Abstract
AIM
To perform an objective, intra-individual comparison of residual colonic fluid volume and attenuation associated with the current front-line laxative magnesium citrate (MgC) versus the former front-line laxative sodium phosphate (NaP) at CT colonography (CTC).
MATERIALS AND METHODS
This retrospective Health Insurance and Portability and Accountability Act-compliant study had institutional review board approval; informed consent was waived. The study cohort included 250 asymptomatic adults (mean age at index 56.1 years; 124 male/126 female) who underwent CTC screening twice over a 5 year interval. Colon catharsis at initial and follow-up screening employed single-dose NaP and double-dose MgC, respectively, allowing for intra-patient comparison. Automated volumetric analysis of residual colonic fluid volume and attenuation was performed on all 500 CTC studies. Colonic fluid volume <200 ml and mean attenuation between 300–900 HU were considered optimal. Paired t-test and McNemar’s test were used to compare differences.
RESULTS
Residual fluid volumes <200 ml were recorded in 192 examinations (76.8%) following MgC and in 204 examinations (81.6%) following NaP (p=0.23). The mean total residual fluid volume was 155±114 ml for MgC and 143±100 ml for NaP (p=0.01). The attenuation range of 300–900 HU was significantly more frequent for MgC (n=220, 88%) than for NaP (n=127, 50.8%; p<0.001). Mean fluid attenuation was significantly lower for MgC (700±165 HU) than for NaP (878±155 HU; p<0.001). Concomitant presence of both optimal fluid volume and attenuation was significantly more frequent for MgC 65.2% than for NaP (38%; p<0.001).
CONCLUSIONS
Objective intra-individual comparison using automated volumetric analysis suggests that the replacement of NaP by MgC as the front-line laxative for CTC has not compromised overall examination quality.
INTRODUCTION
CT colonography (CTC) has been shown to be an effective tool for colorectal cancer screening (1–6). Adequate bowel preparation is paramount to achieve acceptable sensitivity and specificity for polyp detection at CTC (7). Minimizing the residual fluid volume and tagging with positive oral contrast medium produces a higher-quality examination, especially for primary three-dimensional (3D) lesion detection (7–9). The amount of residual fluid depends on the specific bowel regimen and directly impacts lesion detection at time-efficient primary 3D endoluminal fly-through without electronic cleansing (i.e., no digital subtraction of enhanced fluid) because only the gas-filled portions of the colon can be assessed (10, 11). Evaluation of fluid-filled regions is limited to more time-consuming secondary two-dimensional (2D) assessment, which can result in reader fatigue in a high-volume screening setting (11).
The attenuation of the oral contrast medium-enhanced residual fluid also depends on the specific bowel regimen and should ideally be within the optimal range of 300–900 HU (12). This renders the fluid hyperdense relative to submerged soft-tissue lesions, allowing for detection at 2D evaluation (or 3D evaluation with electronic cleansing) (10). Fluid attenuation values <300 HU are considered insufficient, as polyp conspicuity is reduced when “polyp” windowing for 2D evaluation is used (12), whereas values >900 HU are considered excessive, as beam-hardening artefacts can occur at fluid–tissue interfaces (13). Excessively high fluid attenuation can also influence the estimation of polyp size (13) and even completely obscure submerged polyps when electronic cleansing is applied (7).
Historically, bowel preparation protocols employing sodium phosphate (NaP) were used in the seminal studies, demonstrating the excellent diagnostic performance of CTC (4), and initially favoured in early CTC programmes (8). Albeit effective (14, 15), NaP is now seldom used given the rare but finite risk of acute phosphate nephropathy (16–18). In its place, magnesium citrate (MgC) is now considered a front-line laxative for bowel preparation prior to CTC (7). However, the switch from NaP to MgC as a front-line cathartic agent in CTC may yield different results in terms of the volume and attenuation of the residual colonic fluid, which could, in turn, impact performance.
A prior study revealed that both the current standard MgC regimen and the previous NaP regimen resulted in excellent bowel preparation in terms of residual adherent stool, whereas MgC yielded more favourable fluid attenuation than NaP (19). Moreover, using a cumbersome, subjective and insensitive semi-quantitative scoring system, the authors found no significant difference between the residual fluid volumes for the cathartic agents (19). However, this widely used crude fluid scoring system simply grades the percentage of fluid that occupies a defined colonic segment on a randomly selected image (8, 14, 19). Unfortunately, this crosssectional ratio also depends upon the degree of gaseous distention, which biases results (20–23). Moreover, the recent study by Borden et al19 was also biased by the fact that the MgC regimen was given only to a subset of significantly older patients at increased risk for phosphate nephropathy (19). As such, an intra-individual comparison of the two cathartic agents using a more efficient, objective, and sensitive tool would be highly desirable.
To the authors’ knowledge, no intra-individual, objective comparison exists between different bowel regimens at CTC, including the NaP and MgC regimens. An efficient, automated tool would allow comparison between different bowel regimens and different CTC programmes. The purpose of the present study was to use automated volumetric QA software for objective, intra-individual comparison of residual colonic fluid volume and attenuation using the current front-line laxative, MgC, compared with the prior NaP regimen at CTC.
MATERIALS AND METHODS
Study design
The present retrospective study complied with the Health Insurance and Portability and Accountability Act and was approved by the institutional review board; the need for informed consent was waived. Two hundred and seventy-six consecutive asymptomatic adults were identified who underwent CTC for initial colorectal cancer screening using NaP, followed by routine CTC screening at a 5 year minimum interval (mean interval 5.6 years) using MgC as the cathartic agent at our institution between 2004 and 2013. The NaP regimen was administered to all individuals between June 2004 and October 2008 for the initial CTC evaluation and the MgC regimen between May 2012 and May 2013 for the second CTC evaluation. Patients were only included in the final analyses if the entire colon was successfully segmented and processed using the automated CTC software in terms of calculating the total luminal fluid volume for both NaP and MgC regimens. Both examinations of a given patient were excluded when the automated tool did not capture all segments of the luminal fluid, typically related to decreased attenuation from inadequate tagging. This was the case in 26/552 CTC examinations (4.7%). In the remaining 526/552 CTC examinations (95.3%), the CTC software successfully calculated the total residual fluid for both bowel preparations. Examinations were excluded for 14/276 MgC examinations (5.1%) and for 12/276 NaP examinations (4.4%). The affected 26 patients were not included in the final analyses. The mean patient age of the 250 included patients (126 women and 124 men) was 56.1 years (range 45–74 years) at the time of first CTC and 61.8 years (range 51–78 years) at the time of the second CTC.
Cathartic regimes
With the exception of the primary cathartic agent being used, bowel preparation was identical between the two visits. Starting the day before the scheduled CTC examination, patients were restricted to a clear liquid diet and received two 5 mg bisacodyl tablets, which were taken before 11.00 am. Three to 6 h after the bisacodyl tablets, patients ingested either NaP (initial screen) or MgC (follow-up screen), respectively. The single-dose NaP regimen consisted of ingestion of 45 ml NaP solution (Phospho-soda, Fleet laboratories, Lynchburg, VA, USA). The double-dose MgC regimen consisted of two 296 ml bottles of MgC solution (Sun-Mark, San Francisco, CA, USA) separated by 3 h. The evening before CTC, all patient groups were also given 250 ml of (2%) barium sulphate (Readi-Cat 2; Bracco, Princeton, NJ, USA) to tag residual solid stool and 60 ml of sodium diatrizoate/diatrizoate meglumine (Gastrografin; Bracco) to tag residual fluid. In terms of hydration, patients were instructed to take one glass (8 oz.) of a clear liquid with the bisacodyl (step 1), four to six glasses during steps 2 and 3 (cathartic and barium), and one glass with step 4 (Gastrografin).
Colonic distention and CT acquisition protocol
Automated CO2 delivery (PROTOCO2L, Bracco) was used to achieve colonic distention. Spasmolytics were not used. After equilibrium intraluminal pressure was achieved, immediate review of distention was conducted on scout views and 2D axial images with the patient in both supine and prone positions to ensure adequate insufflation of the colon. CT image acquisition was performed on eight-, 16- or 64-section multidetector CT machines (GE Healthcare, Waukesha, WI, USA). Acquisition parameters consisted of 1.25 mm collimation, 1 mm reconstruction interval, 120 kVp, and tube current modulation (noise index set at 50, 30–300 mA range) or 100 mAs.
Data collection and analysis
The total volume and mean attenuation of the residual colonic fluid for each patient were derived using automated quality assessment (QA) software available on a beta version of the CTC software (V3D Colon; Viatronix, Stony Brook, NY, USA). Proper processing required correct centreline generation and colonic segmentation. However, areas of complete collapse were allowed as long as these segments contained no or negligible luminal fluid on visual inspection. The colon segmentation algorithm detects the gas-filled luminal cavity by initial thresholding. After visual confirmation and approval of correct segmentation, the QA software uses a region growing technique to delineate all tagged fluid regions connected to the gas-filled lumen by probing for seed pixels >200 HU along the gravitational direction with defined spatial range constraints. In a final step, the partial volume layer that forms between air and tagged fluid is determined. The processed total colonic lumen consisted of gas, tagged fluid, and partial volume layer regions. A median filtering technique was applied to the extracted regions to remove morphological irregularity caused by image noise and artefacts. Voxels in the colon lumen region <200 HU were labelled as air. Voxels in the colon lumen region ≥200 HU were labelled as tagged fluid. Gas volume, fluid volume, and average fluid attenuation values were calculated by counting the voxels with selected labels. This software algorithm has been validated by the manufacturer on two established phantoms used for their product validation (personal communication, D. Chen). One was a colon physical phantom and the other was a colon mathematical phantom. According to the manufacturer, the accuracy for volume and attenuation measurement is within the range of 98%.
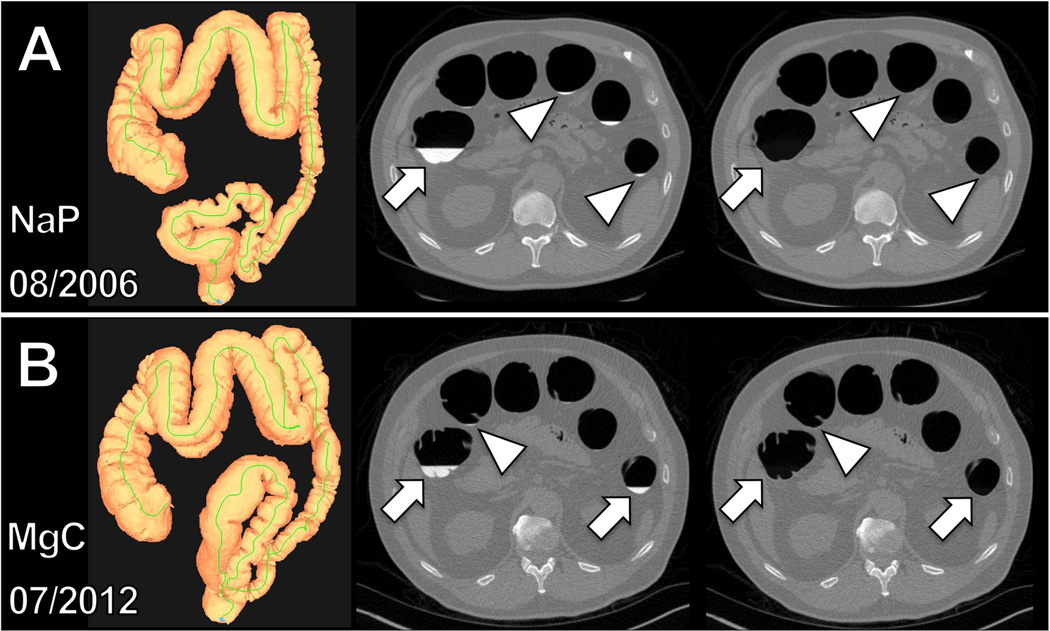
The luminal fluid volume and its average attenuation (CT attenuation number in HU) were recorded. The efficiency of the automated fluid detection was verified online on axial 2D CTC image review with and without digital subtraction of the tagged fluid (electronic cleansing). This step confirmed that all residual tagged colonic luminal fluid was captured by the automated volumetric tool (Fig. 1). As stated above, cases where the software did not properly segment all tagged luminal fluid were excluded.
Figure 1.
Intra-individual, objective comparison of volume and attenuation of residual colonic fluid at CTC following NaP and MgC regimen using automated QA software. (a) Three-dimensional map of the correctly segmented colon (left) following the NaP regimen in a 55-year-old man. Automatically calculated total colonic residual fluid and average attenuation were 111 ml and 979 HU, respectively. (b) Three-dimensional map of the correctly segmented colon (left) following MgC regimen in the same man 6 years later at the age of 61 years. Automatically calculated total colonic residual fluid and average attenuation were 139 ml and 719 HU, respectively. Two-dimensional axial CTC images before (middle) and after (right) automated digital subtraction of residual colonic fluid allowed verification of correct capture. Note that not only large fluid collections (arrows) are captured but also smallest fluid residues (arrowheads).
Statistical analyses
Continuous data were expressed as means±1 SD or 95% confidence intervals (CI). For categorical data, proportions and 95% adjusted Wald CIs were obtained (24). The paired t-test was used for intra-individual comparison of fluid volumes and fluid attenuations between the NaP and MgC regimens. McNemar’s test was performed to compare the total numbers of optimal versus suboptimal NaP und MgC preparations regarding the total volume and/or attenuation of residual fluid. Total residual colonic fluid volumes <200 ml were defined as excellent based on a recent study comparing four different cathartic agents (25). Regarding colonic fluid attenuation, mean values >300 HU were defined as insufficient tagging of residual fluid (12) and mean values >900 HU were defined as too high (13). Pearson’s correlation was obtained between volume and attenuation of residual colonic fluid. Statistical graphics and computations were done in R 3.0.2 (R Foundation for Statistical Computing, Vienna, Austria, http://www.r-project.org.) A p-value of <0.05 indicated a statistically significant difference.
RESULTS
No notable complications were encountered with either the NaP or MgC regimen in the 500 bowel preparations received by the 250 patients. The automated QA software successfully calculated the total residual fluid volumes (mean±SD, 149±107 ml; range 1–853 ml) and the mean fluid attenuation (789±183 HU, range 312–1154 HU) in all 500 CTC examinations. Following verification of colonic segmentation, the calculation time for automated fluid volume and attenuation determination ranged from approximately 5–15 s. Not only were large and obvious fluid collections captured, but also tiny pools of luminal fluid (Fig. 1).
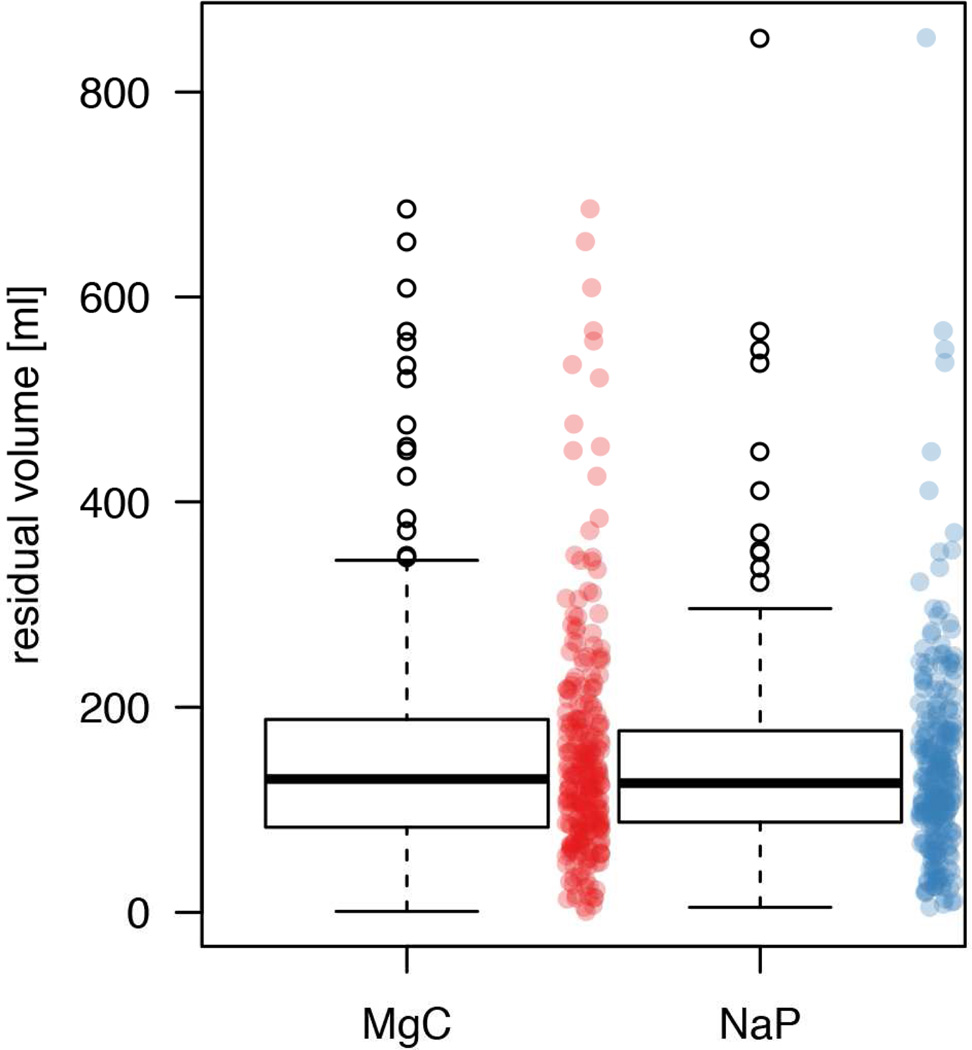
The intra-individual head-to-head comparison revealed that the NaP regimen resulted in less residual fluid in 141 of the 250 patients (56.4%, 95% CI: 50.2–62.4%) examinations, whereas MgC resulted in less residual fluid in the remaining 109 patients (43.6%, 95% CI: 37.6–49.8%; p=0.05). The overall mean residual fluid volume was statistically significantly higher for the MgC regimen (155±114 ml) than for the NaP regimen (143±100 ml; p=0.01; Fig. 2). The average difference between the total residual fluid volumes was only 12 ml (7.7%). In general, both regimes resulted predominantly in excellent bowel preparation, with volumes <200 ml in 204 (81.6%) of NaP cases and 192 (76.8%) MgC examinations (76.8%; p=0.11).
Figure 2.
Residual colonic fluid volume following the NaP regimen versus the MgC regimen in all 250 patients. Box and whisker plots show the median (thick line) and the box spans the interquartile range (IQR; Q1 to Q3); the whiskers extend to the most extreme observation within 1.5 times the IQR, and observations beyond that range are plotted individually and could be considered as outliers. To give a better idea of the distribution of all individual measurements, these are also shown (red for MgC and blue for NaP). Automatically quantified mean residual colonic fluid volume was higher for MgC regimen (155±114 ml) than for NaP regimen (143±100 ml) (p=0.011).
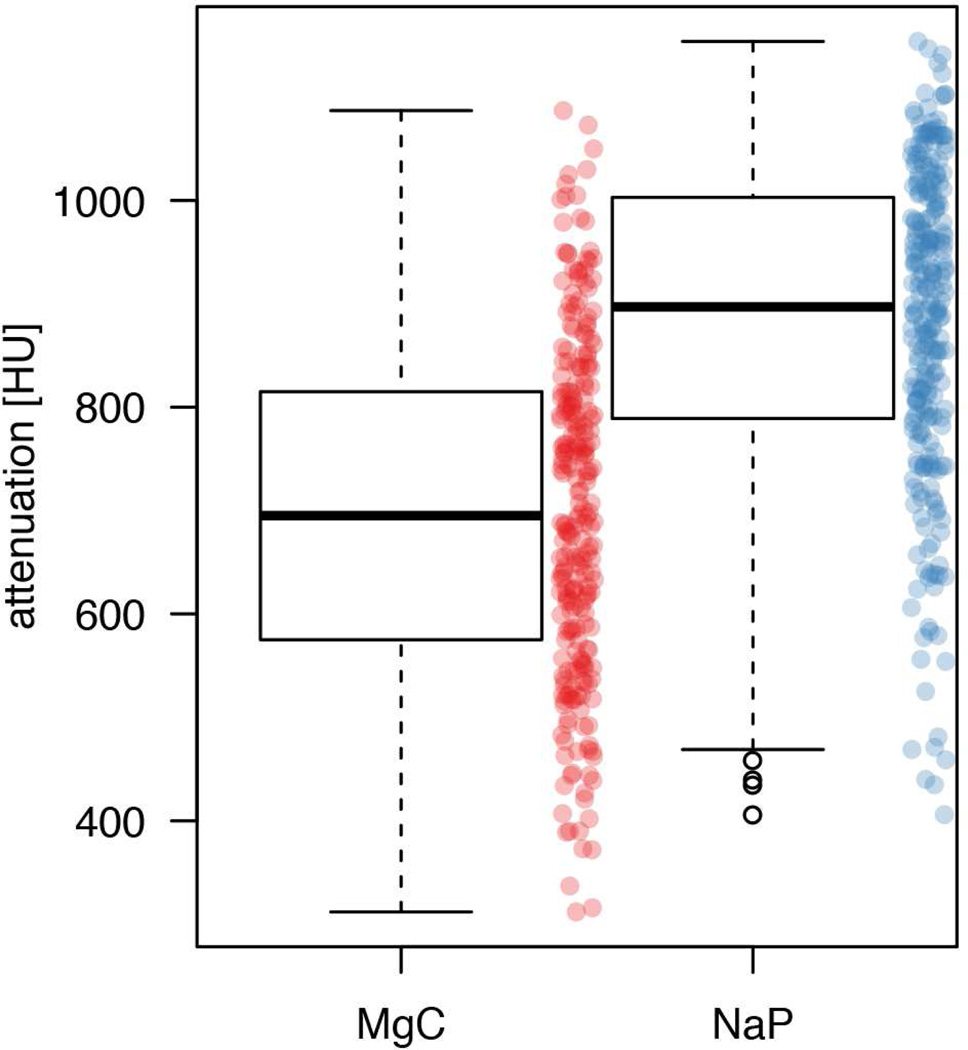
The intra-individual head-to-head comparison revealed that the NaP regimen resulted in higher fluid attenuation in 221 of the 250 patients (88.4%, 95% CI: 83.8–91.8%), whereas MgC resulted in higher fluid attenuation in the remaining 29 patients (11.6%, 95%CI: 8.2–16.2%; p<0.001). The overall mean fluid attenuation was significantly higher for the NaP regimen (878±155 HU) than the MgC regimen (700±165 HU; p<0.001; Fig. 3). The average difference between the mean residual fluid attenuations was 178 HU (20.3%). None of the examinations resulted in attenuation values <300 HU. The MgC regimen resulted in 220 examinations (88%) within the optimal attenuation range of 300–900 HU, whereas the NaP regimen resulted in 127 examinations (50.8%) within that optimal range (p<0.001). The remaining examinations (n=30 for MgC and n=123 for NaP) resulted in attenuation values >900 HU (range 902–1087 HU for MgC and 903–1154 HU for NaP).
Figure 3.
Residual colonic fluid attenuation following the NaP regimen versus the MgC regimen in all 250 patients. Box and whisker plots show the median (thick line) and the box spans the interquartile range (IQR; Q1 to Q3); the whiskers extend to the most extreme observation within 1.5 times the IQR, and observations beyond that range are plotted individually. To give a better idea of the distribution of all individual measurements, these are also shown (red for MgC and blue for NaP). Automatically quantified attenuation of residual colonic fluid was significantly lower for the MgC regimen (700±165 HU) than for the NaP regimen (878±155 HU; p<0.001).
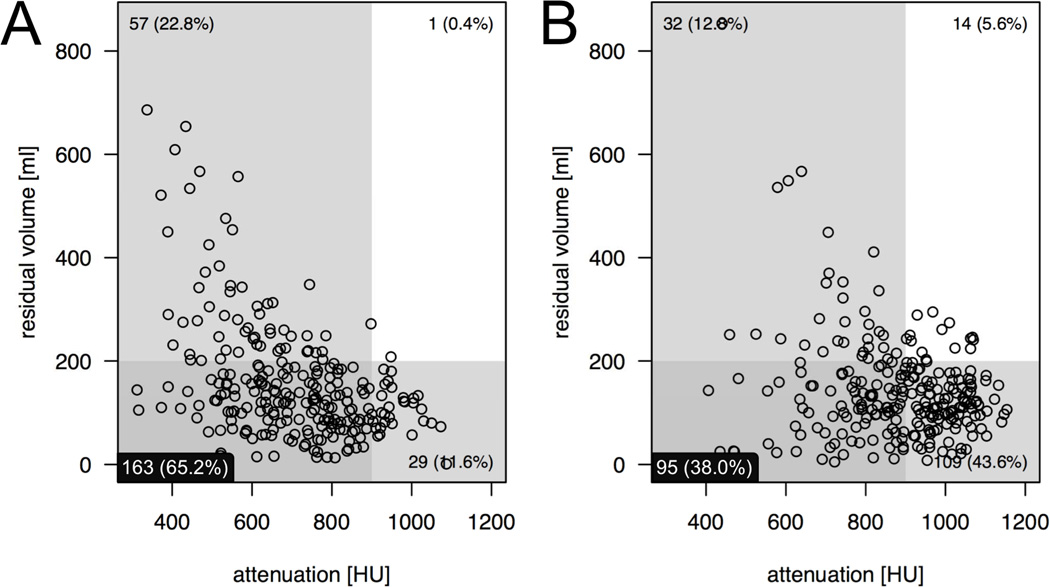
Use of the automated QA software revealed that the volume of residual fluid was negatively correlated with the attenuation of the residual fluid. That is, as residual fluid volume decreased, the degree of fluid attenuation increased. This negative relationship was stronger for the MgC regimen (r=−0.444, p<0.001) than the NaP regimen (r=−0.243, p<0.001). This relationship is illustrated in Fig. 4. The scatterplots also highlight the examinations that are simultaneously optimal in terms of both fluid volume (<200 ml) and attenuation (300–900 HU). The MgC regimen resulted in 163 examinations (65.2%) with minimal fluid and optimal attenuation, whereas NaP regimen resulted in 95 examinations (38%) within that optimal range (p<0.001).
Figure 4.
Scatter plots illustrate the examinations that are optimal in terms of both volume (<200 ml, shaded horizontal box) and attenuation (300–900 HU, shaded vertical box) in the lower left quadrant. Simultaneously optimal fluid volume and attenuation was observed following (a) the MgC regimen in 163 examinations (65.2%) and following (b) the NaP regimen in 95 examinations (38%; p<0.001). There is an inverse correlation between volume and attenuation of residual colonic fluid for both regimens. This negative relationship is stronger for MgC (r=−0.444, p<0.001) than for NaP (r=−0.243, p<0.001).
DISCUSSION
Using the automated volumetric QA software for objective intra-individual comparison showed that both the MgC and NaP regimens resulted in excellent bowel preparation in terms of residual fluid volume, whereas the MgC regimen resulted in more optimal attenuation values.
Minimizing and tagging the residual luminal fluid at CTC produces a higher-quality examination and is critical for achieving acceptable diagnostic performance (7, 8). The amount of residual fluid directly correlates with lesion detection at 3D endoluminal fly-through when electronic cleansing is not performed, which has long been the preferred standard. Major drawbacks to electronic cleansing include the creation of disturbing artefacts that can simulate true polyps and obscure true submerged polyps (10, 11). Evaluation of fluid-filled regions at 2D evaluation is time-consuming and onerous, leading to reader fatigue in a high-volume screening setting. Although the present study showed that the MgC regimen resulted in more residual colonic fluid than the NaP regimen, the actual difference was only 12 ml on average. Although MgC is associated with a statistically significant greater residual fluid volume, a difference of 12 ml is highly unlikely to amount to a clinically significant difference. Most examinations using either NaP (82%) or MgC (77%) resulted in acceptable residual fluid volumes <200 ml.
Using a semi-quantitative scoring system, Borden et al. (19) found that <25% of the colonic lumen was filled with residual fluid in 64% of colonic segments for NaP and in 61% of colonic segments for MgC. The semi-quantitative scoring system and segmental analysis make it difficult to compare with the present findings. Although a slight advantage for the NaP regimen was seen by both studies. However, the subjective semi-quantitative nature of their fluid analysis did not yield a statistically significant difference (p=0.3) (19), whereas the present objective quantitative volumetric comparison did reach statistical significance (p=0.01). Another strength of the present approach was the direct intra-individual comparison methodology.
Using the volumetric QA software tool also allowed for objective quantification of fluid attenuation, which should ideally be within the range 300–900 HU (12). Fluid attenuation values <300 HU are considered insufficient, as polyp conspicuity is reduced (12), whereas values >900 HU are considered excessive, as beam-hardening artefacts can occur at fluid–tissue interfaces and can also artificially diminish polyp size (13). This optimal attenuation range of 300–900 HU was observed significantly more often for the MgC regimen (71%) than for the NaP regimen (28%). Accordingly, the MgC regimen resulted on average in significantly lower attenuation values than the NaP regimen (701 versus 879 HU). This is in accordance with results from Borden et al. (19), who used a limited manual region of interest (ROI) approach and found mean attenuation of 790 HU for MgC and 978 HU for NaP. The offset of ~100 HU between the two studies for both MgC and NaP is likely due to the fact that Borden et al19 randomly sampled fluid only in the ascending and descending colon. In contrast, in the present study, automated QA software was used to calculate the average attenuation of the total fluid in all colonic segments.
The QA software tool used in the present study could be further developed and linked to the 2D view to provide automated setting of the optimal display window according to the actual fluid attenuation. A recent ex-vivo study by Slater et al.12 recommended changing the viewing windows contingent on the attenuation value of tagged fluid to ensure detection of submerged polyps. Using an automatically adjusted viewing window, adjusted to the attenuation of the residual fluid would maximize both detection and measurement accuracy of submerged polyps at 2D evaluation (13).
There have been previous efforts to provide automated assessment of colonic distention and/or residual fluid at CTC (23, 26, 27). However, those attempts provided primarily linear diameter assessment in defined colonic segments and not a true volumetric analysis of the entire colon. As for manual semi-quantitative subjective studies assessing residual fluid (8, 14, 15, 19, 28), the percentage of colonic cross-sectional surface area covered by fluid was used, a value that is highly dependent on luminal distension. The present automatically calculated volumetric data are objective, more robust, less error prone, and more sensitive to change compared with random samples and/or linear measures. Therefore, the automated volumetric QA software tool presented represents an effective means for comparing the performance of different bowel preparation regimes at CTC.
To put the present findings into a clinical context, the results support the use of automated QA software for quality assessment of CTC. The QA software utilized in the present study could be used to compare data sets from different institutions and from different validation trials. This would ensure a reasonable level of uniformity of bowel preparations, or alternatively, highlight deficiencies of a given CTC screening programme or method.
The authors acknowledge that there are limitations to the present study. An inherent limitation of the intra-individual comparison approach was that the effect of fluid volume and attenuation upon actual lesion detection could not be assessed, as the cohort in the present study had no relevant polyps (i.e., ≥6 mm) detected during their initial CTC examination. As only 10–15% of CTC screening examinations will have relevant polyps detected, far larger study cohorts would be needed to reliably detect a statistically significant difference. Another limitation of the automated QA software is that it does not provide segmental data and cannot identify and pinpoint areas that are submerged. However, the software could potentially be developed further and provide volumetric data according to individual colonic segments. Currently, it rapidly and objectively provides total colonic residual fluid volume analysis. The automated method was not directly compared with the subjective reading of residual fluid volume and attenuation, because these methods are prone to sampling and other errors as discussed above.
An inherent limitation of the QA software employed herein is that it does not allow for scoring of residual adherent tagged solid stool. The amount of residual adherent solid stool is another important factor in assessing the fidelity of bowel preparation at CTC, as its presence complicates 3D polyp detection (10, 29, 30). The QA software tool would need to be adapted further for assessment of residual adherent stool. Hitherto, manual scoring by Borden et al19 did not reveal a significant difference between MgC and NaP in terms of residual adherent stool (p=0.8) (19).
In summary, objective intra-individual comparison using automated volumetric analysis suggests that the replacement of NaP by MgC as the front-line laxative for CTC has not compromised overall examination quality.
Highlights.
Automated volumetric analysis provides objective CTC quality assessment
Automated analysis allows for rapid comparison between different bowel regimens
MgC results in higher residual colonic fluid volume (155 ml) than NaP (143 ml)
MgC results in lower attenuation of residual fluid (700 HU) than NaP (878 HU)
Presence of both optimal volume and attenuation is more frequent for MgC than for NaP
ACKNOWLEDGMENTS
Grant Support: This research was supported in part by the National Institutes of Health NCI grants 1R01CA144835-01 and 1R01CA169331-01
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
| 1 guarantor of integrity of the entire study | PJP |
| 2 study concepts and design | PJP |
| 3 literature research | PJP, PB |
| 4 clinical studies | N/A |
| 5 experimental studies / data analysis | PB, JB, PJP |
| 6 statistical analysis | PB, AMR |
| 7 manuscript preparation | PB, PJP |
| 8 manuscript editing | PB, JB, AMR, PJP |
REFERENCES
- 1.Kim DH, Pickhardt PJ, Taylor AJ, et al. CT colonography versus colonoscopy for the detection of advanced neoplasia. N Engl J Med. 2007;357:1403–1412. doi: 10.1056/NEJMoa070543. [DOI] [PubMed] [Google Scholar]
- 2.Pickhardt PJ, Hassan C, Laghi A, Zullo A, Kim DH, Morini S. Cost-effectiveness of colorectal cancer screening with computed tomography colonography — the impact of not reporting diminutive lesions. Cancer. 2007;109:2213–2221. doi: 10.1002/cncr.22668. [DOI] [PubMed] [Google Scholar]
- 3.Hassan C, Pickhardt P, Laghi A, et al. Computed tomographic colonography to screen for colorectal cancer, extracolonic cancer, and aortic aneurysm. Arch Intern Med. 2008;168:696–705. doi: 10.1001/archinte.168.7.696. [DOI] [PubMed] [Google Scholar]
- 4.Pickhardt PJ, Choi JR, Hwang I, et al. Computed tomographic virtual colonoscopy to screen for colorectal neoplasia in asymptomatic adults. N Engl J Med. 2003;349:2191–2200. doi: 10.1056/NEJMoa031618. [DOI] [PubMed] [Google Scholar]
- 5.Johnson CD, Chen MH, Toledano AY, et al. Accuracy of CT colonography for detection of large adenomas and cancers. N Engl J Med. 2008;359:1207–1217. doi: 10.1056/NEJMoa0800996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Graser A, Stieber P, Nagel D, et al. Comparison of CT colonography, colonoscopy, sigmoidoscopy and faecal occult blood tests for the detection of advanced adenoma in an average risk population. Gut. 2009;58:241–248. doi: 10.1136/gut.2008.156448. [DOI] [PubMed] [Google Scholar]
- 7.Pickhardt PJ, Kim DH. CT Colonography: Principles And Practice Of Virtual Colonoscopy. Philadelphia: Saunders; 2010. [Google Scholar]
- 8.Macari M, Lavelle M, Pedrosa I, et al. Effect of different bowel preparations on residual fluid at CT colonography. Radiology. 2001;218:274–277. doi: 10.1148/radiology.218.1.r01ja31274. [DOI] [PubMed] [Google Scholar]
- 9.Pickhardt PJ, Lee AD, Taylor AJ, et al. Primary 2D versus primary 3D polyp detection at screening CT Colonography. AJR Am J Roentgenol. 2007;189:1451–1456. doi: 10.2214/AJR.07.2291. [DOI] [PubMed] [Google Scholar]
- 10.Pickhardt PJ, Choi JHR. Electronic cleansing and stool tagging in CT colonography: advantages and pitfalls with primary three-dimensional evaluation. AJR Am J Roentgenol. 2003;181:799–805. doi: 10.2214/ajr.181.3.1810799. [DOI] [PubMed] [Google Scholar]
- 11.Pickhardt PJ. Screening CT colonography: how I do it. AJR Am J Roentgenol. 2007;189:290–298. doi: 10.2214/AJR.07.2136. [DOI] [PubMed] [Google Scholar]
- 12.Slater A, Taylor SA, Burling D, Gartner L, Scarth J, Halligan S. Colonic polyps: effect of attenuation of tagged fluid and viewing window on conspicuity and measurement—in vitro experiment with porcine colonic specimen. Radiology. 2006;240:101–109. doi: 10.1148/radiol.2401050984. [DOI] [PubMed] [Google Scholar]
- 13.Zalis ME, Perumpillichira JJ, Kim JY, Del Frate C, Magee C, Hahn PF. Polyp size at CT colonography after electronic subtraction cleansing in an anthropomorphic colon phantom. Radiology. 2005;236:118–124. doi: 10.1148/radiol.2361040231. [DOI] [PubMed] [Google Scholar]
- 14.Kim DH, Pickhardt PJ, Hinshaw JL, Taylor AJ, Mukherjee R, Pfau PR. Prospective blinded trial comparing 45 ml and 90 ml doses of oral sodium phosphate for bowel preparation before computed tomographic colonography. J Comp Assist Tomogr. 2007;31:53–58. doi: 10.1097/01.rct.0000230003.61392.2b. [DOI] [PubMed] [Google Scholar]
- 15.Berkelhammer C, Ekambaram A, Silva RG. Low-volume oral colonoscopy bowel preparation: sodium phosphate and magnesium citrate. Gastrointest Endosc. 2002;56:89–94. doi: 10.1067/mge.2002.125361. [DOI] [PubMed] [Google Scholar]
- 16.Markowitz GS, Stokes MB, Radhakrishnan J, D'Agati VD. Acute phosphate nephropathy following oral sodium phosphate bowel purgative: an underrecognized cause of chronic renal failure. J Am Soc Nephrol. 2005;16:3389–3396. doi: 10.1681/ASN.2005050496. [DOI] [PubMed] [Google Scholar]
- 17.Nyberg C, Hendel J, Nielsen OH. The safety of osmotically acting cathartics in colonic cleansing. Nat Rev Gastroenterol Hepatol. 2010;7:557–564. doi: 10.1038/nrgastro.2010.136. [DOI] [PubMed] [Google Scholar]
- 18.Lien YH. Is bowel preparation before colonoscopy a risky business for the kidney? Nat Clin Pract Nephrol. 2008;4:606–614. doi: 10.1038/ncpneph0939. [DOI] [PubMed] [Google Scholar]
- 19.Borden ZS, Pickhardt PJ, Kim DH, Lubner MG, Agriantonis DJ, Hinshaw JL. Bowel preparation for CT colonography: blinded comparison of magnesium citrate and sodium phosphate for catharsis. Radiology. 2010;254:138–144. doi: 10.1148/radiol.09090398. [DOI] [PubMed] [Google Scholar]
- 20.Michel SJ, Pickhardt PJ, Kim DH, Taylor AJ. Effect of colonic distention on superiority of supine versus prone views in screening computed tomographic colonography. Clin Imaging. 2007;31:325–328. doi: 10.1016/j.clinimag.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 21.Burling D, Taylor SA, Halligan S, et al. Automated insufflation of carbon dioxide for MDCT colonography: distension and patient experience compared with manual insufflation. AJR Am J Roentgenol. 2006;186:96–103. doi: 10.2214/AJR.04.1506. [DOI] [PubMed] [Google Scholar]
- 22.Shinners TJ, Pickhardt PJ, Taylor AJ, Jones DA, Olsen CH. Patient-controlled room air insufflation versus automated carbon dioxide delivery for CT colonography. AJR Am J Roentgenol. 2006;186:1491–1496. doi: 10.2214/AJR.05.0416. [DOI] [PubMed] [Google Scholar]
- 23.Hung PW, Paik DS, Napel S, et al. Quantification of distention in CT colonography: development and validation of three computer algorithms. Radiology. 2002;222:543–554. doi: 10.1148/radiol.2222010600. [DOI] [PubMed] [Google Scholar]
- 24.Agresti A, Coull BA. Approximate is better than "exact" for interval estimation of binomial proportions. Am Stat. 1998;52:119–126. [Google Scholar]
- 25.Bannas P, Bakke J, Patrick JL, Pickhardt PJ. Automated volumetric analysis for comparison of oral sulfate solution (SUPREP) with established cathartic agents at CT colonography. Abdom Imaging. 2014 Jun 26; doi: 10.1007/s00261-014-0186-x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deshpande KK, Summers RM, Van Uitert RL, et al. Quality assessment for CT colonography: Validation of automated measurement of colonic distention and residual fluid. AJR Am J Roentgenol. 2007;189:1457–1463. doi: 10.2214/AJR.07.2327. [DOI] [PubMed] [Google Scholar]
- 27.Van Uitert RL, Summers RM, White JM, Deshpande KK, Choi JR, Pickhardt PJ. Temporal and multiinstitutional quality assessment of CT colonography. AJR Am J Roentgenol. 2008;191:1503–1508. doi: 10.2214/AJR.07.3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keedy AW, Yee J, Aslam R, et al. Reduced cathartic bowel preparation for CT colonography: prospective comparison of 2 l polyethylene glycol and magnesium citrate. Radiology. 2011;261:156–164. doi: 10.1148/radiol.11110217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park SH, Ha HK, Kim MJ, et al. False-negative results at multi-detector row CT colonography: multivariate analysis of causes for missed lesions. Radiology. 2005;235:495–502. doi: 10.1148/radiol.2352040606. [DOI] [PubMed] [Google Scholar]
- 30.Pickhardt PJ, Kim DH. CT colonography: pitfalls in interpretation. Radiol Clin North Am. 2013;51:69–88. doi: 10.1016/j.rcl.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]