Abstract
Traumatic brain injury (TBI), either as an isolated injury or in conjunction with other injuries, is an increasingly common occurring event. An estimated 1.7 million injuries occur within the US each year and 10 million people are affected annually worldwide. Indeed, some one-third (30.5%) of all injury-related deaths in the U.S. are associated with TBI, which will soon outstrip many common diseases as the major cause of death and disability. Associated with a high morbidity and mortality, and no specific therapeutic treatment, TBI has become a pressing public health and medical problem. The highest incidence of TBI occurs among young adults (15 to 24 years age) as well as in the elderly (75 years and older) who are particularly vulnerable as injury, often associated with falls, carries an increased mortality and worse functional outcome following lower initial injury severity. Added to this, a new and growing form of TBI, blast injury, associated with the detonation of improvised explosive devices in the war theaters of Iraq and Afghanistan, are inflicting a wave of unique casualties of immediate impact to both military personnel and civilians, for which long-term consequences remain unknown and may potentially be catastrophic. The neuropathology underpinning head injury is becoming increasingly better understood. Depending on severity, TBI induces immediate neuropathological effects that for the mildest form may be transient but with increasing severity cause cumulative neural damage and degeneration. Even with mild TBI, which represents the majority of cases, a broad spectrum of neurological deficits, including cognitive impairments, can manifest that may significantly influence quality of life. In addition, TBI can act as a conduit to longer-term neurodegenerative disorders. Prior studies of glucagon-like peptide-1 (GLP-1) and long-acting GLP-1 receptor agonists have demonstrated neurotrophic/neuroprotective activities across a broad spectrum of cellular and animal models of chronic neurodegenerative (Alzheimer's and Parkinson's diseases) and acute cerebrovascular (stroke) disorders. In line with the commonality in mechanisms underpinning these disorders as well as TBI, the current article reviews this literature and recent studies assessing GLP-1 receptor agonists as a potential treatment strategy for mild to moderate TBI.
Introduction: Traumatic brain injury
Traumatic brain injury (TBI) is a significant cause of disability and death worldwide; but particularly in industrialized countries. Beyond any ensuing physical disabilities and the signature cognitive deficits, predominantly in attention, learning and memory, and higher-order executive functions, TBI is a major conduit for the development of chronic neurodegenerative disorders, especially Alzheimer's disease (AD), Parkinson's disease (PD), amyolateral lateral sclerosis (ALS) and chronic traumatic encephalopathy (CTE) [1]. Within the US alone it is estimated that at least 1.7 million people suffer a TBI event annually, which results in some 235,000 hospitalizations and an excess of 50,000 deaths. Indeed, at least 5.3 million Americans are currently living with a long-term disability associated with a TBI incident [2]. In this regard, the elderly are particularly vulnerable to TBI. Often associated with falls in this increasingly large segment of the population, it carries a higher mortality and worse functional outcome following lower initial injury severity compared to younger adults [3]. Worldwide, the incidence of TBI is approximately 0.5% per year.
TBI is also a much too common occurrence among military forces serving in modern combat operations. Military operations ongoing in Iraq and Afghanistan (Operation Enduring Freedom for the war in Afghanistan, and Operation Iraqi Freedom together with Operation New Dawn - for military operations in Iraq after August 2010) have spanned more than a decade from their initiation in 2001 and have involved more than 2.4 million U.S. and coalition personnel. Bomb explosions, primarily caused by improvised explosive devices, have become ever more frequent in these war theaters, impacting military personnel and increasingly civilians as a consequence of urban terrorist attacks and sectarian violence. Estimates range from approximately 15% [4] to 19.5–22.8% of all returning deployed US troops [5] suffering a blast exposure TBI, with the total number of such injuries estimated as high as 320,000 [4,6]. The vast majority of TBIs experienced by military personnel have been classified as mild injuries, based upon clinical severity [7]. However, many involved likely have repeated injury, where cumulative long-term effects could have particularly serious implications. Emerging evidence suggests possible dose- and frequency-dependent associations between TBI and a risk of neurodegenerative disease; however, a threshold for clinical manifestation remains to be determined and more than likely could be affected by genetic predisposition and environmental factors [8,9].
Some 10% of all concussive TBIs result in significant cognitive and emotional dysfunction, requiring around-the-clock care. The more prevalent mild injuries frequently result in significant long-term effects on an individual's health. TBI can cause meaningful deficiencies across a broad range of brain functions, but mild and moderate TBIs characteristically induce headaches, impairments in sleep, memory, attention and cognitive processes, as well as stress, and affective disorders [10]. Such clinical problems may persist long after the injury occurrence or even permanently. There is a range of similarities and differences in relation to blast exposure TBI, where symptoms include headache, nausea, vomiting, dizziness/balance problems, fatigue, sleep disturbances, together with concentration and cognitive impairments [7,11]. Such symptoms are reported in more than 70% of cases, occur in the absence of overt histological or standard clinical radiological evidence of damage, and are often accompanied by ringing in the ears and sensitivity to light and/or sound. Clearly acutely disabling, particularly in a battlefield environment, such symptoms may resolve or persist for years following initial injury, degrading the subjects’ quality of life and placing an enormous burden on military, veteran and civilian health care systems. Although there are symptomatic treatments to address some of the above-described aspects of mild TBI, the development of effective pharmacological treatments to protect against the injury-induced secondary neurodegeneration that primarily contributes to and largely underpins cognitive dysfunction has been slow and only relatively recently is being evaluated under true battlefield conditions [7].
During recent years, advances in our knowledge of molecular mechanisms that regulate the health and survival of neurons together with an understanding of key pathways induced by TBI that lead to neuronal dysfunction and death 12-14] are being applied to the development of experimental drugs with features potentially beneficial for mild TBI treatment – whether concussive or blast related. Primary brain injury is induced by the immediate insult to the head, likely as a consequence of mechanical forces inducing shearing and compression of neuronal and vascular tissue at the time of impact, and rotational head acceleration. A cascade of pathological events may then follow that leads to further secondary brain injury that takes place from minutes to days following the trauma [15]. Most of the damage apparent in mild injury patients derives from the secondary events of the trauma, which includes brain edema, ischemia, inflammatory responses, free radical generation, elevated excitatory neurotransmitters (e.g., glutamate excitotoxicity), loss/disruption of synaptic connections or altered synaptic physiology and DNA damage [12-16]. This leads to neuronal dysfunction, as manifested by changes in long-term potentiation [17] and dendritic and synaptic loss (personal communication: Dr. Ronald F. Mervis, Center for Aging and Brain Repair, University of South Florida College of Medicine, Tampa, Florida 33612, USA), and when cellular damage is sufficiently profound the pro-apoptotic protein p53 will initiate the process of apoptosis [18-25] – that may then exacerbate inflammatory processes and instigate the development of a self-propagating adverse cycle of events [26-32]. Clinical and experimental research indicates that the hippocampus is particularly vulnerable to secondary damage, underpinning the manifestations of deficits in the hippocampal-dependent functions of learning and memory [33-37]. Such ensuing secondary injury may be amenable to intervention and is worsened by secondary physiological insults. A clear conundrum is that the resulting impairments render the TBI victim less capable of avoiding further potential head injury, thereby increasing the likelihood of repetitive mild TBI to which the brain is yet more vulnerable. Specific risk factors for poor outcome after TBI have been established, some of which recognized at the time of injury, are age, gender, mechanism of injury and presenting signs, whereas others, including hyperglycemia, hypoxia and hypotension, are potential areas for medical intervention [38]. Although prompt and specialist neurocritical care is associated with improved outcome [7,39], to date, no successful drug treatments have been approved for improving patient outcome [38].
Animal models of TBI
Across the clinical spectrum of all TBIs there is significant heterogeneity and thus numerous TBI models have been developed that mimic, to a lesser or greater degree, select aspects of human injury [40]. The classification of human TBI is conventionally based on the presenting symptoms and level of consciousness, routinely using the Glasgow Coma Scale (GCS). Albeit patients may have a similar GCS score, they may have remarkably different radiological appearances (such as contusions, skull fractures, blood-brain barrier disruption, hematoma, axonal injury, etc. – or, indeed, none of these), reiterating that TBI is not a single disorder. Mimicking all aspects of TBI in a single animal model is clearly not feasible, and thus for translational purposes when assessing treatment strategies the use of multiple models would appear prudent. Recent comprehensive reviews of TBI animal models are Marklund & Hillered [40] and Xiong et al [41].
In humans, the form of concussive mild TBI that can occur in traffic accidents, sports injuries and falls can, to a varying degree, be mirrored in a classic weight drop rodent model [42]. In this closed head model, injury is diffuse and occurs throughout the brain, both ipsi- and contralateral to the side of impact. In some TBIs a focal lesion ensues, with the most common in human TBI being a cortical contusion in which destruction of brain tissue with micro hemorrhages can occur [40]. Such contusions frequently arise in frontal and temporal regions, although a contre-coup contusion may occasionally occur within a brain region opposite to initial injury, and such contusions can noticeably expand during early days post-TBI. A widely used model in this context of moderate TBI involves fluid percussion injury where damage is inflicted by a pendulum striking a piston associated with a fluid reservoir in contact with the brain surface via a craniotomy. This generates a fluid pressure pulse to the brain that, depending on craniotomy placement (whether lateral or midline) can result in a local focal cortical contusion and diffuse subcortical neuronal injury to the hippocampus and thalamus [43]. This model can additionally reproduce intracranial hemorrhage, brain swelling and progressive grey matter damage that are classical features of human moderate TBI [43]. A further commonly used more severe contusion TBI model that mimics focal human TBI is the controlled cortical impact model, which generates extensive cortical tissue loss, together with hippocampal and thalamic damage [44]. Additionally, in recent years, interest has grown in the development of blast TBI models, and several have been established in rodents [45-49]. In general, these models of blast injury involve either the use of a compression-driven shock tube to simulate blast effects in a confined laboratory setting or the exposure of animals to a controlled detonation, providing a ‘real world’ model of an improvised explosive device [49].
Potential limitations to currently available animal models are that although there are significant similarities in the neurophysiology between non-human mammals, particularly rodents, and human brain, there are clear differences as well in brain size, complexity and white to grey matter ratio. Whether or not such dissimilarities translate to differences in behavior, neurochemistry and drug responses after TBI is unknown, and thus the importance of assessing more than a single animal model for translational drug studies is reiterated.
The counterbalance between neuronal cell death and survival as a target for TBI therapy
The most widely studied type of programmed cell death in the nervous system, apoptosis [18,24,28,50], is a process regulated by specific cysteine proteases - caspases. Numerous triggers of neuronal apoptosis exist, such as oxidative stress, cell surface receptor engagement (e.g., activation of glutamate receptors or TNF-α receptor engagement), trophic factor insufficiency, DNA damage and accumulation of damaged proteins – all of which have been reported following TBI [51,52]. Several families of proteins and specific biochemical signal-transduction pathways regulate cell death. Cell death signaling can involve plasma membrane death receptors, mitochondrial death proteins, proteases, kinases, and transcription factors. Players in the cell death and cell survival orchestra include the Fas receptor, Bcl-2 and Bax (and their homologues), cytochrome c, caspases, and extracellular signal-regulated protein kinases [18,24,28,50,53]. Other forms require gene activation, RNA synthesis, and protein synthesis. Irrespective of this, many share biochemical cascades that regulate ensuing cell death processes and, thereby, provide targets for intervention.
Counterbalancing this, biochemical cascades can be triggered that activate specific proteins associated with cell survival. For example, numerous growth factors and G-protein coupled receptors activate PI3 kinase, a well-known upstream regulator of cell survival that activates Akt. This has the capacity to phosphorylate a wide variety of substrate proteins, including specific death cascade proteins, such as Bad and caspase 9, to inhibit their ability to induce cell death [54]. It is apparent that Akt promotes cellular survival via a series of distinct pathways that involve the Forkhead family of transcription factors, GSK-3fl, fl-catenin, eIF2B, c-Jun, CREB, Bad, IKK, p53, and JIPs [55]. As apoptosis and opposing cellular survival pathways are finely tuned both within neurons and glial cells and are activated in neurodegenerative diseases and TBI, pharmacological approaches to up or down regulate them provide the opportunity to utilize them as ‘pharmacological tools’ to elucidate the relevance of specific biochemical cascades in TBI and neurodegenerative disorders as well as to test investigational drugs for treatment.
It is, additionally, clear that following any form of brain injury, particularly TBI, new neurons can be generated from stem cells within the subgranular layer of the dentate gyrus and the subventricular zone throughout life [56-61] and some of them are capable of migrating into the granule cell layer of the dentate gyrus [57]. There, they develop granule cell morphology and neuronal markers [62], connect to their target area [63] and become functionally integrated into local circuitry [64], similar to mature cells [65-67]. During TBI, however, there is a loss of neural precursor cells from these neurogenic areas with an accompanying reduction in newly generated neurons [58]. This is associated with learning impairments [68] and an increase in new glial cells [58]. Neural stem cells are exquisitely sensitive to trauma and inflammatory cytokines [69,70], which are known to be elevated in TBI, and to impact their differentiation and survival [58]. The neurogenesis/regenerative process provides an additional intervention strategy to potentially maximize neural stem cell survival and optimize differentiation towards neurons versus glial cells.
Incretin mimetics as a mechanism-based mild TBI treatment strategy to promote neuronal survival
Counterbalancing apoptotic pathways leading to cell death are biochemical cascades that promote cell survival [50,53]. To this end, we and others have been assessing cell survival/neuroprotection consequent to G-protein coupled receptor (GPCR) activation, focusing on the glucagon-like peptide-1 (GLP-1) receptor (R) that is of clinical relevance to type 2 diabetes mellitus (T2DM) and neurodegenerative disorders [71-79]. The incretin GLP-1 (Figure 1) is an endogenous 30-amino acid insulinotropic peptide that controls blood glucose levels via activation of the GLP-1R on pancreatic β-cells [80,81]. GLP-1 derives from the post-translational modification of proglucagon and is secreted from enteroendocrine L cells present throughout the small and large intestine where its basal secretion is rapidly and substantially elevated in response to food intake [81-84]. Interestingly, such cells display a molecular profile that largely overlaps with other gut endocrine cell types and co-express multiple peptide hormones that, depending on their post-translational modification by activities such as prohormone convertase 1/3, can generate a diversity of essential physiological peptides along the length of the gastrointestinal tract [85]. GLP-1 has an array of physiological actions, with its insulinotropic ones being the most notable and well studied [Figure 2]. These are exerted through a distinct GPCR, the GLP-1R that is highly expressed on pancreatic islet β cells. The insulinotropic actions of GLP-1 include glucose-dependent stimulation of insulin secretion and inhibition of glucagon secretion [82]. GLP-1 additionally confers glucose sensitivity to glucose-resistant β cells and acts as a trophic agent, inducing pancreatic β-cell proliferation and neogenesis, as well as inhibiting β-cell apoptosis [80-82]. It is hence a key regulator of β-cell mass that, with its other physiological actions, spurred the development of GLP-1R agonists for treatment of T2DM [80-84]. The biochemical cascades and cellular repertoire responsible for effective GLP-1R signaling within the β-cell to promote GLP-1 mediated trophic actions have been an area of intense study and recently, together with a detailed analysis of the gut-brain GLP-1 axis, have been comprehensively reviewed by Campbell & Drucker [82]. As the insulinotropic actions of GLP-1 and agonists are glucose-dependent, advantageously and unlike other current agents for diabetes treatment, their pharmacological actions are not associated with a high risk of hypoglycemia [80,83,84]. As illustrated in Figure 2, the GLP-1R is also widely expressed in nonislet cells where its activation additionally exerts indirect metabolic actions. Hence there is considerable interest in identifying extrapancreatic actions of GLP-1R activation [86]. GLP-1 is additionally generated within the central nervous system, chiefly in the caudal part of the nucleus of the solitary tract within the brainstem [87-91], from where it can diffuse within brain to induce assorted metabolic, vascular and neuroprotective actions (Figure 2).
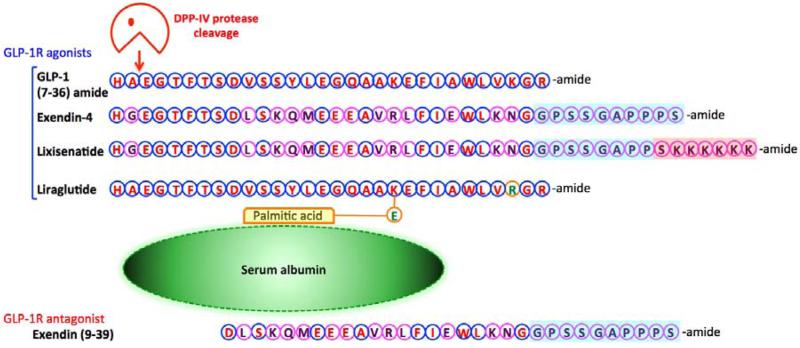
Figure 1.
Amino acid sequence of GLP-1 and that of the long-acting GLP-1 analogs, exendin-4 (Ex-4), lixisenatide and liraglutide. Ex-4 is known clinically as Byetta and Bydureon for subcutaneous (s.c.) twice a day and extended release (once weekly) dosing, respectively. Lixisenatide, known clinically by its trade name Lyxumia, and liraglutide (Victoza) are administered s.c. once daily. Amino acid homology (blue circles) and differences (fuscia circles), in comparison to GLP-1, are highlighted. The peptidase cleavage of GLP-1 by DPP-IV is noted. Of relevance Ex-4 and lixisenatide are close analogues that differ in their tail region. GLP-1 and liraglutide are likewise close analogues, with the latter possessing a C-16 fatty acid (palmitic acid) with a glutamic acid spacer attached to the lysine residue at position 26, permitting its binding to albumin to augment its half-life. By contrast, exendin (9-39) is a widely used pharmacological tool that is an antagonist at the GLP-1R.
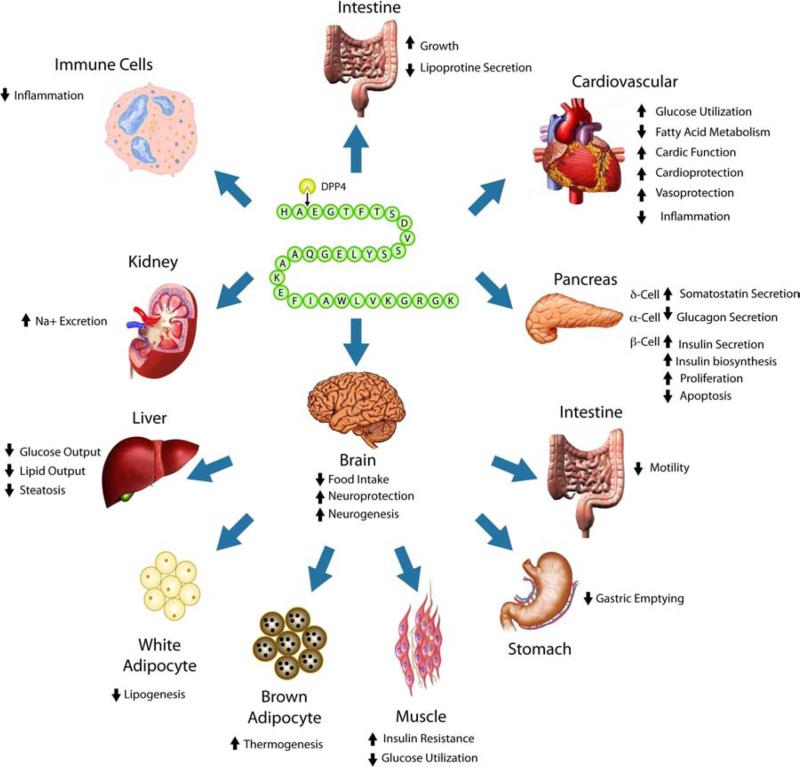
Figure 2.
Direct (upper) and indirect (lower) pharmacological actions of GLP-1R agonists. (Upper) GLP-1 agonists act directly by stimulating the GLP-1R to induce multiple coordinated actions at the level of pancreatic islet cells, within the heart, gastrointestinal tract, on subsets of immune cells and within the brain. (Lower) Within the brain, that GLP-1R agonists appear to freely enter, activation of select GLP-1R signaling pathways instigates biological actions within the gastrointestinal tract, liver and adipose tissue.
Importantly, the activation of GLP-1R signaling cascades within the central and peripheral nervous systems appear to underpin the benefits of GLP-1R agonists described in preclinical animal models of AD, PD, stroke, ALS, Huntington's disease and, more recently TBI (adapted from Campbell and Drucker [82] and Salcedo et al., [75]).
Like most endogenous peptides, however, GLP-1 is short-lived in the circulation following its release [92-94]. It is rapidly degraded by the effective protease dipeptidyl peptidase IV (DPP-IV), rendering it inert at the GLP-1R, and then cleared renally. Elegant studies by Eng and colleagues [95,96] resulted in the discovery and characterization of a naturally occurring DPP-IV resistant GLP-1R agonist, exendin-4 (Ex-4), present within the saliva of a large and heavy-bodied venomous lizard (Gila monster, Heloderma suspectum) that reaches up to 1.25 feet in length and is native to the western and southern foot hills of Arizona through to the Mexican state of Sonora. Purely synthetic forms of Ex-4 as well as more recent GLP-1 and Ex-4 analogues have been generated (Figure 1). These possess substantially longer half-lives than GLP-1, and exenatide (synthetic Ex-4 as a twice daily (Byetta) or once weekly (Bydurion) formulation), liraglutide (Victoza, once daily) and Lixisenatide (Lyxumia, once daily but not yet approved within the US) are now in clinical use as agents for T2DM [80-84].
Although chiefly localized to pancreatic islets [81,82], GLP-1R expression also occurs on neurons throughout the brain and nervous system (primarily localized to dendrites [92]), which GLP-1 and analogues can readily enter after systemic administration [93-96]. Expanding upon our prior interest of GLP-1 and Ex-4 in T2DM, their insulinotropic and antiapoptotic actions [93,94,97,98], the structure-activity relation of their amino acid sequence [99-101], and their translation to clinic [102,75], we hypothesized that GLP-1R stimulation in brain would provide neurotrophic/protective activity as (i) GLP-1 has trophic action on β-cells [103-108], and (ii) the GLP-1R is coupled to the cAMP second messenger pathway, increases in which are well documented to be associated with neuroprotection [109,110] - a function very different from its prior known role in brain in the regulation of food intake and satiety [111,112]. We hence characterized the action of GLP-1 analogs on neuronal cells both in cell culture and animal studies [113-122] demonstrating potent neurotrophic and neuroprotective actions that have been widely confirmed [75-79,123-127].
Our cell culture studies with both immortal PC12 and rat primary hippocampal cells established GLP-1R expression and activation stimulated adenylyl cyclase to elevate intracellular cAMP in a manner similar to pancreatic β-cells [113]. GLP-1 and analogs induced differentiation in PC12 cells in a manner similar to nerve growth factor (NGF), inducing dendrite extension that was reversed by co-incubation with the selective GLP-1R antagonist (Ex 9-39 (Figure 1)). Across a variety of neuronal cell types, coincubation of neurons with GLP-1 and/or Ex-4 enhanced the robustness of their neuronal phenotype, as exemplified by cholinergic cells expressing elevated levels of cholinergic markers (e.g., choline acetyltransferase) [122] and dopaminergic neurons heightened tyrosine hydroxylase activity [118]. GLP-1 analogs enhanced NGF initiated differentiation and rescued degenerating cells from NGF-mediated withdrawal in the absence of cellular dysfunction or toxicity [113]. This indicates that this incretin can provide trophic support in the absence of growth factors, which has been confirmed in other neuronal cell types [122]. The binding affinity of GLP-1 for receptors on hippocampal neurons (EC50 value of 14nM – a concentration apparently achievable [120]) is in the range of its binding to pancreatic β-cells [99,100], and GLP-1 analogs provided complete protection against apoptotic cell death induced by glutamate, amyloid-β (Aβ1-42), hypoxia and a variety of oxidative stressors (e.g., Fe2+, H2O2, 6-hydroxydopamine) [113-115,118,120-122].
Our translational studies and the elegant work of others across numerous independent laboratories have demonstrated that GLP-1R agonists are effective in animal models of (i) AD: reducing levels of amyloid precursor protein (APP), Aβ and tau hyperphosphorylation, augmenting long-term potentiation (LTP), inducing synapogenesis, augmenting neurogenesis, supressing neuroinflammation, reversing brain insulin resistance and improving memory performance [114,115,118,128-140]. (ii) Peripheral neuropathy: promoting neurite outgrowth and inhibiting neuron dysfunction and loss [117,141-144]. (iii) Stroke: reducing stroke volume and neuroinflammation, and augmenting neuronal survival and neurogenesis [118,145-149]. (iv) PD: augmenting dopaminergic cell survival, reducing neuroinflammation, increasing neurogenesis and improving motor coordination [118,150-152], which has recently translated to motor and cognitive improvements in moderately affected PD patients in an open label clinical trial [153]. (v) ALS: promoting motor neuron survival [122,154,155], (vi) Huntington's disease [119] and (vii) across a number of excitotoxic lesions models [114,155]. In defining cellular pathways mediating these neurotrophic/protective actions, thus far our studies implicate participation of PI3-kinase and ERK/MAPK dependent pathways, with additional involvement of protein kinase-A (PKA) signaling [72,121] to divert signaling away from apoptosis towards cell survival. In light of the numerous commonalities existing across these disorders and their occurrence in TBI (neuronal cell death, glutamate excitotoxicity, neuroinflammation, synaptic loss, etc.,) we hypothesized that GLP-1R agonists would be useful to mitigate TBI on two complementary levels: (i) as neuroprotective/neurotrophic agents, and (ii) as anti-hyperglycemic agents – since TBI-induced hyperglycemia is associated with increased mortality and morbidity in humans with moderate to severe injury [38,157-159].
Traumatic brain injury and GLP-1 receptor agonists
Our studies have focused on three complementary rodent models. In one, experimental concussive (weight drop) mild TBI was induced in anesthetized mice using the closed scalp, head trauma device described previously [21,22,25,36,37,42] in which a defined weight (up to 30 g) was dropped through a metal tube of fixed length (80 cm). The opening of the tube was positioned directly over the cushioned animal's head just anterior to the right ear, and the animal held in a manner such that the force of impact to the skull generated anterio-lateral movements without any rotational movements, analogous to that occurring during closed head injury in a car accident. This induced a diffuse injury within the brain in which apoptotic neurons appear throughout the cerebral cortex and hippocampus both ipsi- and contralateral to the injury site [21,22]. Pro- and anti-apoptotic markers (epitomized by p53 and Bcl2, respectively) are up regulated by as little as a 5 g insult when evaluated at 72 h [21,22], with degeneration of neurons and their processes becoming apparent at insults of 15 g within cortical regions and 25 to 30 g in hippocampus and dentate gyrus, as evaluated by silver staining with verification of apoptosis by TUNEL staining [21,22] as well as by Fluoro-Jade B and NeuN immunohistochemistry [25]. Likewise, markers associated with mitochondrial dysfunction and failure, cytochrome-c and Bax, together with markers of inflammation, TNF-α, are found to be elevated within 8 to 72 hours [24,25,160]. Interestingly, when evaluated with a broad neurological battery 1 or 24 hours post-injury, it is not possible to differentiate mild TBI from control (uninjured) mice. However, with the use of selected behavioral paradigms initiated as early as 72 hours (forced swim/Porsolt test [24]) or at 7 and 30 days for learning and memory tests (such as Y-maze and novel object recognition paradigms [161-164]) mild TBI and uninjured animal can readily be differentiated, and such cognitive deficits in spatial and non-spatial tasks have been shown to persist for as long as 90 days in the absence of gross neurological sequelae [42], as occurs in the human condition [1].
The use of the very same mouse strain in a ‘real life’ blast injury mild TBI model [49] allows one to not only assess the potential of experimental treatment strategies but, importantly, to also compare between deficits and associated mechanisms induced by blast and concussive mild TBI. Our blast mild TBI model involves a controlled explosion (500 g TNT) detonated 4 and 7 m from anesthetized mice to expose them to a blast pressure of 5.5 and 2.5 PSI, respectively, in line with human exposure to an improvised explosive device. Similar to our concussive (weight drop) mild TBI model, a diffuse neuronal injury occurs, with axonal and myelin abnormalities, and the development of persistent select cognitive deficits in the absence of alterations in basic neurological assessment or brain gross pathology [49]. Interestingly, whereas both forms of mild TBI are distinctly different regarding the mechanism of trauma induction (blast vs. concussive injury), there are striking similarities in the cognitive and affective status of exposed animals and, similar to humans, the examined indices of cognition and anxiety-like behavior in mice subjected to both forms of TBI were largely alike [165]. Contrasting with these similar mouse behavioral outcomes following either concussive or blast mild TBI, we observed marked differences in gene expression profiles by RNA extracted from intact TBI hippocampal tissue. Although there was a degree of overlap in genes co-regulated by the two distinctly different mechanisms of injury, notably, there were a larger number of genes uniquely expressed in an injury specific manner. Consequently, molecular pathways, particularly associated with ribosomal proteins, the electron transport chain, oxidative stress, inflammation and neurogenesis, displayed highly similar patterns of injury-dependent regulation. However, while these data suggest the involvement of common cascades initiated in response to different types of head injury, there was a divergence in individual gene expression within these pathways, suggesting that the two types of injury (blast vs. concussive mild TBI) are different at the molecular level [165]. Interestingly, pathways associated with AD displayed a markedly different form of regulation depending on the type of TBI [165]. Furthermore, particularly in the blast mild TBI model, changes were evident in other pathways associated with neurological disorders, including the ‘Parkin Pathway’ involving genes associated with PD, as well as genes found to be reduced in major depressive disorders and schizophrenia [165]. This is in line with multiple studies linking TBI to long-term neuropsychiatric/ neurodegenerative disorders as recently reviewed by Walker & Tesco [166].
Remarkably, administration of a clinically translatable dose of Ex-4 to mice with either form of mild TBI (blast or concussive), whether administered prior to or shortly following insult, fully ameliorated the cognitive deficits [161,162] (Figure 3). Importantly, the mild TBI-induced gene expression changes, including genes associated with AD, were largely prevented by Ex-4 both at the pathway analysis level as well as at the individual gene level, as illustrated in Figure 4 for mice exposed to concussive (weight drop) mild TBI. As described, blast mild TBI primarily impacted the very same gene pathways as shown changed for concussive mild TBI, albeit that in large part different individual genes were up and down regulated by blast mild TBI within these pathways [165]. Our studies indicate that Ex-4 likewise ameliorated the majority of these at individual and pathway levels. Hence, taken together, these data suggest a strong beneficial action of Ex-4 in managing secondary events induced by these two very different forms of mild TBI.
Figure 3.
Ex-4 Post-treatment protects against concussive mild TBI (mTBI)-induced cognitive loss, as assessed by novel object recognition at 7 day and 30 day testing post mTBI in two separate groups of mice (a similar protection was provided in a blast-TBI model that closely mimics military personnel trauma). Concussive (30 g weight drop) mTBI induces an impairment in visual memory (red column), as assessed by the novel object recognition paradigm, that was fully ameliorated when Ex-4 was administered (green column) pre-trauma (A (7 days), B (30 days)) and post-trauma (C (7 days), C (30 days)). Mice undergoing cognitive testing initiated on day 7 were euthanized on day 14, and their hippocampus was subjected to gene expression analyses.
The mouse Ex-4 dose was 3.5 pM/kg/min (subcutaneously administered as a steady-state dose), which is 21 ug/kg/day and equivalent to a dose of 1.7 ug/kg/day in a human following normalization of body surface area between mouse and human. This dose compares favorably to once weekly exenatide LAR: 2 mg/week that provides a 60 kg human subject 4.8 ug/kg/day. Significantly impaired behavior compared to sham (uninjured) controls: Fisher's LSD post hoc, *p<0.05, **p<0.01]. Performance of mice was quantitatively assessed 7 and 30 days following mTBI as a preference index, calculated as (time near the new object−time near the old object)/(time near the new object+time near the old object). Values are mean±SEM, [adapted from 161].
Figure 4.
Gene array analyses of the impact of concussive (30 g weight drop) mTBI (versus uninjured controls) on the hippocampus of animals derived from Figure 3, and the ameliorative effects of Ex-4 administration. (A) Pathway analysis: the effects of mild TBI (mTBI) vs. sham on the 10 most down-regulated (green) and up-regulated (red) pathway gene sets in mouse hippocampus are shown (pathway Z-scores are presented). The effects of treatment of mTBI with Ex-4 are shown in the black bars. In these black bars Ex-4 treatment of mTBI induced a change in gene sets relative to the mTBI group. Where no black bar is present, Ex-4 had no effect on gene sets relative to mTBI. In large part, treatment with Ex-4 prevented the down-regulation of the 10 most affected pathways associated with mTBI, while Ex-4 treatment had beneficial effects upon three of 10 up-regulated pathways.
(B) Selecting the ‘Alzheimers disease dn’ pathway as a representative of those whose changes induced by mTBI were ameliorated by Ex-4, the pathway is opened to reveal the individual genes that comprise it (whose gene identities are shown as their gene symbol), presented as a classic heat map: up-regulation (red) and down-regulation (green). Remarkably, across the majority of individual genes mTBI-induced up-regulation is countered by Ex-4 mitigation. APP up-regulation by mTBI is noted, in line with TBI providing a conduit towards AD, which is mitigated by Ex-4. The scale for expression level is shown on the upper right, and the group comparisons are shown at the bottom of each section on the heat map. Adapted from Tweedie et al., [162].
In light of the fact that no single animal TBI model perfectly mimics the human condition [40], additional studies to verify the activity of Ex-4 in moderate TBI were undertaken – specifically, in rat following fluid percussion injury. As the most commonly used rodent model of moderate TBI, fluid percussion injury replicates many of the key cognitive and histological changes evident in human head injury [167,168]. Of the two variations of the model developed, central (i.e., midline) fluid percussion injury was induced in anesthetized animals that produces both focal and diffuse pathology. This generates both primary (a local contusion, edema, and hemorrhage at the gray/white matter interface) and secondary damage (gliosis, inflammation, axonal injury, and delayed cell death) [166,167] and, similar to human TBI, this model adversely impacts hippocampal function and produces long-term cognitive and memory deficits that can last for weeks or months after injury [169,170]. Administration of Ex-4 post injury at a dose comparable to the above described mild TBI mouse studies dramatically mitigated fluid percussion injury-induced cognitive impairments, as assessed in the classical Morris water maze paradigm [171].
Interesting and further supportive evidence for GLP-1R activation as a therapeutic strategy for TBI derives from the elegant studies of Heile and Brinker [172,173], who, together with colleagues, have developed an approach in which encapsulated human mesenchymal stem cells generate and secrete steady-state levels of GLP-1. The implantation of these cells in the lateral ventricle of rats subjected to controlled cortical impact-induced moderate TBI significantly attenuated hippocampal neuronal cell loss as well as cortical glial and neuronal cyto-skeletal abnormalities [172].
Conclusions
In summary activation of the GLP-1R mediated incretin pathway has been demonstrated to be both neurotrophic and neuroprotective across a broad number of cellular and animal neurological models, and to provide functional and behavioral benefits across a wide range of pathophysiological paradigms. These have been seen in independent laboratories, and verified across different animal models of the same disease in the fields of AD, PD, stroke, peripheral neuropathy and, more recently, ALS and TBI (71-79). In large part, commonalities in fundamental biochemical cascades appear to be triggered across diverse acute and chronic neurological disorders that cause neuronal cell populations associated with defined disease to become dysfunctional and undergo apoptosis. The GLP-1 signaling pathway can clearly counter-balance these and, as has been successfully undertaken for GLP-1R activation in pancreatic β-cells, these pathways are now being mapped for the GLP-1R on neurons [72,120]. Not only does the GLP-1R appear to be present across a number of neuronal cell types (e.g., motor neurons, neural stem cells, etc.,) that underlies its diverse actions to provide neurotrophic/protective actions, augment long-term potentiation and synaptic plasticity, and favorably impact neurogenesis, but its appearance on other cells types, for example on multiple immune cell subpopulations [174-176], and glial cells [177] accounts for other positive actions as well, for example suppression of proinflammatory cytokines and reduced microglial activation within the brain. Most likely it is the combination of these actions, rather than any specific and separate one, that supports the current promise of GLP-1R activation as a therapeutic strategy across neurological disorders, including TBI. Maximizing the translational potential of the strategy is clearly crucial, although three GLP-1R agonists are approved and well tolerated in T2DM [80-84] and promising clinical studies in PD [153] support repositioning for neurological disorders. Careful and hypothesis-driven preclinical and clinical studies are warranted [178] to define the optimal neurological use of this promising drug class.
Acknowledgments
NHG, DT and YL are supported by the Intramural Research Program, National Institute on Aging, National Institutes of Health, and in part by the Michael J. Fox Foundation. REB is President of Aristea Translational Medicine Corp., and received no support in relation to this article. CGP, LR and VR are supported by the Sackler School of Medicine, Tel-Aviv University, and in part by a grant from the Israeli science foundation (grant no. 108/09). KS acknowledges support from the Alzheimer's Association (IIRG 10-173-180) and National Institute on Aging (AG022103). DKL acknowledges support from National Institute on Aging grants (AG18379 and AG18884). Jonathan Miller is supported by the Lincoln Scholar Award and Yun-Hsiao Chiang is supported by Taiwan NSC 98-2321-B-038-002-MY3.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Daneshvar DH, Riley DO, Nowinski CJ, McKee AC, Stern RA, Cantu RC. Long-term consequences: effects on normal development profile after concussion. Phys Med Rehabil Clin N Am. 2011;22(4):683–700. doi: 10.1016/j.pmr.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Faul M, Xu L, Wald M, Coronado V. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations and Deaths, 2002-2006. Centers for Disease Control and Prevention, National Ceter for Injury Prevention and Control; Atlanta, GA: 2010. [Google Scholar]
- 3.Susman M, DiRusso SM, Sullivan T, Risucci D, Nealon P, Cuff S, Haider A, Benzil D. Traumatic brain injury in the elderly: increased mortality and worse functional outcome at discharge despite lower injury severity. J Trauma. 2002;53:219–23. doi: 10.1097/00005373-200208000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Hoge CW, McGurk D, Thomas JL, Cox AL, Engel CC, Castro CA. Mild traumatic brain injury in U.S. Soldiers returning from Iraq. N Engl J Med. 2008;358:453–63. doi: 10.1056/NEJMoa072972. [DOI] [PubMed] [Google Scholar]
- 5.Terrio H, Brenner LA, Ivins BJ, Cho JM, Helmick K, et al. Traumatic brain injury screening: preliminary findings in a US Army Brigade Combat Team. J Head Trauma Rehabil. 2009;24:14–23. doi: 10.1097/HTR.0b013e31819581d8. [DOI] [PubMed] [Google Scholar]
- 6.Hosek B. How is deployment to Iraq and Afghanistan affecting U.S. service members and their families? An overview of early RAND research on the topic. RAND Corporation; Santa Monica California, USA: 2011. [PMC free article] [PubMed] [Google Scholar]
- 7.Hoffer ME, Balaban C, Slade MD, Tsao JW, Hoffer B. Amelioration of acute sequelae of blast induced mild traumatic brain injury by N-acetyl cysteine: a double-blind, placebo controlled study. PLoS One. 2013;8(1):e54163. doi: 10.1371/journal.pone.0054163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenfeld JV, McFarlane AC, Bragge P, Armonda RA, Grimes JB, Ling GS. Blast-related traumatic brain injury. Lancet Neurol. 2013;12(9):882–93. doi: 10.1016/S1474-4422(13)70161-3. [DOI] [PubMed] [Google Scholar]
- 9.Duckworth JL, Grimes J, Ling GS. Pathophysiology of battlefield associated traumatic brain injury. Pathophysiology. 2013;20(1):23–30. doi: 10.1016/j.pathophys.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 10.Park E, Bell JD, Baker AJ. Traumatic brain injury: can the consequences be stopped? CMAJ. 2008;178(9):1163–70. doi: 10.1503/cmaj.080282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Multi-National Corps-Iraq policy on mild traumatic brain injury. 2008 May 6; http://www.pdhealth.mil/downloads/mTBI_Policy6may.pdf viewed Sept 10, 2013.
- 12.Maas AIR, Stocchetti N, Bullock R. Moderate and severe traumatic brain injury in adults. Lancet Neurol. 2008;7:728–741. doi: 10.1016/S1474-4422(08)70164-9. [DOI] [PubMed] [Google Scholar]
- 13.Barkhoudarian G, Hovda DA, Giza CC. The molecular pathophysiology of concussive brain injury. Clini Sports Med. 2011;30:33–48. doi: 10.1016/j.csm.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Cornelius C, Crupi R, Calabrese V, Graziano A, Milone P, et al. Traumatic brain injury (TBI): oxidative stress and neuroprotection. Antioxid Redox Signal. 2013 May 10; doi: 10.1089/ars.2012.4981. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 15.Greve MW, Zink BJ. Pathophysiology of traumatic brain injury. Mount Sinai J Med. 2009;76:97–104. doi: 10.1002/msj.20104. [DOI] [PubMed] [Google Scholar]
- 16.Choi DW, Mauluccigedde M, Kriegstein AR. Glutamate neurotoxicity in cortical cell-culture. J Neurosci. 1987;7:357–368. doi: 10.1523/JNEUROSCI.07-02-00357.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldstein LE, Fisher AM, Tagge CA, Zhang XL, Velisek L, Sullivan JA, et al. Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model. Sci Transl Med. 2012;4(134):134ra60. doi: 10.1126/scitranslmed.3003716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raghupathi R. Cell death mechanisms following traumatic brain injury. Brain Pathol. 2004;14(2):215–22. doi: 10.1111/j.1750-3639.2004.tb00056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morrison RS, Kinoshita Y, Johnson MD, Guo W, Garden GA. p53-dependent cell death signaling in neurons. Neurochem Res. 2003;28(1):15–27. doi: 10.1023/a:1021687810103. [DOI] [PubMed] [Google Scholar]
- 20.Hong LZ, Zhao XY, Zhang HL. p53-mediated neuronal cell death in ischemic brain injury. Neurosci Bull. 2010;26(3):232–40. doi: 10.1007/s12264-010-1111-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tashlykov V, Katz Y, Gazit V, Zohar O, Schreiber S, et al. Apoptotic changes in the cortex and hippocampus following minimal brain trauma in mice. Brain Research. 2007;1130:197–205. doi: 10.1016/j.brainres.2006.10.032. [DOI] [PubMed] [Google Scholar]
- 22.Tashlykov V, Katz Y, Volkov A, Gazit V, Schreiber S, et al. Minimal traumatic brain injury induce apoptotic cell death in mice. J Mol Neurosci. 2009;37:16–24. doi: 10.1007/s12031-008-9094-2. [DOI] [PubMed] [Google Scholar]
- 23.Plesnila N, von Baumgarten L, Retiounskaia M, Engel D, Ardeshiri A, et al. Delayed neuronal death after brain trauma involves p53-dependent inhibition of NF-kappa B transcriptional activity. Cell Death Differ. 2007;14:1529–1541. doi: 10.1038/sj.cdd.4402159. [DOI] [PubMed] [Google Scholar]
- 24.Tweedie D, Milman A, Holloway HW, Li Y, Harvey BK, Shen H, et al. Apoptotic and behavioural consequences of mild brain trauma in mice: markers for prospective intervention. J. Neurosci. Res. 2007;85:805–15. doi: 10.1002/jnr.21160. [DOI] [PubMed] [Google Scholar]
- 25.Rachmany L, Tweedie D, Rubovitch V, Yu QS, Li Y, Wang JY, et al. Cognitive impairments accompanying rodent mild traumatic brain injury involve p53-dependent neuronal cell death and are ameliorated by the tetrahydrobenzothiazole PFT-α. PLoS One. 2013;8(11):e79837. doi: 10.1371/journal.pone.0079837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lyeth BG, et al. Prolonged memory impairment in the absence of hippocampal cell death following traumatic brain injury in the rat. Brain Res. 1990;526:249–258. doi: 10.1016/0006-8993(90)91229-a. [DOI] [PubMed] [Google Scholar]
- 27.McIntosh TK, Juhler M, Wieloch T. Novel pharmacologic strategies in the treatment of experimental traumatic brain injury: 1998. J. Neurotrauma. 1998;15:731–769. doi: 10.1089/neu.1998.15.731. [DOI] [PubMed] [Google Scholar]
- 28.Raghupathi R, Graham DI, McIntosh TK. Apoptosis after traumatic brain injury. J. Neurotrauma. 2000;17:927–938. doi: 10.1089/neu.2000.17.927. [DOI] [PubMed] [Google Scholar]
- 29.Frankola KA, Greig NH, Luo W, Tweedie D. Targeting TNF-α to elucidate and ameliorate neuroinflammation in neurodegenerative diseases. CNS Neurol Disord Drug Targets. 2011;10:391–403. doi: 10.2174/187152711794653751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tweedie D, Sambamurti K, Greig NH. New TNF-α inhibitors as drug candidates for neurodegenerative diseases. Curr. Alz. Res. 2007;4:378–385. doi: 10.2174/156720507781788873. [DOI] [PubMed] [Google Scholar]
- 31.McCoy MK, Tansey MG. TNF signaling inhibition in the CNS: implications for normal brain function and neurodegenerative disease. J Neuroinflammation. 2008;5:45. doi: 10.1186/1742-2094-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woodcock T, Morganti-Kossmann MC. The role of markers of inflammation in traumatic brain injury. Front Neurol. 2013;4:18. doi: 10.3389/fneur.2013.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levin HS. Cognitive function outcomes after traumatic brain injury. Curr. Opin. Neurol. 1998;11:643–646. doi: 10.1097/00019052-199812000-00006. [DOI] [PubMed] [Google Scholar]
- 34.Bramlett HM, Green EJ, Dietrich WD. Hippocampally dependent and independent chronic spatial navigational deficits following parasagittal fluid percussion brain injury in the rat. Brain Res. 1997;762:195–202. doi: 10.1016/s0006-8993(97)00387-9. [DOI] [PubMed] [Google Scholar]
- 35.Dixon CE, et al. One-year study of spatial memory performance, brain morphology, and cholinergic markers after moderate controlled cortical impact in rats. J Neurotrauma. 1999;16:109–122. doi: 10.1089/neu.1999.16.109. [DOI] [PubMed] [Google Scholar]
- 36.Zohar O, Schreiber S, Getslev V, Schwartz JP, Mullins PG, et al. Closed-head minimal traumatic brain injury produces long-term cognitive deficits in mice. Neuroscience. 2003;118:949–955. doi: 10.1016/s0306-4522(03)00048-4. [DOI] [PubMed] [Google Scholar]
- 37.Zohar O, Rubovitch V, Milman A, Schreiber S, Pick CG. Behavioral consequences of minimal traumatic brain injury in mice. Acta Neurobiol Exp. 2011;71:36–45. doi: 10.55782/ane-2011-1821. [DOI] [PubMed] [Google Scholar]
- 38.Moppett IK. Traumatic brain injury: assessment, resuscitation and early management. British Journal of Anaesthesia. 2007;99(1):18–31. doi: 10.1093/bja/aem128. [DOI] [PubMed] [Google Scholar]
- 39.Raymont V, Salazar AM, Krueger F, Grafman J. “Studying injured minds” - the Vietnam head injury study and 40-years of brain injury research. Front Neurol. 2011;2:15. doi: 10.3389/fneur.2011.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marklund N, Hillered L. Animal modelling of traumatic brain injury in preclinical drug development: where do we go from here? Br J Pharmacol. 2011;164:1207–29. doi: 10.1111/j.1476-5381.2010.01163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xiong Y, Mahmood A, Chopp M. Animal models of traumatic brain injury. Nat Rev Neurosci. 2013;14(2):128–42. doi: 10.1038/nrn3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Milman A, Rosenberg A, Weizman R, Pick CG. Mild traumatic brain injury induces persistent cognitive deficits and behavioral disturbances in mice. J Neurotrauma. 2005;22:1003–1010. doi: 10.1089/neu.2005.22.1003. [DOI] [PubMed] [Google Scholar]
- 43.Hicks R, Soares H, Smith D, McIntosh T. Temporal and spatial characterization of neuronal injury following lateral fluid-percussion brain injury in the rat. Acta Neuropathol. 1996;91(3):236–46. doi: 10.1007/s004010050421. [DOI] [PubMed] [Google Scholar]
- 44.Saatman KE, Feeko KJ, Pape RL, Raghupathi R. Differential behavioral and histopathological responses to graded cortical impact injury in mice. J Neurotrauma. 2006;23(8):1241–53. doi: 10.1089/neu.2006.23.1241. [DOI] [PubMed] [Google Scholar]
- 45.Cheng J, Gu J, Ma Y, Yang T, Kuang Y, Li B, et al. Development of a rat model for studying blast-induced traumatic brain injury. J Neurol Sci. 2010;294:23–28. doi: 10.1016/j.jns.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 46.Risling M, Plantman S, Angeria M, Rostami E, Bellander BM, Kirkegaard M, et al. Mechanisms of blast induced brain injuries, experimental studies in rats. Neuroimage. 2011;54S1:S89–S97. doi: 10.1016/j.neuroimage.2010.05.031. [DOI] [PubMed] [Google Scholar]
- 47.Readnower RD, Chavko M, Adeeb S, Conroy MD, Pauly JR, McCarron RM, Sullivan PG. Increase in blood-brain barrier permeability, oxidative stress, and activated microglia in a rat model of blast-induced traumatic brain injury. J Neurosci Res. 2010;88(16):3530–9. doi: 10.1002/jnr.22510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huber BR, Meabon JS, Martin TJ, Mourad PD, Bennett R, Kraemer BC, et al. Blast Exposure Causes Early and Persistent Aberrant Phospho- and Cleaved-Tau Expression in a Murine Model of Mild Blast-Induced Traumatic Brain Injury. J Alzheimers Dis. 2013 Jul 3; doi: 10.3233/JAD-130182. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rubovitch V, Ten-Bosch M, Zohar O, Harrison CR, Tempel-Brami C, Stein E, Hoffer BJ, et al. A mouse model of blast-induced mild traumatic brain injury. Exp Neurol. 2011;232:280–289. doi: 10.1016/j.expneurol.2011.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mattson MP, Magnus T. Ageing and neuronal vulnerability. Nat Rev Neurosci. 2006;7(4):278–94. doi: 10.1038/nrn1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Van Den Heuvel C, Thornton E, Vink R. Traumatic brain injury and Alzheimer's disease: a review. Prog Brain Res. 2007;161:303–16. doi: 10.1016/S0079-6123(06)61021-2. [DOI] [PubMed] [Google Scholar]
- 52.Signoretti S, Vagnozzi R, Tavazzi B, Lazzarino G. Biochemical and neurochemical sequelae following mild traumatic brain injury: summary of experimental data and clinical implications. Neurosurg Focus. 2010;29(5):E. doi: 10.3171/2010.9.FOCUS10183. [DOI] [PubMed] [Google Scholar]
- 53.Greig NH, Mattson MP, Perry T, Chan SL, Giordano T, Sambamurti K, et al. New therapeutic strategies and drug candidates for neurodegenerative diseases: p53 and TNF-alpha inhibitors, and GLP-1 receptor agonists. Ann N Y Acad Sci. 2004;1035:290–315. doi: 10.1196/annals.1332.018. [DOI] [PubMed] [Google Scholar]
- 54.Kwon YK. Effect of neurotrophic factors on neuronal stem cell death. J Biochem Mol Biol. 2002;35:87–93. doi: 10.5483/bmbrep.2002.35.1.087. [DOI] [PubMed] [Google Scholar]
- 55.Chong ZZ, Li F, Maiese K. Activating Akt and the brain's resources to drive cellular survival and prevent inflammatory injury. Histol Histopathol. 2005;20:299–315. doi: 10.14670/hh-20.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eriksson PS, et al. Neurogenesis in the adult human hippocampus. Nat. Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 57.Kuhn HG, Dickinson-Anson H, Gage HF. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J. Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rola R, Mizumatsu S, Otsuka S, Morhardt DR, Noble-Haeusslein LJ, Fishman K, et al. Alterations in hippocampal neurogenesis following traumatic brain injury in mice. Exp Neurol. 2006;202(1):189–99. doi: 10.1016/j.expneurol.2006.05.034. [DOI] [PubMed] [Google Scholar]
- 59.Richardson RM, Sun D, Bullock MR. Neurogenesis after traumatic brain injury. Neurosurg Clin N Am. 2007;18(1):169–81. doi: 10.1016/j.nec.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 60.Richardson RM, Singh A, Sun D, Fillmore HL, Dietrich DW, 3rd, Bullock MR. Stem cell biology in traumatic brain injury: effects of injury and strategies for repair. J Neurosurg. 2010;112(5):1125–38. doi: 10.3171/2009.4.JNS081087. [DOI] [PubMed] [Google Scholar]
- 61.Lazarov O, Marr RA. Of mice and men: neurogenesis, cognition and Alzheimer's disease. Front Aging Neurosci. 2013;5:43. doi: 10.3389/fnagi.2013.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cameron HA, Woolley C, McEwen BS, Gould E. Differentiation of newly born neurons and glia in the dentate gyrus of the adult rat. Neuroscience. 1993;56:337–344. doi: 10.1016/0306-4522(93)90335-d. [DOI] [PubMed] [Google Scholar]
- 63.Markakis EA, Gage FH. Adult-generated neurons in the dentate gyrus send axonal projections to field CA3 and are surrounded by synaptic vesicles. J Comp Neurol. 1999;406(4):449–60. [PubMed] [Google Scholar]
- 64.Zhao C, Teng EM, Summers RG, Ming G, Gage FH. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J. Neurosci. 2006;26:3–11. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.van Praag H, Schinder AF, Christie B, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vivar C, Potter MC, Choi J, Lee JY, Stringer TP, Callaway EM, et al. Monosynaptic inputs to new neurons in the dentate gyrus. Nat Commun. 2012;3:1107. doi: 10.1038/ncomms2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lazarov O, Mattson MP, Peterson DA, Pimplikar SW, van Praag H. When neurogenesis encounters aging and disease. Trends Neurosci. 2010;33(12):569–79. doi: 10.1016/j.tins.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shors TJ, Miesegaes G, Beylin A Zhao M, Rydel T, Gould E. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410:372–376. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- 69.Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- 70.Whitney NP, Eidem TM, Peng H, Huang Y, Zheng JC. Inflammation mediates varying effects in neurogenesis: relevance to the pathogenesis of brain injury and neurodegenerative disorders. J Neurochem. 2009;108:1343–59. doi: 10.1111/j.1471-4159.2009.05886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Perry T, Greig NH. The glucagon-like peptides: a new genre in therapeutic targets for intervention in Alzheimer's disease. J Alzheimers Dis. 2002;4(6):487–96. doi: 10.3233/jad-2002-4605. [DOI] [PubMed] [Google Scholar]
- 72.Perry T, Greig NH. The glucagon-like peptides: a double-edged therapeutic sword? Trends Pharmacol Sci. 2003;24(7):377–83. doi: 10.1016/S0165-6147(03)00160-3. [DOI] [PubMed] [Google Scholar]
- 73.Perry T, Greig NH. A new Alzheimer's disease interventive strategy: GLP-1. Curr Drug Targets. 2004;5(6):565–71. doi: 10.2174/1389450043345245. [DOI] [PubMed] [Google Scholar]
- 74.Perry T, Greig NH. Enhancing central nervous system endogenous GLP-1 receptor pathways for intervention in Alzheimer's disease. Curr Alzheimer Res. 2005;2(3):377–85. doi: 10.2174/1567205054367892. [DOI] [PubMed] [Google Scholar]
- 75.Salcedo I, Tweedie D, Li Y, Greig NH. Neuroprotective and neurotrophic actions of glucagon-like peptide-1: an emerging opportunity to treat neurodegenerative and cerebrovascular disorders. Br J Pharmacol. 2012;166(5):1586–99. doi: 10.1111/j.1476-5381.2012.01971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hölscher C. The role of GLP-1 in neuronal activity and neurodegeneration. Vitam Horm. 2010;84:331–54. doi: 10.1016/B978-0-12-381517-0.00013-8. [DOI] [PubMed] [Google Scholar]
- 77.Hölscher C. Potential role of glucagon-like peptide-1 (GLP-1) in neuroprotection. CNS Drugs. 2012;26(10):871–82. doi: 10.2165/11635890-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 78.Mossello E, Ballini E, Boncinelli M, Monami M, Lonetto G, Mello AM, et al. Glucagon-like peptide-1, diabetes, and cognitive decline: possible pathophysiological links and therapeutic opportunities. Exp Diabetes Res. 2011;2011:281674. doi: 10.1155/2011/281674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Duarte AI, Candeias E, Correia SC, Santos RX, Carvalho C, Cardoso S, et al. Crosstalk between diabetes and brain: glucagon-like peptide-1 mimetics as a promising therapy against neurodegeneration. Biochim Biophys Acta. 2013;1832(4):527–41. doi: 10.1016/j.bbadis.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 80.Gallwitz B. Glucagon-like peptide-1 analogues for type 2 diabetes mellitus: current and emerging agents. Drugs. 2011;71:1675–1688. doi: 10.2165/11592810-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 81.Lovshin JA, Drucker DJ. Incretin-based therapies for type 2 diabetes mellitus. Nat Rev Endocrinol. 2009;5:262–269. doi: 10.1038/nrendo.2009.48. [DOI] [PubMed] [Google Scholar]
- 82.Campbell JE, Drucker DJ. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 2013;17(6):819–37. doi: 10.1016/j.cmet.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 83.Drucker DJ. Incretin action in the pancreas: Potential promise, possible perils, and pathological pitfalls. Diabetes. 2013 Jul 1; doi: 10.2337/db13-0822. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Drucker DJ, Sherman SI, Bergenstal RM, Buse JB. The safety of incretin-based therapies--review of the scientific evidence. J Clin Endocrinol Metab. 2011;96(7):2027–31. doi: 10.1210/jc.2011-0599. [DOI] [PubMed] [Google Scholar]
- 85.Habib AM, Richards P, Cairns LS, Rogers GJ, Bannon CA, Parker HE, Morley TC, Yeo GS, Reimann F, Gribble FM. Overlap of endocrine hormone expression in the mouse intestine revealed by transcriptional profiling and flow cytometry. Endocrinology. 2012;153:3054–3065. doi: 10.1210/en.2011-2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Abu-Hamdah R, Rabiee A, Meneilly GS, Shannon RP, Andersen DK, Elahi D. Clinical review: The extrapancreatic effects of glucagon-like peptide-1 and related peptides. J Clin Endocrinol Metab. 2009;94(6):1843–52. doi: 10.1210/jc.2008-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Campos RV, Lee YC, Drucker DJ. Divergent tissue-specific and developmental expression of receptors for glucagon and glucagon-like peptide-1 in the mouse. Endocrinology. 1994;134:2156–2164. doi: 10.1210/endo.134.5.8156917. [DOI] [PubMed] [Google Scholar]
- 88.Calvo JC, Gisolfi CV, Blazquez E, Mora F. Glucagon-like peptide-1(7-36)amide induces the release of aspartic acid and glutamine by the ventromedial hypothalamus of the conscious rat. Brain Res Bull. 1995;38:435–439. doi: 10.1016/0361-9230(95)02010-o. [DOI] [PubMed] [Google Scholar]
- 89.Alvarez E, Roncero I, Chowen JA, Thorens B, Blázquez E. Expression of the glucagon-like peptide-1 receptor gene in rat brain. J Neurochem. 1996;66:920–927. doi: 10.1046/j.1471-4159.1996.66030920.x. [DOI] [PubMed] [Google Scholar]
- 90.Alvarez E, Martínez MD, Roncero I, Chowen JA, García-Cuartero B, Gispert JD, et al. The expression of GLP-1 receptor mRNA and protein allows the effect of GLP-1 on glucose metabolism in the human hypothalamus and brainstem. J Neurochem. 2005;92:798–806. doi: 10.1111/j.1471-4159.2004.02914.x. [DOI] [PubMed] [Google Scholar]
- 91.Larsen PJ, Tang-Christensen M, Holst JJ, Orskov C. Distribution of glucagon-like peptide-1 and other preproglucagon-derived peptides in the rat hypothalamus and brainstem. Neuroscience. 1997;77:257–270. doi: 10.1016/s0306-4522(96)00434-4. [DOI] [PubMed] [Google Scholar]
- 92.Ritzel R, Orskov C, Holst JJ, Nauck MA. Pharmacokinetic, insulinotropic, and glucagonostatic properties of GLP-1 [7-36 amide] after subcutaneous injection in healthy volunteers. Dose-response-relationships. Diabetologia. 1995;38:720–725. doi: 10.1007/BF00401846. [DOI] [PubMed] [Google Scholar]
- 93.Greig NH, Holloway HW, De Ore KA, Jani D, Wang Y, Zhou J, et al. Once daily injection of exendin-4 to diabetic mice achieves long-term beneficial effects on blood glucose concentrations. Diabetologia. 1999;42(1):45–50. doi: 10.1007/s001250051111. [DOI] [PubMed] [Google Scholar]
- 94.De Ore K, Greig NH, Halloway HW, Wang Y, Perfetti R, Egan JM. The effects of GLP-1 on insulin release in young and old rats in the fasting state and during an intravenous glucose tolerance test. J Gerontol B Psychol Sci Soc Sci. 1997;52:B245–B249. doi: 10.1093/gerona/52a.5.b245. [DOI] [PubMed] [Google Scholar]
- 95.Eng J, Kleinman WA, Singh L, Singh G, Raufman JP. Isolation and characterization of exendin-4, an exendin-3 analogue, from Heloderma suspectum venom. Further evidence for an exendin receptor on dispersed acini from guinea pig pancreas. J Biol Chem. 1992;267(11):7402–5. [PubMed] [Google Scholar]
- 96.Göke R, Fehmann HC, Linn T, Schmidt H, Krause M, Eng J, Göke B. Exendin-4 is a high potency agonist and truncated exendin-(9-39)-amide an antagonist at the glucagon-like peptide 1-(7-36)-amide receptor of insulin-secreting beta-cells. J Biol Chem. 1993;268(26):19650–5. [PubMed] [Google Scholar]
- 92.Hamilton A, Hölscher C. Receptors for the incretin glucagon-like peptide-1 are expressed on neurons in the central nervous system. Neuroreport. 2009;20:1161–1166. doi: 10.1097/WNR.0b013e32832fbf14. [DOI] [PubMed] [Google Scholar]
- 93.Kastin AJ, Akerstrom V, Pan W. Interactions of glucagon-like peptide-1 (GLP-1) with the blood–brain barrier. J Mol Neurosci. 2002;18:7–14. doi: 10.1385/JMN:18:1-2:07. [DOI] [PubMed] [Google Scholar]
- 94.Kastin AJ, Akerstrom V. Entry of exendin-4 into brain is rapid but may be limited at high doses. Int J Obes Relat Metab Disord. 2003;27:313–318. doi: 10.1038/sj.ijo.0802206. [DOI] [PubMed] [Google Scholar]
- 95.Banks WA, During MJ, Niehoff ML. Brain uptake of the glucagon-like peptide-1 antagonist exendin(9-39) after intranasal administration. J Pharmacol Exp Ther. 2004;309:469–475. doi: 10.1124/jpet.103.063222. [DOI] [PubMed] [Google Scholar]
- 96.Hunter K, Hölscher C. Drugs developed to treat diabetes, liraglutide and lixisenatide, cross the blood brain barrier and enhance neurogenesis. BMC Neurosci. 2012;13:33. doi: 10.1186/1471-2202-13-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang Y, Perfetti R, Greig NH, Holloway HW, DeOre KA, Montrose-Rafizadeh C, et al. Glucagon-like peptide-1 can reverse the age-related decline in glucose tolerance in rats. J Clin Invest. 1997;99(12):2883–9. doi: 10.1172/JCI119482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Szayna M, Doyle ME, Betkey JA, Holloway HW, Spencer RG, Greig NH, et al. Exendin-4 decelerates food intake, weight gain, and fat deposition in Zucker rats. Endocrinology. 2000;141(6):1936–41. doi: 10.1210/endo.141.6.7490. [DOI] [PubMed] [Google Scholar]
- 99.Doyle ME, Greig NH, Holloway HW, Betkey JA, Bernier M, Egan JM. Insertion of an N-terminal 6-aminohexanoic acid after the 7 amino acid position of glucagon-like peptide-1 produces a long-acting hypoglycemic agent. Endocrinology. 2001;142(10):4462–8. doi: 10.1210/endo.142.10.8410. [DOI] [PubMed] [Google Scholar]
- 100.Doyle ME, Theodorakis MJ, Holloway HW, Bernier M, Greig NH, Egan JM. The importance of the nine-amino acid C-terminal sequence of exendin-4 for binding to the GLP-1 receptor and for biological activity. Regul Pept. 2003;114(2-3):153–8. doi: 10.1016/s0167-0115(03)00120-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Doyle ME, McConville P, Theodorakis MJ, Goetschkes MM, Bernier M, Spencer RG, et al. In vivo biological activity of exendin (1-30). Endocrine. 2005;27(1):1–9. doi: 10.1385/ENDO:27:1:001. [DOI] [PubMed] [Google Scholar]
- 102.Meneilly GS, Greig N, Tildesley H, Habener JF, Egan JM, Elahi D. Effects of 3 months of continuous subcutaneous administration of glucagon-like peptide 1 in elderly patients with type 2 diabetes. Diabetes Care. 2003;26(10):2835–41. doi: 10.2337/diacare.26.10.2835. [DOI] [PubMed] [Google Scholar]
- 103.Xu G, Stoffers DA, Habener JF, Bonner-Weir S. Exendin-4 stimulates both β-cell replication and neogenesis, resulting in increased b-cell mass and improved glucose tolerance in diabetic rats. Diabetes. 1999;48:2270–2276. doi: 10.2337/diabetes.48.12.2270. [DOI] [PubMed] [Google Scholar]
- 104.Tourrel C, Bailbé D, Meile MJ, Kergoat M, Portha B. Glucagon-like peptide-1 and exendin-4 stimulate beta-cell neogenesis in streptozotocin-treated newborn rats resulting in persistently improved glucose homeostasis at adult age. Diabetes. 2001;50(7):1562–70. doi: 10.2337/diabetes.50.7.1562. [DOI] [PubMed] [Google Scholar]
- 105.Zhou J, Pineyro MA, Wang X, Doyle ME, Egan JM. Exendin-4 differentiation of human pancreatic duct cell line into endocrine cells: involvement of PDX-1 and HNF3(transcription factors. J. Cell. Physiol. 2002;192:304–314. doi: 10.1002/jcp.10143. [DOI] [PubMed] [Google Scholar]
- 106.Li Y, Hansotia T, Yusta B, Ris F, Halban PA, Drucker DJ. Glucagon-like peptide-1 receptor signaling modulates β cell apoptosis. J. Biol. Chem. 2003;278:471–478. doi: 10.1074/jbc.M209423200. [DOI] [PubMed] [Google Scholar]
- 107.Urusova IA, Farilla L, Hui H, D'Amico E, Perfetti R. GLP-1 inhibition of pancreatic islet cell apoptosis. Trends Endocrinol Metab. 2004;15(1):27–33. doi: 10.1016/j.tem.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 108.Brubaker PL, Drucker DJ. Minireview: Glucagon-like peptides regulate cell proliferation and apoptosis in the pancreas, gut, and central nervous system. Endocrinology. 2004;145(6):2653–9. doi: 10.1210/en.2004-0015. [DOI] [PubMed] [Google Scholar]
- 109.Walton MR, Dragunow I. Is CREB a key to neuronal survival? Trends Neurosci. 2000;23(2):48–53. doi: 10.1016/s0166-2236(99)01500-3. [DOI] [PubMed] [Google Scholar]
- 110.Cui Q, So KF. Involvement of cAMP in neuronal survival and axonal regeneration. Anat Sci Int. 2004;79(4):209–12. doi: 10.1111/j.1447-073x.2004.00089.x. [DOI] [PubMed] [Google Scholar]
- 111.Yu JH, Kim MS. Molecular mechanisms of appetite regulation. Diabetes Metab J. 2012;36(6):391–8. doi: 10.4093/dmj.2012.36.6.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dailey MJ, Moran TH. Glucagon-like peptide 1 and appetite. Trends Endocrinol Metab. 2013;24(2):85–91. doi: 10.1016/j.tem.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Perry T, Zhou J, Shaw K, Lahiri DK, Egan JM, Greig NH. Induction of differentiation by glucagon-like peptide-1: a novel neurotrophic factor in PC12 cells. J Pharmacol Exp Ther. 2002;300:958–66. doi: 10.1124/jpet.300.3.958. [DOI] [PubMed] [Google Scholar]
- 114.Perry T, Haughey N, Egan JM, Greig NH. Amelioration of cholinergic neuron atrophy by glucagon-like peptide-1. J Pharmacol Exp Ther. 2002;302:881–8. doi: 10.1124/jpet.102.037481. [DOI] [PubMed] [Google Scholar]
- 115.Perry T, Lahiri DK, Sambamurti K, Mattson MP, Egan JM, Greig NH. A role of glucagon-like peptide-1 in the processing of β–amyloid precursor protein. J Neurosci Res. 2003;72:603–12. doi: 10.1002/jnr.10611. [DOI] [PubMed] [Google Scholar]
- 116.Gilman CP, Perry T, Furukawa K, Greig NH, Egan JM, Mattson MP. Glucagon-like peptide 1 modulates calcium responses to glutamate and membrane depolarization in hippocampal neurons. J Neurochem. 2003;87:1137–44. doi: 10.1046/j.1471-4159.2003.02073.x. [DOI] [PubMed] [Google Scholar]
- 117.Perry TA, Holloway HW, Weerasuriya A, Mattison JA, Greig NH. Evidence of GLP-1-mediated neuroprotection in an animal model of pyridoxine-induced peripheral sensory neuropathy. Exp. Neurol. 2007;203:293–301. doi: 10.1016/j.expneurol.2006.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Li Y, Perry T, Kindy MS, Harvey BK, Tweedie D, Holloway HW, et al. GLP-1 receptor stimulation preserves primary cortical and dopaminergic neurons in cellular and rodent models of stroke and Parkinsonism. Proc Natl Acad Sci U S A. 2009;106:1285–1290. doi: 10.1073/pnas.0806720106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Martin B, Golden E, Carlson OD, Pistell P, Zhou J, Kim W, et al. Exendin-4 improves glycemic control, ameliorates brain and pancreatic pathologies, and extends survival in a mouse model of Huntington's disease. Diabetes. 2009;58(2):318–28. doi: 10.2337/db08-0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Li Y, Duffy KB, Ottinger MA, Ray B, Bailey JA, Holloway HW, et al. GLP-1 receptor stimulation reduces amyloid-beta peptide accumulation and cytotoxicity in cellular and animal models of Alzheimer's disease. J Alzheimers Dis. 2010;19:1205–1219. doi: 10.3233/JAD-2010-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Li Y, Tweedie D, Mattson MP, Holloway HW, Greig NH. Enhancing the GLP-1 receptor signaling pathway leads to proliferation and neuroprotection in human neuroblastoma cells. J Neurochem. 2010;113:1621–1631. doi: 10.1111/j.1471-4159.2010.06731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Li Y, Chigurupati S, Holloway HW, Mughal M, Tweedie D, Bruestle DA, et al. Exendin-4 ameliorates motor neuron degeneration in cellular and animal models of amyotrophic lateral sclerosis. PLoS One. 2012;7(2):e32008. doi: 10.1371/journal.pone.0032008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.McIntyre RS, Powell AM, Kaidanovich-Beilin O, Soczynska JK, Alsuwaidan M, Woldeyohannes HO, et al. The neuroprotective effects of GLP-1: possible treatments for cognitive deficits in individuals with mood disorders. Behav Brain Res. 2013;237:164–71. doi: 10.1016/j.bbr.2012.09.021. [DOI] [PubMed] [Google Scholar]
- 124.Bak AM, Egefjord L, Gejl M, Steffensen C, Stecher CW, Smidt K, et al. Targeting amyloid-beta by glucagon-like peptide -1 (GLP-1) in Alzheimer's disease and diabetes. Expert Opin Ther Targets. 2011;15(10):1153–62. doi: 10.1517/14728222.2011.600691. [DOI] [PubMed] [Google Scholar]
- 125.Holst JJ, Burcelin R, Nathanson E. Neuroprotective properties of GLP-1: theoretical and practical applications. Curr Med Res Opin. 2011;27(3):547–58. doi: 10.1185/03007995.2010.549466. [DOI] [PubMed] [Google Scholar]
- 126.Hölscher C, Li L. New roles for insulin-like hormones in neuronal signalling and protection: new hopes for novel treatments of Alzheimer's disease? Neurobiol Aging. 2010;31(9):1495–502. doi: 10.1016/j.neurobiolaging.2008.08.023. [DOI] [PubMed] [Google Scholar]
- 127.Harkavyi A, Whitton PS. Glucagon-like peptide 1 receptor stimulation as a means of neuroprotection. Br J Pharmacol. 2010;159(3):495–501. doi: 10.1111/j.1476-5381.2009.00486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gault VA, Hölscher C. GLP-1 agonists facilitate hippocampal LTP and reverse the impairment of LTP induced by beta-amyloid. Eur J Pharmacol. 2008;587(1-3):112–7. doi: 10.1016/j.ejphar.2008.03.025. [DOI] [PubMed] [Google Scholar]
- 129.Wang XH, Li L, Hölscher C, Pan YF, Chen XR, Qi JS. Val8-glucagon-like peptide-1 protects against Aβ1-40-induced impairment of hippocampal late-phase long-term potentiation and spatial learning in rats. Neuroscience. 2010;170(4):1239–48. doi: 10.1016/j.neuroscience.2010.08.028. [DOI] [PubMed] [Google Scholar]
- 130.Klinge PM, Harmening K, Miller MC, Heile A, Wallrapp C, Geigle P, Brinker T. Encapsulated native and glucagon-like peptide-1 transfected human mesenchymal stem cells in a transgenic mouse model of Alzheimer's disease. Neurosci Lett. 2011;497(1):6–10. doi: 10.1016/j.neulet.2011.03.092. [DOI] [PubMed] [Google Scholar]
- 131.McClean PL, Parthsarathy V, Faivre E, Hölscher C. The diabetes drug liraglutide prevents degenerative processes in a mouse model of Alzheimer's disease. J Neurosci. 2011;31(17):6587–94. doi: 10.1523/JNEUROSCI.0529-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bomfim TR, Forny-Germano L, Sathler LB, Brito-Moreira J, Houzel JC, Decker H, et al. An anti-diabetes agent protects the mouse brain from defective insulin signaling caused by Alzheimer's disease-associated Aβ oligomers. J Clin Invest. 2012;122(4):1339–53. doi: 10.1172/JCI57256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Talbot K, Wang HY, Kazi H, Han LY, Bakshi KP, Stucky A, et al. Demonstrated brain insulin resistance in Alzheimer's disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest. 2012;122(4):1316–38. doi: 10.1172/JCI59903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Han WN, Hölscher C, Yuan L, Yang W, Wang XH, Wu MN, Qi JS. Liraglutide protects against amyloid-β protein-induced impairment of spatial learning and memory in rats. Neurobiol Aging. 2013;34(2):576–88. doi: 10.1016/j.neurobiolaging.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 135.Long-Smith CM, Manning S, McClean PL, Coakley MF, O'Halloran DJ, Hölscher C, et al. The diabetes drug liraglutide ameliorates aberrant insulin receptor localisation and signalling in parallel with decreasing both amyloid-β plaque and glial pathology in a mouse model of Alzheimer's disease. Neuromolecular Med. 2013;15(1):102–14. doi: 10.1007/s12017-012-8199-5. doi: 10.1007/s12017-012-8199-5. [DOI] [PubMed] [Google Scholar]
- 136.Wang XH, Yang W, Hölscher C, Wang ZJ, Cai HY, Li QS, et al. Val8-GLP-1 remodels synaptic activity and intracellular calcium homeostasis impaired by amyloid β peptide in rats. J Neurosci Res. 2013;91(4):568–77. doi: 10.1002/jnr.23181. [DOI] [PubMed] [Google Scholar]
- 137.Parthsarathy V, Hölscher C. Chronic treatment with the GLP1 analogue liraglutide increases cell proliferation and differentiation into neurons in an AD mouse model. PLoS One. 2013;8(3):e58784. doi: 10.1371/journal.pone.0058784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yang Y, Zhang J, Ma D, Zhang M, Hu S, Shao S, Gong CX. Subcutaneous Administration of Liraglutide Ameliorates Alzheimer-Associated Tau Hyperphosphorylation in Rats with Type 2 Diabetes. J Alzheimers Dis. 2013 Jul 9; doi: 10.3233/JAD-130491. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 139.McClean PL, Hölscher C. Liraglutide can reverse memory impairment, synaptic loss and reduce plaque load in aged APP/PS1 mice, a model of Alzheimer's disease. Neuropharmacology. 2013 Aug 21; doi: 10.1016/j.neuropharm.2013.08.005. [Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 140.Xiong H, Zheng C, Wang J, Song J, Zhao G, Shen H, Deng Y. The Neuroprotection of Liraglutide on Alzheimer-Like Learning and Memory Impairment by Modulating the Hyperphosphorylation of Tau and Neurofilament Proteins and Insulin Signaling Pathways in Mice. J Alzheimers Dis. 2013 Sep 4; doi: 10.3233/JAD-130584. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 141.Lim JG, Lee JJ, Park SH, Park JH, Kim SJ, Cho HC, et al. Glucagon-like peptide-1 protects NSC-34 motor neurons against glucosamine through Epac-mediated glucose uptake enhancement. Neurosci Lett. 2010;479(1):13–7. doi: 10.1016/j.neulet.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 142.Liu WJ, Jin HY, Lee KA, Xie SH, Baek HS, Park TS. Neuroprotective effect of the glucagon-like peptide-1 receptor agonist, synthetic exendin-4, in streptozotocin-induced diabetic rats. Br J Pharmacol. 2011;164(5):1410–20. doi: 10.1111/j.1476-5381.2011.01272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Jolivalt CG, Fineman M, Deacon CF, Carr RD, Calcutt NA. GLP-1 signals via ERK in peripheral nerve and prevents nerve dysfunction in diabetic mice. Diabetes Obes Metab. 2011;13(11):990–1000. doi: 10.1111/j.1463-1326.2011.01431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Himeno T, Kamiya H, Naruse K, Harada N, Ozaki N, Seino Y, et al. Beneficial effects of exendin-4 on experimental polyneuropathy in diabetic mice. Diabetes. 2011;60(9):2397–406. doi: 10.2337/db10-1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lee CH, Yan B, Yoo KY, Choi JH, Kwon SH, Her S, et al. Ischemia-induced changes in glucagon-like peptide-1 receptor and neuroprotective effect of its agonist, exendin-4, in experimental transient cerebral ischemia. J Neurosci Res. 2011;89(7):1103–13. doi: 10.1002/jnr.22596. [DOI] [PubMed] [Google Scholar]
- 146.Teramoto S, Miyamoto N, Yatomi K, Tanaka Y, Oishi H, Arai H, et al. Exendin-4, a glucagon-like peptide-1 receptor agonist, provides neuroprotection in mice transient focal cerebral ischemia. J Cereb Blood Flow Metab. 2011;31(8):1696–705. doi: 10.1038/jcbfm.2011.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Briyal S, Gulati K, Gulati A. Repeated administration of exendin-4 reduces focal cerebral ischemia-induced infarction in rats. Brain Res. 2012;1427:23–34. doi: 10.1016/j.brainres.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 148.Darsalia V, Mansouri S, Ortsäter H, Olverling A, Nozadze N, Kappe C, et al. Glucagon-like peptide-1 receptor activation reduces ischaemic brain damage following stroke in Type 2 diabetic rats. Clin Sci (Lond) 2012;122(10):473–83. doi: 10.1042/CS20110374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wang MD, Huang Y, Zhang GP, Mao L, Xia YP, Mei YW, et al. Exendin-4 improved rat cortical neuron survival under oxygen/glucose deprivation through PKA pathway. Neuroscience. 2012;226:388–96. doi: 10.1016/j.neuroscience.2012.09.025. [DOI] [PubMed] [Google Scholar]
- 150.Bertilsson G, Patrone C, Zachrisson O, Andersson A, Dannaeus K, Heidrich J, et al. Peptide hormone exendin-4 stimulates subventricular zone neurogenesis in the adult rodent brain and induces recovery in an animal model of Parkinson's disease. J Neurosci Res. 2008;86(2):326–38. doi: 10.1002/jnr.21483. [DOI] [PubMed] [Google Scholar]
- 151.Harkavyi A, Abuirmeileh A, Lever R, Kingsbury AE, Biggs CS, Whitton PS. Glucagon-like peptide 1 receptor stimulation reverses key deficits in distinct rodent models of Parkinson's disease. J Neuroinflammation. 2008;5:19. doi: 10.1186/1742-2094-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kim S, Moon M, Park S. Exendin-4 protects dopaminergic neurons by inhibition of microglial activation and matrix metalloproteinase-3 expression in an animal model of Parkinson's disease. J Endocrinol. 2009;202(3):431–9. doi: 10.1677/JOE-09-0132. [DOI] [PubMed] [Google Scholar]
- 153.Aviles-Olmos I, Dickson J, Kefalopoulou Z, Djamshidian A, Ell P, Soderlund T, Whitton P, et al. Exenatide and the treatment of patients with Parkinson's disease. J Clin Invest. 2013;123(6):2730–6. doi: 10.1172/JCI68295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Knippenberg S, Thau N, Dengler R, Brinker T, Petri S. Intracerebroventricular injection of encapsulated human mesenchymal cells producing glucagon-like peptide 1 prolongs survival in a mouse model of ALS. PLoS One. 2012;7(6):e36857. doi: 10.1371/journal.pone.0036857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sun H, Knippenberg S, Thau N, Ragancokova D, Körner S, Huang D, et al. Therapeutic potential of N-acetyl glucagon-like peptide-1 in primary motor neuron cultures derived from non-transgenic and SOD1-G93A ALS mice. Cell Mol Neurobiol. 2013;33(3):347–57. doi: 10.1007/s10571-012-9900-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.During MJ, Cao L, Zuzga DS, Francis JS, Fitzsimons HL, Jiao X, et al. Glucagon-like peptide-1 receptor is involved in learning and neuroprotection. Nat Med. 2003;9(9):1173–9. doi: 10.1038/nm919. [DOI] [PubMed] [Google Scholar]
- 157.Prakash A, Matta BF. Hyperglycaemia and neurological injury. Curr Opin Anaesthesiol. 2008;21(5):565–9. doi: 10.1097/ACO.0b013e32830f44e4. [DOI] [PubMed] [Google Scholar]
- 158.Muehlschlegel S, Carandang R, Ouillette C, Hall W, Anderson F, Goldberg R. Frequency and impact of intensive care unit complications on moderate-severe traumatic brain injury: early results of the Outcome Prognostication in Traumatic Brain Injury (OPTIMISM) Study. Neurocrit Care. 2013;18(3):318–31. doi: 10.1007/s12028-013-9817-2. [DOI] [PubMed] [Google Scholar]
- 159.Smith RL, Lin JC, Adelson PD, Kochanek PM, Fink EL, Wisniewski SR, et al. Relationship between hyperglycemia and outcome in children with severe traumatic brain injury. Pediatr Crit Care Med. 2012;13(1):85–91. doi: 10.1097/PCC.0b013e3182192c30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Pan W, Kastin AJ, Rigai T, McLay R, Pick CG. Increased hippocampal uptake of tumor necrosis factor alpha and behavioral changes in mice. Exp Brain Res. 2003;149:195–199. doi: 10.1007/s00221-002-1355-7. [DOI] [PubMed] [Google Scholar]
- 161.Rachmany L, Tweedie D, Li Y, Rubovitch V, Holloway HW, et al. Exendin-4 induced glucagon-like peptide-1 receptor activation reverses behavioral impairments of mild traumatic brain injury in mice. AGE. 2012:1–16. doi: 10.1007/s11357-012-9464-0. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Tweedie D, Rachmany L, Rubovitch V, Lehrmann E, Zhang Y, Becker KG, et al. Exendin-4, a glucagon-like peptide-1 receptor agonist prevents mTBI-induced changes in hippocampus gene expression and memory deficits in mice. Exp Neurol. 2013;239:170–82. doi: 10.1016/j.expneurol.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Baratz R, Tweedie D, Rubovitch V, Luo W, Yoon JS, et al. Tumor necrosis factor-α synthesis inhibitor, 3,6’-dithiothalidomide, reverses behavioral impairments induced by minimal traumatic brain injury in mice. J Neurochem. 2010;118:1032–1042. doi: 10.1111/j.1471-4159.2011.07377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Saykally JN, Rachmany L, Hatic H, Shaer A, Rubovitch V, Pick CG, et al. The nuclear factor erythroid 2-like 2 activator, tert-butylhydroquinone, improves cognitive performance in mice after mild traumatic brain injury. Neuroscience. 2012;223:305–14. doi: 10.1016/j.neuroscience.2012.07.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Tweedie D, Rachmany L, Rubovitch V, Zhang Y, Becker KG, Perez E, et al. Changes in mouse cognition and hippocampal gene expression observed in a mild physical- and blast-traumatic brain injury. Neurobiol Dis. 2013;54:1–11. doi: 10.1016/j.nbd.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Walker KR, Tesco G. Molecular mechanisms of cognitive dysfunction following traumatic brain injury. Front Aging Neurosci. 2013;5:29. doi: 10.3389/fnagi.2013.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Dixon CE, Lighthall JW, Anderson TE. Physiologic, histopathologic, and cineradiographic characterization of a new fluid-percussion model of experimental brain injury in the rat. J. Neurotrauma. 1988;5:91–104. doi: 10.1089/neu.1988.5.91. [DOI] [PubMed] [Google Scholar]
- 168.Dixon CE, Lyeth BG, Povlishock JT, Findling RL, Hamm RJ, Marmarou A, et al. A fluid percussion model of experimental brain injury in the rat. J. Neurosurg. 1987;67:110–119. doi: 10.3171/jns.1987.67.1.0110. [DOI] [PubMed] [Google Scholar]
- 169.Hamm RJ, Lyeth BG, Jenkin LW, O'Dell DM, Pike BR. Selective cognitive impairment following traumatic brain injury in rats. Behav. Brain Res. 1993;59:169–173. doi: 10.1016/0166-4328(93)90164-l. [DOI] [PubMed] [Google Scholar]
- 170.Eakin K, Miller JP. Mild traumatic brain injury is associated with impaired hippocampal spatiotemporal representation in the absence of histological changes. J Neurotrauma. 2012;29(6):1180–7. doi: 10.1089/neu.2011.2192. [DOI] [PubMed] [Google Scholar]
- 171.Eakin K, Li Y, Chiang Y-H, Hoffer BJ, Greig NH, Miller JP. Exendin-4 reduces brain injury in rats. PLoS One. 2013;8(12):e82016. doi: 10.1371/journal.pone.0082016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Heile AM, Wallrapp C, Klinge PM, Samii A, Kassem M, Silverberg G, Brinker T. Cerebral transplantation of encapsulated mesenchymal stem cells improves cellular pathology after experimental traumatic brain injury. Neurosci Lett. 2009;463(3):176–81. doi: 10.1016/j.neulet.2009.07.071. [DOI] [PubMed] [Google Scholar]
- 173.Heile A, Brinker T. Clinical translation of stem cell therapy in traumatic brain injury: the potential of encapsulated mesenchymal cell biodelivery of glucagon-like peptide-1. Dialogues Clin Neurosci. 2011;13(3):279–86. doi: 10.31887/DCNS.2011.13.2/aheile. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hadjiyanni I, Siminovitch KA, Danska JS, Drucker DJ. Glucagon-like peptide-1 receptor signalling selectively regulates murine lymphocyte proliferation and maintenance of peripheral regulatory T cells. Diabetologia. 2010;53(4):730–40. doi: 10.1007/s00125-009-1643-x. [DOI] [PubMed] [Google Scholar]
- 175.Hogan AE, Tobin AM, Ahern T, Corrigan MA, Gaoatswe G, Jackson R, et al. Glucagon-like peptide-1 (GLP-1) and the regulation of human invariant natural killer T cells: lessons from obesity, diabetes and psoriasis. Diabetologia. 2011;54(11):2745–54. doi: 10.1007/s00125-011-2232-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Panjwani N, Mulvihill EE, Longuet C, Yusta B, Campbell JE, Brown TJ, et al. GLP-1 receptor activation indirectly reduces hepatic lipid accumulation but does not attenuate development of atherosclerosis in diabetic male ApoE(−/−) mice. Endocrinology. 2013;154(1):127–39. doi: 10.1210/en.2012-1937. [DOI] [PubMed] [Google Scholar]
- 177.Chowen JA, de Fonseca FR, Alvarez E, Navarro M, García-Segura LM, Blázquez E. Increased glucagon-like peptide-1 receptor expression in glia after mechanical lesion of the rat brain. Neuropeptides. 1999;33(3):212–5. doi: 10.1054/npep.1999.0757. [DOI] [PubMed] [Google Scholar]
- 178.Becker RE, Greig NH. Lost in translation: neuropsychiatric drug development. Sci Transl Med. 2010 Dec 8;2(61):61rv6. doi: 10.1126/scitranslmed.3000446. [DOI] [PMC free article] [PubMed] [Google Scholar]