Abstract
Cancer is a disease in which normal physiological processes are imbalanced, leading to tumour formation, metastasis and eventually death. Recent biological advances have led to the advent of targeted therapies to complement traditional chemotherapy and radiotherapy. However, a major problem still facing modern medicine is resistance to therapies, whether targeted or traditional. Therefore, to increase the survival rates of cancer patients, it is critical that we continue to identify molecular targets for therapeutic intervention. The Inhibitor of Apoptosis (IAP) proteins act downstream of a broad range of stimuli, such as cytokines and extracellular matrix interactions, to regulate cell survival, proliferation and migration. These processes are dysregulated during tumourigenesis and are critical to the metastatic spread of the disease. IAPs are commonly upregulated in cancer and have therefore become the focus of much research as both biomarkers and therapeutic targets. Here we discuss the roles that IAPs may play in cancer, and the potential benefits and pitfalls that targeting IAPs could have in the clinic.
Keywords: IAP, Apoptosis, Cytokines, Extracellular matrix, Cancer therapy, Clinical trials
Introduction
Since their discovery almost 20 years ago, the Inhibitor of Apoptosis (IAP) family of proteins have gathered growing interest as possible drug targets in a wide range of malignancies. IAPs are commonly upregulated in cancer, and although initially thought to only regulate cell death, they are now known to be involved in many aspects of both normal tissue function and tumour development. In this review we will focus on summarising how IAPs affect the signalling pathways dysregulated in cancer and the current IAP-based therapies that are in development.
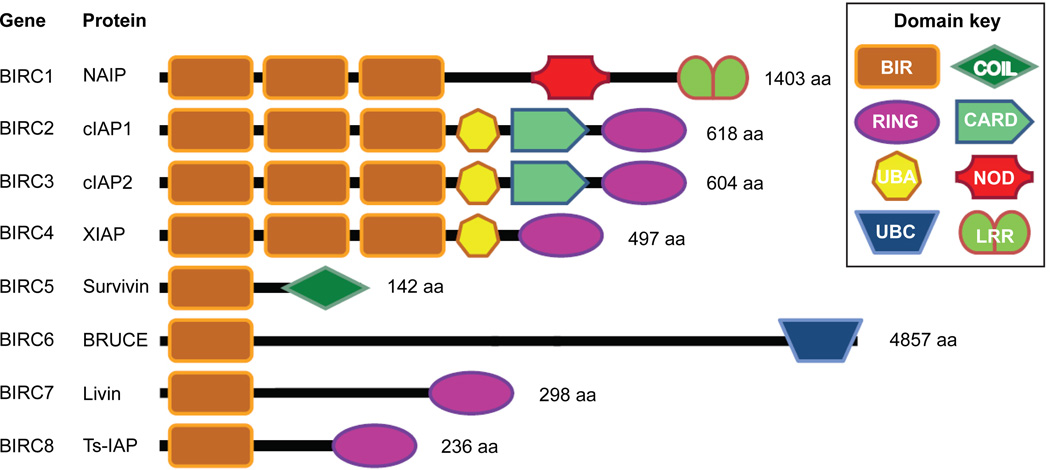
The IAPs were first discovered in baculoviruses, where they were found to encode for proteins (cpIAP, OpIAP) able to inhibit apoptosis in the host cell [1,2]. IAPs are evolutionarily conserved and defined by the presence of at least 1 Baculovirus IAP Repeat (BIR) domain. In humans there are 8 IAPs (genes birc1–8), NAIP, cIAP1, cIAP2, XIAP, Survivin, BRUCE/Apollon, Livin and Ts-IAP (Figure 1). In addition to the BIR domains, IAPs possess a number of other distinct functional domains that impart broader functionality on mammalian IAPs than their viral counterparts [3–5].
Figure 1. Schematic representation of human IAPs.
IAPs contain between one and three Baculovirus IAP repeat (BIR) domains, a 70–80 amino acid Zinc-binding motif. Five of the 8 IAPs possess a carboxy-terminal RING (really interesting new gene) domain that functions as an E3 ligase, capable of self-ubiquitination and ubiquitination of associated proteins. BRUCE lacks a RING domain but possesses an Ubiquitin-Conjugating Domain (UBC) that can induce ubiquitination. XIAP and cIAPs have an Ubiquitin-Associated (UBA) ubiquitin-binding domain that is important for their signalling function [67,98]. In addition cIAP1 and cIAP2 contain a Caspase Recruitment Domain (CARD) that can mediate homotypic interactions [99]. NAIP possesses a LRR (Leucine-Rich Repeat) and a NOD (nucleotide-binding oligomerisation domain), which have been implicated in microbial pathogen recognition. Survivin contains a COIL (coil-coiled) domain, which is involved in binding to chromosomal paasenger proteins INCENP and borealin.
Core Functions of IAPs
From early over-expression studies, it was proposed that IAPs prolong cell survival by inhibiting the activity of initiator (caspase-9) and effector (caspases-3&−7) caspases by binding to the active caspases [6]. However, XIAP is now known to be the only mammalian IAP that is a bona fide caspase inhibitor [7]. XIAP also ubiquitinates caspases via its E3 ubiquitin ligase domain, resulting in caspase degradation or inactivation [8–10].
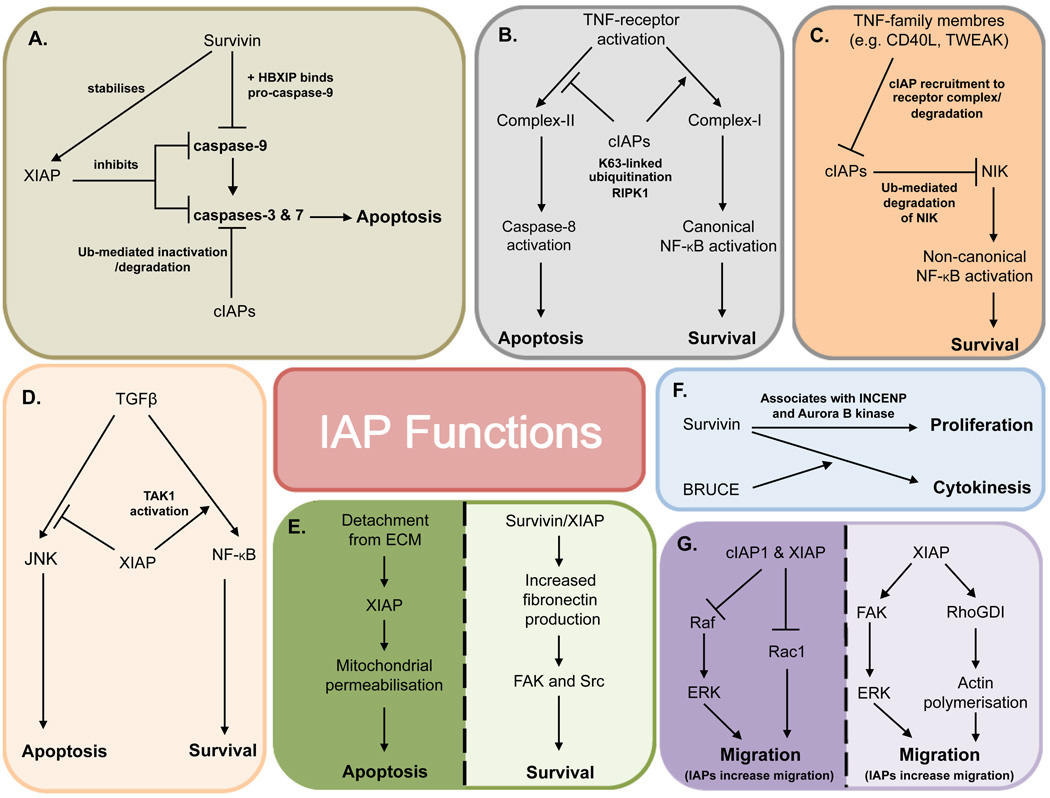
Survivin, in the presence of HBXIP co-factor, binds to and inhibits pro-caspase 9, preventing its recruitment to Apaf1 [11]. In addition Survivin interacts with XIAP, resulting in stabilisation and synergistic inhibition of caspase 9 [12]. The cIAPs, while being able to bind to caspases, do not directly inhibit caspase activity and instead they mediate caspase ubiquitination and degradation [13,14] (Figure 2A).
Figure 2. Summary of IAP functions.
A selection of the pathways in which IAPs function to regulate apoptosis, survival, cell cycle and migration: A –Regulation of caspases, B – TNFα signalling, C – Non-canonical NF-κBD – TGFβ signalling, E –ECM interactions, F – Cell cycle, G – Migration.
It is now known that caspase regulation represents only a small proportion of the mechanisms by which IAPs impact cell longevity. IAPs also regulate cytokine signals and have a role in linking cell-ECM interactions to survival. Moreover, IAPs are signalling effectors in a range of additional cellular processes, including cell cycle and migration (Figure 2B–G).
The role of IAPs in survival signalling
Tumour necrosis factor α (TNFα) is a pleiotropic cytokine, associated with the generation of an inflammatory response. Following TNFα binding to TNF-R1, both TRADD and RIP1 are rapidly recruited to the receptor complex. TRADD then recruits TRAF2, which associates with cIAP1 and 2 to form the survival-inducing “Complex-I”. Polyubiquitination of RIP1 in a non-degradative Lys63 manner by cIAP1 and cIAP2 allows the recruitment of proteins that activate canonical NF-κB signalling, leading to upregulation of survival proteins, such as c-FLIP [15]. In the absence of cIAPs, NF-κB is not activated and the failure to upregulate c-FLIP leads to TNF-induced activation of caspase-8 and apoptosis via formation of a death-inducing “Complex-II” [16,17] (Figure 2B).
As well as influencing the canonical NF-κB pathway, cIAPs affect the non-canonical NF-κB pathway through regulation of NIK [18]. In unstimulated cells, ubiquitin-mediated degradation of NIK by cIAPs prevents non-canonical NF-κB activation. Following activation of receptors belonging to the TNFR superfamily, such as CD40, cIAPs are recruited to the receptor complex, freeing NIK to accumulate and activate non-canonical NF-κB signalling [19] (Figure 2C). Therefore, cIAPs have significant roles in multiple NF-κB pathways.
Downstream of TGFβ receptor activation, NF-κB is activated by 2 main pathways. In the first pathway, XIAP induces transcription of NF-κB responsive genes in a Smad4 dependent manner [20]. In the second pathway XIAP forms a complex with TGFβ-activated kinase 1 (TAK1) and its binding partners TAB1 and TAB2, resulting in TAK1 activation. TAK1 phosphorylates the NF-κB inhibitor, IκB, resulting in its proteasomal degradation and the activation of NF-κB. TGFβ mediated TAK1 activation results in the upregulation of the pro-apoptotic p38 and JNK signalling pathways [21]. However, NF-κB activation induces transcriptional upregulation of XIAP, which then mediates ubiquitination and proteasomal degradation of TAK1 to suppress pro-apoptotic JNK signalling in a pro-survival feedback loop [5,22] (Figure 2D).
IAPs also regulate other pro-survival signalling cascades via their E3 ligase domain. For example, XIAP ubiquitinates PTEN leading to Akt phosphorylation and activation [23]. cIAP1 ubiquitinates the c-myc regulatory protein, MAD1, thereby activating c-myc. In this context, cIAP1 acts synergistically with c-Myc to enhance tumour formation [24]. cIAPs can also promote MAPK-dependent cell proliferation and survival in TNF-stimulated cells. The cIAPs ubiquitinate TRAF3 (TNFR associated factor 3), thereby allowing TRAF2/6:MAPK translocation to the cytosol and activation [25].
The role of IAPs in ECM-mediated survival
Interactions between cells and their surrounding Extracellular Matrix (ECM) mediate the spatial control of cell fate, and are crucial in determining the survival of normal cells [26,27]. Perturbation of ECM-adhesion signals induces apoptosis by activating Bax-driven mitochondrial permeabilisation [28]. Survivin and XIAP cooperate to upregulate several ECM proteins, particularly fibronectin [29]. The resulting fibronectin-activated signalling via FAK and Src kinases promotes survival in response to altered adhesion of cells to ECM [29]. Interestingly, as an early response to altered cell-ECM interactions, XIAP can translocate to mitochondria where it forms a 400 kDa complex and contributes to mitochondrial permeabilisation [30]. Therefore, depending on temporal context, IAPs can both protect cells or promote ECM-regulated apoptosis (Figure 2E).
The role of IAPs in cell cycle
Although cytoplasmic Survivin has a role in promoting cell survival, its primary function is in the nucleus, where it is required along with Aurora B kinase and INCENP to form the chromosomal passenger complex during mitosis. Survivin knockout embryos die at E4.5 due to failed cytokinesis while cells lacking Survivin display a multiploidy phenotype [31,32]. Interestingly, the survival and proliferative functions of Survivin are spatially distinct. Survivin contains a nuclear export sequence and can be found in both the nucleus and the cytosol: while nuclear Survivin regulates proliferation, the cytoplasmic protein acts to suppress apoptosis [33,34]. Moreover, Survivin, along with its family member Bruce, are also implicated, in cytokinesis [35] (Figure 2F).
The role of IAPs in migration
IAPs have a number of roles in cell migration. XIAP is recruited via caveolin-1, to α5-integrin adhesion complexes, where it interacts with focal adhesion kinase (FAK). This α5-integrin:caveolin:FAK complex is required for activation of ERK-dependent shear stress–induced endothelial cell migration [36–38]. Furthermore, XIAP can promote migration and invasion via interactions with RhoGDI and subsequent regulation of actin polymerisation [39].
In contrast to promoting migration, IAPs can also inhibit cellular motion. XIAP and the cIAPs bind to c-Raf, resulting in ubiquitination of c-Raf, in a manner dependent upon the Hsp90-mediated quality control system, but independent of their E3-ligase activity. Knockdown of XIAP resulted in stabilisation of c-Raf and increased c-Raf-dependent cell migration [40]. Additionally, XIAP and cIAP1 can reduce migration by mediating the proteasomal degradation of Rac1 [41]. Therefore, IAPs influence cell migration in a context-dependent manner (Figure 2G).
IAPs contribute to cancer progression
The above discussion reveals that IAPs have a broad portfolio of roles in regulating cell survival, proliferation and migration. Moreover, IAPs are regulated during normal developmental programmes that become subverted in cancer [42]. It is therefore not surprising that there is an ever-expanding body of evidence connecting changes in IAP expression with tumourigenesis [43–46].
Survivin
The survivin gene is among the top 5 cancer-associated genes. It is upregulated in the vast majority of cancers and is associated with resistance to both chemo and radio-therapy, as well as a poor prognosis [47]. The divergent functions of nuclear and cytoplasmic Survivin are highlighted in studies on breast cancer where elevated levels of cytoplasmic Survivin correlate with a poorer patient outcome owing to its anti-apoptotic function, while increased nuclear Survivin is correlated with a better outcome [31,33,48–51].
As a chromosomal passenger protein, Survivin acts to stabilise microtubules, which results in resistance to chemotherapeutics, such as Vinca alkaloids [52]. Survivin, via its interaction with Aurora B kinase, may also function to promote the indefinite proliferation potential of cancer cells by upregulating human telomerase reverse transcriptase [53].
Perhaps surprisingly, Survivin also regulates cancer cell autophagy by interacting with the key autophagy regulator, Beclin [54]. In glioma cells, knockdown of Beclin resulted in decreased Survivin levels, and increased apoptotic sensitivity to TRAIL. In prostate cancer cells, chemokine-mediated protection from autophagic cell death is mediated by upregulation of Survivin [55].
XIAP
XIAP expression is upregulated in a variety of cancers, including breast, lung, renal and bladder carcinoma [56–59]. XIAP may mediate anoikis resistance to contribute to tumour metastasis [60,61]. However, the correlation of XIAP expression and prognosis is unclear. Increased XIAP levels correlate with disease severity in acute myeloid leukaemia and prostate cancer, but not non-small cell lung carcinoma [58,62,63]. In a recent study in which XIAP was stably over-expressed at levels 2–5 times higher than normal, which is similar to levels seen in cancer samples, XIAP only provided chemoresistance when combined with the loss of the XIAP antagonist, Smac/DIABLO. Therefore, elevated levels of XIAP alone may not be a prognostic indicator [64].
cIAPs
Genomic changes in cIAPs are associated with some tumour types. For example chromosomal amplification of 11q21–q23, which encodes both cIAP1 and cIAP2, has been observed in oesophageal squamous cell carcinomas [65,66]. In MALT (mucosal associated lymphoid tissue) B cell lymphomas, cIAP2 gene translocation results in expression of a cIAP2-MALT fusion protein. This drives constitutive NF-κB activation, via a UBA domain dependent binding of NEMO [67]. Similarly, the UBA domain of cIAP1 has also been shown to be essential for cIAP1-mediated oncogenesis [67]. cIAP1 and cIAP2 are often over-expressed in cancers along with YAP, as they are all located within the same genetic locus. In fact, in hepatoma, cIAP and YAP cooperate to induce tumourigenesis [68,69].
IAPs are potential therapeutic targets in the clinical setting
The overwhelming data that IAPs suppress apoptosis, enhance survival signalling and are upregulated in many cancer types argues that they may be excellent therapeutic targets. Particularly appealing is the possibility that IAP antagonists might specifically target cancer cells over normal cells.
Numerous pre-clinical studies have shown that targeting IAPs, with either siRNAs or mimetics of the naturally occurring IAP antagonist Smac/DIABLO, increases sensitivity of cancer cells to therapies that are widely used in the clinic (Table 1). As a consequence IAP drug development has progressed at a rapid pace, such that multiple IAP inhibitors have been developed and some of these have progressed to in- patient clinical trials (Table 2). Discussed below is a selection of the therapeutics and different approaches used to target IAPs in cancer.
Table 1.
Pre-clinical data where IAP inhibition sensitised to anti-cancer therapies.
| IAP | Cancer | Mechanism of inhibition |
Increased sensitivity to | Ref. |
|---|---|---|---|---|
| XIAP | Colorectal | shRNA | TRAIL Taxanes γ-irradiation |
[100] |
| Breast | shRNA | TRAIL Taxanes |
[101] | |
| siRNA | Etoposide Doxorubicin |
[102] | ||
| Lapatinib | [59] | |||
| Lung | Antisense | Doxorubicin Taxol Vinorelbine Etoposide |
[103] | |
| siRNA | Cisplatin | [68] | ||
| Pancreatic | siRNA | Doxorubicin Paclitaxol |
[104] | |
| Melanoma | Dacarbazine TRAIL |
[105] | ||
| Survivin | Lung | siRNA | Adriamycin | [106] |
| Cisplatin Paclitaxol |
[107] | |||
| Breast | siRNA | Adriamycin | [108] | |
| Melanoma | TRAIL | [105] | ||
| Hepatocellular | siRNA | Radiotherapy | [109] | |
| Antisense | TRAIL | [110] | ||
| cIAP2 | Pancreatic | siRNA | Doxorubicin Paclitaxel |
[104] |
| Oral Squamous | siRNA | 5-flurouracil | [111] | |
| Colorectal | [112,113] | |||
| cIAPs | Glioblastoma | Smac mimetic |
Imatinib | [114] |
| Smac mimetic (BV6) |
γ-irradiation | [78] | ||
| Non small cell lung carcinoma |
Smac mimetic (JP1201) |
Doxorubicin Erlotinib Gemcitabine Paclitaxol Vinorelbine |
[115] | |
| Smac mimetc (BV6) |
Radiotherapy | [116] | ||
| Breast | Smac mimetic (SM164) |
TRAIL | [116] | |
| Smac mimetic (Compound C) |
Herceptin | [59] | ||
| Prostate | Smac mimetic (SM164) |
TRAIL | [117] | |
| Colon |
Table 2.
IAP-based therapies in clinical trials.
| IAP | Drug | Company | Mode of action | Clinical Trial | Ref. |
|---|---|---|---|---|---|
| XIAP | AEG35156 | Aegera Therapeutics | Antisense | Phase 1 | [118] |
| Embelin | Small molecule targeting BIR3 domain | Pre-clinical | [119,120] | ||
| Polyphenylureas / Xantags | Burnham Institute | Small molecule targeting BIR2 domain | Pre-clinical | [121] | |
| Arylsulfonamides (TWX006, TWX024) | Novartis | Pre-clinical | [122] | ||
| Survivin | LY2181308 | Eli Lily | Antisense | Phase 2 | [92,93] |
| YM155 | Astellas Pharma Inc | Small molecule antagonist | Phase 2 | [123] | |
| Shepherdin | Small molecule targeting Hsp90 | Pre-clinical | [124,125] | ||
| AICAR | Phase II | [126] | |||
| Anti-Survivin Ab | Antibody | Phase 1 | [85] | ||
| cIAPs and XIAP | TL32711 (Birinapint) Compound A | TetraLogic Pharma | Smac mimetic | Phase 1 /2 | [76] |
| AEG40826 (HGS1029), | Aegera Therapeutics | Phase 1 | [17] | ||
| AEG40730 | Aegera Therapeutics | Phase 2 | |||
| Compound 8, BV6, SM-122, SM-164 | Ascenta Therapeutics | Pre-clinical | [75] | ||
| AT-406 | Ascenta Therapeutics | Phase 1 | [127] | ||
| Compound 3 | University of Texas / Joyant | Phase 1 | [74] | ||
| LBW242, LCL-161 | Novartis | Phase 1 | [128] | ||
| Compound C | Genentech | Phase 1 | [129] | ||
| Compound 11 | Pfizer | Pre-clinical | [130] | ||
| JP-1201 | Pre-clinical | [115] |
Antisense based therapies
An antisense oligonucleotide directed against XIAP (AEG35156) is in phase I/II clinical trials for patients with pancreatic, breast, non-small cell lung cancer, AML, lymphoma and solid tumours in which docetaxel is the drug of choice. Although AEG35156 in its ‘first-in-man’ study was well tolerated, it had little significant effect on patient outcome in pancreatic ductal adenocarcinoma or acute myeloid leukaemia [70,71]. Studies involving patients with non-small cell lung carcinoma were terminated due to unacceptable neurotoxicity in two of the patients.
Smac mimetics
Another method of targeting IAP function is using “Smac mimetics”, which are molecules developed based on the IAP-Binding Motif (IBM) of the potent IAP-antagonist, Smac (also known as DIABLO). Several Smac mimetics are currently in pre-clinical or phase I trials (Table 2). These inhibitors were initially developed as a means of inhibiting XIAP, but it has since been shown that the Smac mimetics also induce the degradation of cIAPs [72]. The loss of cIAPs means that following ligand engagement of the TNF-receptor, Complex I matures into Complex II, leading to caspase-dependent apoptosis [16,17]. Ligand-independent Smac mimetic-induced cIAP degradation causes Ripoptosome (a FADD-caspase 8, RIP1 complex) formation, leading to death via apoptosis or necroptosis [15,73].
At high doses, Smac mimetics induce death in a subset of cancer cell lines in a caspase 8, and TNFα dependent mechanism, but do not induce apoptosis in non-malignant cells [74–77]. Perhaps more importantly, Smac mimetics can work synergistically with other treatments. They sensitise pancreatic cancer cells and glioblastoma cells to γ-irradiation, and breast cancer cells to etoposide, Herceptin, and TRAIL [59,78].
Other small molecule inhibitors
Several small molecule inhibitors directed against Survivin have been developed. YM155, an inhibitor designed to suppress Survivin promoter activity, showed promise in phase I trials, induced stable disease in 9 / 33 patients in one study and significant tumour shrinkage and remission in another phase I study [79,80]. Phase II trials with YM155 showed favourable results in refractory non small cell lung carcinoma and B cell lymphoma but not in melanoma [81–83]. YM155 is also effective in pancreatic cancer cell culture and xenograft models [84].
Hsp90 stabilises Survivin, and targeting Hsp90 can result in proteasomal degradation of Survivin followed by mitochondrial-mediated apoptosis. Therefore drugs that target Hsp90 may also influence Survivin levels and patient outcome. Such drugs include Shepherdin, and AICAR, which are respectively in pre-clinical and phase II clinical trials.
Immune-based therapies
Sera from breast, lung and GI cancer patients contain antibodies to Survivin, suggesting that anti-cancer vaccines may be generated [85]. Recently, a phase I trial in which 9 patients with urothelial cancer were vaccinated against Survivin showed no adverse side effects. Five of the 9 patients had an increase in Survivin peptide specific cytotoxic T cells and one patient showed decreased tumour volume [86]. In a second study, a Survivin minigene DNA vaccine induced a 48 – 52% reduction in tumour volume, weight and metastasis in a syngeneic neuroblastoma mouse model. Therapeutic vaccination of the syngeneic neuroblastoma mice led to eradication of neuroblastoma in 50% of the mice and decreased tumour growth by 80% in the remaining mice [87].
Overall, from the available data on pre-clinical trials and initial ‘in-man’ trials, IAP based therapies may indeed be beneficial in the fight against cancer. Significantly though, targeting IAPs may also help to overcome resistance of cancer to existing therapeutics.
IAPs contribute to the acquired resistance of cancer therapies
IAP levels can increase following the onset of drug treatment. This may provide a mechanism for therapeutic resistance. For example, cisplatin treatment of prostate cancer cells resulted in upregulation of Survivin, XIAP and cIAP2; adriamycin-resistant MCF7 breast cancer cells showed upregulation of XIAP and Survivin; and Lapatinib-resistant BT474 breast cancer cells had elevated Survivin levels. Moreover, survival of adriamycin resistant HL-60 cells were dependent on upregulation of XIAP and MRP (multidrug resistant protein) [88–91]. Importantly, these increases in IAP levels might contribute to acquired drug resistance, one of the major hurdles facing clinicians today. Cancer cells using IAPs as a method to escape chemotherapy highlights another reason why targeting IAPs may be useful to combating the disease.
Notes of caution to targeting IAPs
The majority of current research supports the idea that IAPs are promising therapeutic targets in cancer, but a few notes of caution remain. Therapies targeting Survivin reduce clonogenic survival of cancer cells and increase rates of apoptosis, usually downstream of mitotic catastrophe. However, loss of Survivin can result in the generation of polyploidy cells, which are more susceptible to the accumulation of mutations and genetic instability. Therefore, cancer cells that escape anti-Survivin based therapies may form a more aggressive transformed phenotype than observed in the original cancer [92]. Moreover, although Survivin is not expressed in differentiated adult human cells it is still expressed in adult proliferating cells, such as the cells of the immune system [93]. Thus, as with other chemotherapies, the effect of Survivin antagonists on the immune system would need to be carefully monitored, especially in the megakaryocyte and haempoietic populations.
Therapies aimed at inhibiting XIAP may result in increased rates of apoptosis in sensitised type II cells, such as hepatocytes. In response to death receptor activation, type II cells require the activation of Bid to amplify the apoptosis signal and commit cells to death. Removal of XIAP from this system also removes the requirement for Bid and results in greater rates of apoptosis. Therefore, combining XIAP antagonism with therapies that activate death receptors may result in high liver toxicity in patients [94]. As XIAP can promote the ubiquitination and degradation of c-RAF, XIAP-targeted therapies could also increase cell migration via c-RAF stabilisation and activation of the MAPK signalling cascade [40].
In contrast to many cancers where IAP upregulation occurs, the biallelic deletion of cIAP1 and cIAP2 is associated with a poorer prognosis in multiple myeloma [95,96]. Despite the expectation that cells lacking the cIAPs would be more sensitive to TNFα, this is not the case in multiple myeloma. It is therefore possible that using Smac mimetics in certain specific situations may enhance carcinogenesis. The ability of Smac mimetics to activate NF-κB signalling will also require careful attention. Treatment of mice with the Smac mimetics stimulated osteoclastogenesis and induced osteoporosis by inducing NIK-dependent activation of NF-κB [97]. Bone loss caused by Smac mimetics may be counteracted by administration of bisphosphonates, such as zoledronic acid [97].
Conclusion
IAPs are much more than just “inhibitors of apoptosis”. An involvement with signal transduction cascades regulating apoptosis, proliferation, cell survival and migration strongly implicates IAPs with cancer progression and a growing body of work supports the concept of targeting IAPs to treat cancer. Combining anti-IAP therapies with traditional drug approaches has tremendous promise for the future care of cancer patients.
Acknowledgements
This work is supported by the Wellcome Trust Centre for Cell-Matrix Research (Manchester, UK), which is supported by core funding from the Wellcome Trust (no. 088785/Z/09/Z).
Abbreviations
- BIR
Baculovirus IAP Repeat
- BRUCE
BIR Containing Ubiquitin-Conjugating Enzyme
- c-FLIP
Cellular FLICE Inhibitory Protein
- CARD
Caspase Recruitment Domain
- cIAP
Cellular IAP
- ECM
Extracellular Matrix
- HBXIP
Hepatitis B X-Interacting Protein
- Hsp90
Heat Shock Protein 90
- IAP
Inhibitor of Apoptosis
- IκB
Inhibitor of Kappa B
- INCENP
Inner Centromere Protein
- JNK
cJun N-Terminal Kinase
- MAD1
MAX Dimerisation Protein 1
- MAPK
Mitogen Activated Protein Kinase
- MAX
Myc Associated Factor X
- NAIP
Neuronal Apoptosis Inhibitory Protein
- NEMO
NF-κB Essential Modulator
- NF-κB
Nuclear Factor Kappa B
- NIK
NF-κB-Inducing Kinase
- PTEN
Phosphatase and Tensin Homolog
- RIP
Receptor-Interacting Protein
- Smac/DIABLO
Second Mitochondrial Activator of Caspases/Direct IAP-Binding Protein With Low pI
- TAB1
TAK1 Binding Protein
- TAK1
TGF Beta-Activated Kinase 1
- TGFβ
Transforming Growth Factor Beta
- TNF
Tumour Necrosis Factor
- TNF-R
TNF Receptor
- TRADD
Tumor Necrosis Factor Receptor Type 1-Associated DEATH Domain
- TRAF
TNF Receptor Associated Factor
- TRAIL
TNF-Related Apoptosis-Inducing Ligand
- Ts-IAP
Testis Specific IAP
- UBA
Ubiquitin-Associated
- UBC
Ubiquitin-Binding Domain
- XIAP
X-Linked IAP
References
- 1.Crook NE, Clem RJ, Miller LK. An apoptosis-inhibiting baculovirus gene with a zinc finger-like motif. J Virol. 1993;67:2168–2174. doi: 10.1128/jvi.67.4.2168-2174.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Birnbaum MJ, Clem RJ, Miller LK. An apoptosis-inhibiting gene from a nuclear polyhedrosis virus encoding a polypeptide with Cys/His sequence motifs. J Virol. 1994;68:2521–2528. doi: 10.1128/jvi.68.4.2521-2528.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lopez J, Meier P. To fight or die - inhibitor of apoptosis proteins at the crossroad of innate immunity and death. Curr Opin Cell Biol. 2010;22:872–881. doi: 10.1016/j.ceb.2010.08.025. [DOI] [PubMed] [Google Scholar]
- 4.Srinivasula SM, Ashwell JD. IAPs: what’s in a name. Mol Cell. 2008;30:123–135. doi: 10.1016/j.molcel.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dubrez-Daloz L, Dupoux A, Cartier J. IAPs: more than just inhibitors of apoptosis proteins. Cell Cycle. 2008;7:1036–1046. doi: 10.4161/cc.7.8.5783. [DOI] [PubMed] [Google Scholar]
- 6.LaCasse EC, Cherton-Horvat GG, Hewitt KE, Jerome LJ, Morris SJ, et al. Preclinical characterization of AEG35156/GEM 640, a second-generation antisense oligonucleotide targeting X-linked inhibitor of apoptosis. Clin Cancer Res. 2006;12:5231–5241. doi: 10.1158/1078-0432.CCR-06-0608. [DOI] [PubMed] [Google Scholar]
- 7.Eckelman BP, Salvesen GS, Scott FL. Human inhibitor of apoptosis proteins: why XIAP is the black sheep of the family. EMBO Rep. 2006;7:988–994. doi: 10.1038/sj.embor.7400795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morizane Y, Honda R, Fukami K, Yasuda H. X-linked inhibitor of apoptosis functions as ubiquitin ligase toward mature caspase-9 and cytosolic Smac/DIABLO. J Biochem. 2005;137:125–132. doi: 10.1093/jb/mvi029. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki Y, Nakabayashi Y, Takahashi R. Ubiquitin-protein ligase activity of X-linked inhibitor of apoptosis protein promotes proteasomal degradation of caspase-3 and enhances its anti-apoptotic effect in Fas-induced cell death. Proc Natl Acad Sci U S A. 2001;98:8662–8667. doi: 10.1073/pnas.161506698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galbán S, Duckett CS. XIAP as a ubiquitin ligase in cellular signaling. Cell Death Differ. 2010;17:54–60. doi: 10.1038/cdd.2009.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marusawa H, Matsuzawa S, Welsh K, Zou H, Armstrong R, et al. HBXIP functions as a cofactor of survivin in apoptosis suppression. EMBO J. 2003;22:2729–2740. doi: 10.1093/emboj/cdg263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dohi T, Okada K, Xia F, Wilford CE, Samuel T, et al. An IAP-IAP complex inhibits apoptosis. J Biol Chem. 2004;279:34087–34090. doi: 10.1074/jbc.C400236200. [DOI] [PubMed] [Google Scholar]
- 13.Eckelman BP, Salvesen GS. The human anti-apoptotic proteins cIAP1 and cIAP2 bind but do not inhibit caspases. J Biol Chem. 2006;281:3254–3260. doi: 10.1074/jbc.M510863200. [DOI] [PubMed] [Google Scholar]
- 14.Huang Hk, Joazeiro CA, Bonfoco E, Kamada S, Leverson JD, et al. The inhibitor of apoptosis, cIAP2, functions as a ubiquitin-protein ligase and promotes in vitro monoubiquitination of caspases 3 and 7. J Biol Chem. 2000;275:26661–26664. doi: 10.1074/jbc.C000199200. [DOI] [PubMed] [Google Scholar]
- 15.Feoktistova M, Geserick P, Kellert B, Dimitrova DP, Langlais C, et al. cIAPs block Ripoptosome formation, a RIP1/caspase-8 containing intracellular cell death complex differentially regulated by cFLIP isoforms. Mol Cell. 2011;43:449–463. doi: 10.1016/j.molcel.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Varfolomeev E, Goncharov T, Fedorova AV, Dynek JN, Zobel K, et al. c-IAP1 and c-IAP2 are critical mediators of tumor necrosis factor alpha (TNFalpha)-induced NF-kappaB activation. J Biol Chem. 2008;283:24295–24299. doi: 10.1074/jbc.C800128200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bertrand MJ, Milutinovic S, Dickson KM, Ho WC, Boudreault A, et al. cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination. Mol Cell. 2008;30:689–700. doi: 10.1016/j.molcel.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 18.Varfolomeev E, Goncharov T, Maecker H, Zobel K, Kömüves LG, et al. Cellular inhibitors of apoptosis are global regulators of NF-ΰB and MAPK activation by members of the TNF family of receptors. Sci Signal. 2012;5:ra22. doi: 10.1126/scisignal.2001878. [DOI] [PubMed] [Google Scholar]
- 19.Zarnegar BJ, Wang Y, Mahoney DJ, Dempsey PW, Cheung HH, et al. Noncanonical NF-kappaB activation requires coordinated assembly of a regulatory complex of the adaptors cIAP1, cIAP2, TRAF2 and TRAF3 and the kinase NIK. Nat Immunol. 2008;9:1371–1378. doi: 10.1038/ni.1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Birkey Reffey S, Wurthner JU, Parks WT, Roberts AB, Duckett CS. X-linked inhibitor of apoptosis protein functions as a cofactor in transforming growth factor-beta signaling. J Biol Chem. 2001;276:26542–26549. doi: 10.1074/jbc.M100331200. [DOI] [PubMed] [Google Scholar]
- 21.Lu M, Lin SC, Huang Y, Kang YJ, Rich R, et al. XIAP induces NF-kappaB activation via the BIR1/TAB1 interaction and BIR1 dimerization. Mol Cell. 2007;26:689–702. doi: 10.1016/j.molcel.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaur S, Wang F, Venkatraman M, Arsura M. X-linked inhibitor of apoptosis (XIAP) inhibits c-Jun N-terminal kinase 1 (JNK1) activation by transforming growth factor beta1 (TGF-beta1) through ubiquitin-mediated proteosomal degradation of the TGF-beta1-activated kinase 1 (TAK1) J Biol Chem. 2005;280:38599–38608. doi: 10.1074/jbc.M505671200. [DOI] [PubMed] [Google Scholar]
- 23.Van Themsche C, Leblanc V, Parent S, Asselin E. X-linked inhibitor of apoptosis protein (XIAP) regulates PTEN ubiquitination, content, and compartmentalization. J Biol Chem. 2009;284:20462–20466. doi: 10.1074/jbc.C109.009522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu L, Zhu J, Hu X, Zhu H, Kim HT, et al. c-IAP1 cooperates with Myc by acting as a ubiquitin ligase for Mad1. Mol Cell. 2007;28:914–922. doi: 10.1016/j.molcel.2007.10.027. [DOI] [PubMed] [Google Scholar]
- 25.Karin M, Gallagher E. TNFR signaling: ubiquitin-conjugated TRAFfic signals control stop-and-go for MAPK signaling complexes. Immunol Rev. 2009;228:225–240. doi: 10.1111/j.1600-065X.2008.00755.x. [DOI] [PubMed] [Google Scholar]
- 26.Streuli CH. Integrins and cell-fate determination. J Cell Sci. 2009;122:171–177. doi: 10.1242/jcs.018945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gilmore AP, Metcalfe AD, Romer LH, Streuli CH. Integrin-mediated survival signals regulate the apoptotic function of Bax through its conformation and subcellular localization. J Cell Biol. 2000;149:431–446. doi: 10.1083/jcb.149.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schellenberg B, Wang P, Keeble JA, Rodriguez-Enriquez R, Walker S, et al. Bax exists in a dynamic equilibrium between the cytosol and mitochondria to control apoptotic priming. Mol Cell. 2013;49:959–971. doi: 10.1016/j.molcel.2012.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mehrotra S, Languino LR, Raskett CM, Mercurio AM, Dohi T, et al. IAP regulation of metastasis. Cancer Cell. 2010;17:53–64. doi: 10.1016/j.ccr.2009.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Owens TW, Foster FM, Valentijn A, Gilmore AP, Streuli CH. Role for X-linked Inhibitor of apoptosis protein upstream of mitochondrial permeabilization. J Biol Chem. 2010;285:1081–1088. doi: 10.1074/jbc.M109.072322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wheatley SP, McNeish IA. Survivin: a protein with dual roles in mitosis and apoptosis. Int Rev Cytol. 2005;247:35–88. doi: 10.1016/S0074-7696(05)47002-3. [DOI] [PubMed] [Google Scholar]
- 32.Zangemeister-Wittke U, Simon HU. An IAP in action: the multiple roles of survivin in differentiation, immunity and malignancy. Cell Cycle. 2004;3:1121–1123. [PubMed] [Google Scholar]
- 33.Colnaghi R, Connell CM, Barrett RM, Wheatley SP. Separating the anti-apoptotic and mitotic roles of survivin. J Biol Chem. 2006;281:33450–33456. doi: 10.1074/jbc.C600164200. [DOI] [PubMed] [Google Scholar]
- 34.Connell CM, Colnaghi R, Wheatley SP. Nuclear survivin has reduced stability and is not cytoprotective. J Biol Chem. 2008;283:3289–3296. doi: 10.1074/jbc.M704461200. [DOI] [PubMed] [Google Scholar]
- 35.Pohl C, Jentsch S. Final stages of cytokinesis and midbody ring formation are controlled by BRUCE. Cell. 2008;132:832–845. doi: 10.1016/j.cell.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 36.Ahn S, Park H. XIAP is essential for shear stress-enhanced Tyr-576 phosphorylation of FAK. Biochem Biophys Res Commun. 2010;399:256–261. doi: 10.1016/j.bbrc.2010.07.064. [DOI] [PubMed] [Google Scholar]
- 37.Kim J, Ahn S, Ko YG, Boo YC, Chi SG, et al. X-linked inhibitor of apoptosis protein controls alpha5-integrin-mediated cell adhesion and migration. Am J Physiol Heart Circ Physiol. 2010;299:H300–H309. doi: 10.1152/ajpheart.00180.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahn S, Kim HJ, Chi SG, Park H. XIAP reverses various functional activities of FRNK in endothelial cells. Biochem Biophys Res Commun. 2012;419:419–424. doi: 10.1016/j.bbrc.2012.02.037. [DOI] [PubMed] [Google Scholar]
- 39.Liu J, Zhang D, Luo W, Yu Y, Yu J, et al. X-linked inhibitor of apoptosis protein (XIAP) mediates cancer cell motility via Rho GDP dissociation inhibitor (RhoGDI)-dependent regulation of the cytoskeleton. J Biol Chem. 2011;286:15630–15640. doi: 10.1074/jbc.M110.176982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dogan T, Harms GS, Hekman M, Karreman C, Oberoi TK, et al. X-linked and cellular IAPs modulate the stability of C-RAF kinase and cell motility. Nat Cell Biol. 2008;10:1447–1455. doi: 10.1038/ncb1804. [DOI] [PubMed] [Google Scholar]
- 41.Oberoi TK, Dogan T, Hocking JC, Scholz RP, Mooz J, et al. IAPs regulate the plasticity of cell migration by directly targeting Rac1 for degradation. EMBO J. 2012;31:14–28. doi: 10.1038/emboj.2011.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Owens TW, Foster FM, Tanianis-Hughes J, Cheung JY, Brackenbury L, et al. Analysis of inhibitor of apoptosis protein family expression during mammary gland development. BMC Dev Biol. 2010;10:71. doi: 10.1186/1471-213X-10-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fulda S, Debatin KM. Targeting inhibitor of apoptosis proteins (IAPs) for diagnosis and treatment of human diseases. Recent Pat Anticancer Drug Discov. 2006;1:81–89. doi: 10.2174/157489206775246539. [DOI] [PubMed] [Google Scholar]
- 44.Nachmias B, Ashhab Y, Ben-Yehuda D. The inhibitor of apoptosis protein family (IAPs): an emerging therapeutic target in cancer. Semin Cancer Biol. 2004;14:231–243. doi: 10.1016/j.semcancer.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 45.Reed JC. Apoptosis-targeted therapies for cancer. Cancer Cell. 2003;3:17–22. doi: 10.1016/s1535-6108(02)00241-6. [DOI] [PubMed] [Google Scholar]
- 46.Hunter AM, LaCasse EC, Korneluk RG. The inhibitors of apoptosis (IAPs) as cancer targets. Apoptosis. 2007;12:1543–1568. doi: 10.1007/s10495-007-0087-3. [DOI] [PubMed] [Google Scholar]
- 47.Yamamoto H, Ngan CY, Monden M. Cancer cells survive with survivin. Cancer Sci. 2008;99:1709–1714. doi: 10.1111/j.1349-7006.2008.00870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hinnis AR, Luckett JC, Walker RA. Survivin is an independent predictor of short-term survival in poor prognostic breast cancer patients. Br J Cancer. 2007;96:639–645. doi: 10.1038/sj.bjc.6603616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barnes N, Haywood P, Flint P, Knox WF, Bundred NJ. Survivin expression in in situ and invasive breast cancer relates to COX-2 expression and DCIS recurrence. Br J Cancer. 2006;94:253–258. doi: 10.1038/sj.bjc.6602932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ryan B, O’Donovan N, Browne B, O’Shea C, Crown J, et al. Expression of survivin and its splice variants survivin-2B and survivin-DeltaEx3 in breast cancer. Br J Cancer. 2005;92:120–124. doi: 10.1038/sj.bjc.6602314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Angell H. A study into the potential role of Survivin localization in resistance to drug-induced apoptosis. Bioscience Horizons. 2008;1:85–91. [Google Scholar]
- 52.Cheung CH, Chen HH, Kuo CC, Chang CY, Coumar MS, et al. Survivin counteracts the therapeutic effect of microtubule de-stabilizers by stabilizing tubulin polymers. Mol Cancer. 2009;8:43. doi: 10.1186/1476-4598-8-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Furuya M, Tsuji N, Kobayashi D, Watanabe N. Interaction between survivin and aurora-B kinase plays an important role in survivin-mediated up-regulation of human telomerase reverse transcriptase expression. Int J Oncol. 2009;34:1061–1068. doi: 10.3892/ijo_00000232. [DOI] [PubMed] [Google Scholar]
- 54.Niu TK, Cheng Y, Ren X, Yang JM. Interaction of Beclin 1 with survivin regulates sensitivity of human glioma cells to TRAIL-induced apoptosis. FEBS Lett. 2010;584:3519–3524. doi: 10.1016/j.febslet.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roca H, Varsos Z, Pienta KJ. CCL2 protects prostate cancer PC3 cells from autophagic death via phosphatidylinositol 3-kinase/AKT-dependent survivin up-regulation. J Biol Chem. 2008;283:25057–25073. doi: 10.1074/jbc.M801073200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li M, Song T, Yin ZF, Na YQ. XIAP as a prognostic marker of early recurrence of nonmuscular invasive bladder cancer. Chin Med J (Engl) 2007;120:469–473. [PubMed] [Google Scholar]
- 57.Mizutani Y, Nakanishi H, Li YN, Matsubara H, Yamamoto K, et al. Overexpression of XIAP expression in renal cell carcinoma predicts a worse prognosis. Int J Oncol. 2007;30:919–925. [PubMed] [Google Scholar]
- 58.Tamm I, Kornblau SM, Segall H, Krajewski S, Welsh K, et al. Expression and prognostic significance of IAP-family genes in human cancers and myeloid leukemias. Clin Cancer Res. 2000;6:1796–1803. [PubMed] [Google Scholar]
- 59.Foster FM, Owens TW, Tanianis-Hughes J, Clarke RB, Brennan K, et al. Targeting inhibitor of apoptosis proteins in combination with ErbB antagonists in breast cancer. Breast Cancer Res. 2009;11:R41. doi: 10.1186/bcr2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Glinsky GV. Genomic Models of Metastatic Cancer: Functional Analysis of Death-from-Cancer Signature Genes Reveals Aneuploid, Anoikis-Resistant, Metastasis-Enabling Phenotype with Altered Cell Cycle Control and Activated PcG Protein Chromatin Silencing Pathway. Cell Cycle. 2006;5:1208–1216. doi: 10.4161/cc.5.11.2796. [DOI] [PubMed] [Google Scholar]
- 61.Berezovskaya O, Schimmer AD, Glinskii AB, Pinilla C, Hoffman RM, et al. Increased expression of apoptosis inhibitor protein XIAP contributes to anoikis resistance of circulating human prostate cancer metastasis precursor cells. Cancer Res. 2005;65:2378–2386. doi: 10.1158/0008-5472.CAN-04-2649. [DOI] [PubMed] [Google Scholar]
- 62.Seligson DB, Hongo F, Huerta-Yepez S, Mizutani Y, Miki T, et al. Expression of X-linked inhibitor of apoptosis protein is a strong predictor of human prostate cancer recurrence. Clin Cancer Res. 2007;13:6056–6063. doi: 10.1158/1078-0432.CCR-07-0960. [DOI] [PubMed] [Google Scholar]
- 63.Ferreira CG, van der Valk P, Span SW, Jonker JM, Postmus PE, et al. Assessment of IAP (inhibitor of apoptosis) proteins as predictors of response to chemotherapy in advanced non-small-cell lung cancer patients. Ann Oncol. 2001;12:799–805. doi: 10.1023/a:1011167113067. [DOI] [PubMed] [Google Scholar]
- 64.Seeger JM, Brinkmann K, Yazdanpanah B, Haubert D, Pongratz C, et al. Elevated XIAP expression alone does not confer chemoresistance. Br J Cancer. 2010;102:1717–1723. doi: 10.1038/sj.bjc.6605704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Imoto I, Yang ZQ, Pimkhaokham A, Tsuda H, Shimada Y, et al. Identifcation of cIAP1 as a candidate target gene within an amplicon at 11q22 in esophageal squamous cell carcinomas. Cancer Res. 2001;61:6629–6634. [PubMed] [Google Scholar]
- 66.Hu S, Du MQ, Park SM, Alcivar A, Qu L, et al. cIAP2 is a ubiquitin protein ligase for BCL10 and is dysregulated in mucosa-associated lymphoid tissue lymphomas. J Clin Invest. 2006;116:174–181. doi: 10.1172/JCI25641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gyrd-Hansen M, Darding M, Miasari M, Santoro MM, Zender L, et al. IAPs contain an evolutionarily conserved ubiquitin-binding domain that regulates NF-kappaB as well as cell survival and oncogenesis. Nat Cell Biol. 2008;10:1309–1317. doi: 10.1038/ncb1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cheng YJ, Jiang HS, Hsu SL, Lin LC, Wu CL, et al. XIAP-mediated protection of H460 lung cancer cells against cisplatin. Eur J Pharmacol. 2010;627:75–84. doi: 10.1016/j.ejphar.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 69.Zender L, Spector MS, Xue W, Flemming P, Cordon-Cardo C, et al. Identifcation and validation of oncogenes in liver cancer using an integrative oncogenomic approach. Cell. 2006;125:1253–1267. doi: 10.1016/j.cell.2006.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schimmer AD, Estey EH, Borthakur G, Carter BZ, Schiller GJ, et al. Phase I/II trial of AEG35156 X-linked inhibitor of apoptosis protein antisense oligonucleotide combined with idarubicin and cytarabine in patients with relapsed or primary refractory acute myeloid leukemia. J Clin Oncol. 2009;27:4741–4746. doi: 10.1200/JCO.2009.21.8172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mahadevan D, Chalasani P, Rensvold D, Kurtin S, Pretzinger C, et al. Phase I Trial of AEG35156 an Antisense Oligonucleotide to XIAP Plus Gemcitabine in Patients With Metastatic Pancreatic Ductal Adenocarcinoma. Am J Clin Oncol. 2012 doi: 10.1097/COC.0b013e3182467a13. [DOI] [PubMed] [Google Scholar]
- 72.Lu J, Bai L, Sun H, Nikolovska-Coleska Z, McEachern D, et al. SM-164: a novel, bivalent Smac mimetic that induces apoptosis and tumor regression by concurrent removal of the blockade of cIAP-1/2 and XIAP. Cancer Res. 2008;68:9384–9393. doi: 10.1158/0008-5472.CAN-08-2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tenev T, Bianchi K, Darding M, Broemer M, Langlais C, et al. The Ripoptosome, a signaling platform that assembles in response to genotoxic stress and loss of IAPs. Mol Cell. 2011;43:432–448. doi: 10.1016/j.molcel.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 74.Petersen SL, Wang L, Yalcin-Chin A, Li L, Peyton M, et al. Autocrine TNFalpha signaling renders human cancer cells susceptible to Smac-mimetic-induced apoptosis. Cancer Cell. 2007;12:445–456. doi: 10.1016/j.ccr.2007.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Varfolomeev E, Blankenship JW, Wayson SM, Fedorova AV, Kayagaki N, et al. IAP antagonists induce autoubiquitination of c-IAPs, NF-kappaB activation, and TNFalpha-dependent apoptosis. Cell. 2007;131:669–681. doi: 10.1016/j.cell.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 76.Vince JE, Wong WW, Khan N, Feltham R, Chau D, et al. IAP antagonists target cIAP1 to induce TNFalpha-dependent apoptosis. Cell. 2007;131:682–693. doi: 10.1016/j.cell.2007.10.037. [DOI] [PubMed] [Google Scholar]
- 77.Wu H, Tschopp J, Lin SC. Smac mimetics and TNFalpha: a dangerous liaison? Cell. 2007;131:655–658. doi: 10.1016/j.cell.2007.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Berger R, Jennewein C, Marschall V, Karl S, Cristofanon S, et al. NF-ΰB is required for Smac mimetic-mediated sensitization of glioblastoma cells for Î3-irradiation-induced apoptosis. Mol Cancer Ther. 2011;10:1867–1875. doi: 10.1158/1535-7163.MCT-11-0218. [DOI] [PubMed] [Google Scholar]
- 79.Tolcher AW, Mita A, Lewis LD, Garrett CR, Till E, et al. Phase I and pharmacokinetic study of YM155, a small-molecule inhibitor of survivin. J Clin Oncol. 2008;26:5198–5203. doi: 10.1200/JCO.2008.17.2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Satoh T, Okamoto I, Miyazaki M, Morinaga R, Tsuya A, et al. Phase I study of YM155, a novel survivin suppressant, in patients with advanced solid tumors. Clin Cancer Res. 2009;15:3872–3880. doi: 10.1158/1078-0432.CCR-08-1946. [DOI] [PubMed] [Google Scholar]
- 81.Giaccone G, Zatloukal P, Roubec J, Floor K, Musil J, et al. Multicenter phase II trial of YM155, a small-molecule suppressor of survivin, in patients with advanced, refractory, non-small-cell lung cancer. J Clin Oncol. 2009;27:4481–4486. doi: 10.1200/JCO.2008.21.1862. [DOI] [PubMed] [Google Scholar]
- 82.Lewis KD, Samlowski W, Ward J, Catlett J, Cranmer L, et al. A multicenter phase II evaluation of the small molecule survivin suppressor YM155 in patients with unresectable stage III or IV melanoma. Invest New Drugs. 2011;29:161–166. doi: 10.1007/s10637-009-9333-6. [DOI] [PubMed] [Google Scholar]
- 83.Cheson BD, Bartlett NL, Vose JM, Lopez-Hernandez A, Seiz AL, et al. A phase II study of the survivin suppressant YM155 in patients with refractory diffuse large B-cell lymphoma. Cancer. 2012;118:3128–3134. doi: 10.1002/cncr.26510. [DOI] [PubMed] [Google Scholar]
- 84.Na YS, Yang SJ, Kim SM, Jung KA, Moon JH, et al. YM155 induces EGFR suppression in pancreatic cancer cells. PLoS One. 2012;7:e38625. doi: 10.1371/journal.pone.0038625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yagihashi A, Ohmura T, Asanuma K, Kobayashi D, Tsuji N, et al. Detection of autoantibodies to survivin and livin in sera from patients with breast cancer. Clin Chim Acta. 2005;362:125–130. doi: 10.1016/j.cccn.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 86.Honma I, Kitamura H, Torigoe T, Takahashi A, Tanaka T, et al. Phase I clinical study of anti-apoptosis protein survivin-derived peptide vaccination for patients with advanced or recurrent urothelial cancer. Cancer Immunol Immunother. 2009;58:1801–1807. doi: 10.1007/s00262-009-0691-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fest S, Huebener N, Bleeke M, Durmus T, Stermann A, et al. Survivin minigene DNA vaccination is effective against neuroblastoma. Int J Cancer. 2009;125:104–114. doi: 10.1002/ijc.24291. [DOI] [PubMed] [Google Scholar]
- 88.Nomura T, Yamasaki M, Nomura Y, Mimata H. Expression of the inhibitors of apoptosis proteins in cisplatin-resistant prostate cancer cells. Oncol Rep. 2005;14:993–997. [PubMed] [Google Scholar]
- 89.Shi Z, Liang YJ, Chen ZS, Wang XH, Ding Y, et al. Overexpression of Survivin and XIAP in MDR cancer cells unrelated to P-glycoprotein. Oncol Rep. 2007;17:969–976. [PubMed] [Google Scholar]
- 90.Wang X, Wang C, Qin YW, Yan SK, Gao YR. The association of up-regulation of X-linked inhibitor of apoptosis protein with cell adhesion-mediated drug resistance in U937 cells. Hematol Oncol. 2008;26:21–26. doi: 10.1002/hon.828. [DOI] [PubMed] [Google Scholar]
- 91.Xia W, Bacus S, Hegde P, Husain I, Strum J, et al. A model of acquired autoresistance to a potent ErbB2 tyrosine kinase inhibitor and a therapeutic strategy to prevent its onset in breast cancer. Proc Natl Acad Sci U S A. 2006;103:7795–7800. doi: 10.1073/pnas.0602468103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.LaCasse EC, Mahoney DJ, Cheung HH, Plenchette S, Baird S, et al. IAP-targeted therapies for cancer. Oncogene. 2008;27:6252–6275. doi: 10.1038/onc.2008.302. [DOI] [PubMed] [Google Scholar]
- 93.Pennati M, Folini M, Zaffaroni N. Targeting survivin in cancer therapy: fulflled promises and open questions. Carcinogenesis. 2007;28:1133–1139. doi: 10.1093/carcin/bgm047. [DOI] [PubMed] [Google Scholar]
- 94.Jost PJ, Grabow S, Gray D, McKenzie MD, Nachbur U, et al. XIAP discriminates between type I and type II FAS-induced apoptosis. Nature. 2009;460:1035–1039. doi: 10.1038/nature08229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Keats JJ, Fonseca R, Chesi M, Schop R, Baker A, et al. Promiscuous mutations activate the noncanonical NF-kappaB pathway in multiple myeloma. Cancer Cell. 2007;12:131–144. doi: 10.1016/j.ccr.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Annunziata CM, Davis RE, Demchenko Y, Bellamy W, Gabrea A, et al. Frequent engagement of the classical and alternative NF-kappaB pathways by diverse genetic abnormalities in multiple myeloma. Cancer Cell. 2007;12:115–130. doi: 10.1016/j.ccr.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yang C, Davis JL, Zeng R, Vora P, Su X, et al. Antagonism of inhibitor of apoptosis proteins increases bone metastasis via unexpected osteoclast activation. Cancer Discov. 2013;3:212–223. doi: 10.1158/2159-8290.CD-12-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mace PD, Shirley S, Day CL. Assembling the building blocks: structure and function of inhibitor of apoptosis proteins. Cell Death Differ. 2010;17:46–53. doi: 10.1038/cdd.2009.45. [DOI] [PubMed] [Google Scholar]
- 99.Martin SJ. Dealing the CARDs between life and death. Trends Cell Biol. 2001;11:188–189. doi: 10.1016/s0962-8924(01)01971-7. [DOI] [PubMed] [Google Scholar]
- 100.Connolly K, Mitter R, Muir M, Jodrell D, Guichard S. Stable XIAP knockdown clones of HCT116 colon cancer cells are more sensitive to TRAIL, taxanes and irradiation in vitro. Cancer Chemother Pharmacol. 2009;64:307–316. doi: 10.1007/s00280-008-0872-x. [DOI] [PubMed] [Google Scholar]
- 101.McManus DC, Lefebvre CA, Cherton-Horvat G, St-Jean M, Kandimalla ER, et al. Loss of XIAP protein expression by RNAi and antisense approaches sensitizes cancer cells to functionally diverse chemotherapeutics. Oncogene. 2004;23:8105–8117. doi: 10.1038/sj.onc.1207967. [DOI] [PubMed] [Google Scholar]
- 102.Lima RT, Martins LM, Guimarães JE, Sambade C, Vasconcelos MH. Specifc downregulation of bcl-2 and xIAP by RNAi enhances the effects of chemotherapeutic agents in MCF-7 human breast cancer cells. Cancer Gene Ther. 2004;11:309–316. doi: 10.1038/sj.cgt.7700706. [DOI] [PubMed] [Google Scholar]
- 103.Hu Y, Cherton-Horvat G, Dragowska V, Baird S, Korneluk RG, et al. Antisense oligonucleotides targeting XIAP induce apoptosis and enhance chemotherapeutic activity against human lung cancer cells in vitro and in vivo. Clin Cancer Res. 2003;9:2826–2836. [PubMed] [Google Scholar]
- 104.Lopes RB, Gangeswaran R, McNeish IA, Wang Y, Lemoine NR. Expression of the IAP protein family is dysregulated in pancreatic cancer cells and is important for resistance to chemotherapy. Int J Cancer. 2007;120:2344–2352. doi: 10.1002/ijc.22554. [DOI] [PubMed] [Google Scholar]
- 105.Engesæter BO, Sathermugathevan M, Hellenes T, Engebråten O, Holm R, et al. Targeting inhibitor of apoptosis proteins in combination with dacarbazine or TRAIL in melanoma cells. Cancer Biol Ther. 2011;12:47–58. doi: 10.4161/cbt.12.1.15714. [DOI] [PubMed] [Google Scholar]
- 106.Yonesaka K, Tamura K, Kurata T, Satoh T, Ikeda M, et al. Small interfering RNA targeting survivin sensitizes lung cancer cell with mutant p53 to adriamycin. Int J Cancer. 2006;118:812–820. doi: 10.1002/ijc.21350. [DOI] [PubMed] [Google Scholar]
- 107.Yang H, Fu JH, Hu Y, Huang WZ, Zheng B, et al. Influence of SiRNA targeting survivin on chemosensitivity of H460/cDDP lung cancer cells. J Int Med Res. 2008;36:734–747. doi: 10.1177/147323000803600416. [DOI] [PubMed] [Google Scholar]
- 108.Yang Y, Gao Y, Chen L, Huang Y, Li Y. Downregulation of survivin expression and enhanced chemosensitivity of MCF-7 cells to adriamycin by PDMAE/survivin shRNA complex nanoparticles. Int J Pharm. 2011;405:188–195. doi: 10.1016/j.ijpharm.2010.11.047. [DOI] [PubMed] [Google Scholar]
- 109.Yang W, Sun T, Cao J, Liu F. Survivin downregulation by siRNA/cationic liposome complex radiosensitises human hepatoma cells in vitro and in vivo. Int J Radiat Biol. 2010;86:445–457. doi: 10.3109/09553001003668006. [DOI] [PubMed] [Google Scholar]
- 110.He SQ, Rehman H, Gong MG, Zhao YZ, Huang ZY, et al. Inhibiting survivin expression enhances TRAIL-induced tumoricidal activity in human hepatocellular carcinoma via cell cycle arrest. Cancer Biology & Therapy. 2007;6:1258–1268. doi: 10.4161/cbt.6.8.4444. [DOI] [PubMed] [Google Scholar]
- 111.Nagata M, Nakayama H, Tanaka T, Yoshida R, Yoshitake Y, et al. Overexpression of cIAP2 contributes to 5-FU resistance and a poor prognosis in oral squamous cell carcinoma. Br J Cancer. 2011;105:1322–1330. doi: 10.1038/bjc.2011.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Miura K, Karasawa H, Sasaki I. cIAP2 as a therapeutic target in colorectal cancer and other malignancies. Expert Opin Ther Targets. 2009;13:1333–1345. doi: 10.1517/14728220903277256. [DOI] [PubMed] [Google Scholar]
- 113.Karasawa H, Miura K, Fujibuchi W, Ishida K, Kaneko N, et al. Down-regulation of cIAP2 enhances 5-FU sensitivity through the apoptotic pathway in human colon cancer cells. Cancer Sci. 2009;100:903–913. doi: 10.1111/j.1349-7006.2009.01112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ziegler DS, Wright RD, Kesari S, Lemieux ME, Tran MA, et al. Resistance of human glioblastoma multiforme cells to growth factor inhibitors is overcome by blockade of inhibitor of apoptosis proteins. J Clin Invest. 2008;118:3109–3122. doi: 10.1172/JCI34120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Greer RM, Peyton M, Larsen JE, Girard L, Xie Y, et al. SMAC mimetic (JP1201) sensitizes non-small cell lung cancers to multiple chemotherapy agents in an IAP-dependent but TNF-α-independent manner. Cancer Res. 2011;71:7640–7648. doi: 10.1158/0008-5472.CAN-10-3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Li W, Li B, Giacalone NJ, Torossian A, Sun Y, et al. BV6, an IAP antagonist, activates apoptosis and enhances radiosensitization of non-small cell lung carcinoma in vitro. J Thorac Oncol. 2011;6:1801–1809. doi: 10.1097/JTO.0b013e318226b4a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lu J, McEachern D, Sun H, Bai L, Peng Y, et al. Therapeutic potential and molecular mechanism of a novel, potent, nonpeptide, Smac mimetic SM-164 in combination with TRAIL for cancer treatment. Mol Cancer Ther. 2011;10:902–914. doi: 10.1158/1535-7163.MCT-10-0864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dean E, Jodrell D, Connolly K, Danson S, Jolivet J, et al. Phase I trial of AEG35156 administered as a 7-day and 3-day continuous intravenous infusion in patients with advanced refractory cancer. J Clin Oncol. 2009;27:1660–1666. doi: 10.1200/JCO.2008.19.5677. [DOI] [PubMed] [Google Scholar]
- 119.Mannhold R, Fulda S, Carosati E. IAP antagonists: promising candidates for cancer therapy. Drug Discov Today. 2010;15:210–219. doi: 10.1016/j.drudis.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 120.Dai Y, Desano J, Qu Y, Tang W, Meng Y, et al. Natural IAP inhibitor Embelin enhances therapeutic efficacy of ionizing radiation in prostate cancer. Am J Cancer Res. 2011;1:128–143. [PMC free article] [PubMed] [Google Scholar]
- 121.Schimmer AD, Welsh K, Pinilla C, Wang Z, Krajewska M, et al. Small-molecule antagonists of apoptosis suppressor XIAP exhibit broad antitumor activity. Cancer Cell. 2004;5:25–35. doi: 10.1016/s1535-6108(03)00332-5. [DOI] [PubMed] [Google Scholar]
- 122.Wu TY, Wagner KW, Bursulaya B, Schultz PG, Deveraux QL. Development and characterization of nonpeptidic small molecule inhibitors of the XIAP/caspase-3 interaction. Chem Biol. 2003;10:759–767. doi: 10.1016/s1074-5521(03)00157-1. [DOI] [PubMed] [Google Scholar]
- 123.Iwasa T, Okamoto I, Takezawa K, Yamanaka K, Nakahara T, et al. Marked anti-tumour activity of the combination of YM155, a novel survivin suppressant, and platinum-based drugs. Br J Cancer. 2010;103:36–42. doi: 10.1038/sj.bjc.6605713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Plescia J, Salz W, Xia F, Pennati M, Zaffaroni N, et al. Rational design of shepherdin, a novel anticancer agent. Cancer Cell. 2005;7:457–468. doi: 10.1016/j.ccr.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 125.Gyurkocza B, Plescia J, Raskett CM, Garlick DS, Lowry PA, et al. Antileukemic activity of shepherdin and molecular diversity of hsp90 inhibitors. J Natl Cancer Inst. 2006;98:1068–1077. doi: 10.1093/jnci/djj300. [DOI] [PubMed] [Google Scholar]
- 126.Meli M, Pennati M, Curto M, Daidone MG, Plescia J, et al. Small-molecule targeting of heat shock protein 90 chaperone function: rational identification of a new anticancer lead. J Med Chem. 2006;49:7721–7730. doi: 10.1021/jm060836y. [DOI] [PubMed] [Google Scholar]
- 127.Cai Q, Sun H, Peng Y, Lu J, Nikolovska-Coleska Z, et al. A potent and orally active antagonist (SM-406/AT-406) of multiple inhibitor of apoptosis proteins (IAPs) in clinical development for cancer treatment. J Med Chem. 2011;54:2714–2726. doi: 10.1021/jm101505d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gaither A, Porter D, Yao Y, Borawski J, Yang G, et al. A Smac mimetic rescue screen reveals roles for inhibitor of apoptosis proteins in tumor necrosis factor-alpha signaling. Cancer Res. 2007;67:11493–11498. doi: 10.1158/0008-5472.CAN-07-5173. [DOI] [PubMed] [Google Scholar]
- 129.Li L, Thomas RM, Suzuki H, De Brabander JK, Wang X, et al. A small molecule Smac mimic potentiates TRAIL- and TNFalpha-mediated cell death. Science. 2004;305:1471–1474. doi: 10.1126/science.1098231. [DOI] [PubMed] [Google Scholar]
- 130.Oost TK, Sun C, Armstrong RC, Al-Assaad AS, Betz SF, et al. Discovery of potent antagonists of the antiapoptotic protein XIAP for the treatment of cancer. J Med Chem. 2004;47:4417–4426. doi: 10.1021/jm040037k. [DOI] [PubMed] [Google Scholar]