Abstract
Background
Minimal scientific information is available to inform public health policy on binge drinking prior to pregnancy detection. The nonhuman primate provides a valuable animal model for examining consequences to reproduction and offspring function that may result from this common pattern of alcohol abuse.
Methods
Adult female rhesus monkeys were dosed with 1.5 g/kg-d ethanol by gavage two days/week beginning seven months prior to mating and continuing to pregnancy detection at 19–20 days gestation. Postnatal evaluation of control (n=6) and ethanol treated (n=4) infants included a neonatal neurobehavioral assessment, a visual paired comparison (cognitive) test at 35 days of age and mother-infant interaction at 100–112 days of age.
Results
Alcohol-exposed neonates did not differ from controls in posture and reflex measures. Longer durations of visual fixation, suggesting slower visual processing, and greater novelty preference were seen in the alcohol group. At early weaning age, as infants spent more time away from their dams, more of the reunions between mother and infant were initiated by the mothers in the alcohol-exposed group, suggesting a more immature mother-infant interaction.
Conclusion
Intermittent high dose alcohol exposure (binge drinking) discontinued at early pregnancy detection in rhesus monkey can result in altered behavioral function in the infant. Mediating effects on ovum, reproductive tract and early embryo can be explored in this model. Studies of longer-term consequences in human populations and animal models are needed.
Introduction
Binge drinking continues to increase, especially in young people, and recent data indicates that 28% of 18 to 34 year-olds engage in binge drinking with an average of nearly six drinks per episode for women (CDC, 2012). Heavy alcohol consumption can lead to fetal alcohol syndrome (FAS) and related neurodevelopment effects (Jacobs 2000), but less is known about binge drinking, especially if limited to the peri-conception period. Binge drinking is associated with risky sexual behavior (Chersich and Rees, 2010, Milne et al., 2007), leading to concerns about unintended pregnancies. In a recent study, over 30% of women in the United States engaged in binge drinking before conception and over 10% between conception and prior to pregnancy detection (Tough et al., 2006). Engaging in binge drinking before pregnancy is recognized could lead to lasting effects on the fetus. Human epidemiological studies find it difficult to determine specific risks associated with binge drinking, in part because of dependence on recall or questionnaire design (Conover and Jones, 2012).
While animal studies on developmental consequences of binge drinking are also limited, they have demonstrated that the same daily dose of alcohol, when consumed as a binge, will result in higher peak blood ethanol levels than when alcohol consumption is distributed throughout the day. Further, these studies also found that binge-pattern alcohol is associated with greater effects on neuronal function and behavior (Bonthius and West, 1990, Burden et al., 2011). Early embryonic alcohol exposure has long been known to be relevant to FAS because it is the sensitive period for induction of facial dysmorphology, a marker of FAS. A nonhuman primate (NHP) study found that a critical period of binge alcohol exposure for craniofacial defects occurred on gestation days 19 to 20 (term = 170 days) (Astley et al., 1999).
Oocyte growth and maturation occurs over many months prior to ovulation in primates, including humans. The period of oocyte growth and maturation is becoming recognized as a potentially vulnerable time for effects of maternal nutrition and health, such as obesity (Purcell and Moley, 2011). Binge alcohol consumption may also have the potential to affect oocytes well before the time of ovulation and the menstrual cycle in which pregnancy may be initiated. Because part of the epigenetic reprogramming of oocytes takes place during this phase of oocyte growth (Sabour and Scholer, 2012), it is possible that alcohol can have lasting epigenetic effects that are mediated through oocytes. In this study we evaluated whether binge alcohol intake, over several months prior to conception and until pregnancy was detected, could alter the early behavior of offspring in a nonhuman primate model.
Methods
Adult female rhesus macaques (Macaca mulatta), from the colony at the California National Primate Research Center, were housed as previously described (de Prada and VandeVoort, 2008) except that animals were not socially paired on days when dosing occurred. All procedures for maintenance and handling of the animals were reviewed and approved in advance by the Institutional Animal Use and Care Administrative Advisory Committee of the University of California, Davis. The criteria for selection included age range from 6 to 12 years, history of successful pregnancy, and normal menstrual cycles. Six dams were assigned to each of the experimental groups (ethanol, isocaloric sucrose control). Table 1 details similar characteristics of dams in the control and ethanol groups. Menstrual bleeding was monitored daily and body weights were recorded weekly for the duration of the study. Animals were naturally time-mated and ethanol dosing was discontinued once pregnancy was confirmed by ultrasound examination (Tarantal, 2005) at approximately 19 – 20 days gestation. Two spontaneous abortions were detected by vaginal bleeding and confirmed by ultrasound at 30 days gestation in the ethanol group; a causal relationship with alcohol treatment cannot be assessed in this small sample. On the day of birth, pair housing was discontinued, if applicable, in order to standardize the rearing environment of the infants for the duration of behavioral testing. Group identification was not provided during behavioral testing.
Table 1. Dam background variables.
Background variables. Comparison of Control and Alcohol-exposed dam and pregnancy outcome variables. No statistical group differences were seen.
| CONTROL | ETHANOL | |
|---|---|---|
| Total duration of dosing (days) | 343±18 | 345±10 |
| dosing discontinued (GD) | 20±1* | 19±2 |
| parity | 4.8±1.1 | 3.5±0.6 |
| age at conception (years) | 8.5±1.3 | 8.6±1.1 |
| time in indoor colony (years) | 2.47±0.6 | 0.98±0.5 |
| weight prior to conception (kg) | 8.15±0.6 | 7.54±0.5 |
| weight third trimester (kg)** | 9.94±0.6 | 9.79±0.4 |
| pregnancy weight gain (kg) | 1.80±0.3 | 2.26±0.3 |
| gestation length (d) | 165+3 | 169±3 |
| infant sex | 3 M/3 F | 2 M/2 F |
| infant weight PND2 (g) | 522±34 | 532±42 |
| infant weight PND35 (g) | 680±48 | 640±58 |
| Infant weight gain PND35-2 (g) | 157±27 | 107±33 |
mean ± sem
GD at third trimester weighing = 150±2
Binge drinking effects on oocyte development is a major focus of this research project. Animals received treatment for at least seven months prior to natural mating to assure that all phases of oocyte follicular growth and development were exposed to alcohol. Two days per week animals were hand-caught and administered ethanol in water for the treatment group or isocaloric sucrose in water for the control group via nasogastric tube. A gradually increasing dosing regime was used to initiate treatment in which animals were administered a low dose the first week, a mid-range dose the second week and a high dose for the remainder of the study. The low, middle and high doses of ethanol were 0.75, 1.125, and 1.50 g/kg respectively. The sucrose dose was determined based on equivalent caloric content and the low, middle and high doses were 5.25, 7.90, and 10.5 cal/kg respectively. Dosing continued until the confirmation of pregnancy by ultrasound.
The postnatal infant testing schedule employed tests that have parallels in human infant evaluation and previous nonhuman primate studies of fetal alcohol syndrome. The Neurobehavioral Test (NBT) battery was conducted on postnatal day (PND) 2 ± 1 day. Visual paired comparison test (VPC) was conducted on PND35 ± 3 days, and Mother-Infant (MI) interaction videos were recorded on PND 100, 103, 106, 109 and 112. Dams were lightly sedated with ketamine (5–10 mg/kg i.m.) to remove the infants for NBT and VPC. Infants were placed in a warm incubator and transported to a workroom for testing. At the conclusion of testing, approximately 15 min for NBT and 30 min for VPC, infants were placed back in the incubator for return to the dam in the home cage.
The NBT (Golub et al., 1985, Golub et al., 2006, Golub and Germann, 1998, Golub et al., 1988) measured the quality and maturation of simple reflexes (such as rooting, lipsmack orientation, Moro reflex) and motor patterns (walking, grasping, righting, cling), as well as muscle tone. The test also included a rating of distress behavior or “build-up.” It is similar to the Brazelton Neurobehavioral Test Battery, widely used in humans (Coles et al., 1985) and the Infant Behavioral Assessment Scale (IBAS) previously used in NHP models of FAS (Schneider et al., 1997, Streissguth et al., 1983).
VPC is a test of higher cognitive function used in both human and nonhuman primate infants (Burbacher and Grant, 2012). For VPC, the infant was swaddled in a towel and hand-held with its head free in front of the testing apparatus in a darkened room. The apparatus consisted of a small booth with side panels to shield from outside distractions where the stimuli, black and white pairs of abstract illustrations of varying contrasts (Fagan Test of Infant Intelligence, Infantest Corporation, Cleveland, OH) were mounted to the right or left of a center viewing hole. Four problems (stimulus pairs) were presented in the session, increasing in difficulty (similarity between the novel and familiar stimuli). The infant was held approximately 36 cm away from the stimuli. A second tester behind the booth viewed the infant’s head through a video camera to record fixation times to the right and left. Two identical stimuli were placed on the right and left for the Familiarization Trial which continued until 20 sec total fixation time was reached. For the Preference Trial, one “familiar” stimulus was removed and replaced with a novel stimulus, and the frequency and duration of looking were measured from video for a 10 sec period beginning at the first fixation. The sides of the two stimuli were then exchanged for a second 10 sec period. Novelty preference was calculated from data in the Preference Trials as:
Look duration, which is a measure of speed of visual processing, a basic component of cognition, was also analyzed for ethanol effects (Jacobson et al., 1992, Rose et al., 2002, Rose et al., 1988).
MI videos were recorded in the home cage. A video camera and external light were attached to a tripod and placed in front of the cage. Three adaptation sessions with the inactive camera were conducted prior to recording. The five 30 min videos were recorded at approximately 12:00 pm, and personnel access to the animal room was restricted during this time to minimize disruption. Videos were coded using The Observer software (Noldus Information Technology, Wageningen, The Netherlands) to assess the maturational level of mother-infant interaction with respect to independent activity of the infant (Hinde and Atkinson, 1970). The scoring recorded the discrete number of reunions and separations and the duration of time that the mother-infant pair spent together or apart. Additional modifiers gave details of the interaction, such as who initiated the behavior, the response of the recipient and the final outcome of the event along with the duration of synchronous behavior. Finally, incidences of maternal abuse (Maestripieri et al., 1999) were also scored from the videos.
All statistical analyses utilized ANOVA (GLM Procedure, SAS Inc., Cary, NC) with ethanol vs. isocaloric sucrose as the independent variable. Potential covariates were examined and included in the analysis if they were associated with the independent variable. P<0.05 was used to identify statistical significance.
Results
Background demographics
Details of the background demographics are presented in Table 1. All dams were born at CNPRC. While dams in this study came from heterogeneous prior living conditions, they had been housed indoors for at least six months prior to initiation of dosing and lived in one of four indoor cagerooms during the study. Dams were paired with another cagemate during dosing and pregnancy with the exception of three (two control, one ethanol) for whom no appropriate cagemates were available. The housing variables were not significant confounders but could have added to variability and reduced the sensitivity of the study.
Groups did not differ in background demographics, gestation length or weight gain during pregnancy. All deliveries were live vaginal births and there were no stillbirths or postnatal deaths. There were two male and two female infants in the ethanol group and three males and three females in the control group. The infants’ birthweight (PND2) and one month weight (PND35) were not affected by treatment, although control infants gained more on the average than ethanol infants over the first month. Power calculations indicated that group sizes of n=15 would be needed to identify a difference in weight gain given the effect size.
Neurobehavioral Test Battery
Ethanol infants did not differ from controls in the neonatal examination conducted on the day after birth. Gestational age at birth was used as a covariate in the analysis of these endpoints. All infants displayed normal maturity of basic reflexes and responsiveness in the test situation at that time. For the most sensitive endpoints, observed and elicited muscle tone, group sizes of n=10 and n=9, respectively, would be needed to identify a statistically significant difference given the effect sizes (1.97 and 2.29).
Visual Paired Comparison Test
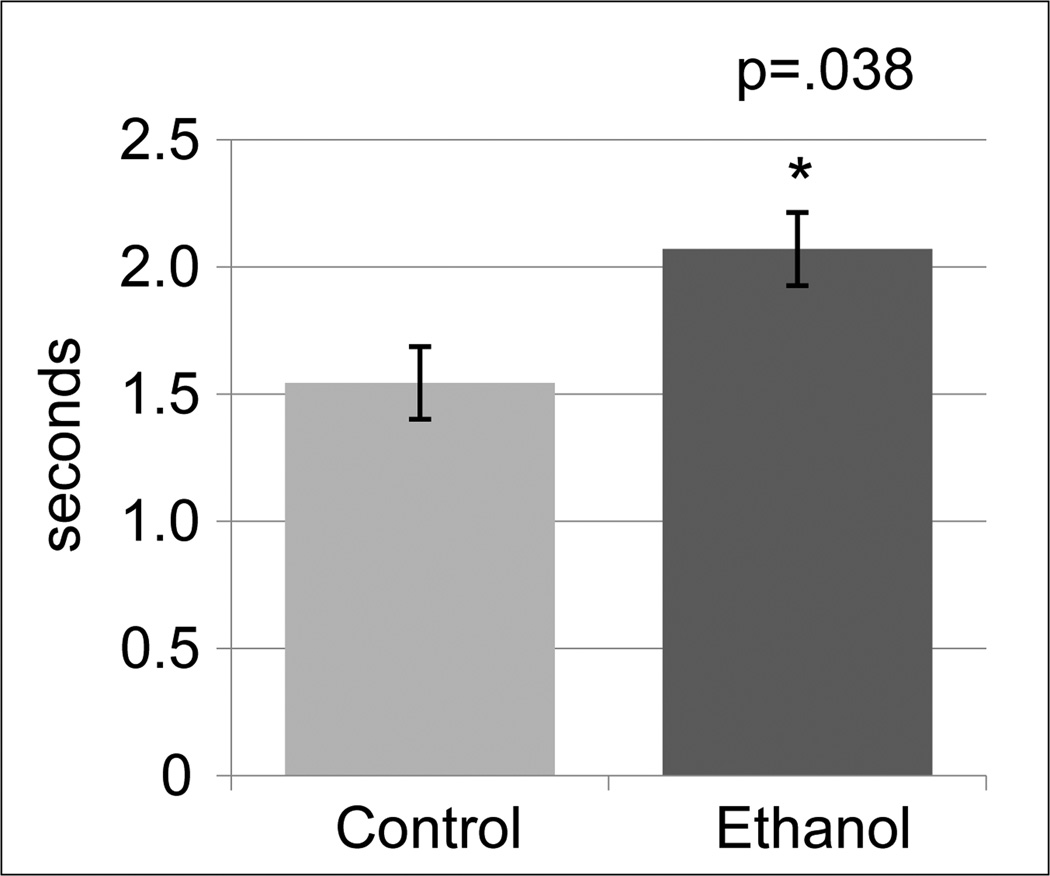
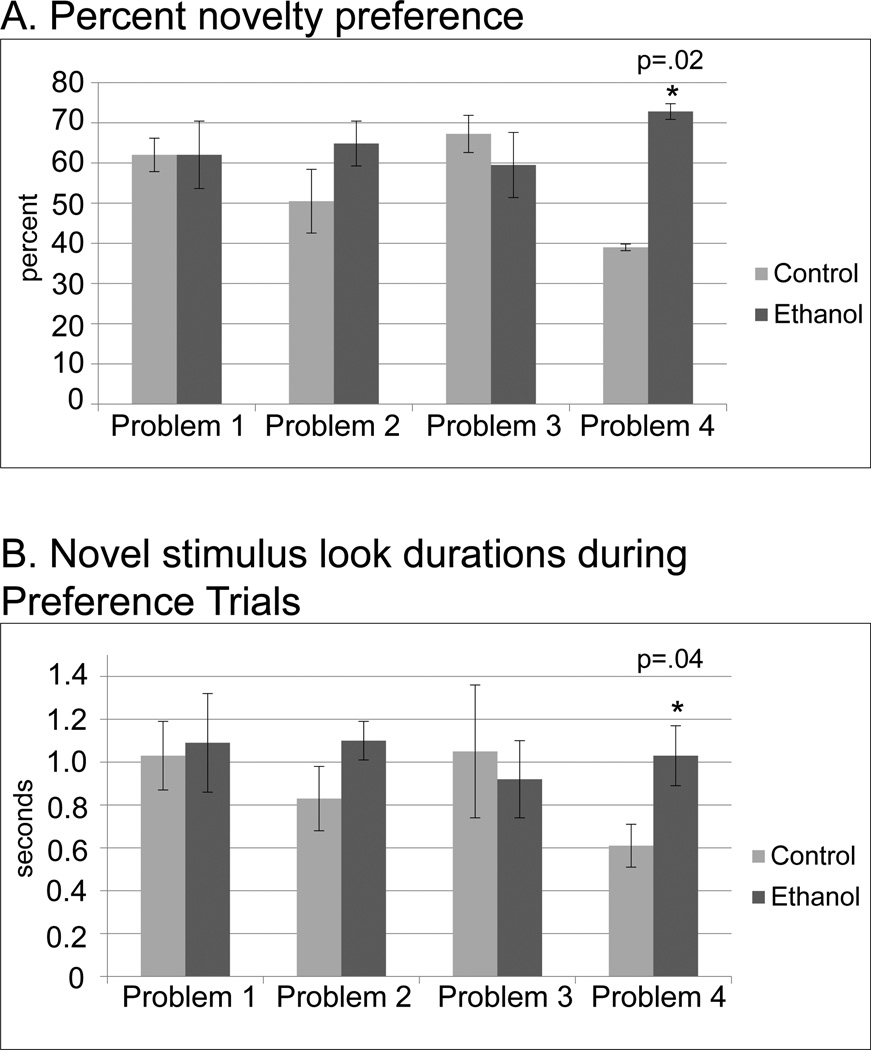
During Familiarization Trials, ethanol group infants demonstrated longer individual look durations than controls (F=6.18, df=1,9, p=0.038) (Figure 1). During Preference Trials, ethanol group infants had greater novelty preference on the most difficult problem (problem 4) (F= 8.44, df=1,9, p=0.02 (Figure 2A)) and for the average of all problems (F=7.96, df=1,9, p=0.02). In addition, longer individual look durations were seen for the novel, but not the familiar stimulus on Problem 4 (F=5.95, df=1,9, p=0.040) (Figure 2B). There was no treatment effect on the number of looks directed at the novel or the familiar stimulus during the Preference Trial.
Figure 1.
Visual Paired Comparison. Average duration of each individual look during the familiarization trials across all four problems. N=6 control (isocaloric sucrose), N=4 ethanol (1.5 g/kg ethanol 2 x/week). Dosing began seven months prior to mating and continued to pregnancy detection at 19–20 days gestation. P-values are from ANOVA.
Figure 2.
Visual Paired Comparison. A. Group comparison of percent novelty preference, the percent time looking at the novel stimulus of total looking time during two preference trials for each stimulus pair. B. The average duration of each individual look at the novel stimulus during two preference trials for each stimulus pair. See legend Figure 1.
Further analysis demonstrated an association between longer novel look durations and novelty preference on Problem 4 (r=.67, t=2.53, df=1,9, p=.035). An integrated interpretation of these data is that slower visual processing during initial exposure to stimuli (Familiarization Trial and novel stimulus during the Preference Trial) resulted in longer individual look durations. Generally, slower visual processing is associated with impaired cognitive function, including lower novelty preference (Rose et al., 2003). However, in the paradigm used here, slower visual processing could affect the preference index independent of recognition memory. For the Preference Trials, the trial length was limited to 10 sec. An average of 2.2 ± 0.2 looks were directed at the novel and familiar stimuli during each of the two 10-sec Preference Trials. Longer look durations for the novel stimulus could potentially lead to a longer total amount of looking at the novel stimulus and a greater novelty preference index.
Mother-Infant Interaction
Early in infancy, the infant spends most of the time clinging to the dam’s ventrum. As it gets older, the infant begins to demonstrate independence by breaking contact with the dam (separation) and returning after an interval of climbing and exploring the environment (reunion); the dam may also break contact with the infant and retrieve it when it is out of contact (Hinde and Atkinson, 1970). Measures of separation were not influenced by ethanol, but reunions reflected an influence of the peri-conceptual exposure. As the infant gains independence there are six types of reunion events which reflect a growing role of the infant in maintaining proximity. The percent of each type of event in the sample as a whole at the ages assessed is shown in parentheses.
Infant initiates, mother accepts (72%)
Infant initiates, mother rejects successfully (13%)
Infant initiates, mother rejects unsuccessfully (2%)
Mother initiates, infant accepts (10%)
Mother initiates, infant rejects successfully (2%)
Mother initiates, infant rejects unsuccessfully (1%)
Infant initiated events accepted by the mother indicate appropriate infant independence. Mother initiated events accepted by the infant indicate a more immature pattern. A larger number of rejections than acceptances indicates conflict. The ethanol mother-infant pairs differed from controls in the number of “mother initiates, infant accepts” reunions (p=.03) (Table 2). This may reflect a delay in the shift in responsibility for maintaining proximity from mother to infant as the infant matures (Hinde and Atkinson, 1970).
Table 2. Percent of mother-infant reunions in each of six categories.
Percent occurrence of each of 6 categories of mother-infant reunion depending on the intiator and receiver of the action and their response.
| CONTROL | ETHANOL | |
|---|---|---|
| Mother initiate-infant accept | 7.3±1.9* | 15.2±2.3** |
| Mother initiate-infant reject-successful | 2.5±1.4 | 0.6±1.7 |
| Mother initiate-infant reject unsuccessful | 0.9±0.4 | 0.2±0.4 |
| Infant initiate-mother accept | 70.4±4.1 | 75.2±5.0 |
| Infant initiate-mother reject-successful | 16.6±3.7 | 7.0±4.6 |
| Infant initiate-mother reject unsuccessful | 2.1±1.0 | 1.8±1.2 |
mean±s.e.m.
p=.03, control vs. ethanol
A second measure that showed a trend for group effects was the duration of synchronous behavior (infant doing the same thing as the mother) during the time that the mother and infant were separated. A relatively small amount of time was spent in synchronous behavior, but this was lower in the ethanol group (13%) than the control group (30%) (F=4.34, df=1.9, p=.071). Power calculations indicate that n=6 monkeys per group, two more than were tested, would be necessary to establish statistical significance.
Scoring of maternal abuse demonstrated a median of three events scored under this category over the 150 min observation time. There were no group differences or indications of individual mother-infant pairs in the ethanol group that were outstanding in this regard.
Discussion
The binge alcohol administration in this study began seven months prior to mating and continued to pregnancy detection for about three weeks (19–20 days) during early embryonic development. The consequences of preconception exposures on conceptus brain development have not been studied to date. Early embryonic alcohol exposure has long been known to be relevant to FAS because it is the sensitive period for induction of facial dysmorphology, a marker of FAS. Animal studies have also demonstrated that early gestation is particularly susceptible to alcohol effects on brain development. Recent research in mice confirms that brain malformations, as well as craniofacial defects, occur in response to alcohol dosing in the period shortly after implantation (Lipinski et al., 2012). Monkey models of behavioral impairment after prenatal alcohol exposure (Schneider et al., 2011), including a chronic low dose exposure model (m. mulatta, 0.6 g/kg daily) and a binge drinking model (m. nemestrima >1.5 g/kg weekly), have used exposures limited to the early embryonic period, beginning at mating.
Evaluation of neonatal brain function with the NBT did not show effects in our peri- conceptual model. Prior studies with a monkey binge-drinking model starting at conception also failed to find effects from exposure over the first three or six weeks of embryonic development on neonatal neurobehavioral function (Clarren et al., 1992). In a continuous low-dose monkey model with longer early embryonic exposure (gestational day (GD) 0 – GD50), effects were found when four infant exams were administered at intervals over the first three weeks of life (Schneider 1997). Effects at birth were not reported separately and many of the sensitive endpoints, such as visual orienting are not yet present at birth. These studies used a similar, but not identical, neonatal neurobehavioral test battery. Longer follow-up, as well as larger group sizes, might provide a more sensitive evaluation in our model. One study in humans assessing neonatal neurobehavior after binge-drinking in the early embryonic period (Coles et al., 1985) found that newborns of mothers who discontinued drinking in the second trimester after identification of pregnancy differed from controls who never drank during pregnancy on incidence of three items of the Brazelton Neonatal Assessment Scale (BNAS), ankle clonus, Moro reflex and rooting reflex. The present study would not have the power to detect a low incidence of abnormal responses.
The current study found longer visual processing time (longer look durations) in the Visual Paired Comparison test. Notably, maternal drinking during pregnancy was associated with longer look durations in a visual paired comparison test (Fagan Test of Infant Intelligence) as well as in a cross-modal recognition task with human infants (Jacobson et al., 1993b). No differences in novelty preference were seen in this study of Detroit children. Later studies in another cohort (Burden et al., 2011) also identified this delayed information processing using visual evoked potentials, rather than look duration, during a Go-NoGo task. In the present study, longer look durations were seen in alcohol-exposed infants than in controls during the Familiarization Trial (both stimuli the same) and for the novel stimulus during the Preference Trial. This finding is consistent with longer visual processing times reported in human studies of prenatal alcohol exposure.
Novelty preference, an early indicator of recognition memory in human and nonhuman primate studies (Burbacher and Grant, 2012), was greater in alcohol-exposed than in control infants in the current study. This test was also used in the NHP binge-drinking model of Clarren (Clarren et al., 1992). The authors reported decreased novelty preference with 6 and 24 weeks of gestational binge-drinking. However, the three-week exposure, most similar to the current study, did not show decreased preference and, in fact, higher novelty preference was reported although a statistical comparison was not given. Visual processing time was not reported.
For problem 4, the most difficult problem, which clearly discriminated alcohol-treated infants and controls, visual processing time and novelty preference were significantly correlated. It is not possible to determine a potential causal direction between these two measures.
We also found effects of the periconceptual binge-drinking on mother-infant interactions during the early weaning period. A more protective, immature pattern was indicated by more frequent mother-initiated retrievals of the infants when they were apart. This pattern is typically interpreted as the infant eliciting and/or the mother discriminating more immaturity or impaired function (Fairbanks, 1996). Mother-infant interaction was not studied in the binge-drinking studies of Clarren et al. (Clarren et al., 1988, Clarren et al., 1992, Clarren et al., 1987) as the infants were separated from their mothers at birth and reared in the primate nursery. Schneider et al. reared infants with dams and observed mother-infant interactions with no difference in time spent in ventral contact during the first week after birth (Schneider et al., 1997). The weaning period was not examined. In humans, mother-infant relationship has been assessed primarily in terms of attachment styles as demonstrated under separation situations (O'Connor et al., 2002). Less secure attachment dependent on the quality of maternal behavior was reported in association with prenatal alcohol consumption. Human studies of mother- infant interaction after gestational alcohol exposure are limited and do not include studies with exclusive early embryonic exposure (Kelly et al., 2000).
Weaning represents a widening of infant exploratory activity as well as a social challenge. Our data also suggested a decrease in synchronous behavior when mother and infant were apart. This may be related to findings of impaired imitative behavior in infants exposed to alcohol prenatally (Kelly et al., 2000, Maestripieri et al., 1999, Jacobson et al., 1993a).
This small, but highly controlled study, in a genetically diverse population with varying prior life experience was able to demonstrate that binge drinking discontinued at early pregnancy detection can result in altered behavioral function in the newborn. It supplements studies in NHP with early embryonic exposure by including a preconception pattern of binge-drinking representative of human alcohol abuse and may offer a model useful in determining the consequences of binge-drinking in young women. Further work is needed to replicate the finding and to identify long term consequences.
Acknowledgements
The authors appreciate the assistance of Research Services personnel who conducted the ethanol dosing, and Dana Hill for dose preparation. Behavioral tests were conducted by the Behavior Assessment Core of CNPRC. Alicia Bulleri assisted with the behavioral testing.
Supported by NIH grants: AA019595 (CAV) and OD011107/RR00169 (CNPRC)
References
- Astley SJ, Magnuson SI, Omnell LM, Clarren SK. Fetal alcohol syndrome: changes in craniofacial form with age, cognition, and timing of ethanol exposure in the macaque. Teratology. 1999;59:163–172. doi: 10.1002/(SICI)1096-9926(199903)59:3<163::AID-TERA8>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Bonthius DJ, West JR. Alcohol-induced neuronal loss in developing rats: increased brain damage with binge exposure. Alcohol Clin Exp Res. 1990;14:107–118. doi: 10.1111/j.1530-0277.1990.tb00455.x. [DOI] [PubMed] [Google Scholar]
- Burbacher TM, Grant KS. Measuring infant memory: Utility of the visual paired-comparison test paradigm for studies in developmental neurotoxicology. Neurotoxicol Teratol. 2012;34:473–480. doi: 10.1016/j.ntt.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burden MJ, Westerlund A, Muckle G, Dodge N, Dewailly E, Nelson CA, Jacobson SW, Jacobson JL. The effects of maternal binge drinking during pregnancy on neural correlates of response inhibition and memory in childhood. Alcohol Clin Exp Res. 2011;35:69–82. doi: 10.1111/j.1530-0277.2010.01323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. Vital signs: binge drinking prevalence, frequecy, and intensity among adults - United States, 2010. MMWR Morb Mortal Wkly Rep. 2012;61:14–19. [PubMed] [Google Scholar]
- Chersich MF, Rees HV. Causal links between binge drinking patterns, unsafe sex and HIV in South Africa: its time to intervene. Int J STD AIDS. 2010;21:2–7. doi: 10.1258/ijsa.2000.009432. [DOI] [PubMed] [Google Scholar]
- Clarren SK, Astley SJ, Bowden DM. Physical anomalies and developmental delays in nonhuman primate infants exposed to weekly doses of ethanol during gestation. Teratology. 1988;37:561–569. doi: 10.1002/tera.1420370605. [DOI] [PubMed] [Google Scholar]
- Clarren SK, Astley SJ, Gunderson VM, Spellman D. Cognitive and behavioral deficits in nonhuman primates associated with very early embryonic binge exposures to ethanol. J Pediatr. 1992;121:789–796. doi: 10.1016/s0022-3476(05)81917-1. [DOI] [PubMed] [Google Scholar]
- Clarren SK, Bowden DM, Astley SJ. Pregnancy outcomes after weekly oral administration of ethanol during gestation in the pig-tailed macaque (Macaca nemestrina) Teratology. 1987;35:345–354. doi: 10.1002/tera.1420350309. [DOI] [PubMed] [Google Scholar]
- Coles CD, Smith I, Fernhoff PM, Falek A. Neonatal neurobehavioral characteristics as correlates of maternal alcohol use during gestation. Alcohol Clin Exp Res. 1985;9:454–460. doi: 10.1111/j.1530-0277.1985.tb05582.x. [DOI] [PubMed] [Google Scholar]
- Conover EA, Jones KL. Safety concerns regarding binge drinking in pregnancy: a review. Birth Defects Res A Clin Mol Teratol. 2012;94:570–575. doi: 10.1002/bdra.23034. [DOI] [PubMed] [Google Scholar]
- de Prada JK, VandeVoort CA. Growth hormone and in vitro maturation of rhesus macaque oocytes and subsequent embryo development. J Assist Reprod Genet. 2008;25:145–158. doi: 10.1007/s10815-008-9208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks L. Individual differences in maternal style: causes and consequences for mothers and offspring. Adv Study Behav. 1996;25:579–611. [Google Scholar]
- Golub MS, Eisele JH, Jr, Donald JM. Obstetric analgesia and infant outcome in monkeys. Neonatal measures after intrapartum exposure to meperidine or alfentanil. Am J Obstet Gynecol. 1988;158:1219–1225. doi: 10.1016/0002-9378(88)90258-x. [DOI] [PubMed] [Google Scholar]
- Golub MS, Germann SL. Perinatal bupivacaine and infant behavior in rhesus monkeys. Neurotoxicol Teratol. 1998;20:29–41. doi: 10.1016/s0892-0362(97)00068-8. [DOI] [PubMed] [Google Scholar]
- Golub MS, Gershwin ME, Hurley LS, Hendrickx AG, Saito WY. Studies of marginal zinc deprivation in rhesus monkeys: infant behavior. Am J Clin Nutr. 1985;42:1229–1239. doi: 10.1093/ajcn/42.6.1229. [DOI] [PubMed] [Google Scholar]
- Golub MS, Hogrefe CE, Germann SL, Capitanio JP, Lozoff B. Behavioral consequences of developmental iron deficiency in infant rhesus monkeys. Neurotoxicol Teratol. 2006;28:3–17. doi: 10.1016/j.ntt.2005.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinde R, Atkinson S. Assessing the roles of social partners in maintaining mutual proximity as exemplified by mother-infant relations in rhesus monkeys. Anim Behav. 1970;18:169–176. [Google Scholar]
- Jacobson JL, Jacobson SW, Sokol RJ, Martier SS, Ager JW, Kaplan-Estrin MG. Teratogenic effects of alcohol on infant development. Alcohol Clin Exp Res. 1993a;17:174–183. doi: 10.1111/j.1530-0277.1993.tb00744.x. [DOI] [PubMed] [Google Scholar]
- Jacobson SW. Specificity of neurobehavioral outcomes associated with prenatal alcohol exposure. Alcohol Clin Exp Res. 1998;22:313–320. doi: 10.1111/j.1530-0277.1998.tb03654.x. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Chiodo LM, Sokol RJ, Jacobson JL. Validity of maternal report of prenatal alcohol, cocaine, and smoking in relation to neurobehavioral outcome. Pediatrics. 2002;109:815–825. doi: 10.1542/peds.109.5.815. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Jacobson JL, O'Neill JM, Padgett RJ, Frankowski JJ, Bihun JT. Visual expectation and dimensions of infant information processing. Child Dev. 1992;63:711–724. [PubMed] [Google Scholar]
- Jacobson SW, Jacobson JL, Sokol RJ, Martier SS, Ager JW. Prenatal alcohol exposure and infant information processing ability. Child Dev. 1993b;64:1706–1721. [PubMed] [Google Scholar]
- Kelly SJ, Day N, Streissguth AP. Effects of prenatal alcohol exposure on social behavior in humans and other species. Neurotoxicol Teratol. 2000;22:143–149. doi: 10.1016/s0892-0362(99)00073-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipinski RJ, Hammond P, O'Leary-Moore SK, Ament JJ, Pecevich SJ, Jiang Y, Budin F, Parnell SE, Suttie M, Godin EA, Everson JL, Dehart DB, Oguz I, Holloway HT, Styner MA, Johnson GA, Sulik KK. Ethanol-induced face-brain dysmorphology patterns are correlative and exposure-stage dependent. PLoS One. 2012;7:e43067. doi: 10.1371/journal.pone.0043067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestripieri D, Tomaszycki M, Carroll KA. Consistency and change in the behavior of rhesus macaque abusive mothers with successive infants. Dev Psychobiol. 1999;34:29–35. [PubMed] [Google Scholar]
- Milne B, Bell J, Lampropoulos B, Towns S. Alcohol, drugs and Australian young people. Int J Adolesc Med Health. 2007;19:245–253. doi: 10.1515/ijamh.2007.19.3.245. [DOI] [PubMed] [Google Scholar]
- O'Connor MJ, Kogan N, Findlay R. Prenatal alcohol exposure and attachment behavior in children. Alcohol Clin Exp Res. 2002;26:1592–1602. doi: 10.1097/01.ALC.0000034665.79909.F0. [DOI] [PubMed] [Google Scholar]
- Purcell SH, Moley KH. The impact of obesity on egg quality. J Assist Reprod Genet. 2011;28:517–524. doi: 10.1007/s10815-011-9592-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose SA, Feldman JF, Jankowski JJ. Processing speed in the 1st year of life: a longitudinal study of preterm and full-term infants. Dev Psychol. 2002;38:895–902. doi: 10.1037//0012-1649.38.6.895. [DOI] [PubMed] [Google Scholar]
- Rose SA, Feldman JF, Jankowski JJ. Infant visual recognition memory: independent contributions of speed and attention. Dev Psychol. 2003;39:563–571. doi: 10.1037/0012-1649.39.3.563. [DOI] [PubMed] [Google Scholar]
- Rose SA, Feldman JF, Wallace IF. Individual differences in infants' information processing: reliability, stability, and prediction. Child Dev. 1988;59:1177–1197. [PubMed] [Google Scholar]
- Sabour D, Scholer HR. Reprogramming and the mammalian germline: the Weismann barrier revisited. Curr Opin Cell Biol. 2012;24:716–723. doi: 10.1016/j.ceb.2012.08.006. [DOI] [PubMed] [Google Scholar]
- Schneider ML, Moore CF, Adkins MM. The effects of prenatal alcohol exposure on behavior: rodent and primate studies. Neuropsychol Rev. 2011;21:186–203. doi: 10.1007/s11065-011-9168-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider ML, Roughton E, Luback G. Moderate alcohol consumption and psychological stress during pregnancy induce attention and neuromotor impairments in primate infants. Child Dev. 1997;68:747–759. doi: 10.1111/j.1467-8624.1997.tb01959.x. [DOI] [PubMed] [Google Scholar]
- Streissguth AP, Barr HM, Martin DC. Maternal alcohol use and neonatal habituation assessed with the Brazelton scale. Child Dev. 1983;54:1109–1118. [PubMed] [Google Scholar]
- Tarantal A. Untrasound imaging in rhesus (Macaca mulatta) and long-tailed (Macaca fasccularis) macaques: Reproductive and research applications. In: Wolfe-Coote S, editor. The Laboratory Primate, The Laboratory Primate. Elsevier Academic Press; 2005. pp. 317–351. [Google Scholar]
- Tough S, Tofflemire K, Clarke M, Newburn-Cook C. Do women change their drinking behaviors while trying to conceive? An opportunity for preconception counseling. Clin Med Res. 2006;4:97–105. doi: 10.3121/cmr.4.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]