Abstract
DMRT1 encodes a conserved transcription factor with an essential role in gonadal function. In the chicken, DMRT1 is located on the Z sex chromosome and is currently the best candidate master regulator of avian gonadal sex differentiation. We previously showed that knockdown of DMRT1 expression during the period of sexual differentiation induces feminisation of male embryonic chicken gonads. This gene is therefore necessary for proper testis development in the chicken. However, whether it is sufficient to induce testicular differentiation has remained unresolved. We show here that over-expression of DMRT1 induces male pathway genes and antagonises the female pathway in embryonic chicken gonads. Ectopic DMRT1 expression in female gonads induces localised SOX9 and AMH expression. It also induces expression of the recently identified Z-linked male factor, Hemogen (HEMGN). Masculinised gonads show evidence of cord-like structures and retarded female-type cortical development. Furthermore, expression of the critical feminizing enzyme, aromatase, is reduced in the presence of over-expressed DMRT1. These data indicate that DMRT1 is an essential sex-linked regulator of gonadal differentiation in avians, and that it likely acts via a dosage mechanism established through the lack of global Z dosage compensation in birds.
Keywords: DMRT1, embryonic chicken gonad, sex determination, SOX9, HEMGN, AMH
Introduction
In most vertebrates, sex is determined genetically by the differential inheritance of sex chromosomes. Sex-linked genes initiate sexually dimorphic pathways during development, the most well known of these being gonadal sex differentiation. In eutherian mammals, the Y chromosome-linked gene SRY controls testicular development during embryogenesis. In the absence of SRY, as in XX embryos, the gonads follow the female pathway and become ovaries, while ectopic expression of an Sry transgene in XX mouse embryos is sufficient to trigger full male somatic development (Koopman et al. 1991; DeFalco and Capel 2009). SRY is therefore considered the “master sex determinant” among therian mammals. However, the gene is absent from monotremes and all non-mammals, indicating that other genes must control gonadal sex differentiation in other vertebrates. Among teleost fishes, some master determinants that are unrelated to SRY have recently been identified. In the Patagonian pejerrey (Odontesthes hatcheri), a duplicated copy of Anti-Mullerian Hormone (amhy) is located on the male-specific Y chromosome, and likely operates as the master sex determinant (Hattori et al. 2012). In other vertebrates, AMH is a downstream component of the gonadal sex differentiation pathway. In the rainbow trout (O. mykiss), an immune-related gene, sdY, is the master sex regulator in the gonads. This gene is located on the male-specific Y chromosome, its knockout induces ovarian development in genetic males and its over-expression induces testicular development in genetic females (Yano et al. 2012).
The data from mammals and fishes indicate that master regulators of gonadal sex differentiation can evolve rapidly. However, one deeply conserved regulator of gonadogenesis is DMRT1 (Doublesex and Mab-3 Related Transcription Factor, #1). This gene encodes a transcription factor with a conserved DNA-binding motif, the DM domain (Raymond et al. (Raymond et al. 1998; Burtis and Baker 1989). DMRT1 is highly expressed in the developing gonads of male embryos, from fishes through to reptiles, birds and mammals (Kettlewell et al. 2000; Raymond et al. 1999; Smith et al. 1999a; De Grandi et al. 2000; Marchand et al. 2000; Moniot et al. 2000; Aoyama et al. 2003; Kim et al. 2003; Kobayashi et al. 2004; Yoshimoto et al. 2010; Masuyama et al. 2012). In the medaka fish, a duplicated copy of DMRT1 translocated to the Y sex chromosome (DMY/ Dmrt1bY) is the master sex determinant. It is required for testicular differentiation, and knockdown induces male-to-female sex reversal (Matsuda et al. 2002; 2007; Nanda et al. 2002; Kobayashi et al. 2004). Similarly, in Xenopus, a divergent copy of DMRT1, DM-W, is located on the female W sex chromosome and antagonises the testis-promoting action of autosomal DMRT1 (Yoshimoto et al. 2008; 2010).
In mammals, DMRT1 plays an essential role in testis development and function (Matson and Zarkower 2012). Deletions of DMRT1 in humans are associated with male-to-female sex reversal and germ cell tumours (Bennett et al. 1993; Veitia et al. 1997; Raymond et al. 1998; Kanetsky et al. 2011). The phenotype seen in humans is consistent with sites of expression in the mouse. In the mouse embryo, DMRT1 expression is restricted to the urogenital system, namely, within the Sertoli cell lineage and in germ cells of the developing testis, with lower expression in developing ovaries. Sertoli cells play an organisational role in the testis, coalescing into seminiferous cords and sending differentiation cues to other cell types. Dmrt1 null mice show aberrant testicular development postnatally, characterised by Sertoli and germ cell dysfunction (Raymond et al. 2000). DMRT1 is required autonomously in both cell types (Kim et al. 2007; Krentz et al. 2009; Matson et al. 2010). Conditional deletion of DMRT1 in adult mice causes trans-differentiation of testes into an ovarian phenotype. Sertoli cells become re-programmed towards a female (granulosa) cell fate. They down-regulate malepromoting transcripts, such as SOX9, and up-regulate key female transcripts, such as FOXL2, and they begin to synthesise estrogens (Matson et al. 2011). DMRT1 is therefore continuously required to maintain the testicular phenotype in adulthood. Despite an essential postnatal role for DMRT1 in mammals, embryonic development of the testis does not require the gene. DMRT1 is therefore considered a downstream component of the molecular hierarchy leading to testicular morphogenesis in mammals.
DMRT1 is autosomal in mammals, but is sex-linked in birds. Birds have a ZZ male: ZW female sex chromosome system, characterised by a lack of global Z dosage compensation. Hence, most Z-linked genes are, on average, expressed 1.5–2 fold higher in males (ZZ) compared to females (ZW) (Itoh et al. 2007; Zhang et al. 2010; Arnold et al. 2008). In the chicken embryo, DMRT1 is expressed in the developing gonads of both sexes but, being Z-linked, is more highly expressed in males versus females (Raymond et al. 1999; Smith et al. 1999b; Shan et al. 2000). The sex-linkage and male up-regulation of DMRT1 makes this gene a prime candidate master regulator of gonadal sex differentiation in the avian embryo. We previously used an RNA interference approach to knock down endogenous DMRT1 expression in early chicken embryos, which caused feminisation of genetic males. The gonads exhibited disrupted testis cord organisation and reduced SOX9 expression, together with upregulation of two key female genes, FOXL2 and aromatase (which synthesises estrogen) (Smith et al. 2009). These findings showed that DMRT1 is necessary for proper testis development in the chicken. However, it is unclear whether DMRT1 is sufficient to engage the male pathway. Furthermore, the possibility exists that other Z-linked genes could act upor downstream of DMRT1. Indeed, most recently, another Z-linked male factor has been identified, Hemogen (HEMGN), which is involved in testicular differentiation in the chicken embryo. Over-expression of HEMGN in female embryos can cause elevation of both DMRT1 and SOX9 expression, together with down-regulation of FOXL2 and aromatase (Nakata et al. 2013). The aim of the current study was to define the temporal relationship between DMRT1 and HEMGN expression and to test the ability of DMRT1 to activate the male developmental pathway. We find that DMRT1 clearly precedes HEMGN expression and that over-expression of DMRT1 in female gonads can induce male pathway genes. In addition, it reduces expression of the critical female gene, aromatase. These data support the view that DMRT1 is a key Z-linked regulator of testicular morphogenesis in the avian model.
Methods
DMRT1 over-expression
To assess the effects of DMRT1 over-expression in chicken embryonic gonads, we used in ovo electroporation of the avian retroviral vector, RCASBP (Fekete and Cepko 1993; Logan and Tabin 1998). This vector is replication competent, so that is can spread horizontally to neighbouring cells as well as vertically to daughter cells following initial infection. The complete chicken DMRT1 open reading frame was obtained by PCR and 5’ RACE, sequence verified and cloned into RCASBP/A strain. This construct robustly expressed DMRT1 protein in vitro, as assessed by immunofluorescence (Smith et al. 2009). High quality RCASBP-DMRT1 DNA was prepared from overnight bacterial cultures using the Nucleobond Maxiprep kit according to the manufacturers instructions. DNA was used at final concentration of 1µg/µL, diluted in electroporation mix (EP) (1.3% Fast Green, 0.16% carboxymethyl cellulose, 1mM MgCl2, in PBS). For in ovo electroporation, embryos were pre-incubated at 37.8°C to day 2.3, equivalent to Hamburger and Hamilton (HH) stage 14 –16 (Hamburger and Hamilton 1992). A small hole was cut in the eggshell and approximately 0.5µL DNA in EP was injected into the left coelomic cavity. Electrodes were placed beneath the left underside of the embryo and dorsolaterally on the upper right side. An electric current was then applied using a BTX square electroporator under the following conditions: 40V, 2 pulses of 150msec each, with a 10 msec pulse interval. Control embryos were either not electroporated or electroporated with RCASBP-EGFP prepared as above. Eggs were sealed with clear tape and incubation was resumed until harvesting. Experiments were repeated at least three times and involved at least 50 embryos for each experiment. Embryo survival was initially high (over 70%) but declined over time to around 40% by day 7.5. Embryos were harvested at embryonic days 5.5 (HH28), 6.5. (HH30) and 7.5 (HH32). Immunostaining tissues with viral p27 antibody, which detects a viral epitope, confirmed RCASBP infection in left (electroporated) gonads but not the right (non-electroporated control) gonads. The left gonad was chosen for electroporation due to the asymmetrical development of female gonads in chicken; only the left gonad develops into a functional ovary, while the right ultimately regresses.
PCR sexing
Control embryos and those over-expressing DMRT1 were dissected at the indicated time points. For genetic sexing of embryos, a small piece of limb tissue was digested in PCR compatible Proteinase K buffer and the genomic DNA was used for rapid PCR sexing (Clinton et al. 2001). By this method, only females show a W-linked repetitive element of the XhoI class. Amplification of 18S rRNA in both sexes served as an internal control.
Immunofluorescence
Control and DMRT1 over-expressing tissues were fixed for 15 minutes in 4% PFA/PBS at room temperature, prior to processing for tissue section immunofluorescence, as described previously (Smith et al. 2003). Controls comprised either uninfected stage-matched embryos, or those infected with RCASBP.EGFP. At least three embryos per time point and/or treatment were examined. Since the embryonic gonad is elongated and sausage-shaped, serial longitudinal sections were cut, which allowed a more thorough analysis of over-expression along the length of the gonad, compared with transverse sections. Briefly, 10 µm sections were cut on a cryostat, permeablised in PBS+1% Triton X-100 and blocked in PBS+2% BSA for 1 hour. Primary antibodies were added overnight at 4°C and were either raised in-house (rabbit anti-chicken aromatase at 1:5000, rabbit anti-chicken DMRT1 at 1:5000; rabbit anti-HEMGN at 1:300), or were obtained commercially (Charles River Services; rabbit anti-p27 (1:1000), Santa Cruz; goat anti-human AMH polyclonal (1:1000), Serotec; mouse antifibronectin (1:500), Millipore; goat anti-GFP (1:500), Millipore; Rabbit anti-human SOX9 (1:6000)). Alexa-fluor secondary antibodies were used (donkey or goat anti-rabbit, mouse or goat 488 or 594; Molecular Probes). Sections were counterstained with DAPI and images collected on a Zeiss microscope equipped with fluorescence optics.
Quantitative reverse transcription-PCR (qRT-PCR)
RNA was extracted from isolated gonads, reverse transcribed as previously described (Smith et al. 2008). Probes were designed using the UPL Assay Design Center (https://www.roche-applied-science.com) and are as follows. DMRT1 probe 59 together with forward primer 5’- AGC CTC CCA GCA ACA TAC AT-3’ and reverse primer 5’-GCG GTT CTC CAT CCC TTT-3’ ; normalizing control HPRT probe 38 together with forward primer 5’-CGC CCT CGA CTA CAA TGAA TA-3’ and reverse primer 5’-CAA CTG TGC TTT CAT GCT TTG- 3’. Analysis was performed using a LightCycler 480 instrument, LC480 master mix and software (Roche). Relative expression was determined using the comparative CT method (ΔΔCT), with samples normalized against HPRT and expressed as fold change relative to the highest expresser. The housekeeping gene HPRT was used to normalize expression data as the levels of this transcript remains constant in both sexes throughout the time points analysed in the current study (data not shown). In addition, the efficiency of each primer/probe combination was determined and only combinations with efficiencies >80% were used.
Results
Chronology of gene expression in developing male gonads
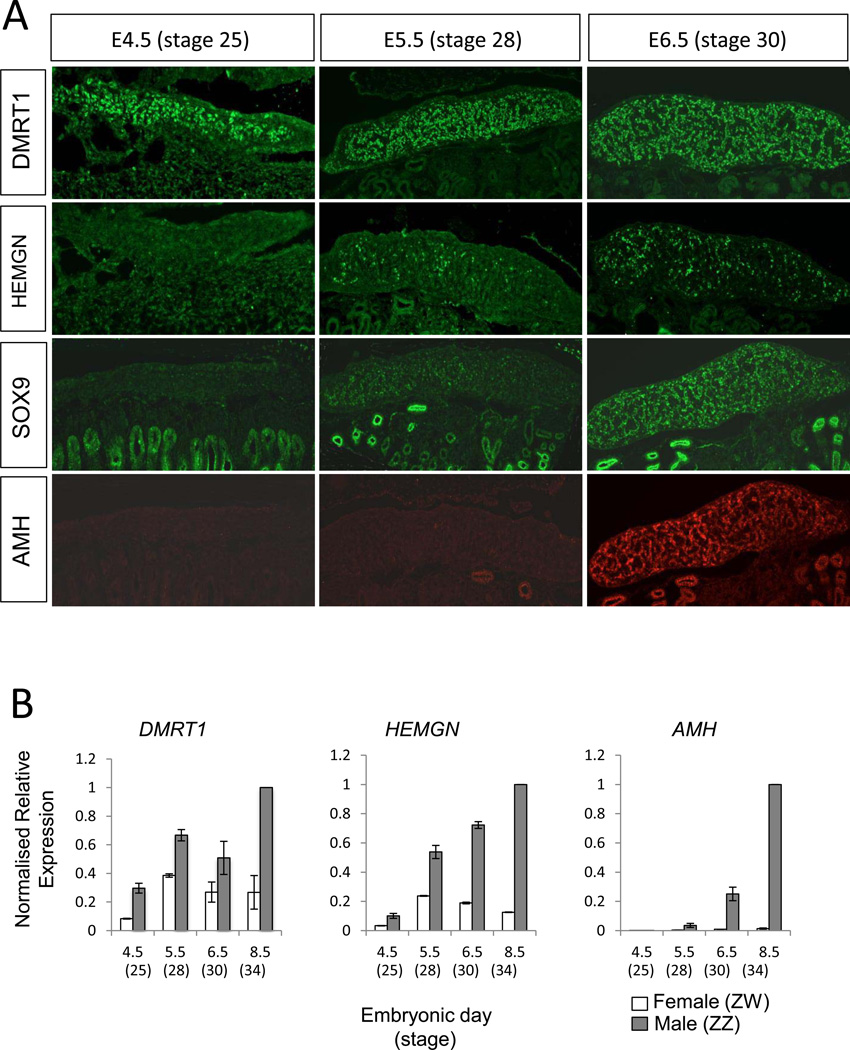
To firstly define the onset of key gene expression in the developing male gonad, staged embryos were assessed by immunofluorescence performed on longitudinal sections. At E4.5 (HH25), the gonad is morphologically undifferentiated and appears identical between the sexes. At this time, DMRT1 protein was already strongly expressed throughout the medulla of male gonads (Fig. 1A). It was also expressed in females, but at a lower level (not shown). None of the other key male markers were expressed at this time. By E5.5 (HH28), DMRT1 expression continued to be widespread, while groups of cells were now expressing HEMGN protein, and there was a low level of SOX9 expression (Fig. 1A). At E6.5 (HH30), the male gonad showed signs of seminiferous cord differentiation (i.e., organising Sertoli cells). DMRT1 was strongly expressed, HEMGN and SOX9 were now strongly and widely expressed and AMH protein was first detectable through the length of the gonad (Fig. 1A). All of these proteins localised to the pre-Sertoli and Sertoli cells of the gonadal medulla. With the exception of DMRT1, none of these proteins were detectable in female gonads. DMRT1 protein was detectable in females throughout all time points, but at significantly lower levels than in males.
Fig. 1.
Chronology of gene expression in developing male gonads. The expression of key testis development genes were analysed across several critical stages of sexual differentiation at both the protein and RNA levels. (A) Immunofluorecent staining of DMRT1 (green), HEMGN (green), SOX9 (green) and AMH (red) expression in E4.5 (HH25), E5.5 (HH28) and E.6.5 (HH32) embryonic gonads. DMRT1 expression shows robust expression from at least as early as E4.5 onwards. Both HEMGN and SOX9 expression were expressed from E.5.5 onwards, and AMH expression was detectable at E6.5. (Note that the SOX and AMH were co-stains on the same sections). (B) Quantitative RT-PCR analysis comparing expression of DMRT1, HEMGN and AMH mRNA in female and male gonads across stages of embryonic development. All transcripts measured were expressed dimorphically with higher levels in the male tissues for each. DMRT1 is expressed in a male-biased fashion from E4.5 onwards. HEMGN mRNA is initially expressed at a lower level, but becomes male biased after DMRT1, and AMH was expressed sexually dimorphically from E6.5. HPRT was used as the normalising control. Error bars represent SEM, n = 3.
A similar pattern was observed when transcripts were analysed. At the mRNA level, DMRT1 was expressed with a male bias throughout the period of gonadal sex differentiation, from at least as early as E4.5 (Fig. 1B). HEMGN mRNA was detected as early as E4.5 (i.e., prior to the protein at E5.5) but at a lower level than DMRT1 expression. HEMGN expression then became clearly male up-regulated from E5.5 through E8.5. AMH mRNA was weakly expressed until E6.5, when expression became strongly male up-regulated.
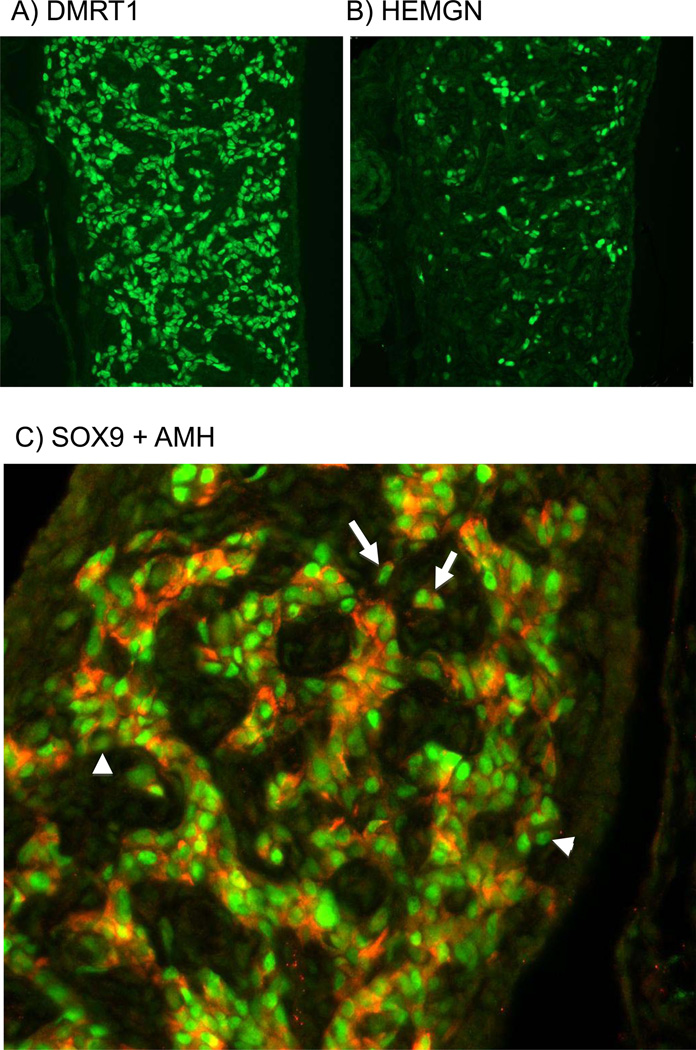
DMRT1 and HEMGN proteins both localised in pre-Sertoli cells. Serial sections of E6.5 gonads showed that DMRT1-positive cells were widely distributed in the male gonads at E6.5. HEMGN -positive cells were also distributed throughout the same developing seminiferous cords (Fig. 2). However, while essentially all pre-Sertoli cells were DMRT1 positive, not all were HEMGN positive, and the level of HEMGN expression varied across cells (compare Fig. 2 A and B; consecutive serial sections are shown). The expression of SOX9 and AMH proteins at the critical time point of E6.5 (stage 30) was also examined more closely. Previous studies have shown the AMH transcripts are first detectable before SOX9 transcripts in the embryonic chicken gonad (Oreal et al. 1998; Smith et al. 1999b). However, in the study described here, at the protein level, SOX9 was detectable prior to AMH (E5.5 versus E6.5; see Fig. 1). High magnification view of male gonads at E6.5 showed that all AMH positive cells within developing seminiferous cords were also SOX9 positive, but not all SOX9 positive cells were AMH positive (Fig. 2C, arrows). In addition, isolated cells were detected that were SOX9/AMH double positive, indicating that pre-Sertoli cells need not be enclosed in membrane-bound defined cords to express these male markers (Fig. 2, arrowheads).
Fig. 2.
Localisation of key testis pathway proteins in developing male gonads. Immunostaining of E7.5 (HH32) male gonads. (A) DMRT1 (green) staining shows widespread expression in nuclei of Sertoli cells. (B) Serial adjacent section showing HEMGN (green) staining. Expression is also localised to Sertoli cell nuclei, but is of variably intensity and limited to a subset of cells. (C) Double staining of SOX9 (green) and AMH (red) in developing Sertoli cells, showing that these proteins are co-expressed in the vast majority of cells (e.g, white arrows). However some cells are SOX9 positive but AMH negative (e.g., white arrowheads).
Over-expression of DMRT1
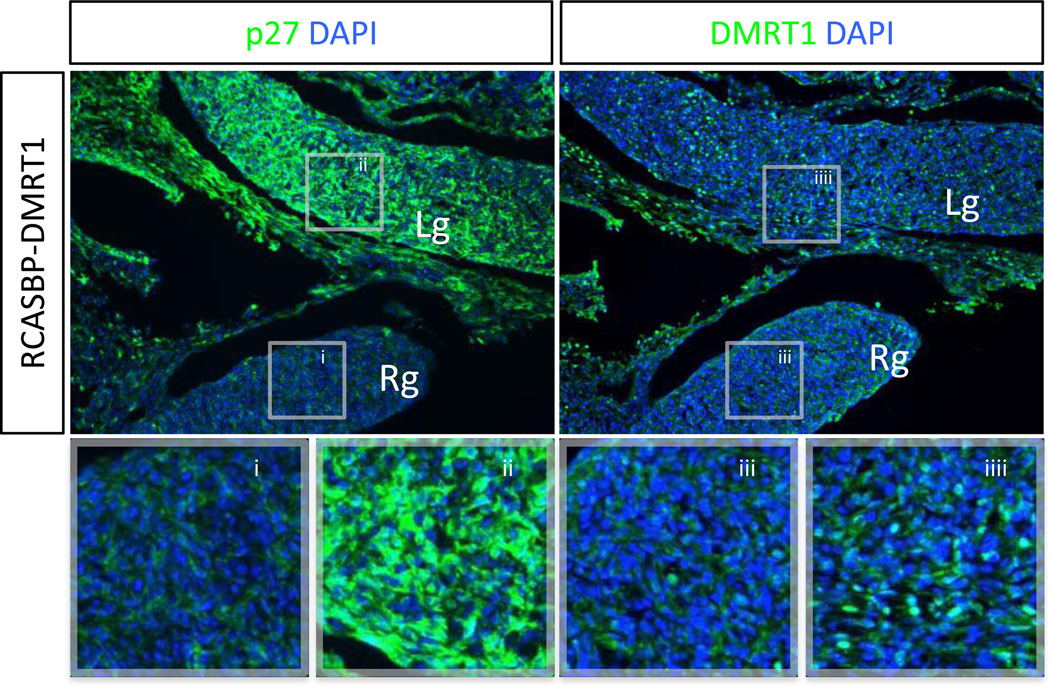
For in ovo overexpression, we used the RCASBP retroviral vector carrying the open reading frame of DMRT1. Robust and specific protein expression was confirmed by DMRT1 immunofluorescence of chicken DF1 cells transfected with RCASBP-DMRT1 DNA (data not shown). To test the efficacy of viral-mediated delivery into the embryonic gonad, E2.5 embryos were electroporated with RCASBP-DMRT1 DNA to specifically target the developing left gonad, and incubated for 4 days. Resultant viral infection was verified by the detection of the viral protein p27 by immunofluorescence in E6.5 gonads, which showed that the virus was present in the left gonad and surrounding tissues but absent from the right gonad, as expected (Fig. 3). Consistent with the appearance of viral p27 protein, localised DMRT1 overexpression was also present in the left gonads females analysed by immunostaining (Fig. 3).
Fig. 3.
DMRT1 over-expression via in ovo electroporation of embryonic female gonads. Immunostaining for the RCASBP viral antigen p27 (green) or DMRT1 (green) on E6.5 left female gonads electroporated with RCASBP-DMRT1 (magnification 10×). The left panel shows widespread infection of RCASBP-DMRT1 throughout the left gonad (Lg) and an absence in the control (un-electroporated) right gonad (Rg). The right panel shows that DMRT1 over expression correlates to the appearance of viral p27 antigen, as the Lg is DMRT1 positive whereas the Rg is negative for DMRT1 protein.
DMRT1 over-expression in female gonads causes activation of male pathway genes
To test the effect of DMRT1 over-expression on female development, the expression of structural and male pathways genes were analysed by immunostaining gonads electroporated with RCASBP-DMRT1. Controls comprised the left gonads of uninfected embryos, or those infected with RCASBP virus expressing GFP. Electroporation of left gonads with RCASBPA-EGFP had no overt effect upon normal gonadal sex differentiation or marker expression in either sex. This is shown in Supplementary Figure 1, and previously reported by Smith et al. (2009) and Lambeth et al. (2013). For clarity, and to avoid complications associated with immunostaining for marker proteins in the presence of GFP fluorescence, only uninfected control gonads are shown.
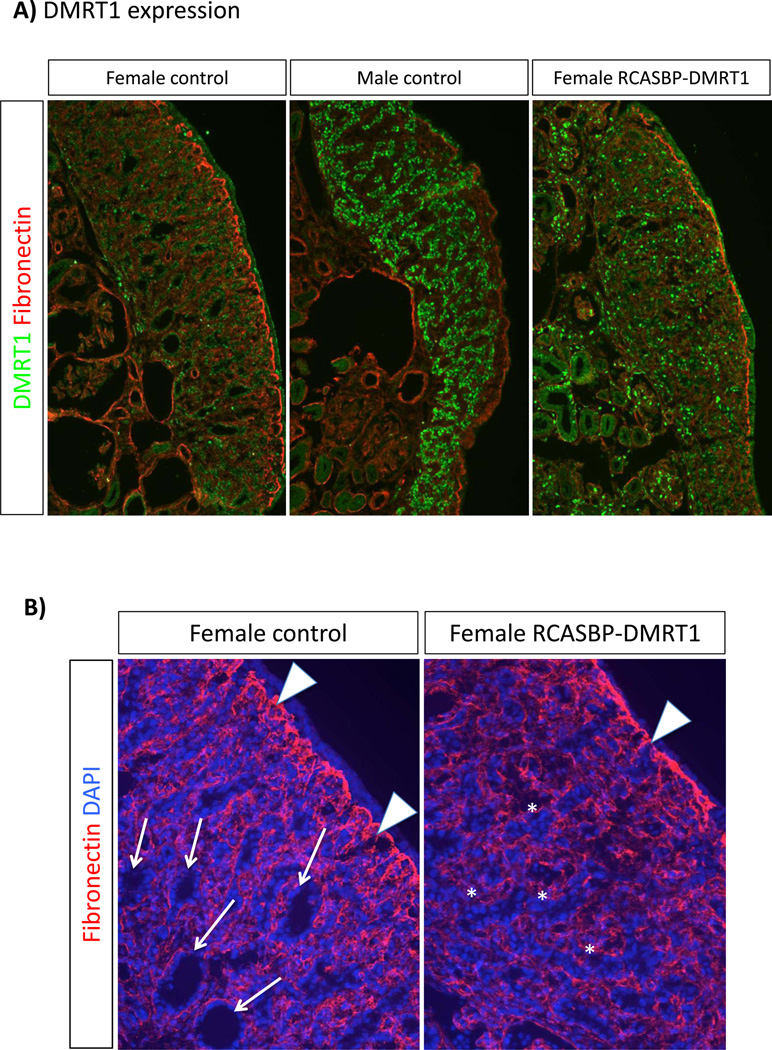
Left gonads electroporated with RCASBP-DMRT1 at E2.3 and examined at E7.5 showed variable over-expression of DMRT1 throughout regions of the gonad (Fig. 4A). Some embryos showed more widespread delivery of the gene than others, which reflects the variable nature of in ovo electroporation. Nevertheless, all electroporated embryos showed some level of DMRT1 over-expression compared to control females. Expression was never as robust as in control males, however (Fig. 3A). Over-expression of DMRT1 in male embryos had no overt effects. However, over-expression of DMRT1 in females induced localised masculinisation. Staining for fibronectin, which delineates the basement membrane, revealed a characteristic thickened cortex in the control females. However, the RCASBP-DMRT1 infected gonads lacked a thickened cortex, with a reduction in the extent of fibronectin expression demarcating the cortical-medullary boundary (Fig. 4B). This reduced expression of fibronectin at the cortical-medullary interface is typical of male gonads. (Also compare the gonads shown in figure 4A, in which the cortical fibronectin expression is robust in the control female, but less well-defined in the control male or female over-expressing DMRT1). In control females at E7.5, the medullary zone is characterised by numerous lacunae, which are fluid filled cavities within the interior part of the gonad (Fig. 4B, arrows). In contrast, in female gonads over-expressing DMRT1, lacunae were far less frequent. Instead, medullary cords took on a solid, male-like anastomosing appearance (Fig. 4B, asterisks).
Fig. 4.
Structural effect of DMRT1 over-expression on gonadal development. Immunostaining on E7.5 left control gonads and left female gonads electroporated with RCASBP-DMRT1. All images were captures at the same exposure. (A) DMRT1 (green) and fibronectin (red) staining. Control females (not electroporated) show a low level of DMRT1 expression throughout the gonad, and a thickened fibronectin positive basement membrane between the outer cortex and underlying medulla. In male controls, DMRT1 expression is higher and localised to developing seminiferous cords, while the fibronectin layer is weaker. DMRT1 is over-expressed in female gonads infected with RCASBP-DMRT1, and the fibronectin layer is less well-developed than in the control female. (B) Fibronectin (red) and DAPI (blue) staining in control versus DMRT1- over-expressing female left gonads. In the control, numerous lacunae are evident in the medulla (e.g., arrows), while the fibronectin+ basement memebrane is extensive (white arrowheads) and the outer cortex beyond the BM is clear. In the female gonad over-expressing DMRT1, medullary lacunae are far less frequent, but medullary cells are arranged instead in a seminiferous cord like pattern (asterisks). Fibronectin+ basement membrane is less well developed (arrowhead) and the cortex itself is noticeably thinner.
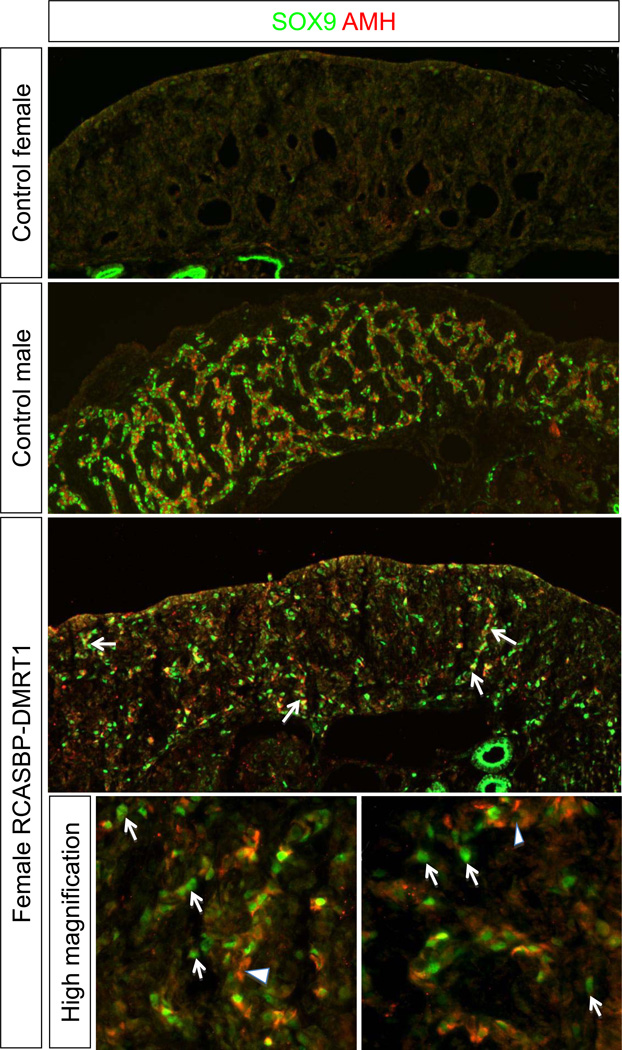
To confirm that DMRT1 over-expression in female gonads can induce the testis pathway genes, SOX9 and AMH were then analysed. Immunostaining on E7.5 control female gonads showed that an absence of both AMH and SOX9 proteins, as expected. In contrast, both proteins were strongly expressed (and co-localised) within developing seminiferous cords of control males. In female gonads overexpressing DMRT1 there was a clear induction in expression of both SOX9 and AMH (Fig. 5). As in genetic males (Fig. 2), both SOX9 and AMH localised in the same cells in the DMRT1-overexpressing female gonads, and there appeared to some cells that were positive for SOX9 only, but few if any cells were expressing AMH only (see Fig. 5, high magnification views).
Fig. 5.
Effect of DMRT1 over-expression on testis development pathway gene expression. Immunostaining for male genes SOX9 (green) and AMH (red) in non-electroporated control versus RCASBP-DMRT1 infected E7.5 gonads (10× magnification). Neither SOX9 nor AMH proteins are detectable in left female control gonads, but both are highly expressed in developing seminiferous cords of control males. In left female gonads electroporated with RCASBP-DMRT1, scattered SOX/AMH positive cells are detected throughout the gonad, some in cord-like arrangement (e.g., white arrows). High magnification views of two regions show that most DMRT1-eletroporated female cells are positive for both SOX9 and AMH, although there are several that are only SOX9+ (arrows). Only occasional cells could be detected that were only potentially AMH+ (arrowheads).
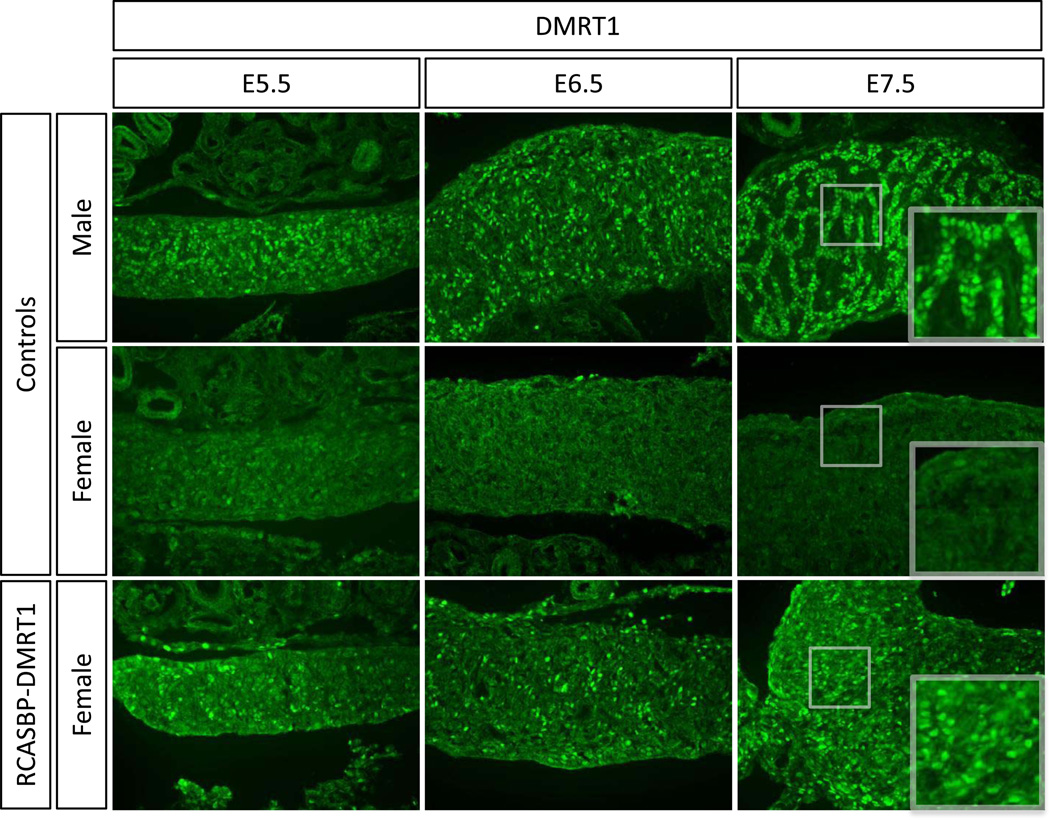
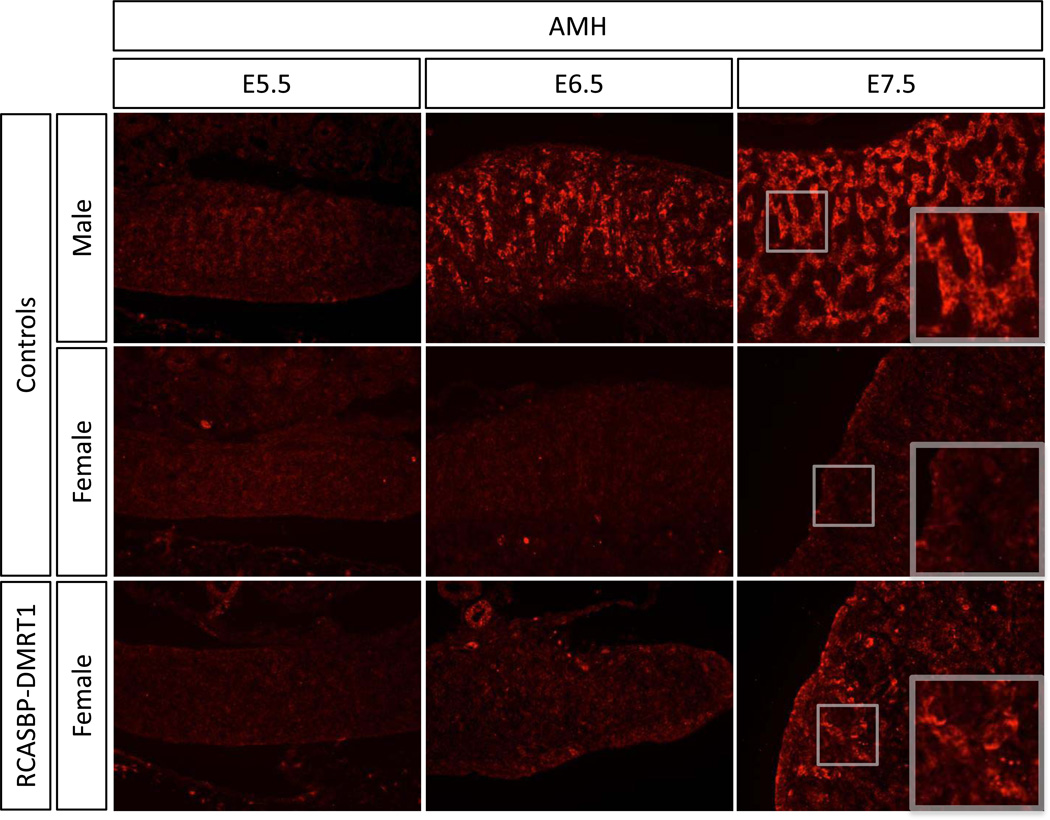
Because the analysis of E7.5 female gonads showed a clear induction of SOX9 and AMH proteins, a time course analysis was carried out on the expression of these genes, as well as the novel testis factor HEMGN and the key ovarian factor aromatase, in the context of DMRT1 over-expression. Embryos were electroporated with RCASBP-DMRT1 at E2.3 and analysed by immunostaining at E5.5, E6.5 and E.7.5 (HH stages 28, 30 and 32). Analysis of DMRT1 expression showed clear over-expression of this protein in female gonads at all embryonic stages tested from E5.5 to E7.5 compared to the control female (Fig. 6). Hence, electroporation at E2.3 can lead to detectable DMRT1 over-expression from at least as early as E5.5, prior to the onset of morphological sex differentiation.
Fig. 6.
Timecourse of DMRT1 over-expression in developing female gonads. Immunostaining of DMRT1 (green) on E5.5, E6.5 and E7.5 female gonads electroporated with RCASBP-DMRT1 (magnification 20×). Over-expression of DMRT1 is evident from E4.5 onwards in female gonads infected with RCASBP-DMRT1, relative to nonelectroporated male and female controls.
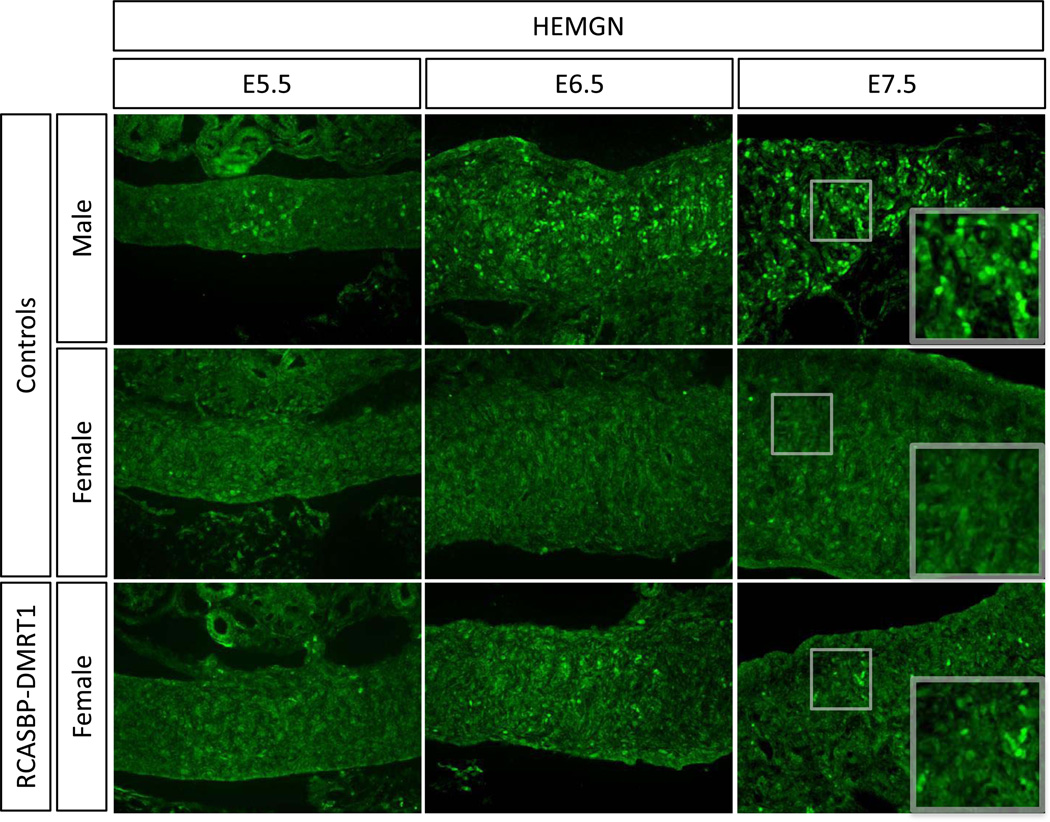
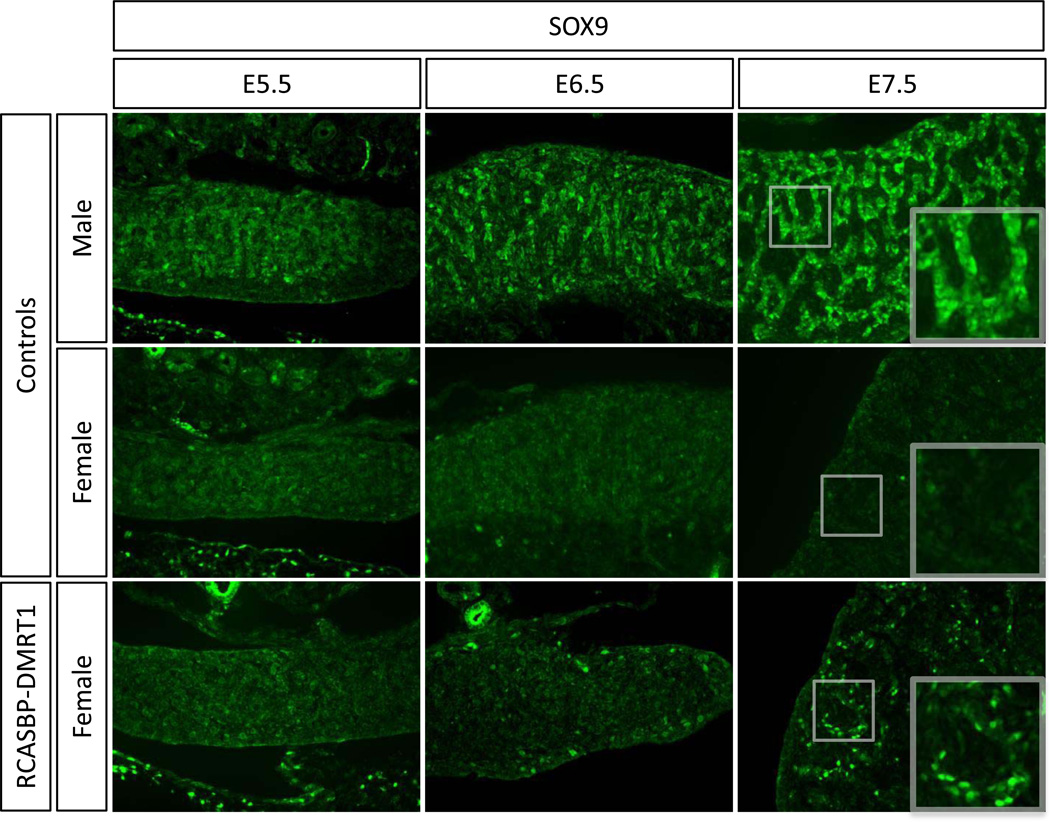
To define the relative timing of male gene expression induced by DMRT1 over-expression in female gonads, we examined HEMGN, SOX9 and AMH. The first appearance of HEMGN protein in male gonads occurred at E.5.5 (Fig. 7). In female gonads over-expressing DMRT1, HEMGN expression was activated in localised patches, detectable from E6.5 (Fig. 7). Like HEMGN, the appearance of SOX9 positive cells occurred from E6.5 onwards in DMRT1 overexpressing female gonads (Fig. 8). Immunostaining for AMH showed that this protein was also induced in E6.5 RCASBP-DMRT1 infected female gonads, however the levels were very low and it was not until E7.5 that more robust expression was detectable (Fig. 9). Altogether, the induction of male pathway genes in genetic female gonads was delayed by approximately one day (two stages) relative to their normal expression in control males.
Fig. 7.
Timecourse of the effect of DMRT1 over-expression on Hemogen expression in developing female gonads. Immunostaining of HEMGN (green) on E5.5, E6.5 and E7.5 female gonads electroporated with RCASBP-DMRT1 (magnification 20×). Induction of some hemogen expression is evident in E6.5 and E7.5 female gonads infected with RCASBP-DMRT1, relative to controls males, in which HEMGN is expressed, and control females, in which no HEMGN protein can be detected.
Fig. 8.
Timecourse of the effect of DMRT1 over-expression on SOX9 expression in developing female gonads. Immunostaining of SOX9 (green) on E5.5, E6.5 and E7.5 female gonads electroporated with RCASBP-DMRT1 (magnification 20×). Induction of SOX9 expression is evident in E6.5 and E7.5 female gonads infected with RCASBP-DMRT1.
Fig. 9.
Timecourse of the effect of DMRT1 over-expression on AMH expression in developing female gonads. Immunostaining of AMH (red) on E5.5, E6.5 and E7.5 female gonads electroporated with RCASBP-DMRT1 (magnification 20×). Induction of AMH expression is evident in E7.5 female gonads infected with RCASBP-DMRT1, relative to nonelectroporated control male and female left gonads.
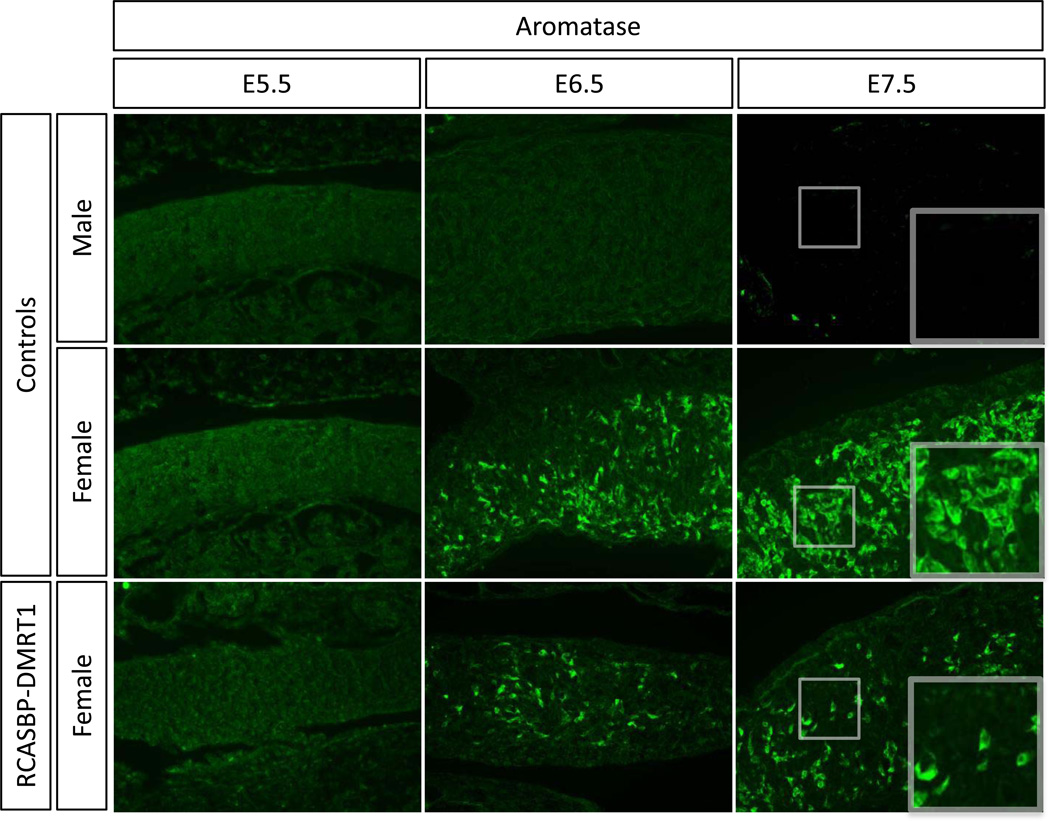
Aromatase enzyme catalyses estrogen synthesis and is essential for ovarian development in chicken. Aromatase is normally only expressed in female and not male embryonic gonads. Aromatase mRNA and protein are expressed from E6.0–6.5 (Stage 29–30) in female gonads and suppression of DMRT1 by RNAi results in an induction of its expression in developing male gonads (Smith et al. 2009). In RCASBP-DMRT1 infected female gonads the expression of aromatase was analysed by immunostaining (Fig. 10). Aromatase enzyme expression was disrupted in both E6.5 and E7.5 gonads, showing clearly reduced levels of expression compared to the female control (Fig. 10).
Fig. 10.
Timecourse of the effect of DMRT1 over-expression on aromatase expression in developing female gonads. Immunostaining of aromatase (green) on E5.5, E6.5 and E7.5 female gonads electroporated with RCASBP-DMRT1 (magnification 20×). A reduction in aromatase expression is evident in E6.5 and E7.5 female gonads infected with RCASBP-DMRT1 compared to the equivalent control female. Aromatase is not expressed in male gonads.
Discussion
The data presented here show that elevated DMRT1 expression is a very early marker of testicular development in the chicken embryo. In female gonads, over-expression of DMRT1 can induce the male pathway, by up-regulating HEMGN, SOX9 and AMH expression, while antagonising the female gene, aromatase. Taken together with previous studies on DMRT1 knockdown, it is clear that DMRT1 is both necessary for testis development and is also capable of inducing this pathway in the avian model, indicating that it is a key Z-linked testis regulator (Smith et al. 2009).
The chronology of protein expression both in male gonads (Fig. 1) and in female gonads over-expressing DMRT1, indicate that the testicular pathway involves the consecutive expression of DMRT1 protein, followed by HEMGN, SOX9 and then AMH proteins. In male gonads, DMRT1 mRNA and protein are already expressed as early as stage 25 (E4.5), prior to the onset of morphological gonadal sex differentiation, which occurs over stages 29–30 (E6.0–6.5) (Van Limborgh 1968; Carlon and Stahl 1985). HEMGN is a nuclear protein involved in proliferation and differentiation of hematopoietic cells and recently implicated in chicken (but not mammalian) testicular development (Yang et al. 2001; Nakata et al. 2013). In the chicken embryo, we found that HEMGN mRNA is first detectable at a low level at E4.5 (stage 25), but the protein is first detectable from E5.5 (stage 28; Fig. 1). Both DMRT1 and HEMGN are expressed in the medullary cord cells of male embryos, within the pre-Sertoli cell lineage. Pre-Sertoli cells are the first cell type to differentiate in the developing testis, and they are thought to send organising signals to other nascent cell types. Hence, this lineage of cells is responsible for directing testis formation. Over-expression of DMRT1 in female gonads caused localised ectopic expression of HEMGN, where it is not normally detected (Fig. 7, and (Nakata et al. 2013)). This indicates that DMRT1 can activate hemogen, either directly or indirectly.
However, comparing adjacent tissue sections of E5.5 and E7.5 gonads, it is clear that DMRT1 is strongly expressed in essentially all pre-Sertoli cells, while HEMGN expression is variable within pre-Sertoli cells (Fig. 2). This heterogeneity in expression level is unlikely to be controlled solely by DMRT1, which is robustly expressed throughout. The early expression of DMRT1 protein at E4.5, at least one day prior to HEMGN at E5.5, also points to other intervening factors also being involved in HEMGN regulation. Nakata et al. (2013) reported that over-expression of HEMGN caused up-regulation of DMRT1 mRNA in female (ZW) gonads, implying a positive regulatory feedback loop between these two male factors. Both DMRT1 and HEMGN are located on the Z sex chromosome, and are therefore candidate master regulators. The earlier onset of DMRT1 expression, together its ectopic activation of HEMGN, suggests that DMRT1 lies upstream of HEMGN in the male pathway. However, this awaits confirmation through the comparative analysis of DMRT1 versus HEMGN knockdown gonads.
Over-expression of DMRT1 in female gonads also induced SOX9 and AMH protein expression, both hallmarks of testis development. In the chicken embryo, SOX9 is normally expressed only in male gonads (Kent et al. 1996; Smith et al. 1999b) where it is presumed to have the same essential role in testis differentiation as it has in mammals. Activation of SOX9 in male gonads is therefore a critical step in the initiation of testis development. Ectopic expression of SOX9 following DMRT1 over-expression provides a functional link between these two genes. However, this is likely to be indirect, given that endogenous DMRT1 protein expression is detected in E4.5 (HH stage 25) gonads, at least 1.5 days and four developmental stages before SOX9 protein induction at day 6.0 (stage 29) in male embryos (Fig. 1, and see also (Smith et al. 2005)). Previous studies have identified a putative DMRT consensus binding site within the enhancer of SOX9, conserved across tetrapods, including chicken (Bagheri-Fam et al. 2010). However, this site was not be stimulated by chicken DMRT1 in vitro (C. Smith, unpubl.). Alternatively, there is evidence that HEMGN may activate SOX9. HEMGN protein expression begins within the appropriate developmental window, after DMRT1 and before SOX9, while over-expression of HEMGN in female gonads induces SOX9 transcription (Nakata et al. 2013).
AMH (Anti-Mullerian Hormone) is a key marker of testis development, being synthesised and secreted from Sertoli cells at the time of sexual differentiation. A number of previous studies have shown that, unlike in mammals, the onset of AMH mRNA expression is initiated before SOX9 mRNA expression in the male chicken gonad (Oreal et al. 1998; Smith et al. 1999b). Furthermore, low level AMH mRNA is also expressed in female gonads, despite the absence of SOX9 expression (Oreal et al. 2002). These data indicate that SOX9 does not activate AMH gene transcription (although it could up-regulate mRNA expression later). In the study described here, we first detected AMH protein at stage 29/30 (day 6.5), well after AMH mRNA is first detected (E4.5) (Smith et al. 1999b) but immediately after the first appearance of SOX9 protein (E6–6.5, see Figure 1; and see also (Smith et al. 2005). Furthermore, over-expression of DMRT1 in female gonads caused activation of SOX9 protein followed by AMH protein. Double staining showed that this occurred in the same cells (see Fig. 5, high magnification images). We could never detect any endogenous AMH protein in female gonads by immunofluorescence. It is possible that the antibody used here was not sensitive enough to detected low-level AMH protein in females or in male gonads earlier than E6.5, although this seems unlikely. Therefore, considering both the mRNA and protein data, it appears that AMH mRNA is transcribed but not translated in the early gonads until SOX9 protein facilitates its translation. The early activator of AMH mRNA expression remains unclear, but DMRT1 is a good candidate, given that both mRNAs are expressed in very early gonads of both sexes, with expression higher in males. In summary, the overexpression data suggest that DMRT1 induces SOX9 protein, which then induces AMH. This may be mediated indirectly via HEMGN. However, this induction in female gonads was delayed relative to the normal temporal expression patterns seen in males. This may reflect the fact that a sufficient level of DMRT1 over-expression must be achieved to induce HEMGN, SOX9 and AMH, which points to a dosage effect.
In addition to facilitating the male pathway, elevated DMRT1 expression acts to repress the female pathway, as assessed by aromatase expression (Fig. 10). Aromatase enzyme catalyses estrogen synthesis from androgenic substrates, and is required for ovarian development in birds (Elbrecht and Smith 1992; Vaillant et al. 2001). Accordingly, aromatase protein is only expressed in female embryonic chicken gonads, from E6.0 (stage 29), the onset of morphological sexual differentiation (Smith et al. 1997; Nomura et al. 1999; Nishikimi et al. 2000; Smith et al. 2005). We have recently reported that the over-expression of the aromatase gene alone is sufficient to induce complete ovarian development in genetically male (ZZ) chicken embryos (Lambeth et al. 2013). DMRT1 knockdown in male gonads causes robust and widespread activation of aromatase expression and feminisation (Smith et al. 2009). This indicates that DMRT1 acts to repress aromatase during testicular development. The results obtained here are consistent with this idea, and in agreement with studies showing a direct link between the two genes in mouse gonads (Matson et al., 2011). Over-expression of DMRT1 in female gonads caused localised reduction in aromatase enzyme expression (Fig. 10). This effect may be direct or indirect, possibly via inhibition of the putative regulator of aromatase, FOXL2 (Govoroun et al. 2004). We did not examine FOXL2 expression, but this is worth testing. There is a mutually antagonistic relationship between DMRT1 and aromatase in the avian embryo. Inhibition of aromatase with the drug fadrozole causes masculinisation of female chicken gonads, characterised by up-regulation of DMRT1 expression and seminiferous cord organisation (Smith et al. 2003).
The exact mechanism underlying avian gonadal sex differentiation is still not completely understood. Although the W chromosome may harbour a dominant acting ovary determinant, no clear candidate gene has emerged, despite our recent thorough RNA-seq analysis of early gonads (Ayers et al. 2013). More likely is a molecular mechanism based on the lack of global Z gene dosage compensation in birds (Clinton et al. 2012). Under this model, gonadal differentiation is controlled by a Z-linked gene/s that escapes dosage compensation and is more highly expressed in males. DMRT1 is such a gene. Indeed, the results described here support the proposal that DMRT1 expression dosage is important. In normal embryos, DMRT1 is expressed in the medullary cord cells of both sexes, but always more highly in males (Fig. 1; see also (Smith et al. 1999b)). Here, we used in ovo electroporation of viral vectors to deliver exogenous DMRT1 expression into female gonads. This resulted in variable over-expression in female gonads, to a level higher than endogenous DMRT1 expression in females but generally lower, or less widespread, than in normal males (Figures 3 and 4.). Electroporation typically yields variable expression of exogenous genes in embryonic chicken tissues, although the RCASBP viral vector used here has the ability to spread horizontally to neighbouring cells following initial transfection. Within female gonads over-expressing DMRT1, areas of higher exogenous DMRT1 delivery correlated with activation of SOX9 and AMH protein. This supports the idea that a threshold level of DMRT1 may be required for activation of male pathway genes. This would be achieved in normal males (ZZ) but not in females (ZW). This could be tested by using a quantitative measure of DMRT1 over-expression in female gonads (qRT-PCR). This was not possible here, because delivery of DMRT1 was always variable, from minor to extensive, and whole gonad PCR of pooled tissues would lack the required sensitivity. Rather, immunofluorescence on serial longitudinal sections through the gonad produced the most informative data, especially with regard to the regional co-localisation of gene expression.
In summary, this study shows that DMRT1 can induce male pathway genes and inhibit female genes in the embryonic chicken gonad. Previous work has shown that DMRT1 is necessary for activation of the male pathway in the chicken embryo. The current study extends this finding by showing that this gene is also able to induce important male pathway genes. Being Z-linked and not subject to dosage compensation, DMRT1 acts as a dose-dependent regulator of avian gonadal sex differentiation.
Supplementary Material
Highlights.
DMRT1 is male up-regulated in embryonic chicken gonads before HEMGN, SOX9 and AMH
Over-expression of DMRT1 in female gonads induces HEMGN, SOX9 and AMH expression.
Expression of Aromatase protein is retarded in female gonads over-expressing DMRT1
DMRT1 can activate the male pathway and antagonize the female pathway in chicken
Acknowledgments
The authors thank Dr. Olivier Serralbo (Australian Regenerative Medicine Institute, Melbourne) for demonstrating the in ovo electroporation technique. LL is supported by the Poultry CRC for Sex Determination. CAS is funded through an Australian Research Council (ARC) Future Fellowship, while KR is funded through an ARC Discovery Project Grant. This research was conducted within the Poultry CRC, established and supported under the Australian Government's Cooperative Research Centres Program and the University of Minnesota Medical School, supported by the NIH (grant GM59152 to DZ) and the Minnesota Medical Foundation. The Murdoch Childrens Research Institute (MCRI) is supported by the Victorian Government's Operational Infrastructure Support Program.
Abbreviations
- PFA/PBS
Paraformaldehyde in PBS
- PCR
Polymerase Chain Reaction
- RCASBP/A
Replication Competent Avian Rous Sarcoma virus, Bryan titre Polymerase, strain A
- DMRT1
Drosophila Doublesex and C. elegans mab-3 Related transcription factor, #1
- SOX9
SRY-like box, number 9
- FOXL2
Forkhead box, subfamily L, number 2
- HEMGN
Hemogen
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aoyama S, Shibata K, Tokunaga S, Takase M, Matsui K, Nakamura M. Expression of Dmrt1 protein in developing and in sex-reversed gonads of amphibians. Cytogenet Genome Res. 2003;101:295–301. doi: 10.1159/000074352. [DOI] [PubMed] [Google Scholar]
- Arnold AP, Itoh Y, Melamed E. A bird's-eye view of sex chromosome dosage compensation. Annu Rev Genomics Hum Genet. 2008;9:109–127. doi: 10.1146/annurev.genom.9.081307.164220. [DOI] [PubMed] [Google Scholar]
- Ayers KL, Davidson NM, Demiya D, Roeszler KN, Grutzner F, Sinclair AH, Oshlack A, Smith CA. RNA sequencing reveals sexually dimorphic gene expression before gonadal differentiation in chicken embryos and allows comprehensive annotation of W-chromosome genes. Genome Biology. 2013 doi: 10.1186/gb-2013-14-3-r26. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagheri-Fam S, Sinclair AH, Koopman P, Harley VR. Conserved regulatory modules in the Sox9 testis-specific enhancer predict roles for SOX, TCF/LEF, Forkhead, DMRT, and GATA proteins in vertebrate sex determination. Int J Biochem Cell Biol. 2010;42:472–477. doi: 10.1016/j.biocel.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Bennett CP, Docherty Z, Robb SA, Ramani P, Hawkins JR, Grant D. Deletion 9p and sex reversal. J Med Genet. 1993;30:518–520. doi: 10.1136/jmg.30.6.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burtis KC, Baker BS. Drosophila doublesex gene controls somatic sexual differentiation by producing alternatively spliced mRNAs encoding related sex-specific polypeptides. Cell. 1989;56:997–1010. doi: 10.1016/0092-8674(89)90633-8. [DOI] [PubMed] [Google Scholar]
- Carlon N, Stahl A. Origin of the somatic components in chick embryonic gonads. Arch Anat Microsc Morphol Exp. 1985;74:52–59. [PubMed] [Google Scholar]
- Clinton M, Haines L, Belloir B, McBride D. Sexing chick embryos: a rapid and simple protocol. Br Poult Sci. 2001;42:134–138. doi: 10.1080/713655025. [DOI] [PubMed] [Google Scholar]
- Clinton M, Zhao D, Nandi S, McBride D. Evidence for avian cell autonomous sex identity (CASI) and implications for the sex-determination process? Chromosome Res. 2012;20:177–190. doi: 10.1007/s10577-011-9257-9. [DOI] [PubMed] [Google Scholar]
- De Grandi A, Calvari V, Bertini V, Bulfone A, Peverali G, Camerino G, Borsani G, Guioli S. The expression pattern of a mouse doublesex-related gene is consistent with a role in gonadal differentiation. Mech Dev. 2000;90:323–326. doi: 10.1016/s0925-4773(99)00282-8. [DOI] [PubMed] [Google Scholar]
- DeFalco T, Capel B. Gonad morphogenesis in vertebrates: divergent means to a convergent end. Annu Rev Cell Dev Biol. 2009;25:457–482. doi: 10.1146/annurev.cellbio.042308.13350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbrecht A, Smith RG. Aromatase enzyme activity and sex determination in chickens. Science. 1992;255:467–470. doi: 10.1126/science.1734525. [DOI] [PubMed] [Google Scholar]
- Fekete DM, Cepko CL. Replication-competent retroviral vectors encoding alkaline phosphatase reveal spatial restriction of viral gene expression/transduction in the chick embryo. Mol Cell Biol. 1993;13:2604–2613. doi: 10.1128/mcb.13.4.2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govoroun MS, Pannetier M, Pailhoux E, Cocquet J, Brillard J-P, Couty I, Batellier F, Cotinot C. Isolation of chicken homolog of the FOXL2 gene and comparison of its expression patterns with those of aromatase during ovarian development. Dev Dyn. 2004;231:859–870. doi: 10.1002/dvdy.20189. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. Dev Dyn. 1992;195:231–272. doi: 10.1002/aja.1001950404. 1951. [DOI] [PubMed] [Google Scholar]
- Hattori RS, Murai Y, Oura M, Masuda S, Majhi SK, Sakamoto T, Fernandino JI, Somoza GM, Yokota M, Strussmann CA. A Y-linked anti-Mullerian hormone duplication takes over a critical role in sex determination. Proc Natl Acad Sci USA. 2012;109:2955–2959. doi: 10.1073/pnas.1018392109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh Y, Melamed E, Yang X, Kampf K, Wang S, Yehya N, Van Nas A, Replogle K, Band MR, Clayton DF, et al. Dosage compensation is less effective in birds than in mammals. J Biol. 2007;6:2. doi: 10.1186/jbiol53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanetsky PA, Mitra N, Vardhanabhuti S, Vaughn DJ, Li M, Ciosek SL, Letrero R, D'Andrea K, Vaddi M, Doody DR, et al. A second independent locus within DMRT1 is associated with testicular germ cell tumor susceptibility. Human Molecular Genetics. 2011;20:3109–3117. doi: 10.1093/hmg/ddr207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent J, Wheatley SC, Andrews JE, Sinclair AH, Koopman P. A male-specific role for SOX9 in vertebrate sex determination. Development. 1996;122:2813–2822. doi: 10.1242/dev.122.9.2813. [DOI] [PubMed] [Google Scholar]
- Kettlewell JR, Raymond CS, Zarkower D. Temperature-dependent expression of turtle Dmrt1 prior to sexual differentiation. genesis. 2000;26:174–178. [PubMed] [Google Scholar]
- Kim S, Bardwell VJ, Zarkower D. Cell type-autonomous and non-autonomous requirements for Dmrt1 in postnatal testis differentiation. Dev Biol. 2007;307:314–327. doi: 10.1016/j.ydbio.2007.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Kettlewell JR, Anderson RC, Bardwell VJ, Zarkower D. Sexually dimorphic expression of multiple doublesex-related genes in the embryonic mouse gonad. Gene Expr Patterns. 2003;3:77–82. doi: 10.1016/s1567-133x(02)00071-6. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Matsuda M, Kajiura-Kobayashi H, Suzuki A, Saito N, Nakamoto M, Shibata N, Nagahama Y. Two DM domain genes, DMY and DMRT1, involved in testicular differentiation and development in the medaka, Oryzias latipes. Dev Dyn. 2004;231:518–526. doi: 10.1002/dvdy.20158. [DOI] [PubMed] [Google Scholar]
- Koopman P, Gubbay J, Vivian N, Goodfellow P, Lovell-Badge R. Male development of chromosomally female mice transgenic for Sry. Nature. 1991;351:117–121. doi: 10.1038/351117a0. [DOI] [PubMed] [Google Scholar]
- Krentz AD, Murphy MW, Kim S, Cook MS, Capel B, Zhu R, Matin A, Sarver AL, Parker KL, Griswold MD, et al. The DM domain protein DMRT1 is a dose-sensitive regulator of fetal germ cell proliferation and pluripotency. Proc Natl Acad Sci USA. 2009;106:22323–22328. doi: 10.1073/pnas.0905431106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambeth LS, Cummins DM, Doran TJ, Sinclair AH, Smith CA. Schubert M, editor. Over-expression of Aromatase Alone is Sufficient for Ovarian Development in Genetically Male Chicken Embryos. PLoS ONE. 2013;8:e68362. doi: 10.1371/journal.pone.0068362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan M, Tabin C. Targeted gene misexpression in chick limb buds using avian replication-competent retroviruses. Methods. 1998;14:407–420. doi: 10.1006/meth.1998.0595. [DOI] [PubMed] [Google Scholar]
- Marchand O, Govoroun M, D'Cotta H, McMeel O, Lareyre JJ, Bernot A, Laudet V, Guiguen Y. DMRT1 expression during gonadal differentiation and spermatogenesis in the rainbow trout, Oncorhynchus mykiss. Biochim Biophys Acta. 2000;1493:180–187. doi: 10.1016/s0167-4781(00)00186-x. [DOI] [PubMed] [Google Scholar]
- Masuyama H, Yamada M, Kamei Y, Fujiwara-Ishikawa T, Todo T, Nagahama Y, Matsuda M. Dmrt1 mutation causes a male-to-female sex reversal after the sex determination by Dmy in the medaka. Chromosome Res. 2012;20:163–176. doi: 10.1007/s10577-011-9264-x. [DOI] [PubMed] [Google Scholar]
- Matson CK, Murphy MW, Griswold MD, Yoshida S, Bardwell VJ, Zarkower D. The mammalian doublesex homolog DMRT1 is a transcriptional gatekeeper that controls the mitosis versus meiosis decision in male germ cells. Dev Cell. 2010;19:612–624. doi: 10.1016/j.devcel.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson CK, Murphy MW, Sarver AL, Griswold MD, Bardwell VJ, Zarkower D. DMRT1 prevents female reprogramming in the postnatal mammalian testis. Nature. 2011;476:101–104. doi: 10.1038/nature10239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson CK, Zarkower D. Sex and the singular DM domain: insights into sexual regulation, evolution and plasticity. Nat Rev Genet. 2012;13:163–174. doi: 10.1038/nrg3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda M, Nagahama Y, Shinomiya A, Sato T, Matsuda C, Kobayashi T, Morrey CE, Shibata N, Asakawa S, Shimizu N, et al. DMY is a Y-specific DM-domain gene required for male development in the medaka fish. Nature. 2002;417:559–563. doi: 10.1038/nature751. [DOI] [PubMed] [Google Scholar]
- Matsuda M, Shinomiya A, Kinoshita M, Suzuki A, Kobayashi T, Paul-Prasanth B, Lau E-L, Hamaguchi S, Sakaizumi M, Nagahama Y. DMY gene induces male development in genetically female (XX) medaka fish. Proc Natl Acad Sci USA. 2007;104:3865–3870. doi: 10.1073/pnas.0611707104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moniot B, Berta P, Scherer G, Sudbeck P, Poulat F. Male specific expression suggests role of DMRT1 in human sex determination. Mech Dev. 2000;91:323–325. doi: 10.1016/s0925-4773(99)00267-1. [DOI] [PubMed] [Google Scholar]
- Nakata T, Ishiguro M, Aduma N, Izumi H, Kuroiwa A. Chicken hemogen homolog is involved in the chicken-specific sex-determining mechanism. Proceedings of the National Academy of Sciences. 2013 doi: 10.1073/pnas.1218714110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanda I, Kondo M, Hornung U, Asakawa S, Winkler C, Shimizu A, Shan Z, Haaf T, Shimizu N, Shima A, et al. A duplicated copy of DMRT1 in the sex-determining region of the Y chromosome of the medaka, Oryzias latipes. Proc Natl Acad Sci USA. 2002;99:11778–11783. doi: 10.1073/pnas.182314699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikimi H, Kansaku N, Saito N, Usami M, Ohno Y, Shimada K. Sex differentiation and mRNA expression of P450c17, P450arom and AMH in gonads of the chicken. Mol Reprod Dev. 2000;55:20–30. doi: 10.1002/(SICI)1098-2795(200001)55:1<20::AID-MRD4>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Nomura O, Nakabayashi O, Nishimori K, Yasue H, Mizuno S. Expression of five steroidogenic genes including aromatase gene at early developmental stages of chicken male and female embryos. J Steroid Biochem Mol Biol. 1999;71:103–109. doi: 10.1016/s0960-0760(99)00127-2. [DOI] [PubMed] [Google Scholar]
- Oreal E, Mazaud S, Picard J-Y, Magre S, Carre-Eusebe D. Different patterns of anti- Mullerian hormone expression, as related to DMRT1, SF-1, WT1, GATA-4, Wnt-4, and Lhx9 expression, in the chick differentiating gonads. Dev Dyn. 2002;225:221–232. doi: 10.1002/dvdy.10153. [DOI] [PubMed] [Google Scholar]
- Oreal E, Pieau C, Mattei MG, Josso N, Picard JY, Carre-Eusebe D, Magre S. Early expression of AMH in chicken embryonic gonads precedes testicular SOX9 expression. Dev Dyn. 1998;212:522–532. doi: 10.1002/(SICI)1097-0177(199808)212:4<522::AID-AJA5>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Raymond CS, Kettlewell JR, Hirsch B, Bardwell VJ, Zarkower D. Expression of Dmrt1 in the genital ridge of mouse and chicken embryos suggests a role in vertebrate sexual development. Dev Biol. 1999;215:208–220. doi: 10.1006/dbio.1999.9461. [DOI] [PubMed] [Google Scholar]
- Raymond CS, Murphy MW, O'Sullivan MG, Bardwell VJ, Zarkower D. Dmrt1, a gene related to worm and fly sexual regulators, is required for mammalian testis differentiation. Genes Dev. 2000;14:2587–2595. doi: 10.1101/gad.834100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond CS, Shamu CE, Shen MM, Seifert KJ, Hirsch B, Hodgkin J, Zarkower D. Evidence for evolutionary conservation of sex-determining genes. Nature. 1998;391:691–695. doi: 10.1038/35618. [DOI] [PubMed] [Google Scholar]
- Shan Z, Zabel B, Trautmann U, Hillig U, Ottolenghi C, Wan Y, Haaf T. FISH mapping of the sex-reversal region on human chromosome 9p in two XY females and in primates. Eur J Hum Genet. 2000;8:167–173. doi: 10.1038/sj.ejhg.5200431. [DOI] [PubMed] [Google Scholar]
- Smith CA, Andrews JE, Sinclair AH. Gonadal sex differentiation in chicken embryos: expression of estrogen receptor and aromatase genes. J Steroid Biochem Mol Biol. 1997;60:295–302. doi: 10.1016/s0960-0760(96)00196-3. [DOI] [PubMed] [Google Scholar]
- Smith CA, Katz M, Sinclair AH. DMRT1 is upregulated in the gonads during femaleto- male sex reversal in ZW chicken embryos. Biol Reprod. 2003;68:560–570. doi: 10.1095/biolreprod.102.007294. [DOI] [PubMed] [Google Scholar]
- Smith CA, McClive PJ, Hudson Q, Sinclair AH. Male-specific cell migration into the developing gonad is a conserved process involving PDGF signalling. Dev Biol. 2005;284:337–350. doi: 10.1016/j.ydbio.2005.05.030. [DOI] [PubMed] [Google Scholar]
- Smith CA, McClive PJ, Western PS, Reed KJ, Sinclair AH. Conservation of a sexdetermining gene. Nature. 1999a;402:601–602. doi: 10.1038/45130. [DOI] [PubMed] [Google Scholar]
- Smith CA, Roeszler KN, Ohnesorg T, Cummins DM, Farlie PG, Doran TJ, Sinclair AH. The avian Z-linked gene DMRT1 is required for male sex determination in the chicken. Nature. 2009;461:267–271. doi: 10.1038/nature08298. [DOI] [PubMed] [Google Scholar]
- Smith CA, Shoemaker CM, Roeszler KN, Queen J, Crews D, Sinclair AH. Cloning and expression of R-Spondin1 in different vertebrates suggests a conserved role in ovarian development. BMC Dev Biol. 2008;8:72. doi: 10.1186/1471-213X-8-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CA, Smith MJ, Sinclair AH. Gene expression during gonadogenesis in the chicken embryo. Gene. 1999b;234:395–402. doi: 10.1016/s0378-1119(99)00179-1. [DOI] [PubMed] [Google Scholar]
- Vaillant S, Dorizzi M, Pieau C, Richard-Mercier N. Sex reversal and aromatase in chicken. J Exp Zool. 2001;290:727–740. doi: 10.1002/jez.1123. [DOI] [PubMed] [Google Scholar]
- Van Limborgh J. The first sign of sexual differentiation of the gonads in chick embryos. Arch Anat Microsc Morphol Exp. 1968;57:79–90. [PubMed] [Google Scholar]
- Veitia R, Nunes M, Brauner R, Doco-Fenzy M, Joanny-Flinois O, Jaubert F, Lortat-Jacob S, Fellous M, McElreavey K. Deletions of distal 9p associated with 46,XY male to female sex reversal: definition of the breakpoints at 9p23.3-p24.1. Genomics. 1997;41:271–274. doi: 10.1006/geno.1997.4648. [DOI] [PubMed] [Google Scholar]
- Yang LV, Nicholson RH, Kaplan J, Galy A, Li L. Hemogen is a novel nuclear factor specifically expressed in mouse hematopoietic development and its human homologue EDAG maps to chromosome 9q22, a region containing breakpoints of hematological neoplasms. Mech Dev. 2001;104:105–111. doi: 10.1016/s0925-4773(01)00376-8. [DOI] [PubMed] [Google Scholar]
- Yano A, Guyomard R, Nicol B, Jouanno E, Quillet E, Klopp C, Cabau C, Bouchez O, Fostier A, Guiguen Y. An immune-related gene evolved into the master sex-determining gene in rainbow trout, Oncorhynchus mykiss. Curr Biol. 2012;22:1423–1428. doi: 10.1016/j.cub.2012.05.045. [DOI] [PubMed] [Google Scholar]
- Yoshimoto S, Ikeda N, Izutsu Y, Shiba T, Takamatsu N, Ito M. Opposite roles of DMRT1 and its W-linked paralogue, DM-W, in sexual dimorphism of Xenopus laevis: implications of a ZZ/ZW-type sex-determining system. Development. 2010;137:2519–2526. doi: 10.1242/dev.048751. [DOI] [PubMed] [Google Scholar]
- Yoshimoto S, Okada E, Umemoto H, Tamura K, Uno Y, Nishida-Umehara C, Matsuda Y, Takamatsu N, Shiba T, Ito M. A W-linked DM-domain gene, DM-W, participates in primary ovary development in Xenopus laevis. Proc Natl Acad Sci USA. 2008;105:2469–2474. doi: 10.1073/pnas.0712244105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SO, Mathur S, Hattem G, Tassy O, Pourquie O. Sex-dimorphic gene expression and ineffective dosage compensation of Z-linked genes in gastrulating chicken embryos. BMC Genomics. 2010;11:13. doi: 10.1186/1471-2164-11-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.