Abstract
We report on the contamination of commercial 15-nitrogen (15N) N2 gas stocks with 15N-enriched ammonium, nitrate and/or nitrite, and nitrous oxide. 15N2 gas is used to estimate N2 fixation rates from incubations of environmental samples by monitoring the incorporation of isotopically labeled 15N2 into organic matter. However, the microbial assimilation of bioavailable 15N-labeled N2 gas contaminants, nitrate, nitrite, and ammonium, is liable to lead to the inflation or false detection of N2 fixation rates. 15N2 gas procured from three major suppliers was analyzed for the presence of these 15N-contaminants. Substantial concentrations of 15N-contaminants were detected in four Sigma-Aldrich 15N2 lecture bottles from two discrete batch syntheses. Per mole of 15N2 gas, 34 to 1900 µmoles of 15N-ammonium, 1.8 to 420 µmoles of 15N-nitrate/nitrite, and ≥21 µmoles of 15N-nitrous oxide were detected. One 15N2 lecture bottle from Campro Scientific contained ≥11 µmoles of 15N-nitrous oxide per mole of 15N2 gas, and no detected 15N-nitrate/nitrite at the given experimental 15N2 tracer dilutions. Two Cambridge Isotopes lecture bottles from discrete batch syntheses contained ≥0.81 µmoles 15N-nitrous oxide per mole 15N2, and trace concentrations of 15N-ammonium and 15N-nitrate/nitrite. 15N2 gas equilibrated cultures of the green algae Dunaliella tertiolecta confirmed that the 15N-contaminants are assimilable. A finite-differencing model parameterized using oceanic field conditions typical of N2 fixation assays suggests that the degree of detected 15N-ammonium contamination could yield inferred N2 fixation rates ranging from undetectable, <0.01 nmoles N L−1 d−1, to 530 nmoles N L−1 d−1, contingent on experimental conditions. These rates are comparable to, or greater than, N2 fixation rates commonly detected in field assays. These results indicate that past reports of N2 fixation should be interpreted with caution, and demonstrate that the purity of commercial 15N2 gas must be ensured prior to use in future N2 fixation rate determinations.
Introduction
Nitrogen (N) is a major nutrient required universally by photosynthetic organisms. Its availability in the environment can directly affect the ecology and productivity of terrestrial and marine ecosystems, with important implications for the regional and global carbon cycles. The natural input of bioavailable N to the biosphere is dominated by nitrogen fixation, the biological reduction of dinitrogen (N2) gas to ammonium (NH4 +). Two methods are commonly utilized to measure N2 fixation rates in the field, the 15N2 tracer assay [1] and the acetylene (C2H2) reduction assay [2,3]. The 15N2 tracer assay was originally developed when artificially 15N-enriched substrate N2 first became available [4]. This approach was then superseded by the acetylene reduction technique, as the cost and availability of high precision isotope ratio measurements proved restrictive [3]. The acetylene reduction assay, however, is associated with variations in the factor used to convert C2H2 reduction into N2 equivalents, and with potentially biasing effects of C2H2 on the physiology of N2 fixing organisms, among other issues [5]–[7]. Interest in the 15N2 tracer assay later regained momentum, owing to the increased affordability of Isotope Ratio Mass Spectrometry (IRMS) instrumentation and to concurrent developments in 15N tracer techniques. Today, it is generally the preferred method to quantify N2 fixation rates in both terrestrial and aquatic environments [1], owing to its high sensitivity, and ability to provide qualitative and quantitative constraints on the translocation and the fate of biologically fixed N [8]–[10].
A salient strength of the 15N2 tracer assay is that 15N-enrichment detected in biomass can be ascribed to the biological reduction of N2 exclusively, as no interfering processes can carry out the reduction of 15N2 gas concurrently. This premise requires that the 15N2 stock be devoid of any contaminant 15N-species that could be assimilated into biomass simultaneously. However, during recent research projects on N2 fixation conducted independently in our laboratories at the University of Connecticut Avery Point and the University of Massachusetts Dartmouth, convergent observations indicated that some commercial 15N2 stocks could be contaminated with 15N-enriched N-species other than N2, including nitrate, nitrite and/or ammonium. These reactive forms of N would be readily assimilated by microorganisms, leading to significantly biased (i.e., overestimated) N2 fixation rate measurements.These observations motivated the current study, with the goal of testing whether commercially available 15N2 stocks contain 15N-contaminants at levels that would interfere with 15N2 tracer N2 fixation assays, particularly in the open ocean, and to assess if such contaminants are prevalent among 15N2 stocks from different suppliers. We thus uncovered substantial contamination of one of three brands of commercial 15N2 gas with bioavailable inorganic 15N-species. Our findings raise important concerns regarding the pervasiveness of reactive 15N contamination of the 15N2 stocks, and the extent to which these contaminants may have affected the magnitude of the N2 fixation rate estimates reported in the literature. We outline steps to contend with this issue to ensure the veracity of future N2 fixation estimates.
Methods
Reagents
Four 33 mL lecture bottles of 98+ at% 15N-labeled N2 gas were purchased from Sigma-Aldrich (produced by their subsidiary, Isotec Stable Isotopes; St. Louis, MO; Stock Keeping Unit 364584), three from lot # SZ1670V, synthesized in 2010, and one from lot # MBBB0968V, synthesized in 2014. Two 1L lecture bottles of 98+ at% 15N2 were purchased from Cambridge Isotopes (Tewksbury, MA, part # NLM-363-1-LB) from respective lot #’s I1-11785A and I-16727. One 1L lecture bottle of 98+ at% 15N2 was purchased from Campro Scientific (Berlin, Germany; catalogue # CS01-185_261) from lot # EB1169V. Ammonium and nitrate solutions were prepared with salts or with solutions obtained from different distributors: sodium nitrate (NaNO3: CAS 7631-99-4), potassium nitrate (KNO3: CAS 7757-79-1), and ammonium chloride (NH4Cl: CAS 12125-02-9) from Fisher Scientific (Pittsburgh, PA); analytical-grade potassium nitrate (CAS 7757-79-1) from Fluka Analytical and a gravimetric solution of ammonium chloride (catalogue # AS-NH3N9-2Y) from SPEX CertiPrep (Metuchen, NJ).
Preparation of nitrate and ammonium solutions equilibrated with 15N2 gas
In order to determine whether the 15N2 gas stocks contained 15N-labeled ammonia (NH3) or nitrate and/or nitrite (NOx) contaminants, aqueous solutions of natural abundance (unlabeled) ammonium and nitrate salts were equilibrated overnight with an air headspace supplemented with an injection of 15N2 gas. After equilibration, the 15N/14N ratio of ammonium and the 15N/14N and 18O/16O ratios of nitrate/nitrite in solution were measured, as well as the 15N/14N ratio of N2 gas in the headspace, as described below. The isotope ratios of nitrate and ammonium were compared to those in control solutions, which were not supplemented with 15N2 gas. Experiments with the Campro Scientific 15N2 stock were verified for 15N-nitrate/nitrite contaminants only (and not for 15N-ammonium).
Initial experiments consisted of 40 mL or 100 mL solutions of 10, 50, 100, 200, or 300 µmol L−1 nitrate and 5 µmol L−1 ammonium chloride in 60 mL or 120 mL serum vials that were sealed with Thermo Scientific gas-impermeant stoppers (part # C4020-30) or with Bellco Glass septum stoppers (catalogue # 2048-11800). The 20 mL of air headspace in each of the treatment vials was supplemented with 0.1 mL of 15N2 gas from respective bottles from each of the three suppliers (three lecture bottles from Sigma-Aldrich lot # SZ1670V and one bottle from lot # MBBB0968V, two bottles from Cambridge Isotopes lot # I1-11785A and lot # I-16727, and one bottle from Campro Scientific lot # EB1169V). The solutions were equilibrated overnight on a shaker, after which the 15N/14N and 18O/16O isotope ratios of nitrate were analyzed as described below. The 15N/14N isotope ratio of ammonium was also analyzed (described below) in experimental solutions treated with the Sigma-Aldrich and Cambridge Isotopes stocks, but not the Campro Scientific stock.
The experimental sensitivity to 15N-contaminants was increased in subsequent experiments involving 15N2 stocks that did not show clear evidence of contamination in the experiments described above (see Results ) by increasing the volume of 15N2 gas injections and decreasing solution volumes. Experiments were initiated in which 2 mL 15N2 gas was equilibrated overnight in 20 mL serum vials containing 10 mL solutions of 10 µmol L−1 sodium nitrate, after which the 15N/14N and 18O/16O ratios of nitrate were measured as described below. Similarly, 10 mL solutions of 5 µmol L−1 ammonium chloride were dispensed in 20 mL serum vials and equilibrated overnight with 2 mL 15N2 gas, after which the 15N/14N isotope ratios of ammonium were analyzed (described below).
The measured 18O/16O ratios of nitrate/nitrite in solutions equilibrated with 15N2 gas from some stocks suggested the presence of 46N2O contamination. As our analyte for isotope ratio analysis is N2O, and 46N2O can be explained by both 15N15N16O and 14N14N18O, N2O that is doubly labeled with 15N is falsely detected as δ18ONO3 enrichment. The presence of 46N2O contamination in 15N2 gas was verified directly for one of the Sigma-Aldrich stocks (Lot # SZ1670V) by adding 0.0125, 0.020, or 0.025 mL of 15N2 stock to 20 mL serum vials containing 10 nmoles of reference N2O in helium. The N and O isotopic composition of the N2O was analyzed as described below, and compared to unamended N2O injections.
Dunaliella tertiolecta cultures
The marine green alga Dunaliella tertiolecta was cultured in growth media equilibrated with 15N2 gas in order to ascertain the susceptibility of 15N-labeled gas contaminants to assimilation by non-N2-fixing organisms. Culture medium was prepared from filtered Long Island Sound sea water supplemented with 50 µmol L−1 NaNO3, 36.3 µmol L−1 NaH2PO4*H2O, and 107 µmol L−1 Na2SiO3*9H2O, as well as f/2 trace metals and f/2 vitamins [11], added from filter sterilized stock solutions. Medium (200 mL) was dispensed in 250 mL stoppered glass bottles. Experimental treatment bottles were equilibrated overnight with 0.2 mL 15N2 gas from either a Cambridge Isotopes (lot #I-16727) or Sigma-Aldrich (lot # SZ1670V) lecture bottle. Following inoculation, cultures were left loosely capped and placed on a windowsill with exposure to natural light. Nitrate concentrations were monitored daily. Upon the complete depletion of nitrate, 8 days after inoculation, the cultures were harvested on pre-combusted GF/F filters. Filters were dried at 60°C for 18 h pending N isotopic analysis of the particulate nitrogen (described below).
Nitrate and ammonium concentrations
Nitrate concentrations in the experimental solutions were verified via reduction to nitric oxide in hot vanadium (III) solution followed by detection with a chemiluminescence NOx analyzer (model T200 Teledyne Advanced Pollution Instrumentation) [12]. Ammonium concentrations were measured by derivatization with orthophthaldialdehyde (OPA) and fluorometric detection on an AJN Scientific f-2500 Fluorescence Spectrophotometer [13].
Nitrate N and O isotope ratio analyses
Nitrate/nitrite nitrogen (15N/14N) and oxygen (18O/16O) isotope ratios were measured using the denitrifier method [14,15]. Nitrate (and nitrite) in experimental samples was converted stoichiometrically to nitrous oxide (N2O) by a denitrifying bacterial strain (Pseudomonas chlororaphis f. sp. aureofaciens, ATCC 13985) that lacks nitrous oxide reductase. The N and O isotopic composition of N2O was then measured on a Delta V Advantage Isotope Ratio Mass Spectrometer (IRMS) interfaced with a modified Gas Bench II gas chromatograph (Thermo Fisher) purge and trap system. The isotope ratio measurements are reported in the conventional delta (δ) notation in per mille (‰) units, defined for N and O by the following equations:
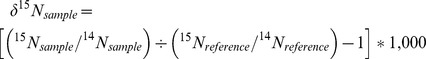 |
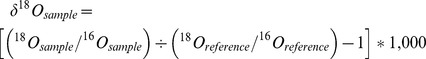 |
The 15N/14N reference is N2 in air, and the 18O/16O reference is Vienna Standard Mean Ocean water (V-SMOW). Individual analyses on the GC-IRMS were referenced to injections of N2O from a pure N2O gas cylinder, and then standardized through comparison to the international nitrate standards USGS-34 (δ15N of −1.8‰ vs. air; δ18O of −27.9‰ vs. V-SMOW), USGS-32 (δ 15N of +180‰ vs. air; δ18O of +25.7‰ vs. V-SMOW), and IAEA-NO-3 (δ15N of +4.7‰ vs. air; δ18O of +25.6‰ vs. V-SMOW) [16]–[18], using standard bracketing techniques. Nitrate samples from experiments with Campro Scientific 15N2 were standardized with USGS-32 and IAEA-NO-3, and an additional internal lab nitrate standard (UBN-1; δ15N of 14.15‰ vs. air; δ18O of +25.7‰ vs. V-SMOW). Precision for analytical replicates was ≤0.2‰ for δ15NNO3 and ≤0.2‰ for δ18ONO3 for isotope ratio amplitudes encompassed by the standards. Above 200‰, the precision decreased in proportion to δ15NNO3 amplitude, with standard deviations of 7.5‰ for δ15NNO3 at or above 1000‰. Similarly, precision decreased with increasing δ18ONO3, with standard deviations of 1.3‰ to 6.1‰ for δ18ONO3 values ≥80‰. The poor precision of the higher range measurements is likely due to the variable contribution of a trace NOX contaminant in denitrifier preparations with δ15N and δ18O values that are in the range of natural abundance samples [19].
Nitrous oxide N and O isotope ratio analyses
N2O isotope ratios were measured directly on the GC-IRMS, and referenced against the N2O tank, which was standardized indirectly by comparison to the δ15N and δ18O of nitrate standards.
Ammonium N isotope ratio analyses
The ammonium δ15NNH4 was measured using the hypobromite-azide method [20]. Ammonium in basic solution was converted to N2O via oxidation to nitrite (NO2 −) with hypobromite, followed by reduction of nitrite to N2O with sodium azide in acetic acid. The δ15N of the N2O analyte was measured on the GC-IRMS, as outlined above. Measurements were calibrated using solutions made from the international standard ammonium salts, IAEA-N1 and IAEA-N2, with assigned δ15N values of +0.4‰, +20.3‰ vs. air, respectively [16,17,21,22]. Our standard error for analytical replicates was ≤0.6‰ at relatively low 15N-abundances, but increased substantially for δ15NNH4 from 100‰ to 9000‰, varying from 2.9‰ to as high as 59.7‰. As with the nitrate analyses, the low precision of higher range measurements likely stems from the variable contribution of a trace ammonium or nitrite contaminant with a natural abundance δ15N value, inadvertently introduced during the analyses.
Headspace N2 isotope ratio analyses
To measure the δ15N of N2 gas in the headspace of experimental samples, 75 µL of headspace was injected into 12 mL Exetainer vials previously flushed with helium, then analyzed on a Gas Bench II GC-IRMS (Delta V Advantage Plus) operated in continuous flow mode. N2 and (O2+ Ar) were separated on a 5-Å mole-sieve capillary gas chromatography column. The analyses were standardized with parallel analyses of ambient N2 gas in air. These direct N2 gas measurements were carried out for experiments conducted using two of three lecture bottles from Sigma-Aldrich lot # SZ1670V, and for experiments conducted using the lecture bottle from Cambridge Isotopes lot # I1–11785A. The 15N2 concentration in the headspace of other experiments was estimated from the tracer injection volume rather than from direct measurements.
Particulate nitrogen isotope ratio analyses
The δ15N of particulate nitrogen (PN) was analyzed using a Costech Instruments elemental combustion system (model 4010) coupled to a Thermo Scientific Delta V Advantage IRMS. Analyses were standardized using L-glutamic acid reference materials, USGS-40 (δ15N of −4.52‰ vs. air) and USGS-41 (δ15N of +47.57‰ vs. air) [23].
Results
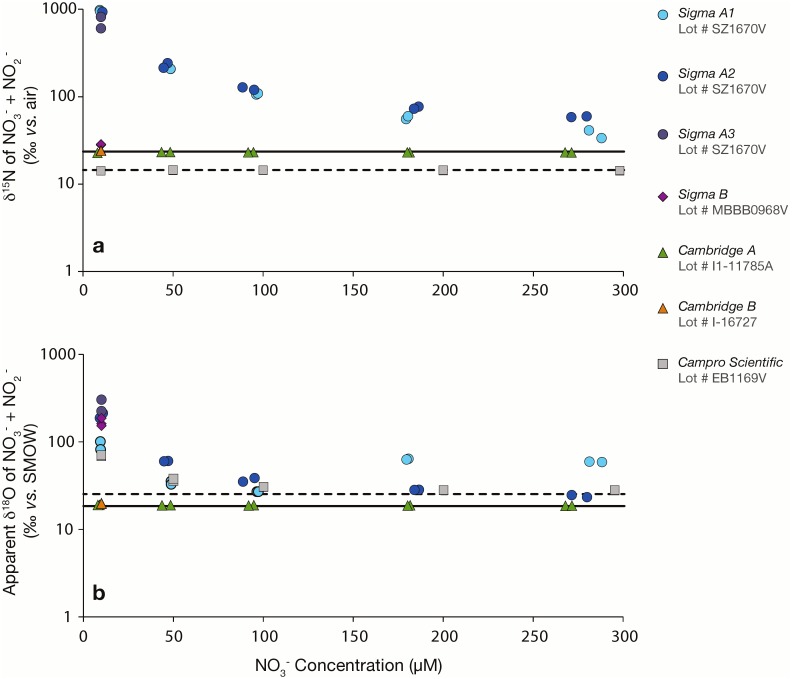
Nitrate solutions equilibrated with any of three 15N2 gas stocks from Sigma-Aldrich lot # SZ1670V (referred to hereafter as ‘Sigma A1, A2 and A3’) showed a substantial increase in the δ15N of nitrate (and possibly nitrite) compared to control solutions in the lower sensitivity nitrate dilutions (Fig. 1a). Respective 15N enrichments evidenced by the δ15NNO3+NO2 were inversely proportional to the concentration of nitrate in the solutions, from nearly 1000‰ at 10 µmol L−1 nitrate to 30‰ at 290 µmol L−1 nitrate, compared to a δ15NNO3 of 23.5±0.5‰ in the corresponding potassium nitrate control solutions. The 15N enrichments imparted on the nitrate solutions were comparable among the three lecture bottles from this lot (# SZ1670V). The δ15NNO3+NO2 resulting from equilibration with a single Sigma-Aldrich gas stock from lot # MBBB0968V (‘Sigma B’) was relatively modest, but still significant, averaging 28.4±0.3‰ at 10 µmol L−1 nitrate, compared to 23.5±0.5‰ in the corresponding control solutions (Fig. 1a). When tested at the more sensitive experimental dilution, equilibrations of nitrate solutions with the Sigma B stock resulted in a δ15NNO3+NO2 of 200.2±70.9‰, compared to a δ15NNO3 of 1.3±0.1‰ in control solutions (Fig. 2a). These measurements thus indicate that N2 gas stocks sourced from lot # SZ1670V contained 410±80 µmoles of 15N-nitrate and/or nitrite per mole of 15N2, whereas the bottle from lot # MBBB0968V contributed 1.8±0.6 µmoles of 15N-nitrate and/or nitrite per mole of 15N2 (Table 1). The 15N-nitrate additions were determined by a mass balance calculation:
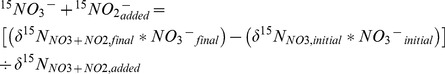 |
where 15NO3 − + NO2 − added is the moles of 15N-labeled nitrate and/or nitrite added by the 15N2 gas injection, δ15NNO3+NO2,added is presumed to be equivalent to the δ15N of 15N2 tracer gas (266,540‰), NO3 − initial and NO3 − final refer to the moles of nitrate in solution before and after 15N2 equilibration, and δ15NNO3,initial and δ15NNO3+NO2,final refer to the δ15NNO3 of nitrate solutions before and after 15N2 equilibration. The quantity of 15N2 gas added to experimental treatments was measured explicitly in Sigma A1 and A2 bottles, and was calculated from the 15N2 injection volumes for experiments treated with Sigma A3 and B stocks.
Figure 1. (a) δ15NNO3+NO2 (log scale) of nitrate solutions (10–300 µmol L−1) following equilibration with 0.1 mL 15N2 gas from lecture bottles procured from three distributors.
Solutions were 40 mL for Sigma-Aldrich and Campro Scientific equilibrations, and 100 mL for Cambridge Isotopes equilibrations. The solid line corresponds to the δ15NNO3 of the control solutions for Sigma-Aldrich and Cambridge Isotopes experiments (δ15NNO3 = 23.5±0.5‰); the dashed line corresponds to controls for Campro Scientific experiments (δ15NNO3 = 14.15±0.1‰). Paired symbols identify replicate experimental treatments. (b) Corresponding apparent δ18ONO3+NO2 of the experimental nitrate solutions. The solid line corresponds to the δ18ONO3 of control solutions for the Sigma-Aldrich and Cambridge Isotope experiments (δ18ONO3 = 18.9±0.3‰); the dashed line corresponds to controls for Campro Scientific experiments (25.4±0.3‰).
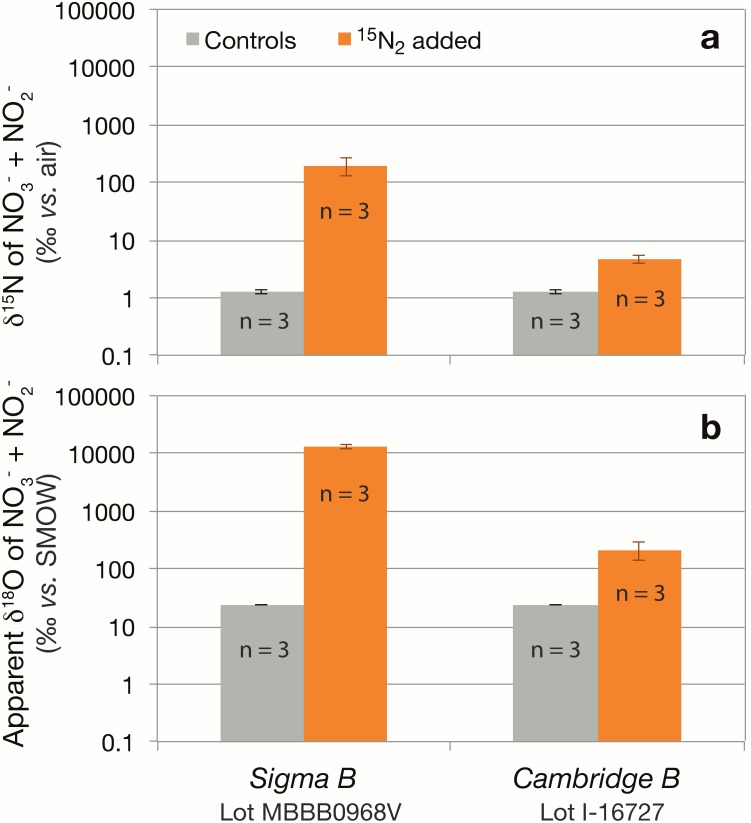
Figure 2. (a) δ15NNO3+NO2 (log scale) of higher sensitivity equilibrations of 10 µmol L−1 nitrate solutions (10 mL) with 2 mL of 15N2 gas from a Cambridge Isotopes or a Sigma-Aldrich bottle.
(b) Corresponding apparent δ18ONO3 (log scale) of higher sensitivity equilibrations of the two stocks. n = the number of experimental replicates.
Table 1. The quantity of 15N-labeled contaminants detected relative to 15N2 additions.
| µmoles 15NX per mole 15N2 | |||
| 15NO3 −/NO2 − | 15NH4 + | 46N2O | |
| Sigma A1lot # SZ1670V | 420±110 | 34±11 | ≥21±3 |
| Sigma A2lot # SZ1670V | 420±40 | 520±30 | 109±5§ |
| Sigma A3 †lot # SZ1670V | 350±80 | N/A | ≥63±15 |
| Sigma B †lot # MBBB0968V | 1.8±0.6 | 1900±560 | ≥49±17 |
| Cambridge Alot # I1-11785A | n.d.* | 0.052±0.020 | n.d.* |
| Cambridge B †lot # I-16727 | 0.024±0.006 | 0.014±0.004 | ≥0.81±0.24 |
| Campro Scientific†lot # EB1169V | n.d.* | N/A | ≥11±3 |
The µmoles of 15N contaminants (NO3 −+NO2 −, NH4 +, and N2O) detected per mole of 15N2 gas from lecture bottles provided by different suppliers. N/A = not available; n.d. = not detected.
*Not explicitly tested in high sensitivity 15N2 dilutions.
Moles of 15N2 estimated from the injection volume rather than direct measurements.
46N2O measured directly.
In contrast to the Sigma-Aldrich stocks, 15NNO3+NO2 contaminants were significantly lower, or possibly absent, in Cambridge Isotopes and Campro Scientific 15N2 stocks. The δ15NNO3+NO2 values of solutions treated with Cambridge Isotopes 15N2 gas (lots # I1–11785A and I-16727, hereafter referred to as Cambridge A and Cambridge B, respectively) and with Campro Scientific 15N2 gas (lot # EB1169V) were indistinguishable from those of control solutions at all experimental nitrate concentrations in the lower sensitivity tests (Fig. 1a). In the more sensitive experimental treatments, however, solutions treated with Cambridge B 15N2 gas (lot # I-16727) had a δ15NNO3+NO2 of 4.8±0.8‰, compared to a δ15NNO3 of 1.3±0.1‰ in control solutions (Fig. 2a). This stock thus contributed trace contaminants on the order of 0.024±0.006 µmoles of 15N-nitrate and/or nitrite per mole of 15N2 (Table 1). Nitrate isotope ratios in the Cambridge A (lot # I1–11785A) and Campro Scientific 15N2 gas stocks were not tested at these lower experimental dilutions.
In treatments using several 15N2 gas stocks, δ18ONO3 was found to be elevated relative to control solutions. The denitrifier method, employed for δ18ONO3 measurements, involves the bacterial reduction of NO3 − and NO2 − to N2O, and the subsequent analysis of N2O using an IRMS. However, the elevated δ18ONO3 values detected within experimental treatments are expressly not explained by the formation of 14N14N18O during bacterial reduction of 15N-enriched nitrate, which could only account for a negligible portion of the observed δ18ONO3+NO2 increase. Instead, the values are best explained by the presence of doubly-labeled 15N–N2O (i.e., 46N2O) in the 15N2 gas stocks. The apparent δ18ONO3+NO2 of nitrate solutions equilibrated with all of the Sigma-Aldrich stocks, the Campro Scientific stock, and the Cambridge B 15N2 stock proved to be greater than that of control solutions in the low sensitivity treatments (Fig. 1b). At 10 µmol L−1 nitrate, the apparent δ18ONO3+NO2 of treated solutions was 188.5±83.8 among the Sigma A1-A3 stocks (lot # SZ1670V), 169.8±17.9‰ for the Sigma B stock (lot # MBBB0968V), and 20.1±0.2 for the Cambridge B stock, compared to 18.9±0.3‰ in corresponding control solutions. The apparent δ18ONO3+NO2 of the Campro Scientific stock at 10 µmol L−1 nitrate was 70.4±1.4‰, compared to 25.7±0.1‰ in corresponding control solutions. The apparent δ18ONO3+NO2 of the samples decreased coherently with increasing nitrate concentrations for respective stocks. The apparent δ18ONO3+NO2 values of solutions equilibrated with the Cambridge A stock, at 19.1±0.2‰, were not distinguishable from the control solutions. In the more sensitive equilibrations, nitrate solutions equilibrated with Sigma B 15N2 gas had a δ18ONO3+NO2 of 13,129±1186‰ compared to 23.9±0.2‰ in control solutions, whereas the δ18ONO3+NO2 of the Cambridge B stock was 216.7±78.4‰ (Fig. 2b). Given that the apparent δ18ONO3 enrichments are explained by the presence of 46N2O, the inverse relationship between δ18O values and nitrate concentration stems from the fact that the detected 46N2O derives from 46N2O dissolved in the nitrate solutions, and solutions containing higher nitrate concentrations require lower sample volume injections when using the denitrifier method for IRMS analysis. The observed excess 46N2O levels indicate 15N15N16O contaminants (µmole 46N2O per mole of 15N2) on the order of 41±21 among the Sigma A1-A3 bottles, 49±17 in Sigma B, 11±3 in Campro Scientific, and 0.81±0.24 in Cambridge B (Table 1). The Cambridge A bottle was not tested in higher sensitivity dilutions that could have revealed the presence of some N2O therein. The presence of 46N2O contaminant was verified directly for the Sigma A2 lecture bottle from analyses of N2O amended with injections of 15N2 gas. Among four experimental samples, 109±5 µmoles of 46N2O were detected per mole of Sigma A2 15N2 added, more than double the 46N2O that was detected in samples analyzed by the denitrifier method (39±8 µmole 46N2O per mole of 15N2). This discrepancy likely resulted because samples analyzed by the denitrifier method were uncapped immediately prior to their injection into P. aureofaciens denitrifier cultures, allowing N2O to escape to the atmosphere. As contaminant N2O was not the target analyte of the denitrifier measurements, precautions were not taken to prevent N2O gas loss at this step. The 46N2O concentrations derived from solution equilibrations of respective 15N2 stocks thus constitute lower limits (Table 1).
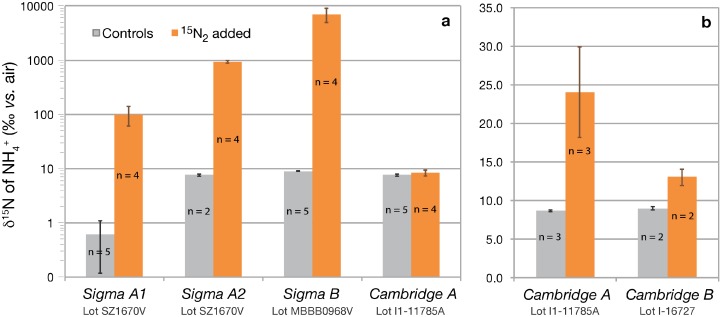
Solutions equilibrated with Sigma-Aldrich 15N2 gas showed substantial 15N-enrichments of ammonium compared to control solutions (Fig. 3a): Equilibration with 15N2 from the Sigma A1 lecture bottle (lot # SZ1670V) resulted in a δ15NNH4 of 99±39‰, compared to 0.6±0.5‰ for the control solution (NH4Cl, SPEX CertiPrep); equilibration with 15N2 from the Sigma A2 bottle (lot # SZ1670V) yielded a δ15NNH4 of 940±60‰, compared to 7.6±0.3‰ for the control solution (NH4Cl salt, Fisher Scientific); equilibration with 15N2 from the Sigma B bottle (lot # MBBB0968V) resulted in a δ15NNH4 of 7030±2100‰, compared to 9.0±0.06‰ for the corresponding control solutions (NH4Cl salt, Fisher Scientific). Mass balance calculations based on these isotope ratio values thus evidence the presence of 34±11, 518±26, and 1890±560 µmoles of 15N-ammonium per mole of 15N2 injected from Sigma A1, A2, and B 15N2 bottles, respectively (Table 1). Unlike 15N-labelled nitrate/nitrite contaminants, the 15N-ammonium contaminants appeared to be variable among bottles of lot # SZ1670V (Fig. 3a). The Sigma A3 lecture bottle was not tested for 15N-ammonium.
Figure 3. (a) δ15NNH4 (log scale) of 5 µmol L−1 ammonium solutions after equilibration with 0.1 mL 15N2 gas from respective Sigma-Aldrich and Cambridge Isotopes lecture bottles vs. control solutions.
Sigma-Aldrich treatments utilized 40 mL ammonium solutions, whereas Cambridge Isotopes treatments utilized 100 mL ammonium solutions. (b) δ15NNH4 of higher sensitivity equilibrations of 5 µmol L−1 ammonium solutions (10 mL) with 2.0 mL 15N2 gas from Cambridge Isotopes lecture bottles vs. control solutions. n = the number of experimental replicates.
In contrast to Sigma-Aldrich stocks, ammonium solutions equilibrated with 15N2 from the Cambridge A bottle had a δ15NNH4 of 8.3±1.0‰, comparable to that of the corresponding control solution of 7.6±0.3‰ (NH4Cl salt, Fisher Scientific) in the lower sensitivity experiments (Fig. 3a). In the more sensitive dilutions, however, 15N-ammonium contaminants were detected in both of the Cambridge A and B stocks (Fig. 3b). Solutions equilibrated with Cambridge A had a δ15NNH4 of 24.0±5.9‰, compared to 8.7±0.1‰ for the control solutions (NH4Cl salt, Fisher Scientific) and solutions equilibrated with Cambridge B had a δ15NNH4 of 13.1±1.1‰, compared to 9.0±0.1‰ for the control solutions ((NH4Cl salt, Fisher Scientific). The enrichment relative to control solutions invariably originates from a 15N-ammonium contaminant, and cannot be attributed to a trace N2O (15N14N16O) contaminant, because the samples were purged when conducting 15N-ammonium analyses, following the oxidation of ammonium to nitrite with hypobromite. These more sensitive treatments thus reveal the presence of minuscule 15N-ammonium concentrations in the Cambridge Isotopes stocks, on the order 0.052±0.020 and 0.014±0.004 µmoles of 15N-ammonium per mole of 15N2 gas in lots # I1–11785A and I-16727, respectively (Table 1).
The control solutions in the 15N-ammonium experiments prepared from a single Fisher Scientific NH4Cl salt stock revealed progressively heavier mean δ15NNH4 values among experiments, at 7.6±0.3‰, 8.7±0.1‰, or 9.0±0.06‰. The solutions with a δ15NNH4 estimated at 7.6±0.3‰ and 8.7±0.1‰ were made fresh from the salts for each experiment, such that the 15N-enrichment of ammonium cannot be attributed to the progressive degassing of ammonia in solution during storage. In turn, isotopic standards for NH4 + (IAEA-N1 and N2) were stored in acidic solution, and thus were not subject to progressive degassing. Moreover, degassing of the isotopic standards would manifest as progressively lower δ15N values measured for control solutions. Inter-batch variability intrinsic to the hypobromite-azide method [20] is plausible, as this technique is relatively recent, such that subtle sensitivities may not yet be apparent.
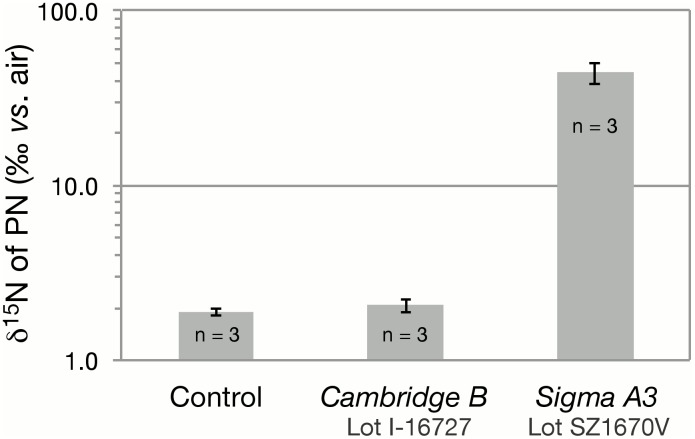
D. tertiolecta cultures grown in medium equilibrated with 15N2 gas from the Sigma A3 bottle expectedly showed substantial 15N enrichment of particulate nitrogen (δ15NPN), averaging 44.3±6.1‰ among triplicate treatment cultures compared to 1.9±0.1‰ in control cultures (Fig. 4). Conversely, the δ15NPN of cultures equilibrated with Cambridge B 15N2 gas was 2.1±0.2‰, and thus not detectably different from that of control cultures, at 1.9±0.1‰ (Fig. 4).
Figure 4. The δ15N of particulate nitrogen (δ15NPN) of D. tertiolecta harvested in stationary phase following growth in media containing sodium nitrate (and no ammonium) and equilibrated with 15N2 gas from Sigma Aldrich or Cambridge Isotopes.
n = the number of experimental replicates.
Discussion
This study reveals that some commercial 15N2 gas stocks contain contaminant 15N-labeled bioavailable nitrogen species, including nitrate/nitrite, ammonium and nitrous oxide. Substantial levels of 15N-labeled nitrate/nitrite, ammonium, and nitrous oxide were detected in Sigma-Aldrich stocks from lot # SZ1670V. Another Sigma-Aldrich stock from a different lot (# MBBB0968V) contained considerably less, but still significant, 15N-nitrate/nitrite contaminants, similar nitrous oxide concentrations, and a greater concentration of 15N-ammonium. Cambridge Isotopes stocks, in turn, contained relatively low concentrations of 15N-nitrate/nitrite, 15N-ammonium and 15N-nitrous oxide. A 15N2 stock from Campro Scientific contained no detected 15N-nitrate/nitrite contaminants in low sensitivity experiments, but measurable 15N-nitrous oxide. Indeed, a certificate of analysis provided by Campro Scientific attests that stocks may contain up to 15 ppm N2O. 15N-ammonium was not analyzed in Campro Scientific gas, nor was the stock tested at more sensitive 15N2 dilutions, which could have revealed trace 15N-nitrate or ammonium contaminants in the stock. In any case, 15N-contamination with nitrous oxide is of no obvious consequence for biological 15N2 applications, such as N2 fixation rate measurements. However, the presence of 15N-nitrate, nitrite and ammonium has serious implications for measurements of N2 fixation, as these contaminants could lead to the detection of false positives or inflated rates.
The propensity of the detected 15N-labeled contaminants to be assimilated into biomass was verified directly from cultures of D. tertiolecta, which acquired elevated δ15N of PN in media equilibrated with a Sigma-Aldrich 15N2 stock. Expectedly, media equilibrated with 15N2 from a Cambridge Isotopes stock did not cause detectable 15N-enrichment of biomass. At the given experimental conditions, however, the complete assimilation of the contaminant 15N-nitrate/nitrite from the Sigma-Aldrich stock should have yielded greater δ15NPN values than those observed, of at least 56.3±5.4‰ (vs. control δ15NPN values of ∼1.9‰), notwithstanding the additional contribution of any 15N-ammonium contaminant (15N-ammonium was not measured explicitly in the Sigma A3 stock). This discrepancy is difficult to reconcile. We tentatively posit that 15N-nitrite comprises a substantial fraction of the trace 15N-nitrate/nitrite contaminant, and that D. tertiolecta may not be able to transport nitrite at nanomolar to sub-nanomolar concentrations. Indeed, such trace nitrite concentrations are likely below the thresholds achievable by micro-algal nitrite transport systems [24].
The contaminants in the 15N2 stocks ostensibly derive from the method of 15N2 gas production. 15N2 gas is generally produced by the catalytic oxidation of 15N ammonia (15NH3) gas with cupric oxide [25]. The bulk of the oxidation product is N2 gas, although more oxidized N species are also produced in lesser quantities, specifically N2O and NO [26]. Thus, potential contaminants in a 15N2 gas stock would expectedly consist of unreacted ammonia gas, N2O, and nitric oxide (NO). In contact with any oxygen and water vapor, NO would inadvertently be oxidized to nitric and nitrous acid [27], which would, in turn, dissociate to nitrate and nitrite upon dissolution in water, respectively. Purification of the 15N2 gas from unreacted ammonia and from the generated nitrogen oxides involves sequential acid and alkaline scrubbing, respectively [28] and/or cryo-trapping of ammonia and NOx gases. Upon personal communication, Cambridge Isotopes and Campro Scientific did not provide details on their method of 15N2 production, whereas Sigma-Aldrich reported that the company’s subsidiary, Isotec, produces 15N2 gas by the catalytic oxidation of 15N-ammonia gas with cupric oxide, followed by sequential rounds of cryo-trapping and alkaline scrubbing of the N2 gas to increase purity.
In order to gauge the extent to which the observed 15N-ammonium contamination of Sigma-Aldrich and Cambridge Isotopes 15N2 gas could skew estimates of N2 fixation in incubations with 15N2 gas, we modeled a field incubation experiment in which microorganisms assimilate the 15N-ammonium contaminant rather than reduce 15N2 gas. A simple finite-differencing model of a ‘typical’ oceanic N2 fixation assay was devised, in which 0.1 mL of 15N2 gas was equilibrated in two different water sample volumes, 0.25 L or 4.5 L, then incubated for 24 hours. Prescribed biomass and growth rates were characteristic of those at the oligotrophic surface ocean, namely, a particulate N stock of 0.2 µmol L−1 assimilating ammonium at a specific growth rate coefficient (µ) of 0.1 d−1 to 0.3 d−1, with a recycling rate (the rate at which particulate N is returned to the ammonium pool) equivalent to the respective growth rate. The prescribed δ15N of the initial particulate N was 0‰ [29], and the δ15N of ambient ammonium was −2‰ [30]. Incremental initial concentrations of ambient ammonium were prescribed, from 1 nmol L−1 to 1 µmol L−1. Ammonium concentrations in surface oligotrophic waters are typically very low (≤10 nmol L−1), however, ammonium is a pervasive contaminant that could easily be introduced during sample preparation, as well as leached from incubation vial septa. We note that the 15N-ammonium introduced by the 15N2 gas, while substantial in terms of the 15N/14N ratio of ammonium, is on the order of ∼20 nanomolar at most (under the modeled conditions), and thus has minimal effect on ambient ammonium concentrations. 14N-ammonium contamination is expected to be negligible, given the method of 15N2 gas synthesis. Finally, 15N-ammonium assimilation was simulated for the broad range of 15N-ammonium contaminant concentrations observed among Sigma-Aldrich and Cambridge Isotopes lecture bottles. N2 fixation rates inferred from the simulated δ15N increase of particulate N were computed based on the formulation of Montoya (1996):
[PN]Δ is the change in particulate nitrogen concentration, [PN]f is the final particulate nitrogen concentration, A PNf is the final 15N enrichment of particulate nitrogen, A PN0 is the initial 15N enrichment of particulate nitrogen, A N2 is the 15N enrichment of the N2 available for fixation, V is the specific rate of N2 uptake, and ρ is the volumetric rate of N2 fixation.
The model-derived ‘N2 fixation rates’ resulting from Sigma-Aldrich 15N-ammonium contaminant levels ranged from undetectable, <0.01 nmol N L−1 d−1, to as high as 530 nmol N L−1 d−1 under the modeled conditions (Table 2). Rates were clearly sensitive to the concentration of 15N-contaminant, the ambient ammonium concentration, the incubation volume, and the specific growth rate. At the lower level of 15N-ammonium contaminant observed in the Sigma-Aldrich stocks, N2 fixation rates were comparable to rates observed in situ for nearly all parameter permutations, from<0.01 to 9 nmoles N L−1 d−1. N2 fixation rates reported for marine environments cover a broad range, from 0.01 nmoles L−1 d−1 to tens of nmoles N L−1 d−1 [31]–[33]. Rates simulated with the highest observed level of contaminant, in smaller incubation volumes at given 15N2 additions, and/or with low ambient ammonium concentrations, tended to surpass rates observed in situ by 10 to 100 fold. The N2 fixation rates modeled using the minute contaminant level detected in a Cambridge Isotopes stock ranged from undetectable to 0.02 nmoles N L−1 d−1 (Table 2), approximating the lower limit of some N2 fixation rates reported in the literature [31],[34]–[38]. These simulated rates can be deemed conservative since the model does not account for any assimilation of contaminant 15N-nitrate/nitrite, and the 0.1 mL 15N2 injection volume used in the model is on the lower end of 15N2 injection volumes typically used in open ocean N2 fixation rate measurements.
Table 2. Inferred N2 fixation rates (nmoles N L−1 d−1) resulting from 15N-labeled contaminants.
| Ambient [NH4+] | Cambridge Isotopes | Sigma-Aldrich | Sigma-Aldrich | |||||||
| (µmol L−1) | Cambridge A | Sigma A1 | Sigma B | |||||||
| Lot # I1–11785A | Lot # SZ1670V | Lot # MBBB0968V | ||||||||
| 0.52 µmol 15NH4 +/mol 15N2 | 25 µmol 15NH4 +/mol 15N2 | 1900 µmol 15NH4 +/mol 15N2 | ||||||||
| µ = 0.1 | µ = 0.2 | µ = 0.3 | µ = 0.1 | µ = 0.2 | µ = 0.3 | µ = 0.1 | µ = 0.2 | µ = 0.3 | Incubation Volume (L) | |
| 0.001 | 0.019 | 0.019 | 0.019 | 9.0 | 9.0 | 9.0 | 310 | 460 | 530 | 0.25 |
| 0.01 | 0.014 | 0.016 | 0.016 | 7.5 | 8.5 | 8.6 | 250 | 400 | 470 | |
| 0.1 | n.d. | n.d. | n.d | 1.6 | 2.7 | 3.6 | 90 | 170 | 220 | |
| 1 | n.d. | n.d. | n.d. | 0.17 | 0.32 | 0.45 | 13 | 24 | 34 | |
| 0.001 | n.d. | n.d. | n.d. | 0.50 | 0.50 | 0.50 | 38 | 38 | 38 | 4.5 |
| 0.01 | n.d. | n.d. | n.d. | 0.42 | 0.47 | 0.48 | 30 | 35 | 36 | |
| 0.1 | n.d. | n.d. | n.d. | 0.08 | 0.15 | 0.19 | 6.5 | 11 | 15 | |
| 1 | n.d. | n.d. | n.d. | n.d. | 0.012 | 0.017 | 0.7 | 1.4 | 1.9 | |
N2 fixation rates that would be inferred from 24-h field N2 fixation assays conducted with 15N2 stocks containing the respective concentrations 15N-ammonium contaminants detected in Sigma-Aldrich and Cambridge Isotopes 15N2 gas. In the simulations, microbial plankton assimilate 15N-ammonium rather than fix 15N2. Incubations are simulated in volumes of 0.25 L or 4.5 L equilibrated with 0.1 mL of 15N2 gas, with 2.0×10−7 µmol L−1 of plankton nitrogen (with a δ15N = 0‰) assimilating at a range of specific growth rates, µ (d−1), countered by equivalent recycling rates, at incremental concentrations of ambient ammonium (δ15NNH4 = −2‰). Inferred rates of <0.01 nmoles N L−1 d−1 are considered undetectable (n.d.).
Based on the simulations above, the likelihood of N2 fixation rates being inflated when using contaminated 15N2 gas stocks is high. It is surprising, then, that contamination of the 15N2 stocks has not been reported previously. While growth solely upon N from N2 fixation would eliminate the effect of 15N-labeled bioavailable contaminants, it is expected that nitrate and ammonium assimilation would be rapid relative to N2 fixation due to the prohibitive energetic cost of N2 fixation [39]. A review of pertinent literature reveals that soil scientists were once aware of the possible contamination of 15N2 with bioavailable N, and took steps to mitigate it [28,40]. However, to the best of our knowledge, there is no mention of potential contamination of 15N2 stocks in the marine literature, or in more recent terrestrial literature. The fact that this issue has gone unnoticed could mean that major contamination of 15N2 gas stocks, such as that observed here in Sigma-Aldrich stocks, could be limited to the current lots. Supporting the notion that contamination is rare is the observation of undetectable N2 fixation rates at the surface ocean, where phytoplankton readily assimilate ammonium [38] – even in investigations utilizing the Sigma-Aldrich (Isotec) 15N2 gas [31]. However, a representative at Isotec stated that their procedures for synthesis and purification of 15N2 gas have not changed in past decades, which suggests that 15N-contaminants may have been pervasive in previous lots. Failure to detect interferences from 15N-contaminants in previous studies may then stem from incubation conditions conspiring to yield expected rates of apparent N2 fixation in spite of the presence of 15N contaminants (Table 2). Interference of 15N contaminants on N2 fixation rate measurements may then be relatively minor in systems where bioavailable N assimilation rates are low and/or where ambient nitrate and ammonium concentrations are relatively elevated (≥100 nmol L−1; Table 2), as ambient assimilable N effectively diminishes 15N enrichment resulting from 15N-labeled contaminants.
It is difficult, if not impossible, to discern whether N2 fixation rate estimates in previous studies may have been confounded due to the assimilation of 15N contaminants in 15N2 gas stocks. Given that 15N2 stocks from only one of the three suppliers tested here contained contaminants to an extent that would interfere with any but the lowest reported N2 fixation measurements, there is a strong likelihood that published estimates performed with 15N2 from the other two suppliers have not been significantly inflated by labeled contaminants. In fact, many estimates may be lower than reality due to the incomplete equilibration of 15N2 gas with the incubation medium, a pervasive problem with aqueous 15N2 fixation assays that was diagnosed only recently [41]–[43]. Nevertheless, it is advisable at this point to analyze commercial 15N2 stocks prior to their use to ensure their relative purity. In doing so, particular attention must be paid to the lower limit of detection for N2 fixation rates. In recent years, workers have reported estimates of very low rates (≤0.1 nmol L−1 d−1) in environments where N2 fixation is otherwise unexpected, which include oxygen-deplete regions of the water column at Pacific margins [34,35,38], as well as in the Beaufort Gyre of the Arctic Ocean [36]. Such minimal rates are questionable, considering that the relatively clean Cambridge Isotopes 15N2 gas was found to contain enough 15N-ammonium to infer N2 fixation rates of up to 0.02 nmoles N L−1 d−1. Campro Scientific and other commercially available 15N2 gas stocks could similarly contain minute, but significant, concentrations of 15N-nitrate or ammonium. Therefore, it behooves investigators to not only verify the purity of their commercial 15N2 prior to use, but also to generate constraints as to the lower limit of detection, allowing for the possibility that a trace-level 15N-contaminant could interfere with the detection of diminutive N2 fixation rates.
Steps toward mitigation
The catalytic synthesis of 15N2 gas from 15N-ammonia gas invariably entails the incidence of 15N-ammonium and 15N–NOx contaminants, the removal of which is dependent on the stringency of scrubbing procedures to which a given batch is subjected. The consistency of 15N-nitrate/nitrite measurements among bottles from an individual lot from Sigma-Aldrich (Sigma A1-A3), in contrast to the lower 15N-nitrate/nitrite detected in a subsequent lot (Sigma B), supports the premise that the levels of 15N-contaminants are associated with discrete batch syntheses, identified by lot numbers, rather than with individual lecture bottles. The variability in 15N-ammonium among lecture bottles of the same lot suggests that ammonia gas does not disperse homogeneously in compressed N2 gas. In any case, large-scale batch syntheses of 15N2 occur relatively infrequently, on the order of every 2 years at Isotec (subsidiary of Sigma-Aldrich). We currently have a verbal agreement with Isotec to perform nitrate and ammonium isotopic analyses of 15N2 batches, toward providing a certificate of analysis ensuring adequate purity for N2 fixation assays. In the meantime, we advise that workers procure low-contaminant stocks from lots that we tested here. The very high purity of the batches from these suppliers suggests stringent and efficacious purification protocols, such that batches synthesized by these groups in the future are likely to be equally pure, notwithstanding the potential for human error during synthesis or subsequent purification.
Regardless of ‘expected’ purity, we recommend that workers explicitly test new batches availed by respective suppliers for 15N-nitrate and ammonium prior to using them in N2 fixation assays, and actively disseminate the results to targeted web-based forums. To test a given batch, 15N2 gas can be equilibrated with nitrate and ammonium solutions following protocols akin to the low and high sensitivity equilibrations herein. A number of laboratories perform commercial nitrate isotope analyses routinely at a modest cost per sample. Ammonium isotope analyses are substantially more involved, but are also performed routinely by a number of laboratories.
We further recommend that pertinent publications include not only the brand of 15N2 stock, but also the associated lot number, and references to reported contaminants. With continued testing, our understanding of the prevalence of commercial 15N2 contamination will grow and shed light on this problem, which may have plagued N2 fixation estimates in the past.
Acknowledgments
We wish to thank Nicole Chang for assistance with 15N2 measurements, David Cady at the University of Connecticut for assistance with particulate nitrogen and carbon δ15N and δ14C measurements, and Mark Rollog and Thomas Kuhn for assistance in the lab at the University of Basel.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. Data files are available from Figshare using the link http://dx.doi.org/10.6084/m9.figshare.1170194.
Funding Statement
This work was supported by the following sources of funding: National Science Foundation Ocean Sciences, 1233897, JG; National Science Foundation Ocean Sciences, 1130495, PHM, MAA (http://www.nsf.gov/div/index.jsp?div=OCE); University of Connecticut Research Funding Large Grant, 461510, JG (http://research.uconn.edu); and Swiss National Science Foundation R’Equip 121258, MFL (http://www.snf.ch/en/Pages/default.aspx). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Montoya JP, Voss M, Kahler P, Capone DG (1996) A simple, high-precision, high-sensitivity tracer assay for N2 fixation. Appl Environ Microbiol 62(3): 986–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stewart WDP, Fitzgerald GP, Burris RH (1967) In situ studies on N2 fixation using the acetylene reduction technique. Proc Natl Acad Sci USA 58(5): 2071–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hardy RWF, Holsten RD, Jackson EK, Burns RC (1968) The acetylene-ethylene assay for N2 fixation: laboratory and field evaluation. Plant Physiol 43(8): 1185–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Burris RH, Miller CE (1941) Application of N15 to the study of biological nitrogen fixation. Science 93(2405): 114–5. [DOI] [PubMed] [Google Scholar]
- 5. Hardy RWF, Burns RC, Holsten RD (1973) Applications of the acetylene-ethylene assay for measurement of nitrogen fixation. Soil Biol Biochem 5(1): 47–81. [Google Scholar]
- 6. Giller KE (1987) Use and abuse of the acetylene-reduction assay for measurement of associative nitrogen fixation. Soil Biology & Biochemistry 19(6): 783–4. [Google Scholar]
- 7. Staal M, Lintel-Hekkert ST, Harren F, Stal L (2001) Nitrogenase activity in cyanobacteria measured by the acetylene reduction assay: A comparison between batch incubation and on-line monitoring. Environ Microbiol 3(5): 343–51. [DOI] [PubMed] [Google Scholar]
- 8. Belay N, Sparling R, Choi B, Roberts M, Roberts J, et al. (1988) Physiological and 15N–Nmr analysis of molecular nitrogen fixation by methanococcus-thermolithotrophicus, methanobacterium-bryantii and methanospirillum-hungatei. Biochim Biophys Acta 971(3): 233–45. [DOI] [PubMed] [Google Scholar]
- 9. Scharff A, Egsgaard H, Hansen P, Rosendahl L (2003) Exploring symbiotic nitrogen fixation and assimilation in pea root nodules by in vivo 15N nuclear magnetic resonance spectroscopy and liquid chromatography-mass spectrometry. Plant Physiol 131(1): 367–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Addison SL, McDonald IR, Lloyd-Jones G (2010) Identifying diazotrophs by incorporation of nitrogen from 15N2 into RNA. Appl Microbiol Biotechnol 87(6): 2313–22. [DOI] [PubMed] [Google Scholar]
- 11. Guillard RRL, Ryther JH (1962) Studies of marine planktonic diatoms: I. Cyclotella nana Hustedt, and Detonula confervacea (cleve) Gran. Can. J. Microbiol. 8: 229–239. [DOI] [PubMed] [Google Scholar]
- 12. Braman R, Hendrix S (1989) Nanogram nitrite and nitrate determination in environmental and biological-materials by vanadium(III) reduction with chemi-luminescence detection. Anal Chem 61(24): 2715–8. [DOI] [PubMed] [Google Scholar]
- 13. Holmes R, Aminot A, Kerouel R, Hooker B, Peterson B (1999) A simple and precise method for measuring ammonium in marine and freshwater ecosystems. Can J Fish Aquat Sci 56(10): 1801–8. [Google Scholar]
- 14. Sigman D, Casciotti K, Andreani M, Barford C, Galanter M, et al. (2001) A bacterial method for the nitrogen isotopic analysis of nitrate in seawater and freshwater. Anal Chem 73(17): 4145–53. [DOI] [PubMed] [Google Scholar]
- 15. Casciotti K, Sigman D, Hastings M, Böhlke J, Hilkert A (2002) Measurement of the oxygen isotopic composition of nitrate in seawater and freshwater using the denitrifier method. Anal Chem 74(19): 4905–12. [DOI] [PubMed] [Google Scholar]
- 16.Gonfiantini R (1984) Report on an advisory group meeting on stable isotope reference samples for geochemical and hydrochemical investigations. Vienna, 19–21 Sept. 1983. IAEA, Vienna.
- 17.Böhlke JK, Coplen TB (1995) Interlaboratory comparison of reference materials for nitrogen isotope ratio measurements. Proceedings of a consultants meeting held in Vienna, 1–3. Dec. 1993, IAEA-TECDOC-825, 51–66.
- 18. Böhlke J, Mroczkowski S, Coplen T (2003) Oxygen isotopes in nitrate: new reference materials for 18O:17O:16O measurements and observations on nitrate-water equilibration. Rapid Commun Mass Spectrom 17(16): 1835–46. [DOI] [PubMed] [Google Scholar]
- 19. McIlvin MR, Casciotti KL (2011) Technical updates to the bacterial method for nitrate isotopic analyses. Anal Chem 83(5): 1850–6. [DOI] [PubMed] [Google Scholar]
- 20. Zhang L, Altabet MA, Wu T, Hadas O (2007) Sensitive measurement of NH4 +15N/14N (δ15NH4 +) at natural abundance levels in fresh and saltwaters. Anal Chem 79(14): 5297–303. [DOI] [PubMed] [Google Scholar]
- 21. Böhlke J, Gwinn C, Coplen T (1993) New reference materials for nitrogen-isotope-ratio measurements. Geostand Newsl 17(1): 159–64. [Google Scholar]
- 22. Kendall C, Grim E (1990) Combustion tube method for measurement of nitrogen isotope ratios using calcium-oxide for total removal of carbon-dioxide and water. Anal Chem 62(5): 526–9. [Google Scholar]
- 23. Qi H, Coplen T, Geilmann H, Brand W, Bohlke J (2003) Two new organic reference materials for δ13C and δ15N measurements and a new value for the δ13C of NBS 22 oil. Rapid Communications in Mass Spectrometry 17(22): 2483–7. [DOI] [PubMed] [Google Scholar]
- 24. Cordoba F, Cardenas J, Fernandez E (1986) Kinetic characterization of nitrite uptake and reduction by chlamydomonas-reinhardtii. Plant Physiol 82(4): 904–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bergersen FJ (1980) Measurements of nitrogen fixation by direct means. In: Bergersen FJ, editor. Methods for evaluating biological nitrogen fixation. Wiley-Interscience, Chichester, pp. 5–110.
- 26. Il’chenko N (1976) Catalytic-oxidation of ammonia. Usp Khim 45(12): 2168–95. [Google Scholar]
- 27. Ashmore PG, Burnett MG, Tyler BJ (1962) Reaction of nitric oxide and oxygen. Trans Faraday Soc 58: 685–691. [Google Scholar]
- 28. Ohyama T, Kumazawa K (1981) A simple method for the preparation, purification and storage of 15N2 gas for biological nitrogen fixation studies. Soil Sci Plant Nutr 27(2): 263–5. [Google Scholar]
- 29. Altabet M (1988) Variations in nitrogen isotopic composition between sinking and suspended particles - implications for nitrogen cycling and particle transformation in the open ocean. Deep-Sea Research Part A-Oceanographic Research Papers 35(4): 535–54. [Google Scholar]
- 30. Fawcett SE, Lomas M, Casey JR, Ward BB, Sigman DM (2011) Assimilation of upwelled nitrate by small eukaryotes in the Sargasso Sea. Nat Geosci 4(10): 717–22. [Google Scholar]
- 31. Dore J, Brum J, Tupas L, Karl D (2002) Seasonal and interannual variability in sources of nitrogen supporting export in the oligotrophic subtropical North Pacific Ocean. Limnol Oceanogr 47(6): 1595–607. [Google Scholar]
- 32. Montoya J, Holl C, Zehr J, Hansen A, Villareal T, et al. (2004) High rates of N2 fixation by unicellular diazotrophs in the oligotrophic Pacific Ocean. Nature 430(7003): 1027–31. [DOI] [PubMed] [Google Scholar]
- 33. Needoba JA, Foster RA, Sakamoto C, Zehr JP, Johnson KS (2007) Nitrogen fixation by unicellular diazotrophic cyanobacteria in the temperate oligotrophic North Pacific Ocean. Limnol Oceanogr 52(4): 1317–27. [Google Scholar]
- 34. Fernandez C, Farias L, Ulloa O (2011) Nitrogen fixation in denitrified marine waters. PLoS One 6(6): e20539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hamersley MR, Turk KA, Leinweber A, Gruber N, Zehr JP, et al. (2011) Nitrogen fixation within the water column associated with two hypoxic basins in the Southern California Bight. Aquat Microb Ecol 63(2): 193–205. [Google Scholar]
- 36. Blais M, Tremblay J, Jungblut AD, Gagnon J, Martin J, et al. (2012) Nitrogen fixation and identification of potential diazotrophs in the Canadian Arctic. Global Biogeochem Cycles 26: GB3022. [Google Scholar]
- 37. Halm H, Lam P, Ferdelman TG, Lavik G, Dittmar T, et al. (2012) Heterotrophic organisms dominate nitrogen fixation in the South Pacific Gyre. Isme Journal 6(6): 1238–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dekaezemacker J, Bonnet S, Grosso O, Moutin T, Bressac M, et al. (2013) Evidence of active dinitrogen fixation in surface waters of the eastern tropical South Pacific during El Nino and La Nina events and evaluation of its potential nutrient controls. Global Biogeochem Cycles 27(3): 768–79. [Google Scholar]
- 39. Stam H, Stouthamer A, Vanverseveld H (1987) Hydrogen metabolism and energy costs of nitrogen-fixation. FEMS Microbiol Lett 46(1): 73–92. [Google Scholar]
- 40. De-Polli H, Matsui E, Döbereine J, Salati E (1977) Confirmation of nitrogen fixation in two tropical grasses by 15N2 incorporation. Soil Biol and Biochem 9: 119–123. [Google Scholar]
- 41. Mohr W, Grosskopf T, Wallace DWR, LaRoche J (2010) Methodological underestimation of oceanic nitrogen fixation rates. PLoS One 5(9): e12583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Grosskopf T, Mohr W, Baustian T, Schunck H, Gill D, et al. (2012) Doubling of marine dinitrogen-fixation rates based on direct measurements. Nature 488(7411): 361–4. [DOI] [PubMed] [Google Scholar]
- 43. Wilson ST, Boettjer D, Church MJ, Karl DM (2012) Comparative assessment of nitrogen fixation methodologies, conducted in the oligotrophic North Pacific Ocean. Appl Environ Microbiol 78(18): 6516–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. Data files are available from Figshare using the link http://dx.doi.org/10.6084/m9.figshare.1170194.