Abstract
BACKGROUND
Modification of cryoprotective medium (CPM) R18S3 (18% raffinose and 3% skim milk) by addition of monothioglycerol (MTG) or L-glutamine (Glu) has been shown to improve in vitro fertilization (IVF) using mouse sperm cryopreserved in cryostraws. However, whether these CPMs can be applied effectively to sperm cryopreserved in cryovials is unknown.
OBJECTIVE
The study was to determine the comparative effectiveness of using R18S3, R18S3+Glu (100mM and 87 mM), or R18S3+MTG (477 µM) to cryopreserve various sample volumes of mouse sperm in cryovials and cryostraws.
METHODS
This study compared the effects of different CPMs on motility of fresh and frozen-thawed C57BL/6J sperm and on IVF rate of C57BL/6J sperm cryopreserved in different CPMs and containers with different volumes, and then used technologies developed to cryopreserve and recover sperm of knockout mouse lines on inbred C57BL/6 backgrounds.
RESULTS
Glutamine at 100 mM inhibited, but MTG at 477 µM protected, fresh sperm motility significantly (P<0.05). Sperm cryopreserved in R18S3+MTG had significantly better (P<0.05) post-thaw progressive motility and IVF rate than when cryopreserved in R18S3 alone, R18S3+Glu (100 mM), or RSGlu87 (15.7% raffinose, 2.6% skim milk, and 87 mM L-glutamine). There was no significant difference in IVF rates among sperm cryopreserved with R18S3+MTG in cryovials or in cryostraws (P>0.05). Sperm from 63 knockout mouse lines on C57BL/6 backgrounds cryopreserved using R18S3+MTG in cryovials were all recovered successfully to genotypically-confirmed offspring.
CONCLUSION
Mouse sperm on C56BL/6 backgrounds can be successfully cryopreserved in cryovials using R18S3+MTG.
Keywords: Mouse, sperm, cryopreservation, cryovial, cryostraw, IVF
INTRODUCTION
Cryopreservation of mouse sperm was first achieved successfully in 1990 using raffinose and skim milk (14, 24, 32). Since then, researchers have reported success using various cryoprotectants and procedures. Eventually, cryoprotective medium (CPM) composed of 18% raffinose and 3% skim milk (R18S3) became widely accepted (12, 13, 15, 25). This CPM was successfully used to cryopreserve sperm in cryostraws (10 µl in each straw) using a rapid freezing method (cooling in LN vapor for 10 min followed by plunging into LN) (12). Unfortunately, this technique is less effective for many inbred strains, including C57BL/6 on which most genetically modified (GM) mouse lines are developed. Using this CPM, in vitro fertilization (IVF) rates of thawed sperm from GM mouse lines on a C57BL/6 background is only a few percent (15, 28). Therefore, protocols using more reliable and efficient CPMs are urgently needed.
Attempts have been made to improve the IVF rate using cryopreserved C57BL/6 sperm. The CPM containing R18S3 plus a reducing agent monothioglycerol (R18S3+MTG) has attracted the most attention (15). The use of R18S3+MTG can substantially increase post-thaw IVF rates using frozen-thawed mouse sperm in straws on many genetic backgrounds including C57BL/6 (15). Two years later, it was reported that the addition of 100 mM L-glutamine enhanced cryoprotection of mouse sperm preserved in straws compared to R18S3 alone (26). Unfortunately, the latter report did not determine whether R18S3 with either MTG or Glu could be used more effectively for cryopreservation of mouse sperm. Nor was there any discussion of whether this CPM could be used as effectively in cryovials as in plastic straws. Although plastic straws are widely used, there are several advantages using cryovials over cryostraws. For example, cryovials are stronger and more resistant to damage than some cryostraws, depending on manufacturer. For this reason, cryovials are an important and useful alternative for storage, shipping, and transport of sperm samples. In addition, it is easier to use cryovials than cryostraws for handling and loading sperm, facilitating the cryopreservation processing.
Several studies have tried to cryopreserve mouse sperm in cryovials. Using traditional CPM, preservation of sperm from Balb/C, 129S3/SvIm and C57BL/6 strains in cryovials was not successful (22, 28). On the other hand, cryopreserving sperm from hybrid strains B6D2F1 (23), (C3H/HeH × BALB/c)F1 (28) and B6129SF1 (22) in cryovials containing 100 or 200 µl sperm in R18S3 has been successful. A recent paper (7) comparing post-thaw motility of ICR and C57BL/6Jcl mouse sperm cryopreserved in cryovials containing 1, 10 and 50 µl sperm in R18S3 found the highest post-thaw sperm motility when cryovials with conical bottom containing 10 µl of sperm each were used. Unfortunately, IVF rates were not compared in that study.
Therefore, this study was to determine the comparative effectiveness of using R18S3, R18S3+Glu, or R18S3+MTG to cryopreserve various sample volumes of mouse sperm in cryovials and cryostraws. To make this determination, we measured and compared sperm motility and fertilization rates using sperm harvested from wild-type C57BL/6 strain and from 63 different GM mouse lines on C57BL/6 genetic backgrounds.
MATERIALS AND METHODS
Animals
Male mice for 63 unique GM mouse lines on inbred C57BL/6J and C57BL/6N genetic backgrounds were obtained from the KOMP Repository and the Mutant Mouse Regional Resource Center (MMRRC) at the University of California (Davis, CA). Wild-type C57BL/6J mice were purchased from the Jackson Laboratory (Bar Harbor, ME) and wild-type C57BL/6NTac mice from Taconic (Hudson, NY). Mice were housed in a specific pathogen free vivarium with a 14h on/10h off light cycle (7am on and 9pm off). Euthanasia was performed by CO2 asphyxiation according to the 2013 AVMA guidelines on euthanasia. The care, use, and disposition of all mice used in this study were reviewed and approved by the Institutional Animal Care and Use Committee of the University of California Davis.
Reagents and media
BD Difco™ skim milk was purchased from Voigt Global Distribution (Lawrence, KS), and equine chorionic gonadotropin (eCG) was from the National Hormone and Peptide Program (Harbor-UCLA Medical Center, Torrance, CA). Human chorionic gonadotropin (hCG), D-(+)-raffinose pentahydrate, α-monothioglycerol (MTG), L-glutamine (Glu), methyl-β-cyclodextrin (MBCD), reduced L-glutathione (GSH), bovine serum albumin (BSA, embryo tested), and embryo tested water were purchased from Sigma-Aldrich Corp. (St. Louis, MO). M2 medium was purchased from Zenith Biotech, and Research Vitro Fert (RVF) medium from Cook Medical, Inc. (Bloomington, IN, U.S.A.).
CPM preparation
Four different CPMs were tested: R18S3, R18S3+MTG (477µM), R18S3+Glu (100 mM) and RSGlu87 (raffinose 15.7%, skim milk 2.6%, and L-glutamine 87 mM). R18S3 was prepared by dissolving 18 g raffinose and 3 g skim milk in embryo tested water at 50–60°C with final volume 100 ml. R18S3+Glu (100 mM glutamine) was prepared by dissolving 1.4614 g glutamine, 18 g raffinose, and 3 g skim milk in embryo tested water (50–60°C) with final volume 100 ml. Both CPMs were centrifuged at 18,500 × g and 20°C for 3 hours until clear, and then filter-sterilized through a 0.2 µm filter, aliquoted and stored at 8°C prior to use.
RSGlu87 was prepared according to the European Mouse Mutant Archive (EMMA) online protocol (strains.emmanet.org/protocols/cryopreservation-of-mouse-sperm_MBCD-and-GSH-protocol_Feb-2013.pdf) based on previous publications (26, 27) by dissolving 0.584 g glutamine, 7.2 g raffinose and 1.2 g skim milk in 40 ml embryo tested water (50–60°C), and the final volume was 46 ml, and therefore the concentrations of the 3 components in RSGlu87 were 15.7% raffinose, 2.6% skim milk, and 87 mM glutamine. CPM was centrifuged at 18,500 × g and 20°C for 3 h, and sterile-filtrated by a 0.2 µm filter and stored at 8°C prior to use.
R18S3+MTG was prepared freshly on the day of use by adding an appropriate volume of filter-sterilized 100 mM MTG in M2 medium to an aliquot of R18S3 to achieve a final MTG concentration of 477 µM (15). The osmolality as measured by a freezing point osmometer (Model 3300, Advanced Instruments, Norwood, MA) was 480–500, 480–500, 510–530 and 617–640 mOsm/kg for R18S3, R18S3+MTG, RSGlu87 and R18S3+Glu, respectively.
Sperm cryopreservation
Cauda epididymal sperm were collected and pooled from 4 to 6 male wild-type C57BL/6J mice (3–4 months old) for each experiment unless stated otherwise to reduce male to male variation in sperm quality. Sperm were collected in R18S3 (0.5 ml per male or two epididymides) and incubated for 10 min at 37°C for dispersion. Pooled sperm were mixed and distributed equally into 1.5 ml microcentrifuge tubes. After samples were centrifuged at 400×g for 3 min, the supernatants were removed and the sperm pellets were resupended in different CPMs with the same volume as that before centrifugation.
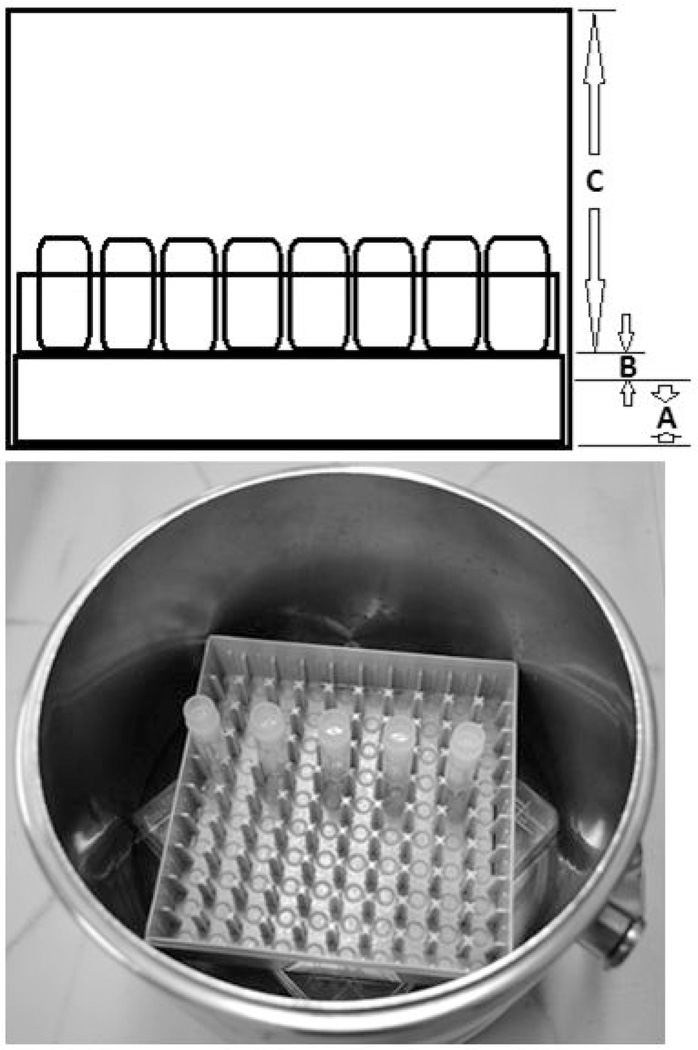
Using wide-bore tips, sperm suspended in 4 CPMs were pipetted to the bottom of 1.8ml cryovial (50, 100 or 150 µl) (round bottom with internal threads, NUNC #377267, Nalge NUNC International, Rochester, NY). Cryovials were capped and placed into LN) vapor in a 3L stainless steel Dewar (Cole Parmer, Vernon Hills, IL) for cooling 10 min (Figure 1). The sperm cooling apparatus was made by placing the lid of a Nalgene polycarbonate cryobox (133mm length ×133mm width × 52mm height) to the Dewar bottom with its opening facing down, and then the bottom part of cryobox was placed on the top of the lid for holding cryovials. LN was poured into the Dewar so that the height of LN level was 2.0~2.5 cm at the bottom of the Dewar (A), the distance between the bottom of the cryovials and the LN surface was 0.5~1.0 cm (B), and the distance between cryovial bottom and Dewar opening was 11 cm (C). After 10 min in LN vapor with the Dewar lid covered, more LN was poured into the Dewar so that cryovial bottoms were submerged in LN liquid phase. Frozen samples in cryovials were transferred and stored in LN storage tanks.
Figure 1.
LN Dewar setup for freezing. A, the depth of LN at the Dewar bottom, 2.0~2.5cm; B, the distance between cryovial bottom and LN surface, 0.5~1.0cm; C, the distance between cryovial bottom and the opening of the Dewar, 11 cm. The Dewar was covered with a lid during sperm cooling.
For cryopreservation in cryostraws (0.25ml, IMV Technology, Maple Grove, MN), the straw was loaded with 7 cm of RVF medium as a weight followed by 1.5 cm air, and then 10, 25 or 50 µl sperm suspension in CPM using a straw aspirator. Both ends of the straw were heat sealed using an impulse sealer. Straws were placed into a freezing canister (13) with the ends containing the sperm facing down. The freezing canister was floated on the LN surface in the neck of a 34 liter Dewar (33 cm depth of LN, Model 34HC, Taylor-Wharton, Theodore, AL) for 10 min before plunging into LN.
The cooling rate of a cryovial (1.8ml Nunc cryovial, NUNC #377267) containing 100 µl of sperm in R18S3+MTG placed inside LN vapor was determined by placing the wire lead of a HH506RA thermometer thermocouple (Omega, Laval, Canada) into the sperm sample through a small hole on the cryovial cap. The cryovial was placed into the cooling apparatus as described above, the temperature recorded every 3 sec and analyzed by linear regression using GraphPad Prism 5 software (GraphPad Software, Inc., San Diego, CA, USA). Cooling rates were defined by the calculated slopes during different period of time of the 10 min inside LN vapor.
Sperm motility assessment
Cauda epididymal sperm were collected and pooled from 4 to 6 male wild-type C57BL/6J mice for each experiment as described above. Sperm were collected in 0.5 ml R18S3 per male, and the pooled sperm were mixed and distributed equally into microcentrifuge tubes, and then centrifuged at 400×g for 3 min before the supernatants were removed and the sperm resupended in different CPMs with the same volume as that before centrifugation.
After resuspension in different CPMs, the sperm samples were incubated at ~23°C, and their motility was assessed at different time points post centrifugation and resuspension using an IVOS computerized sperm analyzer (Hamilton Thorne, Beverly, MA, USA) after the sperm suspension was diluted in M2 medium at room temperature. Total (% of motile sperm) and progressive (% of progressively swimming sperm with average path velocity ≥ 50 µm/s) motility were obtained from reading 8 –10 fields (2000–3000 sperm) at 37°C.
In vitro fertilization
For IVF using sperm cryopreserved in cryovials, sperm were thawed in 37°C water bath for 10 min. The thawed cryovial was swirled gently for 2 times to mix the sperm prior to motility assessment and IVF. Two different IVF methods, GSH IVF and MBCD/GSH IVF, were used in this study. Addition of GSH to the fertilization medium increases fertilization rate and pre-incubation of sperm with MBCD enhances sperm capacitation (25–27).
The GSH IVF method was used to compare IVF rates using sperm cryopreserved in different CPMs. Thawed sperm (25 µl) from cryovials was added to an IVF drop of 250 µl RVF medium containing 5.1 mM CaCl2 and 1 mM GSH, and then incubated for 30 min at 37°C with 5.5% CO2 and humidified air (rh >90%) before the cumulus-oocyte complexes (COCs) from 5–8 superovulated females (80– 150 oocytes) was added to the IVF drop.
To compare GSH IVF rates of sperm cryopreserved in cryovials and cryostraws with different CPM volumes, 10 µl thawed sperm from different samples were added to each IVF drop. Cryostraws were thawed by immersing in 37°C water bath for 10 min. The straw was removed from water, wiped dry, and disinfected with 70% ethanol. The sperm suspension was expelled into an IVF drop directly (if 10 µl) or into an empty dish (if 25 µl or more) and then 10 µl of sperm was transferred into an IVF drop using a wide-bore pipette tip.
For MBCD/GSH IVF, 25 µl thawed sperm was added to a 90 µl of MBCD medium drop (BSA-free TYH medium containing 1 mg/ml PVA and 0.75 mM MBCD) (27, 29) and incubated for 30 min at 37°C in 5.5% CO2 and humidified air prior to insemination. COCs from 5–8 superovulated females (80–150 oocytes) were added into an IVF drop of 250 µl RVF medium containing 5.14 mM CaCl2 and 1 mM GSH. Immediately thereafter, 25 µl motile sperm were slowly collected from the peripheral part of a MBCD medium drop using a P20 pipette and a regular tip, and then expelled gently and directly onto each of the COCs under a dissecting microscope with minimum medium. As a control, MBCD/ GSH IVF using fresh (non-cryopreserved) C57BL/6J sperm was performed by adding 25 µl sperm collected in R18S3+MTG as described above to a 90-µl MBCD medium drop and incubated for 30 min before sperm were used for IVF.
Oocytes were collected from superovulated females 14–16 hours post hCG injection. Superovulation was induced by IP injection of 7.5 IU eCG followed by IP injection of 7.5 IU hCG 47 hours later. COCs from a group of 5–8 females were collected in 2–3 ml pre-equilibrated RVF medium with 2.04 mM CaCl2 by rupturing the ampulla with fine sterile forceps and a syringe needle, and then transferred into IVF drops using a wide-bore pipette tip under a dissecting microscope.
Sperm and oocytes were co-cultured at 37°C in 5.5% CO2 and humidified air for 5–6 hours without disturbance, then the oocytes were picked up on a warm stage (37°C) and distributed into 3×150µl drops of RVF medium with 2.01 mM CaCl2, and then incubated overnight. Fertilization rate was calculated as the 2-cell embryo percent of the oocytes used.
Surgical embryo transfer
For in vivo study 2-cell stage embryos for all 63 mutant mouse lines were transferred into the oviducts (10–13 for each oviduct, 20–25 per recipient) of 0.5 days post coitum pseudo-pregnant CD-1 recipient female mice anesthetized with 1.25% Avertin (Sigma). After 45–60 min anesthesia, 0.1 ml Buprenex (0.03 mg/ml; Western Medical Supply, Arcadia, CA, USA) was administered subcutaneously in the flank of each mouse postoperatively. Recipients were kept warm on a heating pad until fully recovered. All pregnant recipients were allowed to go to term and give birth to litters for health and genotyping analysis. The health, number, and genotype of pups born per litter were determined at 21 days after birth.
Statistical analysis
GraphPad Prism 5 software (GraphPad Software, Inc., San Diego, CA, USA) was used for statistical analysis. Sperm motility and fertilization rate data were arcsine transformed (15), and treatment differences were detected by two-way ANOVA and Bonferroni post-tests (for data in Figures 2 and 3) or by one-way ANOVA followed by Tukey HSD tests (data in Figures 4–7). P<0.05 was chosen as an indication of statistical significance. Data are expressed as mean ± standard error of the mean (S.E.M).
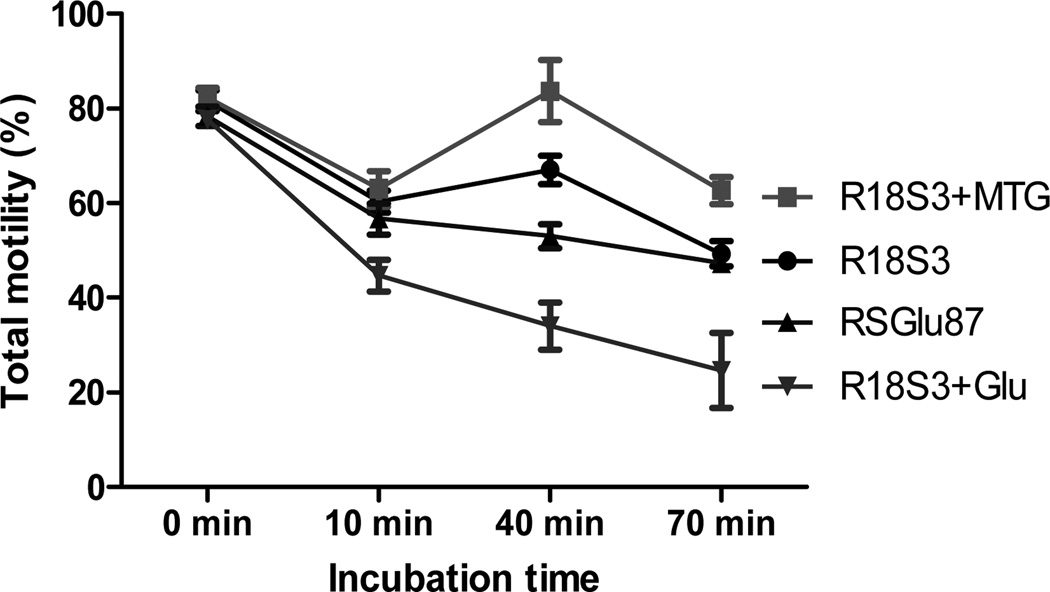
Figure 2.
Changes in total motility of C57BL/6J sperm during incubation in 4 CPMs at ~23°C. Experiments were repeated 3 times using sperm pooled from 4 to 6 males each experiment.
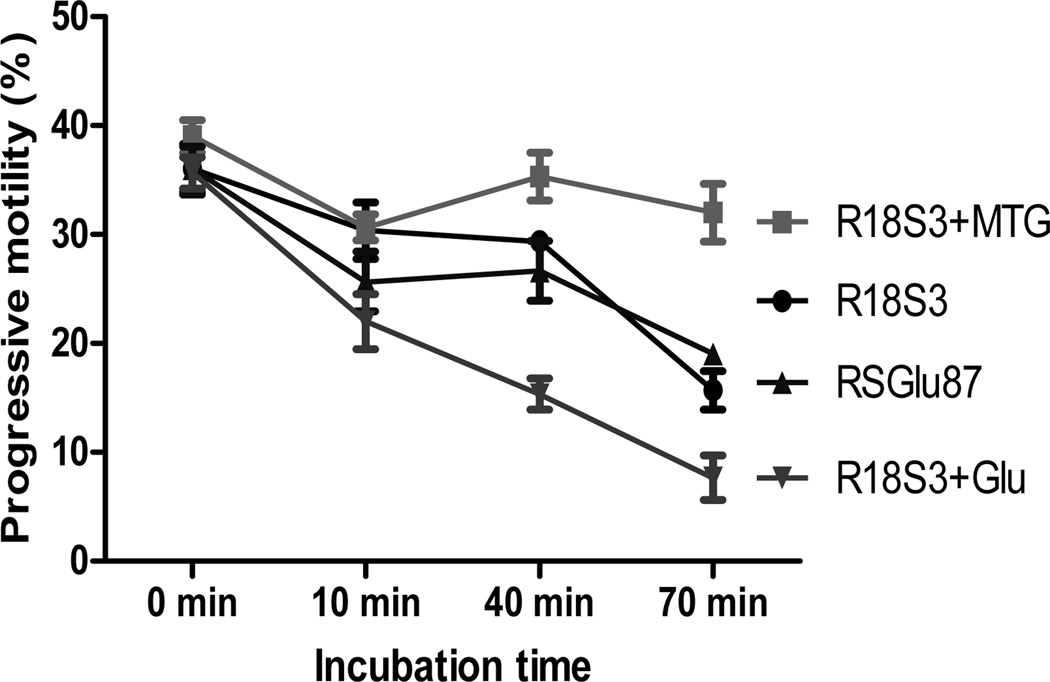
Figure 3.
Changes in progressive motility of C57BL/6J sperm during incubation in 4 CPMs at ~23°C. Experiments were repeated 3 times using sperm pooled from 4 to 6 males each experiment.
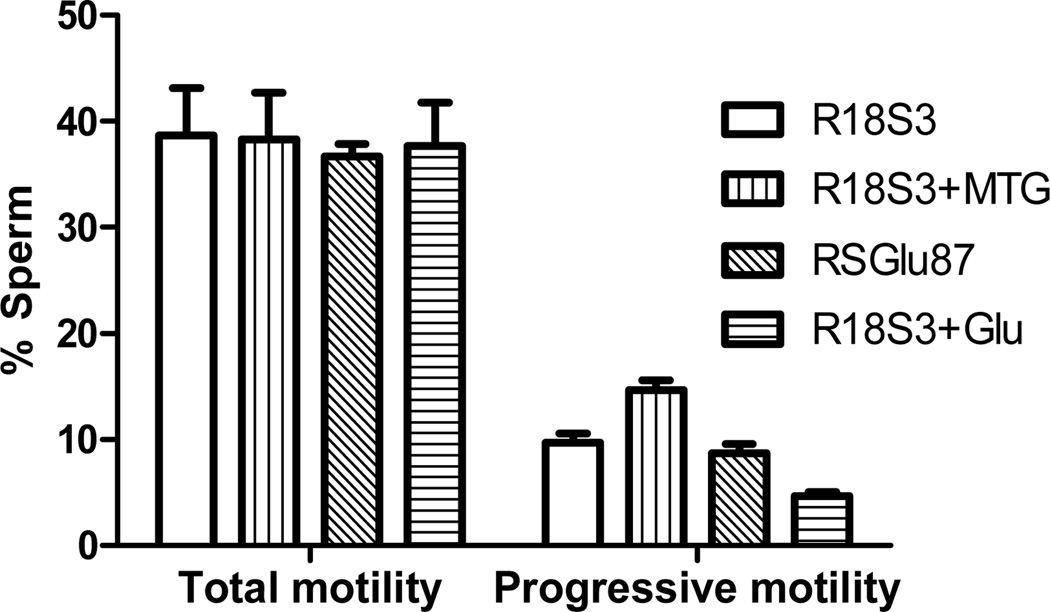
Figure 4.
Effects of CPMs on post-thaw total and progressive motility of C57BL/6J sperm preserved in cryovials. Experiments were repeated 3 times using sperm pooled from 4~6 males.
Figure 7.
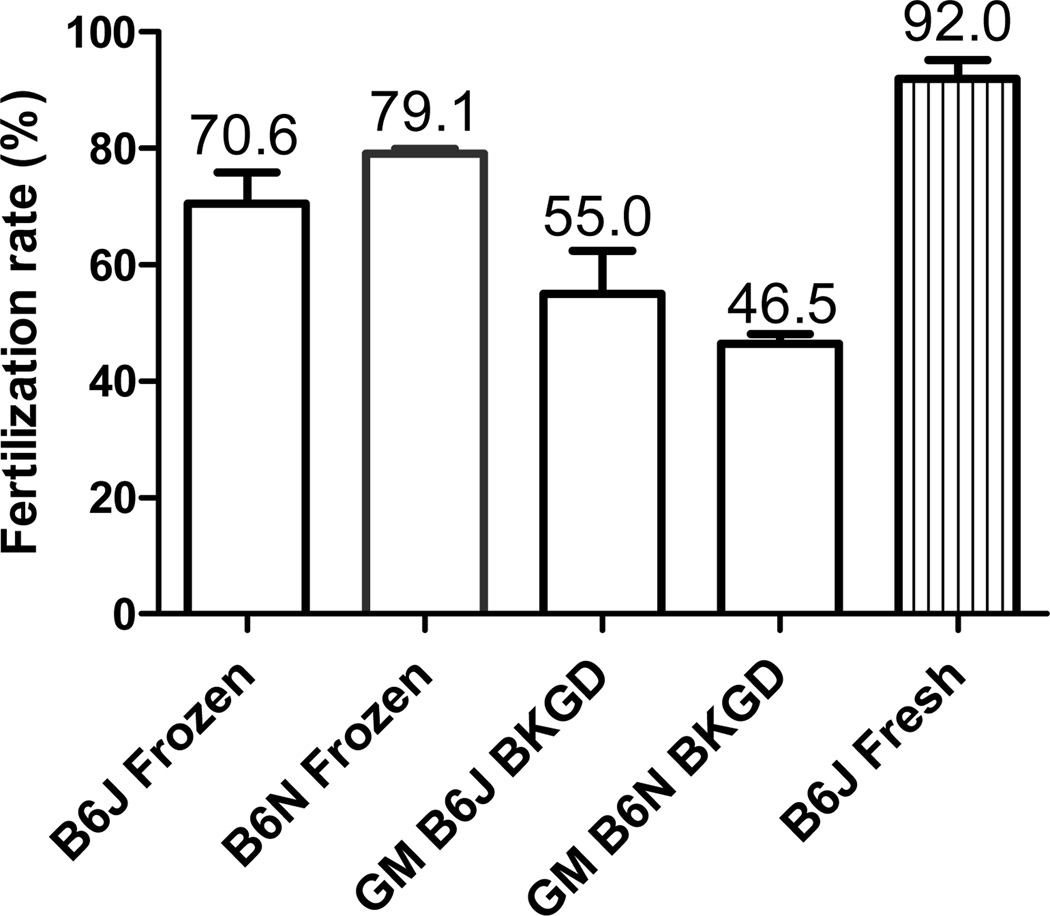
IVF rates of frozen-thawed sperm of C57BL/6J (n=3), C57BL/6N (n=3), GM lines on C57BL6J background (n=9), and GM lines on C57BL/6N background (n=54), compared with that of fresh C57BL/6J sperm (n=3). B6J = C57BL/6J; B6N = C57BL/6N; BKGD = background; n =number of sperm cryopreservation (SC) procedures = number of IVF procedures. Each SC or IVF procedure used sperm from 1 male in GM mouse lines or pooled from 4 to 6 males in B6J and B6N wild-type strains. Sperm were collected in R18S3+MTG (0.5 ml per male) and cryopreserved in cryovials with 50 µl sperm.
RESULTS
Effects of different CPM on fresh sperm motility
To compare motility of fresh C57BL/6J sperm at room temperature (23°C) in 4 different CPMs (R18S3, R18S3+MTG, R18S3+Glu and RSGlu87), total and progressive motility were analyzed after 0, 10, 40 and 70-min incubation. As shown in Figures 2 and 3, the 4 sperm treatments in different CPMs were not significantly different in both the total motility and progressive motility at 0 min of incubation (P>0.05). During the first 10 min of incubation, sperm motility decreased, especially the sperm in R18S3+Glu decreased significantly more in both total and progressive motility than that of sperm in other three CPMs (P<0.05). At 40 min of incubation, sperm total and progressive motility in R18S3+MTG became higher than that at 10 min of incubation, and they were the highest when sperm were incubated at 40 and 70 min among the 4 CPMs tested. Such differences in both sperm total and progressive motility between R18S3+MTG and the other 3 CPMs increased proportionately with incubation time which became highly significant at 70 min of incubation (P<0.001). Sperm motility in R18S3+Glu was the lowest in all of the time points of incubation tested except the 0 min, and both the total and progressive motility of sperm in R18S3+Glu were significantly lower than that of sperm in the other 3 CPMs (at 10 min, P<0.05; 40 min, P<0.001; and 70 min, P<0.05).
There was no significant differences in both total and progressive motility found between sperm incubated in RSGlu87 or in R18S3 alone at any of the 4 time points tested (P>0.05). The results indicate that MTG has a protective effect on sperm motility, whereas 87 mM glutamine had no effect. In contrast, 100 mM glutamine significantly inhibit sperm motility.
Effects of different CPM on post-thaw motility
C57BL6J sperm were cryopreserved in cryovials with 50 µl each in different CPM. Sperm motility was analyzed immediately after thawing at 37°C for 10 min. As shown in Figure 4, there was no significant difference in post-thaw total motility found among the sperm cryopreserved in the 4 different CPMs (R18S3, R18S3+MTG, RSGlu87, and R18S3+Glu). In contrast, sperm cryopreserved in R18S3+MTG had significantly (P<0.05) higher post-thaw progressive motility (14.7±0.9%) than that in R18S3 (9.7±0.9%), RSGlu87 (8.7±0.9%) and R18S3+Glu (4.7±0.3%). Progressive motility was no different between RSGlu87 and R18S3 (P>0.05), but was significantly lower in R18S3+Glu compared to R18S3 (P<0.05). The decrease in progressive motility from sperm cryopreserved in R18S3+MTG (14.7±0.9%) to that cryopreserved in R18S3+Glu (4.7±0.3%), was highly significant (P<0.001). Data indicate that post-thaw progressive motility is protected and preserved by MTG, while it is inhibited by glutamine at 100 mM concentration.
IVF rates of sperm cryopreserved in 4 CPMs
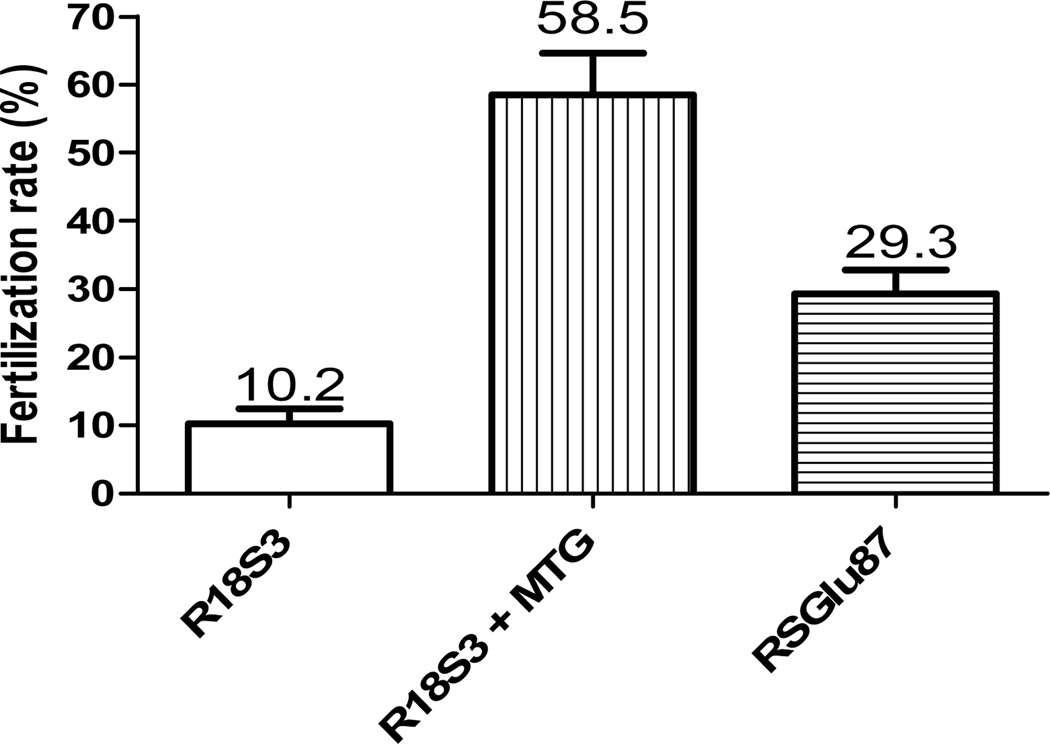
To determine the difference in fertilization rates using either R18S3+MTG or RSGlu87, the GSH IVF rates of frozen-thawed C57BL/6J sperm were compared. As shown in Figure 5, the IVF rate of sperm cryopreserved in R18S3+MTG (58.5±6.1%) was about 5 times higher (P<0.001) than that of R18S3 (control 10.1±2.2%), and about 2 times higher than that of sperm cryopreserved in RSGlu87 (29.3±3.5%, P<0.05). Compared with sperm cryopreserved in CPM R18S3, the IVF rate was significantly greater after addition of 87 mM glutamine (CPM RSGlu87, P<0.05), but less than that after addition of MTG (P<0.001).
Figure 5.
Comparisons of GSH IVF rates of C57BL/6J sperm cryopreserved in cryovials with 50 µl sperm each in R18S3, R18S3+MTG and RSGlu87. The mean of each IVF rate is shown on the top of each bar. Experiments were repeated 3 times using sperm pooled from 4~6 males.
IVF rates of sperm cryopreserved in different containers with different volumes
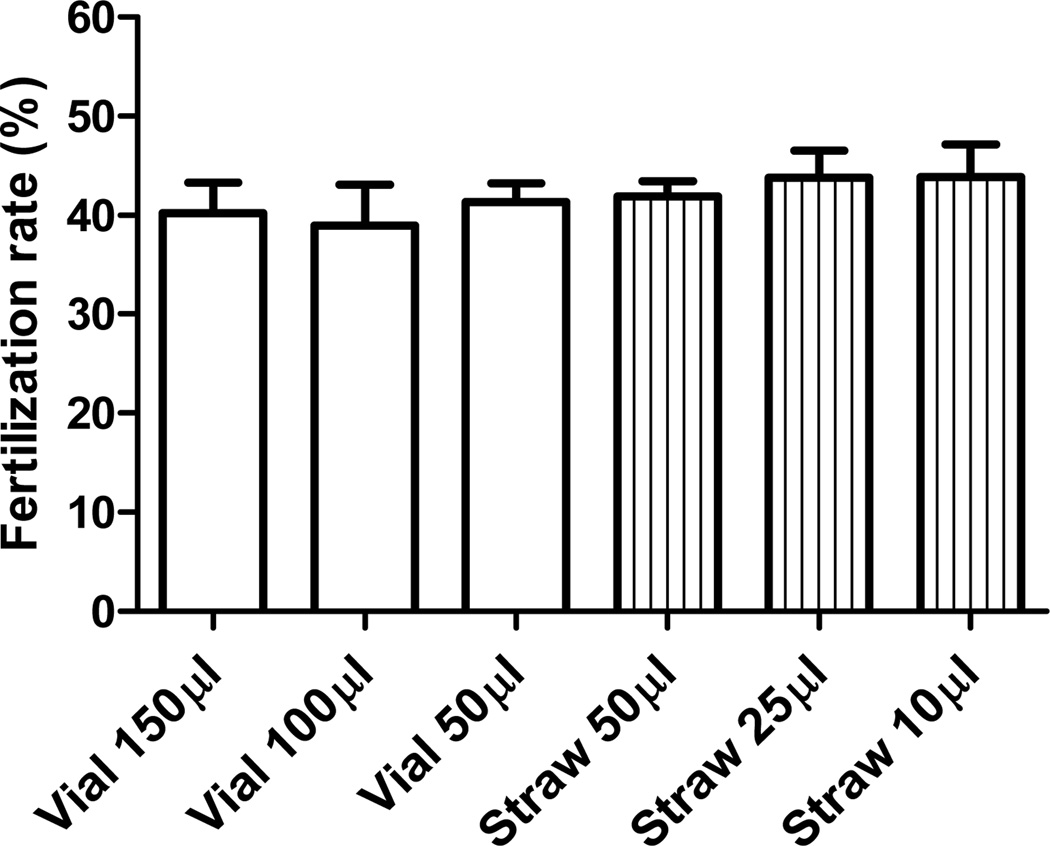
To determine if the type of containers and volume of sperm cryopreserved affect IVF rate of frozen-thawed sperm, sperm from cryovials with 150, 100 and 50 µl sperm in R18S3+MTG and from cryostraws with 10, 25 and 50 µl sperm in the same CPM were compared by GSH IVF. There was no significant difference in fertilization rates among sperm cryopreserved in different containers with different volumes of sperm (Figure 6, P>0.05). These results indicate that, within the range of volumes analyzed, mouse sperm can be cryopreserved equally well in cryovials and cryostraws.
Figure 6.
Comparisons of GSH IVF rates of C57BL/6J sperm cryopreserved in cryovials with 150, 100 and 50 µl sperm each and cryostraws with 50, 25 and 10 µl sperm each. R18S3+MTG was used as CPM, and experiments were repeated 3 times using sperm pooled from 4 to 6 males.
Recovery of GM mouse sperm cryopreserved in cryovials using R18S3+MTG
MBCD and GSH were used to enhance sperm capacitation and to increase IVF rate for cryopreserving mouse sperm in cryostraws containing 10 µl sperm each (27). In this study, we modified the MBCD/GSH IVF protocol for resuscitation of sperm cryopreserved in cryovials or in cryostraws with more than 10 µl sperm. Using this IVF protocol combined with cryovial cryopreservation with R18S3+MTG, a large number of GM mouse lines were cryopreserved and recovered to live pups.
The fertilization rates of frozen-thawed sperm from 9 GM mouse lines on a C57BL/6J background (54.97 ± 7.46%) and from 54 GM mouse lines on a C57BL/6N background (46.48 ± 1.65%) compared with that of frozen-thawed wild-type C57BL/6J (70.57 ± 5.29%) and C57BL/6N (79.10 ± 0.80%) mice as well as that of fresh C57BL/6J sperm (92.0 ± 3.24%), respectively, are summarized in Figure 7.
Although significantly (P<0.05) lower, IVF success rate using the MBCD/GSH method with frozen-thawed C57BL/6J sperm (70.6%) was ~77% of that using fresh sperm (92.0%). No significant (P<0.05) difference in IVF rates was found between frozen-thawed C57BL/6J and C57BL/6N sperm from wild-type mice, nor between frozen-thawed sperm from GM mouse lines on C56BL/6J and C57BL/6N genetic backgrounds. Compared with post-thaw IVF rate using sperm from wild-type mouse strains, the post-thaw IVF rate using sperm from GM mouse lines was lower. This difference was highly significant in GM mice on the C57BL/6N background (P<0.001), although it was not significant in GM mice on the C57BL/6J background lines.
Two-cell stage embryos derived by MBCD/GSH IVF using thawed sperm from heterozygous males on C57BL/6 background and oocytes from wild-type C57BL/6J or C57BL/6NTac females were transferred into oviducts of pseudopregnant CD-1 recipients for in vivo studies. The results summarized in Table 1 shows that GM embryos derived by IVF using sperm on both C57BL/6J and C57BL/6N backgrounds cryopreserved with R18S3+MTG in cryovials developed normally to pups (48.4 ± 3.0% and 41.8 ± 1.7% birth rates, respectively) with expected Mendelian inheritance of the mutant allele (~50%).
Table 1.
In vivo development of embryos of GM mouse lines derived by MBCD/GSH IVF using sperm cryopreserved with R18S3+MTG in cryovials
| Background | No. GM mouse lines used |
No. embryos transferred |
No. pups born | Pup birth rate (%) |
Mutant pup ratio (%) |
|---|---|---|---|---|---|
| C57BL/6J | 9 | 372 | 179 | 48.4±3.0 | 51.2±2.8 |
| C57BL/6N | 54 | 2,110 | 893 | 41.8±1.7 | 52.5±1.9 |
Pup birth rate = (pups born/embryos transferred) × 100;
Mutant pup ratio = (mutant pups/pups born) × 100.
Cooling rates of sperm sample inside a cryovial
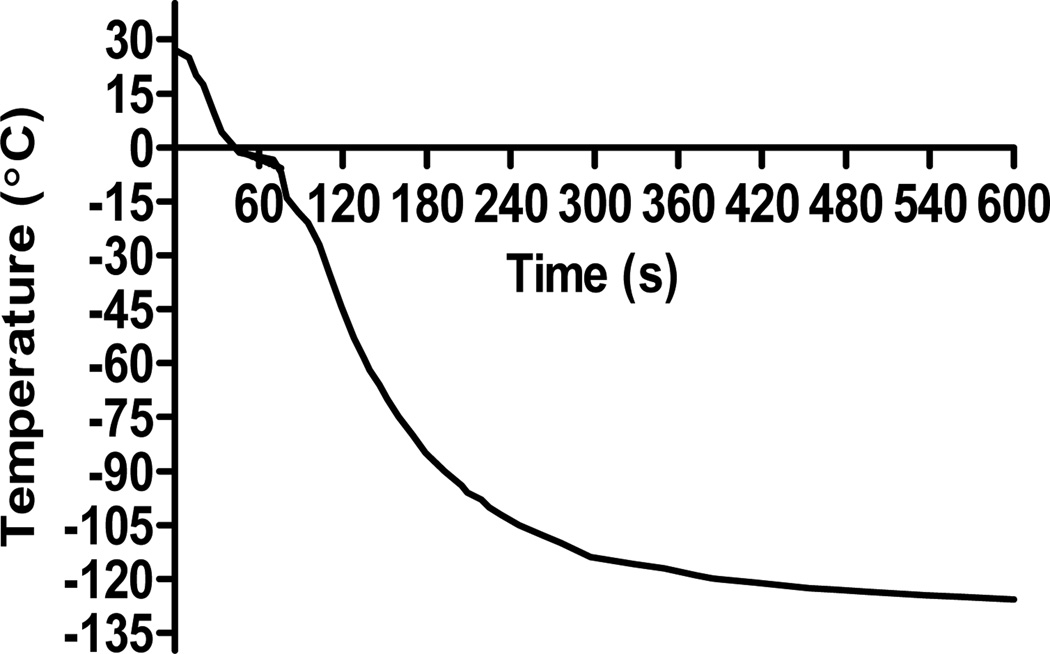
The cooling curve of a sperm sample (100 µl in R18S3+MTG) in a 1.8ml round bottom Nunc cryovial submerged in LN vapor for 10 min is shown in Figure 8. The CPM temperature decreased immediately after placing the cryovial into the LN vapor; the cooling rate was 41.2 ± 3.5 °C/min during the first 46 s in which the sample temperature was cooled to −1.3°C from starting temperature (27.1°C). The cooling rate slowed to 17.5 ± 6.9°C/min during the time from 46 to 80 s because of the phase change at temperature between −1.3 to −14°C. Then the cooling rate increased to 37.4 ± 1. °C/min during the time from 80 to 225 s in which the temperature dropped from −14 to −100 °C. The cooling rate during the final period of time from 225 to 600 s in LN vapor when the sample temperature decreased from −100°C to −126°C was 3.9 ± 0.4°C/min.
Figure 8.
The cooling curve of a sperm sample in a cryovial with 100 µl sperm in R18S3+MTG submerged in LN vapor for 10 min.
DISCUSSION
In contrast to previous reports (26, 27) of improved fertilization rates using R18S3 supplemented with 100 mM L-glutamine (designated as mR18S3) to preserve C57BL/6 mouse sperm in cryostraws, we found that both the total and progressive motility of fresh sperm incubated in R18S3+Glu (100 mM L-glutamine) were significantly inhibited compared with that of sperm incubated in glutamine-free R18S3 and R18S3+MTG (P<0.05). Progressive motility of thawed sperm cryopreserved in R18S3+Glu was also significantly lower than that of R18S3 (P<0.05) and R18S3+MTG (P<0.001). We have no explanation for the discrepancy between our results and those from earlier reports other than the possibility that the final concentration of glutamine in those earlier reports was less than 100 mM. In fact, upon reconstitution of this CPM using previously published information (12, 13, 26, 27), our measurements and calculations indicate that the final concentration in mR18S3 were 15.7% raffinose, 2.6% skim milk, and 87 mM glutamine (not 100 mM glutamine). Thus, we used 87 mM glutamine in our CPM, and designated it as RSGlu87.
We found no significant differences in either total or progressive motility between fresh or frozen sperm incubated in RSGlu87 and that incubated in R18S3 (P>0.05). Total and progressive motility of fresh sperm incubated in R18S3+MTG were the highest among the 4 CPMs tested. Furthermore, sperm cryopreserved with R18S3+MTG had significantly higher post-thaw progressive motility than that cryopreserved with R18S3, RSGlu87, or R18S3+Glu (P<0.05), indicating that MTG had a protective effect on sperm motility, which is probably the mechanism by which the IVF rate of sperm cryopreserved in R18S3+MTG was significantly higher than that of sperm cryopreserved in R18S3 (P<0.001) and in RSGlu87 (P<0.05). On the other hand, 87 mM glutamine had no effect, while 100 mM glutamine inhibited sperm motility.
R18S3 with 477 µM MTG is significantly more reliable and efficient for preserving mouse sperm than with either 87 or 100 mM glutamine. The study demonstrated that mouse sperm of C57BL/6 genetic background are cryopreserved equally well using R18S3+MTG in cryovials and cryostraws. There was no significant difference in IVF rates among tested volumes with R18S3+MTG in either cryovials or cryostraws. Additionally, this study indicates that sperm of GM mouse lines on C57BL/6J or C57BL/6N backgrounds can be successfully cryopreserved using R18S3+MTG in cryovials. Further, GM embryos derived by IVF using cryopreserved sperm develop normally to pups and express the expected Mendelian inheritance of the mutant allele.
Our data show that the cooling rate of 100 µl CPM+MTG in cryovials placed in LN vapor is 37.4 ± 1.8 °C/min between −14 and −100 °C, which is similar to the cooling rate (37 ± 1 °C/min) of a 10 µl sperm cryostraw on a raft floating on LN (15). While it is generally accepted that sperm survive better in plastic straws than in cryovials or cryotubes (7), our results refute this assumption. A recent study (7) reported that round bottom cryovials have a faster cooling rate than conical bottom cryovials, and the post-thaw motility of ICR and C57BL/6Jcl mouse sperm were the highest when 10 µl sperm was cryopreserved in a conical bottom cryovial compared with that of 1 and 50 µl of sperm cryopreserved in the same type of container. However, that study did not use round bottom cryovials to compare post-thaw sperm motility or IVF rates. In the present study, we found that various volumes of mouse sperm can be cryopreserved equally well in round bottom cryovials and cryostraws.
Stacy et al. (21) measured the cooling rates of a cryostraw with 10 µl Dulbecco’s modified Eagle’s medium (but unfortunately not the CPM R18S3) located at different locations above LN and concluded that the samples reached a steady temperature in about 3 min, and therefore the additional 7 min of exposure to LN vapor is probably unnecessary. Our data show that 10 min is necessary and sufficient to reach the final steady temperature of a 100 µl R18S3 sample.
As a reducing agent (19), MTG is thought to improve the fertilizing ability of cryopreserved sperm. Sperm are particularly susceptible to oxidative stress due to their high unsaturated fatty acid content, limited availability of intracellular antioxidant enzymes, and lack of a DNA repair system (1, 2). Excessive reactive oxygen species (ROS) affects the fluidity of the sperm plasma membrane, impairs sperm motility, and destroys the integrity of sperm DNA. In addition, oxidative stress inflicts damage to mitochondria, which in turn produces more ROS (6, 17). Oxidative stresses, such as chilling and freezing, increase the generation of ROS in sperm (5). Increased ROS levels have been correlated with decreased sperm motility in humans (4, 9). Antioxidant supplementation to inhibit lipid peroxidation and maintain sperm membrane integrity has been documented in mammalian species (10, 11, 16, 20).
Mechanism of cryoprotective effect of L-glutamine on mouse sperm is not known. In stallion and human sperm 30~80 mM glutamine was found to improve sperm motility, but glutamine at a higher concentration (>100 mM) showed osmotic toxicity (8, 18, 30, 31), which is consistent with our finding in this study that R18S3 plus 100 mM glutamine (osmolality 617–640 mOsm) inhibited significantly sperm motility. Motility of bull sperm decreases with >80 mM glutamine (3), suggesting that high glutamine concentration might be toxic.
Acknowledgements
The study was funded by NIH awards (R24# RR018934 and U42# OD012210) supporting the Mutant Mouse Regional Resource Center (MMRRC) and by the UC Davis Mouse Biology Program.
REFERENCES
- 1.Aitken RJ, Baker MA. Molecular and Cellular Endocrinology. 2006;250:66–69. doi: 10.1016/j.mce.2005.12.026. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez JG, Storey BT. Biol Reprod. 1984;30:833–841. doi: 10.1095/biolreprod30.4.833. [DOI] [PubMed] [Google Scholar]
- 3.Amirat-Briand L, Bencharif D, Vera-Munoz O, et al. Theriogenology. 2009;71:1209–1214. doi: 10.1016/j.theriogenology.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 4.Armstrong JS, Rajasekaran M, Chamulitrat W, et al. Free Radic Biol Med. 1999;26:869–880. doi: 10.1016/s0891-5849(98)00275-5. [DOI] [PubMed] [Google Scholar]
- 5.Chatterjee S, Gagnon C. Mol Reprod Dev. 2001;59:451–458. doi: 10.1002/mrd.1052. [DOI] [PubMed] [Google Scholar]
- 6.De Iuliis GN, Wingale JK, Koppers AJ. Journal of Clinical Endocrinology and Metabolism. 2006;91:1968–1975. doi: 10.1210/jc.2005-2711. [DOI] [PubMed] [Google Scholar]
- 7.Hasegawa A, Yonezawa K, Ohta A, et al. J Reprod Dev. 2012;58:156–161. doi: 10.1262/jrd.11-097n. [DOI] [PubMed] [Google Scholar]
- 8.Khlifaoui M, Battut I, Bruyas JF, et al. Theriogenology. 2005;63:138–149. doi: 10.1016/j.theriogenology.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 9.Lenzi A, Lombardo F, Gandini L, et al. J Endocrinol Invest. 1993;16:683–686. doi: 10.1007/BF03348911. [DOI] [PubMed] [Google Scholar]
- 10.McNiven MA, Richardson GF. Cell Preservation Technology. 2002;1:165–174. [Google Scholar]
- 11.McNiven MA, Richardson GF. Cell Preservation Technology. 2006;4:169–177. [Google Scholar]
- 12.Nakagata N. Mamm Genome. 2000;11:572–576. doi: 10.1007/s003350010109. [DOI] [PubMed] [Google Scholar]
- 13.Nakagata N. Methods Mol Biol. 2011;693:57–73. doi: 10.1007/978-1-60761-974-1_4. [DOI] [PubMed] [Google Scholar]
- 14.Okuyama M, Isogai S, Saga M, et al. J Fertil Implant. 1990;7:116–119. [Google Scholar]
- 15.Ostermeier GC, Wiles MV, Farley JS, et al. PLoS ONE. 2008;3:e2792. doi: 10.1371/journal.pone.0002792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Purdy PH, Ericsson SA, Dodson RE, et al. Small Ruminant Research. 2004;55:239–243. [Google Scholar]
- 17.Raha S, Robinson B. Trends Biochem Sci. 2000;25:502–508. doi: 10.1016/s0968-0004(00)01674-1. [DOI] [PubMed] [Google Scholar]
- 18.Renard P, Grizard G, Griveau JF, et al. Cryobiology. 1996;33:311–319. doi: 10.1006/cryo.1996.0031. [DOI] [PubMed] [Google Scholar]
- 19.Rich IN, Kubanek B. British Journal of Huernatology. 1982;52:579–588. doi: 10.1111/j.1365-2141.1982.tb03934.x. [DOI] [PubMed] [Google Scholar]
- 20.Roca J, Gil MA, Hernandez M, et al. Journal of Andrology. 2004;25:397–405. doi: 10.1002/j.1939-4640.2004.tb02806.x. [DOI] [PubMed] [Google Scholar]
- 21.Stacy R, Eroglu A, Fowler A, et al. Cryobiology. 2006;52:99–107. doi: 10.1016/j.cryobiol.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 22.Sztein JM, Farley JS, Mobraaten LE. Biol Reprod. 2000;63:1774–1780. doi: 10.1095/biolreprod63.6.1774. [DOI] [PubMed] [Google Scholar]
- 23.Sztein JM, Farley JS, Young AF, et al. Cryobiology. 1997;35:46–52. doi: 10.1006/cryo.1997.2024. [DOI] [PubMed] [Google Scholar]
- 24.Tada N, Sato M, Yamanoi J, at al. J Reprod Fertil. 1990;89:511–516. doi: 10.1530/jrf.0.0890511. [DOI] [PubMed] [Google Scholar]
- 25.Takeo T, Hoshii T, Kondo Y, et al. Biol Reprod. 2008;78:546–551. doi: 10.1095/biolreprod.107.065359. [DOI] [PubMed] [Google Scholar]
- 26.Takeo T, Nakagata N. Lab Anim. 2010;44:132–137. doi: 10.1258/la.2009.009074. [DOI] [PubMed] [Google Scholar]
- 27.Takeo T, Nakagata N. Biol Reprod. 2011;85:1066–1072. doi: 10.1095/biolreprod.111.092536. [DOI] [PubMed] [Google Scholar]
- 28.Thornton CE, Brown SD, Glenister PH. Mamm Genome. 1999;10:987–992. doi: 10.1007/s003359901145. [DOI] [PubMed] [Google Scholar]
- 29.Toyoda Y, Yokoyama M, Hosi T. Jpn J Anim Reprod. 1971;16:147–151. [Google Scholar]
- 30.Trimeche A, Renard P, Le Lannou D, at al. Theriogenology. 1996;45:1015–1027. doi: 10.1016/0093-691x(96)00029-5. [DOI] [PubMed] [Google Scholar]
- 31.Trimeche A, Yvon JM, Vidament M, et al. Theriogenology. 1999;52:181–191. doi: 10.1016/s0093-691x(99)00120-x. [DOI] [PubMed] [Google Scholar]
- 32.Yokoyama M, Akiba H, Katsuki M, Nomura T. Jikken Dobutsu. 1990;39:125–128. doi: 10.1538/expanim1978.39.1_125. [DOI] [PubMed] [Google Scholar]