Abstract
Although all of the DNA in an eukaryotic cell replicates during the S-phase of cell cycle, there is a significant difference in the actual time in S-phase when a given chromosomal segment replicates. Methods are described here for generation of high-resolution temporal maps of DNA replication in synchronized human cells. This method does not require amplification of DNA before microarray hybridization and so avoids errors introduced during PCR. A major advantage of using this procedure is that it facilitates finer dissection of replication time in S-phase. Also, it helps delineate chromosomal regions that undergo biallelic or asynchronous replication, which otherwise are difficult to detect at a genome-wide scale by existing methods. The continuous TR50 (time of completion of 50% replication) maps of replication across chromosomal segments identify regions that undergo acute transitions in replication timing. These transition zones can play a significant role in identifying insulators that separate chromosomal domains with different chromatin modifications.
Keywords: Replication timing, BrdU labeling, tiling arrays, TR50
1. Introduction
DNA replication is a key event in the cell cycle, occurring within a confined period termed S-phase (1). Analysis of replication time for individual genes or chromosomal regions has historically relied on either fractionation of S-phase followed by semi-quantitative PCR for synthesis-induced increase in copy number (2, 3) or the counting of FISH signals in S-phase nuclei (4). Although, these methods show local variation in replication timing, the laborious nature of the methods have restricted high-resolution analysis to small regions of the chromosomes (3) or lower-resolution analysis to single chromosomal segments (2). The completion of the human genome sequence and the advent of genome-tiling microarrays have provided an opportunity to study time of replication at a much finer resolution. Here, we detail methods for studying temporal behavior of replication at 25 bp resolution by using nucleotide analog incorporation, density centrifugation, and hybridization. This approach relies on the synchronization of cells to obtain the replication pattern from multiple discrete intervals of S-phase. This strategy has advantages over the existing S:G1 ratio based method of mapping replication timing. In a typical S:G1 method, DNA content is examined at the end and beginning of S-phase and the ratio between these measurements is used to estimate the time of replication. Thus an early replicating segment has a ratio that is closer to 2, while a late replicating segment has a ratio that is closer to 1 (5). In such an experiment, segments showing biallelic replication appear to replicate near the middle of S-phase and provide misleading results. In the method described here, computing signal enrichment for multiple time intervals in S-phase allows a finer dissection of the temporal profile of replication and also identifies biallelic or asynchronously replicating regions of the genome more accurately (6).
Most genomic methods depend on amplification of the experimental material prior to hybridization; this can change relative amounts of DNA in a complex mixture, and can hence provide unreliable signal values. In the strategy described here, there is no amplification step prior to hybridization, thus freeing the method from any artifacts that could arise due to amplification bias.
We also provide a section in this chapter on the algorithms used to generate continuous TR50 curves along the length of the chromosome. TR50 plots can be used to segregate discrete regions of early, mid, late, and Pan-S replication. Additionally, these curves provide information on genomic regions that undergo acute transition in replication time. These replication domains can be valuable indicators of chromatin structure, as we have observed them to have different levels of gene expression as well as activating and repressing histone marks (6). Finally, the local minima of the TR50 curve show areas that replicate earlier than the flanking regions and so are likely to contain origins of replication, as has been shown previously in Saccharomyces cerevisiae, (7). Thus, the hundreds of minima in the TR50 profile are likely to be at or near origins of replication.
2. Materials
2.1. Cell Culture, Synchronization, BrdU Labeling, and FACS
Cell culture: Dulbecco's Modified Eagle's Medium (DMEM; CELLGRO) supplemented with 10% iron supplemented donor calf serum (CELLECT) and 1% Penicillin-Streptomycin (GIBCO).
Synchronization: 1 M Thymidine (Sigma) prepared in phosphate-buffered saline (PBS). 1 μg/μl Aphidicolin (Sigma) dissolved in DMSO.
Bromodeoxyuridine (BrdU; Sigma) is dissolved in PBS at 10 mM concentration. Filter sterilized using 0.2-μm filter (CORNING) and stored in aliquots in dark at -−20°C.
Propidium iodide FACS: Dilute 1 mg propidium iodide (Sigma) in 10 ml sterile water, 25 μl of 20% NP40, and 10 μl of 10 mg/ml RNase A (Roche Applied Sciences); store wrapped in aluminum foil.
2.2. Genomic DNA Extraction
Cell lysis solution: 0.5% SDS, 10 mM Tris-HCl pH 8.0, 300 mM NaCl, 5 mM EDTA, and 200 μg/ml Proteinase K.
Phenol/chloroform/isoamyl-alcohol.
Cholorform/isoamyl-alcohol.
DNase-free ribonuclease (RNase A).
Ethanol, 100% and 70%.
2.3. Purification of Heavy/Light DNA by CsCl Density Centrifugation
Restriction endonucleases EcoRI and HindIII (NEB).
CsCl solution: Use 1 g CsCl (Sigma) per ml of TE buffer (10 mM Tris-HCl pH 8.0, 1 mM EDTA pH 8.0). After the CsCl is dissolved in solution, the refractive index should be 1.4052 (see Note 1).
OptiSeal tubes (Beckman).
Quick-Seal tubes (Beckman).
2.4. BrdU ELISA
2X SE: 0.8 M NaOH, 20 mM EDTA.
96-well ELISA plate (BD BioCoat™).
2 M Ammonium acetate (pH 7.0).
Non-fat dried milk.
Anti-BrdU (Monoclonal antibody to the thymidine-analogue 5-bromo-2’-deoxyuridine Fab fragments with peroxidase (POD) conjugated; Roche Applied Sciences).
TMB substrate (PIERCE).
2 M H2SO4.
2.5. Fragmentation and Labeling of H/L DNA
DNaseI (Epicenter).
10X One-Phor-All buffer (Amersham-Pharmacia).
5X TdT buffer (Roche).
25 mM CoCl2 (Roche).
1 mM bio-ddATP (Enzo Life Sciences).
Terminal deoxytransferase (400 U/ml; Roche).
2.6. Genome-Tiling Array Hybridization
Microarrays
To generate replication time profiles ENCODE01-Forward (Affymetrix, Santa Clara, CA) tiling arrays were used. These arrays are designed to study the pilot ENCODE regions of DNA, comprised of 30 Mb of DNA, or approximately 1% of the human genome. These pilot regions were selected by a committee of the National Human Genome Research Institute (NHGRI). Half of the content on the ENCODE01 Array was manually selected by the NHGRI committee, while the remaining 50% were randomly selected (8). A total of 14.82 Mb of sequence constituted the manually selected regions and included 14 target regions ranging in size from 500 kb to 2 Mb. The randomly selected content includes 30, 500 kb regions selected based on gene density and level of non-exonic conservation.
3. Methods
To generate temporal maps of replication, cell populations are synchronized at G1/S by thymidine/aphidicolin block and released into S-phase as described in the following section. After release from block, replicating DNA is pulse-labeled with 5-bromo-2’-deoxyuridine (BrdU) for successive 2 h intervals of S-phase, thereby dividing 10 h of S-phase into five time intervals (Fig. 14.1B). The efficiency of the block and release is ascertained by propidium iodide based FACS (flow cytometry) (Fig. 14.1A). Genomic DNA is isolated from all five time intervals (0–2, 2–4, 4–6, 6–8, and 8–10 h) representing 10 h of the entire S-phase (Fig. 14.1B). Genomic DNA is digested with EcoRI/HindIII restriction enzymes (Fig. 14.1C) and then the BrdU-incorporated heavy/light (H/L) DNA is purified by CsCl density gradient (Fig. 14.1D). Success of purification is assayed by BrdU ELISA (Fig. 14.2). Purified DNA from each time interval is fragmented to 50–200 bp by DNaseI as described later. The DNA fragments are end-labeled with biotinylatedddATP using terminal transferase (Fig. 14.1E). This labeled DNA is then hybridized to the high-density genome-tiling Affymetrix array.
Fig. 14.1. Schematic of methods used to map replication timing.
(A) Synchronous progression of HeLa cell through S-phase and harvesting of timed replication pools. HeLa cells released from a G1-S block followed by FACS for DNA content ((9); “Copyright (2005) National Academy of Sciences, USA”). (B) Cells synchronously released in the same way as in (A) were pulsed with BrdU at indicated time intervals. (C) Isolation of DNA (BrdU substituted; in gray) and digestion to produce fragments that resolve well H/L and L/L DNA. (D) Top black band represents LL DNA while the lower gray/black band represents H/L DNA. (E) Purified H/L DNA was fragmented with DNaseI and labeled with biotin-ddATP by terminal transferase and hybridized to the ENCODE arrays.
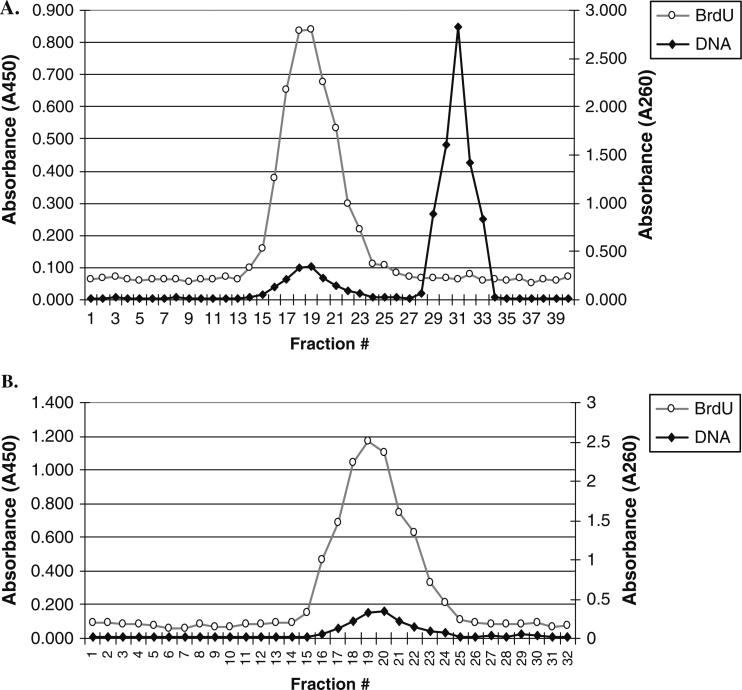
Fig. 14.2. BrdU ELISA to check fractionation of H/L DNA.
(A) Genomic DNA labeled during 0–2 h of S-phase. Gray line is the Anti-BrdU reactivity in an ELISA (measured as absorbance at 450 nm) and black line is the absorbance at 260 nm for total DNA. The H/L DNA forms a peak away from the bulk of the unreplicated DNA. (B) Second purification of H/L fractions. Complete overlap of BrdU peak with total DNA peak indicates purity of H/L DNA.
During computational processing of the data, it is important to identify two separate classes of replication, temporally specific replication (TSR) and temporally non-specific replication (TNSR). An area undergoes TSR when all alleles at that locus replicate synchronously in S-phase. A non-trivial portion of a given genome may replicate alleles in a non-synchronous manner, with some replicating early and others later. For a given probe on the array, the algorithm to classify it as temporally specific versus non-specific considers the signal of that probe across all time points. If there is evidence that significant replication is occurring in two non-adjacent time points, then the probe is classified as temporally non-specific. In the absence of such evidence, where significant replication is isolated to a single time point or two adjacent time points, the probe is classified as temporally specific. Later, broad regions of TSR and TNSR are segregated using a majority algorithm where the ratio of temporally specific to temporally non-specific probes is considered.
3.1. Cell Synchronization and Pulse Labeling
Treat 10–30 × 15 cm plates (10 plates for 2–4 h, 4–6 h, and 6–8 h time points and 30 plates each for 0–2 h and 8–10 h time points) of cells at 60% confluency with 2 mM thymidine for 12 h.
Release cells from thymidine block by removing the media and washing three times with PBS.
Add 20 ml of fresh media and incubate for 12 h.
Add 1 μg/ml aphidicolin and incubate for 12 h.
Release the cells from aphidicolin block by removing the media and washing three times with PBS.
Add 20 ml of fresh media to release the cells into S-phase of cell cycle. Label cells with 100 μM BrdU for 2 h.
Remove the media and wash cells thrice with PBS and then trypsinize them.
Harvest the cells by centrifugation at 200g for 5 min in Eppendorf centrifuge 5810 (or equivalent). Save 5 × 105 cells for FACS (fix cells in 70% ethanol for at least 1 h at 4°C. Stain in 1 ml propidium iodide solution for 1 h).
Proceed to genomic DNA extraction step. If the genomic DNA extraction is not to be performed the same day, freeze the cell pellets at −80°C.
3.2. Genomic DNA Extraction
Collect 108 cells (monolayer cells) in a 15 ml tube. Then centrifuge at 200g for 5 min.
Wash 2X with 10 ml PBS.
Add 10 ml cell lysis buffer to cell pellet and resuspend the cells by gently tapping the tube with finger and incubate at 55°C for 2 h.
Add 10 ml of Phenol/Chloroform/IAA (25:24:1), rotate 10 min at room temperature. Centrifuge at 1,731g for 10 min, transfer supernatant to a new tube.
Add equal volume of chloroform/isoamyl alcohol (24:1). Rotate 10 min at room temperature. Centrifuge at 1,731g for 10 min, and transfer the supernatant to a new tube.
Add DNase-free ribonuclease A (RNase A) to a final concentration of 25 μg/ml and incubate for 1 h at 37°C.
Repeat Steps 4 and 5 and transfer the aqueous phase to SS-34 rotor Sorvall tubes.
Add 2 volumes ethanol, mix by inverting the tube. Leave on ice for 10 min.
Centrifuge at 12,000g at room temperature for 30 min in Sorvall RC5-B centrifuge (or equivalent tubes/rotor). Decant the supernatant by gently inverting it.
Wash the pellet with 10 ml 70% ethanol. Centrifuge at 12,000g for 10 min in RC5-B centrifuge.
Decant the supernatant by gently inverting it.
Air dry the pellet for about 5–10 min (see Note 2).
Resuspend the pellet in 1 ml sterile water and incubate at 37°C for an hour till DNA is completely dissolved.
Record concentration and yield by reading the absorbance at 260 nm.
3.3. H/L DNA Purification by CsCl Gradient
Digest genomic DNA with 5 U/μg each of EcoRI and HindIII restriction enzymes for 5 h. Add EDTA to the reaction at 1 mM concentration.
Check for completion of digestion by running 10 μl of the reaction volume on a gel. Typically, the spread of digested DNA will be more abundant in the 2–5 kb range. If this is not the case then add more enzyme and leave it longer for digestion.
Prepare 1 g/ml CsCl solution in TE buffer pH 8.0 (Tris-HCl 10 mM, EDTA 1 mM).
Add 1 g CsCl to the digested DNA (400 μg) and make up the volume to 1 ml (see Note 3).
Carefully pour DNA-CsCl solution into OptiSeal tubes. Fill rest of the tube with CsCl solution.
Centrifuge the density gradients at 25°C (see Note 2) in Beckman VTi50 rotor (or equivalent tubes/rotor) at 167,200g for 48 h, no brake (i.e., deceleration = 0).
After ultracentrifugation, carefully collect fractions at flow rate of 1 ml/min, starting from bottom of the tube.
Check for the H/L peak by performing BrdU ELISA (Fig. 14.2A).
If density banding worked fine then pool the fractions representing H/L peak in Quick-Seal tubes and ultracentrifuge again using NVT90 rotor (or equivalent tubes/rotor) for 18 h at 292,200g, no brake (i.e., deceleration = 0).
Collect the fractions at flow rate of 200 μl/min from bottom and perform BrdU ELISA to ascertain separation of H/L DNA (Fig. 14.2B). This second centrifugation step further cleans up the H/L DNA from any contaminating LL DNA.
Pool the fractions representing H/L DNA, dialyze for 5 h (or overnight) in TE buffer.
3.4. BrdU ELISA
Spot 2.5 μl H/L DNA + 12.5 μl water + 15 μl of 2X SE on poly-lysine 96-well ELISA plate. Make sure to have no-DNA control wells to evaluate the background. Also perform the whole assay in triplicate.
Denature DNA for 10 min on 100°C hot plate.
Add 30 μl of 2 M ammonium acetate (pH 7.0) and 40 μl of TE (pH 8.0) to each well and leave it on shaker for 30 min at RT.
Change the solution with 120 μl each of 5% non-fat milk in PBS containing 10 mM EDTA and shake for 30 min at RT.
Rinse the wells three times with 150 μl each of PBS.
Prepare 1:100 dilution of Anti-BrdU in PBS. Add 100 μl in each well and shake the plate at RT for 30 min (see Note 5).
Wash the plates three times with 150 μl of PBS. Each wash should be 10 min with shaking at RT.
Develop the color (blue) using 100 μl of TMB substrate. Developing time can vary from 1 min to 30 min (see Note 6).
Quench with 100 μl of 2 M H2SO4 (color turns yellow).
Read the absorbance at 450 nm.
3.5. Fragmentation and Labeling of H/L DNA
Dilute DNaseI 1:16 with 1X One-Phor-All buffer and keep it on ice. Do not vortex any solutions containing DNaseI.
Set up the reaction in 40 μl as detailed in the Table 14.1.
Check on 2% agarose gel, the bulk DNA should be 50–100 bp. If not then incubate the tube again at 37°C (see Note 7).
If the digestion is complete to desired fragment length then inactivate DNaseI at 99°C for 10 min.
For labeling assemble the reaction as in Table 14.2.
The labeled (ds) DNA is now ready to be hybridized to the array. Store labeled DNA at −80°C till further use.
Table 1.
Conditions for setting up DNaseI digestion of H/L DNA
| Reagents | Final concentraion or amount in reaction | Reaction conditions |
|---|---|---|
| 10X One-Phor-All buffer | 4 μl | 37°C for 4 min and then hold on ice |
| DNase diluted 1:16 with 1X One-Phor-All buffer | 5 μl | |
| DNA | 12 μl | |
| Water | To 40 μl | |
| Total volume | 40 μl |
Table 2.
Conditions for labeling of H/L DNA using terminal transferase
| Reagents | Volume | Reaction conditions |
|---|---|---|
| DNA from fragmentation | 34 jd(~9 μg) | Incubate 37°C for 2 h |
| 5X TdT buffer | 14 μl | |
| 25 mM CoCl2 | ||
| 1 mM bio-ddATP | 5 μl | |
| Terminal deoxytransferase (400 U/ml) | 3 μl | |
| Total volume | 70 μ1 | |
3.6. Hybridization and Washing of Microarrays
Hybridize, wash, and stain the chips as per manufacturer's protocol (FS450_0001).
Scan and analyze each microarray for signal intensities using GeneChIP® Scanner 3000 and GeneChIP Operating Software (GCOS) from Affymetrix.
3.7. Time of Replication of 50% (TR50) Calculation
Calculate a signal value for each probe of each array as Max [PM (Perfect Match) – MM (Mis Match), 0]. This means that if PM–MM is <0, it is set as 0.
Calculate a TR50 value for each probe of the array set by linearly interpolating the point at which 50% of signal is accumulated across all time points. Any probe of the array set that shows 0 signal in every time point is excluded from further analysis.
- Classify each probe of the array set as TSR or TNSR by the following criteria:
- Find the minimum signal of all time points, call it MIN.
- Subtract MIN from each time point to adjust their signal.
- Call the number of expected alleles at each locus of the cell line in question N.
- Sum the total signal across all time points, call it TOTAL.
- Find the maximum signal value of all time points, call it MAX.
- Find the maximum sum of all sets of two adjacent time points, call it MAXSUM.
- Find the maximum sum of all sets of two adjacent time points where neither time point in the sum has signal equal to MAX, call it MAXSUMNOT.
- If MAXSUM > (1 – 1/N) * Total, then classify the probe as TSR.
- Otherwise, if MAXSUMNOT ≥ (1/N) * Total, then classify the probe as TNSR.
- Otherwise, if both Steps h and i fail to classify the probe, then classify it as TSR.
3.8. Segregation of TSR and Pan-S Regions
Process the probes with a sliding window of 60,000 bp. When the number of probes in the window is ≥ 600, generate an interval. If the number of TSR probes is greater than the number of TNSR probes, generate a TSR interval. If the number of TSR probes is less than the number of TNSR probes, generate a Pan-S interval. If they are equal, use the next probe to break the tie. Whenever the ratio changes, switch to generating the opposite interval. If the number of probes in the window drops below 600, end the current interval.
The mutually exclusive TSR intervals and Pan-S intervals divide the area on the array with the required minimum probe density into TSR regions and Pan-S regions. Remove intervals with length less than 10,000 bp. Then within each set, join intervals whose endpoints are less than 10,000 bp apart.
3.9. TR50 Smoothing
Perform a Lowess smoothing on the set of TSR probes TR50 values with the smoother set to consider a window of 60,000 bp.
The resulting smoothed curve is called the smoothed TR50 and is paired with the segregation intervals to comprise the replication profile.
3.10. Discrete Temporally Specific Timing Categories
Collect the set of smoothed TR50 probe values that fall into TSR regions.
Sort the smoothed TR50 values of these probes.
Choose the one-third point of the distribution as the early-mid cutoff and the two-thirds point of the distribution as the mid-late cutoff.
Break the TSR regions up into subregions based on the smoothed TR50 values. A smoothed TR50 value less than the early-mid cutoff gives rise to an early interval. A smoothed TR50 value less than the mid-late cutoff but later than the early-mid cutoff gives rise to a mid interval. A smoothed TR50 value greater than the mid-late cutoff gives rise to a late interval.
Remove intervals with length less than 10,000 bp from each of these sets: early intervals, mid intervals, and late intervals.
Join intervals in each set that are separated by less than 10,000 bp.
The final replication timing profile is attained by pairing the smoothed TR50 curve with the discrete non-overlapping segregation intervals: early, mid, late, and Pan-S. Figure 14.3 shows an example of the replication timing profile for part of human chromosome 21 in HeLa cell line.
Fig. 14.3.
Smoothed TR50 plot and segregation profile from the 1.9 Mb part of human chromosome 21 in HeLa cell line. (A) Segregation of replication timing profile into early, middle, late, and Pan-S replicating regions along the length of the chromosome. (B) TR50 plot. The lowest point (line plot) in each valley indicates a site that is replicated before its adjoining segments and so is likely to contain origins of replication. The solid circles represent these putative origins. The gaps in the TR50 plots indicate the presence of repeats. In order to minimize cross-hybridization of oligonucleotides, repeat regions of the genome are not spotted on the tiling arrays.
Acknowledgments
This work was supported by National Institutes of Health Grant HG003157 (to A.D.)
Footnotes
Before estimating the refractive index of the CsCl solution, calibrate the instrument with distilled water (refractive index of 1.3330).
Do not dry the DNA completely: dessicated DNA is very difficult to dissolve. To get genomic DNA in solution, first dissolve at 37°C for at least an hour and then leave at 4°C overnight.
Do not forget to set refractive index of the DNA with CsCl. It should be 1.4052.
Always run the CsCl gradients at RT (25°C). Running the gradient at lower temperature can cause crystallization of CsCl, which will affect the gradient and can also be detrimental to rotor and the ultracentrifuge.
We have found that Anti-BrdU-POD antibody to be excellent for this assay. It also negates the use of secondary antibody.
Do not let the color turn dark blue after addition of TMB substrate and green after addition of H2SO4: this is the sign of signal saturation and hence can give misleading results.
DNaseI digestion: Since efficiency of DNaseI digestion is sensitive to a number of conditions (e.g., purity of DNA, lot and vendor of DNaseI) it is necessary to titrate the DNaseI amount. The conditions mentioned here therefore should only be considered as guidelines that may have to be adjusted for a particular DNAseI prep.
References
- 1.Bell SP, Dutta A. DNA replication in eukaryotic cells. Annu Rev Biochem. 2002;71:333–374. doi: 10.1146/annurev.biochem.71.110601.135425. [DOI] [PubMed] [Google Scholar]
- 2.Watanabe Y, Fujiyama A, Ichiba Y, Hattori M, Yada T, Sakaki Y, Ikemura T. Chromosome-wide assessment of replication timing for human chromosomes 11q and 21q: disease-related genes in timing-switch regions. Hum Mol Genet. 2002;11:13–21. doi: 10.1093/hmg/11.1.13. [DOI] [PubMed] [Google Scholar]
- 3.Sinnett D, Flint A, Lalande M. Determination of DNA replication kinetics in synchronized human cells using a PCR-based assay. Nucleic Acids Res. 1993;21:3227–3232. doi: 10.1093/nar/21.14.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Selig S, Okumura K, Ward DC, Cedar H. Delineation of DNA replication time zones by fluorescence in situ hybridization. Embo J. 1992;11:1217–1225. doi: 10.1002/j.1460-2075.1992.tb05162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woodfine K, Fiegler H, Beare DM, Collins JE, McCann OT, Young BD, Debernardi S, Mott R, Dunham I, Carter NP. Replication timing of the human genome. Hum Mol Genet. 2004;13:191–202. doi: 10.1093/hmg/ddh016. [DOI] [PubMed] [Google Scholar]
- 6.Karnani N, Taylor C, Malhotra A, Dutta A. Pan-S replication patterns and chromosomal domains defined by genome-tiling arrays of ENCODE genomic areas. Genome Res. 2007;17:865–876. doi: 10.1101/gr.5427007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raghuraman MK, Winzeler EA, Collingwood D, Hunt S, Wodicka L, Conway A, Lockhart DJ, Davis RW, Brewer BJ, Fangman WL. Replication dynamics of the yeast genome. Science. 2001;294:115–121. doi: 10.1126/science.294.5540.115. [DOI] [PubMed] [Google Scholar]
- 8.ENCODE project consortium The ENCODE (ENCyclopedia Of DNA Elements) Project. Science. 2004;306:636–640. doi: 10.1126/science.1105136. [DOI] [PubMed] [Google Scholar]
- 9.Jeon Y, Bekiranov S, Karnani N, Kapranov P, Ghosh S, MacAlpine D, Lee C, Hwang DS, Gingeras TR, Dutta A. Temporal profile of replication of human chromosomes. Proc Natl Acad Sci USA. 2005;102:6419–6424. doi: 10.1073/pnas.0405088102. [DOI] [PMC free article] [PubMed] [Google Scholar]