Abstract
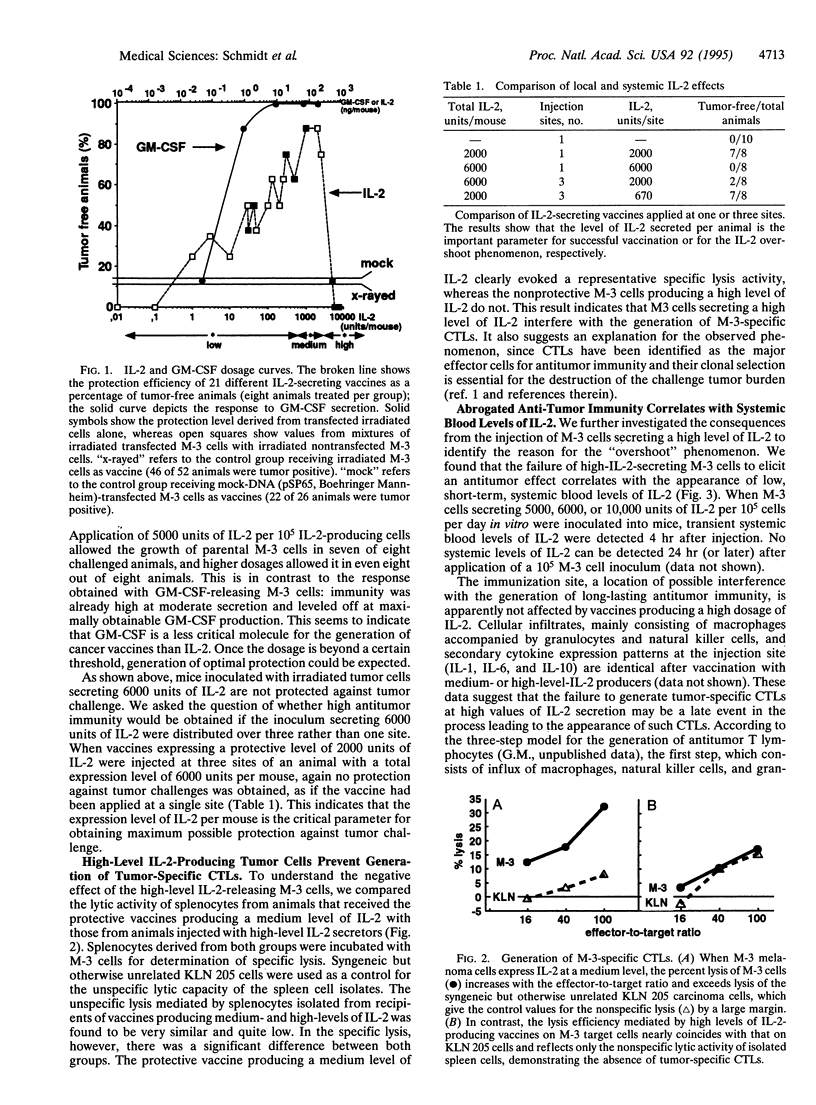
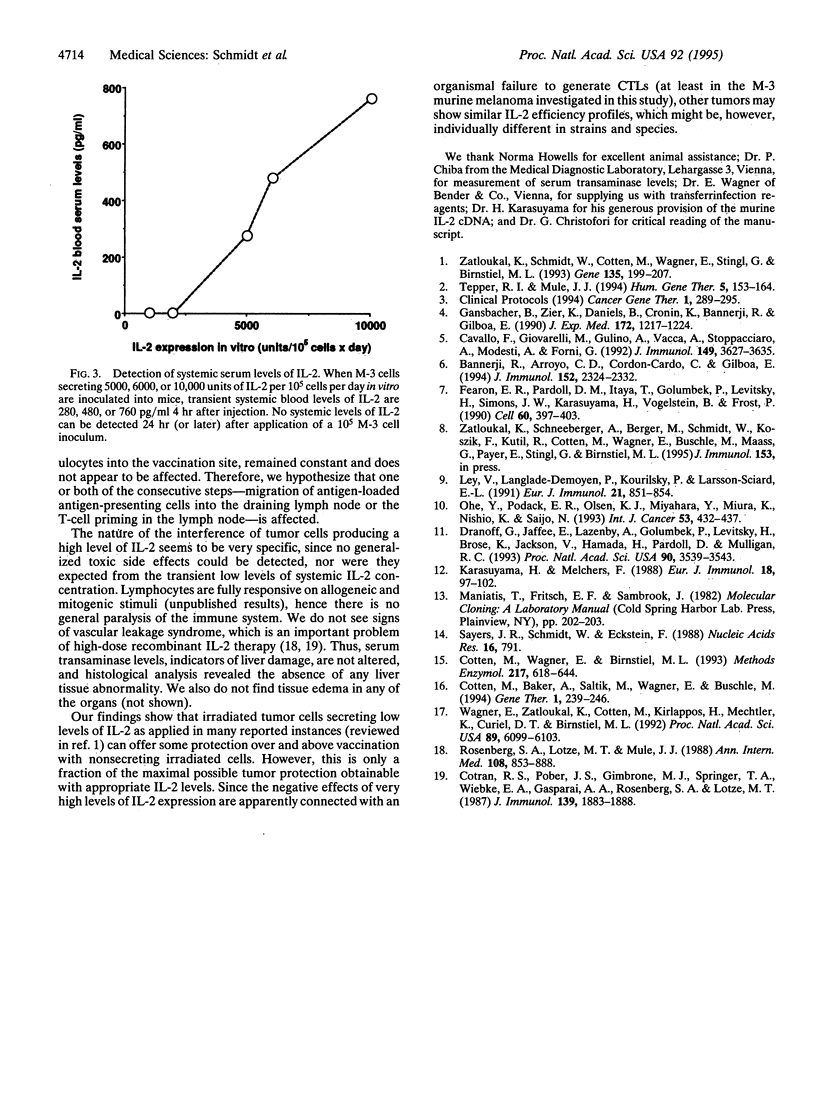
Cancer vaccines genetically engineered to produce interleukin 2 have been investigated intensively in a series of animal models and are at the point of entering into clinical trials. In this study we demonstrate a strong correlation between the rate of interleukin 2 production and the protection efficiency of murine S91 melanoma cell (clone M-3) vaccines. Best immunization is achieved with vaccines producing medium interleukin 2 levels of 1000-3000 units per 10(5) cells per day. Reduced interleukin 2 production evokes a corresponding decline in the number of successfully treated animals. Unexpectedly, when interleukin 2 expression is raised to high levels of 5000-7500 units per 10(5) cells per day, protection is completely absent because of impaired generation of tumor-specific cytotoxic T lymphocytes. In comparison, granulocyte-macrophage colony-stimulating factor as immunomodulator induces substantial immunization even at a moderate level of secretion and protects all animals at the maximal obtainable level of secretion. Our findings demonstrate the importance of the interleukin 2 level produced by genetically modified tumor cells and may have substantial impact for the clinical application of cancer vaccines.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bannerji R., Arroyo C. D., Cordon-Cardo C., Gilboa E. The role of IL-2 secreted from genetically modified tumor cells in the establishment of antitumor immunity. J Immunol. 1994 Mar 1;152(5):2324–2332. [PubMed] [Google Scholar]
- Cavallo F., Giovarelli M., Gulino A., Vacca A., Stoppacciaro A., Modesti A., Forni G. Role of neutrophils and CD4+ T lymphocytes in the primary and memory response to nonimmunogenic murine mammary adenocarcinoma made immunogenic by IL-2 gene. J Immunol. 1992 Dec 1;149(11):3627–3635. [PubMed] [Google Scholar]
- Cotten M., Baker A., Saltik M., Wagner E., Buschle M. Lipopolysaccharide is a frequent contaminant of plasmid DNA preparations and can be toxic to primary human cells in the presence of adenovirus. Gene Ther. 1994 Jul;1(4):239–246. [PubMed] [Google Scholar]
- Cotten M., Wagner E., Birnstiel M. L. Receptor-mediated transport of DNA into eukaryotic cells. Methods Enzymol. 1993;217:618–644. doi: 10.1016/0076-6879(93)17092-j. [DOI] [PubMed] [Google Scholar]
- Dranoff G., Jaffee E., Lazenby A., Golumbek P., Levitsky H., Brose K., Jackson V., Hamada H., Pardoll D., Mulligan R. C. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3539–3543. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon E. R., Pardoll D. M., Itaya T., Golumbek P., Levitsky H. I., Simons J. W., Karasuyama H., Vogelstein B., Frost P. Interleukin-2 production by tumor cells bypasses T helper function in the generation of an antitumor response. Cell. 1990 Feb 9;60(3):397–403. doi: 10.1016/0092-8674(90)90591-2. [DOI] [PubMed] [Google Scholar]
- Gansbacher B., Zier K., Daniels B., Cronin K., Bannerji R., Gilboa E. Interleukin 2 gene transfer into tumor cells abrogates tumorigenicity and induces protective immunity. J Exp Med. 1990 Oct 1;172(4):1217–1224. doi: 10.1084/jem.172.4.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasuyama H., Melchers F. Establishment of mouse cell lines which constitutively secrete large quantities of interleukin 2, 3, 4 or 5, using modified cDNA expression vectors. Eur J Immunol. 1988 Jan;18(1):97–104. doi: 10.1002/eji.1830180115. [DOI] [PubMed] [Google Scholar]
- Ley V., Langlade-Demoyen P., Kourilsky P., Larsson-Sciard E. L. Interleukin 2-dependent activation of tumor-specific cytotoxic T lymphocytes in vivo. Eur J Immunol. 1991 Mar;21(3):851–854. doi: 10.1002/eji.1830210350. [DOI] [PubMed] [Google Scholar]
- Ohe Y., Podack E. R., Olsen K. J., Miyahara Y., Ohira T., Miura K., Nishio K., Saijo N. Combination effect of vaccination with IL2 and IL4 cDNA transfected cells on the induction of a therapeutic immune response against Lewis lung carcinoma cells. Int J Cancer. 1993 Feb 1;53(3):432–437. doi: 10.1002/ijc.2910530314. [DOI] [PubMed] [Google Scholar]
- Rosenberg S. A., Lotze M. T., Mulé J. J. NIH conference. New approaches to the immunotherapy of cancer using interleukin-2. Ann Intern Med. 1988 Jun;108(6):853–864. doi: 10.7326/0003-4819-108-6-853. [DOI] [PubMed] [Google Scholar]
- Sayers J. R., Schmidt W., Eckstein F. 5'-3' exonucleases in phosphorothioate-based oligonucleotide-directed mutagenesis. Nucleic Acids Res. 1988 Feb 11;16(3):791–802. doi: 10.1093/nar/16.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper R. I., Mulé J. J. Experimental and clinical studies of cytokine gene-modified tumor cells. Hum Gene Ther. 1994 Feb;5(2):153–164. doi: 10.1089/hum.1994.5.2-153. [DOI] [PubMed] [Google Scholar]
- Wagner E., Zatloukal K., Cotten M., Kirlappos H., Mechtler K., Curiel D. T., Birnstiel M. L. Coupling of adenovirus to transferrin-polylysine/DNA complexes greatly enhances receptor-mediated gene delivery and expression of transfected genes. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):6099–6103. doi: 10.1073/pnas.89.13.6099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatloukal K., Schmidt W., Cotten M., Wagner E., Stingl G., Birnstiel M. L. Somatic gene therapy for cancer: the utility of transferrinfection in generating 'tumor vaccines'. Gene. 1993 Dec 15;135(1-2):199–207. doi: 10.1016/0378-1119(93)90066-c. [DOI] [PubMed] [Google Scholar]



