Abstract
In the present study, a specific and sensitive liquid chromatography-triple quadrupole mass spectrometry method was developed and validated for the determination of SP-141, a novel pyrido[b]indole anticancer agent. After a liquid-liquid extraction with n-hexane–dichloromethane-2-propanol (20:10:1, v/v/v) mixture, the analyte was separated on a Kinetex C18 column (50 × 2.1 mm, 2.6 μm) with mobile phases comprising of water (0.1% formic acid, v/v) and acetonitrile (0.1% formic acid, v/v) at a flow rate of 0.4 mL/min. The test compound (SP-141) and the internal standard (SP-157) were analyzed in the multiple reaction-monitoring mode using the mass transitions m/z 325.1 → 282.0. The method was linear in the concentration range of 0.648-162 ng/mL with coefficients of determination (R2) of 0.999 in mouse plasma. The lower limit of quantification was 0.648 ng/mL. The intra- and inter-day assay precisions (coefficient of variation, %CV) were less than 4.2% and accuracies (relative error, %RE) ranged from −6.1% to 2.1%. The extraction recoveries were between 97.1-103.1% and the relative matrix effect was minimal. In addition, SP-141 was found to be stable in the plasma after three freeze-thaw cycles, at 37°C and 4°C for 24 h, and at −80 °C for 4 weeks. It was also stable in the stock solution at room temperature for 24 h and after preparation in the autosampler for 36 h. The validated method was successfully applied to an initial pharmacokinetic study of SP-141 in CD-1 mice following intraperitoneal and intravenous administrations.
Keywords: SP-141, Pyrido[b]indole, LC-MS/MS, Mouse plasma, Pharmacokinetics
1. Introduction
Cancer is one of the leading causes of disease-related deaths in human beings [1]. Chemotherapy and radiation therapy have remained as the major types of non-surgical treatments for cancer [1, 2]. Despite their use, survival rate has not dramatically improved and severe adverse effects are frequently reported [2, 3]. Therefore, developing specific targeted therapeutics with minimal side effects for cancer is urgently needed.
MDM2 overexpression is a critical molecular alteration in several types of human cancer [4, 5]. It has been reported to play an important role in tumor growth, metastasis, and resistance to treatment [6-8]. We and others have demonstrated that MDM2 is a promising molecular target for cancer therapy [8-11]. Indeed, several specific MDM2 inhibitors have been developed [12-16]; the majority of these inhibitors target the MDM2-p53 binding, which consequently activate the p53 pathway in cancer cells harboring wild-type p53, such as nutlin-3a [17], RITA [18], and MI219 [19]. However, most of these MDM2 inhibitors show limited or no efficacy in p53 mutant or null human tumors.
We have recently developed a highly specific small molecule inhibitor of the MDM2 oncoprotein, SP-141 {6-methoxy-1-(naphthalen-1-yl)-9H-pyrido[3,4-b]indole} [20] (Fig. 1A). This compound is different from conventional MDM2 inhibitors in the fact that it directly binds to the MDM2 protein, induces its degradation, and inhibits its oncogenic functions in cancer cells. Further studies have also indicated that SP-141 has high potency against human breast and pancreatic cancers, without any observable toxicity at doses leading to tumor regression [20]. Therefore, we see that SP-141 shows good promise for development as a novel anticancer therapeutic agent. However, in order to study the pharmacokinetics and other clinical aspects of the compound, a rapid and sensitive quantitative method for the evaluation of SP-141 in vitro and in vivo is needed. In the present study, we firstly developed and validated a liquid chromatography-triple quadrupole mass spectrometry method for the determination of SP-141 in mouse plasma. This method was also applied to the pharmacokinetic studies of SP-141 in CD-1 mice after intraperitoneal and intravenous administration.
Fig. 1.
Chemical structures of SP-141(A) and SP-157 (B).
2. Experimental
2.1. Chemicals and reagents
The structures of SP-141 (Fig. 1A) and the internal standard (IS) SP-157 {7-methoxy-1-(naphthalen-2-yl)-9H-pyrido[3,4-b]indole} (Fig. 1B) were confirmed by IR, MS and NMR spectroscopy. The purity of the compound was determined to be greater than 99%. LC-MS grade acetonitrile was obtained from Fisher Scientific (Fair Lawn, NJ, USA). LC-MS grade water was obtained from the Elga Purelab Ultra® water purifier system. Formic acid (LC-MS grade) was purchased from Sigma (St Louis, MO, USA). Blank mouse plasma (heparinized, non-Swiss albino) was obtained from Lampire Biological Laboratories (Pipersville, PA, USA) or from untreated mouse plasma samples generated in our lab. n-Hexane (HPLC grade), dichloromethane (HPLC grade), and isopropyl alcohol (LC-MS grade) were purchased from Sigma (St Louis, MO, USA).
2.2. Instrumentation
The HPLC-MS/MS instrument consisted of an AB SCIEX QTRAP® 5500 mass spectrometer equipped with a Turbo IonSpray source (Foster City, CA, USA), and attached to a Nexera HPLC system from Shimadzu Corporation (Columbia, MD). The HPLC system consisted of a Sil-30AC autosampler, LC-30AD pumps, a CBM-20A controller, a DGA-20A degasser, and a CTO-30A column oven. Analyst and MultiQuant software were used for data acquisition and quantitation, respectively. A triple quadrupole mass spectrometer was used to analyze the analytes by monitoring their m/z transitions with the help of Analyst software (Applied Biosystems/MDS Sciex).
2.3. Liquid chromatographic and mass spectrometric conditions
The chromatographic separation of analytes was performed at 40°C on a Kinetex C18 column (50 mm × 2.1 mm i.d., 2.6 μm particle size, 100Å pore size), preceded by a Security Guard ULTRA guard column (Phenomenex; Torrance, CA, USA). The elution of the analytes (drug + IS) was based on a gradient method using mobile phases of water: formic acid (99.9:0.1, % v/v) (A) and (B) acetonitrile: formic acid (99.9:0.1, % v/v), which was run at a flow rate of 0.4 mL/min. The elution started with 10% B, which was increased to 80% B over 3 min and kept constant until 3.1 min. At 3.1 min, the mobile phase was switched to 90% B and maintained until 5 min, which was followed by return to the initial condition (at 5.1 min). The total run time was 7 min, but the mass spectrometer data was recorded only from 1 to 3 min. For all assays, 1 μL of each sample was loaded onto the column. The autosampler injection needle was washed with both mobile phases B and A to eliminate carry-over after each injection. The ionization source was through electrospray ionization (Turboion Spray), and the analytes were detected using multiple reaction-monitoring (MRM) operated in the positive mode. The source/gas and compound parameters were optimized to obtain the highest [M+H]+ ion abundance by infusing the standard solutions of the analyte of interest via a syringe pump into the mass spectrometer. The optimized source/gas parameters were as follows: nitrogen as curtain gas, 35 psi; collision gas, high; ion spray voltage, 4500 V; temperature, 600°C. The ion source gas 1 (nebulizer gas) and ion source gas 2 (turbo gas) were both set at 60 psi. A summary of the ion transitions, collision energies, and capillary voltages for both the analyte (SP-141) and the IS (SP-157) are presented in Table 1.
Table 1. Optimized mass-spectrometric conditions.
| Analyte | Parention (m/z) |
Daughter ions (m/z) |
Collision energy (V) |
Capillary voltage (V) |
Ionization mode |
|---|---|---|---|---|---|
| SP-141 | 325.2 | 282, 279 | 50 | 80 | Positive |
| SP-157 (IS) | 325.2 | 282, 279 | 50 | 55 | Positive |
2.4. Preparation of standards and quality control
The stock solutions for both SP-141 and SP-157 at 10 mM (3.24 mg/mL) were prepared in acetonitrile and stored at −80°C. An intermediate stock solution for SP-141 at a concentration of 324 μg/mL was prepared by diluting the primary stock solution with acetonitrile: water (1:1, v/v); this intermediate stock solution was utilized for preparing the calibration standards and quality control (QC) samples. Samples for testing dilution integrity were prepared from a 32400-ng/mL SP-141 intermediate stock solution and diluted to 129.6 ng/mL via a serial dilution with extracted blank matrix. A 162 ng/mL working IS solution (SP-157) was prepared by dilution of the SP-157 intermediate stock solution (324 μg/mL) with acetonitrile: water (1:1, v/v). The SP-141 stock solutions and working IS solutions were kept at −20°C. The SP-141 calibration standard samples at various concentrations (0.648, 1.94, 3.24, 6.48, 16.2, 32.4, 81.0, and 162.0 ng/mL) were prepared by serial dilution with mouse plasma from the primary stock solution, with each sample containing 16.2 ng/mL of the IS (SP-157). The calibration curves were prepared at concentrations of 0.648-162 ng/mL in CD-1 mouse plasma. The QC samples of low (1.94 ng/mL), medium (81.0 ng/mL), and high (129.6 ng/mL) concentrations were prepared in a similar manner to assess intra-day and inter-day precision and accuracy, as well as recovery and stability.
2.5. Sample preparation
For construction of the calibration curves and analysis of QC samples, 40 μL of blank plasma was spiked with 5 μL of SP-141 working solutions at various concentrations and 5 μL of IS (SP-157 at 162 ng/mL) to yield a final volume of 50 μL. The mixture was then extracted with 375 μL n-hexane–dichloromethane-2-propanol (20:10:1, v/v/v). The mixture was vortexed for 2 min and then shaken for 10 min, and centrifuged at 3500 rpm for 5 min. The organic phase was then transferred to another glass tube and evaporated to dryness at 40°C under a gentle stream of nitrogen. The residue was then reconstituted in 50 μL of ACN-water (50:50, v/v) with 1 μL injected into the LC-MS/MS system. The same sample preparation procedure was followed for incurred samples analysis. The test samples were diluted appropriately with extracted blank plasma matrix to yield samples containing a final analyte concentration within the calibration curve range, with each sample containing a final IS concentration of 16.2 ng/mL.
2.6. Method validation
The developed LC-MS/MS method was validated for linearity, accuracy, precision, recovery, and matrix effect, according to USA FDA guidelines [20]. Firstly, the specificity of the method was assessed by analyzing blank plasma spiked with SP-141 to observe the possible endogenous interference from plasma with the analyte. A structurally-related compound, SP-157, was chosen as the IS due to its similarity to SP-141 in terms of extractability and chromatographic behavior. The linearity of the relationship between the detector response and SP-141 concentrations was confirmed within the concentration range of 0.648-162 ng/mL for mouse plasma. The plasma calibration curves were constructed by plotting the peak area ratios (y) of SP-141 to the IS vs the nominal concentrations (x) in standard plasma by the 1/x2 weighted least-square linear regression. According to the FDA guidance for determining the lower limit of quantification (LLOQ), the lowest standard should be selected with an analyte response which is at least 5 times the blank response, has a precision of 20% or less, and allows accuracy of 80-120% [21, 22]. In order to evaluate the precision and accuracy of the method, QC samples at four concentration levels, i.e., 0.648 ng/mL (LLOQ), 1.94 ng/mL (low), 81.0 ng/mL (medium), and 129.6 ng/mL (high), were analyzed in five replicates on four separate days. Means, standard deviations (SD) and the ratio of the standard deviation to the mean (% coefficient of variation (CV)) were calculated and used to evaluate the precision. The intra-day and inter-day precisions (represented by % CV) were not to exceed 15%. The accuracy of the assay was assessed by comparing the calculated mean concentrations to the actual concentrations of serial dilutions. The accuracy was calculated as the % relative error (RE) and required to be within ± 15%. The percentage recoveries of SP-141 at LLOQ and three QC levels (1.94 (low), 81.0 (medium), and 129.6 (high) ng/mL) (n = 3) from mouse plasma was determined by comparing the mean peak area of the QC samples extracted from mouse plasma with those of pure compound prepared in the mobile phase. Matrix effects were evaluated by comparing peak areas of SP-141 and IS reconstituted in blank plasma extracts at LLOQ and QC concentrations (1.94 (low), 81.0 (medium), and 129.6 (high) ng/mL) with peak areas obtained by direct injection of standard solutions of SP-141 and IS at corresponding concentrations. A dilution integrity experiment was performed with the aim of validating the dilution test to be carried out on higher analyte concentrations above the ULOQ during analysis of real subject samples. For assays of accuracy and precision, the dilution was tested by analyses of five replicates on four separate days of QC samples at concentrations of 3240 (UHQC-1), and 6480 (UHQC-2) ng/mL, with 25- and 50-fold dilution, respectively.
2.7. Stability Studies
The stability of SP-141 in mouse plasma was evaluated under various conditions (time and temperature) by analyzing triplicates of the samples at three concentrations within the calibration curve range (1.94, 81.0, and 129.6 ng/mL), respectively. These results were compared with those obtained for freshly prepared samples to calculate the percentages of intact drug remaining. The freeze-thaw cycle involved the following procedure: 1) the samples were stored at −20°C for 24 h after which they were allowed to thaw unassisted at room temperature; 2) upon complete thaw, the samples were again frozen at −20°C for 24 h; and 3) this freeze-thaw cycle was repeated for two more times, making the samples undergo a total of three freeze-thaw cycles. The short-term stability in plasma was assessed by analyzing samples kept at 37°C for 2, 4, 8, and 24 h, and analyzing samples kept at 4 °C for 2, 4, 8, and 24 h. The long-term stability was determined by analyzing samples after storage at −80°C for 1, 2, 3, and 4 weeks. The bench-top stability of SP-141 in mouse plasma was assessed by incubating samples at ambient temperature (25°C) for 2, 4, 8, and 24 h. The stability of stock solutions was determined for 6, 12, and 24 h after stored at room temperature (25°C). The autosampler stability was evaluated by comparing the extracted plasma samples that were injected immediately (time 0), with samples that were re-injected after storage at 4°C (in the autosampler) for 36 h.
2.8. Pharmacokinetics of SP-141 in CD-1mice
To demonstrate the utility of the LC-MS/MS method, the pharmacokinetics of SP-141 in mouse plasma were determined in CD-1 mice following intraperitoneal and intravenous administration. The animal study protocol was approved by the Institutional Animal Care and Use Committee of Texas Tech University Health Sciences Center. The animals were randomly divided into groups of three each, and 40 mg/kg of SP-141 formulated in PEG400: EtOH: saline (57.1: 14.3: 28.6, v/v/v) was administrated. Plasma samples were collected at 0 (pre-dose), 5, 10, 30 and 60 min, and 2, 4, 6, 8, and 24 h after drug administration. Plasma was obtained from the retro-orbital plexus of the anesthetized mice. The blood samples were immediately centrifuged at 14000×g (4°C) for 10 min to separate the plasma. The samples were then processed as described above, and concentration–time curves were obtained.
3. Results and discussion
3.1. Optimization of mass spectrometric parameters
Both the positive and negative ionization modes were investigated for the detection of SP-141 and SP-157 and better response was achieved in the positive ionization mode. Data from the MRM mode were considered to obtain better selectivity. The most sensitive mass transition was monitored from m/z 325.1 to 282.0 for both SP-141 and SP-157 (Table 1). Considering both the compounds are structural isomers of each other, it is reasonable that they showed a similar pattern of mass transition.
3.2. Optimization of chromatographic conditions
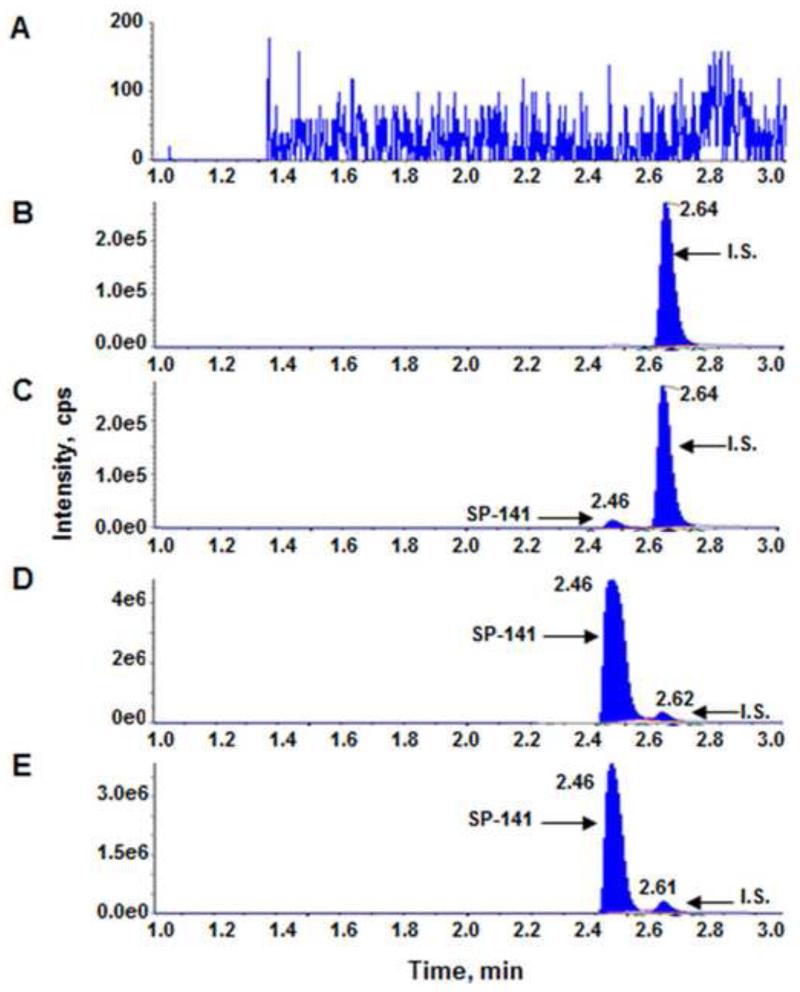
We first tested the isocratic mobile phases with different concentrations of methanol or acetonitrile and found that they were unsatisfactory due to the peak broadening and carry-over effect. Various linear gradients of water (A) and methanol (B) or water (A) and acetonitrile (B) were also evaluated which provided sharper peaks; however, the carry-over issue remained. Further studies indicated that the better peak shape could be obtained by adding 0.1% formic acid to water phase and 0.1% formic acid to acetonitrile phase. Thus, a gradient elution with modified mobile phases was chosen to give optimal performance with no carry-over. The specificity of the assay was examined using the described mass spectrometric parameters and chromatographic conditions. Representative MRM chromatograms of blank mouse plasma (Fig. 2A), mouse plasma spiked with SP-157 (Fig. 2B), mouse plasma spiked with SP-141 and SP-157 (Fig. 2C and 2D), and plasma samples from CD-1 mouse (Fig. 2E) are shown. The chromatograms were free from any endogenous interference at the retention time of the peaks of interest in plasma matrix. The retention times for SP-141 and the IS were around 2.5 and 2.6 min, respectively.
Fig. 2.
Representative multiple reaction monitoring chromatograms of (A) blank mouse plasma, (B) mouse plasma spiked with SP-157 (IS, 16.2 ng/mL), (C) mouse plasma spiked with SP-141 (0.648 ng/mL, LLOQ) and SP-157 (16.2 ng/mL), (D) mouse plasma spiked with SP-141 (64.8 ng/mL) and SP-157 (16.2 ng/mL), and (E) plasma sample from CD-1 mouse 0.083 h (5 mins) after single i.v. dose of 40 mg/kg SP-141 plus SP-157 (16.2 ng/mL).
3.3. Optimization of extraction solvent
Different organic solvents were investigated to extract analytes from the plasma samples. Compared with the most commonly used method of extraction in the literature (i.e. protein precipitation), n-hexane–dichloromethane–2-propanol (20:10:1, v/v/v) mixture yielded better results, and was selected as the liquid–liquid extraction solvent, which improved the extraction recovery of SP-141 to more than 90% with little background noise.
3.4. Method validation
The calibration curves for SP-141 were linear over the concentration range of 0.648–162.0 ng/mL. The typical regression equation for SP-141 was y = 0.0171x + 0.0559 (R2 = 0.9988). The LLOQ for the determination of SP-141 in plasma was found to be 0.648 ng/mL. The intra-day and inter-day precision and accuracy for detection of SP-141 in mouse plasma at four concentrations (0.648 (LLOQ), 1.94 (low), 81.0 (medium), and 129.6 (high) ng/mL) are presented in Table 2. Precision was defined as the variation between replicate samples. Accuracy was defined as the percentage of the absolute difference of observed concentration compared with the theoretical value of the prepared samples. The intra-day precision values were in the range of 0.5% to 1.6% while inter-day precision values were in the range of 1.4% to 4.2%, the accuracy of the quantitative analysis of the compound ranged from −6.1% to −0.5% for intra-day and −6.0% to −3.0% for inter-day analyses in mouse plasma samples. These parameters were well within the acceptable range as described by the US FDA. We next determined the effects of multiple dilutions on our QC samples. Since the QC samples at concentrations as high as 6480 ng/mL were determined with sufficient accuracy and precision (Table 2), the samples with concentrations above 162.0 ng/mL (ULOQ) can be reliably measured by dilution with blank plasma extract. The intra-day and inter-day precision/accuracy were acceptable. For the intra-day assay, the precision values (%CV) were 0.4% and 0.6%, respectively, and the accuracy (%RE) values were 2.1% and 1.3%, respectively. For the inter-day assay, the precision (%CV) values were 0.8% and 1.2%, respectively, and the accuracy (%RE) were −0.9% and 0.8%, respectively.
Table 2. Accuracy and precision of the method for detection of SP-141 in mouse plasma samples.
| Nominal Concentration (ng/mL) |
Intra-Day (n=5) |
Inter-Day (n=4) |
||||
|---|---|---|---|---|---|---|
| Measured (ng/mL) |
Precision (%CV) |
Accuracy (%RE) |
Measured (ng/mL) |
Precision (%CV) |
Accuracy (%RE) |
|
| LLOQ (0.648) | 0.645±0.010 | 1.6 | −0.5 | 0.611±0.025 | 4.2 | −5.6 |
| LQC (1.94) | 1.89±0.02 | 1.3 | −2.6 | 1.82±0.07 | 3.9 | −6.0 |
| MQC (81.0) | 76.0±1.2 | 1.6 | −6.1 | 78.3±2.4 | 3.1 | −3.3 |
| HQC (129.6) | 123.9±0.6 | 0.5 | −4.4 | 125.8±1.8 | 1.4 | −3.0 |
| UHQC1 (3240.0: 25-fold) | 3310.1±11.8 | 0.4 | 2.1 | 3215.0±22.4 | 0.8 | −0.9 |
| UHQC2 (6480.0: 50-fold) | 6595.1±35.8 | 0.6 | 1.3 | 6607.5±47.5 | 1.2 | 0.8 |
3.5. Matrix effects and extraction recovery
As shown in Table 3, extraction recoveries of the compound were determined at the LLOQ (0.648 ng/mL), low (1.94 ng/mL), medium (81.0 ng/mL), and high (129.6 ng/mL) concentrations (in triplicate) in plasma and the recoveries obtained were greater than 102.1 ± 1.8%, 97.1 ± 2.3%, 101.2 ± 0.7%, and 103.1 ± 6.3%, respectively. The matrix effect factors were 92.0 ± 1.2%, 96.3 ± 0.8%, 96.5 ± 1.4%, and 99.1 ± 1.0% at the LLOQ and the three QC concentrations, respectively, and the CV values from different lots of plasma were less than 1.4% (Table 3), indicating minimal matrix effect under the current conditions.
Table 3. Extraction recoveries and matrix factor effects for the detection method of SP-141 in mouse plasma samples.
| Concentration (ng/mL ) |
% Recovery | % Matrix Effect Factor | ||
|---|---|---|---|---|
|
| ||||
| Recovery (%) ± SD (%) | %CV | MF (%) ± SD (%) | %CV | |
| 0.648 | 102.1±1.8 | 1.7 | 92.0±1.2 | 1.3 |
| 1.94 | 97.1±2.3 | 2.3 | 96.3±0.8 | 0.8 |
| 81.0 | 101.2±0.7 | 0.7 | 96.5±1.4 | 1.4 |
| 129.6 | 103.1±6.3 | 0.6 | 99.0±1.0 | 1.0 |
3.6. Stability
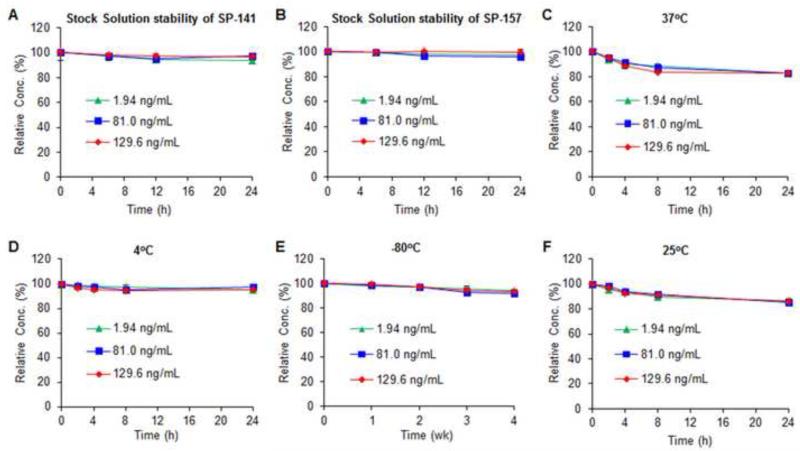
The stock solutions of both SP-141 and SP-157 were found to be stable at room temperature (25°C) for at least 24 h (Figs. 3A and 3B). SP141 was also found to have excellent freeze-thaw stability in mouse plasma samples. Our results showed that, after three freeze-thaw cycles, the percentages of intact compound remaining were 96.4% ± 0.6% at 1.94 ng/mL, 96.2% ± 2.4% at 81.0 ng/mL, and 99.3% ± 2.5% at 129.6 ng/mL of SP-141. SP-141 was also relatively stable in mouse plasma at 37°C, with more than 83.0% of the compound remaining after a 24-h incubation for all the QC concentrations (Fig. 3C). SP-141 in mouse plasma samples was stable under tested storage conditions. After a 24-h storage at 4°C, more than 95.3% of the compound remained intact for all the QC concentrations (Fig. 3D); after stored at −80°C for 4 weeks, more than 92.2% of the intact drug remained at all the concentrations tested (Fig. 3E). As shown in Fig. 3F, the test for the stability of SP-141 in plasma for up to 24 h at room temperature (25°C), prior to extraction and analysis, indicated that SP-141 was relatively stable. Additionally, negligible degradation occurred in the autosampler (at 4°C) for 36 h; the percentage of intact compound were 96.3% ± 2.7% at 1.94 ng/mL, 98.5% ± 3.1% at 81.0 ng/mL, and 96.8% ± 0.6% at 129.6 ng/mL of SP-141, respectively.
Fig. 3.
Stability of the stock solutions of SP-141 (A) and SP-157 (B). Stability of SP-141 in mouse plasma at 37°C (C), 4°C (D), −80°C (E), and 25°C (F).
3.8. Pharmacokinetics application
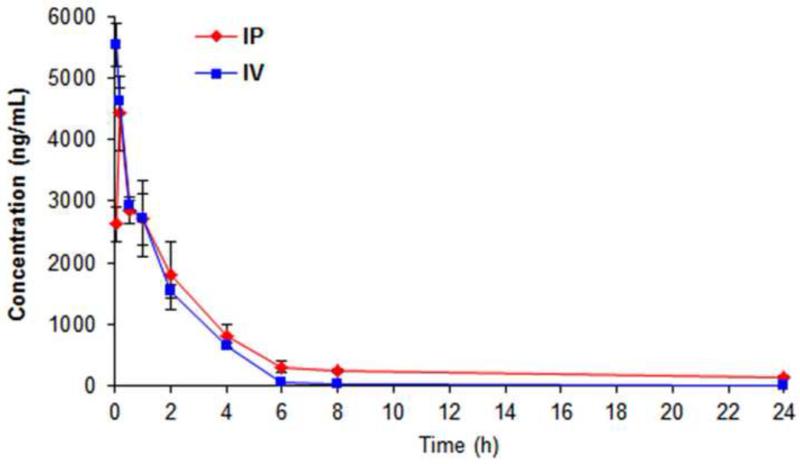
The concentrations of SP-141 in mouse plasma were successfully determined after administration of a single dose of 40 mg/kg to CD-1 mice through both intraperitoneal and intravenous injection. The mean plasma concentration–time profiles of SP-141 via both intraperitoneal and intravenous routes are shown in Fig. 4. Following administration, the concentration of the compound in plasma decreased rapidly, from 4422 ng/mL at 10 min to less than 150 ng/mL at 24 h (intraperitoneal route) and from 5531 ng/mL at 5 min to less than 20 ng/mL at 24 h (intravenous route). The pharmacokinetic parameters were estimated for both the routes using the WinNonlin software (Phoenix, USA) and the results are summarized in Table 4. For the intraperitoneal route, the maximum plasma concentration (Cmax) was 4422 ng/mL and occurred at 10 min (Tmax) after administration, indicating that the drug was rapidly absorbed. The elimination half-life (T1/2) was 1.78 h. Compared with IP administration, the IV administration yield a higher Cmax, a shorter T1/2, and smaller AUCs (Table 4). These data provided a basis for future pharmacology studies of this compound as anticancer agent.
Fig. 4.
SP-141 plasma time-concentration curves following intraperitoneal and intravenous administration of 40 mg/kg of the compound to CD-1 mice.
Table 4. Pharmacokinetic parameters of SP-141 in CD-1 mice after administrations of single dose of 40 mg/kg via intraperitoneal (IP) and intravenous (IV) routes.
| Routes | T1/2 (h) | Tmax (mins) | Cmax (ng/mL) | AUC0–t (h ng/mL) | AUC0-∞ (h ng/mL) |
|---|---|---|---|---|---|
| IP | 1.78 | 10 | 4422 | 12650 | 13170 |
| IV | 0.98 | Not applicable | 5531 | 8340 | 8541 |
4. Conclusions
In summary, a highly sensitive LC-MS/MS method has been successfully developed for the determination of SP-141 in mouse plasma. This method is highly sensitive and selective with quantitative and reproducible recoveries for both analyte and IS. The analysis requires only a small volume of sample. The validation data demonstrates good precision and accuracy of the method. Under the described conditions, no matrix effect influences the quantification. We also characterized the in vitro stability of SP-141 in mouse plasma under various temperature and storage conditions, and found the drug to be stable under the analytical conditions. Finally, the pharmacokinetic results show that SP-141 has a short plasma half-life and reaches maximum concentration in the mouse plasma within 10 minutes of administration by the intraperitoneal route.
Highlights.
This is the first report of a LC-MS/MS technique for the determination of SP-141 in mouse plasma
The developed method was demonstrated to meet FDA Bio Analytical Method Guidelines
The validated method was successfully applied to pharmacokinetic studies in CD-1 mice
Acknowledgements
This work was supported by NIH grant R01 CA186662 (to Dr. R.Z.). Dr. J.K.B. was supported by NIH grant R15 CA100102. The contents of the paper are solely the responsibility of the authors, and do not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- %CV
percent coefficient of variation
- RE
relative error
- IS
internal standard
- LLOQ
lower limit of quantification
- MRM
multiple reaction monitoring
- QC
quality control
- SD
standard deviation
- SP-141
6-methoxy-1-(naphthalen-1-yl)-9H-pyrido[3,4-b]indole
- SP-157
7-methoxy-1-(naphthalen-2-yl)-9H-pyrido[3,4-b]indole
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare no actual or potential competing financial interests.
References
- [1].Siegel R, Naishadham D, Jemal A. CA: Cancer J. Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- [2].James ND, Hussain SA, Hall E, Jenkins P, Tremlett J, Rawlings C, Crundwell M, Sizer B, Sreenivasan T, Hendron C, Lewis R, Waters R, Huddart RA. N. Engl. J. Med. 2012;366:1477–1488. doi: 10.1056/NEJMoa1106106. [DOI] [PubMed] [Google Scholar]
- [3].Bentzen SM. Nat. Rev. Cancer. 2006;6:702–713. doi: 10.1038/nrc1950. [DOI] [PubMed] [Google Scholar]
- [4].Boersma BJ, Howe TM, Goodman JE, Yfantis HG, Lee DH, Chanock SJ, Ambs S. J. Natl. Cancer Inst. 2006;98:911–919. doi: 10.1093/jnci/djj245. [DOI] [PubMed] [Google Scholar]
- [5].Santarius T, Shipley J, Brewer D, Stratton MR, Cooper CS. Nat. Rev. Cancer. 2010;10:59–64. doi: 10.1038/nrc2771. [DOI] [PubMed] [Google Scholar]
- [6].Zhang Z, Zhang R. Curr. Cancer Drug Targets. 2005;5:9–20. doi: 10.2174/1568009053332618. [DOI] [PubMed] [Google Scholar]
- [7].Bouska A, Eischen CM. Trends Biochem. Sci. 2009;34:279–286. doi: 10.1016/j.tibs.2009.02.006. [DOI] [PubMed] [Google Scholar]
- [8].Nag S, Qin J, Srivenugopal KS, Wang M, Zhang R. J. Biomed. Res. 2013;27:254–271. doi: 10.7555/JBR.27.20130030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Rayburn ER, Ezell SJ, Zhang R. Anticancer Agents Med. Chem. 2009;9:882–903. doi: 10.2174/187152009789124628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Qin JJ, Nag S, Voruganti S, Wang W, Zhang R. Curr. Med. Chem. 2012;19:5705–5725. doi: 10.2174/092986712803988910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Shangary S, Wang S. Clin. Cancer Res. 2008;14:5318–5324. doi: 10.1158/1078-0432.CCR-07-5136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wang W, Zhang X, Qin JJ, Voruganti S, Nag SA, Wang MH, Wang H, Zhang R. PloS One. 2012;7:e41586. doi: 10.1371/journal.pone.0041586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wang H, Nan L, Yu D, Agrawal S, Zhang R. Clin. Cancer Res. 2001;7:3613–3624. [PubMed] [Google Scholar]
- [14].Zhang Z, Li M, Wang H, Agrawal S, Zhang R. Proc. Natl. Acad. Sci. U. S. A. 2003;100:11636–11641. doi: 10.1073/pnas.1934692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wang W, Rayburn ER, Velu SE, Nadkarni DH, Murugesan S, Zhang R. Clin. Cancer Res. 2009;15:3511–3518. doi: 10.1158/1078-0432.CCR-08-2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wang W, Rayburn ER, Zhao Y, Wang H, Zhang R. Cancer Lett. 2009;278:241–248. doi: 10.1016/j.canlet.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Vassilev LT, Vu BT, Graves B, Carvajal D, Podlaski F, Filipovic Z, Kong N, Kammlott U, Lukacs C, Klein C, Fotouhi N, Liu EA. Science. 2004;303:844–848. doi: 10.1126/science.1092472. [DOI] [PubMed] [Google Scholar]
- [18].Issaeva N, Bozko P, Enge M, Protopopova M, Verhoef LG, Masucci M, Pramanik A, Selivanova G. Nat. Med. 2004;10:1321–1328. doi: 10.1038/nm1146. [DOI] [PubMed] [Google Scholar]
- [19].Shangary S, Qin D, McEachern D, Liu M, Miller RS, Qiu S, Nikolovska-Coleska Z, Ding K, Wang G, Chen J, Bernard D, Zhang J, Lu Y, Gu Q, Shah RB, Pienta KJ, Ling X, Kang S, Guo M, Sun Y, Yang D, Wang S. Proc. Natl. Acad. Sci. U. S. A. 2008;105:3933–3938. doi: 10.1073/pnas.0708917105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Wang W, Qin JJ, Voruganti S, Wang MH, Sharma H, Patil S, Zhou J, Wang H, Mukhopadhyay D, Buolamwini JK, Zhang R. Gastroenterology. 2014 doi: 10.1053/j.gastro.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].FDA US Food and Drug Administration: Guidance for Industry: Bioanalytical Method Validation. 2001 http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM070107.pdf
- [22].Jusko WJ. Pharma. Res. 2012;29:2628–2631. doi: 10.1007/s11095-012-0805-6. [DOI] [PMC free article] [PubMed] [Google Scholar]