Abstract
Candida albicans is an important commensal of mucosal surfaces that is also an opportunistic pathogen. This organism colonizes a wide range of host sites that differ in pH; thus, it must respond appropriately to this environmental stress to survive. The ability to respond to neutral-to-alkaline pHs is governed in part by the RIM101 signal transduction pathway. Here we describe the analysis of C. albicans Rim13p, a homolog of the Rim13p/PalB calpain-like protease member of the RIM101/pacC pathway from Saccharomyces cerevisiae and Aspergillus nidulans, respectively. RIM13, like other members of the RIM101 pathway, is required for alkaline pH-induced filamentation and growth under extreme alkaline conditions. Further, our studies suggest that the RIM101 pathway promotes pH-independent responses, including resistance to high concentrations of lithium and to the drug hygromycin B. RIM13 encodes a calpain-like protease, and we found that Rim101p undergoes a Rim13p-dependent C-terminal proteolytic processing event at neutral-to-alkaline pHs, similar to that reported for S. cerevisiae Rim101p and A. nidulans PacC. However, we present evidence that suggests that C. albicans Rim101p undergoes a novel processing event at acidic pHs that has not been reported in either S. cerevisiae or A. nidulans. Thus, our results provide a framework to understand how the C. albicans Rim101p processing pathway promotes alkaline pH-independent processes.
Candida albicans is a commensal fungus of humans that can become pathogenic in susceptible hosts. In fact, C. albicans is the fourth most common cause of nosocomial bloodstream infections (3, 30, 31). As a commensal, C. albicans colonizes the mucosal surfaces of the oral-pharyngeal, gastrointestinal, and urogenital tracts. As a pathogen, C. albicans generally infects these tissues; however, it can also enter the bloodstream and disseminate to virtually any tissue of the body. Thus, during both commensal and pathogenic growth, C. albicans must be able to adapt to diverse environments.
One environmental factor that C. albicans must respond to is extracellular pH (4). For example, acidic environments are encountered in the gastrointestinal tract and vaginal cavities; neutral-to-alkaline environments are encountered in the oral-pharyngeal tract and bloodstream. The ability to respond to environmental pH is critical for virulence, as demonstrated by the fact that mutants unable to grow at a given pH in vitro are limited in their sites of infections in vivo. For example, a mutant unable to grow at acidic pHs in vitro is less virulent in a vaginal model but not in a systemic model (8). Further, a mutant unable to grow at alkaline pHs in vitro is less virulent in the systemic model but not in the vaginal model (8, 15). Thus, the ability of C. albicans to successfully respond to the extracellular pH is critical for its success as a pathogen.
The response of C. albicans to the extracellular pH is controlled by a conserved fungal pH response pathway that is governed by the zinc finger transcription factor Rim101p (also previously called Prr2p and Hrm101p) (6, 33, 34, 41). This pathway has been characterized in Saccharomyces cerevisiae (RIM101 pathway) and Aspergillus nidulans (pacC pathway) (9, 29). In these organisms, Rim101p/PacC activity is controlled by proteolytic processing. At acidic pHs, Rim101p exists in a full-length form that has no known activity. At alkaline pHs, Rim101p is processed through removal of a C-terminal glutamate-aspartate-rich domain to an active short form that governs changes in gene expression (11, 18-20, 28, 39). Processing requires the activity of several gene products, including Rim8p/PalF, Rim13p/PalB, Rim9p/PalI, Rim20p/PalA, and Rim21p/PalH (2, 10, 14, 18, 20, 21, 26, 27, 38, 43).
We previously described the C. albicans homologs of Rim101p, Rim20p, and Rim8p (6). In C. albicans, these gene products govern alkaline pH-induced filamentation, induction of alkaline response genes, repression of an acidic response gene, and virulence in a model of hematogenously disseminated systemic candidiasis (4-6, 33, 35). However, these gene products were not required for growth at pH 8 but were required for growth at more extreme alkaline pHs. Rim8p and Rim20p appear to act upstream of Rim101p, as expression of Rim101-405p, which lacks the C-terminal domain, rescues the phenotypes seen for rim8−/− and rim20−/− mutants (6). Thus, the pH responses governed by the RIM101 pathway in C. albicans are essential in vivo.
Here we describe the C. albicans homolog of RIM13, a calpain-like protease thought to cleave full-length Rim101p into the active form. We describe the role of RIM13 in the known RIM101 pathway functions and describe new phenotypes associated with mutants in this pathway. Finally, we identified a novel processed form of Rim101p at acidic pHs that suggests the possibility that C. albicans Rim101p has functions independent of alkaline pHs.
MATERIALS AND METHODS
Strains and plasmids.
The strains used in this study are described in Table 1 and are derivatives of BWP17 (41). The creation of the rim13−/− transposon mutant was described previously (7). In short, a Tn7-UAU1 mutagenized plasmid was identified in which the Tn7-UAU1 insertion occurred at position 239 of the RIM13 coding sequence. Flanking genomic DNA and the Tn7-UAU1 insertion were released via NotI restriction digestion and transformed into BWP17. Homozygous rim13−/− transposon mutants were generated and identified as previously described (7).
TABLE 1.
C. albicans strains used in this study
| Name | Genotype | Reference |
|---|---|---|
| BWP17 | ura3Δ::limm434 his1::hisGarg4::hisG | 41 |
| ura3Δ::limm434 his1::hisG arg4::hisG | ||
| DAY5 | ura3Δ::limm434his1::hisGarg4::hisGrim101::URA3 | 41 |
| ura3Δ::limm434 his1::hisG arg4::hisG rim101::ARG4 | ||
| DAY23 | ura3Δ::limm434his1::hisGarg4::hisGrim20::URA3 | 6 |
| ura3Δ::limm434 his1::hisG arg4::hisG rim20::ARG4 | ||
| DAY25 | ura3Δ::limm434pHIS1::his1::hisGarg4::hisGrim101::URA3 | 5 |
| ura3Δ::limm434his1::hisGarg4::hisG rim101::ARG4 | ||
| DAY61 | ura3Δ::limm434his1::hisGarg4::hisGrim8::URA3 | 6 |
| ura3Δ::limm434 his1::hisG arg4::hisG rim8::ARG4 | ||
| DAY62 | ura3Δ::limm434his1::hisGarg4::hisGrim8::URA3pRIM101-405::HIS1 | 6 |
| ura3Δ::limm434 his1::hisG arg4::hisG rim8::ARG4 | ||
| DAY67 | ura3Δ::limm434his1::hisGarg4::hisGrim13::URA3 | This study |
| ura3Δ::limm434 his1::hisG arg4::hisG RIM13 | ||
| DAY106 | ura3Δ::limm434pHIS1::RIM8::his1::hisGarg4::hisGrim8::URA3 | 5 |
| ura3Δ::limm434 his1::hisG arg4::hisG rim8::ARG4 | ||
| DAY117 | ura3Δ::limm434pHIS1::his1::hisGarg4::hisGrim8::URA3 | 5 |
| ura3Δ::limm434 his1::hisG arg4::hisG rim8::ARG4 | ||
| DAY128 | ura3Δ::limm434his1::hisGarg4::hisGrim13::URA3pRIM101::HIS1 | This study |
| ura3Δ::limm434 his1::hisG arg4::hisG rim13::ARG4 | ||
| DAY132 | ura3Δ::limm434his1::hisGarg4::hisGrim13::URA3pRIM101-405::HIS1 | This study |
| ura3Δ::limm434 his1::hisG arg4::hisG rim13::ARG4 | ||
| DAY185 | ura3Δ::limm434pHIS1::his1::hisGpARG4::URA3::arg4::hisG | 5 |
| ura3Δ::limm434 his1::hisG arg4::hisG | ||
| DAY224 | ura3Δ::limm434pHIS1::his1::hisGarg4::hisGrim13::URA3 | This study |
| ura3Δ::limm434 his1::hisG arg4::hisG rim13::ARG4 | ||
| DAY226 | ura3Δ::limm434pHIS1::RIM13::his1::hisGarg4::hisGrim13::URA3 | This study |
| ura3Δ::limm434 his1::hisG arg4::hisG rim13::ARG4 | ||
| DAY286 | ura3Δ::limm434his1::hisGpARG4::URA3::arg4::hisG | 7 |
| ura3Δ::limm434 his1::hisG arg4::hisG | ||
| DAY349 | ura3Δ::limm434his1::hisGarg4::hisGrim13::URA3 | This study |
| ura3Δ::limm434 his1::hisG arg4::hisG rim13::ARG4 | ||
| DAY492 | ura3Δ::limm434his1::hisGarg4::hisGpHIS1::RIM101-V5-AgeI::rim101::ARG4 | This study |
| ura3Δ::limm434 his1::hisG arg4::hisG rim101::URA3 | ||
| DAY499 | ura3Δ::limm434his1::hisGarg4::hisGpHIS1::RIM101-V5-BstEII::rim101::ARG4 | This study |
| ura3Δ::limm434 his1::hisG arg4::hisG rim101::URA3 | ||
| DAY504 | ura3Δ::limm434his1::hisGarg4::hisGpHIS1::RIM101-V5-NgoMI::rim101::ARG4 | This study |
| ura3Δ::limm434 his1::hisG arg4::hisG rim101::URA3 | ||
| DAY610 | ura3Δ::limm434his1::hisGarg4::hisGrim20::URA3pHIS1::RIM101-V5-AgeI | This study |
| ura3Δ::limm434 his1::hisG arg4::hisG rim20::ARG4 | ||
| DAY615 | ura3Δ::limm434his1::hisGarg4::hisGrim8::ARG4pHIS1::RIM101-V5-AgeI | This study |
| ura3Δ::limm434 his1::hisG arg4::hisG rim8::URA3 | ||
| DAY626 | ura3Δ::limm434his1::hisGarg4::hisGrim13::URA3pHIS1::RIM101-V5-BstEII | This study |
| ura3Δ::limm434 hisI::hisG arg4::hisG rim13::ARG4 | ||
| DAY643 | ura3Δ::limm434his1::hisGarg4::hisGrim13::URA3pHIS1::RIM101-V5-AgeI | This study |
| ura3Δ::limm434 hisI::hisG arg4::hisG rim13::ARG4 | ||
| DAY665 | ura3Δ::limm434his1::hisGarg4::hisGrim13::Tn7-UAU1 | This study |
| ura3Δ::limm434 his1::hisG arg4::hisG RIM13 | ||
| DAY666 | ura3Δ::limm434his1::hisGarg4::hisGrim13::Tn7-UAU1 | This study |
| ura3Δ::limm434 his1::hisG arg4::hisG RIM13 | ||
| DAY667 | ura3Δ::limm434his1::hisGarg4::hisGrim13::Tn7-UAU1 | This study |
| ura3Δ::limm434 his1::hisG arg4::hisG rim13::Tn7-URA3 | ||
| RIM13 | ||
| DAY668 | ura3Δ::limm434his1::hisGarg4::hisGrim13::Tn7-UAU1 | This study |
| ura3Δ::limm434 his1::hisG arg4::hisG rim13::Tn7-URA3 | ||
| GKO88 | ura3Δ::limm434his1::hisGarg4::hisGrim13::Tn7-UAU1 | 7 |
| ura3Δ::limm434 his1::hisG arg4::hisG rim13::Tn7-URA3 | ||
| GKO89 | ura3Δ::limm434his1::hisGarg4::hisGrim13::Tn7-UAU1 | 7 |
| ura3Δ::limm434 his1::hisG arg4::hisG rim13::Tn7-URA3 |
The rim13−/− mutant (DAY349) was generated as follows. Strain BWP17 was subjected to consecutive rounds of transformation with rim13::URA3 and rim13::ARG4 with primers RIM13-5DR and RIM13-3DR as described previously (41). The primers used in this study are listed in Table 2. This deletes sequences from 604 to 1023 of the open reading frame (ORF), deleting residues 202 to 341 of the predicted protein. DAY67 is a Ura+ RIM13+/− heterozygous transformant that was used to generate the Arg+ rim13−/− homozygous transformant DAY349. Correct integration was demonstrated by PCR with the primers rim13 5-detect and rim13 3-detect, which respectively bind 112 nucleotides upstream and 66 nucleotides downstream of the DR homology region used to target integration.
TABLE 2.
Primers used in this study
| Name | Sequence |
|---|---|
| RIM13-5DR | 5′-TCCCAAAAGTACAAAGTTTCACTACGATTTAATGGGGCATTAAGAAACGTAATTGTTGATTTTCCCAGTCACGACGTT |
| RIM13-3DR | 5′-AAAATAGTGTTGGTAAGGTTTATGGTCTGGTTTCCAATTGACATAGAGATACTTGAAATGGTGGAATTGTGAGCGGATA |
| rim13 5-detect | 5′-ATTGGGACAATGATATCACTCAC |
| rim13 3-detect | 5′-GTGCCGTTCAAGGAGTATCCAC |
| RIM13 5′ | 5′-AATTGTACAACGGGAAAGTC |
| RIM13 3′ | 5′-AGTAAGAGGCACAGTCTGAG |
| RIM101promoter | 5′-ACAAGTATGCAGTGAATGAC |
| RIM101 3′ | 5′-GTCAGCGATAAGAATTATGAG |
| AgeI 5′ V5 | 5′-CATTCATCCCGTAACATACTTAAATGCTGATAGCAATACCGGTAAGCCTATCCCTAACCC |
| AgeI 3′ V5 | 5′-TCTTGGAACCATGGTGACTTGCAGTACTCTCACTTGCACCATGGTGATGGTGATGATGAC |
| BstEII 5′ V5 | 5′-AACTACCGCATCCGATTTGCAATTGAACTACTATTCCGGTGGTAAGCCTATCCCTAACCC |
| BstEII 3′ V5 | 5′-TTCTGGAGGTGTCGTCGTAGTTCAATCCATCAGCAGGGTTATGGTGATGGTGATGATGAC |
| NgoMI 5′ V5 | 5′-CGCAGGTTCTGCTGAGTTCACCACCAAGAGGATGAAAGCCGGTAAGCCTATCCCTAACCC |
| NgoMI 3′ V5 | 5′-GATTCAACTTGTTAAACACATCAATGTTATACTCAGTGCCATGGTGATGGTGATGATGAC |
Strains DAY128 and DAY132 were created by integration of plasmids pDDB61 and pDDB71, respectively (6). pDDB61 and pDDB71 were digested with PpuMI and transformed into DAY349 to create strains DAY128, which contains an extra copy of RIM101, and DAY132, which contains the RIM101-405 allele.
The complemented rim13−/− RIM13 strain DAY226 was created as follows. The wild-type RIM13 sequence from −876 to 2036 was amplified from genomic DNA with primers RIM13 5′ and RIM13 3′ and ligated into pGEM-T Easy (Promega) to create pDDB98. pDDB98 was digested with PvuII, and the RIM13-containing fragment was cotransformed into S. cerevisiae with NotI-digested pDDB78 to generate pDDB111 by in vivo recombination (23). Plasmid pDDB111 was digested with NruI (NEB) and transformed into DAY349 to generate DAY226. DAY224 was created by transformation of NruI-digested pGEM-HIS1 (41).
V5-tagged RIM101 mutant strains were created as follows. Plasmid pDDB233 was digested with NruI and transformed into strains DAY5, DAY23, DAY61, and DAY349 to create strains DAY492, DAY610, DAY615, and DAY643, respectively. Plasmids pDDB235 and pDDB236 were digested with NruI and transformed into DAY5 to generate strains DAY499 and DAY504. Plasmid pDDB235 was also digested with NruI and transformed into DAY349 to generate DAY626. These strains carry the V5-tagged RIM101 construct at a his1::hiG locus. Both pDDB233 and pDDB209 (no V5 tag) complement the rim101−/− mutant (data not shown), which suggests that Rim101p and Rim101-V5p are functional when expressed from the his1::hisG locus.
Plasmids pDDB233, pDDB235, and pDDB236 were constructed as follows. RIM101 plus flanking sequence was amplified in a PCR with primers RIM101promoter and RIM101 3′. The resulting PCR product was cloned into pGEM-T Easy (Promega) to make pDDB200. Plasmid pDDB200 was digested with PvuII (NEB) and transformed into a trp1− S. cerevisiae strain with NotI/SpeI-digested pDDB78 to generate pDDB209 by in vivo recombination. The V5 epitope was amplified from pTRACER-EF (Invitrogen) in a PCR with primer pairs AgeI 5′ V5 and AgeI 3′V5, BstEII 5′ V5 and BstEII 3′V5, and NgoMI 5′ V5 and NgoMI 3′V5. The resulting PCR products and pDDB209 were digested with AgeI, BstEII, or NgoMI, respectively, and transformed into DAY414 to generate pDDB233, PDDB235, and pDDB236, respectively. pDDB209, pDDB233, pDDB235, and pDDB236 were purified from S. cerevisiae into Escherichia coli by electroporation.
Media and growth conditions.
Candida cells were routinely grown in YPD plus Uri (1% yeast extract, 2% Bacto Peptone, 2% dextrose, 80 μg of uridine per ml). For transformations, cells were plated on synthetic media lacking the appropriate amino acid as described previously (1). Bacteria were grown in Luria broth (LB) containing 100 μg of ampicillin per ml. All solid media contained 2% Bacto Agar.
For filamentation assays, cells were grown in YP (1% yeast extract, 2% Bacto Peptone) containing 20% bovine calf serum (Sigma) or in M199 medium (Gibco BRL) buffered with 150 mM HEPES to pH 4.0 or 8.0. Filamentation assays were done in triplicate, and results were compared by analysis of variance.
For determination of growth phenotypes, cells were grown on YPD plates containing 150 mM LiCl, 150 μg of hygromycin B (Sigma) per ml, 10 μM unbuffered calcofluor (Sigma), and 10 μM calcofluor buffered at pH 8.0 with 150 mM HEPES and on YPD plates buffered with 150 mM HEPES at pH 10.
Protein preparation and Western blot analyses.
Overnight cultures were grown to saturation in YPD plus Uri. Cells were diluted 40-fold into fresh M199 medium buffered with 150 mM HEPES to pH 4.0 or 7.0 and grown for 4 h at 30°C. Cells were pelleted and stored at −80°C prior to protein extraction. Cell pellets were resuspended in ice-cold radioimmunoprecipitation assay buffer containing 1 μg of leupeptin per ml, 2 μg of aprotinin per ml, 1 μg of pepstatin per ml, 1 mM phenylmethylsulfonyl fluoride, and 10 mM dithiothreitol and transferred to glass test tubes containing acid-washed glass beads. Cells were lysed by vortexing for 2 min, followed by 2 min on ice. This regimen was repeated four times. Cell debris was removed by centrifugation, and supernatants were removed and stored at −80°C. For Western blot assays, 20 μl of 2× sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer was added to 20 μl of supernatant and samples were boiled for 5 min. Samples were loaded onto an SDS-8% PAGE gel and run overnight at 35 V. Proteins were transferred to nitrocellulose and blocked. Anti-V5-horseradish peroxidase antibody (Invitrogen) in 30 ml of 5% nonfat milk in TBS-T (50 mM Tris [pH 7.6], 150 mM NaCl, 0.05% Tween 20) solution (1:7,500 dilution) was added to the blot, which was incubated for 4 h at 4°C. Blots were washed in TBST, incubated with ECL reagent (Amersham Biosciences), and exposed to film.
RESULTS
Identification of C. albicans Rim13p.
We recently described the construction of a set of 217 homozygous C. albicans insertion mutants (7). In order to understand how C. albicans adapts to changes in the extracellular pH, we screened this collection of mutants for growth at alkaline pHs. Of the 217 homozygous mutants tested, two showed poor growth on YPD medium buffered at pH 10 (Fig. 1 and data not shown). One strain carried a mutation in MDS3, as described previously (7), and one carried a mutation in ORF 6.7408. ORF 6.7408 can encode a 718-residue protein with 60 to 65% similarity to S. cerevisiae Rim13p/Cpl1p and A. nidulans PalB (9, 11, 14, 43). Thus, this ORF appears to encode the C. albicans homolog of Rim13p.
FIG. 1.
Growth of rim13::Tn7 mutants at alkaline pH. The wild type (WT; DAY286), the rim101−/− mutant strain (DAY5), rim13::Tn7::UAU1/rim13::URA3 homozygous strains (GKO88, GKO89, and DAY668), rim13::Tn7::UAU1/RIM13 heterozygous strains (DAY665 and DAY666), and the rim13::Tn7::UAU1/rim13::URA3/RIM13 triplication strain (DAY667) were grown for 2 days at 37°C on YPD (A) and on YPD at pH 10 (B).
We considered the possibility that a secondary mutation occurred during the creation of the rim13−/− homozygous transposon mutant that confers the alkaline growth defect. We addressed this possibility in two ways. First, we analyzed independent rim13::Tn7::UAU1/rim13::URA3 homozygotes and found that they also grew poorly at pH 10 (Fig. 1, GKO88, GKO89, and DAY668). However, a triplication isolate, which retains a wild-type copy of RIM13 (rim13::Tn7::UAU1/rim13::URA3/RIM13), grew like the wild type (Fig. 1, DAY667). Second, we constructed a rim13 mutant by a PCR-mediated gene disruption approach to make a defined mutation (Fig. 2). Deletion of one RIM13 allele did not confer alkaline sensitivity; deletion of both copies of RIM13 did confer alkaline sensitivity (data not shown and Fig. 3). Also, reintroduction of a wild-type copy of RIM13 into the defined rim13−/− mutant restored the ability to grow at pH 10, although not quite to wild-type levels. In total, these results demonstrate that C. albicans RIM13 is required for wild-type rates of growth at alkaline pH 10.
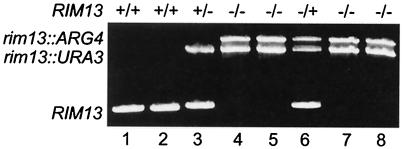
FIG. 2.
Generation of RIM13 strains. Genomic DNAs from BWP17 and its derivatives were purified and used for PCRs with rim13 5-detect and rim13 3-detect. Lanes: 1, wild-type auxotrophic strain (BWP17); 2, wild-type prototrophic strain (DAY185); 3, RIM13+/− strain (DAY67); 4, rim13−/− strain (DAY349); 5, rim13−/− strain (DAY224); 6, rim13−/− RIM13 strain (DAY226); 7, rim13−/− RIM101 strain (DAY128); 8, rim13−/− RIM101-405 strain (DAY132).
FIG. 3.
Growth phenotypes of RIM101 pathway mutants. The wild type (DAY185), rim13−/− homozygous mutant, complemented, and RIM101-405-rescued strains (DAY224, DAY226, and DAY132, respectively), the rim101−/− mutant strain (DAY25), and rim8−/− homozygous mutant, complemented, and RIM101-405-rescued strains (DAY117, DAY106, and DAY62, respectively) were grown overnight at 30°C in YPD plus Uri. Cells were diluted 10-fold in water, and 3-μl volumes of fivefold serial dilutions were spotted onto YPD at pH 8, YPD at pH 10, YPD plus 10 μM calcofluor, YPD plus 10 μM calcofluor at pH 8, YPD plus 150 mM LiCl, and YPD plus 150 μg of hygromycin B per ml. Growth was measured after 2 days at 30°C, except for the 10 μM calcofluor pH 8 plates and the 150 mM LiCl plates, which were measured after 4 days at 30°C.
We had previously analyzed several mutants affecting the RIM101 pathway and found no growth defects at pH 8 (6). Thus, we asked if other members of the RIM101 pathway, in addition to RIM13, are required for growth at pH 10. We found that rim101−/−, rim8−/−, and rim20−/− mutants showed a growth defect at pH 10 similar to that of the rim13−/−mutant (Fig. 3 and data not shown). Further, the rim13−/− mutant grew as well as the wild type and the RIM101 pathway mutants at pH 8 (Fig. 3). Thus, in C. albicans, the RIM101 pathway is required for growth under extreme alkaline conditions.
The RIM101 pathway is required for alkaline pH-induced filamentation. To determine if RIM13 governs this response, we assayed the rim13−/− mutant for the ability to filament in M199 medium buffered at pH 8. Similar to the rim8−/− mutant, the rim13−/−mutant did not filament in liquid M199 medium buffered at pH 8 (Table 3). Although the RIM101 pathway is required for filamentation under some conditions, it is not essential for filamentation under all conditions, such as in liquid serum (6). Similar to other RIM101 pathway mutants, the rim13−/− mutant did form filaments in liquid serum (Table 3). However, there was a quantitative difference from the wild type (P < 0.0005). This defect was also observed for the other RIM101 pathway mutants. Thus, RIM13 is required for alkaline pH-induced filamentation and plays a limited role in serum-induced filamentation.
TABLE 3.
Filamentation of RIM101 pathway mutants
| Strain | Genotype | Plasmid | Medium | % Filamentationa |
|---|---|---|---|---|
| DAY1 | WTc (auxotrophic) | M199, pH 8 | 96 | |
| DAY185 | WT (prototrophic) | M199, pH 8 | 94 | |
| DAY67 | RIM13/rim13− | M199, pH 8 | 96 | |
| DAY349 | rim13−/rim13− | M199, pH 8 | <1b | |
| DAY128 | rim13−/rim13− | pRIM101 | M199, pH 8 | <1b |
| DAY132 | rim13−/rim13− | pRIM101-405 | M199, pH 8 | 95 |
| DAY224 | rim13−/rim13− | M199, pH 8 | <1b | |
| DAY226 | rim13−/rim13− | pRIM13 | M199, pH 8 | 98 |
| DAY1 | WT (auxotrophic) | Serum | 95 | |
| DAY185 | WT (prototrophic) | Serum | 96 | |
| DAY67 | RIM13/rim13− | Serum | 97 | |
| DAY349 | rim13−/rim13− | Serum | 76b | |
| DAY128 | rim13−/rim13− | pRIM101 | Serum | 69b |
| DAY132 | rim13−/rim13− | pRIM101-405 | Serum | 89 |
| DAY224 | rim13−/rim13− | Serum | 76b | |
| DAY226 | rim13−/rim13− | pRIM13 | Serum | 96 |
Standard deviations were ≤5%, except for DAY128 in serum in which case the standard deviation was 15%.
Significantly different from the wild type.
WT, wild type.
To test the possibility that RIM13 is indeed an upstream member of the RIM101 pathway, we introduced the truncated RIM101-405 allele into the rim13−/− mutant. This constitutively active RIM101 allele bypasses the requirement for the upstream RIM101 pathway regulators (6). Introduction of the RIM101-405 allele into the rim13−/− mutant restored alkaline growth to levels similar to that achieved by introduction of the complementing RIM13 allele. Introduction of a wild-type RIM101 allele did not restore growth (Fig. 3 and data not shown). Further, the RIM101-405 allele rescued the filamentation defect in both M199 at pH 8 and serum medium, whereas the wild-type RIM101 allele did not (Table 3). Thus, these results demonstrate that RIM13 encodes an upstream member of the RIM101 pathway.
New phenotypes associated with defects in the RIM101 pathway.
Since many of the known targets of the RIM101 pathway encode cell wall proteins, such as PHR1 and PRA1, and given that the RIM101 pathway governs the yeast-to-hyphal morphological transition, we predicted that the RIM101 pathway may play a role in cell wall stability. To test this possibility, wild-type, rim13−/− homozygous mutant, complemented rim13−/− + RIM13, and rescued rim13−/− + RIM101-405 strains were plated on medium to test for potential cell wall defects (Fig. 3). All strains grew equally well on YPD at pH 8 and on YPD plus 10 μM calcofluor. Wild-type cells grew slightly less well on YPD plus 10 μM calcofluor at pH 8 than on either YPD at pH 8 or YPD plus 10 μM calcofluor. However, the rim13−/− mutant had pronounced growth defects on YPD plus 10 μM calcofluor at pH 8 compared to the wild-type strain. The rim13−/− mutant was at least partially rescued by introduction of either a wild-type copy of RIM13 or the truncated RIM101-405 allele. These results suggest that although the rim13−/− mutant grows well at pH 8, the cell walls of this mutant may be more sensitive to stress.
Recent work with S. cerevisiae revealed a role for the RIM101 pathway in resistance to high concentrations of lithium and the drug hygromycin B (19). We found that C. albicans rim13−/− mutants also had growth defects on YPD plus 150 mM LiCl and on YPD plus 150 μg of hygromycin B per ml and that these defects were at least partially rescued by the wild-type RIM13 or truncated RIM101-405 allele. Similar results were also obtained with the rim101−/− and rim8−/− mutants (Fig. 3) (7). Sensitivity to lithium chloride and hygromycin B is often seen in mutants with cell wall defects. Thus, these results support the idea that the C. albicans RIM101 pathway is required for cell wall metabolism.
Rim13p governs Rim101p processing in C. albicans.
Rim13p is predicted to encode a calpain-like protease, and in S. cerevisiae and A. nidulans, Rim101p activity is controlled by proteolytic processing. Genetic evidence suggests that proteolytic processing also governs Rim101p activity in C. albicans (6, 12, 34). To test the possibility that C. albicans Rim101p is processed and that this processing requires Rim13p, we introduced the V5 epitope, which inserts a 23-residue V5-His6 epitope, into the RIM101 gene by in vivo recombination (23). The V5 epitope was introduced into three sites of the RIM01 sequence, i.e., the AgeI, BstEII, and NgoMI sites, which inserted the V5 epitope after residues 17, 348, and 436, respectively (Fig. 4A). To determine if the V5-tagged RIM101 constructs were functional, these constructs were introduced into a rim101−/− strain and tested for complementation. All three constructs rescued the growth and filamentation defects associated with the rim101−/− mutation (data not shown). Thus, the presence of the V5 tag at these locations does not disrupt Rim101p function.
FIG. 4.
Rim101-V5p Western blots. (A) Cartoon of the Rim101p sequence showing the three zinc finger domains, the D/E-rich C-terminal domain, and the position of the V5 insertion. The AgeI (A, top), BstEII (B, middle), and NgoMI (N, bottom) constructs are shown with the last residue before the insertion in parentheses. (B) Western blot assays of rim101−/− mutant strains containing the RIM101-V5-AgeI (DAY492, lanes 1 and 4), RIM101-V5-BsteII (DAY499, lanes 2 and 5), and RIM101-V5-NgoMI (DAY504, lanes 3 and 6) constructs. (C) Western blot assays of RIM13+/+ (DAY499, lanes and 1 and 2) and rim13−/− (DAY626, lanes 3 and 4) strains containing the RIM101-V5-BsteII construct. (D) Western blot assays of wild-type (DAY492, lanes 1 and 2), rim20−/− (DAY610, lanes 3 and 4), rim8−/− (DAY615, lanes 5 and 6), and rim13−/− (DAY643, lanes 7 and 8) strains containing the RIM101-V5-AgeI construct. All strains were grown for 4 h at pH 4 or 7, and proteins were purified and separated by SDS-8% PAGE. Gels were transferred to nitrocellulose and probed with anti-V5-horseradish peroxidase (Invitrogen). WT, wild type. The values on the left of panels B, C, and D are molecular sizes in kilodaltons.
Since Rim101p governs neutral-to-alkaline pH responses, we predicted that Rim101p would be processed in a pH-specific fashion. Thus, cells expressing the RIM101-V5 constructs were grown at pH 4 or 7, protein purified, and analyzed by Western blotting. At pH 4, Western blot assays of cells carrying the RIM101-V5-AgeI construct revealed two bands of ∼85 and 65 kDa (Fig. 4B, lane 1). Three independent transformants also gave rise to these two bands, and cells lacking the V5 tag had no bands (data not shown). The predicted size of Rim101-V5p is ∼75 kDa; however, in S. cerevisiae a hemagglutinin-tagged form of Rim101p, Rim1-HA2p, is predicted to run at ∼82 kDa but runs at 98 kDa (20). Thus, the 85-kDa band likely represents the full-length form of Rim101p and the 65-kDa band may represent a processed form of Rim101p.
At pH 4, cells carrying the RIM101-V5-BsteII construct had three major bands of 85, 80, and 63 kDa (Fig. 4B, lane 2). The 85-kDa band ran in a position similar to that seen for the RIM101-V5-AgeI construct and thus likely represents the same product. However, the 63-kDa band had a slightly faster mobility than the 65-kDa band seen in the RIM101-V5-AgeI construct. Finally, the 80-kDa band was notably absent from the RIM101-V5-AgeI construct (Fig. 4B, lane 1). Thus, these results support the idea that Rim101p may be processed at acidic pHs to give rise to an ∼63- to 65-kDa band.
Finally, at pH 4, cells carrying the RIM101-V5-NgoMI construct had two predominant bands of 85 and 80 kDa similar to those seen in the RIM101-V5-BsteII construct (Fig. 4B, lane 3). A faint 65-kDa band could also be seen that may represent the same processing event seen with the RIM101-V5-AgeI construct. Thus, with three distinct V5 constructs, we have identified three forms of Rim101p at acidic pHs, the 85-kDa form, which likely represents the full-length form of Rim101p, and the 80-kDa and 63- to 65-kDa forms, which may represent two distinct processed forms of Rim101p. This represents the first evidence that suggests that any member of the Rim101p/PacC family is processed at acidic pHs.
We next analyzed Rim101p processing at pH 7. Cells expressing the RIM101-V5-AgeI construct had three major bands, including the 85- and 65-kDa bands seen at pH 4. An additional band of 74 kDa was also seen with this construct (Fig. 4B, lane 4). The levels of the 65-kDa form at pH 7 were reduced compared to those seen at pH 4, apparently in favor of the 74-kDa form (Fig. 4B, compare lanes 1 and 4). Cells expressing the RIM101-V5-BsteII construct also had the bands seen at pH 4 and an additional band of 72 kDa (Fig. 4B, lane 5), similar to the results obtained with the RIM101-V5-AgeI construct. A faint 80-kDa band could be seen in the RIM101-V5-BsteII construct with prolonged exposures. Finally, cells expressing the RIM101-V5-NgoMI construct had the 85- and 80-kDa bands seen at pH 4 and an additional band of 74 kDa (Fig. 4B, lane 6). Thus, at pH 7, when Rim101p is thought to be active, a specific processed form, of 72 to 74 kDa, is observed that is absent at pH 4.
If the different forms of Rim101-V5p are in fact processed by Rim13p, then they should be absent in rim13−/− strains. To test this hypothesis, the RIM101-V5-BsteII construct was introduced into the rim13−/− mutant and analyzed for processing at pHs 4 and 7 by Western blotting (Fig. 4C). In wild-type cells, the 85-, 80-, and 63-kDa bands were observed at pH 4 and the 85-, 74-, and 63-kDa bands were observed at pH 7 (Fig. 4C, lanes 1 and 2). However, in the rim13−/− background, the 85- and 80-kDa bands were observed at pHs 4 and 7, but the 63- and the 74-kDa bands were absent at pHs 4 and 7 (Fig. 4C, lanes 3 and 4). Similar results were observed for the RIM101-V5-AgeI construct (Fig. 4D). Wild-type cells had two bands at pH 4 of 85 and 65 kDa and two bands at pH 7 of 85 and 74 kDa (Fig. 4D, lanes 1 and 2). However, in rim20−/−, rim8−/−, and rim13−/− cells, only the 85-kDa band was seen regardless of the pH (Fig. 4D, lanes 3 to 8). Thus, the 63- to 65-kDa and 72- to 74-kDa forms of Rim101-V5p require the presence of Rim13p and the other upstream members of the RIM101 pathway and thus represent Rim101p processing events.
DISCUSSION
The RIM101 pathway of C. albicans governs alkaline responses in vitro and is required for virulence in vivo. In this report, we describe the identification of a new component of this pathway, RIM13, which is essential for alkaline pH-induced filamentation and plays a limited role in liquid serum-induced filamentation. With V5 epitope tags, we have demonstrated biochemically that C. albicans Rim101p is proteolytically processed in a pH-dependent fashion.
The RIM101 pathway and the cell wall.
The RIM101 pathway is required for growth of S. cerevisiae and A. nidulans at alkaline pHs. However, previous work showed that this pathway is not required for growth of C. albicans at alkaline pH 8 (6). Here we show that the RIM101 pathway is in fact required for growth at alkaline pH 10. Further, our studies revealed a pH-dependent calcofluor white sensitivity in RIM101 pathway mutants. Calcofluor white binds to chitin and prevents its incorporation into the cell wall (17). In S. cerevisiae, sensitivity to calcofluor white is often associated with the amount of chitin in the cell wall. For example, mutants that have less chitin in the cell wall, such as those with changes in SKT5/CHS4/CSD4/CAL2, are more resistant to calcofluor white (40); mutants that have more chitin in the cell wall, such as those with changes in ERG3/PSO6 and GAS1, are more sensitive to calcofluor white (32, 37). We found that wild-type cells are more sensitive to calcofluor white at alkaline pHs, which suggests either that C. albicans may require more cell wall chitin under alkaline growth conditions than under acidic growth conditions or that the amount of chitin does not change but has a function for growth that is more sensitive to perturbation. It is also possible that calcofluor is simply more soluble at alkaline pHs. However, we found that RIM101 pathway mutants were more sensitive than wild-type cells to calcofluor white at alkaline pHs. It is noteworthy that the RIM101 pathway regulates the expression of PHR1 and PHR2, which encode 1,3-β-glucanosyltransferases that are homologous to that encoded by S. cerevisiae GAS1 (6, 13, 24, 33, 35, 36). Thus, one model to explain this rim13−/− calcofluor sensitivity is that the RIM101 pathway regulates the expression of cell wall components that result in increased chitin levels compared to that of the wild type and thus increased sensitivity to calcofluor white.
In S. cerevisiae, the RIM101 pathway is required for resistance to high concentrations of lithium and hygromycin B and we found a similar role for the C. albicans RIM101 pathway (19). It was suggested that the S. cerevisiae RIM101 pathway functions in ion homeostasis. For example, S. cerevisiae ENA1, which is positively regulated by Rim101p, encodes a plasma membrane sodium pump required for lithium chloride resistance (16, 19). Calcineurin pathway mutants are also sensitive to lithium chloride, presumably owing to defects in ENA1 expression (22). Genes involved in ion homeostasis, such as HAL5, TRK1/2, and PMT1, and the calcineurin pathway are required for hygromycin B resistance (25, 42). Increased hygromycin B sensitivity, which acts to inhibit the translational machinery, is likely due to increased uptake when ion homeostasis is disrupted. Thus, these two phenotypes are attributed to changes in the expression of plasma membrane proteins required for ion homeostasis. In C. albicans, microarray analysis of rim101−/− mutants has demonstrated that the RIM101 pathway governs the expression of many genes that encode ion transporters and pumps (E. Bensen et al., submitted for publication). Thus, a conserved function for the RIM101 pathway in fungi may be ion homeostasis.
C. albicans Rim101p processing.
With V5 epitope tags, we found that C. albicans Rim101p exists in several distinct forms (Fig. 4). One form, the 85-kDa form, is seen under all conditions and appears to be the full-length form of Rim101p. Two additional forms arise from RIM101 pathway-dependent processing events. Under neutral conditions, Rim101p is processed to an ∼74-kDa protein; under acidic conditions, Rim101p is processed to an ∼65-kDa protein. Both processed forms are detected when the V5 epitope is inserted at residue 17; thus, these almost certainly arise from loss of C-terminal sequences. Both the 74- and 65-kDa forms are dependent on the upstream members of the RIM101 pathway, including the calpain-like protease Rim13p. Thus, these two forms represent processed molecules.
An 80-kDa band is also seen with the RIM101-V5-BsteII and RIM101-V5-NgoMI constructs. However, this molecule differs from the 74- and 65-kDa molecules in two ways. First, the 80-kDa form is not seen when the V5 epitope is inserted at residue 17 but is apparent when the V5 epitope is inserted at residue 348 or 436. This suggests that the 80-kDa form arises from loss of N-terminal sequences. Second, the 80-kDa band is not dependent on upstream members of the RIM101 pathway. Since this fragment is absent in the RIM101-V5-AgeI construct, yet the 74- and 65-kDa processed forms are seen with this construct, it seems likely the processed forms of Rim101p have an intact N terminus. This idea is supported by the fact that the processed forms of Rim101p-V5 seen with the RIM101-V5-BstEII and RIM101-V5-NgoMI constructs run with the same mobility or slightly slower than those seen with the RIM101-V5-AgeI construct. Thus, the 74- and 65-kDa processed forms of Rim101p contain intact N-terminal sequences.
On the basis of these studies, we propose the following model (Fig. 5A and B). In alkaline environments, the RIM101 pathway is activated, which results in Rim13p-dependent processing at the ALK site of the full-length 85-kDa Rim101p protein to yield the 74-kDa form (Fig. 5A). This form then governs induction of alkaline response genes, such as PHR1 and PRA1, and repression of acidic response genes, such as PHR2. In acidic environments, the RIM101 pathway promotes Rim13p-dependent processing at the ACID site of full-length Rim101p to yield the 65-kDa form (Fig. 5A). While Rim101p processing has not been described in other fungi at acidic pHs, our work suggests the possibility that this event occurs in these organisms.
FIG. 5.
Model of Rim101p processing. (A) Cartoon of C. albicans Rim101p showing the locations of the V5 insertions (A, AgeI; B, BstEII; N, NgoMI) and the predicted C-terminal processing sites at alkaline pHs (C-proc ALK) and acidic pHs (C-proc ACID) (arrows). (B) Scale model of Rim101p/PacC processing in C. albicans, S. cerevisiae, and A. nidulans showing predicted processing forms and pH and Rim13p/PalB dependence. In C. albicans, the 65- and 74-kDa forms are pH and Rim13p dependent. The possibility that the CaRim101p 65-kDa form arises from the 74-kDa intermediate is shown. The functional activities of these CaRim101p forms has not been determined. In S. cerevisiae, the single pH- and Rim13p-dependent processing step is shown. This is the only ScRim101p processing event described. In A. nidulans, PacC is processed in a pH- and PalB-dependent step to a 53-kDa intermediate. This intermediate is then a substrate for a PalB-independent processing event to the active 27-kDa form.
How does the 65-kDa form of Rim101p arise? One possibility is that full-length Rim101p is directly processed to the 65-kDa form. However, another possibility is that full-length Rim101p is first processed to the 74-kDa form, which is then a substrate for processing to the 65-kDa form under the appropriate conditions (Fig. 5B). In both S. cerevisiae and A. nidulans, Rim101p/PacC undergoes a single Rim13p/PalB-dependent processing event at neutral-to-alkaline pHs. However, in A. nidulans, the PalB-processed form of PacC undergoes a second processing event that is PalB independent and results in a further C-terminal truncation to yield the active PacC molecule at neutral-to-alkaline pHs (Fig. 5B) (11). Since Rim13p/PalB in S. cerevisiae and A. nidulans only processes Rim101p/PacC at a single site, it seems likely that C. albicans Rim13p also carries out a single processing cleavage that results in the 74-kDa form. At acidic pHs, the 74-kDa form would then be a substrate for a Rim13p-independent processing event resulting in the 65-kDa form. In the absence of Rim13p, no 74-kDa product, and thus no 65-kDa product, is formed.
What is the role of the 65-kDa form of Rim101p? One possibility is that as conditions become more acidic, the 74-kDa form that arises is inactivated by processing to the 65-kDa form. This would allow a rapid response to a shift to acidic conditions. However, RIM101 alleles that result in premature stop codons after residues 463 and 486 yield Rim101 proteins proficient in alkaline responses (6, 12). On the basis of the mobility of full-length Rim101p, these truncated Rim101p molecules are predicted to run at ∼60 kDa, which suggests that the 65-kDa protein may not be completely inactive. Further, overnight pregrowth at pH 4 did not change the levels of the 65-kDa band (unpublished data). Another possibility is that the 65-kDa form is functional, but the difference between the 74- and 65-kDa forms is one of specificity. At alkaline pHs, the 74-kDa form is seen, which governs alkaline pH-specific changes in gene expression. At acidic pHs, the 65-kDa form is seen, which governs alkaline pH-independent changes in gene expression. On the basis of microarray analysis of wild-type and rim101−/− cells, Rim101p is dispensable for acidic pH transcriptional responses (Bensen et al., submitted). Thus, it seems unlikely that the 65-kDa form governs acidic-response gene expression. However, the RIM101 pathway is also required for responses independent of alkaline pH, including resistance to LiCl and hygromycin B (Fig. 3), and Rim101p governs the expression of 70 genes that are not regulated by pH (Bensen et al., submitted). Thus, the 65-kDa form may promote changes in gene expression that govern these responses. On the basis of this model, we suggest that the region of Rim101p between the 65- and 74-kDa processing sites may define an alkaline pH specificity domain. For example, this domain may inhibit Rim101p interaction with an activator required for the alkaline pH-independent responses.
The ability to respond and adapt to changes in extracellular pH is critical for colonization and survival in the host. In C. albicans, this response is controlled by the RIM101 processing pathway, which results in a novel Rim101p processing product not observed in other fungi. The RIM101 pathway plays an important role in pathogenesis; thus, this new processing event may represent an adaptation specific to the human host environment.
Acknowledgments
We thank P. Bohjanen and D. Williams for providing plasmids and technical assistance. We also thank A. Kullas and other members of the Davis laboratory for helpful criticism of the manuscript and many useful discussions during the course of this work.
This research was supported by startup funds provided by the University of Minnesota Medical School to D.A.D. and Public Health Service grants R01 AI50931 and T32 AI07161 to A.P.M.
REFERENCES
- 1.Adams, A., D. E. Gotschling, C. A. Kaiser, and T. Stearns. 1997. Methods in yeast genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 2.Arst, H. N., Jr., E. Bignell, and J. Tilburn. 1994. Two new genes involved in signalling ambient pH in Aspergillus nidulans. Mol. Gen. Genet. 245:787-790. [DOI] [PubMed] [Google Scholar]
- 3.Calderone, R. A. 2002. Candida and candidiasis. ASM Press, Washington, D.C.
- 4.Davis, D. 2003. Adaptation to environmental pH in Candida albicans and its relation to pathogenesis. Curr. Genet. 44:1-7. [DOI] [PubMed] [Google Scholar]
- 5.Davis, D., J. E. Edwards, Jr., A. P. Mitchell, and A. S. Ibrahim. 2000. Candida albicans RIM101 pH response pathway is required for host-pathogen interactions. Infect. Immun. 68:5953-5959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis, D., R. B. Wilson, and A. P. Mitchell. 2000. RIM101-dependent and -independent pathways govern pH responses in Candida albicans. Mol. Cell. Biol. 20:971-978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis, D. A., V. M. Bruno, L. Loza, S. G. Filler, and A. P. Mitchell. 2002. Candida albicans Mds3p, a conserved regulator of pH responses and virulence identified through insertional mutagenesis. Genetics 162:1573-1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Bernardis, F., F. A. Muhlschlegel, A. Cassone, and W. A. Fonzi. 1998. The pH of the host niche controls gene expression in and virulence of Candida albicans. Infect. Immun. 66:3317-3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Denison, S. H. 2000. pH regulation of gene expression in fungi. Fungal Genet. Biol. 29:61-71. [DOI] [PubMed] [Google Scholar]
- 10.Denison, S. H., M. Orejas, and H. N. Arst, Jr. 1995. Signaling of ambient pH in Aspergillus involves a cysteine protease. J. Biol. Chem. 270:28519-28522. [DOI] [PubMed] [Google Scholar]
- 11.Diez, E., J. Alvaro, E. A. Espeso, L. Rainbow, T. Suarez, J. Tilburn, H. N. Arst, Jr., and M. A. Penalva. 2002. Activation of the Aspergillus PacC zinc finger transcription factor requires two proteolytic steps. EMBO J 21:1350-1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El Barkani, A., O. Kurzai, W. A. Fonzi, A. Ramon, A. Porta, M. Frosch, and F. A. Muhlschlegel. 2000. Dominant active alleles of RIM101 (PRR2) bypass the pH restriction on filamentation of Candida albicans. Mol. Cell. Biol. 20:4635-4647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fonzi, W. A. 1999. PHR1 and PHR2 of Candida albicans encode putative glycosidases required for proper cross-linking of β-1,3- and β-1,6-glucans. J. Bacteriol. 181:7070-7079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Futai, E., T. Maeda, H. Sorimachi, K. Kitamoto, S. Ishiura, and K. Suzuki. 1999. The protease activity of a calpain-like cysteine protease in Saccharomyces cerevisiae is required for alkaline adaptation and sporulation. Mol. Gen. Genet. 260:559-568. [DOI] [PubMed] [Google Scholar]
- 15.Ghannoum, M. A., B. Spellberg, S. M. Saporito-Irwin, and W. A. Fonzi. 1995. Reduced virulence of Candida albicans PHR1 mutants. Infect. Immun. 63:4528-4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haro, R., B. Garciadeblas, and A. Rodriguez-Navarro. 1991. A novel P-type ATPase from yeast involved in sodium transport. FEBS Lett. 291:189-191. [DOI] [PubMed] [Google Scholar]
- 17.Herth, W. 1980. Calcofluor white and Congo red inhibit chitin microfibril assembly of Poterioochromonas: evidence for a gap between polymerization and microfibril formation. J. Cell Biol. 87:442-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lamb, T. M., and A. P. Mitchell. 2003. The transcription factor Rim101p governs ion tolerance and cell differentiation by direct repression of the regulatory genes NRG1 and SMP1 in Saccharomyces cerevisiae. Mol. Cell. Biol. 23:677-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lamb, T. M., W. Xu, A. Diamond, and A. P. Mitchell. 2001. Alkaline response genes of Saccharomyces cerevisiae and their relationship to the RIM101 pathway. J. Biol. Chem. 276:1850-1856. [DOI] [PubMed] [Google Scholar]
- 20.Li, W., and A. P. Mitchell. 1997. Proteolytic activation of Rim1p, a positive regulator of yeast sporulation and invasive growth. Genetics 145:63-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maccheroni, W., Jr., G. S. May, N. M. Martinez-Rossi, and A. Rossi. 1997. The sequence of palF, an environmental pH response gene in Aspergillus nidulans. Gene 194:163-167. [DOI] [PubMed] [Google Scholar]
- 22.Mendoza, I., F. Rubio, A. Rodriguez-Navarro, and J. M. Pardo. 1994. The protein phosphatase calcineurin is essential for NaCl tolerance of Saccharomyces cerevisiae. J. Biol. Chem. 269:8792-8796. [PubMed] [Google Scholar]
- 23.Muhlrad, D., R. Hunter, and R. Parker. 1992. A rapid method for localized mutagenesis of yeast genes. Yeast 8:79-82. [DOI] [PubMed] [Google Scholar]
- 24.Muhlschlegel, F. A., and W. A. Fonzi. 1997. PHR2 of Candida albicans encodes a functional homolog of the pH-regulated gene PHR1 with an inverted pattern of pH-dependent expression. Mol. Cell. Biol. 17:5960-5967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mulet, J. M., M. P. Leube, S. J. Kron, G. Rios, G. R. Fink, and R. Serrano. 1999. A novel mechanism of ion homeostasis and salt tolerance in yeast: the Hal4 and Hal5 protein kinases modulate the Trk1-Trk2 potassium transporter. Mol. Cell. Biol. 19:3328-3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Negrete-Urtasun, S., S. H. Denison, and H. N. Arst, Jr. 1997. Characterization of the pH signal transduction pathway gene palA of Aspergillus nidulans and identification of possible homologs. J. Bacteriol. 179:1832-1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Negrete-Urtasun, S., W. Reiter, E. Diez, S. H. Denison, J. Tilburn, E. A. Espeso, M. A. Penalva, and H. N. Arst, Jr. 1999. Ambient pH signal transduction in Aspergillus: completion of gene characterization. Mol. Microbiol. 33:994-1003. [DOI] [PubMed] [Google Scholar]
- 28.Orejas, M., E. A. Espeso, J. Tilburn, S. Sarkar, H. N. Arst, Jr., and M. A. Penalva. 1995. Activation of the Aspergillus PacC transcription factor in response to alkaline ambient pH requires proteolysis of the carboxy-terminal moiety. Genes Dev. 9:1622-1632. [DOI] [PubMed] [Google Scholar]
- 29.Penalva, M. A., and H. N. Arst, Jr. 2002. Regulation of gene expression by ambient pH in filamentous fungi and yeasts. Microbiol. Mol. Biol. Rev. 66:426-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pfaller, M. A., D. J. Diekema, R. N. Jones, H. S. Sader, A. C. Fluit, R. J. Hollis, and S. A. Messer. 2001. International surveillance of bloodstream infections due to Candida species: frequency of occurrence and in vitro susceptibilities to fluconazole, ravuconazole, and voriconazole of isolates collected from 1997 through 1999 in the SENTRY antimicrobial surveillance program. J. Clin. Microbiol. 39:3254-3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pfaller, M. A., R. N. Jones, S. A. Messer, M. B. Edmond, and R. P. Wenzel. 1998. National surveillance of nosocomial blood stream infection due to Candida albicans: frequency of occurrence and antifungal susceptibility in the SCOPE program. Diagn. Microbiol. Infect. Dis. 31:327-332. [DOI] [PubMed] [Google Scholar]
- 32.Popolo, L., M. Vai, E. Gatti, S. Porello, P. Bonfante, R. Balestrini, and L. Alberghina. 1993. Physiological analysis of mutants indicates involvement of the Saccharomyces cerevisiae GPI-anchored protein gp115 in morphogenesis and cell separation. J. Bacteriol. 175:1879-1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Porta, A., A. M. Ramon, and W. A. Fonzi. 1999. PRR1, a homolog of Aspergillus nidulans palF, controls pH-dependent gene expression and filamentation in Candida albicans. J. Bacteriol. 181:7516-7523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Porta, A., Z. Wang, A. Ramon, F. A. Muhlschlegel, and W. A. Fonzi. 2001. Spontaneous second-site suppressors of the filamentation defect of prr1Δ mutants define a critical domain of Rim101p in Candida albicans. Mol. Genet Genomics 266:624-631. [DOI] [PubMed] [Google Scholar]
- 35.Ramon, A. M., A. Porta, and W. A. Fonzi. 1999. Effect of environmental pH on morphological development of Candida albicans is mediated via the PacC-related transcription factor encoded by PRR2. J. Bacteriol. 181:7524-7530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saporito-Irwin, S. M., C. E. Birse, P. S. Sypherd, and W. A. Fonzi. 1995. PHR1, a pH-regulated gene of Candida albicans, is required for morphogenesis. Mol. Cell. Biol. 15:601-613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmidt, C. L., M. Grey, M. Schmidt, M. Brendel, and J. A. Henriques. 1999. Allelism of Saccharomyces cerevisiae genes PSO6, involved in survival after 3-CPs+UVA induced damage, and ERG3, encoding the enzyme sterol C-5 desaturase. Yeast 15:1503-1510. [DOI] [PubMed] [Google Scholar]
- 38.Su, S. S., and A. P. Mitchell. 1993. Molecular characterization of the yeast meiotic regulatory gene RIM1. Nucleic Acids Res. 21:3789-3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tilburn, J., S. Sarkar, D. A. Widdick, E. A. Espeso, M. Orejas, J. Mungroo, M. A. Penalva, and H. N. Arst, Jr. 1995. The Aspergillus PacC zinc finger transcription factor mediates regulation of both acid- and alkaline-expressed genes by ambient pH. EMBO J. 14:779-790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trilla, J. A., T. Cos, A. Duran, and C. Roncero. 1997. Characterization of CHS4 (CAL2), a gene of Saccharomyces cerevisiae involved in chitin biosynthesis and allelic to SKT5 and CSD4. Yeast 13:795-807. [DOI] [PubMed] [Google Scholar]
- 41.Wilson, R. B., D. Davis, and A. P. Mitchell. 1999. Rapid hypothesis testing in Candida albicans through gene disruption with short homology regions. J. Bacteriol. 181:1868-1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Withee, J. L., R. Sen, and M. S. Cyert. 1998. Ion tolerance of Saccharomyces cerevisiae lacking the Ca2+/CaM-dependent phosphatase (calcineurin) is improved by mutations in URE2 or PMA1. Genetics 149:865-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu, W., and A. P. Mitchell. 2001. Yeast PalA/AIP1/Alix homolog Rim20p associates with a PEST-like region and is required for its proteolytic cleavage. J. Bacteriol. 183:6917-6923. [DOI] [PMC free article] [PubMed] [Google Scholar]