Abstract
Various antioxidant strategies such as supplementation of antioxidants, limiting oxygen concentration with Oxyrase, and reducing reactive oxygen species (ROS) through mild mitochondrial uncoupling had significant beneficial effects on sperm cryopreservation from rhesus monkeys with low cryoresistant ejaculates. Individuals or species that have higher sensitivity to cryodamage may derive the most benefit from these treatments.
Keywords: Macaca mulatta, semen, reactive oxygen species, electron transport
The process of sperm cryopreservation leads to an increase in ROS production (1, 2) and a decrease in the antioxidants levels in sperm (3, 4). Thus, oxidative stress or lipid peroxidation associated with increased ROS production during the freezing-thawing process is now considered as one of the major causes of sperm damage in addition to cold-shock and osmotic stress (5). In response to the evidence for ROS-mediated sperm damage, many approaches have been used to prevent ROS production and thus potentially reduce sperm damage during the freeze-thaw process. One of the most popular methods applied so far is antioxidant supplementation of extenders and/or medium. While straightforward in approach, antioxidant supplementation has produced widely varying results on post-thaw sperm quality depending on the species, and the type and dose of antioxidants. (6). Another approach for eliminating ROS production is to reduce the oxygen level in media by supplementation of an Escherichia coli membrane fraction (Oxyrase) which has been shown to be beneficial for frozen-thawed mouse sperm (7). A third strategy to minimize oxidative stress is to treat sperm with mitochondrial uncoupling agents. Incomplete or mild mitochondrial uncoupling has been shown to reduce oxidative damage (8) and improve in vitro embryo development (9, 10). However, this approach has not been explored for its use in sperm cryopreservation.
The possibility of ROS-mediated damage in cryopreserved monkey sperm has not been studied. To evaluate whether the three different approaches stated above for ROS damage amelioration will produce beneficial effects in post-thaw survival for monkey sperm, we studied the effect of various antioxidant additives, oxyrase, and 2,4-dinitrophenol (DNP, mitochondrial uncoupler) on sperm cryopreservation of rhesus macaques.
Ejaculates were collected from 10 adult males that were individually caged at the CNPRC with lights on from 06:00 to 18:00 h at 25–27 °C. All procedures for maintenance and handling of the animals were reviewed and approved in advance by the Institutional Animal Use and Care Administrative Advisory Committee at the University of California at Davis. The males were trained to chair restraint and semen was collected by direct penile stimulation with a Grass 6 stimulator equipped with ECG pad electrodes (30–50 V, 20 ms duration, 18 pulses s−1). Samples were allowed to liquefy for 30 min before processing. Sperm suspensions were washed twice with Tyrode’s medium supplemented with bovine serum albumin (TL-BSA) at 300 g for 10 min, and resuspended to 2 × 108 cells/mL of total motile sperm (sperm density × initial motility) with TEST solution (43.25 g TES, 10.265 g Tris, 10g glucose in 1L distilled water, pH 7.4, 350 mOsm/kg) before being subjected to various treatments. Sperm were frozen at a final concentration of 5 × 107 cells/mL based on our previous method (11). Except for vitamin E (DL-α-tocopherol acetate, Alexis Biochemicals, Farmingdale, New York) and Oxyrase (Oxyrase, Inc. Mansfield, Ohio), all other chemicals were purchased from Sigma (Sigma Chemical Corporation, St. Louis, Missouri).
After a preliminary screening, glutathione (reduced GSH and oxidized GSSH), superoxide dimutase (SOD), catalase (CAT), Oxyrase, and vitamin E were selected for addition to the cryopreservation medium for freezing trials. Sperm samples from eight ejaculates (one ejaculate per male) were used in this trial, and samples were suspended in TEST-yolk with 3% glycerol. For testing with the mitochondrial uncoupler, 10 ejaculates from 7 males were compared for post-thaw survival when DNP was supplemented in TEST-yolk-glycerol medium at 0.01, 0.1, 1, 10, and 50 μM. Motility was evaluated immediately after thawing. Data were analyzed using paired t-test (SAS 9.1). Percent motility was arcsine-square root transformed (=asin(sqrt(number))) and means of 3 straws per treatment were used for analysis. Values are presented as means ± SEM.
When data were analyzed across all ejaculates, significant improvement in post-thaw motility was found in vitamin E treatment group (P = 0.011), but not for samples with the additives of GSH, GSSH, SOD, CAT and Oxyrase. However, when data were allocated into two categories based on post-thaw motility of the controls (< and ≥ 60%), there were significant improvements in post-thaw motility for 200 U/ml SOD (P = 0.049), 200 U/ml CAT (P = 0.007), and 0.3 U/ml Oxyrase (P = 0.004) in ejaculates with low post-thaw survival (n = 4) (Fig. 1a), but not in ejaculates with high post-thaw survival (n = 4) (Fig. 1b). For DNP treatment, the sperm from most males exhibited improved post-thaw motility, however, the optimal dose varied considerably among ejaculates. When motility values associated with the optimal dose of DNP for each ejaculate were compared to the ethanol controls, post thaw motility of DNP treatment was significantly higher than that of solvent control for ejaculates with low post-thaw survival (< 50%, n = 3), but not in ejaculates with high post-thaw survival (≥ 50%, n = 7) (Fig. 1).
Fig. 1.
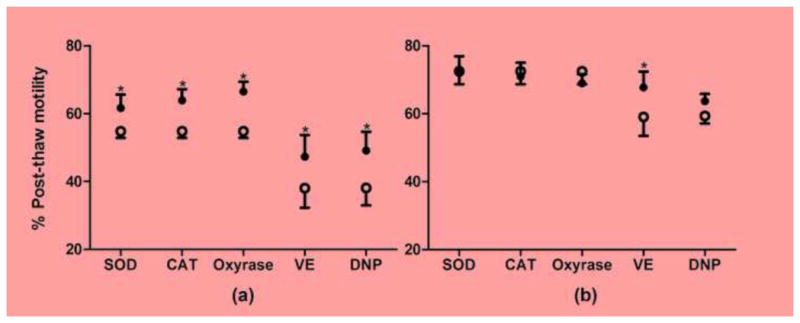
Post-thaw motility of rhesus monkey sperm samples frozen in TEY-3% glycerol with additives (closed circles) of 200 U/ml superoxide dimutase (SOD), 200 U/ml catalase (CAT), 0.3 U/ml oxyrase, 10 mM vitamin E (VE), and dinitrophenol (DNP) at the concentration range of 0.01, 0.1, 1, 10, 50 μM vs. controls without these additives (open circles). Controls for VE and DNP additives were 0.1% ethanol solvent control. Males with low post-thaw motility in controls (less than 60% for SOD, CAT, and oxyrase or less than 50% for VE and DNP groups) were shown in Panel (a), and males with high post-thaw motility in controls (equal or above 60% for SOD, CAT, and oxyrase groups or 50% for VE and DNP groups) were shown in Panel (b). Asterisks indicate significant difference between controls and treatment groups.
Antioxidants play an important role in preventing the formation and scavenging of free radicals. They usually can be classified as enzymatic and non-enzymatic compounds. The enzymatic compounds include SOD, glutathione peroxidase, glutathione reductase, and CAT. Previous studies with bulls (3) and rams (4) found that glutathione peroxidase and glutathione reductase activities were less affected by cryopreservation, while a significant decrease was observed for SOD in samples after freeze-thaw (4, 12). As SOD dismutates the free radical O2− into H2O2, CAT is responsible for converting the still highly disruptive oxidant H2O2 into H2O and O2. Catalase has also been found to be beneficial for post-thaw sperm quality in boars (6), dogs (2), and buffalo bulls (13). The present study revealed significant beneficial effects for these two enzymes in post-thaw survival of monkey sperm from ejaculates with low post-thaw survival in the controls, but only marginal beneficial effects for ejaculates with high post-thaw survival.
Non-enzymatic antioxidant compounds include vitamin C (ascorbic acid), vitamin E (or Trolox), glutathione (reduced GSH and oxidized GSSH), cysteine, taurine, alpha monothioglycerol etc. Of these, vitamins C, E, glutathione, and cysteine are the most commonly tested compounds in previous studies. In general, vitamin C was found to have no positive effect on post-thaw survival, while vitamin E or its analogue Trolox has usually been reported as beneficial (see 6 for review). The present study revealed no significant beneficial effect in post-thaw survival with vitamin C, GSH, GSSH but significant improvement in post-thaw survival with vitamin E when these compounds were supplemented in the freezing media.
Oxyrase is an enzyme derived from the cytoplasmic membrane of E. coli, and is often used to produce anaerobic conditions in a wide variety of environments (14). The sperm cryopreservation handling process is often conducted without controlling the oxygen level; in contrast, sperm within the epididymides or female reproductive tract are restricted to low oxygen concentrations (15). Therefore, it is possible that the relatively high oxygen level in the ambient air may induce substantial free radical formation, and thus partially contribute to the motility loss in frozen-thawed sperm. Studies with mouse sperm revealed that Oxyrase provides substantial protection to motility of sperm exposed to cryoprotectants and the freezing process (7). In the present study, post-thaw survival in the treatment group supplemented with 0.3 U/ml Oxyrase was significantly higher than controls for ejaculates with low post-thaw survival. In addition, supplementation of Oxyrase in the post-thaw suspension medium prolonged post-thaw motility up to 24 h (data not shown), which suggests that high oxygen concentration in the ambient environment may lead to high ROS production, which in turn may contribute to the rapid decline of sperm motility after thawing.
The mitochondrial uncoupling agent DNP allows protons to cross the inner mitochondrial membrane in a manner not coupled to oxidative phosphorylation, resulting in increased electron transport and oxygen consumption rates (16). Though severe uncoupling could reduce ATP production significantly, mild uncoupling has been shown to decrease mitochondrial ROS dramatically through many mechanisms (17). A recent study with DNP on mitochondrial function in mice further supported the theory of marked ROS reduction associated with mild uncoupling (8), and supports the theory that preventing mitochondrial generation of ROS by uncoupling may be a far more effective antioxidant strategy than attempting to remove or neutralize these species with supplemental antioxidants. In the present study, DNP treatment yielded significantly higher post-thaw survival than ethanol solvent controls. Similar to treatments with antioxidant and Oxyrase supplementation, ejaculates with low post-thaw survival usually benefit more from DNP treatment than those with high post-thaw motility. In addition, we also found that a higher DNP dose (50 μM) was necessary for the beneficial effect to occur when samples were frozen in the absence of egg yolk compared with the presence of egg yolk (with a mode of 10 μM) (data not shown). As the absence of egg yolk in the freezing medium represents less optimal freezing conditions (18), the requirement of high DNP dose may compensate the protective effect associated with egg yolk as if it were present. These findings suggest that the beneficial effect of DNP treatment could be due to reduction of ROS through mitochondrial uncoupling, however, the beneficial DNP dose varied widely among ejaculates.
Our findings of the most beneficial effects observed with antioxidant additives, Oxyrase, or DNP on ejaculates with low post-thaw survival in controls supports the view that individuals or species with low resistance to cryodamage will benefit most from optimized freezing conditions (19, 20). It is possible that these various treatments could have additional beneficial effects on other aspects of sperm function that were not measured in this study. In addition, findings in this study may have important clinical applications for men requiring sperm cryopreservation.
Acknowledgments
This work was supported by NIH grants RR00169 and RR13439.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chatterjee S, Gagnon C. Production of reactive oxygen species by spermatozoa undergoing cooling, freezing, and thawing. Mol Reprod Dev. 2001;59:451–8. doi: 10.1002/mrd.1052. [DOI] [PubMed] [Google Scholar]
- 2.Michael A, Alexopoulos C, Pontiki E, Hadjipavlou-Litina D, Saratsis P, Boscos C. Effect of antioxidant supplementation on semen quality and reactive oxygen species of frozen-thawed canine spermatozoa. Theriogenology. 2007;68:204–12. doi: 10.1016/j.theriogenology.2007.04.053. [DOI] [PubMed] [Google Scholar]
- 3.Bilodeau JF, Chatterjee S, Sirard MA, Gagnon C. Levels of antioxidant defenses are decreased in bovine spermatozoa after a cycle of freezing and thawing. Mol Reprod Dev. 2000;55:282–8. doi: 10.1002/(SICI)1098-2795(200003)55:3<282::AID-MRD6>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 4.Marti E, Marti JI, Muino-Blanco T, Cebrian-Perez JA. Effect of the cryopreservation process on the activity and immunolocalization of antioxidant enzymes in ram spermatozoa. J Androl. 2008;29:459–67. doi: 10.2164/jandrol.107.003459. [DOI] [PubMed] [Google Scholar]
- 5.Thuwanut P, Chatdarong K, Techakumphu M, Axnér E. The effect of antioxidants on motility, viability, acrosome integrity and DNA integrity of frozen-thawed epididymal cat spermatozoa. Theriogenology. 2008;70:233–40. doi: 10.1016/j.theriogenology.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 6.Großfeld R, Sieg B, Struckmann C, Frenzel A, Maxwell WMC, Rath D. New aspects of boar semen freezing strategies. Theriogenology. 2008;70:1225–33. doi: 10.1016/j.theriogenology.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 7.Koshimoto C, Gamlie E, Mazur P. Effect of osmolality and oxygen tension on the survival of mouse sperm frozen to various temperatures in various concentrations of glycerol and raffinose. Cryobiology. 2000;41:204–31. doi: 10.1006/cryo.2000.2281. [DOI] [PubMed] [Google Scholar]
- 8.Caldeira da Silva CC, Cerqueira FM, Barbosa LF, Medeiros MH, Kowaltowski AJ. Mild mitochondrial uncoupling in mice affects energy metabolism, redox balance and longevity. Aging Cell. 2008;7:552–60. doi: 10.1111/j.1474-9726.2008.00407.x. [DOI] [PubMed] [Google Scholar]
- 9.Machaty Z, Thompson JG, Abeydeera LR, Day BN, Prather RS. Inhibitors of mitochondrial ATP production at the time of compaction improve development of in vitro produced porcine embryos. Mol Reprod Dev. 2001;58:39–44. doi: 10.1002/1098-2795(200101)58:1<39::AID-MRD6>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 10.Thompson JG, McNaughton C, Gasparrini B, McGowan LT, Tervit HR. Effect of inhibitors and uncouplers of oxidative phosphorylation during compaction and blastulation of bovine embryos cultured in vitro. J Reprod Fertil. 2000;118:47–55. [PubMed] [Google Scholar]
- 11.Dong Q, Rodenburg SE, Huang C, VandeVoort CA. Effect of pre-freezing conditions on semen cryopreservation of rhesus monkey. Theriogenology. 2008;70:61–9. doi: 10.1016/j.theriogenology.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lasso JL, Noiles EE, Alvarez JG, Storey BT. Mechanism of superoxide dismutase loss from human sperm cells during cryopreservation. J Androl. 1994;15:255–65. [PubMed] [Google Scholar]
- 13.El-Sisy GA, El-Nattat WS, El-Sheshtawy Effect of superoxide dismutase and catalase on viability of cryopreserved Buffalo spermatozoa. Global Veterinaria. 2008;2:56–61. [Google Scholar]
- 14.Kressin MD, Schreuders PD, Mazur P. Effects on motility and aster formation of mouse spermatozoa from a reduction in oxygen concentration by Oxyrase, an Escherichia Coli membrane preparation. Cryobiology. 1997;35:353. [Google Scholar]
- 15.Maas DHA, Storey BT, Mastroianni L. Oxygen tension in the oviduct of the Rhesus monkey (Macaca mulatta) Fertil Steril. 1976;27:1312–8. doi: 10.1016/s0015-0282(16)42201-6. [DOI] [PubMed] [Google Scholar]
- 16.Harper JA, Dickinson K, Brand MD. Mitochondrial uncoupling as a target for drug development for the treatment of obesity. Obes Rev. 2001;2:255–65. doi: 10.1046/j.1467-789x.2001.00043.x. [DOI] [PubMed] [Google Scholar]
- 17.Skulachev VP. Uncoupling: new approaches to an old problem of bioenergetics. Biochim Biophys Acta. 1998;1363:100–24. doi: 10.1016/s0005-2728(97)00091-1. [DOI] [PubMed] [Google Scholar]
- 18.Dong Q, Correa LM, VandeVoort CA. Rhesus monkey sperm cryopreservation with TEST-yolk extender in the absence of permeable cryoprotectant. Cryobiology. 2009;58:20–7. doi: 10.1016/j.cryobiol.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dong Q, Huang C, Eudeline B, Tiersch T. Systematic factor optimization for cryopreservation of shipped sperm samples of diploid Pacific Oysters, Crassostrea gigas. Cryobiology. 2005;51:176–97. doi: 10.1016/j.cryobiol.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 20.Hernandez M, Roca J, Gil MA, Vazquez JM, Martinez EA. Adjustments on the cryopreservation conditions reduce the incidence of boar ejaculates with poor sperm freezability. Theriogenology. 2007;67:1436–45. doi: 10.1016/j.theriogenology.2007.02.012. [DOI] [PubMed] [Google Scholar]


