz GA, Kok FJ, Sacks FM, Speizer FE, Rosner B, Hennekens CH. Relation of moderate alcohol consumption and risk of systemic hypertension in women. Am J Cardiol. 1990;65(9):633–637. doi: 10.1016/0002-9149(90)91043-6. [DOI] [PubMed] [Google Scholar]
- Wu G. Amino acids: metabolism, functions, and nutrition. Amino Acids. 2009;37(1):1–17. doi: 10.1007/s00726-009-0269-0. [DOI] [PubMed] [Google Scholar]
- Wu G, Meininger CJ. Regulation of nitric oxide synthesis by dietary factors. Annu Rev Nutr. 2002;22:61–86. doi: 10.1146/annurev.nutr.22.110901.145329. [DOI] [PubMed] [Google Scholar]
- Wu G, Meier SA, Knabe DA. Dietary glutamine supplementation prevents jejunal atrophy in weaned pigs. J Nutr. 1996;126(10):2578–2584. doi: 10.1093/jn/126.10.2578. [DOI] [PubMed] [Google Scholar]
- Wu G, Haynes TE, Li H, Yan W, Meininger CJ. Glutamine metabolism to glucosamine is necessary for glutamine inhibition of endothelial nitric oxide synthesis. Biochem J. 2001;353(Pt 2):245–252. doi: 10.1042/0264-6021:3530245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Wu Z, Dai Z, Yang Y, Wang W, Liu C, Wang B, Wang J, Yin Y. Dietary requirements of “nutritionally non-essential amino acids” by animals and humans. Amino Acids. 2013;44(4):1107–1113. doi: 10.1007/s00726-012-1444-2. [DOI] [PubMed] [Google Scholar]
- Xi P, Jiang Z, Dai Z, Li X, Yao K, Zheng C, Lin Y, Wang J, Wu G. Regulation of protein turnover by L-glutamine in porcine intestinal epithelial cells. J Nutr Biochem. 2012;23(8):1012–1017. doi: 10.1016/j.jnutbio.2011.05.009. [DOI] [PubMed] [Google Scholar]
- Xu H, Pearl RG. Effect of L-glutamine on pulmonary hypertension in the perfused rabbit lung. Pharmacology. 1994;48(4):260–264. doi: 10.1159/000139188. [DOI] [PubMed] [Google Scholar]
- Zehtabchi S, Sinert R, Baron BJ, Paladino L, Yadav K. Does ethanol explain the acidosis commonly seen in ethanol-intoxicated patients? Clin Toxicol (Phila) 2005;43(3):161–166. [PubMed] [Google Scholar]
- Zygmunt M, Herr F, Munstedt K, Lang U, Liang OD. Angiogenesis and vasculogenesis in pregnancy. Eur J Obstet Gynecol Reprod Biol. 2003;110(Suppl 1):S10–S18. doi: 10.1016/s0301-2115(03)00168-4. [DOI] [PubMed] [Google Scholar]