Abstract
Studies investigating mechanisms controlling gene regulation frequently examine specific DNA sequences using chromatin immunoprecipitation (ChIP) assays to determine whether specific regulatory factors or modified histones are present. While use of primary cells or cell line models for differentiating or differentiated tissue is widespread, the ability to assess factor binding and histone modification in tissue defines the events that occur in vivo and provides corroboration for studies in cultured cells. Many tissues can be analyzed with minimal modification to existing ChIP protocols that are designed for cultured cells; however, some tissues, such as skeletal muscle, are problematic in that accessibility of the cross-linking agent is limited.
We describe a method to isolate skeletal muscle tissue nuclei suitable for use in ChIP protocols. Furthermore, we utilize a simple fractionation of digested skeletal muscle tissue that can separate mature myofibers from satellite cells, which are responsible for postnatal skeletal muscle regeneration, thereby allowing simultaneous preparation of nuclei from both cell types.
Keywords: Skeletal muscle, Satellite cells, Nuclei isolation, Chromatin immunoprecipitation, Brgl, Brahma
1. Introduction
In general, the chromatin structure that exists in eukaryotic cells is refractory to processes that utilize the DNA, including transcription, replication, and recombination. In response to this problem, two large families of enzymes that can alter chromatin structure in manners that facilitate access of regulatory factors to the DNA have evolved. These include the ATP-dependent chromatin remodeling enzymes, which utilize the hydrolysis of ATP to alter histone–DNA contacts (1, 2), and histone modifying enzymes, which posttranslationally modify specific histone residues by acetylation, methylation, phosphorylation, ubiquitylation, ADP-ribosylation, and sumoylation (3–5).
The conventional chromatin immunoprecipitation (ChIP) assay is a powerful tool that has allowed investigators to cross-link proteins to DNA, immuno-purify complexes of protein bound to fragmented DNA, and recover the DNA to identify specific DNA sequences that were interacting with the protein of interest in the cell at the time of cross-linking. This assay has been widely used to identify sequences bound by regulatory factors that directly bind DNA, regulatory factors that interact with DNA indirectly through protein–protein interactions, including chromatin remodeling and modifying enzymes, and the structural components of chromatin, including specifically modified histones (6, 7). Typically, the cross-linking agent can be added directly to cell culture media to cross-link proteins to DNA in cultured cells. Protein–DNA interactions in many tissues can be similarly cross-linked by immersion of minced tissue pieces in media containing the cross-linking agent (8). While some investigators have successfully performed ChIP experiments from cross-linked skeletal muscle (9), cross-linking of skeletal muscle tissue is relatively inefficient compared to most other tissues, presumably due to the myofiber structure presenting a physical barrier that limits access of the cross-linking agent to the nuclei. As an alternative, we have determined that brief enzymatic digestion of skeletal muscle and subsequent purification of nuclei from the tissue allow for efficient cross-linking and highly reproducible ChIP results.
A further advantage of this methodology is that the enzymatic digestion of skeletal muscle tissue releases satellite cells from between the basal lamina and sarcolemma of myofibers. Satellite cells are the resident muscle stem cell population present in muscle fibers. Upon activation by injury, they exit the microenvironment provided by the myofiber, enter a proliferative stage, migrate to the site of injury, and differentiate (10, 11). Separation of satellite cells from mature myofibers permits analysis of protein–DNA interactions in both populations, which will increase our understanding of similarities and differences in gene activation that occur in these distinct cell types.
It is important to note that while satellite cells in noninjured tissue are quiescent, the act of isolating the tissue will cause activation of some of the cells, thus the pool of nuclei that is obtained represents a mixture of activated and quiescent cells. While myogenic regulators of differentiation can therefore be detected in the satellite cell sample as well as in the myofiber sample, assessment of the separation of satellite cells from myofibers can still be monitored by quantification of specific mRNAs from the isolated cells or nuclei, as will be discussed.
The reader should also note that although the satellite cell pool is devoid of myofibers, it is not a homogenous population of satellite cells. Fibroblasts, in particular, are present in large numbers. Thus, investigations are necessarily restricted to the activation and maintenance of skeletal muscle-specific gene expression. This is because the presence of nuclei from other cell types does not impact the results since the relevant myogenic genes are silent in those nuclei.
2. Materials
Prepare reagents using double-distilled deionized water and analytical or molecular biology grade chemicals.
2.1. Isolation and Digestion of Tissue
95% ethanol in a spray bottle.
Tray of ice.
Plastic wrap.
Scalpel, forceps, and scissors suitable for animal dissection.
Phosphate-buffered saline (PBS): For 1 L of 1× PBS, add 8 g sodium chloride, 0.2 g potassium chloride, 1.44 g disodium hydrogen phosphate, and 0.24 g potassium dihydrogen phosphate to 800 mL of deionized water. Adjust pH to 7.4 with hydrochloric acid and make up the volume to 1 L with deionized water.
Plastic 10 cm petri dishes.
2.2. Separation of Satellite Cells and Myofibers
Collagenase type II (Invitrogen).
1 M calcium chloride (CaCl2) stock solution: Autoclave and store at room temperature.
Collagenase type II in PBS supplemented with 1 mM CaCl2: Dissolve 10,000 units of collagenase type II in 100 mL of 1× PBS and filter sterilize by passing through a 0.22 µm filter to obtain 100 units/mL. Store in 5 mL aliquots in −20°C. Add 5 µL of 1 M CaCl2 stock solution to the 5 mL aliquot of collagenase type II before use to generate a final concentration of 1 mM CaCl2.
Temperature-controlled incubator/shaker.
70 µm cell strainer (Becton Dickinson).
50 mL plastic tubes.
14 mL round bottom polypropylene tubes (Falcon).
1.5 mL microcentrifuge tubes.
Liquid nitrogen.
Dewar flask.
Forceps.
1 M 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES-KOH) (pH 7.3) stock solution: To 700 mL of deionized water, add 238.3 g of HEPES, stir to dissolve, and set the pH to 7.3 using potassium hydroxide crystals. Make up the volume to 1,000 mL using deionized water. Sterilize using a 0.22 µm filter and store at 4°C.
1 M potassium chloride (KCl) stock solution.
1 M magnesium chloride (MgCl2) stock solution.
1 M dithiothreitol (DTT) stock solution: Store in 0.5 or 1 mL aliquots at −20°C.
0.1 M phenylmethanesulfonylfluoride (PMSF) stock solution: Add 17 4 mg of PMSF to 10 mL of isopropanol and dissolve by vortexing or rotation on a rotating platform.
10% Nonidet P-40 (NP-40) stock solution.
1 mg/mL leupeptin stock solution: Dissolve 10 mg of leupeptin into 10 mL of deionized water and store in aliquots at −20°C (stable up to 6 months).
3 mg/mL cytochalasin B (Calbiochem) stock solution: Dissolve 30 mg in 10 mL of dimethyl sulfoxide (DMSO) and store as aliquots at −20°C.
Lysis buffer (10 mM HEPES-KOH (pH 7.3), 10 mM KCl, 5 mM MgCl2, 0.5 mM DTT, 0.2 mM PMSF, and 10 µg/mL leupeptin): Prepare 25 mL of lysis buffer by adding 250 µL of 1 M HEPES-KOH, 250 µL of 1 M KCl, 125 µL of l M MgCl2, 12.5 µL of 1 M DTT, 50 µL of 100 mM PMSF, 25 µL of 1 mg/mL of leupeptin in 15 mL deionized water and make up the volume to 25 mL with deionized water. Add the DTT, PMSF, and Leupeptin just before use.
Lysis buffer plus cytochalsin B (10 mM HEPES-KOH (pH 7.3), 10 mM KCl, 5 mM MgCl2, 0.5 mM DTT, 0.2 mM PMSF, 10 µg/mL leupeptin, 3 µg/mL cytochalasin B): Prepare 25 mL of lysis buffer as described in Subheading 2.2, item 20, then add 25 µL of 3 mg/mL cytochalasin B just before use.
Lysis buffer +0.1% NP-40: Prepare 25 mL of lysis buffer as described in Subheading 2.2, item 20, except that 250 µL of 10% NP-40 is added prior to the addition of DTT, PMST, and leupeptin.
Tissue homogenizer.
10 mL Dounce with pestle A.
Hoechst 33258 dye.
1 M Triethanolamine pH 7.5: For 100 mL, add 13.3 mL of triethanolamine (Sigma; the stock is 7.5 M) to 80 mL of deionized water and adjust the pH to 7.5 with HCI. Add deionized water to raise the volume to 100 mL.
10-STM buffer (10% sucrose, 10 mM triethanolamine pH 7.5, 5 mM MgCl2, 10 µg/mL leupeptin): For 500 mL, add 5 mL of 1 M triethanolamine pH 7.5 stock solution, 2.5 mL of 1 M MgCl2, and 50 g sucrose to 300 mL of deionized water and dissolve by stirring. Raise the volume to 500 mL using deionized water. Sterilize using a 0.22 µm filter and store at 4°C. Just before use, add leupeptin to a final concentration of 10 µg/mL.
1 M Tris–hydrochloride (Tris–HCI), pH 7.4: For 100 mL, add 12.11 g Tris to 80 mL of deionized water and dissolve using a magnetic stirrer. Adjust the pH to 7.4 with HCl and make up the volume to 100 mL using deionized water. Autoclave and store at room temperature.
2.0 M Sucrose/10 mM Tris–HCl/5 mM MgCl2 buffer: For 500 mL, add 342.3 g of sucrose to 300 mL of water. Dissolve using a magnetic stirrer. Add 5 mL of 1 M Tris-HCI pH 7.4 and 2.5 mL of 1 M MgCl2 Make up the volume to 500 mL using deionized water. Sterilize using a 0.22 µm filter and store at 4°.
Ultracentrifuge with hanging bucket rotor that holds 5 mL ultracentrifuge tubes.
5 mL ultracentrifuge tubes.
2.3. Preparation of Cross-linked Nuclei for Subsequent Use in a Chromatin lmmunoprecipitation
Formaldehyde.
1 M glycine stock solution.
Lysis buffer (see Subheading 2.2, item 20).
3. Methods
3.1. Isolation and Digestion of Tissue
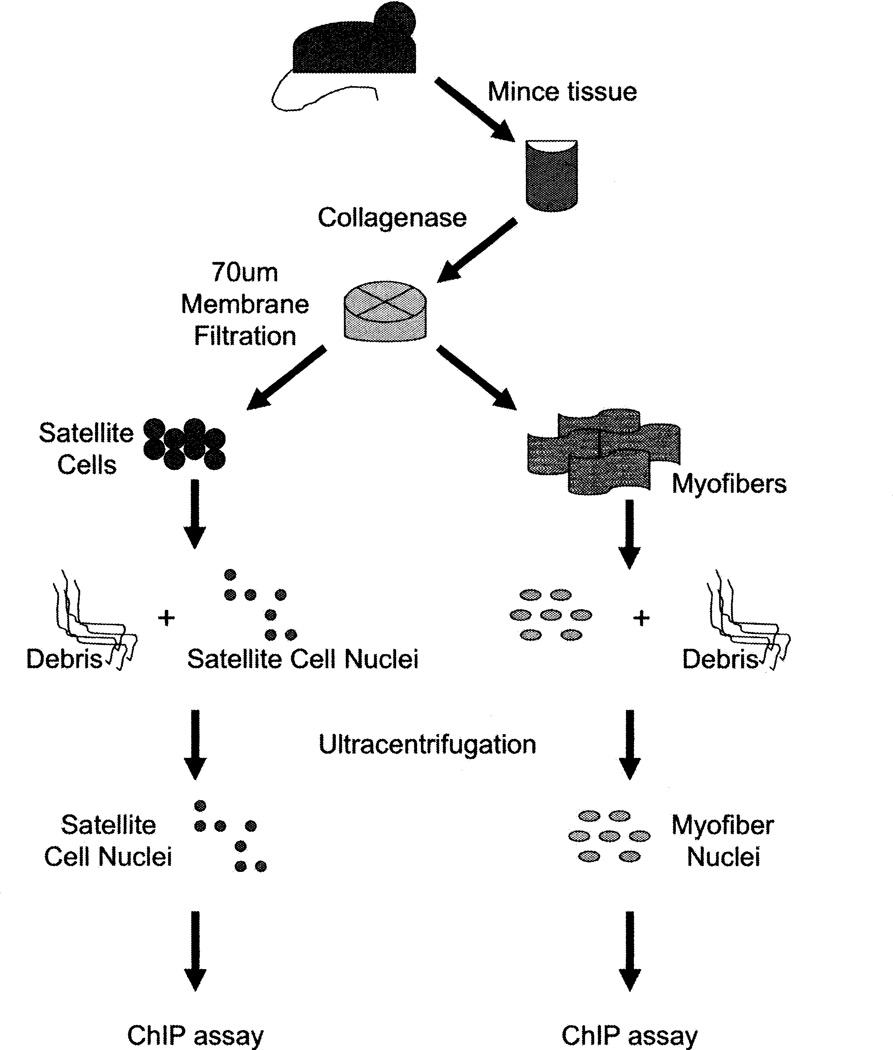
A schematic of the protocol is presented in Fig. 1. The procedure described below is for isolation of upper hindlimb skeletal muscle from young adult (4–8 week old) mice. This procedure should be applicable to any skeletal muscle from mice of any age; however, the number of satellite cells present varies depending upon the specific muscle and the age of the organism (12).
Wash the forceps, scissors, and scalpel with detergent. Wrap in aluminum foil or suitable autoclave bag. Autoclave to sterilize.
Sacrifice the mice in accordance with your institutionally approved protocol (see Note 1).
Spray the mouse with 95% ethanol and place back up on Styrofoam tray on ice to slow autolysis.
Lift the dermis with the forceps above the base of the tail (Fig. 2a).
Make a small subcutaneous incision with the scissors above the base of the tail (Fig. 2b).
Using the scissors, cut along the midline toward the head (Fig. 2c).
Use the scissors to cut the dermis perpendicular to the midline and to separate the dermis from the underlying musculature (Fig. 2d).
Use the forceps to grasp the dermis and pull away from the back and the limbs to reveal the musculature (Fig. 2e, f).
Use the scalpel to dissect skeletal muscle from both hindlimbs.
Use the forceps to place skeletal muscle in cold 1× PBS in a petri dish on ice.
To isolate liver tissue for use as a control, create a V-shaped incision in the parietal peritoneum to reveal the underlying visceral peritoneum (see Note 2).
Make an additional V-shaped incision in the visceral peritoneum to expose the liver.
Dissect several lobes of the liver and place in a different petri dish containing cold 1× PBS on ice.
Using the scalpel and forceps, remove contaminating tissues such as adipose and connective tissues from the skeletal muscle.
Using the scalpel and forceps, mince the skeletal muscle to approximately 1 mm3 pieces and transfer to a prechilled 50 mL conical tube on ice.
Repeat 3.1 steps 14 and 15 with the control tissue. Transfer to a prechilled 50 mL conical tube and place on ice. Control tissues are not subjected to the collagenase digestion or filtration steps described below (see Subheading 3.2). Mincing is sufficient for subsequent steps.
Fig. 1.
Schematic of protocol.
Fig. 2.
(a–f) Sequential images of the exposure of hindlimb musculature to facilitate isolation of skeletal muscle.
3.2. Separation of Satellite Cells and Myofibers (see Note 3)
Partially digest the minced skeletal muscle tissue by resuspension in 100 units/mL collagenase type II in PBS supplemented with 1 mM CaCl2.
Incubate the tissue with agitation at 37°C for 1 h (13, 14) in a temperature-controlled incubator/shaker (see Note 4).
Following the collagenase treatment, separate the satellite cells from the mature myofibers by filtration by gravity through a 70 µm nylon cell strainer into a 50 mL conical tube at room temperature (15). The flow through material is enriched for satellite cells.
To isolate the fraction retained on the membrane (myofibers), turn the filtration unit upside-down and tap over a 50 mL conical tube to facilitate transfer of the myofibers into the tube.
Place both tubes on ice along with the control tissue samples.
Centrifuge at 300×g at 4°C for 5 min.
Remove the supernatant above the pellet by aspiration or with a pipette.
If some of the tissue is to be used for RNA analysis, transfer small aliquots of each tissue sample into 1.5 mL microcentrifuge tubes using a wide-mouth P1000 pipetman tip. Freeze by placing the tubes into a Dewar flask (or other suitable container) with liquid nitrogen (see Notes 5 and 6). Remove the tubes from the flask with forceps and place samples in a −70°C freezer.
Estimate the volume of the pellet in each 50 mL conical tube.
Resuspend the pellet in 7 volumes of lysis buffer (10 mM HEPES-KOH (pH 7.3), 10 mM KCl, 5 mM MgCl2, 0.5 mM DTT) containing freshly added protease inhibitors (0.2 mM PMSF, and 10 µg/mL leupeptin). The lysis buffer for the myofiber fraction, but not the satellite cell or control tissue samples, also includes 3 µg/mL cytochalasin B (see Note 7).
Incubate the samples for 30 min on ice.
Transfer the samples to 14 mL round bottom tubes.
Homogenize the samples using a tissue homogenizer (see Note 8) to disrupt larger fragments of tissue and to create a more homogenous single cell suspension to ease subsequent douncing. Place homogenized sample on ice.
Between samples, clean the homogenizer with 95% ethanol, then sonicate in a fresh tube of deionized water. Repeat the water sonications twice more, each time with fresh water.
Centrifuge the samples at 3,000×g for 5 min at 4°C.
Estimate the volume of the pellet.
Resuspend the pellet in 2.5 volumes of 10-STM buffer (10% sucrose, 10 mM triethanolamine pH 7.5, 5 mM MgCl2, 10 µg/mL leupeptin).
Add twice the original volume of the pellet of 2.0 M sucrose/10 mM Tris–HCl/5 mM MgCl2 and mix the sample by gentle pipeting.
Transfer the sample to a prechilled 10 mL Dounce homogenizer by pouring.
Dounce homogenize each sample on ice for 7 slow strokes using pestle A to release the nuclei. Move the pestle slowly enough to avoid foaming of the sample solution. Rinse the Dounce homogenizer well with cold 1× PBS between samples.
Check the release and integrity of nuclei under a light microscope after staining of a small aliquot (1–2 µL) with an equal volume of Hoechst 33258 dye; nuclei will stain blue while any remaining unlysed cells will clear the dye and appear unstained. If lysis is insufficient (<90% cells lysed), repeat Subheading 3.2, step 20.
Transfer the sample from the Dounce homogenizer to a prechilled 14 mL tube.
Prepare the samples for ultracentrifugation. Aliquot 750 µL of the 2.0 M sucrose/10 mM Tris-HCl/5 mM MgCl2 buffer into the bottom of a 5 mL ultracentrifuge tube, and gently overlay with the Dounce homogenized nuclei mixture from Subheading 3.2, step 22.
Ultracentrifuge the samples at 116, 100 ×g for 1 h at 4 °C.
Aspirate or pi pet off the supernatant.
Resuspend the pellet in 500 µL of lysis buffer +0.1% NP-40.
If desired, an aliquot can be taken for RNA preparation (see Notes 5 and 6).
Use 1–2 µL to determine the A260 reading using a spectrophotometer or spectrofluorometer (see Note 1).
3.3. Preparation of Cross-linked Nuclei for Subsequent Use in a Chromatin lmmunoprecipitation Assay
Cross-link the sample by adding formaldehyde at a final concentration of 1 % for 5 min at room temperature (see Note 9).
Quench the cross-linking reaction by the addition of glycine to a final concentration of 0.125 M for 5 min at room temperature.
Pellet the cross-linked nuclei by centrifugation for 1 min at room temperature at 13,500 ×g.
Aspirate or remove the supernatant.
Freeze the pellet in liquid nitrogen. Store the samples at −70°C for later use or thaw on ice for immediate use.
Resuspend the cross-linked nuclei in 400 µL of lysis buffer.
We have made use of the Millipore ChIP protocol (http://www.millipore.com/immunodetection/id3/immunoprecipitationprotocols) to detect cross-linked protein-DNA interactions.
3.4. Examples of Results Achieved with Isolated Satellite Cell, Myofiber, and Liver (Control) Nuclei
Subheading 3.2, steps 8 and 27 indicate that aliquots of sample can be reserved for RNA isolation. We have utilized the Trizol reagent (Sigma) and followed the manufacturer’s directions to isolate RNA, although any standard RNA isolation method can be utilized. We looked for the presence or absence of specific mRNAs to examine the quality of the separation of satellite cells and myofibers.
Pax3 mRNA is expressed in satellite cells but not mature myofibers (16), while the dystrophin gene is inactive in myoblasts but expressed in mature myofibers (17). Experimental analysis of Pax3 and dystrophin mRNA expression in the separated tissue prior to isolation of nuclei (Fig. 3) or following isolation of nuclei (data not shown) revealed clean tissue separation with little to no cross-contamination.
Fig. 3.
mRNA analysis indicates clean separation of satellite cells from myofibers. mRNA quantification of (a) Pax3, a satellite cell marker, and (b) dystrophin, a marker of mature skeletal muscle tissue, by real-time PGR in satellite cells, myofibers, and liver tissue. Expression levels were normalized to EF-1a mRNA levels, and the relative expression in liver cells was set at 1. A representative experiment is shown.
We utilized cross-linked satellite cell, myofiber, and liver nuclei for ChIP experiments to examine the interaction of Brahma (Brm) and Brgl (Brahma-related gene 1) with the promoter sequences controlling the expression of the myogenin gene, which encodes a myogenic regulatory protein that is expressed early in the differentiation process (18, 19). Brgl and Brm are highly related DNA-dependent ATPases that act as the catalytic subunit for the SWI/SNF family of mammalian chromatin remodeling enzymes (20, 21). These enzymes alter histone–DNA contacts to permit structural alterations in local chromatin structure and are essential for most cellular differentiation programs (2, 22). Previous work has demonstrated that the Brgl protein is essential for activation of the myogenin gene and for skeletal muscle differentiation (23–25), and that it is likely targeted to the myogenin promoter by a DNA-bound complex containing Pbx/Meis proteins and MyoD (24, 26).
Affinity-purified antibodies isolated from polyclonal antisera generated against either the Brgl or Brm protein (27) were used for the immunoprecipitation of cross-linked chromatin fragments derived from all three nuclei preps (see Note 10). Real-time PCR was used with primers to the myogenin promoter to amplify the DNA isolated by the ChIP procedure. The results from this experiment are presented in Fig. 4. Brgl was associated with the myogenin promoter in both satellite cells and in myofibers, which correlates with transcriptional expression of myogenin in these tissues. In contrast, Brgl was not associated with the myogenin promoter in liver cells, where the gene is transcriptionally inactive. The results for the related ATPase, Brm, were different. Brm was present at the myogenin promoter in myofibers, but not in satellite cells or the control liver cells. These experiments indicate that only Brgl is present at the myogenin locus in satellite cells and suggest that Brgl is the relevant SWI/SNF ATPase associated with myogenin activation in these cells. Both Brgl and Brm were present at the myogenin locus in mature myofiber tissue. Since Brgl and Brm are mutually exclusive in formation of SWI/SNF enzyme complexes (28); these data suggest that the myogenin promoter in myofiber nuclei is either occupied by multiple SWI/SNF complexes or that individual loci can be occupied by SWI/SNF complexes containing either ATPase.
Fig. 4.
Brg1 and Brm bind to the myogenin promoter in skeletal muscle tissues. Representative ChlP experiments performed on nuclei isolated from satellite cells, myofibers, and liver. The data reflect relative Brg1 or Brm recruitment to the myogenin promoter and normalized to input as determined by real-time PCR. Primers (31) spanned −143 to −5 relative to the start site of transcription; this region includes binding sites for TFllD, MyoD, Mef2, and Pbx. Recruitment is relative to that in liver cells, which was assigned a value of 1. Negative controls included immunoprecipitation with preimmune antisera or purified lgG and amplification of the lgH enhancer, where no binding of SWl/SNF proteins is evident (24). Background signals were obtained for all control experiments.
In conclusion, the data clearly demonstrate the feasibility of identifying protein–DNA interactions that occur at specific regions of DNA in satellite cell and myofibers isolated from skeletal muscle tissue.
Acknowledgments
We thank C. Baron for assistance with the figures and OH Cho for manuscript comments. This work was supported by NIH grant GM56244 to ANI and by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan to YO.
Footnotes
Typically, we have pooled tissue from four mice per isolation to isolate nuclei. We do not count the number of isolated nuclei; instead, we quantify recovery based on a simple A260 reading. An average preparation yields approximately 100 µg of satellite cell DNA and approximately 600 µg of myofiber DNA.
Liver tissue is suggested as a control because it is a large tissue and is easy to isolate. We have also utilized brain tissue as well as adipose from various depots as control tissues. Any non-muscle tissue should act as an adequate control.
Separating the satellite cells from the myofibers is an option, but not a requirement. Total skeletal muscle nuclei can be obtained by omitting the separation step.
Do not overdigest. If the solution becomes sticky and very viscous, it means that overdigestion has occurred and the isolation should be abandoned.
As an alternative to reserving aliquots of tissue for subsequent RNA isolation, aliquots of the purified nuclei (see Subheading 3.2, steps 27) can be utilized for RNA isolation.
RNA can be prepared subsequently using any standard RNA isolation procedure.
Inclusion of cytochalasin B is important because it inhibits actin polymerization, which promotes actin depolymerization, instability of actin-based filaments, and nuclei release.
Settings for the homogenizer will vary by manufacturer and by tissue, so the investigator will need to empirically determine the minimum amount of homogenization required. The objective is to create a single cell suspension.
Cross-linking conditions may be optimized by the investigator. Typical ranges for formaldehyde concentration are 0.5–2%. Typical time ranges are 4–20 min, and some investigators perform the cross-linking at 37°C.
The nature of ChIP experiments makes the procedure largely dependent upon the quality of the antibody being used. Thus, there is no absolute amount of nuclei necessary for a particular ChIP experiment. We routinely use 10–20 µg of nuclei for experiments. We suspect that even less can be used; prior experiments with embryonic tissue (29, 30) or nuclei isolated from embryonic tissue (data not shown) has yielded such small quantities that we did not sacrifice any for quantification purposes and instead simply used the samples for the desired ChIP experiments. However, antibodies that work poorly in ChIP experiments where tissue culture cells are the starting material usually also work poorly, if at all, with tissue nuclei, even with larger amounts of starting material. Therefore, we recommend that the investigator determine the minimal amount of starting material empirically.
References
- 1.Racki LR, Narlikar GJ. ATP-dependent chromatin remodeling enzymes: two heads are not better, just different. Curr Opin Genet Dev. 2008;18:137–144. doi: 10.1016/j.gde.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saha A, Wittmeyer J, Cairns BR. Mechanisms for nucleosome movement by ATP-dependent chromatin remodeling complexes. Results Probl Cell Differ. 2006;41:127–148. doi: 10.1007/400_005. [DOI] [PubMed] [Google Scholar]
- 3.Berger SL. The complex language of chromatin regulation during transcription. Nature. 2007;447:407–412. doi: 10.1038/nature05915. [DOI] [PubMed] [Google Scholar]
- 4.Bhaumik SR, Smith E, Shilatifard A. Covalent modifications of histones during development and disease pathogenesis. Nat Struct Mol Biol. 2007;14:1008–1016. doi: 10.1038/nsmb1337. [DOI] [PubMed] [Google Scholar]
- 5.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 6.Massie CE, Mills IG. ChIPping away at gene regulation. EMBO Rep. 2008;9:337–343. doi: 10.1038/embor.2008.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Minard ME, Jain AK, Barton MC. Analysis of epigenetic alterations to chromatin during development. Genesis. 2009;47:559–572. doi: 10.1002/dvg.20534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wells J, Farnham PJ. Characterizing transcription factor binding sites using formaldehyde crosslinking and immunoprecipitation. Methods. 2002;26:48–56. doi: 10.1016/S1046-2023(02)00007-5. [DOI] [PubMed] [Google Scholar]
- 9.Mejat A, Ramond F, Bassel-Duby R, Khochbin S, Olson EN, Schaeffer L. Histone deacetylase 9 couples neuronal activity to muscle chromatin acetylation and gene expression. Nat Neurosci. 2005;8:313–321. doi: 10.1038/nn1408. [DOI] [PubMed] [Google Scholar]
- 10.Le Grand F, Rudnicki MA. Skeletal muscle satellite cells and adult myogenesis. Curr Opin Cell Biol. 2007;19:628–633. doi: 10.1016/j.ceb.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi X, Garry DJ. Muscle stem cells in development, regeneration, and disease. Genes Dev. 2006;20:1692–1708. doi: 10.1101/gad.1419406. [DOI] [PubMed] [Google Scholar]
- 12.Conboy IM, Rando TA. Aging, stem cells and tissue regeneration: lessons from muscle. Cell Cycle. 2005;4:407–410. doi: 10.4161/cc.4.3.1518. [DOI] [PubMed] [Google Scholar]
- 13.Asakura A, Komaki M, Rudnicki M. Muscle satellite cells are multipotential stem cells that exhibit myogenic, osteogenic, and adipogenic differentiation. Differentiation. 2001;68:245–253. doi: 10.1046/j.1432-0436.2001.680412.x. [DOI] [PubMed] [Google Scholar]
- 14.Cornelison DD, Wold BJ. Single-cell analysis of regulatory gene expression in quiescent and activated mouse skeletal muscle satellite cells. Dev Biol. 1997;191:270–283. doi: 10.1006/dbio.1997.8721. [DOI] [PubMed] [Google Scholar]
- 15.Olguin HC, Olwin BB. Pax-7 up-regulation inhibits myogenesis and cell cycle progression in satellite cells: a potential mechanism for self-renewal. Dev Biol. 2004;275:375–388. doi: 10.1016/j.ydbio.2004.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Conboy IM, Rando TA. The regulation of Notch signaling controls satellite cell activation and cell fate determination in postnatal myogenesis. Dev Cell. 2002;3:397–409. doi: 10.1016/s1534-5807(02)00254-x. [DOI] [PubMed] [Google Scholar]
- 17.Lev AA, Feener CC, Kunkel LM, Brown RH., Jr Expression of the Duchenne’s muscular dystrophy gene in cultured muscle cells. J Biol Chem. 1987;262:15817–15820. [PubMed] [Google Scholar]
- 18.Edmondson DG, Olson EN. A gene with homology to the myc similarity region of MyoD1 is expressed during myogenesis and is sufficient to activate the muscle differentiation program. Genes Dev. 1989;3:628–640. doi: 10.1101/gad.3.5.628. [DOI] [PubMed] [Google Scholar]
- 19.Wright WE, Sassoon DA, Lin VK. Myogenin, a factor regulating myogenesis, has a domain homologous to MyoD. Cell. 1989;56:607–617. doi: 10.1016/0092-8674(89)90583-7. [DOI] [PubMed] [Google Scholar]
- 20.Khavari PA, Peterson CL, Tamkun JW, Mendel DB, Crabtree GR. BRGl contains a conserved domain of the SWI2/SNF2 family necessary for normal mitotic growth and transcription. Nature. 1993;366:170–174. doi: 10.1038/366170a0. [DOI] [PubMed] [Google Scholar]
- 21.Muchardt C, Yaniv M. A human homologue of Saccharomyces cerevisiae SNF2/SWI2 and Drosophila brm genes potentiates transcriptional activation by the glucocorticoid receptor. Embo J. 1993;12:4279–4290. doi: 10.1002/j.1460-2075.1993.tb06112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de la Serna IL, Ohkawa Y, Imbalzano AN. Chromatin remodelling in mammalian differentiation: lessons from ATP-dependent remodellers. Nat Rev Genet. 2006;7:461–473. doi: 10.1038/nrg1882. [DOI] [PubMed] [Google Scholar]
- 23.de la Serna IL, Carlson KA, Imbalzano AN. Mammalian SWI/SNF complexes promote MyoD-mediated muscle differentiation. Nat Genet. 2001;27:187–190. doi: 10.1038/84826. [DOI] [PubMed] [Google Scholar]
- 24.de la Serna IL, Ohkawa Y, Berkes CA, Bergstrom DA, Dacwag CS, Tapscott SJ, Imbalzano AN. MyoD targets chromatin remodeling complexes to the myogenin locus prior to forming a stable DNA-bound complex. Mol Cell Biol. 2005;25:3997–4009. doi: 10.1128/MCB.25.10.3997-4009.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simone C, Forcales SV, Hill DA, Imbalzano AN, Latella L, Puri PL. p38 pathway targets SWI-SNF chromatin-remodeling complex to muscle-specific loci. Nat Genet. 2004;36:738–743. doi: 10.1038/ng1378. [DOI] [PubMed] [Google Scholar]
- 26.Berkes CA, Bergstrom DA, Penn BH, Seaver KJ, Knoepfler PS, Tapscott SJ. Pbx marks genes for activation by MyoD indicating a role for a homeodomain protein in establishing myogenic potential. Mol Cell. 2004;14:465–477. doi: 10.1016/s1097-2765(04)00260-6. [DOI] [PubMed] [Google Scholar]
- 27.de La Serna IL, Carlson KA, Hill DA, Guidi CJ, Stephenson RO, Sif S, Kingston RE, Imbalzano AN. Mammalian SWI-SNF complexes contribute to activation of the hsp70 gene. Mol Cell Biol. 2000;20:2839–2851. doi: 10.1128/mcb.20.8.2839-2851.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang W, Cote J, Xue Y, Zhou S, Khavari PA, Biggar SR, Muchardt C, Kalpana GV, Goff SP, Yaniv M, Workman JL, Crabtree GR. Purification and biochemical heterogeneity of the mammalian SWI-SNF complex. Embo J. 1996;15:5370–5382. [PMC free article] [PubMed] [Google Scholar]
- 29.Ohkawa Y, Marfella CG, Imbalzano AN. Skeletal muscle specification by myogenin and Mef2D via the SWI/SNF ATPase Brgl. Embo J. 2006;25:490–501. doi: 10.1038/sj.emboj.7600943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ohkawa Y, Yoshimura S, Higashi C, Marfella CG, Dacwag CS, Tachibana T, Imbalzano AN. Myogenin and the SWI/SNF ATPase Brgl maintain myogenic gene expression at different stages of skeletal myogenesis. J Biol Chem. 2007;282:6564–6570. doi: 10.1074/jbc.M608898200. [DOI] [PubMed] [Google Scholar]
- 31.Lu J, McKinsey TA, Zhang CL, Olson EN. Regulation of skeletal myogenesis by association of the MEF2 transcription factor with class II histone deacetylases. Mot Cell. 2000;6:233–244. doi: 10.1016/s1097-2765(00)00025-3. [DOI] [PubMed] [Google Scholar]