Abstract
Introduction
Adipose tissue is responsible for triggering chronic systemic inflammatory response and these changes may be involved in the pathophysiology of preeclampsia.
Objective
To characterize the lipid profile in the placenta and plasma of patients with preeclampsia.
Methodology
Samples were collected from placenta and plasma of 10 pregnant women with preeclampsia and 10 controls. Lipids were extracted using the Bligh–Dyer protocol and were analysed by MALDI TOF-TOF mass spectrometry.
Results
Approximately 200 lipid signals were quantified. The most prevalent lipid present in plasma of patients with preeclampsia was the main class Glycerophosphoserines-GP03 (PS) representing 52.30% of the total lipid composition, followed by the main classes Glycerophosphoethanolamines-GP02 (PEt), Glycerophosphocholines-GP01 (PC) and Flavanoids-PK12 (FLV), with 24.03%, 9.47% and 8.39% respectively. When compared to the control group, plasma samples of patients with preeclampsia showed an increase of PS (p<0.0001), PC (p<0.0001) and FLV (p<0.0001). Placental analysis of patients with preeclampsia, revealed the PS as the most prevalent lipid representing 56.28%, followed by the main class Macrolides/polyketides-PK04 with 32.77%, both with increased levels when compared with patients control group, PS (p<0.0001) and PK04 (p<0.0001).
Conclusion
Lipids found in placenta and plasma from patients with preeclampsia differ from those of pregnant women in the control group. Further studies are needed to clarify if these changes are specific and a cause or consequence of preeclampsia.
Introduction
Preeclampsia is a systemic disease characterized by intense inflammatory response, endothelial injury, platelet aggregation, coagulation system activation and increase vascular resistance. It affects about 5–8% of all pregnant women [1]–[3]. The diagnosis of preeclampsia is based on the development of hypertension (≥140/90 mmHg) and significant proteinuria (≥300 mg/24 hours) after 20 weeks of gestation [4].
The systemic complications of preeclampsia are not limited to the gestational period and recent studies have shown long-term adverse outcomes, such as increased risk for developing chronic hypertension, ischemic heart disease, acute myocardial infarction and venous thromboembolism, requiring longer follow-up and surveillance of these patients throughout their lives [4], [5]. Despite its relevance, preeclampsia pathogenesis is not completely understood. It has been established that the trophoblast has a key role in this process and many other conditions related to chronic inflammation can be relevant in different stages of the disease [3].
Obesity and Preeclampsia
Obesity, defined by the World Health Organization (WHO) through the body mass index above 30 kg/m2, is a growing epidemic problem and it affects 500 million adults across the world [6], [7]. It represents an important health problem and it has an enormous impact on modern obstetrics.
Adipose tissue is responsible for triggering chronic systemic inflammatory response, with increased levels of inflammatory cytokines such as TNF-α, IL-6 and MCP-1. The inflammatory response related to obesity has been considered as the link between this condition and preeclampsia [6], [8]–[13].
Although the link between obesity and inflammatory response is well recognized, the roles of lipids in the cell function are even more extended. These molecules are responsible for the control of important cellular processes, including proliferation, apoptosis, metabolism and migration. They also assist in the transmission of biological information across cell membranes, directly contributing to proper cell functioning [14]–[16]. An impairment in lipid signaling pathways may contribute to the progression of chronic inflammatory diseases, such as autoimmune, allergic, neoplastic, atherosclerosis, hypertension, myocardial hypertrophy and metabolic degenerative diseases [17], [18] and may be also related to preeclampsia pathophysiology.
Lipid molecules are defined by the International Committee for the Classification and Nomenclature of Lipids (ICCNL) in eight categories, based on their chemical functions: Fatty Acyls (FA), Glycerolipids (GL), Glycerophospholipids (GP), Sphingolipids (SP), Sterol Lipids (ST), Prenol Lipids (PR), Saccharolipids (SL) and Polyketides (PK). Each category is further subdivided into lipid main classes and subclasses [19], [20].
Historically, the study of the function and properties of lipids was always extremely complicated due to their structural diversity and large number of isomorphic species. Technically, the distinction between pathogenic and nonpathogenic lipid molecules represents a challenge that has become possible through lipidomics [18], [21], [22].
Lipidomic analysis
Lipidomic analysis is a global characterization of all kinds of lipid molecules in biological system. The methodology used is mass spectrometry (MS) [22]–[24]. A technique known as Matrix-Assisted Laser Desorption/Ionization - Mass Spectrometry (MALDI-MS), has been the preferred method to evaluate lipidomics because it is relatively easy to handle [25], [26]. MALDI is an ionization technique enabled by a laser beam (light amplification by stimulated emission of radiation) that acts upon a sample mixed with a matrix. This process generates ionized molecules. For complete separation, the most widely used technology is the time of flight (TOF), which consists of a long pipe (tube flight) capable of separating the ionized molecules according to a ratio of mass to charge (m/z) [25].
Lipidomic analysis in preeclampsia is a new research line. Recently, we demonstrated that women with early-onset preeclampsia have particular lipids in their plasma when compared to those with healthy pregnancy [26]. Additionally, Baig et al. published their findings evaluating samples of syncytiotrophoblast microvesicles from human placenta [27]. These authors also demonstrated that there was a significant increase of some classes of lipids as well as a reduction of others in samples from preeclamptic women.
Given the strong association between obesity and early dyslipidemia with preeclampsia and the first reports associating distinct lipid species with the disease, this study aimed to find a specific lipid profile that may be characteristic for these patients. Here we evaluated plasma samples and placental tissues of women with early-onset preeclampsia and established an interesting panel of lipids in these different settings.
Material and Methods
Ethics Statement
All participants in this study have provided their written informed consent. This study was approved by the Research Ethics Committee of the Federal University of São Paulo with the number 297/027 and by the Research Ethics Committee of the School Maternity Vila Nova Cachoeirinha with the number 34/2011, linked with the National database (CEP/CONEP) under the protocol number CAAE - 18100813.1.0000.5505.
Study Population
This is a case-control study involving 20 pregnant women (10 women with early-onset preeclampsia and 10 women with healthy pregnancy). All samples were collected at the School Maternity Vila Nova Cachoeirinha from October 2011 to April 2013. Early-onset preeclampsia was defined as blood pressure ≥140/90 mmHg and significant proteinuria (≥300 mg/24 hours) after 20 weeks of gestation and before 34 weeks.
Sample collection
Blood
Five milliliters of peripheral blood were drawn in EDTA-tube at the time of delivery. Immediately after collection, blood samples were centrifuged at 2000 rpm for 5 minutes and supernatants aliquoted and stored at −80°C for subsequent lipid extraction.
Placenta
Immediately after cesarean delivery, one fragment was removed from the central region of the basal plate of the placenta, with a wedge shape of approximately 3.0 cm in diameter at its greatest diameter, obtained with a sterile scalpel blade N°15. This fragment was divided in 3 smaller pieces of 1×1 cm, washed in saline solution and immediately frozen and stored at −80°C for further processing and lipid extraction. For lipid extraction the frozen placental sample of 1×1 cm was plunged into liquid nitrogen for 1 minute and crushed using marble stone until obtaining small fragments (powder). Powder was then placed in a dry tube containing 300 µl of Milli-Q water. This material was subjected to further mixing and homogenization in a mechanical processor for five minutes and the resulting material subjected to lipid extraction.
Lipid extraction
Lipids were extracted from each sample using the Bligh–Dyer protocol [28]. Immediately after thawing, each 50 µL of plasma and placental homogenated (described above) were dissolved in a mixture of chloroform–methanol (125∶250 µL) and vortexed well. After vortexing, 125 µL of chloroform and 100 µL of deionized water were added to supernatant and centrifuged at 1000 rpm in a table-top centrifuge for 5 min at room temperature. Following this protocol a two-phase system (aqueous top, organic bottom) was achieved. The bottom phase containing lipids was gently recovered using a Pasteur pipette; they were dried and sealed to be stored at −80°C.
Reagents
All chemicals were of analytical reagent grade and they were used as received. Chloroform (CHCl3) and methanol (MeOH) were purchased from Burdick & Jackson (Muskegon, MI, USA). 2,5-Dihydroxybenzoic acid (DHB) was purchased from ICN Biomedicals (Aurora, OH, USA). Distilled water was deionized on a Millipore Milli-Q water reagent system (Millipore, Bedford, MA, USA). EDTA-tubes were purchased from Sigma-Aldrich (St. Louis, MO).
Mass spectrometry analysis
MALDI-MS spectra were acquired in the positive ion and reflector modes using the equipment MALDI TOF-TOF - Ultraflex model - Bruker and matrix 2,5 - DHB - White (2,5-Dihydroxy benzoic acid - 40 mg/ml in acetonitrile). The main operating condition used was 10 V (sample plate). The laser irradiation consisted of diverse shots during 60–90 seconds in the region where the sample had been placed.
Data processing
Raw data were analyzed by MarkerLynx (Waters, UK) for peak detection and alignment. The parameters were set as follows: mass tolerance was set at 100 ppm (suggested more than twice the instrument mass accuracy considering extreme value in signal saturation condition); peak width at 5% height and peak-to-peak baseline noise were calculated automatically by the software; mass window was set at 0.1 amu (atomic mass units); retention time window was set at 1 min (considering the maximum variation obtainable by a CapLC system); noise elimination level was 5; minimum intensity was set at 5%; peak intensity and retention time were normalized with the signal of the internal standard. This procedure allowed deconvolution alignment, and data reduction to give a table of mass and relative retention time pairs with associated relative intensities for all the detected peaks. Then data matrix was exported for partial least squares discriminant analysis (PLS-DA). In order to find differential circulating lipids, a VIP parameter (Variable Importance in the Projection) was employed to reflect the variable importance in the discriminant analysis. The major discriminant variables returned by the PLS-DA model were selected and underwent the Mann–Whitney U test to confirm the differential expression between groups. Those peaks showing p<0.05 were considered as having statistically significant differences.
Statistical Analysis
The lipid composition of the samples was established by the area of the peaks obtained for the main lipids identified. The data were normally distributed and statistical analysis was performed using the Student's t-test, using the GraphPad Prism version 6. Differences between the groups were considered statistically significant when p<0.05.
Results
Table 1 shows the demographic and obstetric characteristics of study participants.
Table 1. Demographic and obstetric characteristics of study subjects.
| Control | Preeclampsia | P | |
| (n = 10) | (n = 10) | ||
| Maternal age (years) | 25.3±6.237 | 22.4±7.152 | 0.372 |
| Gestational age (weeks) | 38.6±1.174 | 35.5±3.504 | 0.026 |
| Weight gain (Kg) | 10.77±5.094 | 20.22±5.037 | 0.001 |
| BMI (Kg/m2) | 22.59±6.912 | 26.73±5.379 | 0.154 |
| Blood Pressure | |||
| Systolic (mmHg) | 116±10.666 | 140.4±4.971 | <0.0001 |
| Diastolic (mmHg) | 70.6±7.306 | 91±5.436 | <0.0001 |
| Birth Weights (g) | 3190.6±581.115 | 2607±881.328 | 0.0975 |
| Proteinuria (mg) | NE | 1624.4±1072.915 | NE |
Results are expressed as mean ± standard deviation. Significant at P<0.05.
BMI – Body Mass Index.
NE - not evaluated.
Mass Spectrometry
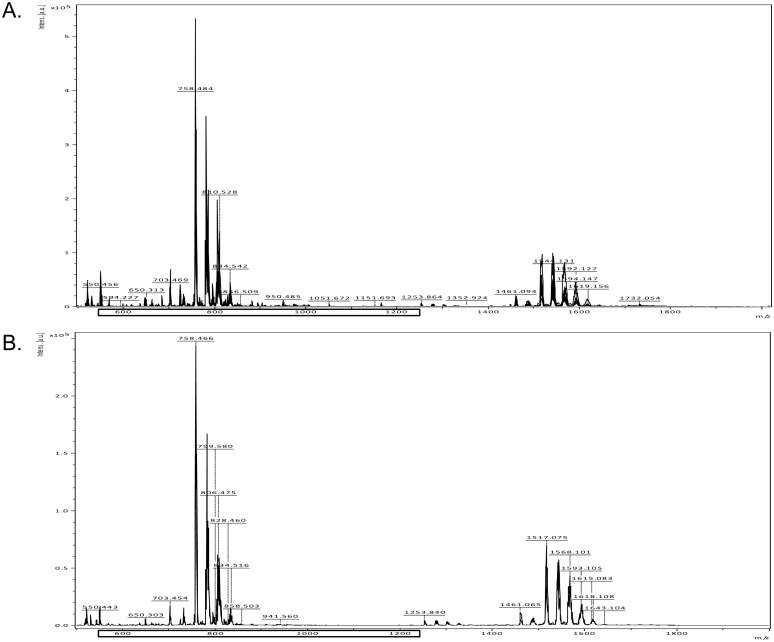
Approximately 200 signals were identified between the lipid tracks acquisition from 600 to 1200 m/z ( Figure 1 ). The identification of the different lipids found was carried out through the Lipid Database Search (http://www.lipidmaps.org), using the results of m/z analyzes.
Figure 1. Representative lipid spectrum showing signals in plasma samples of control and preeclamptic patients.
The signals were identified between the lipid tracks acquisition from 600 to 1200 m/z.
Plasma samples
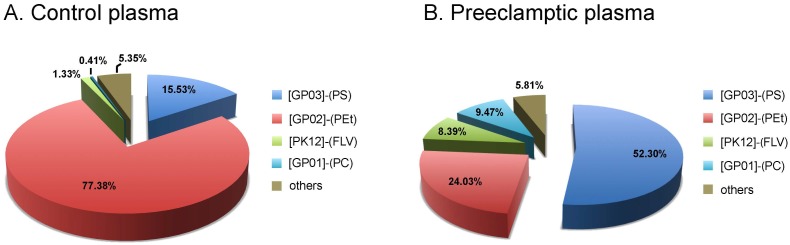
Table 2 and Figure 2 show the lipid composition found in plasma samples from both groups. Plasma analysis of patients with preeclampsia revealed the main class of Glycerophosphoserines-GP03 (PS) as the most prevalent lipid, representing 52.30% of the total lipid composition, followed by Glycerophosphoethanolamines-GP02 (PEt), Glycerophosphocholines-GP01 (PC) and Flavanoids-PK12 (FLV). When compared to the control group, plasma samples of patients with preeclampsia showed an increased proportion of PS (p<0.0001), PC (p<0.0001) and FLV (p<0.0001).
Table 2. Main classes of lipids in plasma samples.
| Plasma | Group | P | |
| [Main class]-(Common name) | Control (n = 10) | Preeclampsia (n = 10) | |
| Glycerophosphocholines [GP01]-(PC) | 0.41% | 9.47% | <0.0001 |
| Glycerophosphoethanolamines [GP02]-(PEt) | 77.38% | 24.03% | <0.0001 |
| Glycerophosphoserines [GP03]-(PS) | 15.53% | 52.30% | <0.0001 |
| Glycerophosphoglycerols [GP04]-(PG) | 3.14% | 0.90% | 0.003 |
| Glycerophosphoinositols [GP06]-(PI) | 0.62% | 0.13% | NS |
| Glycerophosphates [GP10]-(PAc) | 0.09% | 1.40% | <0.0001 |
| CDP-Glycerols [GP13] | 0.02% | 0.00% | NE |
| Triradylglycerols [GL03]-(TG) | 0.22% | 0.16% | NS |
| Phosphosphingolipids [SP03]-(SM) | 0.00% | 0.37% | <0.0001 |
| Neutral glycosphingolipids [SP05] | 0.00% | 1.72% | <0.0001 |
| Sterols [ST01] | 0.31% | 0.03% | <0.0001 |
| Steroid conjugates [ST05] | 0.00% | 0.65% | 0.0002 |
| Polyprenols [PR03] | 0.02% | 0.00% | NE |
| Acylaminosugars [SL01] | 0.00% | 0.21% | NS |
| Other acyl sugars [SL05] | 0.38% | 0.00% | <0.0001 |
| Flavonoids [PK12] | 1.33% | 8.39% | <0.0001 |
| Fatty esters [FA07] | 0.55% | 0.24% | NS |
Results are expressed as percentage. Significant at P<0.05.
NE - not evaluated.
NS – not significant.
Common names used: (PC) Phosphatidylcholines, (PEt) Phosphatidylethonolamines, (PS) Phosphatidylserines, (PG) Phosphatidylglycerols, (PI) Phosphatidylenositos, (PAc) Phosphatidic acid, (TG) Triradylglycerols, (SM) Sphingomyelins.
Figure 2. Lipid composition detected in plasma from control and preeclamptic patients.
Comparison of relative distribution of main class of lipids in plasma of normal (A) and preeclamptic (B) patients, established by the area of the peaks obtained for the main lipids identified. N = 10 in each group. [GP03]-(PS): Glycerophosphoserines or Phosphatidylserines; [GP02]-(PEt): Glycerophosphoethanolamines or Phosphatidylethonolamines; [PK12]-(FLV): Flavonoids; [GP01]-(PC): Glycerophosphocholines or Phosphatidylcholines.
Although in smaller proportion, other increased lipids in patients with preeclampsia were Glycerophosphates-GP10 (PAc) (p<0.0001), Phosphosphingolipids-SP03 (SM) (p<0.0001), Neutral glycosphingolipids-SP05 (p<0.0001) and Steroid conjugates-ST05 (p<0.0002). The main class PEt, was reduced in patients with preeclampsia when compared to the control group (p<0.0001). Other main lipid classes reduced in preeclampsia group were: Glycerophosphoglycerols-GP04 (PG) (p<0.003), Sterols-ST01 (p<0.0001) and Other acyl sugars-SL05 (p<0.0001).
Placental samples
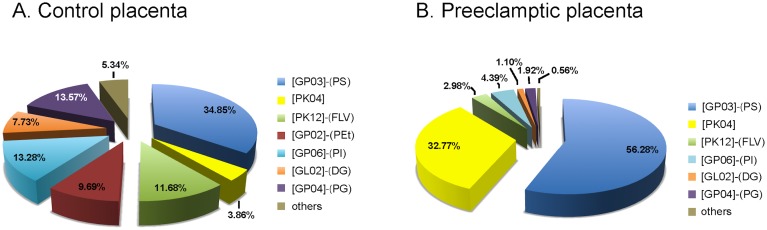
Table 3 and Figure 3 show the lipid composition found in placental samples from both groups. The placental analysis of patients with preeclampsia revealed the main class PS as the most prevalent lipid, representing 56.28% of the total composition. Other main classes found were: Macrolides and lactone polyketides-PK04 with 32.77%, both were increased in preeclamptic placentas when compared to the control group, PS (p<0.0001) and PK04 (p<0.0001). Some lipids found in placentas from patients with preeclampsia were reduced when compared to control group; PEt (p<0.0001), PG (p<0.0001), Glycerophosphoinositols-GP06 (PI) (p<0.0001), Glycerophosphoinositol monophosphates-GP07 (p<0.0001), Diradylglycerols-GL02 (p<0.0001), Triradylglycerols-GL03 (p<0.0001), Acidic glycosphingolipids-SP06 (GM3) (p<0.0001), Steroid conjugates-ST05 (p<0.0001), Other acyl sugars-SL05 (p<0.0001) and Flavonoids-PK12 (p<0.0001).
Table 3. Main classes of lipids in placental samples.
| Placenta | Group | P | |
| [Main class]-(Common name) | Control (n = 10) | Preeclampsia (n = 10) | |
| Glycerophosphocholines [GP01]-(PC) | 0.00% | 0.02% | NE |
| Glycerophosphoethanolamines [GP02]-(PEt) | 9.69% | 0.00% | <0.0001 |
| Glycerophosphoserines [GP03]-(PS) | 34.85% | 56.28% | <0.0001 |
| Glycerophosphoglycerols [GP04]-(PG) | 13.57% | 1.92% | <0.0001 |
| Glycerophosphoinositols [GP06]-(PI) | 13.28% | 4.39% | <0.0001 |
| Glycerophosphoinositol monophosphates [GP07] | 0.57% | 0.00% | <0.0001 |
| Glycerophosphates [GP10]-(PAc) | 1.30% | 0.24% | NS |
| Diradylglycerols [GL02]-(DG) | 7.73% | 1.10% | <0.0001 |
| Triradylglycerols [GL03] | 1.04% | 0.08% | <0.0001 |
| Neutral glycosphingolipids [SP05] | 0.07% | 0.00% | NE |
| Acidic glycosphingolipids [SP06]- (GM3) | 0.26% | 0.06% | <0.0001 |
| Sterols [ST01] | 0.00% | 0.12% | NS |
| Steroid conjugates [ST05] | 1.68% | 0.00% | <0.0001 |
| Other acyl sugars [SL05] | 0.42% | 0.04% | <0.0001 |
| Flavonoids [PK12] | 11.68% | 2.98% | <0.0001 |
| Macrolide lactone polyketide [PK04] | 3.86% | 32.77% | <0.0001 |
Results are expressed as percentage. Significant at P<0.05.
NE - not evaluated; NS – not significant.
Common names used: (PC) Phosphatidylcholines, (PEt) Phosphatidylethonolamines, (PS) Phosphatidylserines, (PG) Phosphatidylglycerols, (DG) Diradylglycerols; (PI) Phosphatidylenositos, (PAc) Phosphatidic acid, (TG) Triradylglycerols, (SM) Sphingomyelins, (GM3) Gangliosides.
Figure 3. Lipid composition detected in placenta from control and preeclamptic women.
Comparison of relative distribution of main class of lipids in placenta of normal (A) and preeclamptic (B) patients, established by the area of the peaks obtained for the main lipids identified. N = 10 in each group [GP03]-(PS): Glycerophosphoserines or Phosphatidylserines; [PK04] = Macrolide and lactone polyketides; [PK12]-(FLV): Flavonoids; [GP02]-(PEt): Glycerophosphoethanolamines or Phosphatidylethonolamines; [GP06]-(PI): Glycerophosphoinositols or Phosphatidylinositols; [GL02]-(DG): Diradylglycerols; [GP04]-(PG): Glycerophosphoglycerols or Phosphatidylglycerols.
Discussion
The pathogenesis of preeclampsia has its roots on deficient trophoblastic invasion and failure in spiral artery remodeling. This incomplete transformation of the spiral arteries leads to inadequate placental perfusion and consequently to placental oxidative stress [29]. The altered placenta then releases great amount of microparticles, debris and antiangiogenic factors into the maternal circulation [30]–[32]. All these factors are supposed to act in synergy to initiate and to maintain an intense inflammatory response and an antiangiogenic state.
Maternal obesity has been considered to have important impact on the genesis of preeclampsia as obese women have higher risk for developing the disease. In addition, women with body-mass-index lower than 20 have lower risk to develop preeclampsia [33]. The complete link between preeclampsia and obesity has not been defined. However, it is known that the inflammatory aspect that characterize the lipotoxicity of adipose tissues leads to maternal endothelial dysfunction, decreases trophoblastic invasion and influences placental metabolism and function. In addition, the chronic inflammatory response of the “metabolic syndrome” can contribute to the systemic inflammation seen in preeclampsia [13].
Actually, lipids play important roles in cellular function as they are the main components of biological membranes [14]. Therefore, lipids can participate in the constitution of membrane receptors, ion channels and in cell signaling mechanisms. In addition, many lipids can act as endogenous ligands, binding to specific receptors and then initiating several immunological responses [34].
In this study we investigated and established the main composition of the lipid profile identified in plasma and placental tissue of normal pregnant and preeclamptic women. Either placental tissues or plasma from patients with preeclampsia expressed different lipid profile when compared to normal pregnant women. These findings suggest that specific lipid species may be more associated with risk of developing preeclampsia than others. Additional functional studies will be necessary to clarify the involvement of these lipids in the pathophysiology of preeclampsia.
PS were the most prevalent species of lipids in preeclamptic women group. These lipids belong to Glicophospholipids category, representing the major lipid constituent of cell membranes and lipoproteins. They play different biological roles, acting as signaling molecules involved in the processes of oxidative stress, apoptosis and coagulation, all exacerbated in preeclampsia [35]–[39].
PC species also belong to Glicophospholipids category and were increased in plasma of patients with preeclampsia. PC species are precursors of several molecules that act as lipid second messengers, including phosphatidic acid. Increased levels of PC have been associated with increased cell proliferation. These lipids have been recently correlated with different tumor behaviors and cancer progressions. They are probably important for treatment considerations [40]–[42].
Oxidative stress generally affects lipid function due to changes in their native behavior. It can generate numerous different lipids that have diverse biological activities [43], [44]. Glicerophospholipids, when oxidized, induce platelet aggregation, monocyte adhesion to endothelial cells, present in atherosclerotic lesions and they play an important role in signaling inflammatory response [45], [46]. Thus, this increase in PS and PC in preeclampsia suggests a role in the inflammatory and oxidative phenomena observed in these patients.
There was a curious reduction of PEt in plasma samples of the preeclampsia group, compared to the control group. Apparently the reduction of PEt in the endoplasmic reticulum is associated with araquidonic acid release [47], [48]. This is the precursor of prostaglandins, thromboxanes and prostacyclins by the cyclooxygenase pathway and leukotrienes by the lipoxygenase pathway. Prostaglandins cause vasodilation, inhibition of platelet aggregation and pain. Thromboxane A2 promotes vasoconstriction and platelet aggregation. It is possible that these lipids act in opposing mechanisms in different patients.
Although also associated with apoptotic processes, cell proliferation and differentiation, the PI lipid class was found in small amounts in the plasma lipid composition in both groups. The SM lipid species, found only in plasma samples from patients with preeclampsia, act in vascular reactivity and mediate cell growth due to intrinsic properties of these vasoactive species [49]. Experiments on hypertensive mice have showed significant increase in SM [50]. Apparently it is involved in processes of endothelial dysfunction, increased production of angiotensin II, elevated levels of thromboxane A2 and hence hypertensive disorders, which could explain its exclusively occurrence in the population of patients with preeclampsia [50]–[52]. The predominant lipid in preeclampsia placental samples was PS, and this aspect can be correlated with the oxidation process.
The Flavonoids are known for their antioxidant properties [53], [54]. These lipids are not present in mammalian lipid composition, and their presence in our study is probably derived from the diet. These lipids interact in the signaling pathways of apoptotic processes, operating in both promotion and inhibition [55], [56]. Evidences support the Flavonoids as protective factors against cardiac ischemic [57], [58]. In our study, we found a reduction in the amount of Flavonoids in samples of placentas from patients with preeclampsia when compared to controls. This did not happen when we performed the analysis of plasma samples that identified an increase of these lipids in patients with preeclampsia.
The Macrolides polyketides-lactone-PK04 have shown greater incidence in placental samples of preeclampsia group. They are not a natural part of mammalian lipid composition and they can be found in bacteria, fungi and plants. Rapamycin, also called Sirolimus, is an important and known polyketide with many biological and pharmacological activities, including antifungal, immunosuppressive, antitumor, neuroprotective and antiaging activities [59]–[63]. Recently, rapamycin has attracted interest for the clinical treatment of organ transplant rejection and autoimmune diseases [64].
Rapamicyn use has been associated with the development or exacerbation of proteinuria [65]–[67]. The pathogenesis of proteinuria is likely multifactorial and may involve tubular and glomerular contributions. Recent data derived from biopsy sub studies of clinical trials, which compared cyclosporine with rapamycin, demonstrated that rapamycin use is associated with tubular damage and tubular proteinuria [68]. In the glomerular compartment, other ones have demonstrated reduced nephrin expression [69] and reduced VEGF, particularly in patients with significant proteinuria [70], [71].
The immunosuppressive effects of rapamycin result from its ability to inhibit proliferation by interfering with the function of the mammalian target of rapamycin (mTOR) [72]. mTOR is a serine/threonine protein kinase which controls the cellular processes of growth, proliferation, transcription, protein biosynthesis and ribosomal biogenesis [59], [73]. mTOR exists in two distinct protein complexes referred to as mTOR complex 1 and mTOR complex 2. The inactivation of mTOR complex 1 kinase activity by rapamycin results in the inhibition of the activities of ribosomal S6 kinase and the eukaryotic translation initiation factor 4E-binding proteins, which have roles in ribosome biogenesis and protein translation, respectively. In contrast, apoptosis and autophagy are also stimulated by rapamycin [72], [74].
Studies in immortalized cell lines originating from human trophoblast suggest a key role for mTOR in the regulation of trophoblast proliferation and it is suggested that the mTOR pathway is a regulator of invasive trophoblast differentiation [75], [76]. In the mature placenta mTOR is expressed at the mRNA level, however, the cellular localization of mTOR and the functional role of this signaling pathway in the placenta after implantation and early placental development remains unknown [75], [77], [78].
Our study did not include other groups, such as gestational hypertension or intrauterine growth restriction, which would be important to evaluate the specificity of this association. In addition it would be interesting to compare the lipid profile between obese pregnant patients with normal outcomes and patients with preeclampsia with and without obesity to better understand if the lipid changes are a reflection of obesity, increased BMI or true PE.
Conclusion
We identified a different pattern of lipids and distinct concentrations of some lipid species in plasma and placenta samples of preeclamptic patients. Further studies are needed to clarify if these changes are specific to preeclampsia and whether and how they could be related to its pathogenesis.
Acknowledgments
We thank the Department of Obstetrics of Federal University of Sao Paulo, the Laboratory of Clinical and Experimental Investigation from School Maternity Vila Nova Cachoeirinha, the Department of Immunology of University of São Paulo and the Department of Gynecology of Federal University of Sao Paulo.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.
Funding Statement
Funding provided by National Council for Scientific and Technological Development; CNPq Number: 476486/2011-4, http://www.cnpq.br/en and Foundation for Research Support of the State of São Paulo; FAPESP Number: 12/02270-2, http://www.fapesp.br/en/. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Rana S, Cerdeira AS, Wenger J, Salahuddin S, Lim KH, et al. (2012) Plasma concentrations of soluble endoglin versus standard evaluation in patients with suspected preeclampsia. PLoS One 7: e48259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Borzychowski AM, Sargent IL, Redman CW (2006) Inflammation and pre-eclampsia. Semin Fetal Neonatal Med 11: 309–316. [DOI] [PubMed] [Google Scholar]
- 3. de Oliveira LG, Karumanchi A, Sass N (2010) [Preeclampsia: oxidative stress, inflammation and endothelial dysfunction]. Rev Bras Ginecol Obstet 32: 609–616. [DOI] [PubMed] [Google Scholar]
- 4. Ghulmiyyah L, Sibai B (2012) Maternal mortality from preeclampsia/eclampsia. Semin Perinatol 36: 56–59. [DOI] [PubMed] [Google Scholar]
- 5. Bellamy L, Casas JP, Hingorani AD, Williams DJ (2007) Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ 335: 974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Walsh SW (2007) Obesity: a risk factor for preeclampsia. Trends Endocrinol Metab 18: 365–370. [DOI] [PubMed] [Google Scholar]
- 7. Kral JG, Kava RA, Catalano PM, Moore BJ (2012) Severe Obesity: The Neglected Epidemic. Obes Facts 5: 254–269. [DOI] [PubMed] [Google Scholar]
- 8.(2000) Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser 894: i–xii, 1–253. [PubMed]
- 9. Hogan JL, Maguire P, Farah N, Kennelly MM, Stuart B, et al. (2011) Body mass index and blood pressure measurement during pregnancy. Hypertens Pregnancy 30: 396–400. [DOI] [PubMed] [Google Scholar]
- 10. Bodnar LM, Catov JM, Klebanoff MA, Ness RB, Roberts JM (2007) Prepregnancy body mass index and the occurrence of severe hypertensive disorders of pregnancy. Epidemiology 18: 234–239. [DOI] [PubMed] [Google Scholar]
- 11. Sohlberg S, Stephansson O, Cnattingius S, Wikstrom AK (2012) Maternal body mass index, height, and risks of preeclampsia. Am J Hypertens 25: 120–125. [DOI] [PubMed] [Google Scholar]
- 12. Pou KM, Massaro JM, Hoffmann U, Vasan RS, Maurovich-Horvat P, et al. (2007) Visceral and subcutaneous adipose tissue volumes are cross-sectionally related to markers of inflammation and oxidative stress: the Framingham Heart Study. Circulation 116: 1234–1241. [DOI] [PubMed] [Google Scholar]
- 13. Jarvie E, Hauguel-de-Mouzon S, Nelson SM, Sattar N, Catalano PM, et al. (2010) Lipotoxicity in obese pregnancy and its potential role in adverse pregnancy outcome and obesity in the offspring. Clin Sci (Lond) 119: 123–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gross RW, Han X (2011) Lipidomics at the interface of structure and function in systems biology. Chem Biol 18: 284–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shevchenko A, Simons K (2010) Lipidomics: coming to grips with lipid diversity. Nat Rev Mol Cell Biol 11: 593–598. [DOI] [PubMed] [Google Scholar]
- 16. Yetukuri L, Ekroos K, Vidal-Puig A, Oresic M (2008) Informatics and computational strategies for the study of lipids. Mol Biosyst 4: 121–127. [DOI] [PubMed] [Google Scholar]
- 17. Wymann MP, Schneiter R (2008) Lipid signalling in disease. Nat Rev Mol Cell Biol 9: 162–176. [DOI] [PubMed] [Google Scholar]
- 18. Bou Khalil M, Hou W, Zhou H, Elisma F, Swayne LA, et al. (2010) Lipidomics era: accomplishments and challenges. Mass Spectrom Rev 29: 877–929. [DOI] [PubMed] [Google Scholar]
- 19. Fahy E, Subramaniam S, Brown HA, Glass CK, Merrill AH Jr, et al. (2005) A comprehensive classification system for lipids. J Lipid Res 46: 839–861. [DOI] [PubMed] [Google Scholar]
- 20. Fahy E, Subramaniam S, Murphy RC, Nishijima M, Raetz CR, et al. (2009) Update of the LIPID MAPS comprehensive classification system for lipids. J Lipid Res 50 Suppl: S9–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Han X, Gross RW (2005) Shotgun lipidomics: electrospray ionization mass spectrometric analysis and quantitation of cellular lipidomes directly from crude extracts of biological samples. Mass Spectrom Rev 24: 367–412. [DOI] [PubMed] [Google Scholar]
- 22. Schmelzer K, Fahy E, Subramaniam S, Dennis EA (2007) The lipid maps initiative in lipidomics. Methods Enzymol 432: 171–183. [DOI] [PubMed] [Google Scholar]
- 23. Harkewicz R, Dennis EA (2011) Applications of mass spectrometry to lipids and membranes. Annu Rev Biochem 80: 301–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Want EJ, Cravatt BF, Siuzdak G (2005) The expanding role of mass spectrometry in metabolite profiling and characterization. Chembiochem 6: 1941–1951. [DOI] [PubMed] [Google Scholar]
- 25. Postle AD (2012) Lipidomics. Curr Opin Clin Nutr Metab Care 15: 127–133. [DOI] [PubMed] [Google Scholar]
- 26. De Oliveira L, Camara NO, Bonetti T, Lo Turco EG, Bertolla RP, et al. (2012) Lipid fingerprinting in women with early-onset preeclampsia: a first look. Clin Biochem 45: 852–855. [DOI] [PubMed] [Google Scholar]
- 27. Baig S, Lim JY, Fernandis AZ, Wenk MR, Kale A, et al. (2013) Lipidomic analysis of human placental syncytiotrophoblast microvesicles in adverse pregnancy outcomes. Placenta 34: 436–442. [DOI] [PubMed] [Google Scholar]
- 28. Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37: 911–917. [DOI] [PubMed] [Google Scholar]
- 29. Burton GJ, Woods AW, Jauniaux E, Kingdom JC (2009) Rheological and physiological consequences of conversion of the maternal spiral arteries for uteroplacental blood flow during human pregnancy. Placenta 30: 473–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Redman CW, Sargent IL (2008) Circulating microparticles in normal pregnancy and pre-eclampsia. Placenta 29 Suppl A: S73–77. [DOI] [PubMed]
- 31. Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, et al. (2004) Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med 350: 672–683. [DOI] [PubMed] [Google Scholar]
- 32. Rajakumar A, Cerdeira AS, Rana S, Zsengeller Z, Edmunds L, et al. (2012) Transcriptionally active syncytial aggregates in the maternal circulation may contribute to circulating soluble fms-like tyrosine kinase 1 in preeclampsia. Hypertension 59: 256–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Duckitt K, Harrington D (2005) Risk factors for pre-eclampsia at antenatal booking: systematic review of controlled studies. BMJ 330: 565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Leavy O (2012) Inflammation: Trauma kicks up a storm. Nat Rev Immunol 12: 3. [DOI] [PubMed] [Google Scholar]
- 35. Hopkins PN, Heiss G, Ellison RC, Province MA, Pankow JS, et al. (2003) Coronary artery disease risk in familial combined hyperlipidemia and familial hypertriglyceridemia: a case-control comparison from the National Heart, Lung, and Blood Institute Family Heart Study. Circulation 108: 519–523. [DOI] [PubMed] [Google Scholar]
- 36. Leventis PA, Grinstein S (2010) The distribution and function of phosphatidylserine in cellular membranes. Annu Rev Biophys 39: 407–427. [DOI] [PubMed] [Google Scholar]
- 37. Domingues MR, Reis A, Domingues P (2008) Mass spectrometry analysis of oxidized phospholipids. Chem Phys Lipids 156: 1–12. [DOI] [PubMed] [Google Scholar]
- 38. Kagan VE, Borisenko GG, Tyurina YY, Tyurin VA, Jiang J, et al. (2004) Oxidative lipidomics of apoptosis: redox catalytic interactions of cytochrome c with cardiolipin and phosphatidylserine. Free Radic Biol Med 37: 1963–1985. [DOI] [PubMed] [Google Scholar]
- 39. Li M, Huang SJ (2009) Innate immunity, coagulation and placenta-related adverse pregnancy outcomes. Thromb Res 124: 656–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nakagami K, Uchida T, Ohwada S, Koibuchi Y, Suda Y, et al. (1999) Increased choline kinase activity and elevated phosphocholine levels in human colon cancer. Jpn J Cancer Res 90: 419–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hernando E, Sarmentero-Estrada J, Koppie T, Belda-Iniesta C, Ramirez de Molina V, et al. (2009) A critical role for choline kinase-alpha in the aggressiveness of bladder carcinomas. Oncogene 28: 2425–2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jagannathan NR, Kumar M, Seenu V, Coshic O, Dwivedi SN, et al. (2001) Evaluation of total choline from in-vivo volume localized proton MR spectroscopy and its response to neoadjuvant chemotherapy in locally advanced breast cancer. Br J Cancer 84: 1016–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fruhwirth GO, Loidl A, Hermetter A (2007) Oxidized phospholipids: from molecular properties to disease. Biochim Biophys Acta 1772: 718–736. [DOI] [PubMed] [Google Scholar]
- 44. Spickett CM, Dever G (2005) Studies of phospholipid oxidation by electrospray mass spectrometry: from analysis in cells to biological effects. Biofactors 24: 17–31. [DOI] [PubMed] [Google Scholar]
- 45. Leitinger N (2003) Oxidized phospholipids as modulators of inflammation in atherosclerosis. Curr Opin Lipidol 14: 421–430. [DOI] [PubMed] [Google Scholar]
- 46. Zhang W, Salomon RG (2005) Oxidized phospholipids, isolevuglandins, and atherosclerosis. Mol Nutr Food Res 49: 1050–1062. [DOI] [PubMed] [Google Scholar]
- 47. Meikle PJ, Christopher MJ (2011) Lipidomics is providing new insight into the metabolic syndrome and its sequelae. Curr Opin Lipidol 22: 210–215. [DOI] [PubMed] [Google Scholar]
- 48. Andreyev AY, Fahy E, Guan Z, Kelly S, Li X, et al. (2010) Subcellular organelle lipidomics in TLR-4-activated macrophages. J Lipid Res 51: 2785–2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hannun YA, Obeid LM (2008) Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol 9: 139–150. [DOI] [PubMed] [Google Scholar]
- 50. Spijkers LJ, van den Akker RF, Janssen BJ, Debets JJ, De Mey JG, et al. (2011) Hypertension is associated with marked alterations in sphingolipid biology: a potential role for ceramide. PLoS One 6: e21817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Berry C, Touyz R, Dominiczak AF, Webb RC, Johns DG (2001) Angiotensin receptors: signaling, vascular pathophysiology, and interactions with ceramide. Am J Physiol Heart Circ Physiol 281: H2337–2365. [DOI] [PubMed] [Google Scholar]
- 52. Fenger M, Linneberg A, Jorgensen T, Madsbad S, Sobye K, et al. (2011) Genetics of the ceramide/sphingosine-1-phosphate rheostat in blood pressure regulation and hypertension. BMC Genet 12: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Terao J, Kawai Y, Murota K (2008) Vegetable flavonoids and cardiovascular disease. Asia Pac J Clin Nutr 17 Suppl 1 291–293. [PubMed] [Google Scholar]
- 54. Boots AW, Haenen GR, Bast A (2008) Health effects of quercetin: from antioxidant to nutraceutical. Eur J Pharmacol 585: 325–337. [DOI] [PubMed] [Google Scholar]
- 55. Ramos S (2007) Effects of dietary flavonoids on apoptotic pathways related to cancer chemoprevention. J Nutr Biochem 18: 427–442. [DOI] [PubMed] [Google Scholar]
- 56. Mandel S, Weinreb O, Amit T, Youdim MB (2004) Cell signaling pathways in the neuroprotective actions of the green tea polyphenol (−)-epigallocatechin-3-gallate: implications for neurodegenerative diseases. J Neurochem 88: 1555–1569. [DOI] [PubMed] [Google Scholar]
- 57. Akhlaghi M, Bandy B (2009) Mechanisms of flavonoid protection against myocardial ischemia-reperfusion injury. J Mol Cell Cardiol 46: 309–317. [DOI] [PubMed] [Google Scholar]
- 58. Akhlaghi M, Bandy B (2012) Preconditioning and acute effects of flavonoids in protecting cardiomyocytes from oxidative cell death. Oxid Med Cell Longev 2012: 782321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Park SR, Yoo YJ, Ban YH, Yoon YJ (2010) Biosynthesis of rapamycin and its regulation: past achievements and recent progress. J Antibiot (Tokyo) 63: 434–441. [DOI] [PubMed] [Google Scholar]
- 60. Sehgal SN, Baker H, Vezina C (1975) Rapamycin (AY-22,989), a new antifungal antibiotic. II. Fermentation, isolation and characterization. J Antibiot (Tokyo) 28: 727–732. [DOI] [PubMed] [Google Scholar]
- 61. Calne RY, Collier DS, Lim S, Pollard SG, Samaan A, et al. (1989) Rapamycin for immunosuppression in organ allografting. Lancet 2: 227. [DOI] [PubMed] [Google Scholar]
- 62. Steiner JP, Connolly MA, Valentine HL, Hamilton GS, Dawson TM, et al. (1997) Neurotrophic actions of nonimmunosuppressive analogues of immunosuppressive drugs FK506, rapamycin and cyclosporin A. Nat Med. 3: 421–428. [DOI] [PubMed] [Google Scholar]
- 63. Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, et al. (2009) Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 460: 392–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kahan BD (2000) Efficacy of sirolimus compared with azathioprine for reduction of acute renal allograft rejection: a randomised multicentre study. The Rapamune US Study Group. Lancet 356: 194–202. [DOI] [PubMed] [Google Scholar]
- 65. Ko HT, Yin JL, Wyburn K, Wu H, Eris JM, et al. (2013) Sirolimus reduces vasculopathy but exacerbates proteinuria in association with inhibition of VEGF and VEGFR in a rat kidney model of chronic allograft dysfunction. Nephrol Dial Transplant 28: 327–336. [DOI] [PubMed] [Google Scholar]
- 66. Letavernier E, Pe'raldi MN, Pariente A, Morelon E, Legendre C (2005) Proteinuria following a switch from calcineurin inhibitors to sirolimus. Transplantation 80: 1198–1203. [DOI] [PubMed] [Google Scholar]
- 67. Chapman JR, Rangan GK (2010) Why do patients develop proteinuria with sirolimus? Do we have the answer? Am J Kidney Dis 55: 213–216. [DOI] [PubMed] [Google Scholar]
- 68. Franz S, Regeniter A, Hopfer H, Mihatsch M, Dickenmann M (2010) Tubular toxicity in sirolimus- and cyclosporine-based transplant immunosuppression strategies: an ancillary study from a randomized controlled trial. Am J Kidney Dis 55: 335–343. [DOI] [PubMed] [Google Scholar]
- 69. Biancone L, Bussolati B, Mazzucco G, Barreca A, Gallo E, et al. (2010) Loss of nephrin expression in glomeruli of kidney-transplanted patients under m-TOR inhibitor therapy. Am J Transplant 10: 2270–2278. [DOI] [PubMed] [Google Scholar]
- 70. Vogelbacher R, Wittmann S, Braun A, Daniel C, Hugo C (2007) The mTOR inhibitor everolimus induces proteinuria and renal deterioration in the remnant kidney model in the rat. Transplantation 84: 1492–1499. [DOI] [PubMed] [Google Scholar]
- 71. Vuiblet V, Birembaut P, Francois A, Cordonnier C, Noel LH, et al. (2012) Sirolimus-based regimen is associated with decreased expression of glomerular vascular endothelial growth factor. Nephrol Dial Transplant 27: 411–416. [DOI] [PubMed] [Google Scholar]
- 72. Sehgal SN (2003) Sirolimus: its discovery, biological properties, and mechanism of action. Transplant Proc 35: 7S–14S. [DOI] [PubMed] [Google Scholar]
- 73. Dowling RJ, Topisirovic I, Fonseca BD, Sonenberg N (2010) Dissecting the role of mTOR: lessons from mTOR inhibitors. Biochim Biophys Acta 1804: 433–439. [DOI] [PubMed] [Google Scholar]
- 74. Abraham RT, Eng CH (2008) Mammalian target of rapamycin as a therapeutic target in oncology. Expert Opin Ther Targets 12: 209–222. [DOI] [PubMed] [Google Scholar]
- 75.Wen HY, Abbasi S, Kellems RE, Xia Y (2005) mTOR: a placental growth signaling sensor. Placenta 26 Suppl A: S63–69. [DOI] [PubMed]
- 76.Pollheimer J, Knofler M (2005) Signalling pathways regulating the invasive differentiation of human trophoblasts: a review. Placenta 26 Suppl A: S21–30. [DOI] [PubMed]
- 77. Kim DH, Sarbassov DD, Ali SM, King JE, Latek RR, et al. (2002) mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell 110: 163–175. [DOI] [PubMed] [Google Scholar]
- 78. Roos S, Jansson N, Palmberg I, Saljo K, Powell TL, et al. (2007) Mammalian target of rapamycin in the human placenta regulates leucine transport and is down-regulated in restricted fetal growth. J Physiol 582: 449–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.