Abstract
Background
The gastrointestinal tract (GI) is important for detection and transport of consumed nutrients and has been implicated in susceptibility to diet-induced obesity in various rat strains.
Aims
The current studies investigated the regulation of CD36, a receptor which facilitates uptake of long-chain fatty acids, in the GI tract of obesity-prone Osborne–Mendel and obesity-resistant S5B rats fed a high-fat diet.
Methods
Osborne–Mendel and S5B rats consumed a high-fat diet (HFD, 55 % kcal from fat) or a low-fat diet (10 % kcal from fat) for either 3 or 14 days. CD36 messenger RNA (mRNA) levels were measured from circumvallate papillae of the tongue and from duodenal enterocytes.
Results
In Osborne–Mendel rats, consumption of HFD for 3 and 14 days led to an increase in CD36 mRNA on circumvallate papillae and in duodenal enterocytes. CD36 mRNA levels were positively correlated with body weight gain and kilocalories consumed at 3 days. In S5B rats, consumption of HFD for 3 days did not alter CD36 mRNA levels on circumvallate papillae or in the duodenum. Duodenal CD36 levels were elevated in S5B rats following 14 days of HFD consumption. CD36 mRNA levels in the duodenum were positively correlated with body weight gain and kilocalories consumed at 14 days.
Conclusions
These data support the differential sensing of nutrients by two regions of the GI tract of obesity-prone and obesity-resistant rats consuming HFD and suggest a role for CD36 in the strain-specific susceptibility to obesity.
Keywords: Obesity-prone, Obesity-resistant, CD36, Taste bud, Duodenum, High-fat diet
Introduction
The role of the gastrointestinal tract (GI) in detection and absorption of nutrients is an increasingly important component for understanding the increase in the prevalence of obesity. There are numerous hormones and receptor systems produced in the GI tract which are affected by the intake of macronutrients. The release of hormones and the activation of these receptors alter the physiology of the GI tract (i.e., motility), are important modulators of nutrient detection and absorption, alter pancreatic function, and produce effects on the continued ingestion of nutrients (i.e., hunger, satiety). The combined action of cues in the mouth (taste) and gut controls what an individual consumes and may alter an individual’s susceptibility to developing obesity. Investigation of the mechanisms by which the GI tract detects and responds to specific nutrients enhances our knowledge of this region and its role in obesity.
Evidence from animal models of diet-induced obesity suggests that there are differences in the response of the GI tract to the detection of dietary fat in obesity-prone and obesity-resistant strains. Obesity-prone Osborne–Mendel (OM) and obesity-resistant S5B/Pl (S5B) rats are animal models used to assess the mechanisms underlying food intake and obesity [1–8]. OM rats are susceptible to diet-induced obesity and gain more weight and adiposity when eating a high-fat diet (HFD) than S5B rats, which are resistant to diet-induced obesity.
Several aspects of the GI tract have been investigated in obesity-prone (OM) and obesity-resistant (S5B) rats. Using these animal models, Greenberg et al. [2] reported that duodenal infusions of Intralipid and sodium linoleate into sham-fed animals suppressed food intake more completely and for a longer period of time in S5B rats compared with OM rats, suggesting that the obesity-resistant S5B rats were more sensitive to the presence of dietary fat in the duodenum than the OM rats. A recent study examined the role of glucagon-like peptide-1 (GLP-1), a circulating hormone released from the GI tract in response to a meal, in OM and S5B rats. Peripheral administration of a GLP-1 agonist (Exendin 4) led to greater suppression of HFD intake in obesity-resistant S5B rats compared with obesity-prone OM rats, indicating increased sensitivity to Exendin 4 in S5B rats [8]. The response to fatty acids on taste receptors from the tongues of OM and S5B rats also differ and has been investigated using patch clamp techniques [9]. A dose analysis of the effects of linoleic acid on the potassium current of delayed rectifying potassium channels demonstrated greater suppression in OM rats, indicating suppression of the channel’s ability to repolarize following an action potential, suggesting prolonged depolarization of the taste receptors. The data from these studies indicate that the GI tract plays a role in the response to fat and fatty acids in these obesity-prone and obesity-resistant strains and supports a role for the GI tract in the susceptibility to obesity in these animals.
In the GI tract, there are various cellular proteins capable of detecting and transporting fatty acids across the cell membrane. One mechanism by which fatty acids are detected in the GI tract is the long-chain fatty acid receptor, CD36. CD36 is an integral membrane glycoprotein which has been identified in numerous tissues including taste buds, small intestine, muscle, heart, brain, liver, and adipose tissue [6, 10, 11]. A major function of CD36 is to facilitate uptake of long-chain fatty acids. In addition, CD36 is a receptor for several ligands, including thrombospondin 1, collagen, and Gram-negative bacteria [12, 13]. CD36 knockout mice exhibit decreased preference for fatty acids and HFD, decreased cephalic response to fatty acids, and decreased food intake, body weight, and body adiposity, and are protected from weight gain when eating a HFD [14–20]. In the mouth, CD36 has been proposed as the taste receptor for dietary fat and/or fatty acids and is primarily located on the circumvallate papillae of the tongue. As an extension of the mouth, the small intestine expresses many of the same receptors as found on the tongue [21–24]. CD36 is highly expressed in the small intestine and presents a proximal to distal gradient that would be consistent with its function in lipid transport. High-fat diets rich in long-chain fatty acids increase CD36 mRNA levels in the duodenum [25].
Quantitative differences in CD36 expression on the circumvallate papillae and duodenum may be fundamental for sensing of fat in the GI tract, and the current experiments tested the hypothesis that these differences are related to the susceptibility to diet-induced obesity in obesity-prone OM and obesity-resistant S5B rats. Basal expression of CD36 mRNA was evaluated in the circumvallate papillae of the tongue and the duodenal enterocytes of obesity-prone OM and obesity-resistant S5B rats. In experiment 2, the effect of HFD consumption on CD36 mRNA levels on the circumvallate papillae and in the duodenal enterocytes was evaluated in OM and S5B rats. Due to temporal changes in the adaptation to a HFD, two time points (3 versus 14 days) were assessed in this experiment.
Materials and Methods
Animals
The male obesity-prone Osborne–Mendel (OM) and obesity-resistant S5B/Pl (S5B) rats (8–9 weeks old) used in these studies were bred in the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC)-approved Pennington Biomedical Research Center vivarium. Rats were individually housed on a 12/12 h light/dark cycle (lights on at 0700) with food and water available ad libitum. All procedures were approved by the Pennington Biomedical Research Center Institutional Animal Care and Use Committee.
Experiment 1: Basal Levels of CD36 Receptor mRNA on the Circumvallate Papillae of the Tongue and in the Duodenum
OM (n = 5) and S5B (n = 5) rats were given ad libitum access to a standard laboratory chow prior to sacrifice. Average daily food intake was assessed over 3 days prior to sacrifice. For the circumvallate papillae of the tongue, the tongue was removed and cleaned, and the epithelial layer of the tongue encompassing the circumvallate papillae was excised using a sterile scalpel blade. For the duodenum, a 2.5-cm section of the duodenum was removed and cleaned, and the duodenal enterocytes were excised by gentle scraping with a clean metal spatula. Samples were immediately frozen on dry ice and stored at −80 °C until further processing. Body weight was measured immediately prior to sacrifice.
Experiment 2: CD36 Receptor mRNA on the Circumvallate Papillae and in the Duodenum Following 3 or 14 Days Consumption of High-Fat or Low-Fat Diet
OM and S5B rats were given ad libitum access to one of two custom semisynthetic, pelleted rodent diets as previously described [26–28]. The high-fat diet (HFD) and low-fat diet (LFD) differed in their quantity of dietary fat (HFD: 55 % kilocalories from fat; LFD: 10 % kilocalories from fat). Rats consumed either the HFD or the LFD for 3 days (early response; n = 5/group) or 14 days (adaptive response; n = 10/group). Average daily food intake over 3 days was assessed for the 3-day group, and average daily food intake over the last 4 days of diet access in the 14-day group. Body weight was measured prior to dietary manipulation and at the time of sacrifice. Upon sacrifice, the epithelial layer of the tongue containing the circumvallate papillae and the duodenal enterocytes were collected as described in experiment 1. Excised tissue was immediately frozen on dry ice and stored at −80 °C until further processing.
Real-Time Polymerase Chain Reaction (PCR)
RNA was isolated from the circumvallate papillae and the duodenal enterocytes using Tri-Reagent (Molecular Research Ctr, Cincinnati, OH USA) and RNeasy Minikit procedures (Qiagen, Valencia, CA USA) and based on previous experiments [27, 29]. Briefly, enterocytes were homogenized in Tri-Reagent using a motorized tissue homogenizer, chloroform was added to the lysate, and the mixture was centrifuged (12,000×g) in phase lock tubes to separate RNA. Ethanol (70 %) was added to the upper aqueous phase, applied to column, and filtered by centrifugation (8,000×g). Following multiple washes, the samples were subjected to an elution step using RNAase-free water. Reverse transcription (RT) was conducted using the High-Capacity cDNA Reverse Transcriptase Kit (Applied Biosystems, Foster City, CA, USA). For RT, 2.0 μg RNA from each sample was added to random primers (10×), dNTP (25×), MultiScribe Reverse Transcriptase (50U/μl), and RT buffer (10×) and incubated in a thermal cycler (PTC-100; MJ Research, Inc., Watertown, MA, USA) for 10 min at 27 °C, then for 120 min at 37 °C. Primers were designed using Primer Express (Applied Biosystems, Foster City, CA, USA). The following primers were used for CD36: 5′-GAGGTCCTTACACATACAGAGTTCGTT-3′ and 5′-ACAGACAGTGAAGGCTCAAAGATG-3′ and cyclophilin: 5′-CCCACCGTGTTCGACAT-3′ and 5′-CTGTCTTTGGAACTTTGTCTGC-3′. For real-time PCR, SYBR Green 2× Master Mix (Applied Biosystems), forward and reverse primers (10 μM), and RT product (10 ng) were added to 384-well plates. The cycling parameters consisted of an initial 2 min incubation at 50 °C, followed by 10 min at 95 °C, then 15 s at 95 °C, and a 1 min annealing/extension step at 60 °C (40 cycles). The levels of CD36 receptor mRNA were based on a standard curve and normalized to cyclophilin levels and are shown in arbitrary units (a.u.) (ABI Prism 7900 Sequence Detection System, Applied Biosystems).
Statistical Analyses
In experiment 1, two-tailed, between-subjects t tests were used to compare average daily food intake and CD36 receptor mRNA levels between OM and S5B rats fed a standard chow diet. In experiment 2, a between-subjects analysis of variance (ANOVA) (strain × diet) was conducted to assess differences in average daily food intake (kcal), body weight change, CD36 mRNA levels in the circumvallate papillae, and duodenal CD36 receptor mRNA levels for each time point (3 and 14 days). Bonferroni post hoc tests were used to assess differences between individual groups. Correlation analyses were performed to determine the relationship between CD36 mRNA levels in the duodenum and tongue with body weight change and food intake. A significance level of p <.05 was used for all tests.
Results
Experiment 1: Basal Levels of CD36 Receptor mRNA on the Circumvallate Papillae of the Tongue and in the Duodenum
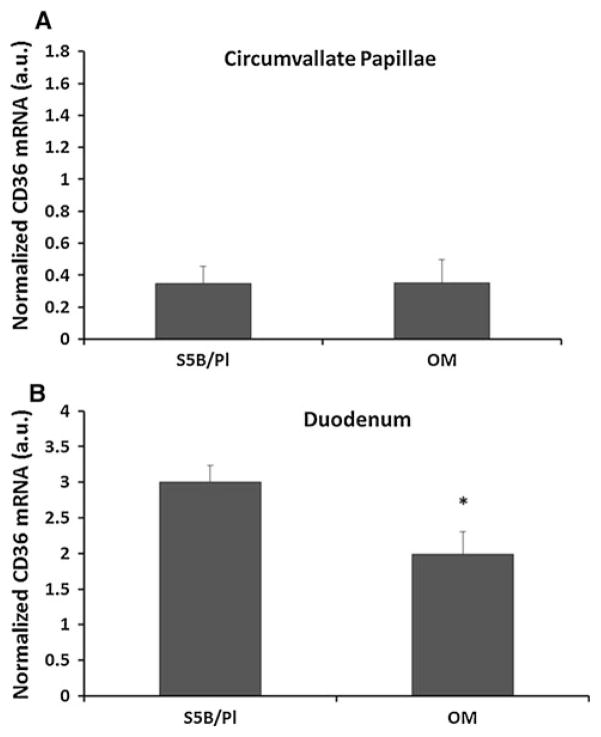
Obesity-prone OM rats consuming a standard chow diet consumed more chow than the obesity-resistant S5B rats (t = 2.41, p <.05, data not shown). Basal CD36 mRNA levels on the circumvallate papillae did not differ between OM and S5B rats (Fig. 1a). Basal CD36 receptor mRNA levels in the duodenal enterocytes were elevated in S5B rats compared with OM rats fed a chow diet (t = 2.27, p <.05; Fig. 1b).
Fig. 1.
Basal levels of CD36 mRNA expression in two regions of the GI tract were measured in OM and S5B rats fed a standard chow diet. a CD36 mRNA levels on the circumvallate papillae of the tongue did not differ between OM and S5B rats. b Duodenal CD36 mRNA levels were higher in the obesity-resistant S5B rats, compared with the OM rats (n = 4–5/group). Data shown as mean ± standard error of the mean (SEM). *p <.05; arbitrary units (a.u.)
Experiment 2: CD36 Receptor mRNA on the Circumvallate Papillae and in the Duodenum Following 3 or 14 Days Consumption of High-Fat or Low-Fat Diet
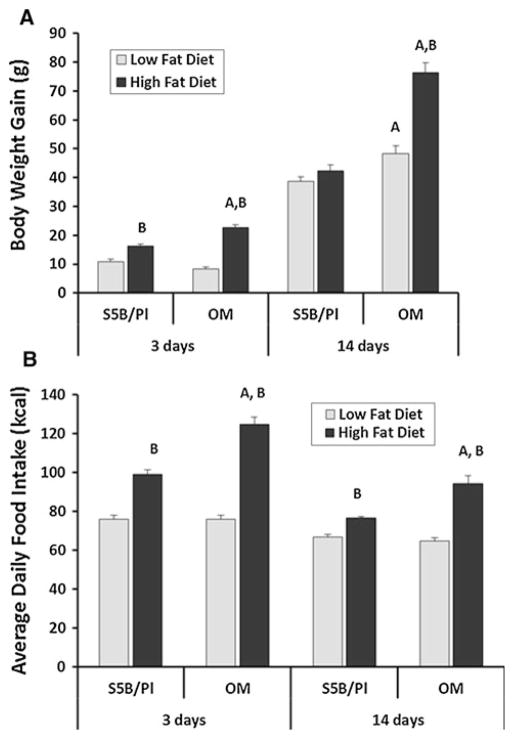
OM and S5B rats consumed either HFD or LFD for 3 or 14 days. A significant diet × strain interaction was detected for body weight gain following 3 days (F = 27.66, p <.05) and following 14 days (F = 20.28, p <.05). As expected, consumption of the HFD increased body weight in S5B and OM rats, compared with rats consuming the LFD. Additionally, OM rats consuming the HFD gained more weight than S5B rats eating the HFD (Fig. 2a). A significant diet × strain interaction was detected for average daily food intake of HFD and LFD following 3 days (F = 24.45, p <.05) and 14 days (F = 17.36, p <.05). Overall, the rats consumed more HFD than LFD, and OM rats consumed more HFD than the S5B rats (Fig. 2b).
Fig. 2.
OM and S5B rats were fed the HFD or LFD diet for 3 or 14 days. a At 3 days, the HFD increased weight gain in OM and S5B rats. Following 14 days of HFD intake, body weight increased in OM rats only. b OM and S5B rats consuming the HFD consumed more kilocalories per day than rats consuming the LFD (n = 5/group for 3 days; n = 10/group for 14 days). Data shown as mean ± SEM. A strain difference; B diet difference within strain; p <.05
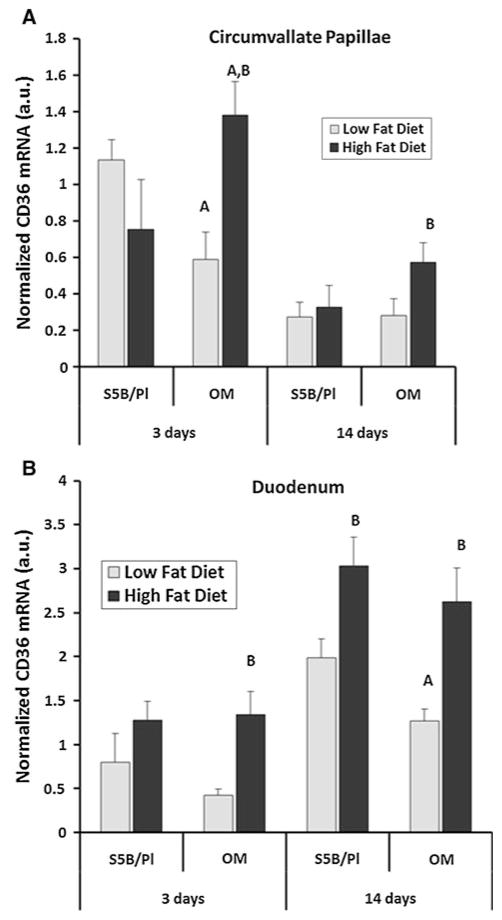
CD36 mRNA levels on the circumvallate papillae and in the duodenum were assessed following 3 or 14 days of HFD or LFD consumption. CD36 mRNA levels on the circumvallate papillae were significantly altered by diet following 3 days of HFD consumption (F = 5.08, p <.05) and 14 days of HFD consumption (F = 4.41, p <.05). In OM rats, consumption of the HFD for 3 days increased expression of CD36 mRNA, compared with OM rats fed the LFD or S5B rats fed the HFD or LFD. Consumption of the HFD for 14 days increased CD36 mRNA levels on the circumvallate papillae of OM rats. Following 3 days of LFD intake, S5B rats exhibited a short-lived increase in CD36 mRNA on the circumvallate papillae compared with OM rats (Fig. 3a).
Fig. 3.
CD36 mRNA expression was assessed in OM and S5B rats fed the HFD or LFD for 3 or 14 days in two regions of the GI tract. a On the circumvallate papillae of the tongue, CD36 mRNA levels were selectively increased by HFD consumption for 3 days and for 14 days in the OM rats. CD36 mRNA levels on the circumvallate papillae were not altered by HFD intake in the S5B rats. b Duodenal CD36 mRNA levels were increased by consumption of the HFD for 3 days in the OM rats and following 14 days consumption in the OM and S5B rats (n = 4–5/group for 3 days; n = 8–9/group for 14 days). Data shown as mean ± SEM. A strain difference; B diet difference within strain; p <.05; arbitrary units (a.u.)
In the duodenum, a significant main effect of diet on CD36 mRNA levels was detected following 3 days of HFD (F = 8.44, p <.05) and following 14 days of HFD consumption (F = 20.51, p <.05). Consumption of HFD for 3 days increased duodenal expression of CD36 mRNA in OM rats, but not S5B rats. Consumption of HFD for 14 days increased duodenal CD36 mRNA expression in both S5B and OM rats. S5B rats consuming the LFD expressed higher levels of CD36 mRNA than OM rats consuming the LFD (Fig. 3b).
A correlational analysis was conducted to determine the relationship between CD36 mRNA expression in the duodenum and on the circumvallate papillae with body weight gain and kilocalories consumed in S5B and OM rats (Table 1). Following 3 days of HFD or LFD intake in OM rats, CD36 mRNA levels in the duodenum were positivity correlated with body weight change (r = .71, p <.05) and kilocalories consumed (r = .90, p <.05). In addition, CD36 mRNA levels on the circumvallate papillae were positively correlated with body weight gain (r = .80, p <.05) and kilocalories consumed (r = .75, p <.05). Following 14 days consumption of the HFD or LFD, CD36 mRNA in the GI tract was not correlated with either body weight gain or kilocalories consumed in OM rats. In the S5B rats, CD36 mRNA levels in the duodenum were positively correlated with body weight gain (r = .67, p <.05) and kilocalories consumed (r = .46, p <.05) following 14 days access to HFD or LFD.
Table 1.
Correlational analyses conducted in OM and S5B rats consuming the HFD or LFD for 3 or 14 days
| CD36 mRNA | Body weight gain | Kilocalories consumed | CD36 mRNA duodenum | CD36 mRNA tongue |
|---|---|---|---|---|
| Osborne–Mendel—3 days | ||||
| Duodenum | .712* | .904* | 1 | .530 |
| Tongue | .804* | .753* | .530 | 1 |
| Osborne–Mendel—14 days | ||||
| Duodenum | .471 | .392 | 1 | .491 |
| Tongue | .405 | .500 | .491 | 1 |
| S5B/Pl—3 days | ||||
| Duodenum | .325 | .377 | −.363 | 1 |
| Tongue | −.552 | −.262 | 1 | −.363 |
| S5B/Pl—14 days | ||||
| Duodenum | .668* | .463* | 1 | −.299 |
| Tongue | −.181 | .004 | −.299 | 1 |
p <.05
Discussion
There are a number of molecular candidates for the detection and transport of fat/fatty acids in the GI tract, and the mechanisms by which fatty acids are detected in the GI tract are of great interest to understanding obesity. One potential mechanism is the long-chain fatty acid receptor, CD36, which is expressed on the tongue and in the small intestine. The purpose of the current experiments was to examine both strain- and diet-induced differences in the expression of CD36 in the GI tract of obesity-prone and obesity-resistant rats. Two time points (early response and adaptive response) were selected for analyses due to strain differences in the overconsumption of HFD at these time points. Our previous studies have shown a significant increase in HFD intake in S5B for 3 days following introduction to the HFD and for 14 days in the OM rats. It was hypothesized that the susceptibility to obesity would be related to the expression of CD36 on the tongue and in the duodenum.
Previous reports have suggested that the obesity-prone OM and obesity-resistant S5B rats differ neurochemically, behaviorally, and physiologically in their response to consumption of dietary fat [1, 3, 4, 8, 29–39]. Several studies have impacted the focus of the current experiments and led to the investigation of differences in the GI tract of these rats. Greenberg et al. [2] reported that duodenal infusions of Intralipid and sodium linoleate produced greater suppression of food intake in obesity-resistant S5B rats than in obesity-prone OM rats. These data suggested that there were strain differences in the detection of fatty acids in the gut. Gilbertson et al. [9, 40] reported strain differences in the response to the application of fatty acids on the taste receptors of OM and S5B rats using patch clamp techniques. Their data suggested that, at the level of the tongue, S5B rats were more sensitive to the effects of fatty acids than OM rats and that OM rats had fewer fatty acid-sensitive delayed rectifying potassium channels expressed on the tongue than S5B rats. Recently, we provided evidence that the response to the satiety hormone, GLP-1, was decreased in OM rats, again suggesting that S5B rats were more responsive to signals from the GI tract. This increased sensitivity may ultimately decrease their susceptibility to obesity [8].
On the circumvallate papillae, CD36 has been proposed as a fat taste receptor [17, 19, 20, 41–49]. These reports have provided compelling evidence for the detection of dietary fat by CD36 on the tongue. As the first step in the GI tract, CD36 on the tongue has the potential to affect both positive and negative feedback responses to dietary fat, thereby increasing or decreasing the intake of dietary fat, in addition to altering the sensitivity to the “taste” of dietary fat. The relative differences in expression of CD36 on the tongue in response to short-term exposure to a HFD versus the adaptation to the consumption of HFD may provide insight into the ways by which these two rat strains alter their intake patterns of HFD.
In the current experiments, the data indicate that, though there are no basal differences in the expression of CD36 mRNA on the circumvallate papillae of obesity-prone OM and obesity-resistant S5B rats, the consumption of the HFD produced significant strain effects on CD36 expression. CD36 mRNA levels on the circumvallate papillae were increased by consumption of the HFD in the OM rats only. OM rats consumed significantly more HFD than S5B rats at both time points, suggesting that this hyperphagia was associated with an increase in CD36. In the obesity-prone OM rats, CD36 mRNA levels were positively correlated with body weight gain and kilocalories consumed following 3 days access to HFD or LFD, but not at the 14-day time point. These data suggest that CD36 mRNA expression on the tongue plays a role in the early response to HFD intake in the obesity-prone rats, likely due to an increase in the rewarding aspects of the HFD. This relationship appears to be short-lived and is strain-specific.
The data reported in experiment 2 differ from a previous report which suggested that HFD intake led to a decrease in the expression of CD36 mRNA on the circumvallate papillae of rats [49]. These differences are likely due to a variety of factors, most importantly the use of different rat strains. In the current experiment, a possible explanation for the increase in CD36 mRNA levels found in the OM rats consuming the HFD is that these receptors are part of a positive feedback response to the HFD, which is specific to this strain and may signal an increase in the rewarding effects of HFD. Additionally, though CD36 mRNA levels were increased in this study, CD36 protein levels were not evaluated in the current studies and may not be increased to the same extent as the mRNA levels in the OM rats. Further studies are needed to explore this possibility. A study conducted by Gilbertson et al. [40] reported that, though the expression of delayed rectifying potassium receptors was elevated in OM rats, these rats had fewer fatty acid-sensitive receptors compared with S5B rats. Therefore, it is possible that gene expression and protein levels of CD36 do not correlate. More studies are needed to determine the role of CD36 on the tongue of OM and S5B rats.
Duodenal expression of CD36 was also investigated as the duodenum plays a role in fat detection and absorption in the GI tract. Interestingly, S5B rats expressed higher basal levels of duodenal CD36 mRNA than OM rats. This potentially reflects an increased sensitivity to the presence of dietary fat/fatty acids in S5B rats, as suggested by Greenberg et al. [2]. Though CD36 is involved in fat absorption, which may lead to weight gain, duodenal levels of CD36 in S5B rats likely play a role as a molecular sensor linking fat ingestion with satiety to initiate a release in satiety hormones [50–52]. In experiment 2, duodenal CD36 expression was increased by 3 days access to HFD in the OM rats, but not in the S5B rats. This demonstrates the rapid response to the HFD in the OM rats and the apparent resistance to the HFD in the S5B rats, with respect to changes in CD36 levels. After 14 days of HFD intake, duodenal CD36 mRNA levels were increased in the OM and S5B rats. Consumption of a HFD rich in long-chain fatty acids has been shown to increase CD36 mRNA levels in the duodenum [25]; therefore, the increase in duodenal CD36 mRNA levels following 14 days of consumption of HFD as the sole source of food was expected. More studies are needed to fully understand the role of CD36 in the duodenum of OM and S5B rats. Correlational analyses suggest that CD36 mRNA levels are positively correlated with body weight gain and kilocalories consumed following 3 days access to HFD or LFD in OM rats, but not S5B rats. These relationships are not detected once the OM rats have become adapted to the HFD or LFD; however, a positive relationship is detected between body weight gain and kilocalories consumed and CD36 mRNA expression in S5B rats following 14 days.
There are various cellular proteins which are capable of detecting and transporting fatty acids across the cell membrane. The current experiments examined the mRNA expression of CD36, which has been linked to detection and transport of long-chain fatty acids in the brain, muscle, tongue, and intestines. Overall, these experiments suggest that differential expression of CD36 following consumption of HFD in obesity-prone OM and obesity-resistant S5B rats contributes to the differential sensing of nutrients by two regions of the GI tract in these rat strains. There are apparent alterations in the topographical expression of CD36 suggesting a difference in the response to HFD on the tongue and in the duodenum. The increase in CD36 expression on the tongues of the OM rats suggests an increase in positive feedback or the rewarding aspects of the HFD through taste-sensitive pathways and supports previous reports with CD36 KO mice on fat preference and intake [14–20]. Alterations in the duodenum are representative of the postingestive effects of the HFD in our study. Prolonged consumption of the HFD increased the expression of CD36 in the S5B and OM rats, which may influence the detection of fatty acids in these strains. Previous reports have suggested that S5B rats are more sensitive to the satiating effects of a HFD, and therefore the increase in CD36 expression in S5B rats may reflect this increase in sensitivity and increase in satiety [2, 8]. However, the OM rats have an apparent dysregulation of the satiety signals released by the GI tract [2, 8]. Therefore, the increased expression of duodenal CD36 may not be able to adequately detect and signal the duodenum to produce and release satiety hormones. Overall, the increased potential for diet-induced obesity in OM rats may be influenced by the ability of CD36 to adequately detect the presence of the HFD at various topographical levels in the GI tract.
Acknowledgments
This research was supported by NIDDK 32089 to G.A. Bray. The authors would like to thank Christine Blackmon, Katherine Pyburn, and Daniel Shaheen for their assistance on this project. This work was supported in part by P20-RR021945 from the National Center for Research Resources and NIH Center Grant 1P30 DK072476.
Footnotes
Conflict of interest None.
Contributor Information
Stefany D. Primeaux, Email: sprime@lsuhsc.edu, Joint Diabetes, Endocrinology and Metabolism Program, Louisiana State University System, Louisiana State University Health Science Center-New Orleans, New Orleans, LA 70112, USA. Internal Medicine-Endocrinology, Diabetes & Metabolism, LSUHSC-NO, 1542 Tulane Ave, Box T4 M-2, New Orleans, LA 70112, USA
H. Douglas Braymer, Pennington Biomedical Research Center, Baton Rouge, LA 70808, USA.
George A. Bray, Pennington Biomedical Research Center, Baton Rouge, LA 70808, USA
References
- 1.Barnes MJ, Holmes G, Primeaux SD, York DA, Bray GA. Increased expression of mu opioid receptors in animals susceptible to diet-induced obesity. Peptides. 2006;27:3292–3298. doi: 10.1016/j.peptides.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 2.Greenberg D, McCaffery J, Potack JZ, Bray GA, York DA. Differential satiating effects of fats in the small intestine of obesity-resistant and obesity-prone rats. Physiol Behav. 1999;66:621–626. doi: 10.1016/s0031-9384(98)00336-9. [DOI] [PubMed] [Google Scholar]
- 3.Ishihara Y, White CL, Kageyama H, Kageyama A, York DA, Bray GA. Effects of diet and time of the day on serum and CSF leptin levels in Osborne-Mendel and S5B/Pl rats. Obes Res. 2004;12:1067–1076. doi: 10.1038/oby.2004.134. [DOI] [PubMed] [Google Scholar]
- 4.Liu X, York DA, Bray GA. Regulation of Ghrelin gene expression in stomach and feeding response to a Ghrelin analogue in two strains of rats. Peptides. 2004;25:2171–2177. doi: 10.1016/j.peptides.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 5.Petrescu O, Cheema AF, Fan X, Bradbury MW, Berk PD. Differences in adiposyte long chain fatty acid uptake in Osborne-Mendel and S5B/Pl rats in response to high-fat diets. Int J Obes. 2008;32:853–862. doi: 10.1038/sj.ijo.0803792. [DOI] [PubMed] [Google Scholar]
- 6.Pittman D, Smith KR, Crawley ME, et al. Orosensory detection of fatty acids by obesity-prone and obesity-resistant rats: strain and sex differences. Chem Senses. 2008;33:449–460. doi: 10.1093/chemse/bjn012. [DOI] [PubMed] [Google Scholar]
- 7.Primeaux SD, Barnes MJ, Bray GA. Olfactory bulbectomy increases food intake and hypothalamic neuropeptide Y in obesity-prone, but not obesity-resistant rats. Behav Brain Res. 2007;180:190–196. doi: 10.1016/j.bbr.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Primeaux SD, Barnes MJ, Braymer HD, Bray GA. Sensitivity to the satiating effects of Exendin 4 is decreased in obesity-prone Osborne-Mendel rats compared to obesity-resistant S5B/Pl rats. Int J Obes. 2010;34:1427–1433. doi: 10.1038/ijo.2010.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilbertson TA, Liu L, York DA, Bray GA. Dietary fat preferences are inversely correlated with peripheral gustatory fatty acid sensitivity. Ann N Y Acad Sci. 1998;855:165–168. doi: 10.1111/j.1749-6632.1998.tb10560.x. [DOI] [PubMed] [Google Scholar]
- 10.Bonen A, Holloway GP, Tandon NN, et al. Cardiac and skeletal muscle faty acid transport and transporters and triacylglycerol and fatty acid oxidation in lean and Zucker diabetic fatty rats. Am J Physiol Regul Integrat Comp Physiol. 2009;297:R1202–R1212. doi: 10.1152/ajpregu.90820.2008. [DOI] [PubMed] [Google Scholar]
- 11.Moore KJ, El Khoury J, Medeiros LA, et al. A CD36-initiated signaling cascade mediate inflammatory effects of beta-amyloid. J Biol Chem. 2002;277:47373–47379. doi: 10.1074/jbc.M208788200. [DOI] [PubMed] [Google Scholar]
- 12.Coburn CT, Knapp FF, Febbraio M, Beets AL, Silverstein RL, Abumrad NA. Defective uptake and utilization of long chain fatty acids in muscle and adipose tissues of CD36 knockout mice. J Biol Chem. 2000;275:32523–32529. doi: 10.1074/jbc.M003826200. [DOI] [PubMed] [Google Scholar]
- 13.Febbraio M, Silverstein RL. CD36: implications in cardiovascular disease. Int J Biochem Cell Biol. 2007;39:2012–2030. doi: 10.1016/j.biocel.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abumrad NA. CD36 may determine our desire for dietary fats. J Clin Invest. 2005;115:2965–2967. doi: 10.1172/JCI26955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Febbraio M, Abumrad NA, Hajjar DP, et al. A null mutation in murine CD36 reveals an importnt role in fatty acid and lipoprotein metabolism. J Biol Chem. 1999;274:19055–19062. doi: 10.1074/jbc.274.27.19055. [DOI] [PubMed] [Google Scholar]
- 16.Febbraio M, Guy E, Coburn C, et al. The impact of overexpression and deficiency of fatty acid traslocase (FAT)/CD36. Mol Cell Biochem. 2002;239:193–197. [PubMed] [Google Scholar]
- 17.Gaillard D, Laugerette F, Darcel N, et al. The gustatory pathway is involved in CD36-mediated orosensory perception of long-chain fatty acids in the mouse. FASEB J. 2008;22:1458–1468. doi: 10.1096/fj.07-8415com. [DOI] [PubMed] [Google Scholar]
- 18.Hajri T, Hall AM, Jensen DR, et al. CD36-facilitated fatty acid uptake inhibits leptin production and signaling in adipose tissue. Diabetes. 2008;56:1872–1880. doi: 10.2337/db06-1699. [DOI] [PubMed] [Google Scholar]
- 19.Laugerette F, Passilly-Degrace P, Patris B, et al. CD36 involvement in orosensory detection of dietary lipids, spontaneous fat preference, and digestive secretions. J Clin Invest. 2005;115:3177–3184. doi: 10.1172/JCI25299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sclafani A, Ackroff K, Abumrad NA. CD36 gene deletion reduces fat preference and intake but not post-oral fat conditioning in mice. Am J Physiol Regulat Integ Comp Physiol. 2007;293:R1823–R1832. doi: 10.1152/ajpregu.00211.2007. [DOI] [PubMed] [Google Scholar]
- 21.Hofer D, Puschel B, Drenckhahn D. Taste receptor-like cells in the rat gut identified by expression of alpha-gustducin. Proc Natl Acad Sci U S A. 1996;93:6631–6634. doi: 10.1073/pnas.93.13.6631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jang HJ, Kokrashvili Z, Theodorakis MJ, et al. Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide 1. Proc Natl Acad Sci U S A. 2007;104:15069–15074. doi: 10.1073/pnas.0706890104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kokrashvili Z, Mosinger B, Margolskee RF. Taste signaling elements expressed in gut enteroendocrine cells regulate nutrient-responsive secretion of gut hormones. Am J Clin Nutr. 2009;90:822S–825S. doi: 10.3945/ajcn.2009.27462T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Margolskee RF, Dyer J, Kokrashvili Z, et al. T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose co-transporter 1. Proc Natl Acad Sci U S A. 2007;104:15075–15080. doi: 10.1073/pnas.0706678104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen M, Yang Y, Braunstein E, Georgeson KE, Harmon CM. Gut expression and regulation of FAT/CD36: possible role in fatty acid transport in rat enterocytes. Am J Physiol Endocrinol Metab. 2001;281:E916–E923. doi: 10.1152/ajpendo.2001.281.5.E916. [DOI] [PubMed] [Google Scholar]
- 26.Lin L, Chen J, York DA. Chronic ICV enterostatin preferentially reduced fat intake and lowered body weight. Peptides. 1997;18:657–661. doi: 10.1016/s0196-9781(97)00128-9. [DOI] [PubMed] [Google Scholar]
- 27.Primeaux SD. QRFP in female rats: effects on high fat food intake and hypothalamic gene expression across the estrous cycle. Peptides. 2011;32:1270–1275. doi: 10.1016/j.peptides.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Primeaux SD, York DA, Bray GA. Neuropeptide Y administration into the amygdala alters high fat food intake. Peptides. 2006;27:1644–1651. doi: 10.1016/j.peptides.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 29.Primeaux SD, Blackmon C, Barnes MJ, Braymer HD, Bray GA. Central administration of the RF amide peptides, QRFP-26 and QRFP-43, increases high fat food intake in rats. Peptides. 2008;29:1994–2000. doi: 10.1016/j.peptides.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin L, Martin RJ, Schaffhauser AO, York DA. Acute changes in the response to peripheral leptin with alteration in the diet composition. Am J Physiol Regulat Integrat Comp Physiol. 2001;280:R504–R509. doi: 10.1152/ajpregu.2001.280.2.R504. [DOI] [PubMed] [Google Scholar]
- 31.Lin L, York DA. Comparisons of the effects of enterostatin on food intake and gastric emptying in rats. Brain Res. 1997;745:205–209. doi: 10.1016/s0006-8993(96)01152-3. [DOI] [PubMed] [Google Scholar]
- 32.Madiehe AM, Schaffhauser AO, Braymer DH, Bray GA, York DA. Differential expression of leptin receptor in high- and low-fat-fed Osborne-Mendel and S5B/Pl rats. Obes Res. 2000;8:467–474. doi: 10.1038/oby.2000.58. [DOI] [PubMed] [Google Scholar]
- 33.Okada S, York DA, Bray GA, Mei J, Erlanson-Albertsson C. Differential inhibition of fat intake in two strains of rat by the peptide enterostatin. Am J Physiol. 1992;262:R1111–R1116. doi: 10.1152/ajpregu.1992.262.6.R1111. [DOI] [PubMed] [Google Scholar]
- 34.Ookuma K, Barton C, York DA, Bray GA. Differential response to kappa-opioidergic agents in dietary fat selection between Osborne-Mendel and S5B/P1 rats. Peptides. 1998;19:141–147. doi: 10.1016/s0196-9781(97)00255-6. [DOI] [PubMed] [Google Scholar]
- 35.Schaffhauser AO, Madiehe AM, Braymer HD, Bray GA, York DA. Effects of a high-fat diet and strain on hypothalamic gene expression in rats. Obes Res. 2002;10:1188–1196. doi: 10.1038/oby.2002.161. [DOI] [PubMed] [Google Scholar]
- 36.Schemmel RA, Teague RJ, Bray GA. Obesity in Osborne-Mendel and S5B/Pl rats: effects of sucrose solutions, castration, and treatment with estadiol or insulin. Am J Physiol. 1982;243:R347–R353. doi: 10.1152/ajpregu.1982.243.3.R347. [DOI] [PubMed] [Google Scholar]
- 37.Thanos PK, Cho J, Kim R, et al. Bromocriptine increased operant responding for high fat food but decreased chow intake in both obesity-prone and resistant rats. Behav Brain Res. 2011;217:165–170. doi: 10.1016/j.bbr.2010.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thanos PK, Kim R, Cho J, et al. Obesity-resistant S5B rats showed greater cocaine conditioned place preference than the obesity-prone OM rat. Physiol Behav. 2010;101:713–718. doi: 10.1016/j.physbeh.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.White CL, Ishii Y, Mendoza T, et al. Effect of a selective OX1R antagonist on food intake and body weight in two strains of rats that differ in susceptibility to dietary-induced obesity. Peptides. 2005;26:2331–2338. doi: 10.1016/j.peptides.2005.03.042. [DOI] [PubMed] [Google Scholar]
- 40.Gilbertson TA, Liu L, Kim I, Burks KA, Hansen DR. Fatty acid responses in taste cells from obesity-prone and -resistant rats. Physiol Behav. 2005;86:681–690. doi: 10.1016/j.physbeh.2005.08.057. [DOI] [PubMed] [Google Scholar]
- 41.El-Yassimi A, Hichami A, Besnard P, Khan NA. Linoleic acid induces calcium signaling, Src kinase phosphorylation and neurotransmitter release in mouse CD36-positive gustatory cells. J Biol Chem. 2008;283:12949–12959. doi: 10.1074/jbc.M707478200. [DOI] [PubMed] [Google Scholar]
- 42.Gaillard D, Passilly-Degrace P, Besnard P. Molecular mechanisms of fat preference and overeating. Ann N Y Acad Sci. 2008;1141:163–175. doi: 10.1196/annals.1441.028. [DOI] [PubMed] [Google Scholar]
- 43.Khan NA, Besnard P. Oro-sensory preception of dietary lipids: new insights into the fat taste transduction. Biochim Biophys Acta. 2009;1791:149–155. doi: 10.1016/j.bbalip.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 44.Martin C, Passilly-Degrace P, Merlin JF, Chevrot M, Besnard P. The lipid-sensor candidates CD36 and GPR120 are differentially regulated by dietary lipids in mouse taste buds: impact on spontaneous fat preference. PLoS ONE. 2011;6:e24014. doi: 10.1371/journal.pone.0024014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mizushige T, Inoue K, Fushiki T. Why is fat so tasty? Chemical reception of fatty acid on tongue. J Nutr Sci Vitaminol (Tokyo) 2007;53:1–4. doi: 10.3177/jnsv.53.1. [DOI] [PubMed] [Google Scholar]
- 46.Passilly-Degrace P, Gaillard D, Besnard P. Orosensory perception of dietary lipids in mammals. Results Probl Cell Differ. 2009;47:221–238. doi: 10.1007/400_2008_7. [DOI] [PubMed] [Google Scholar]
- 47.Simons PJ, Boon L. Lingual CD36 and obesity: a matter of fat taste? Acta Histochem. 2011;113:765–767. doi: 10.1016/j.acthis.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 48.Simons PJ, Kummer JA, Luiken JJ, Boon L. Apical CD36 immunolocalization in human and procine taste buds from circumvallate and foliate papillae. Acta Histochem. 2011;113:839–843. doi: 10.1016/j.acthis.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 49.Zhang XJ, Zhou LH, Ban X, Liu DX, Jiang W, Liu XM. Decreased expression of CD36 in circumvallate taste buds of high-fat induced obese rats. Acta Histochem. 2011;113:663–667. doi: 10.1016/j.acthis.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 50.Guijarro A, Fu J, Astarita G, Piomelli D. CD36 gene deletion decreases oleoylethanolamide levels in small intestine of free-feeding mice. Pharmacol Res. 2010;61:27–33. doi: 10.1016/j.phrs.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schwartz GJ, Fu J, Astarita G, et al. The lipid messenger OEA links dietary fat intake to satiety. Cell Metab. 2008;8:281–288. doi: 10.1016/j.cmet.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang Y, Chen M, Loux TJ, Harmon CM. Regulation of FAT/ CD36 mRNA gene expression by long chain fatty acids in the differentiated 3T3-L1 cells. Pediatr Surg Int. 2007;23:675–683. doi: 10.1007/s00383-007-1942-6. [DOI] [PubMed] [Google Scholar]