Abstract
Background
The androgen-receptor isoform encoded by splice variant 7 lacks the ligand-binding domain, which is the target of enzalutamide and abiraterone, but remains constitutively active as a transcription factor. We hypothesized that detection of androgen-receptor splice variant 7 messenger RNA (AR-V7) in circulating tumor cells from men with advanced prostate cancer would be associated with resistance to enzalutamide and abiraterone.
Methods
We used a quantitative reverse-transcriptase–polymerase-chain-reaction assay to evaluate AR-V7 in circulating tumor cells from prospectively enrolled patients with metastatic castration-resistant prostate cancer who were initiating treatment with either enzalutamide or abiraterone. We examined associations between AR-V7 status (positive vs. negative) and prostate-specific antigen (PSA) response rates (the primary end point), freedom from PSA progression (PSA progression–free survival), clinical or radiographic progression–free survival, and overall survival.
Results
A total of 31 enzalutamide-treated patients and 31 abiraterone-treated patients were enrolled, of whom 39% and 19%, respectively, had detectable AR-V7 in circulating tumor cells. Among men receiving enzalutamide, AR-V7–positive patients had lower PSA response rates than AR-V7–negative patients (0% vs. 53%, P = 0.004) and shorter PSA progression–free survival (median, 1.4 months vs. 6.0 months; P<0.001), clinical or radiographic progression–free survival (median, 2.1 months vs. 6.1 months; P<0.001), and overall survival (median, 5.5 months vs. not reached; P = 0.002). Similarly, among men receiving abiraterone, AR-V7–positive patients had lower PSA response rates than AR-V7–negative patients (0% vs. 68%, P = 0.004) and shorter PSA progression–free survival (median, 1.3 months vs. not reached; P<0.001), clinical or radiographic progression–free survival (median, 2.3 months vs. not reached; P<0.001), and overall survival (median, 10.6 months vs. not reached, P = 0.006). The association between AR-V7 detection and therapeutic resistance was maintained after adjustment for expression of full-length androgen receptor messenger RNA.
Conclusions
Detection of AR-V7 in circulating tumor cells from patients with castration-resistant prostate cancer may be associated with resistance to enzalutamide and abiraterone. These findings require large-scale prospective validation. (Funded by the Prostate Cancer Foundation and others.)
It is now accepted that castration-resistant prostate cancer is not androgen-independent and continues to rely on androgen signaling.1 Owing to this new understanding, several drugs have recently emerged for the treatment of castration-resistant prostate cancer; these agents either suppress the synthesis of extragonadal androgens or target the androgen receptor directly.2 Enzalutamide is an inhibitor of androgen-receptor signaling that exerts its activity by binding avidly to the ligand-binding domain of the androgen receptor, competing with and displacing the natural ligands of this receptor (testosterone and dihydrotestosterone) while also inhibiting translocation of the androgen receptor into the nucleus and impairing transcriptional activation of androgen-responsive target genes.3,4 Abiraterone is an inhibitor of cytochrome P450 17A1 (CYP17A1) that impairs androgen-receptor signaling by depleting adrenal and intratumoral androgens.5,6 After studies showed improved survival with these drugs,7-9 both agents were approved by the Food and Drug Administration for the treatment of metastatic castration-resistant prostate cancer.
Although enzalutamide and abiraterone represent breakthroughs in the treatment of metastatic castration-resistant prostate cancer, approximately 20 to 40% of patients have no response to these agents with respect to prostate-specific antigen (PSA) levels (i.e., they have primary resistance).4,7-9 Among patients who initially have a response to enzalutamide or abiraterone, virtually all eventually acquire secondary resistance. One plausible explanation for the resistance to both agents may involve the presence of androgen-receptor splice variants.10,11 These alternatively spliced variants encode a truncated androgen-receptor protein that lacks the C-terminal ligand-binding domain but retains the transactivating N-terminal domain.12,13 Although the resultant truncated proteins are unable to bind ligand, they are constitutively active as transcription factors and capable of promoting activation of target genes.
Because enzalutamide exerts its antitumor activity through its interaction with the ligand-binding domain of the androgen receptor, it would be expected that the presence of the protein encoded by the androgen-receptor variant (which lacks the ligand-binding domain) may be associated with enzalutamide resistance. Furthermore, this protein is ligand-independent and yet constitutively active, and its activity would not be expected to be inhibited by ligand-depleting agents such as abiraterone. Although these hypotheses are supported by preclinical studies,10,11,14,15 the clinical significance of androgen-receptor variants in patients receiving enzalutamide or abiraterone is unknown.
To investigate the clinical relevance of androgen-receptor variants in castration-resistant prostate cancer, we prospectively evaluated androgen-receptor splice variant 7 messenger RNA (AR-V7) in circulating tumor cells from patients receiving enzalutamide or abiraterone. Although multiple androgen-receptor variants have been discovered, we focused on AR-V7 because it is the only known androgen-receptor variant encoding a functional protein product that is detectable in clinical specimens.13,16 We hypothesized that detection of AR-V7 in circulating tumor cells may be associated with resistance to enzalutamide and abiraterone in patients with castration-resistant prostate cancer.
Methods
Patients
We prospectively enrolled men with metastatic castration-resistant prostate cancer who were beginning standard-of-care treatment with enzalutamide or abiraterone. Patients were required to have histologically confirmed prostate adenocarcinoma, progressive disease despite “castration levels” of serum testosterone (<50 ng per deciliter [1.73 nmol per liter]) with continued androgen-deprivation therapy, and documented metastases, as confirmed on computed tomography (CT) or bone scanning with technetium-99m–labeled methylene diphosphonate. Patients had to have three or more rising serum PSA values obtained 2 or more weeks apart, with the last value being 2.0 ng per milliliter or higher — criteria for PSA progression that are consistent with Prostate Cancer Clinical Trials Working Group 2 (PCWG2) guidelines.17 Patients were excluded if they planned to receive additional concurrent anticancer therapies. Prior chemotherapy was permitted, as was previous treatment with the alternative agent directed at the androgen receptor (i.e., prior abiraterone use in enzalutamide-treated patients and vice versa). All enrolled patients provided written informed consent.
Study Design and Assessments
This was a prospective study evaluating the ability of baseline (pretreatment) AR-V7 status (positive vs. negative) in circulating tumor cells to predict a response or resistance to agents directed at the androgen receptor. The study was approved by the institutional review board at Johns Hopkins University. All the authors vouch for the completeness and integrity of the data and for the fidelity of the study to the clinical protocol (available with the full text of this article at NEJM.org). Peripheral-blood samples, for analysis of circulating tumor cells, were obtained from eligible patients at three prespecified time points: baseline, the time of a clinical or biochemical response (if a response occurred), and the time of clinical or radiographic progression. In addition, patients were encouraged to undergo core-needle biopsies of metastatic tumors at baseline and at the time of progression. Enzalutamide was given at a dose of 160 mg daily; abiraterone was given at a dose of 1000 mg daily, with prednisone at a dose of 5 mg twice daily.
The times of follow-up assessments were prospectively defined: PSA measurements were obtained every 1 to 2 months, and CT of the chest, abdomen, and pelvis and technetium-99m bone scanning were performed every 2 to 4 months. Therapy with enzalutamide or abiraterone was continued until PSA progression, clinical or radio-graphic progression, or the occurrence of unmanageable drug-related toxic effects.
All the clinical investigators were unaware of the AR-V7 status of the participants. All the laboratory investigators were unaware of clinical information when determining AR-V7 status. The study statisticians were the first to unblind the data, after at least 30 patients had been enrolled per cohort.
Analysis of Circulating Tumor Cells and Tumor Tissue
Descriptions of the methods used for the capture of circulating tumor cells and of messenger RNA (mRNA) analysis for full-length androgen receptor and AR-V7 are provided in the Supplementary Appendix, available at NEJM.org. Quantitative reverse-transcriptase–polymerase-chain-reaction (RT-PCR) assays were used for mRNA detection.
The analysis of AR-V7 in metastatic tumor tissue is also described in the Supplementary Appendix. RNA in situ hybridization assays were used.
Clinical Outcomes
The primary end point was the proportion of patients with a PSA response (≥50% decline in PSA level from baseline, maintained for ≥4 weeks) at any time after the initiation of therapy; the end point was assessed separately for enzalutamide-treated patients and abiraterone-treated patients. The best PSA response (maximal percentage decrease in PSA level from baseline) for each patient was also determined.
Secondary end points were freedom from PSA progression (PSA progression–free survival), freedom from clinical or radiographic progression (clinical or radiographic progression–free survival), and overall survival. PSA progression was defined as an increase in the PSA level of 25% or more above the nadir (and by ≥2 ng per milliliter), with confirmation 4 or more weeks later (PCWG2 criteria).17 Clinical or radiographic progression was defined as symptomatic progression (worsening disease-related symptoms or new cancer-related complications), radio-graphic progression (≥20% increase in the sum of the diameters of soft-tissue target lesions on CT scanning [according to the Response Evaluation Criteria in Solid Tumors18] or ≥2 new bone lesions on bone scanning), or death, whichever occurred first.17 Overall survival was defined as the time to death from any cause.
Statistical Analysis
Statistical analyses were performed separately in the enzalutamide and abiraterone cohorts. The sample size was determined on the basis of the primary end point of PSA response. We assumed that AR-V7 would be detectable from baseline samples of circulating tumor cells in 50% of enzalutamide-treated patients and 50% of abiraterone-treated patients. In both cohorts, we hypothesized that PSA response rates would be 10% or less in AR-V7–positive patients and 60% or more in AR-V7–negative patients.7,8 With this assumption, we calculated that a sample of 30 patients per cohort would give the study 85% power to detect a difference of 50 percentage points in PSA response rates (i.e., a rate of 10% in AR-V7–positive men and 60% in AR-V7–negative men), with the use of a two-sided test at an alpha level of 0.10.
In each cohort, clinical outcomes were compared between AR-V7–positive patients and AR-V7–negative patients. PSA response rates were compared with the use of Fisher's exact test. Time-to-event outcomes (i.e., PSA progression– free survival, clinical or radiographic progression– free survival, and overall survival) were evaluated with the use of Kaplan–Meier methods, and survival-time differences were compared with the use of the log-rank test. Univariate and multivariable Cox regressions were used to assess the effect of AR-V7 status on the prediction of time-to-event outcomes. Owing to the small sample size and the limited number of events, each multivariable model included only three variables (AR-V7 status, the level of expression of full-length androgen receptor, and prior use of the alternative therapy directed at the androgen receptor), to prevent overfitting.
We also performed propensity-score–weighted multivariable Cox analyses for PSA progression–free survival and clinical or radiographic progression–free survival, in which the propensity score (the probability of being AR-V7–positive) was calculated from logistic regression with the use of variables including the Gleason score, the baseline PSA level, the number of prior hormonal treatments, the presence or absence of visceral metastases, the Eastern Cooperative Oncology Group (ECOG) score, prior use of abiraterone or enzalutamide, and the level of full-length androgen receptor. All tests were two-sided, and P values of 0.05 or less were considered to indicate statistical significance. Statistical analyses were performed with the use of R software, version 2.15.1.
Results
AR-V7 Detection in Circulating Tumor Cells
We first demonstrated our ability to detect AR-V7 transcript in cells by looking for AR-V7 in normal human blood spiked with VCaP cells (Fig. S1A in the Supplementary Appendix), a prostate-cancer cell line known to express both full-length androgen receptor and AR-V7.13 We then assayed the patient samples; examples of positive and negative detection of AR-V7 in blood samples from two patients are shown in Figure S1B in the Supplementary Appendix. After the validity of the assay was established (not shown), AR-V7 positivity was defined as detection of the AR-V7 transcript by means of a quantitative RT-PCR assay at 36 or fewer PCR cycles, corresponding to detection of one or more copies of AR-V7 complementary DNA as determined by the relationship between cycle number and serial dilutions of prequantified AR-V7 (Fig. S2 in the Supplementary Appendix).
Patient Characteristics
From December 2012 through September 2013, we prospectively enrolled 62 patients with detectable circulating tumor cells, of whom 31 received enzalutamide and 31 received abiraterone (Table S1 in the Supplementary Appendix). A total of 35 enzalutamide-treated men were screened to identify 31 with detectable circulating tumor cells (89% yield); 36 abiraterone-treated men were screened to identify 31 with detectable circulating tumor cells (86% yield). The 9 men with no detectable circulating tumor cells were excluded from further analysis. The median follow-up time was 5.4 months (range, 1.4 to 9.9) among enzalutamide-treated patients and 4.6 months (range, 0.9 to 8.2) among abiraterone-treated patients. A total of 39% of enzalutamide-treated patients (12 of 31 patients) and 19% of abiraterone-treated patients (6 of 31 patients) had detectable AR-V7 mRNA in baseline samples of circulating tumor cells. Among the 18 men with detectable AR-V7 from the entire study cohort, the median ratio of AR-V7 to full-length androgen receptor mRNA was 21.0% (range, 1.8 to 208.0) (Fig. 1); detection of AR-V7 was associated with increased expression of full-length androgen receptor mRNA (P<0.001).
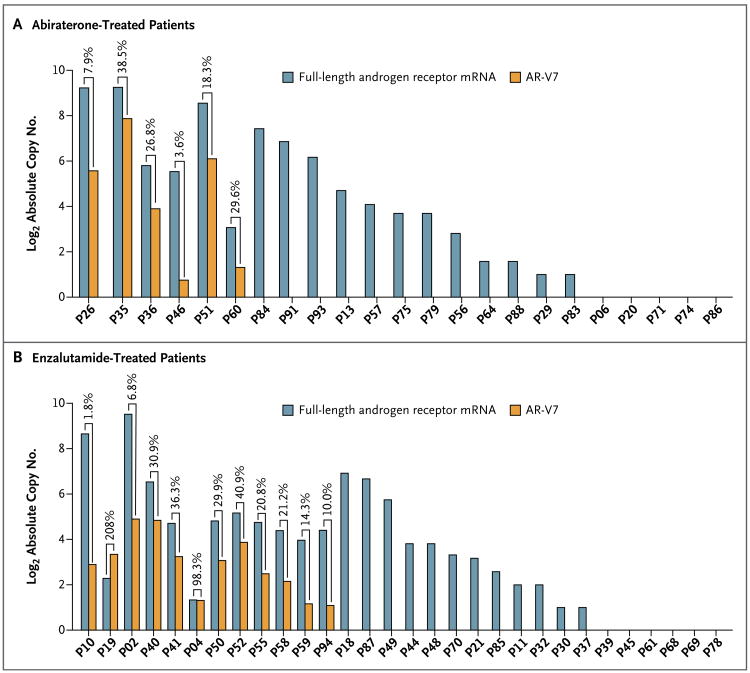
Figure 1. Transcript Levels of Full-Length Androgen Receptor mRNA and AR-V7 in Circulating Tumor Cells from Patients with Castration-Resistant Prostate Cancer.
Absolute transcript copy numbers of full-length androgen receptor messenger RNA (mRNA) and androgen-receptor splice variant 7 mRNA (AR-V7) detected in circulating tumor cells are shown for the 6 abiraterone-treated patients (Panel A) and the 12 enzalutamide-treated patients (Panel B) who were positive for AR-V7 at baseline (i.e., in pretreatment samples of circulating tumor cells). Ratios of AR-V7 to full-length androgen receptor mRNA are expressed as percentages. Levels of full-length androgen receptor mRNA are also shown for AR-V7–negative samples. Eight abiraterone-treated patients who were negative for full-length androgen receptor mRNA (not shown) were also negative for AR-V7. One enzalutamide-treated patient was negative for both full-length androgen receptor mRNA and AR-V7 (not shown).
In the enzalutamide cohort, AR-V7–positive patients had higher levels of full-length androgen receptor mRNA and PSA than did AR-V7– negative patients and were more likely than AR-V7–negative patients to have an ECOG performance-status score of 1 or 2 (scores range from 0 to 5, with 0 indicating no symptoms and higher scores indicating increasing disability), visceral metastases, and six or more bone metastases and to have had prior docetaxel treatment and prior abiraterone treatment (Table S1A in the Supplementary Appendix). A total of 55% of the patients who had previously received abiraterone (11 of 20 patients) had detectable AR-V7, as compared with 9% of the patients who had not previously received the drug (1 of 11 patients). Table S2A in the Supplementary Appendix shows clinical outcomes separately for patients who had previously received abiraterone and those who had not previously received the drug.
In the abiraterone cohort, AR-V7–positive patients had higher levels of full-length androgen receptor mRNA, PSA, and alkaline phosphatase, and a higher number of prior hormonal therapies than did AR-V7–negative patients and were more likely than AR-V7–negative patients to have an ECOG performance-status score of 1 or 2 and prior enzalutamide treatment (Table S1B in the Supplementary Appendix). A total of 50% of patients who had previously received enzalutamide (2 of 4 patients) had detectable AR-V7, as compared with 15% of patients who had not previously received the drug (4 of 27 patients). Table S2B in the Supplementary Appendix shows clinical outcomes separately for patients who had previously received enzalutamide and those who had not previously received the drug.
Primary End Point
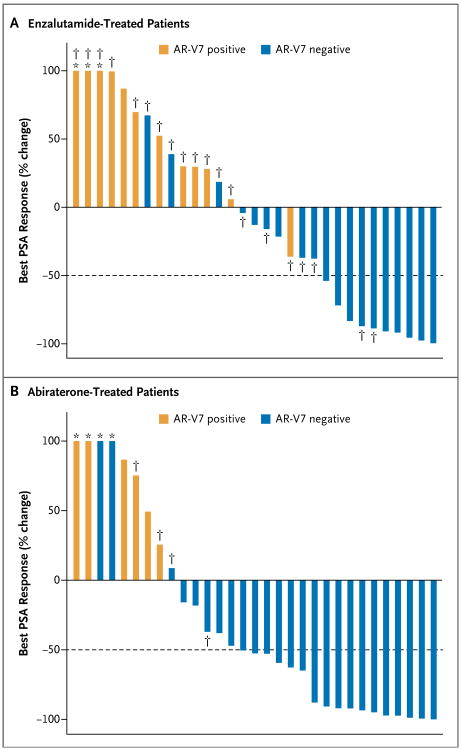
The overall proportion of patients who had a PSA response while receiving enzalutamide was 32% (95% confidence interval [CI], 17 to 51; 10 of 31 men). In the enzalutamide cohort, the PSA response rate among AR-V7–positive patients was 0% (95% CI, 0 to 26; 0 of 12 men), and the rate among AR-V7–negative patients was 53% (95% CI, 29 to 76; 10 of 19 men; P = 0.004). The best PSA responses are shown in Figure 2A. In linear regression modeling, AR-V7 status remained predictive of PSA response after adjustment for the expression of full-length androgen receptor mRNA (P<0.001).
Figure 2. Waterifall Plots of Best Prostate-Specific Antigen (PSA) Responses According to AR-V7 Status.
Panel A shows the 31 enzalutamide-treated patients, and Panel B the 31 abiraterone-treated patients. The dotted line shows the threshold for defining a PSA response (≥50% reduction in PSA level from baseline). Asterisks indicate an increase of more than 100% in best PSA response. Daggers indicate patients in the enzalutamide cohort who had previously received abiraterone and patients in the abiraterone cohort who had previously received enzalutamide.
The overall proportion of patients who had a PSA response while receiving abiraterone was 55% (95% CI, 36 to 73; 17 of 31 men). In the abiraterone cohort, the PSA response rate among AR-V7–positive patients was 0% (95% CI, 0 to 46; 0 of 6 men), and the rate among AR-V7–negative patients was 68% (95% CI, 46 to 85; 17 of 25 men; P = 0.004). The best PSA responses are shown in Figure 2B. In linear regression modeling, AR-V7 status remained predictive of PSA response after adjustment for the expression of full-length androgen receptor mRNA (P = 0.02).
Secondary End Points
PSA Progression–free Survival
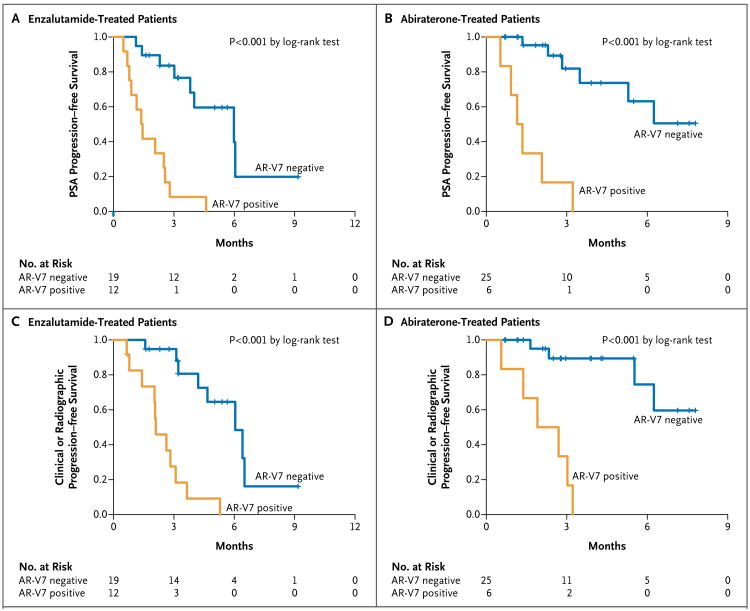
Among enzalutamide-treated patients, PSA progression–free survival was shorter among men with detectable AR-V7 at baseline than among those with undetectable AR-V7 (P<0.001 in a univariate analysis) (Fig. 3A). In a multivariable Cox model adjusted for the expression of full-length androgen receptor mRNA and prior abiraterone use, the detection of AR-V7 remained independently predictive of shorter PSA progression–free survival (hazard ratio for PSA progression, 3.1; 95% CI, 1.0 to 9.2; P = 0.046); the level of full-length androgen receptor mRNA was also predictive of shorter PSA progression–free survival (hazard ratio, 1.4; 95% CI, 1.0 to 1.9), but previous abiraterone use was not (hazard ratio, 2.5; 95% CI, 0.4 to 14.5). Results of the propensity-score–weighted multivariable model are shown in Table S3A in the Supplementary Appendix.
Figure 3. Kaplan–Meier Analysis of PSA Progression–free Survival and Clinical or Radiographic Progression–free Survival According to AR-V7 Status.
The median PSA progression–free survival in enzalutamide-treated patients (Panel A) was 1.4 months (95% CI, 0.9 to not reached) in AR-V7–positive patients and 6.0 months (95% CI, 3.8 to not reached) in AR-V7–negative patients (hazard ratio for PSA progression with AR-V7 positivity, 7.4; 95% CI, 2.7 to 20.6; P<0.001 by the log-rank test). The median PSA progression–free survival in abiraterone-treated patients (Panel B) was 1.3 months (95% CI, 0.9 to not reached) in AR-V7–positive patients and more than 5.3 months (95% CI, 5.3 to not reached) in AR-V7–negative patients (hazard ratio for PSA progression with AR-V7 positivity, 16.1; 95% CI, 3.9 to 66.0; P<0.001 by the log-rank test). The median clinical or radiographic progression–free survival in enzalutamide-treated patients (Panel C) was 2.1 months (95% CI, 2.0 to not reached) in AR-V7–positive patients and 6.1 months (95% CI, 4.7 to not reached) in AR-V7–negative patients (hazard ratio for clinical or radiographic progression with AR-V7 positivity, 8.5; 95% CI, 2.8 to 25.5; P<0.001 by the log-rank test). The median clinical or radiographic progression–free survival in abiraterone-treated patients (Panel D) was 2.3 months (95% CI, 1.4 to not reached) in AR-V7–positive patients and more than 6.3 months (95% CI, 6.3 to not reached) in AR-V7–negative patients (hazard ratio for clinical or radiographic progression with AR-V7 positivity, 16.5; 95% CI, 3.3 to 82.9; P<0.001 by the log-rank test).
Among abiraterone-treated patients, PSA progression–free survival was shorter among men with detectable AR-V7 at baseline than among those with undetectable AR-V7 (P<0.001 in a univariate analysis) (Fig. 3B). In a multivariable Cox model adjusted for the expression of full-length androgen receptor mRNA and prior enzalutamide use, the detection of AR-V7 was the only independent predictor of shorter PSA progression–free survival (hazard ratio for PSA progression, 15.7; 95% CI, 2.1 to 117.5; P = 0.007); neither the level of full-length androgen receptor mRNA (hazard ratio, 1.0; 95% CI, 0.8 to 1.2) nor previous enzalutamide use (hazard ratio, 0.9; 95% CI, 0.1 to 5.2) was predictive. Results of the propensity-score–weighted multivariable model are shown in Table S3C in the Supplementary Appendix.
Clinical or Radiographic Progression–free Survival
Among enzalutamide-treated patients, clinical or radiographic progression–free survival was shorter among men with detectable AR-V7 at baseline than among those with undetectable AR-V7 (P<0.001 in a univariate analysis) (Fig. 3C). In a multivariable Cox model adjusted for the expression of full-length androgen receptor mRNA and prior abiraterone use, the detection of AR-V7 remained marginally predictive of shorter clinical or radiographic progression–free survival (hazard ratio for clinical or radiographic progression, 3.0; 95% CI, 0.9 to 9.6; P = 0.06); the level of full-length androgen receptor mRNA was also predictive (hazard ratio, 1.7; 95% CI, 1.1 to 2.6), but previous abiraterone use was not (hazard ratio, 2.6; 95% CI, 0.2 to 27.6). Table S3B in the Supplementary Appendix shows the results of the propensity-score–weighted multivariable model.
Among abiraterone-treated patients, clinical or radiographic progression–free survival was shorter among men with detectable AR-V7 at baseline than among those with undetectable AR-V7 (P<0.001 in a univariate analysis) (Fig. 3D). In a multivariable Cox model adjusted for the expression of full-length androgen receptor mRNA and prior enzalutamide use, the detection of AR-V7 was the only factor that was independently predictive of shorter clinical or radiographic progression–free survival (hazard ratio for clinical or radiographic progression, 7.6; 95% CI, 1.0 to 57.6; P = 0.05); the level of full-length androgen receptor mRNA (hazard ratio, 1.1; 95% CI, 0.9 to 1.5) and previous enzalutamide use (hazard ratio, 1.9; 95% CI, 0.4 to 10.0) were not predictive. Table S3D in the Supplementary Appendix shows the results of the propensity-score–weighted multivariable model.
Overall Survival
A preliminary survival analysis was conducted at 32% maturity in the enzalutamide-treated cohort (i.e., after 32% of the patients [10 patients] had died) (median follow-up, 8.4 months) and at 16% maturity in the abiraterone-treated cohort (i.e., after 16% of the patients [5 patients] had died) (median follow-up. 9.3 months). Overall survival was shorter in men with detectable AR-V7 at baseline than among those with undetectable AR-V7 both in the enzalutamide cohort (median, 5.5 months vs. not reached; hazard ratio for death, 6.9; 95% CI, 1.7 to 28.1; P = 0.002 by the log-rank test) (Fig. S3A in the Supplementary Appendix) and in the abiraterone cohort (median, 10.6 months vs. not reached; hazard ratio for death, 12.7; 95% CI, 1.3 to 125.3; P = 0.006 by the log-rank test) (Fig. S3B in the Supplementary Appendix). Owing to the small number of events in each cohort, multivariable models were not constructed.
Combined Analysis
As an exploratory analysis, we evaluated PSA responses, PSA progression–free survival, clinical or radiographic progression–free survival, and overall survival in the combined patient population of all 62 participants. The effect of AR-V7 status on these outcomes remained significant (Fig. S4 in the Supplementary Appendix).
Conversion From AR-V7–Negative to AR-V7– Positive Status
Of 42 men with undetectable AR-V7 at baseline who had at least one follow-up sample available, 6 patients (4 receiving enzalutamide and 2 receiving abiraterone) subsequently converted to AR-V7– positive status during the course of treatment. All 16 patients with detectable AR-V7 at baseline who had at least one follow-up sample available remained AR-V7–positive during treatment. Clinical outcomes for all patients according to AR-V7 conversion status are summarized in Table S4 in the Supplementary Appendix. Changes in levels of AR-V7 expression during the course of treatment are summarized in Figure S5 in the Supplementary Appendix.
Tissue-Based Analyses
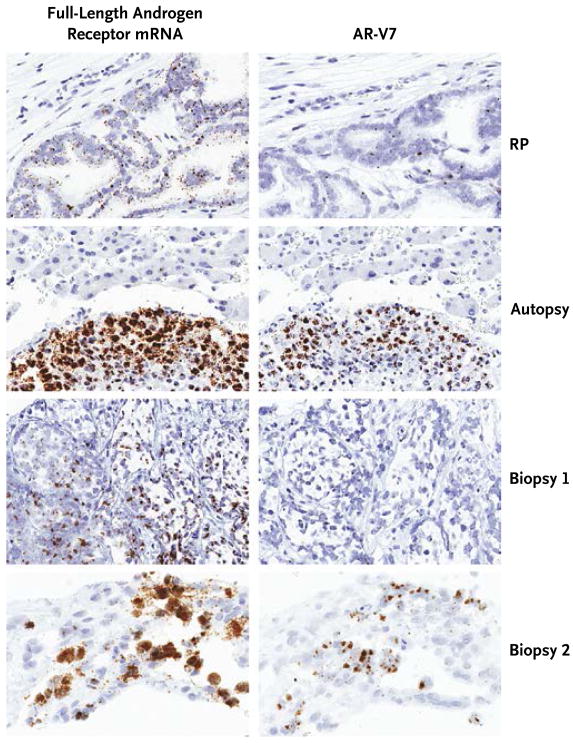
Seven patients consented to additional tissue-based studies: five underwent biopsies of metastatic tumors, and two consented to allow research autopsies to be performed after their death. Three of the seven patients had detectable AR-V7 in circulating tumor cells; these three patients also had detectable AR-V7 in metastatic tumor tissue according to RNA in situ hybridization analysis (Fig. 4). In addition, AR-V7 and full-length androgen receptor were detected at the protein level with the use of Western blot analysis in these patients (Fig. S7 in the Supplementary Appendix). Conversely, none of the four patients with undetectable AR-V7 in circulating tumor cells had detectable AR-V7 in metastatic tissue on RNA in situ hybridization, a finding that suggests good concordance. Finally, sequencing of the AR transcript with the use of RNA sequencing in metastatic lesions from two AR-V7–positive patients (autopsy specimens) did not identify mutations in the androgen-receptor gene that could explain resistance but did confirm the presence of AR-V7 splice junctions in both patients (Fig. S9 in the Supplementary Appendix).
Figure 4. Detection of Full-Length Androgen Receptor mRNA and AR-V7 in Metastatic Prostate-Cancer Tissue.
In situ detection of full-length androgen receptor mRNA and AR-V7 in prostate-cancer tumor specimens was performed with the use of RNA in situ hybridization analysis. The tumor-tissue specimens shown are a radical-prostatectomy (RP) specimen that lacks AR-V7 expression from a patient (not enrolled in this study) who had not received hormonal treatment, an autopsy-derived specimen of a liver metastasis from a patient whose circulating tumor cells were shown to express AR-V7 (Autopsy), and core-needle biopsy specimens from a patient in whom AR-V7 was not detected (Biopsy 1) and a patient in whom AR-V7 was detected (Biopsy 2) in circulating tumor cells. All the tumor specimens show expression of full-length androgen receptor mRNA. The prostate-cancer cell lines that served as positive and negative controls for AR-V7 detection by means of RNA in situ hybridization are shown in Figure S6 in the Supplementary Appendix.
Relationship Between Full-Length Androgen Receptor mRNA and AR-V7
In all patients with detectable AR-V7, full-length androgen receptor mRNA was also expressed and at higher levels (with one exception) than the levels of AR-V7; increased expression of AR-V7 was generally (but not always) coupled with that of full-length androgen receptor mRNA (Fig. 1). Although expression of PSA (an indicator of canonical androgen-receptor signaling) was generally suppressed in AR-V7–negative patients during treatment with enzalutamide or abiraterone, PSA expression did not decrease in post-treatment samples of circulating tumor cells from men with detectable AR-V7 at baseline (Fig. S8 in the Supplementary Appendix). This observation is consistent with continued androgen-receptor signaling despite potent inhibition of full-length androgen receptor when AR-V7 coexists with full-length androgen receptor and contrasts with previous findings from a cell-line model of prostate cancer.19
In addition, genomewide comparisons of two AR-V7–negative and two AR-V7–positive metastatic tumor samples by means of gene-set enrichment analysis of RNA sequencing data (Figs. S9 and S10 in the Supplementary Appendix) or by means of targeted analysis of a set of genes regulated by the canonical androgen receptor (Table S5 in the Supplementary Appendix) revealed alterations consistent with a shift toward AR-V7–driven transcription in AR-V7–positive samples. Finally, the addition of AR-V7 status to regression models that included only levels of full-length androgen receptor mRNA resulted in significant improvements in model fit across all clinical outcomes evaluated, confirming the added value of AR-V7 status in predicting outcomes with enzalutamide or abiraterone (Table S6 in the Supplementary Appendix.
Discussion
Enzalutamide and abiraterone, two new therapies directed at the androgen receptor, represent important advances in the management of castration-resistant prostate cancer.4,7-9 However, a proportion of men do not benefit from these agents, and a clearer understanding of the mechanisms underlying resistance to these drugs would facilitate selection of alternative therapies (e.g., chemotherapies) for such patients. We found that AR-V7 can be detected reliably from circulating tumor cells and that detection of A R-V7 in tumor cells appears to be associated with resistance to both enzalutamide and abiraterone. This conceptually simple model is biologically plausible, because the protein encoded by AR-V7 lacks the ligand-binding domain of the androgen receptor (the direct target of enzalutamide and the indirect target of abiraterone) while remaining constitutively active as a transcription factor in a ligand-independent manner.13,16
In our study, no AR-V7–positive patient had any appreciable clinical benefit from enzalutamide or abiraterone therapy. Moreover, although AR-V7 detection was associated with increased expression of full-length androgen receptor mRNA, the prognostic effect of AR-V7 was maintained after adjustment for levels of full-length androgen receptor mRNA. Finally, although prior treatment with abiraterone or enzalutamide was associated with AR-V7 positivity, AR-V7 status remained prognostic after adjustment for this factor. Therefore, the current study shows a strong association between the presence of AR-V7 and resistance to enzalutamide and abiraterone. If this finding is substantiated by others, it is possible that AR-V7 could be used as a biomarker to predict resistance to enzalutamide and abiraterone and to facilitate treatment selection. However, our study does not prove a causal role for AR-V7 in mediating resistance to enzalutamide or abiraterone, and it remains possible that AR-V7 is a marker of more advanced disease or a higher disease burden.
Preclinical studies have shown that androgen-receptor variants are much more common in castration-resistant prostate cancer than in hormone-sensitive prostate cancer,13 that they represent one potential mechanism driving the emergence of the castration-resistant phenotype,10 and that they may be responsible for the progression of castration-resistant prostate cancer.14 Studies involving patients with castration-resistant prostate cancer have shown that androgen-receptor variants are often expressed in metastases20,21 and that high levels of these variants in metastatic tissue are associated with faster disease progression and shorter cancer-specific survival than are low or undetectable levels.13,16,20 However, all these studies have been retrospective in nature, and none have obtained serial specimens across time or investigated the clinical significance of androgen-receptor variants in patients receiving enzalutamide or abiraterone.
Several studies have shown that although the protein isoforms encoded by androgen-receptor splice variants are constitutively active, their function may be dependent on the activity of full-length androgen receptor.19 Therefore, despite the fact that the protein isoforms encoded by androgen-receptor splice variants cannot be targeted directly by currently available drugs, it has been hypothesized that inhibition of full-length androgen receptor by enzalutamide or abiraterone could partially reverse resistance mediated by androgen-receptor variants. Our clinical data do not support this claim, because we did not observe any PSA responses in men harboring AR-V7 (all of whom also expressed full-length androgen receptor mRNA). An alternative treatment approach for AR-V7–positive patients would be to design agents targeting the N-terminal domain of the androgen receptor,22-24 which would theoretically inhibit both full-length androgen receptor and androgen-receptor isoforms that lack the ligand-binding domain; such inhibitors are in early stages of drug development.23,24
There are likely to be multiple additional explanations for primary or acquired resistance to enzalutamide and abiraterone. For instance, overexpression of CYP17A1 (or other steroidogenic enzymes) leading to increased synthesis of intracrine or paracrine androgens has been shown to occur in patients receiving these agents.25-28 In addition, point mutations affecting the ligand-binding domain of the androgen receptor have been shown to confer agonistic activity to enzalutamide.29,30 Furthermore, expression of androgen-regulated genes may be driven by alternative steroid receptors, such as the glucocorticoid receptor.31,32 Finally, inhibition of the androgen receptor may lead to reciprocal up-regulation of other oncogenic pathways, such as the PI3K– AKT pathway.33 It is unlikely that all cases of enzalutamide or abiraterone resistance will be explained by a single cause.
In conclusion, our data support an association between AR-V7 and resistance to both enzalutamide and abiraterone in patients with castration-resistant prostate cancer. These findings require large-scale prospective validation.
Supplementary Material
Acknowledgments
Supported by a Prostate Cancer Foundation (PCF) Young Investigator Award (to Dr. Antonarakis), a PCF Challenge Award, grants from the Department of Defense Prostate Cancer Research Program (W81XWH-10-2-0056 and W81XWH-10-2-0046, to the Prostate Cancer Biorepository Network [PCBN]; and W81XWH-12-1-0605, to Dr. Luo), a Johns Hopkins Prostate Cancer Specialized Program of Research Excellence grant (P50 CA058236), and a grant from the National Institutes of Health (P30 CA006973).
We thank the entire PCBN team at Johns Hopkins University School of Medicine, including Drs. Bruce Trock and Karen Sfanos, for providing valuable input and organizational support; Drs. Carla Ellis, Christine Iacobuzio-Donahue, and Barbara Crain for assistance with the research autopsies; Dr. Nate Brennen and Ms. Jessica Hicks for technical assistance; and Ms. Medha Darshan and Ms. Guifang Yan for assistance in preparing the cryostat sections; and the patients and their families who participated in this study.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Longo DL. New therapies for castration-resistant prostate cancer. N Engl J Med. 2010;363:479–81. doi: 10.1056/NEJMe1006300. [DOI] [PubMed] [Google Scholar]
- 2.Ryan CJ, Tindall DJ. Androgen receptor rediscovered: the new biology and targeting the androgen receptor therapeutically. J Clin Oncol. 2011;29:3651–8. doi: 10.1200/JCO.2011.35.2005. [DOI] [PubMed] [Google Scholar]
- 3.Tran C, Ouk S, Clegg NJ, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324:787–90. doi: 10.1126/science.1168175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scher HI, Beer TM, Higano CS, et al. Antitumour activity of MDV3100 in castration-resistant prostate cancer: a phase 1-2 study. Lancet. 2010;375:1437–46. doi: 10.1016/S0140-6736(10)60172-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O'Donnell A, Judson I, Dowsett M, et al. Hormonal impact of the 17alpha-hydroxylase/C(17,20)-lyase inhibitor abiraterone acetate (CB7630) in patients with prostate cancer. Br J Cancer. 2004;90:2317–25. doi: 10.1038/sj.bjc.6601879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Attard G, Reid AH, Yap TA, et al. Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. J Clin Oncol. 2008;26:4563–71. doi: 10.1200/JCO.2007.15.9749. [DOI] [PubMed] [Google Scholar]
- 7.Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–97. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 8.de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368:138–48. doi: 10.1056/NEJMoa1209096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nadiminty N, Tummala R, Liu C, et al. NF-κB2/p52 induces resistance to enzalutamide in prostate cancer: role of androgen receptor and its variants. Mol Cancer Ther. 2013;12:1629–37. doi: 10.1158/1535-7163.MCT-13-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mostaghel EA, Marck BT, Plymate SR, et al. Resistance to CYP17A1 inhibition with abiraterone in castration-resistant prostate cancer: induction of steroidogenesis and androgen receptor splice variants. Clin Cancer Res. 2011;17:5913–25. doi: 10.1158/1078-0432.CCR-11-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dehm SM, Schmidt LJ, Heemers HV, Vessella RL, Tindall DJ. Splicing of a novel androgen receptor exon generates a constitutively active androgen receptor that mediates prostate cancer therapy resistance. Cancer Res. 2008;68:5469–77. doi: 10.1158/0008-5472.CAN-08-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu R, Dunn TA, Wei S, et al. Ligand-independent androgen receptor variants derived from splicing of cryptic exons signify hormone-refractory prostate cancer. Cancer Res. 2009;69:16–22. doi: 10.1158/0008-5472.CAN-08-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu R, Lu C, Mostaghel EA, et al. Distinct transcriptional programs mediated by the ligand-dependent full-length androgen receptor and its splice variants in castration-resistant prostate cancer. Cancer Res. 2012;72:3457–62. doi: 10.1158/0008-5472.CAN-11-3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Y, Chan SC, Brand LJ, Hwang TH, Silverstein KA, Dehm SM. Androgen receptor splice variants mediate enzalutamide resistance in castration-resistant prostate cancer cell lines. Cancer Res. 2013;73:483–9. doi: 10.1158/0008-5472.CAN-12-3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo Z, Yang X, Sun F, et al. A novel androgen receptor splice variant is upregulated during prostate cancer progression and promotes androgen depletion-resistant growth. Cancer Res. 2009;69:2305–13. doi: 10.1158/0008-5472.CAN-08-3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scher HI, Halabi S, Tannock I, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148–59. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors: European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 19.Watson PA, Chen YF, Balbas MD, et al. Constitutively active androgen receptor splice variants expressed in castration-resistant prostate cancer require full-length androgen receptor. Proc Natl Acad Sci U S A. 2010;107:16759–65. doi: 10.1073/pnas.1012443107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hörnberg E, Ylitalo EB, Crnalic S, et al. Expression of androgen receptor splice variants in prostate cancer bone metastases is associated with castration-resistance and short survival. PLoS One. 2011;6(4):e19059. doi: 10.1371/journal.pone.0019059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang X, Morrissey C, Sun S, et al. Androgen receptor variants occur frequently in castration resistant prostate cancer metastases. PLoS One. 2011;6(11):e27970. doi: 10.1371/journal.pone.0027970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sadar MD. Small molecule inhibitors targeting the “Achilles' heel” of androgen receptor activity. Cancer Res. 2011;71:1208–13. doi: 10.1158/0008-5472.CAN_10-3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ravindranathan P, Lee TK, Yang L, et al. Peptidomimetic targeting of critical androgen receptor-coregulator interactions in prostate cancer. Nat Commun. 2013;4:1923. doi: 10.1038/ncomms2912. [DOI] [PubMed] [Google Scholar]
- 24.Andersen RJ, Mawji NR, Wang J, et al. Regression of castrate-recurrent prostate cancer by a small-molecule inhibitor of the amino-terminus domain of the androgen receptor. Cancer Cell. 2010;17:535–46. doi: 10.1016/j.ccr.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 25.Mitsiades N, Sung CC, Schultz N, et al. Distinct patterns of dysregulated expression of enzymes involved in androgen synthesis and metabolism in metastatic prostate cancer tumors. Cancer Res. 2012;72:6142–52. doi: 10.1158/0008-5472.CAN-12-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Efstathiou E, Titus M, Wen S, et al. Molecular characterization of enzalutamide-treated bone metastatic castration-resistant prostate cancer. Eur Urol. 2014 May 29; doi: 10.1016/j.eururo.2014.05.005. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Efstathiou E, Titus M, Tsavachidou D, et al. Effects of abiraterone acetate on androgen signaling in castrate-resistant prostate cancer in bone. J Clin Oncol. 2012;30:637–43. doi: 10.1200/JCO.2010.33.7675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang KH, Li R, Kuri B, et al. A gain-of-function mutation in DHT synthesis in castration-resistant prostate cancer. Cell. 2013;154:1074–84. doi: 10.1016/j.cell.2013.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Balbas MD, Evans MJ, Hosfield DJ, et al. Overcoming mutation-based resistance to antiandrogens with rational drug design. eLife. 2013;2:e00499. doi: 10.7554/eLife.00499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joseph JD, Lu N, Qian J, et al. A clinically relevant androgen receptor mutation confers resistance to second-generation antiandrogens enzalutamide and ARN-509. Cancer Discov. 2013;3:1020–9. doi: 10.1158/2159-8290.CD-13-0226. [DOI] [PubMed] [Google Scholar]
- 31.Sahu B, Laakso M, Pihlajamaa P, et al. FoxA1 specifies unique androgen and glucocorticoid receptor binding events in prostate cancer cells. Cancer Res. 2013;73:1570–80. doi: 10.1158/0008-5472.CAN-12-2350. [DOI] [PubMed] [Google Scholar]
- 32.Arora VK, Schenkein E, Murali R, et al. Glucocorticoid receptor confers resistance to antiandrogens by bypassing androgen receptor blockade. Cell. 2013;155:1309–22. doi: 10.1016/j.cell.2013.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carver BS, Chapinski C, Wongvipat J, et al. Reciprocal feedback regulation of PI3K and androgen receptor signaling in PTEN-deficient prostate cancer. Cancer Cell. 2011;19:575–86. doi: 10.1016/j.ccr.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.