Abstract
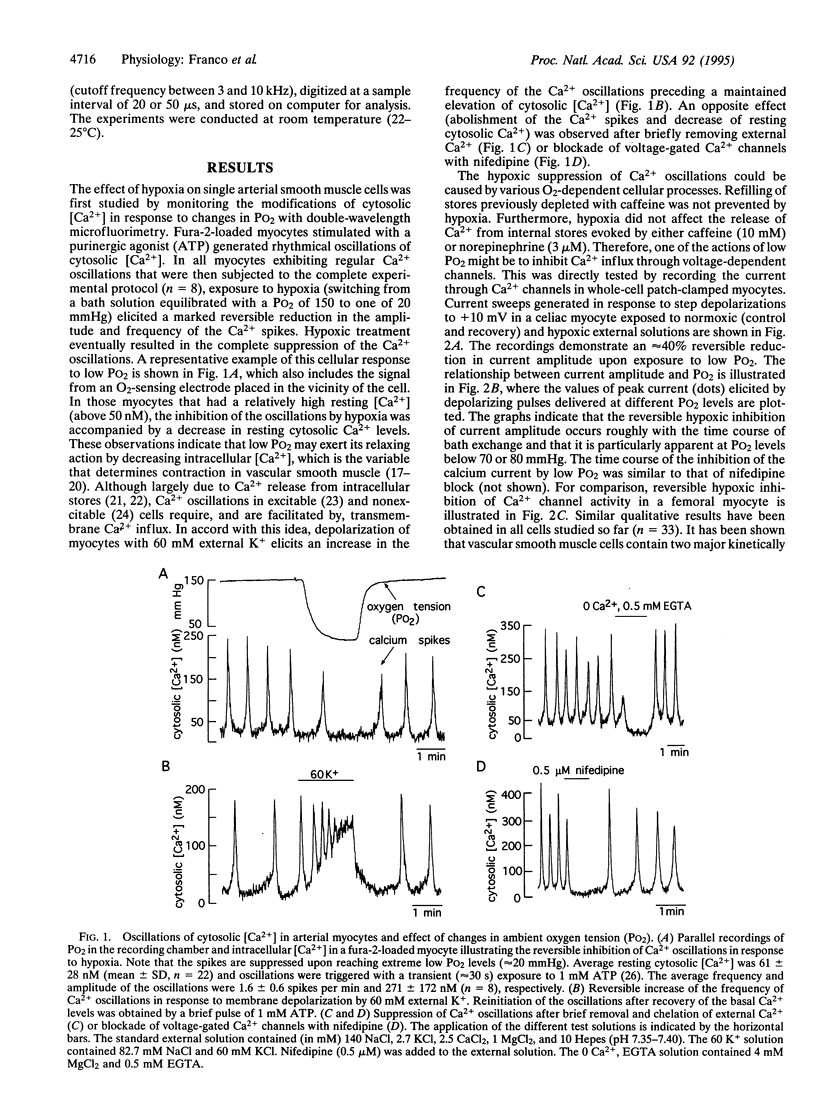
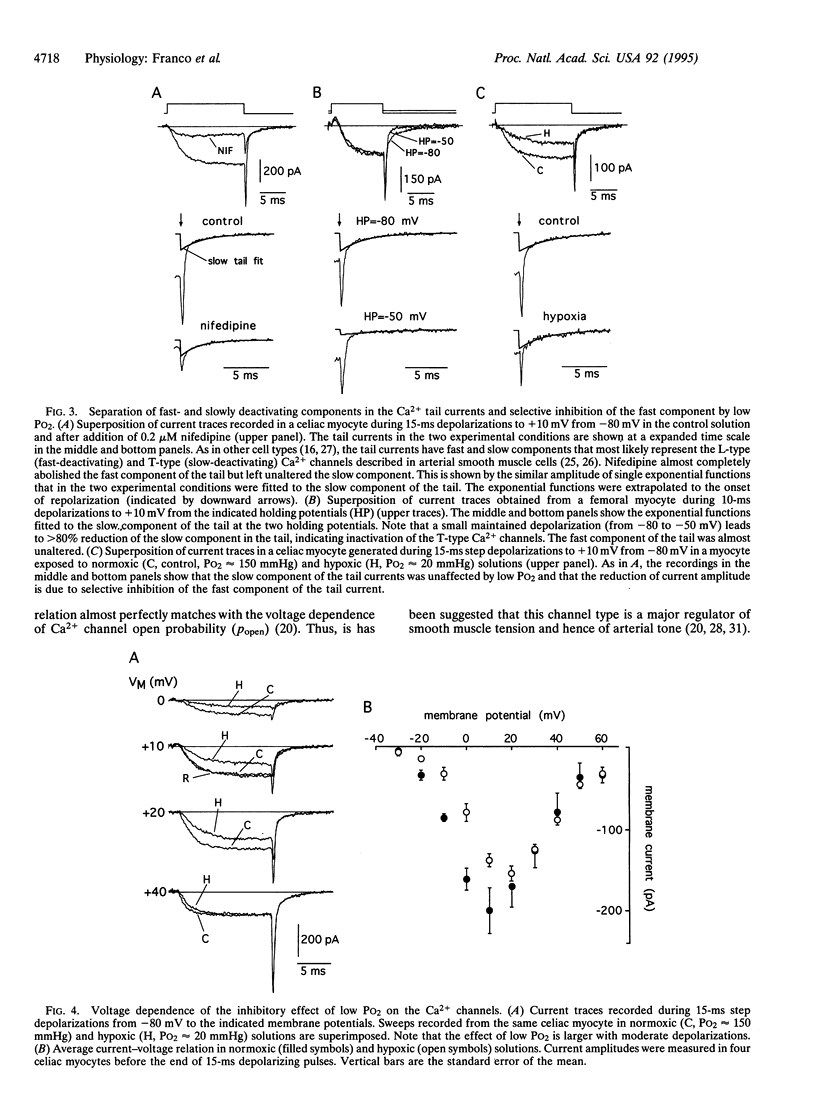
We have investigated the modifications of cytosolic [Ca2+] and the activity of Ca2+ channels in freshly dispersed arterial myocytes to test whether lowering O2 tension (PO2) directly influences Ca2+ homeostasis in these cells. Unclamped cells loaded with fura-2 AM exhibit oscillations of cytosolic Ca2+ whose frequency depends on extracellular Ca2+ influx. Switching from a PO2 of 150 to 20 mmHg leads to a reversible attenuation of the Ca2+ oscillations. In voltage-clamped cells, hypoxia reversibly reduces the influx of Ca2+ through voltage-dependent channels, which can account for the inhibition of the Ca2+ oscillations. Low PO2 selectively inhibits L-type Ca2+ channel activity, whereas the current mediated by T-type channels is unaltered by hypoxia. The effect of low PO2 on the L-type channels is markedly voltage dependent, being more apparent with moderate depolarizations. These findings demonstrate the existence of O2-sensitive, voltage-dependent, Ca2+ channels in vascular smooth muscle that may critically contribute to the local regulation of circulation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almers W., Neher E. The Ca signal from fura-2 loaded mast cells depends strongly on the method of dye-loading. FEBS Lett. 1985 Nov 11;192(1):13–18. doi: 10.1016/0014-5793(85)80033-8. [DOI] [PubMed] [Google Scholar]
- Amundson J., Clapham D. Calcium waves. Curr Opin Neurobiol. 1993 Jun;3(3):375–382. doi: 10.1016/0959-4388(93)90131-h. [DOI] [PubMed] [Google Scholar]
- Benndorf K., Bollmann G., Friedrich M., Hirche H. Anoxia induces time-independent K+ current through KATP channels in isolated heart cells of the guinea-pig. J Physiol. 1992 Aug;454:339–357. doi: 10.1113/jphysiol.1992.sp019267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatter L. A., Wier W. G. Agonist-induced [Ca2+]i waves and Ca(2+)-induced Ca2+ release in mammalian vascular smooth muscle cells. Am J Physiol. 1992 Aug;263(2 Pt 2):H576–H586. doi: 10.1152/ajpheart.1992.263.2.H576. [DOI] [PubMed] [Google Scholar]
- Castellano A., López-Barneo J. Sodium and calcium currents in dispersed mammalian septal neurons. J Gen Physiol. 1991 Feb;97(2):303–320. doi: 10.1085/jgp.97.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A. E., Detar R. Oxygen and vascular smooth muscle contraction revisited. Am J Physiol. 1980 May;238(5):H716–H728. doi: 10.1152/ajpheart.1980.238.5.H716. [DOI] [PubMed] [Google Scholar]
- Daut J., Maier-Rudolph W., von Beckerath N., Mehrke G., Günther K., Goedel-Meinen L. Hypoxic dilation of coronary arteries is mediated by ATP-sensitive potassium channels. Science. 1990 Mar 16;247(4948):1341–1344. doi: 10.1126/science.2107575. [DOI] [PubMed] [Google Scholar]
- Detar R. Mechanism of physiological hypoxia-induced depression of vascular smooth muscle contraction. Am J Physiol. 1980 Jun;238(6):H761–H769. doi: 10.1152/ajpheart.1980.238.6.H761. [DOI] [PubMed] [Google Scholar]
- Ebeigbe A. B., Pickard J. D., Jennett S. Responses of systemic vascular smooth muscle to hypoxia. Q J Exp Physiol Cogn Med Sci. 1980 Oct;65(4):273–292. doi: 10.1113/expphysiol.1980.sp002517. [DOI] [PubMed] [Google Scholar]
- Friel D. D., Tsien R. W. Phase-dependent contributions from Ca2+ entry and Ca2+ release to caffeine-induced [Ca2+]i oscillations in bullfrog sympathetic neurons. Neuron. 1992 Jun;8(6):1109–1125. doi: 10.1016/0896-6273(92)90132-w. [DOI] [PubMed] [Google Scholar]
- Ganfornina M. D., López-Barneo J. Potassium channel types in arterial chemoreceptor cells and their selective modulation by oxygen. J Gen Physiol. 1992 Sep;100(3):401–426. doi: 10.1085/jgp.100.3.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard S., Clapham D. Acceleration of intracellular calcium waves in Xenopus oocytes by calcium influx. Science. 1993 Apr 9;260(5105):229–232. doi: 10.1126/science.8385801. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Gustafsson H., Nilsson H. Rhythmic contractions of isolated small arteries from rat: role of calcium. Acta Physiol Scand. 1993 Nov;149(3):283–291. doi: 10.1111/j.1748-1716.1993.tb09623.x. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hellstrand P., Johansson B., Norberg K. Mechanical, electrical, and biochemical effects of hypoxia and substrate removal on spontaneously active vascular smooth muscle. Acta Physiol Scand. 1977 May;100(1):69–83. doi: 10.1111/j.1748-1716.1977.tb05923.x. [DOI] [PubMed] [Google Scholar]
- López-Barneo J. Oxygen-sensitive ion channels: how ubiquitous are they? Trends Neurosci. 1994 Apr;17(4):133–135. doi: 10.1016/0166-2236(94)90084-1. [DOI] [PubMed] [Google Scholar]
- Marriott J. F., Marshall J. M. Differential effects of hypoxia upon contractions evoked by potassium and noradrenaline in rabbit arteries in vitro. J Physiol. 1990 Mar;422:1–13. doi: 10.1113/jphysiol.1990.sp017968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matteson D. R., Armstrong C. M. Properties of two types of calcium channels in clonal pituitary cells. J Gen Physiol. 1986 Jan;87(1):161–182. doi: 10.1085/jgp.87.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald T. F., Pelzer S., Trautwein W., Pelzer D. J. Regulation and modulation of calcium channels in cardiac, skeletal, and smooth muscle cells. Physiol Rev. 1994 Apr;74(2):365–507. doi: 10.1152/physrev.1994.74.2.365. [DOI] [PubMed] [Google Scholar]
- Nelson M. T., Patlak J. B., Worley J. F., Standen N. B. Calcium channels, potassium channels, and voltage dependence of arterial smooth muscle tone. Am J Physiol. 1990 Jul;259(1 Pt 1):C3–18. doi: 10.1152/ajpcell.1990.259.1.C3. [DOI] [PubMed] [Google Scholar]
- Nelson M. T., Standen N. B., Brayden J. E., Worley J. F., 3rd Noradrenaline contracts arteries by activating voltage-dependent calcium channels. Nature. 1988 Nov 24;336(6197):382–385. doi: 10.1038/336382a0. [DOI] [PubMed] [Google Scholar]
- Post J. M., Hume J. R., Archer S. L., Weir E. K. Direct role for potassium channel inhibition in hypoxic pulmonary vasoconstriction. Am J Physiol. 1992 Apr;262(4 Pt 1):C882–C890. doi: 10.1152/ajpcell.1992.262.4.C882. [DOI] [PubMed] [Google Scholar]
- Toledo-Aral J., Castellano A., Ureña J., López-Barneo J. Dual modulation of K+ currents and cytosolic Ca2+ by the peptide TRH and its derivatives in guinea-pig septal neurones. J Physiol. 1993 Dec;472:327–340. doi: 10.1113/jphysiol.1993.sp019949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. W., Tsien R. Y. Calcium channels, stores, and oscillations. Annu Rev Cell Biol. 1990;6:715–760. doi: 10.1146/annurev.cb.06.110190.003435. [DOI] [PubMed] [Google Scholar]
- Weissberg P. L., Little P. J., Bobik A. Spontaneous oscillations in cytoplasmic calcium concentration in vascular smooth muscle. Am J Physiol. 1989 May;256(5 Pt 1):C951–C957. doi: 10.1152/ajpcell.1989.256.5.C951. [DOI] [PubMed] [Google Scholar]
- Yuan X. J., Goldman W. F., Tod M. L., Rubin L. J., Blaustein M. P. Ionic currents in rat pulmonary and mesenteric arterial myocytes in primary culture and subculture. Am J Physiol. 1993 Feb;264(2 Pt 1):L107–L115. doi: 10.1152/ajplung.1993.264.2.L107. [DOI] [PubMed] [Google Scholar]
- van Breemen C., Saida K. Cellular mechanisms regulating [Ca2+]i smooth muscle. Annu Rev Physiol. 1989;51:315–329. doi: 10.1146/annurev.ph.51.030189.001531. [DOI] [PubMed] [Google Scholar]
- von Beckerath N., Cyrys S., Dischner A., Daut J. Hypoxic vasodilatation in isolated, perfused guinea-pig heart: an analysis of the underlying mechanisms. J Physiol. 1991 Oct;442:297–319. doi: 10.1113/jphysiol.1991.sp018794. [DOI] [PMC free article] [PubMed] [Google Scholar]



