Abstract
Objectives:
The presence of neck metastases represents one of the most important prognostic factors for carcinomas of the anterior tongue, the five-year survival rate being under 20% in patients with regional metastases. The aim of this study was to demonstrate the efficacy of prophylactic selective neck dissection in patients without detectable nodal metastases.
Materials and Methods:
A matched case-control study with prospective follow up was conducted in ENT Department of Coltea Clinical Hospital for 86 patients with T1-T2N0 stage carcinoma of the anterior tongue surgically treated between January 2000 and January 2005 with or without concurrent selective supraomohyoid neck dissection (SND). The patients were divided in two groups, comparable in age and sex distribution. Descriptive statistics, risk of recurrences, Kaplan Maier five-year survival curves and the global and specific mortality rates were performed using EpiInfo software. The level of significance was established at p<0.05.
Results:
After a mean follow-up time of 90.5 months, for all variables considered as outcomes of SND efficacy evaluation, significance differences (p < 0.05) were registered between groups: the frequency of patients who developed neck metastases was lower in the group of subjects who underwent prophylactic selective neck dissection; the all-cause mortality rate at the end of the follow-up period was three times lower in SND study group compared with controls; the specific mortality rate due to regional recurrences was five times lower in test-group compared with controls.
Conclusions:
Our study suggest that prophylactic selective neck dissection could be indicated for patients with T1-T2N0 carcinomas of the anterior tongue in order to increase both overall and free of recurrence survival time, respectivelly.
Keywords: Anterior tongue carcinoma, N0 neck, neck dissection
Introduction
The presence of neck metastases represents the most important prognostic factor for carcinomas of the anterior tongue¹. The survival rate is 50% lower in patients with neck metastases than in patients without neck metastases, the five-year survival rate being under 20% in this category of patients².
The management of cervical lymph nodes in N0 patients is extremely controversial. The results of the numerous studies trying to reach a therapeutic consensus are contradictory, similar results being reported only in a small number of trials3-6.
The management of N0 patients consists of one of the following options: watchful waiting, followed by neck dissection when regional metastases are detected, or prophylactic selective neck dissection with histopathological examination of the removed specimen.
Each of these methods has pro and con arguments, although most authors acknowledge that prophylactic selective neck dissection has an important diagnostic and therapeutic role. The existence of a non-invasive diagnostic method with a high sensitivity and specificity for the detection of cervical lymph node metastases would probably simplify the decision regarding the best therapeutic strategy for N0 patients.
Clinical examination (palpation) has a sensitivity of 75% and a specificity of 81% in detecting affected lymph nodes7, the reported lower limit of palpability being 0.5 cm in superficial areas and 1 cm in deeper areas8. Stuckensen et al have compared the efficiency of positron-emission tomography (PET), ultrasonography, computed tomography (CT) and magnetic resonance imaging (MRI) in the detection of cervical lymph node metastases. They found that ultrasonography demonstrated the best sensitivity, whereas PET showed the best specificity. They also found that a clinical examination done in conjunction with a CT scan raised the detection rate of neck metastases from 75% to 91%9.
There are also a number of pro arguments for watchful waiting: a rise in local morbidity after neck dissection and regional metastases can be easily detected in compliant and well-monitored patients. Moreover, occult metastases are detected in approximately 35% of all N0 patients10,11, so one can conclude that neck dissection would represent an unnecessary surgical procedure in 2/3 of all N0 patients.
A meta-analysis of multiple randomized studies done by Weiss et al showed that watchful waiting is preferable to surgery when the probability of occult metastasis is under 20%, a situation which is commonly encountered in T1 or T2 anterior carcinomas of the tongue with a tumor girth of less than 4 mm12.
One of the most important pro arguments for prophylactic neck dissection is that it can be useful for better neck staging. Andersen et al have shown that 77% of all N0 patients had regional lymph node metastases after histopathological analysis of the excised specimen. 49% of these patients had extracapsular dissemination, which is an important negative prognostic factor13. This category of patients could benefit much earlier from a number of more aggressive therapeutic strategies (such as external cervical radiotherapy).
Material and Methods
This study comprised of 86 patients with T1N0M0 and T2N0M0 carcinomas of the anterior tongue, who were diagnosed between January 2000 and January 2005 and managed by a multidisciplinary team of experts.
The following inclusion criteria were adopted: patients with a newly-diagnosed and histopathologically confirmed carcinoma of the anterior tongue, patients with this diagnosis who had never received treatment, patients who presented with a single malignant tumor at the time of diagnosis, patients without distant metastases at the time of diagnosis, patients without local and/or general contraindications for surgery.
The following categories of patients were excluded from this study: patients who could not be monitored for the whole duration of the study, patients with distant metastases at the time of diagnosis.
The diagnostic protocol for all patients included a thorough clinical examination, panendoscopy for the exclusion of synchronous malignant tumors, cervical and thoracic CT and biopsy of the lingual tumor. Tumors were staged according to the criteria put forth in 1988 by the American Joint Committee on Cancer (AJCC) – Otorhinolaryngology - Head and Neck Surgery. Lymph nodes were staged by corroboration of the data obtained from clinical examination and cervical CT scans.
The therapeutic protocol consisted of: surgery for the primary tumor (partial glossectomies or hemiglossectomies), selective neck dissection (SND) levels I, II and III in some of the N0 patients. We preferred this type of selective neck dissection because most cervical neck metastases from anterior lingual carcinomas can be found at levels Ib and IIa of lymphatic drainage. External beam radiotherapy of the neck was performed in patients with neck metastases proved by histopathological exams of the removed specimen. The total radiation dose was between 60 and 75 Gy.
The research was conducted as a matched case-control study, control to case ratio: 10 to 12. The test-group (SND+), 48 px, was obtained by two steps randomized selection from pool of patients with T1-T2N0 stage carcinoma of the anterior tongue surgically treated with concurrent prophylactic selective neck dissection; the sample size was calculated according to the number and distributions by age and sex of three years previous undergoing SND patients group (maximum acceptable margin of error: 5%; 95% confidence interval, minimum sample size necessary: 20 px). Thus the results could be applied to any patient having similar demographic characteristic as studied sample, being admitted in a clinic with similar standard of medical practice. The subjects of control group (SND-), 38 px, were enrolled from the pool of patients with T1-T2 N0 carcinoma of the anterior tongue surgically treated without prophylactic selective neck dissection, by stratified-random- selection to match the demographic characteristics of the test group. The allocation process was done by computer, from January 2000 to January 2005; the patients were prospectively followed until the final analysis point in 2010. The follow-up time ranged from 24 to 120 months (mean of 96, 4 months for SND+, and 82, 8 months for SND-, respectively).
For both groups results were presented as means and standard deviation for continuous variables and were compared using Student’s t-test; categorical variables were described by absolute or relative frequency and compared by chi-square or Fisher’s exact test. The level of significance was established at p < 0.05. Kaplan-Meier survival analysis was performed at 5 years of follow-up. Differences in time to death between goups were estimated using two-sided log rank test. Global and specific mortality rates were calculated at the end of the follow-up by dividing the number of deaths to the number of the interest subjects group.
Results
Eighty six patients with stage T1-T2 N0 malignant tumors of the anterior tongue participated in this study, 69 (80.23%) of whom were male and 17 (19.76%) of whom were female, aged between 35 and 73 years. The patients were enrolled in two comparable (p > 0.05) by age and sex distribution groups (Table 1).
Table 1. Descriptive statistics regarding age, for the two groups that underwent (SND+) or not (SND-) selective neck dissection (in years).

SND+: selective neck dissection group, SND-: watchful waiting group (clinical observation of the neck).
Resection of the primary tumor and unilateral selective neck dissection levels I, II and III was performed in 48 patients (55.81%), and we named this group “selective neck dissection group” (SND+). In the other 38 patients (44.18%) included in this study, resection of the primary tumor was followed by clinical observation of the neck, and we named this group “watchful waiting group” (SND-). The patients included in the second group were examined at every 3 months and radical or modified radical neck dissection was performed when neck metastases were detected. The diagnosis of cervical metastases was made by clinical examination (palpation) and confirmed by CT scans.
Neck irradiation was carried out in every patient with positive cervical lymph nodes - i.e. in patients with a positive histopathological diagnosis of neck metastasis after SND (“false N0 patients”) and in patients from the watchful waiting group who developed clinically positive nodes, and were subsequently dissected by radical or modified radical neck dissection. The received radiation dose was between 60 and 75 Gy.
Histopathological examination of the removed tissue in patients from the SND+ group demonstrated the presence of metastasis in 4 of the 20 patients with T1 tumors and in 9 of the 28 patients with T2 tumors. This means that 20% of patients with T1 tumors and 32.14% of patients with T2 tumors had been falsely evaluated as N0 at the time of diagnosis.
At the end of the study period, 41 patients from the SND group (85.41%) and 23 patients from the watchful waiting group (62,16%) were alive and there is a statistically significant difference between groups (p <0.001)(Table 2). Also, in SND group, we found fewer regional recurrences, lower global and specific death rates, as well as an increase in the median survival time. In SND group, there were only 8 regional recurrences compared to 18 regional recurrences in the watchful waiting group. The frequency of regional recurrences was therefore significantly lower in the SND group (p<0.05), and the risk of regional recurrences was five times greater in patients from the watchful waiting group (Table 3).
Table 2. The number and distribution of alive patients at the end of the study period.
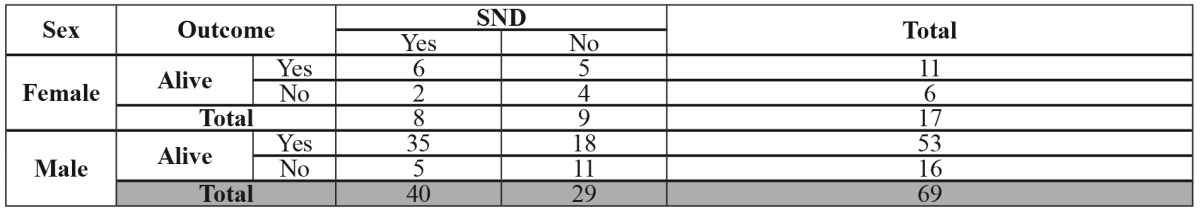
SND: selective neck dissection.
Table 3. Risks of recurrence according to patient submission to selective neck dissection or not.
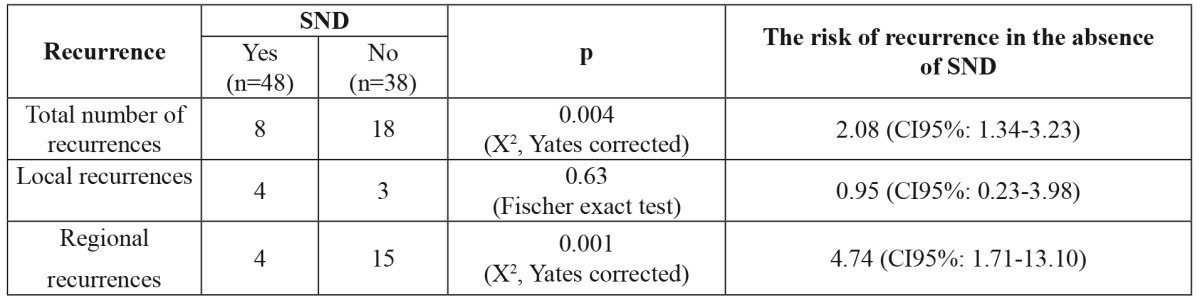
SND: selective neck dissection.
A total of 3 cases of metachronous malignant tumors with a different localization were noted during this study in two patients from the SND group and in one patient from the watchful waiting group. The second malignant tumor was located in lungs (2 cases) and to the left vocal fold (1 case). These 3 patients were heavy smokers and they did not receive external radiotherapy. We can conclude that smoking was the major factor that caused the second malignant tumor (the field cancerization theory). The second tumor was diagnosed after 8- 26 months.
The total number of deaths recorded throughout this study was 22, including 7 patients from the SND group (14.6%) and 15 patients from the watchful waiting group (39.5%). The analysis of deaths registered at the end of the follow-up reveal the influence of SND in the decrease of mortality: global and specific deaths rates were significantly lower in SND+ group (p=0.05). The main cause of death in both groups was related to regional recurrences. The specific death rate (related to regional recurrences) was 6.25% in SND+ group, statistically significant lower (p<0.05) than 31.6% registered in SND- group (Table 4). No statistically significant differences were noted for local recurrences related deaths.
Table 4. Global and specific death rate at the end of study according to patient submission to selective neck dissection or not.
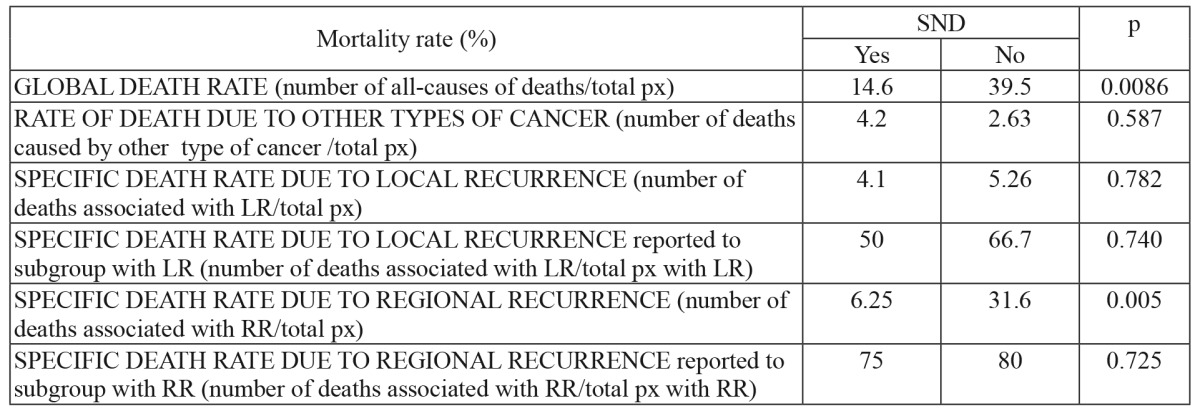
SND: selective neck dissection, px: number of patients, LR: local recurrence, RR: regional recurrence.
The median survival time at the end of the study is higher in patients from the SND group, compared to that of patients from the watchful waiting group (Table 5).
Table 5. Impact of prophylactic selective neck dissection, on the survival time registered at the end of the monitoring period (in years).
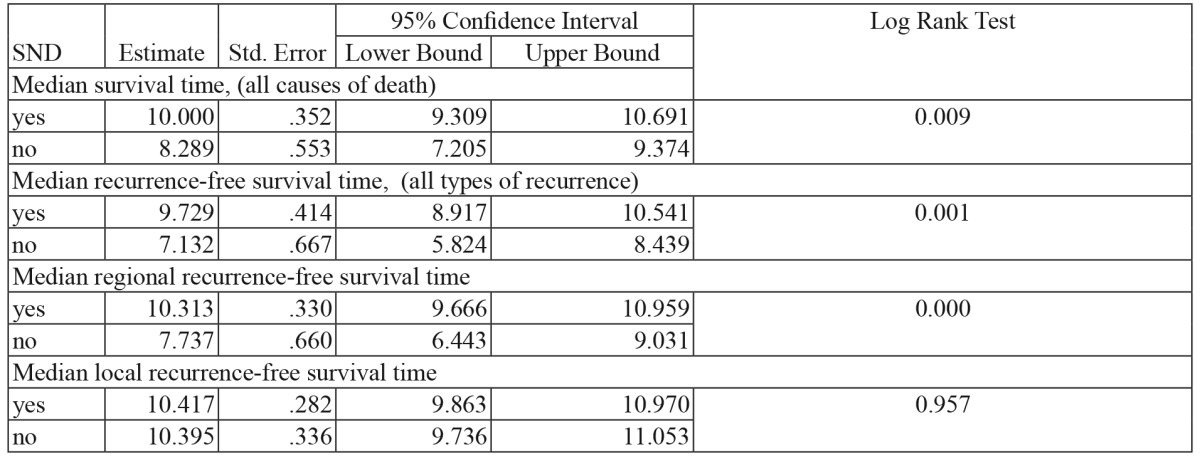
SND: selective neck dissection. Significant differences between SND yes/no group for Log Rank Test <0.05.
Discussion
Obtaining adequate regional control represents a great challenge for surgical oncologists due to its crucial influence on the patients’ survival rate. However, specific therapeutic protocols which facilitate regional control are often controversial.
The present study tries to demonstrate the efficacy of prophylactic selective neck dissection in patients with carcinoma of the anterior tongue with a clinically negative neck. A very important aspect is represented by the presence of occult metastases or false-negative nodes. The number of occult cervical metastases is directly-proportional to the tumor stage. In the present study, occult metastases were found in 20% of patients with T1 tumors and in 32.14% of patients with T2 tumors. The treatment of these patients was completed with supplementary irradiation of the neck. Prophylactic neck dissection is therefore necessary for the identification and subsequent adequate treatment of patients with false-negative cervical lymph nodes. Other studies have published similar data, demonstrating that prophylactic lymphadenectomy is effective because it leads to more accurate staging14,15. In another study, approximately 25% of all clinically occult metastases seemed to be too small to be detected by any imaging technique available16. Occult cervical metasases were found in patients with T1N0 and T2N0 squamous cell carcinoma of the oral cavity in 13-33% and 37-53% respectively at the time of diagnosis13,17,18.
The rate of regional recurrences is lower in patients with prophylactic SND (8.3%) compared to the much higher rate found in the watchful waiting group (39.47%). So, the regional control is better obtained in patients with selective neck dissection. The surgical treatment of regional recurrences after radiotherapy is often difficult, with high intraoperative risks and significant postoperative morbidity at the surgical site.
Regional recurrences were also the main cause of death in this patient group, namely in 6.25% of the patients from the lymphadenectomy group and 31.6% of the patients in the watchful waiting group.
The efficiency of prophylactic SND is also supported by the fact that the mean survival rate of the patients in the lymphadenectomy group (9.7 years) was superior to that of the patients in the watchful waiting group (7.13 years).
Several studies have demonstrated that prophylactic SND is efficient in decreasing the regional recurrence rate in patients with low-stage carcinoma of the anterior tongue, but does not necessarily prolong the life of these patients19-23. Kligerman et al have shown that prophylactic lymphadenectomy has a beneficial effect not only on the rate of regional recurrences, but also on the survival rate of patients with T1-T2 malignant tumors of the anterior tongue2. Yuen et al, demonstrated that patients who have been subjected to prophylactic lymphadenectomy benefit from a lower local recurrence rate and, consequently, from a reduction in the mortality associated with such local recurrences24.
On the other hand, regional recurrence is more correlated with tumor thickness, considered the only independent predictor of neck failure25-28. Selective neck dissection is indicated only in T1N0 carcinoma of the anterior tongue patients with tumor depth greater than 3 mm29.
Conclusion
Selective neck dissection levels I, II and III could be necessary in patients with T1-T2 N0 carcinomas of the anterior tongue in order to identify the occult metastases and in obtain a better regional control. This is reflected by the improved survival time of SND+ patients, as the most frequent cause of death in this patient group is related to the presence of regional recurrences.
Conflict of interest
Authors declare that no funding was received for this research and therefore there is no conflict of interest.
Acknowledgement
The authors thank to Stroe Felicia from The National Health Institute Bucharest for the statistical data processing.
References
- 1.Shah JP. Patterns of cervical lymph nodes metastasis from squamous carcinomas of the upper aerodigestive tract. Am J Surg. 1990;160:405–409. doi: 10.1016/s0002-9610(05)80554-9. [DOI] [PubMed] [Google Scholar]
- 2.Kligerman J, Lima RA, Soares JR, Prado L, Dias F, Freitas E, et al. Supraomohyoid neck dissection in the treatment of T1/T2 squamous cell carcinoma of oral cavity. Am J Surg. 1994;168:391–394. doi: 10.1016/s0002-9610(05)80082-0. [DOI] [PubMed] [Google Scholar]
- 3.Duvvuri U, Simental AA Jr, D'Angelo G, Johnson JT, Ferris RL, Gooding W, et al. Elective neck dissection and survival in patients with squamous cell carcinoma of the oral cavity and oropharynx. Laryngoscope. 2004;114:2228–2234. doi: 10.1097/01.mlg.0000149464.73080.20. [DOI] [PubMed] [Google Scholar]
- 4.Dias FL, Kligerman J, Matos de Sá G, Arcuri RA, Freitas EQ, Farias T, et al. Elective neck dissection versus observation in stage I squamous cell carcinomas of the tongue and floor of the mouth. Otolaryngol Head Neck Surg. 2001;125:23–29. doi: 10.1067/mhn.2001.116188. [DOI] [PubMed] [Google Scholar]
- 5.Keski-Säntti H, Atula T, Törnwall J, Koivunen P, Mäkitie A. Elective neck treatment versus observation in patients with T1/T2 N0 squamous cell carcinoma of oral cavity. Oral Oncol. 2006;42:96–101. doi: 10.1016/j.oraloncology.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 6.Yuen AP, Ho CM, Chow TL, Tang LC, Cheung WY, Ng RW, et al. Prospective randomized study of selective neck dissection versus observation for N0 neck of early tongue carcinoma. Head Neck. 2009;31:765–772. doi: 10.1002/hed.21033. [DOI] [PubMed] [Google Scholar]
- 7.Merritt RM, Williams MF, James TH, Porubsky ES. Detection of cervical metastasis. A meta-analysis comparing computer tomography with physical examination. Arch Otolaryngol Head and Neck Surg. 1997;123:149–152. doi: 10.1001/archotol.1997.01900020027004. [DOI] [PubMed] [Google Scholar]
- 8.Sako K, Pradier RN, Marchetta FC, Pickren JW. Fallibility of palpation in the diagnosis of metastases of cervical lymph nodes. Surg Gynecol Obstet. 1964;118:989–990. [PubMed] [Google Scholar]
- 9.Stuckensen T, Kovács AF, Adams S, Baum RP. Staging of the neck in patients with oral cavity squamous cell carcinomas: a prospective comparison of PET, ultrasound, CT and MRI. J Craniomaxillofac Surg. 2000;28:319–324. doi: 10.1054/jcms.2000.0172. [DOI] [PubMed] [Google Scholar]
- 10.Byers RM, El-Naggar AK, Lee YY, Rao B, Fornage B, Terry NH, et al. Can we detect or predict the presence of occult nodal metastases in patients with squamous cell carcinoma of the oral tongue? Head Neck. 1998;20:138–144. doi: 10.1002/(sici)1097-0347(199803)20:2<138::aid-hed7>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 11.Po Wing Yuen A, Lam KY, Lam LK, Ho CM, Wong A, Chow TL, et al. Prognostic factors of clinically stage I and II oral tongue carcinoma-A comparative study of stage, thickness, shape, growth pattern, invasive front malignancy grading, Martinez-Gimeno score, and pathologic features. Head Neck. 2002;24:513–520. doi: 10.1002/hed.10094. [DOI] [PubMed] [Google Scholar]
- 12.Weiss MH, Harrison LB, Isaacs RS. Use of decision analysis in planning a management strategy for the stage N0 neck. Arch Otolaryngol Head and Neck Surg. 1994;120:699–702. doi: 10.1001/archotol.1994.01880310005001. [DOI] [PubMed] [Google Scholar]
- 13.Andersen PE, Cambronero E, Shaha AR, Shah JP. The extent of neck disease after regional failure during observation of the N0 neck. Am J Surg. 1996;172:689–691. doi: 10.1016/s0002-9610(96)00290-5. [DOI] [PubMed] [Google Scholar]
- 14.Houck JR, Medina JE. Management of cervical lymph nodes in squamous carcinomas of the head and neck. Semin Surg Oncol. 1995;11:228–239. doi: 10.1002/ssu.2980110308. [DOI] [PubMed] [Google Scholar]
- 15.Rao RS. Deshmane VH, Parikh HK, Parikh DM, Sukthankar PS. Extent of lymph node dissection in T3/T4 cancer of the alveolo-buccal complex. Head Neck. 1995;17:199–203. doi: 10.1002/hed.2880170306. [DOI] [PubMed] [Google Scholar]
- 16.van den Brekel MW, Castelijns JA, Snow GB. Diagnostic evaluation of the neck. Otolaryngol Clin North Am. 1998;31:601–620. doi: 10.1016/s0030-6665(05)70075-x. [DOI] [PubMed] [Google Scholar]
- 17.Haddadin KJ, Soutar DS, Oliver RJ, Webster MH, Robertson AG, MacDonald DG. Improved survival for patients with clinically T1/T2, N0 tongue tumors undergoing a prophylactic neck dissection. Head Neck. 1999;21:517–525. doi: 10.1002/(sici)1097-0347(199909)21:6<517::aid-hed4>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 18.Teichgraeber JF, Clairmont AA. The incidence of occult metastases for cancer of the oral tongue and floor of the mouth: treatment rationale. Head Neck Surg. 1984;7:15–21. doi: 10.1002/hed.2890070105. [DOI] [PubMed] [Google Scholar]
- 19.Hughes CJ, Gallo O, Spiro RH, Shah JP. Management of occult neck metastases in oral cavity squamous cell carcinoma. Am J Surg. 1993;166:380–383. doi: 10.1016/s0002-9610(05)80337-x. [DOI] [PubMed] [Google Scholar]
- 20.O'Brien CJ, Urist MM. Current status of neck dissection in the management of squamous carcinoma of the head and neck. Aust N Z J Surg. 1987;57:501–509. doi: 10.1111/j.1445-2197.1987.tb01411.x. [DOI] [PubMed] [Google Scholar]
- 21.Franceschi D, Gupta R, Spiro RH, Shah JP. Improved survival in the treatment of squamous carcinoma of the oral tongue. Am J Surg. 1993;166:360–365. doi: 10.1016/s0002-9610(05)80333-2. [DOI] [PubMed] [Google Scholar]
- 22.McGuirt WF Jr, Johnson JT, Myers EN, Rothfield R, Wagner R. Floor of mouth carcinoma: the management of the clinically negative neck. Arch Otolaryngol Head Neck Surg. 1995;121:278–282. doi: 10.1001/archotol.1995.01890030020004. [DOI] [PubMed] [Google Scholar]
- 23.Ho CM, Lam KH, Wei WI, Lau SK, Lam LK. Occult node metastasis in small oral tongue cancers. Head Neck. 1992;14:359–363. doi: 10.1002/hed.2880140504. [DOI] [PubMed] [Google Scholar]
- 24.Yuen AP, Wei WI, Wong YM, Tang KC. Elective neck dissection versus observation in the treatment of early oral tongue carcinoma. Head Neck. 1997;19:583–588. doi: 10.1002/(sici)1097-0347(199710)19:7<583::aid-hed4>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 25.Ganly I, Goldstein D, Carlson DL, Patel SG, O’Sullivan B, Lee N, et al. Long term regional control and survival in patients with “low-risk” early stage oral tongue cancer managed by partial glossectomy and neck dissection without postoperative radiotherapy. Cancer. 2013;119:1168–1176. doi: 10.1002/cncr.27872. [DOI] [PubMed] [Google Scholar]
- 26.Sparano A, Weinstein G, Chalian A, Yodul M, Weber R. Multivariate predictors of occult neck metastasis in early oral tongue cancer. Otolaryngol Head Neck Surg. 2004;131:472–476. doi: 10.1016/j.otohns.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 27.Fukano H, Matsuura H, Hasegawa Y, Nakamura S. Depth of invasion as a predictive factor for cervical lymph node metastasis in tongue carcinoma. Head Neck. 1997;19:205–210. doi: 10.1002/(sici)1097-0347(199705)19:3<205::aid-hed7>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 28.O'Brien CJ, Lauer CS, Fredricks S, Clifford AR, McNeil EB, Bagia JS, et al. Tumor thickness influences prognosis of T1 and T2 oral cavity cancer--but what thickness? Head Neck. 2003;25:937–945. doi: 10.1002/hed.10324. [DOI] [PubMed] [Google Scholar]
- 29.Zhang T, Lubek J, Salama A, Dyalram D, Liu X, Ord RA. Treatment of cT1N0 tongue cancer: outcome and prognostic parameters. J Oral Maxillofac Surg. 2014;72:406–414. doi: 10.1016/j.joms.2013.05.028. [DOI] [PubMed] [Google Scholar]


