Abstract
Tubulin partition between soluble and polymeric forms is tightly regulated in cells. Stathmin and tubulin tyrosine ligase (TTL)a each form stable complexes with tubulin and inhibit tubulin polymerization. Here we explore the mutual relationship between these proteins in vitro and demonstrate that full-length stathmin and TTL compete for binding to tubulin and fail to make a stable tubulin:stathmin:TTL triple complex in solution. Moreover, stathmin depresses TTL tubulin tyrosination activity in vitro. These results suggest that TTL and stathmin have either a partially overlapping footprint on the tubulin dimer or that stathmin induces a tubulin conformation incompatible with stable TTL binding.
The morphology and dynamics of the microtubule network are tightly regulated through interactions with a vast array of cellular factors as well as the post-translational modification of tubulin 1,2. Stathmin/Op18a is a highly conserved microtubule dynamics regulator that is ubiquitously expressed at high levels 3,4. TTL adds a C-terminal tyrosine to α-tubulin. TTL is essential and also ubiquitously expressed. Both TTL and stathmin were isolated from cell extracts in complex with tubulin 4,5,6 and were each shown to interact preferentially with unpolymerized tubulin subunits 3,4,7, however with different stoichiometries. TTL forms a 1:1 complex with tubulin 5,8 while stathmin forms a complex by sequestering two tubulin dimers 3. TTL and stathmin each inhibit tubulin growth rates in vitro and in vivo 3,4,8,9. Solution studies revealed that TTL binds at the edge of the tubulin dimer, making contacts mostly with α-tubulin and possibly the α-β protomer junction where it can interfere with both longitudinal and lateral tubulin polymerization interfaces 8. Consistent with this, TTL inhibits tubulin polymerization in vitro 8. Stathmin strings two curved, longitudinally arranged tubulin dimers (α1-β1-α2-β2) using an extended α-helix that makes interactions with structural elements in the nucleotide binding (the S3-H3 loop, the C-terminus of helix H4 and the N-terminus of the H4-S5 turn) and C-terminal domains (the turn immediately preceding helix H12, the C-terminal helix leading into the disordered tubulin tails) of each of the α and β-tubulin protomers. In addition, stathmin caps the α-tubulin longitudinal interface at one end of the complex via an N-terminal β-hairpin that extends a preexisting β-sheet in α-tubulin containing conserved residues important for longitudinal interactions 10. Stathmin is disordered in solution in the absence of tubulin 3.
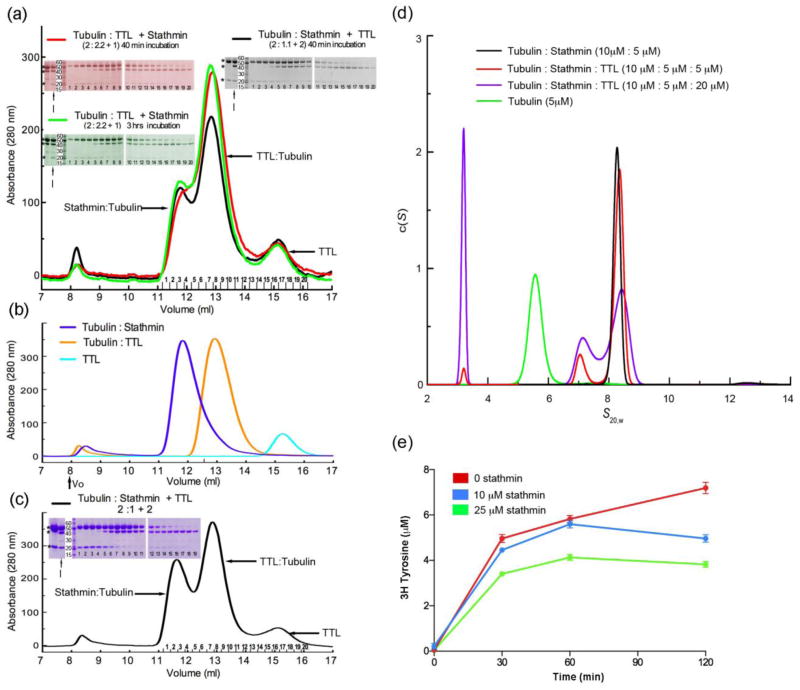
Since stathmin and TTL were each isolated complexed with tubulin and both inhibit tubulin polymerization, we investigated whether binding of full-length stathmin and TTL to tubulin are mutually exclusive and whether TTL can form a triple complex with tubulin:stathmin and tyrosinate it efficiently. Figure 1 shows that under conditions where stable tubulin:stathmin and tubulin:TTL complexes are detected by size exclusion chromatography, when all three components are incubated together, no tubulin:stathmin:TTL triple complex is detected while binary tubulin:stathmin: and tubulin:TTL complexes are (Fig. 1a and b). This result is independent of the order of addition of the three components. Pre-incubation of stathmin and tubulin for various times followed by addition of TTL, or vice versa did not alter the result, implying that the formation of a stable tubulin:stathmin:TTL triple complex in solution is unfavorable over the binary complexes, regardless of the order of addition of the components. The inability to detect a triple complex is unlikely due to dilution during chromatography because the same result holds when three times as much sample is loaded on the same column (Fig. 1c). The concentration of soluble tubulin in the cytoplasm is ~5 – 10 μM 11. Stathmin levels comparable to those of tubulin have been detected (Ref. 4, 11 and references therein) while TTL levels have been estimated to be lower in the cell 5,6 (~ 1:150 TTL:tubulin molar ratio 5). Even at concentrations more than five times higher than these, we fail to observe any triple complex formation in our assay. We complemented our gel filtration results with analytical ultracentrifugation (AUC) studies of tubulin-stathmin-TTL mixtures (Fig. 1d). The sedimentation coefficient distribution (c(s)) of tubulin:stathmin complexes to which increasing amounts of TTL were added show three peaks, a small s value peak representing free TTL 8, a peak with an s value of 7.1 representing tubulin:TTL and a large s value peak of 8.3 representing the tubulin:stathmin complex. No peaks with higher s values were detected, consistent with the absence of a tubulin:stathmin:TTL triple complex under these conditions. Addition of 5 and 20 μM TTL is accompanied by a 7% and 48% decrease in the area of the stathmin:tubulin peak, respectively (Fig. 1d) indicating the competition between TTL and stathmin for tubulin binding. Consistent with our size exclusion and AUC data that show a competition between stathmin and TTL for binding to tubulin, stathmin mildly inhibits tubulin tyrosination by TTL in a dose-dependent manner (Fig. 1e). Together, these results suggest that stathmin and TTL either compete for a partially overlapping binding site on tubulin or that stathmin induces a tubulin conformation that is not conducive to stable binding by TTL. While this manuscript was in review, a crystal structure of a TTL:tubulin:RB3 complex (the latter being a stathmin-like homolog) was reported 12 that shows one copy of a TTL molecule binding to two copies of the tubulin dimer in the tubulin:RB3 complex despite the fact that the tubulin:RB3 complex appears to contain two equivalent TTL binding sites. However, the authors did not present data supporting the existence and stoichiometry of such a complex in solution (Supplementary Materials). The interplay between TTL and stathmin activities in vivo remains to be explored.
Fig. 1.
Solution studies of TTL and stathmin binding to tubulin. (a) Complex formation between TTL, stathmin and tubulin in various orders of addition of TTL and stathmin monitored by size exclusion chromatography. Tubulin and TTL were pre-incubated for 40 minutes, followed by incubation with stathmin for another 40 minutes resulting in final concentrations of 47, 52 and 23 μM for tubulin, TTL and stathmin respectively (red) or 3 hours (green); tubulin and stathmin were pre-incubated for 2 hours followed by incubation with TTL for 40 minutes, resulting in final concentrations of 44, 24 and 44 μM for tubulin, stathmin and TTL respectively (black). The gels correspond to the indicated peak fractions. Molar ratios of the components are indicated. * indicate SDS-PAGE mobility of tubulin, TTL and stathmin (top to bottom); I is input. (b) Size exclusion chromatograms of binary complexes of tubulin:stathmin (blue) injected at 2:1.1 molar ratio, tubulin:TTL (orange) at 1:1.1 and TTL alone (cyan). (c) Size exclusion chromatography of tubulin, stathmin, and TTL mixed at indicated molar ratios; three times as much sample as in panel (a) was injected onto the same column. (d) Analytical ultracentrifugation analyses of tubulin-stathmin-TTL mixtures showing the absence of a tubulin:stathmin:TTL triple complex and the competition between TTL and stathmin for tubulin binding. (e) Tubulin tyrosination by TTL is depressed by stathmin. 10 μM tubulin was pre-incubated with 0, 10 and 25 μM stathmin for 40 minutes before starting the tyrosination reaction (Supplementary Material).
Supplementary Material
TTL and stathmin are essential microtubule dynamics regulators
TTL and stathmin bind to tubulin and inhibit polymerization
TTL, stathmin and tubulin do not form a stable triple complex in solution
TTL and stathmin compete for tubulin binding in vitro
Acknowledgments
We thank V. Kormendi (NIH) for purifying stathmin, M. Sirajuddin and R. Vale (UCSF) for the stathmin expression plasmid and A. Ferre-D’Amare and S. Gottesman for useful discussions. This work was supported by the intramural program of the National Institutes of Health (NIH) and a grant to A.R-M from the Searle Scholars Program.
Footnotes
Abbreviations: tubulin tyrosine ligase (TTL), oncoprotein 18 (Op18)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers. we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Desai A, Mitchison TJ. Microtubule polymerization dynamics. Annu Rev Cell Dev Biol. 1997;13:83–117. doi: 10.1146/annurev.cellbio.13.1.83. [DOI] [PubMed] [Google Scholar]
- 2.Garnham CP, Roll-Mecak A. The chemical complexity of cellular microtubules: Tubulin post-translational modification enzymes and their roles in tuning microtubule functions. Cytoskeleton. 2012;69:442–463. doi: 10.1002/cm.21027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steinmetz MO. Structure and thermodynamics of the tubulin-stathmin interaction. J Struct Biol. 2007;158:137–147. doi: 10.1016/j.jsb.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 4.Belmont LD, Mitchison TJ. Identification of a protein that interacts with tubulin dimers and increases the catastrophe rate of microtubules. Cell. 1996;84:623–631. doi: 10.1016/s0092-8674(00)81037-5. [DOI] [PubMed] [Google Scholar]
- 5.Raybin D, Flavin M. Enzyme which specifically adds tyrosine to the alpha chain of tubulin. Biochemistry. 1977;16:2189–2194. doi: 10.1021/bi00629a023. [DOI] [PubMed] [Google Scholar]
- 6.Murofushi H. Purification and characterization of tubulin-tyrosine ligase from porcine brain. J Biochem. 1980;87:979–984. doi: 10.1093/oxfordjournals.jbchem.a132828. [DOI] [PubMed] [Google Scholar]
- 7.Raybin D, Flavin M. An enzyme tyrosylating alpha-tubulin and its role in microtubule assembly. Biochem Biophys Res Commun. 1975;65:1088–1095. doi: 10.1016/s0006-291x(75)80497-9. [DOI] [PubMed] [Google Scholar]
- 8.Szyk A, Deaconescu AM, Piszczek G, Roll-Mecak A. Tubulin tyrosine structure reveals adaptation of an ancient fold to bind and modify tubulin. Nature Struct & Molec Biol. 2011;8:1250–8. doi: 10.1038/nsmb.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ringhoff DN, Cassimeris L. Stathmin regulates centrosomal nucleation of microtubules and tubulin dimer/polymer partitioning. Mol Biol Cell. 2009;20:3451–3458. doi: 10.1091/mbc.E09-02-0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ravelli RB, Gigant B, Curmi PA, Jourdain I, Lachkar S, Sobel A, Knossow M. Insight into tubulin regulation from a complex with colchicine and stathmin-like domain. Nature. 2004;428:198–202. doi: 10.1038/nature02393. [DOI] [PubMed] [Google Scholar]
- 11.Hiller G, Weber K. Radioimmunoassay for tubulin: a quantitative comparison of the tubulin content of different established tissue culture cells and tissues. Cell. 1978;14:795–804. doi: 10.1016/0092-8674(78)90335-5. [DOI] [PubMed] [Google Scholar]
- 12.Prota AE, Magiera MM, Kuijpers M, Bargsten K, Frey D, Wieser M, Jaussi R, Hoogenraad CC, Kammerer RA, Janke C, Steinmetz MO. Structural basis for tubulin tyrosination by tubulin tyrosine ligase. J Cell Biol. 2013;200(3):259–70. doi: 10.1083/jcb.201211017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.