Abstract
Prions are notorious for their extraordinary resistance to traditional methods of decontamination, rendering their transmission a public health risk. Iatrogenic Creutzfeldt–Jakob disease (iCJD) via contaminated surgical instruments and medical devices has been verified both experimentally and clinically. Standard methods for prion inactivation by sodium hydroxide or sodium hypochlorite have failed, in some cases, to fully remove prion infectivity, while they are often impractical for routine applications. Prion accumulation in peripheral tissues and indications of human-to-human bloodborne prion transmission, highlight the need for novel, efficient, yet user-friendly methods of prion inactivation. Here we show both in vitro and in vivo that homogenous photocatalytic oxidation, mediated by the photo-Fenton reagent, has the potential to inactivate the pathological prion isoform adsorbed on metal substrates. Photocatalytic oxidation with 224 μg mL−1 Fe3+, 500 μg mL−1 h−1 H2O2, UV-A for 480 min lead to 100% survival in golden Syrian hamsters after intracranial implantation of stainless steel wires infected with the 263K prion strain. Interestingly, photocatalytic treatment of 263K infected titanium wires, under the same experimental conditions, prolonged the survival interval significantly, but failed to eliminate infectivity, a result that we correlate with the increased adsorption of PrPSc on titanium, in comparison to stainless steel. Our findings strongly indicate that our, user- and environmentally friendly protocol can be safely applied to the decontamination of prion infected stainless steel surfaces.
Keywords: photocatalytic, photo-Fenton, prion, inactivation, decontamination, stainless steel
Introduction
Prions are the causative agents of fatal neurodegenerative disorders known as transmissible spongiform encephalopathies (TSEs), including Creutzfeldt-Jakob disease (CJD) in humans, bovine spongiform encephalopathy (BSE) in cattle and scrapie in sheep and goats.1 The agent that causes TSEs was termed “prion,” to describe “a proteinaceous infectious particle resistant to inactivation by most procedures that modify nucleic acids.”2 Prions are certainly a unique class of infectious pathogens. The only known component of the prion agent is a modified form of the cellular glycoprotein, PrPC, encoded by the PRNP gene,3 termed PrPSc, with unknown physiological function.4 The central event in prion pathogenesis is the conformational conversion of PrPC into PrPSc, an insoluble and partially protease-resistant isoform that propagates itself by imposing its abnormal conformation onto PrPC molecules.1
The unique characteristics of prions correlate with their extraordinary resistance to inactivation processes5 and the potential nosocomial transmission of CJD after medical treatments with prion-contaminated surgical tools or medical devices. In this context, a considerable amount of study has been conducted aiming to efficient, applicable methods of inactivation. Several chemical decontaminants and physical methods including UV-C, ionising irradiation and heat have been characterized as ineffective or partially effective against prion infectivity.6 Standard methods for prion decontamination include treatment with 20 g L−1 sodium hypochlorite or 1 M sodium hydroxide.7 However, in many cases these methods failed to fully eliminate infectivity8 and were detrimental to non-disposable surgical instruments.9 The World Health Organization7 still recommends the use of disposable surgical items in highly infectious tissues, in confirmed or suspected cases of CJD, but also recognizes the need for non-disposable instruments and devices, even, in such cases. A generalized use of single-use instruments in operating theaters would increase costs and introduce severe ergonomic deficiencies during the operating process. In addition, the existence of potentially asymptomatic carriers, the long incubation time of the disease and the variety of tissues which may harbor infectivity,10 underline the necessity for effective methods of prion inactivation in surgical practice.
Additionally, several studies have demonstrated that prion infectivity persists over years upon exposure to extraordinary environmental conditions.11,12 Incineration of hamster brain, infected with the 263K prion strain, at 600 °C, has been reported to completely ash the sample, but failed to fully eliminate prion infectivity.13 This fact poses important questions concerning the disposal of thousands of contaminated or potentially contaminated animal carcasses and the risks of transmission to other animals and humans from water runoff originating from contaminated landfills, meat processing facilities, biochemical laboratories and hospitals.14 Thus, prion inactivation remains a prominent topic of prion disease research.
Our group demonstrated for the first time that TiO2-mediated photocatalytic oxidation may significantly reduce prion infectivity,15 and that the photo-Fenton reagent can degrade PrPSc in aqueous media.16 Both methods belong to the group of Advanced Oxidation Processes (AOPs), employed mainly in the detoxification and disinfection of water and air. Other oxidation processes, more importantly those employing gas plasma technologies, have been developed, aiming to address the problem of prion inactivation, requiring, however, sophisticated equipment.17,18 Here, we investigate the potential of homogenous photocatalysis, a simple, user-friendly process that takes place under mild environmental conditions, mediated by the photo-Fenton reagent, to inactivate prions adsorbed on stainless steel and titanium substrates, by in vitro techniques and bioassays in suitable prion models. According to our knowledge, this is also the first report of photo-Fenton based removal of organic targets adsorbed on solid substrates.
Results and Discussion
In the present study, photocatalytic decontamination of prion-contaminated metal substrates is mediated by the photo-Fenton reagent (Fe3+/H2O2/UV-A,Vis). The Fenton reagent, a mixture of Fe+2 and H2O2, is an attractive oxidative system well known for its potential to oxidize organic compounds.19 The oxidation mechanism is based on the generation of potent oxidizing species, hydroxyl radicals (OH•). The efficiency of this process can be greatly enhanced by UV/VIS irradiation20 (artificial or natural), which results to the production of additional OH• radicals and to the regeneration of the catalyst (Fe2+). These transients, with a reduction potential of 2.8 V, can attack and degrade organic compounds21 or inactivate viruses and microorganisms22 non-selectively, leading to their mineralization. Our group was the first to demonstrate that the photo-Fenton reagent has the potential to rapidly degrade recombinant prion proteins as well as PrPSc in brain homogenates from different TSE affected mammalian species.16
However, it is well known that prions exhibit high binding affinity to metal surfaces and that when adsorbed, they are significantly more resistant to inactivation than prion proteins present in brain homogenates.23 Thus, in order to investigate the potential of homogenous photocatalytic oxidation to inactivate PrPSc adsorbed on metal surfaces, two substrates commonly used in the manufacture of non-disposable surgical instruments, were employed in the study: stainless steel and titanium. Although stainless steel continues to serve as the most popular material for this purpose, the use of titanium increases rapidly, due to its excellent corrosion resistance, its lack of magnetic properties, its biocompatibility and its lower specific weight.24 When exposed to environmental conditions titanium is passivated, forming a protective layer consisting of TiO2 in the TiO2 in the rutile crystalline form, known for its ability to strongly adsorb organic molecules.25 Thus, in the present study TiO2 (rutile) particles served as substrates for contamination with the RML prion strain, by incubation with a 10% w/v mouse brain homogenate. The contaminated particles were then treated photocatalytically with the photo-Fenton reagent. After 120 min of UV-A irradiation in the presence of 56 μg mL−1 Fe3+ and 600 μg mL−1 h−1 H2O2 (pH: 3.5), complete degradation of the total protein organic load was observed (Fig. 1A). Elimination of the PrP signal occurred within 60 min of photocatalytic treatment under the same experimental conditions (Fig. 1B). Visualization of PrP was performed employing the highly sensitive West Femto substrate.16 Control experiments, performed in combinations in which at least one of the photo-Fenton components was absent, showed no or partial protein degradation, as expected, in the case of Fe3+/H2O2 in the absence of UV-A irradiation (data not shown).
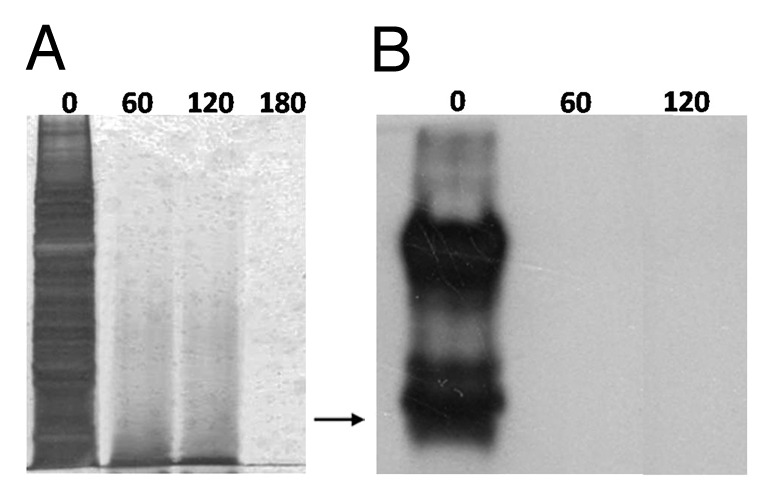
Figure 1. Photo-Fenton treatment of TiO2 (rutile) particles contaminated with the RML scrapie strain. 0.2 g of TiO2 were incubated with 10% w/v RML mouse brain homogenate and were then treated with 56 μg mL−1 Fe3+, 600 μg mL−1 h−1, UV-A, pH: 3.5. (A) SDS-PAGE, following AgNO3 staining of adsorbed proteins after treatment with photo-Fenton at the indicated time points (min). (B) western blotting of adsorbed PrP after treatment with photo-Fenton at the indicated time points (min). Primary antibody: 12F10, visualization: West Femto substrate (Pierce). Arrow: 32.5 kDa relative molecular mass marker.
Several studies, however, have demonstrated that elimination of the PrP signal verified by immunoblotting, often does not correlate with complete removal of infectivity.26 Therefore, a bioassay was conducted, based on the intraperitoneal inoculation of C57BL mice with RML-contaminated TiO2 particles. As expected all mice inoculated with 20 mg of RML-contaminated TiO2 (which corresponds to approximately 140 cm2 of contaminated titanium surface/animal) were clinically affected and sacrificed at the terminal stage of the scrapie illness, 250 d post inoculation. On the other hand, 67% of the animals that were inoculated with prion-contaminated TiO2 particles following photo-Fenton treatment for 180 or 360 min under the experimental conditions previously described remained asymptomatic even 453 d.p.i. (Table 1). Furthermore, the difference in the incubation period of the clinically affected mice (2/6) of the two photo-Fenton treated groups, in comparison to the positive control group, was statistically significant (P < 0.01). Although a 100% survival rate was not accomplished by the applied photocatalytic protocol, it should be stressed that the proposed method of exposure to prion infectivity introduces an important advantage, since it enables the administration of a significantly higher amount of PrP, in comparison to a wire or a single sphere also inoculated i.p.27 We found that the amount of PrP adsorbed on TiO2 that was inoculated per animal, was approximately 240 ng (data not shown), whereas a steel wire (0.15–0.25 mm diameter, 5 mm length) adsorbs approximately 15–25 pg of PrP.28
Table 1. Efficiency of the following photo-Fenton decontamination of scrapie infected TiO2 particles based on a bioassay employing C57BL/6J mice.
| Group No | Description | Survival rate (%) | Clinically affected/ Total number | Survival interval ± standard error (days) |
|---|---|---|---|---|
| 1 | aPositive control, 1% brain homogenate | 0 | 4/4 | 236.8 ± 2.3 |
| 2 | aPositive control, contaminated rutile | 0 | 5/5 | 249.8 ± 8.6 |
| 3 | bphoto-Fenton, 180 min | 67 | 2/6b | 269.0 ± 32.0 |
| 4 | photo-Fenton, 360 min | 67 | 2/6 | 278.5 ± 22.5 |
Animals were inoculated with 0.02 g RML-contaminated TiO2 particles following photo-Fenton treatment (56 μg mL−1 Fe3+, 600 μg mL−1 h−1 H2O2, UV-A, pH: 3.5). Asymptomatic animals were sacrificed 453 d.p.i. Only clinically affected animals were taken into account in the calculation of the survival interval. aThe difference in the incubation period between the two positive control groups was not statistically significant, demonstrating similar initial infectivity titers. bAn asymptomatic individual was found with PK-resistant cerebral PrP, 453 d post inoculation (see Fig. S1).
Further investigation of the potential of homogenous photocatalytic oxidation to inactivate prions adsorbed on metal substrates was conducted employing the well accepted prion infectivity assay, based on the intracranial implantation of steel wires.29 Groups of stainless steel or titanium wires (n: 30) were contaminated with the 263K prion strain and were then, treated with 56 μg mL−1 Fe3+, 300 μg mL−1h−1 H2O2, UV-A (pH: 3.5). Elimination of the PrP signal was achieved, within 60 min or 120 min in the case of stainless steel and titanium respectively, under the employed conditions, as demonstrated by immunoblotting (Fig. 2). Densitometric analysis of the PrP bands in Figure 2, prior to the photo-Fenton treatment, demonstrated that titanium wires adsorb approximately 2.7 times more PrP than stainless steel.
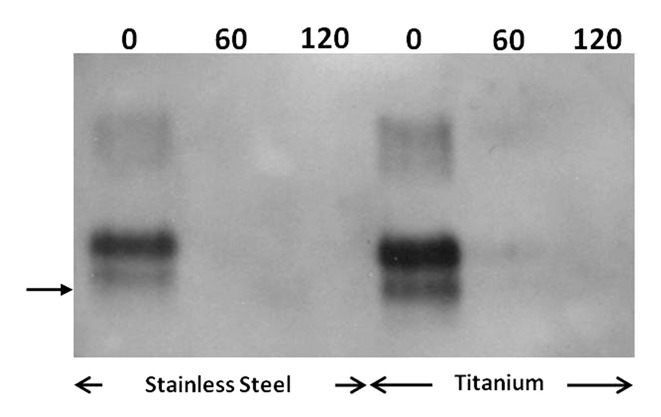
Figure 2. Photo-Fenton treatment of stainless steel and titanium wires contaminated with the 263K scrapie strain. Western blotting of PrP adsorbed on the surface of wires previously incubated with 10% w/v 263K hamster brain homogenate and then, treated with 56 μg mL−1 Fe3+, 300 μg mL−1 h−1 H2O2, UV-A, pH: 3.5, at the indicated time points (min). Primary antibody: 6Η4 (Prionics, AG), visualization: CDP-Star substrate (New England Biolabs). Arrow: 32.5 kDa relative molecular mass marker.
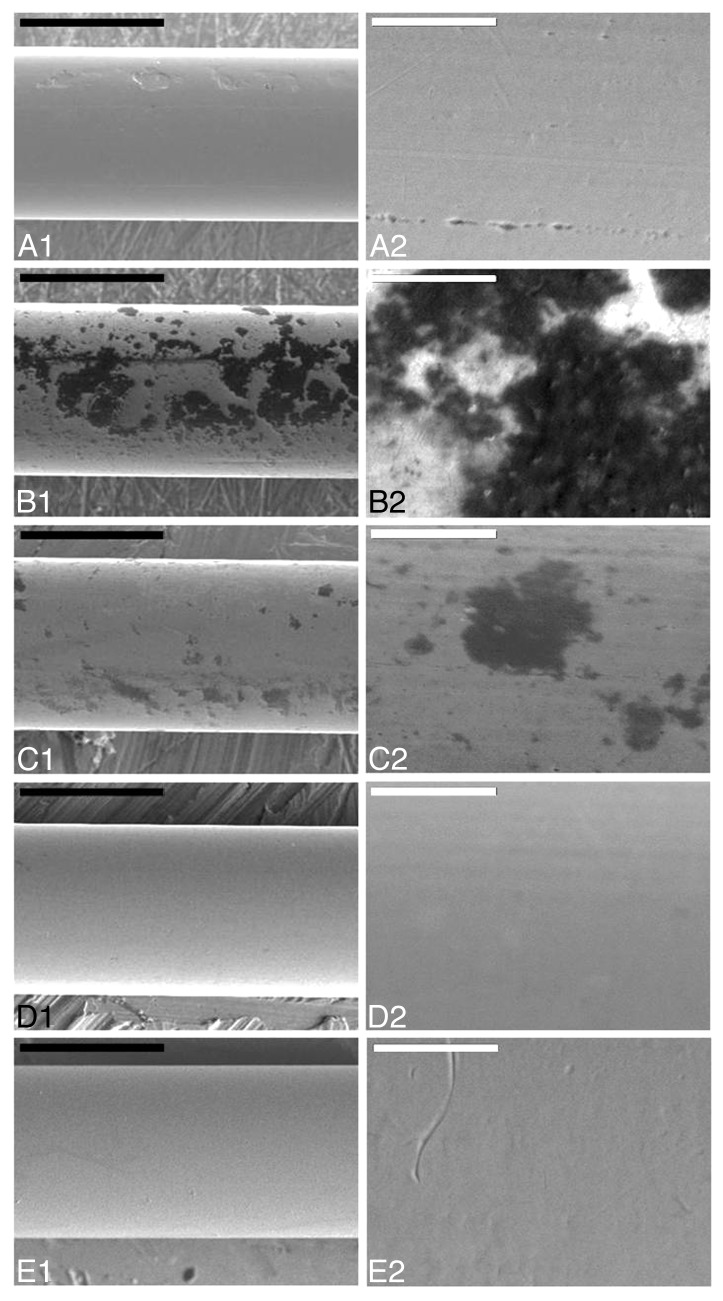
Additionally, SEM was employed in order to investigate the efficiency of photocatalysis in the removal of adsorbed organic contamination from the surface of stainless steel wires. Based on the results obtained by immunoblotting, groups of wires (n: 8) were contaminated with the 263K scrapie strain and were then treated with the photo-Fenton reagent under the conditions previously described. Interestingly, areas of remaining organic contamination could be detected, even after 480 min of illumination in the presence of 56 μg mL−1 Fe3+ and 300 μg mL−1 h−1 H2O2 (see Fig. S2). By applying more drastic treatment conditions (112 μg mL−1 Fe3+ and 400 μg mL−1 h−1 H2O2, UV-A) photocatalytic treatment resulted in a significant reduction of the surface area and the depth of the organic deposits. Careful monitoring, however, revealed residual organic contamination (see Fig. S3). In contrast, the use of 224 μg mL−1 Fe3+ and 500 μg mL−1 h−1 H2O2, UV-A for 480 min, resulted in complete elimination of organic contaminants within 480 min of treatment (Fig. 3). On the other hand, in the case of titanium, detection of residual contamination based on SEM was often hindered, due to the presence of the rough protective layer of TiO2, especially when the organic deposits were limited in size and depth (see Fig. S4).
Figure 3. Scanning Electron Microscopy of stainless steel wires. a: uncontaminated, b-e: incubated with 10% hamster brain homogenate infected with the 263K prion strain and treated with 224 μg mL−1 Fe3+, 500 μg mL−1 h−1 H2O2, pH = 3.5, UV-A for 0, 240, 360, and 480 min respectively. White bar: 200μm, black bar: 20 μm. Panels to the right (A2-E2) are close ups of respective areas of the left column (A1-E1).
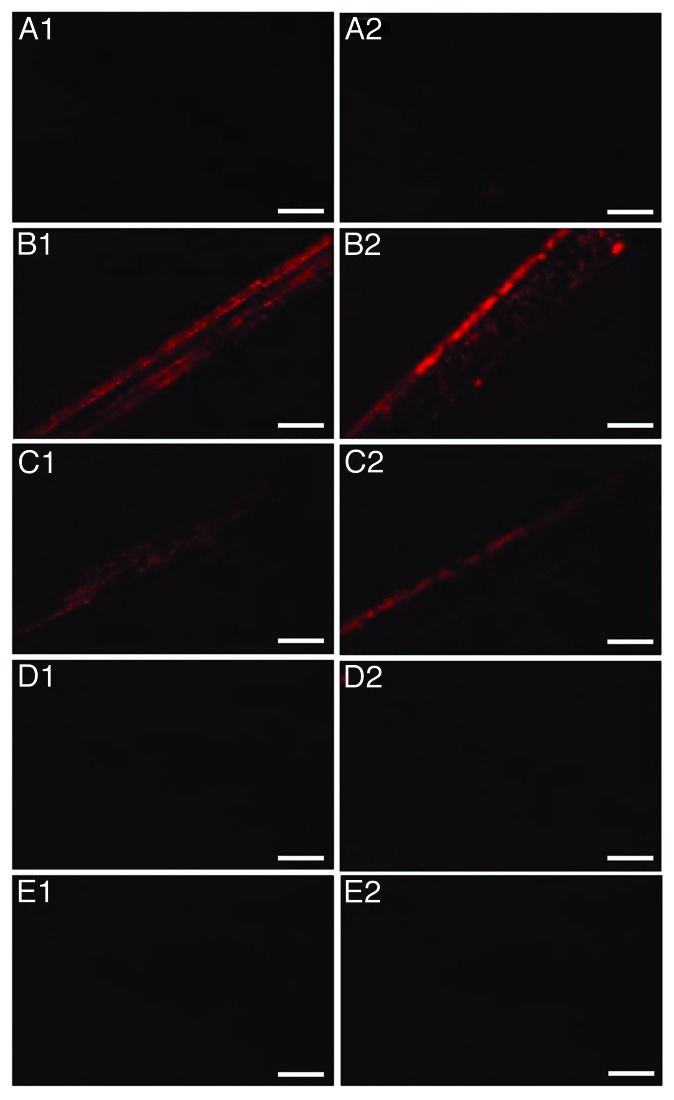
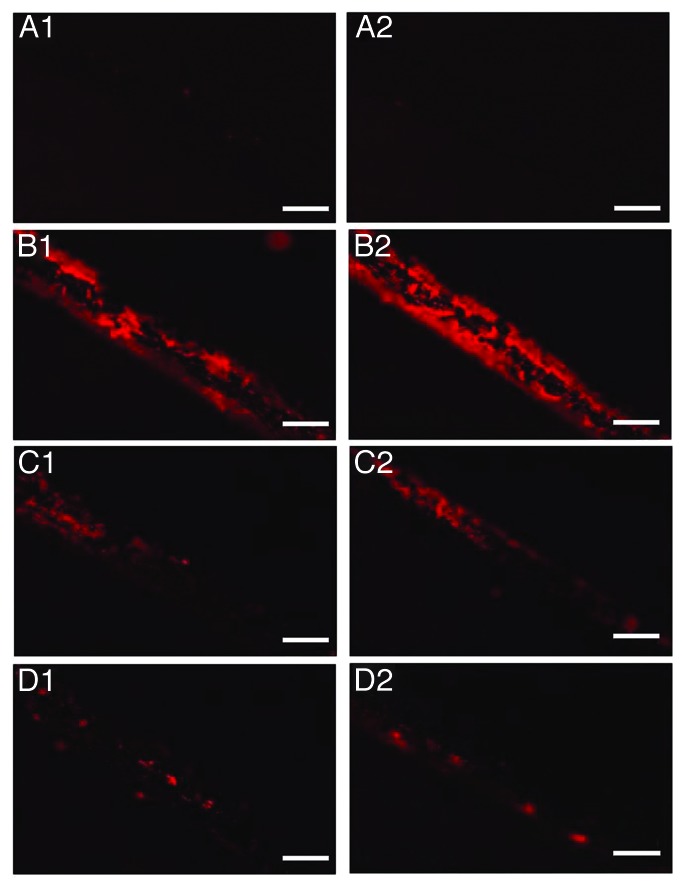
Fluorescent microscopy was employed in order to monitor the removal of protein contamination from the surface of the wires. Groups of wires (n: 8) were contaminated with the 263K scrapie strain and were then treated with the photo-Fenton reagent (224 μg mL−1 Fe3+ and 500 μg mL−1 h−1 H2O2, UV-A). Similarly to SEM, elimination of the fluorescent signal was observed within 480 min of photocatalytic treatment of stainless steel wires (Fig. 4). However, when decontamination was held under identical experimental conditions on titanium, the fluorescent signal was significantly reduced but not completely eliminated (Fig. 5).
Figure 4. Fluorescence Microscopy of stainless steel wires. (A) uncontaminated, (B–E) incubated with 10% hamster brain homogenate infected with the 263K prion strain and treated with 224 μg mL−1 Fe3+, 500 μg mL−1 h−1 H2O2, pH = 3.5 and UV-A for 0, 240, 360, and 480 min respectively. Each row of panels (1–2) demonstrates areas of wires of the same group. Bar: 200 μm
Figure 5. Fluorescence Microscopy of titanium wires. (A) uncontaminated, (B–D) incubated with 10% hamster brain homogenate infected with the 263K prion strain and treated with 224 μg mL−1 Fe3+, 500 μg mL−1 h−1 H2O2, pH = 3.5 and UV-A for 0, 240, and 480 min respectively. Each row of panels (1–2) demonstrates areas of wires of the same group. Bar: 200 μm
Estimation of the area of protein contamination detected by fluorescence microscopy was conducted by densitometric analysis. The amount of protein contamination on titanium, prior to the photocatalytic treatment, was approximately 3 times higher in comparison to the stainless steel wires (Fig. 6). This finding is in good agreement with the increased adsorption of PrP on titanium as already demonstrated by immunoblotting (Fig. 2). Photo-Fenton treatment of stainless steel for 360 min lead to almost complete elimination of the fluorescent signal (Fig. 6A); however, under the same experimental conditions, areas of protein deposits could still be detected on the surface of titanium wires (Fig. 6B).
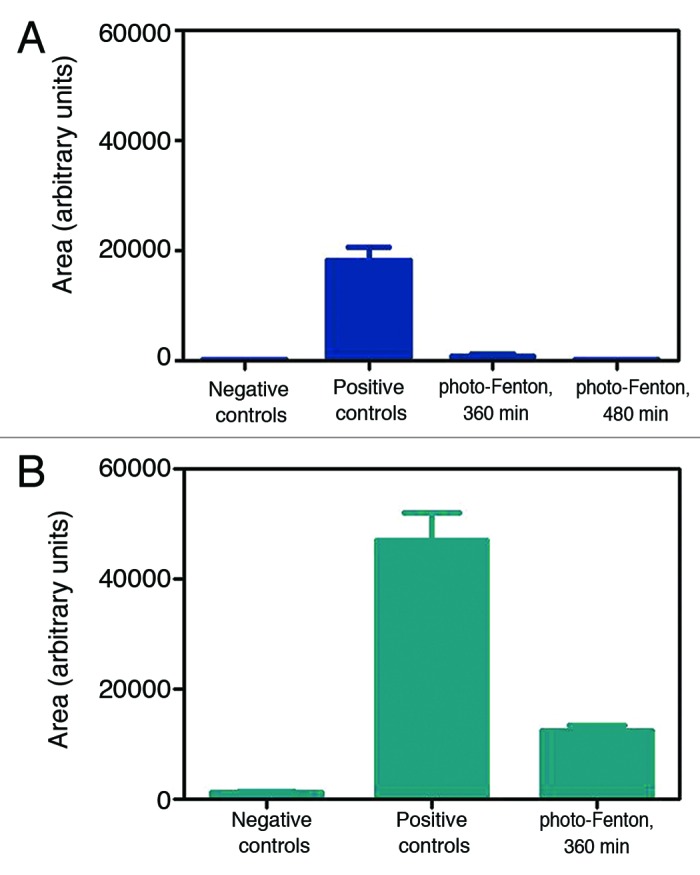
Figure 6. Surface protein contamination in groups of wires (n = 8), after densitometric analysis of fluorescence micrographs. (A) Stainless steel, (B) Titanium. Homogenous photocatalytic treatment was performed in the presence of 224 μg mL−1 Fe3+, 500 μg mL−1 h−1 H2O2, and UV-A (pH = 3.5).
Based on the findings of both scanning electron and fluorescence microscopy, a bioassay was conducted, involving intracranial implantation of both types of wires in golden Syrian hamsters, in order to further investigate the potential of photo-Fenton to efficiently inactivate adsorbed PrPSc. As expected, all animals in the positive control groups (Table 2) were clinically affected. Interestingly, the difference in the survival interval between these two groups was found to be statistically significant (t test, P < 0.05). This finding indicates that titanium not only adsorbs a higher amount of PrP, organic and protein load as demonstrated by immunoblotting, scanning and fluorescence microscopy respectively, but it also binds a significantly higher amount of initial prion infectivity. Furthermore, the increased amount of initial infectivity on the surface of titanium resulted in differentiated survival rates when both types of wires were subjected to photocatalytic treatment under the same experimental conditions for 360 min (Table 2, groups 3 and 7). A 62.5% survival rate was observed in the case of stainless steel, whereas all animals implanted with 263K-contaminated titanium wires were clinically affected. However, the extension in the survival interval of the individuals of group 7, in comparison to the positive control group 6, was statistically significant (P < 0.001).
Table 2. Bioassay of wires contaminated with the 263K scrapie strain.
| Group No | Treatment | Survival interval ± standard error (days) | Clinically affected/ Total |
|---|---|---|---|
| 1 | Negative controls, stainless steel | >505.0 | 0/3 |
| 2 | Positive controls, stainless steel | 135.0 ± 8.0 | 3/3 |
| 3 | 360 min photo-Fenton, stainless steel | 302.0 ± 29.3 | 3/8 |
| 4 | 480 min photo-Fenton, stainless steel | >505.0 | 0/8 |
| 5 | Negative controls, titanium | >505.0 | 0/4 |
| 6 | Positive controls, titanium | 113.8 ± 3.5 | 4/4 |
| 7 | 360 min photo-Fenton, titanium | 202.9 ± 5.8 | 8/8 |
After photo-Fenton treatment (224 μg mL−1 Fe3+, 500 μg mL−1 h−1 H2O2, UV-A) wires were intracranially implanted in male golden Syrian hamsters. The survival interval of group 3 was calculated taking into account only the scrapie affected animals.
Prolonged photocatalytic treatment (224 μg mL−1 Fe3+, 500 μg mL−1 h−1 H2O2, UV-A, 480 min) of prion-contaminated stainless steel resulted in complete elimination of the initial infectivity (Table 2, group 4). All individuals demonstrated no sign of the scrapie illness and were euthanized 505 d.p.i. The absence of PrPSc in the brains of all asymptomatic animals was verified by immunoblotting (see Fig. S5).
In conclusion, the present paper demonstrates for the first time the potential of homogenous photocatalytic oxidation, mediated by the photo-Fenton reagent, to effectively degrade and inactivate prions adsorbed on metal surfaces. The mechanism of inactivation is based on the oxidative attack of powerful transitory species. Moreover, a novel method of prion transmission, via intraperitoneal inoculation of prion-contaminated TiO2 particles in C57BL mice, is presented. This method significantly increases the amount of inoculated PrP and could offer an alternative or a supplement in future prion inactivation studies. The current study also investigates for the first time the behavior of titanium, a material commonly used for the manufacture of surgical instruments, in terms of prion adsorption and following decontamination efficiency. Both in vitro and in vivo analyses reveal that titanium adsorbs more protein and PrP, thus more intensive treatment conditions are required in comparison to stainless steel. This finding raises questions concerning the adequacy of current protocols of decontamination of non-disposable metallic surgical instruments in healthcare facilities, since the vast majority of decontamination studies and the optimization of protocols have been conducted employing stainless steel substrates. The specific type of the stainless steel used in the manufacture of instruments may affect PrP adsorption,30 while even the prolonged age of instruments is known to aid the accumulation of deposits onto wearing sites.31 Furthermore, contrary to immunoblotting, SEM and fluorescence microscopy have served as particularly sensitive tools, aiding the standardization of our photocatalytic decontamination protocols, prior to the bioassay. This finding could perhaps contribute to the reduction of the number of animals used in future prion decontamination studies employing metal substrates.
Indeed, further work is required, under real conditions, before this process could evolve into a mature application, both for the decontamination of surgical instruments and the treatment of contaminated biological waste, including the design of a practical setup and the reduction of treatment times by employing even more drastic treatment conditions. However, this proposed homogenous photocatalytic decontamination process, among others, bears another very significant advantage: It may also be efficient for parts of the instruments not directly illuminated, since its’ main reaction (Fenton reaction), responsible for the production of the highly oxidative species is, in fact, a dark reaction.16 Furthermore, the simplicity of the technique, the mild, instrument-compatible and user-friendly operational conditions, the low cost of the reagents and equipment and the potential of catalyst reuse, render homogenous photocatalytic oxidation a promising tool for decontamination applications, which could be exploited alone or in combination with traditional disinfection processes.
Materials and Methods
Contamination materials
Brain homogenates (10% w/v) were prepared in phosphate buffered saline (PBS) and 5 mM phenylmethylsulfonyl fluoride (PMSF), using a FastPrep (Thermo Scientific) apparatus. Brain tissue originated from terminally ill C57BL/6J female mice infected with the RML scrapie strain, as well as from male golden Syrian hamsters infected with the 263K scrapie strain. A 10% w/v brain homogenate from a healthy hamster was similarly prepared. The presence of protease-resistant prion isoform in scrapie infected brains was verified by digestion with protease K, followed by western blot immunodetection.32
Contamination of surfaces
TiO2 particles (rutile, Alfa-Aesar, 1–2 μm diameter, 7092 cm2 g−1 surface area) as well as stainless steel and titanium wires (Alfa-Aesar, 5.0 mm length, 0.25 mm diameter) served as substrates for scrapie contamination. TiO2 particles were incubated with 10% w/v RML brain homogenate for 3 h (h) at room temperature under constant agitation, washed with PBS and dried for 16 h at room temperature, prior to the photocatalytic treatment. Wires were cleaned by ultrasonication in a 2% w/v Triton X-100 solution, rinsed in distilled water and dried at 37 °C. The wires were artificially contaminated by immersion in 10% w/v scrapie brain homogenate for 1 h at room temperature (positive controls) or in normal hamster brain homogenate (negative controls). Wires were then dried for 16 h at room temperature. Excess of unbound infectivity was removed by rinsing for 5 min in PBS.
Photo-Fenton decontamination
Analytical grade FeCl3•6H20 and H2O2 were used in the study. Photocatalytic treatment of prion-contaminated TiO2 particles and wires, was performed at room temperature in polystyrene plates and the reaction mixture in each well was gently stirred during the treatment. Irradiation was performed using 5 parallel black light blue fluorescent tubes (TLD 8W/08; Philips, UV-A region, 340–400 nm), placed 10 cm above the solution surface. The light intensity was 9.1 ± 0.2 mW cm−2 as measured by a PMA 2100 radiometer (Solar Light Co) equipped with a UV-A S/N 8773 detector. Substrates were treated in the presence of UV-A with Fe3+ and H2O2 at pH 3.5. Control reactions were performed in combinations in which at least one of the photo-Fenton components (Fe3+/H2O2/UV-A) was absent.
Electrophoresis and immunoblotting
After photocatalytic processing, the substrates were incubated at 100 °C with O’Farrell loading buffer,33 to remove any remaining protein contamination. Degradation of total protein adsorbed on the substrates’ surface was monitored by SDS-PAGE34 following silver staining,32 whereas degradation of adsorbed PrPSc was followed by immunodetection.32 For the immunodetection of RML, blots were probed with the anti-PrP monoclonal antibody 12F10 (Cayman chemical), whereas for the 263K strain the anti-PrP monoclonal antibody 6H4 (a generous gift from Prionics) was used.
Scanning electron (SEM) and fluorescence microscopy
SEM inspection of the wires’ surface was performed using a JEOL, JSM-840A microscope operating at 21 kV. SYPRO Ruby fluorescent protein staining of wires was conducted as described elsewhere.35 Fluorescence microscopy was performed using a Nikon Eclipse TE2000-4 microscope, equipped with a HQ TexasRedrhodamine filter. Estimation of the fluorescent area of the wires was performed with the ImageJ software (version 1.37v, http://rsb.info.nih.gov/ij/).
Bioassays
The hamster-adapted scrapie strains 263K and RML were propagated in golden Syrian hamsters and C57BL/6J mice, respectively. Bioassays were conducted according to the regulations of the local ethics committee (reference number 13/7225). TiO2 particles were inoculated intraperitoneally into C57BL/6J female mice (Charles Rivers Laboratories). Wires were individually implanted into the right frontal parietal lobe of anesthetized 8 week-old male golden Syrian hamsters (Harlan Laboratories). Animals were regularly monitored for clinical signs of scrapie and were euthanized at terminal stage of the disease. Asymptomatic mice and hamsters were euthanized 453 and 505 d post inoculation (d.p.i.), respectively. Diagnosis of TSE was confirmed by detection of PrPSc in brain tissue by immunoblotting, according to a previously described protocol.32
Supplementary Material
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
Part of this work was supported by the NeuroPrion-Network of Excellence. The authors also thank Assistant Professor Eleni Pavlidou for experimental assistance on SEM (Solid State Section, Department of Physics, AUTh), Dr Evgenia Salta (Laboratory for the Research of Neurodegenerative Diseases, Leuven) for experimental assistance and useful discussions and Dr Roza Lagoudaki (AHEPA University Hospital, Aristotle University of Thessaloniki) for assistance on animal inoculations.
References
- 1.Aguzzi A, Calella AM. Prions: protein aggregation and infectious diseases. Physiol Rev. 2009;89:1105–52. doi: 10.1152/physrev.00006.2009. [DOI] [PubMed] [Google Scholar]
- 2.Prusiner SB. Prions. Proc Natl Acad Sci U S A. 1998;95:13363–83. doi: 10.1073/pnas.95.23.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stahl N, Borchelt DR, Hsiao K, Prusiner SB. Scrapie prion protein contains a phosphatidylinositol glycolipid. Cell. 1987;51:229–40. doi: 10.1016/0092-8674(87)90150-4. [DOI] [PubMed] [Google Scholar]
- 4.Aguzzi A, Baumann F, Bremer J. The prion’s elusive reason for being. Annu Rev Neurosci. 2008;31:439–77. doi: 10.1146/annurev.neuro.31.060407.125620. [DOI] [PubMed] [Google Scholar]
- 5.Taylor DM. Inactivation of prions by physical and chemical means. J Hosp Infect. 1999;43(Suppl):S69–76. doi: 10.1016/S0195-6701(99)90067-1. [DOI] [PubMed] [Google Scholar]
- 6.Macalister GO, Buckley RJ. The risk of transmission of variant Creutzfeldt-Jakob disease via contact lenses and ophthalmic devices. Cont Lens Anterior Eye. 2002;25:104–36. doi: 10.1016/S1367-0484(02)00008-5. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization. WHO Infection Control Guidelines for Transmissible Spongiform Encephalopathies: Report of a WHO Consultation (WHO/CDS/CSR/APH/2000.3). Geneva: WHO. 1999. [Google Scholar]
- 8.Rutala WA, Weber DJ. Creutzfeldt-Jakob disease: recommendations for disinfection and sterilization. Clin Infect Dis. 2001;32:1348–56. doi: 10.1086/319997. [DOI] [PubMed] [Google Scholar]
- 9.Brown SA, Merritt K, Woods TO, Busick DN. Effects on instruments of the World Health Organization--recommended protocols for decontamination after possible exposure to transmissible spongiform encephalopathy-contaminated tissue. J Biomed Mater Res B Appl Biomater. 2005;72:186–90. doi: 10.1002/jbm.b.30125. [DOI] [PubMed] [Google Scholar]
- 10.Wadsworth JD, Joiner S, Hill AF, Campbell TA, Desbruslais M, Luthert PJ, Collinge J. Tissue distribution of protease resistant prion protein in variant Creutzfeldt-Jakob disease using a highly sensitive immunoblotting assay. Lancet. 2001;358:171–80. doi: 10.1016/S0140-6736(01)05403-4. [DOI] [PubMed] [Google Scholar]
- 11.Johnson CJ, Phillips KE, Schramm PT, McKenzie D, Aiken JM, Pedersen JA. Prions adhere to soil minerals and remain infectious. PLoS Pathog. 2006;2:e32. doi: 10.1371/journal.ppat.0020032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seidel B, Thomzig A, Buschmann A, Groschup MH, Peters R, Beekes M, Terytze K. Scrapie Agent (Strain 263K) can transmit disease via the oral route after persistence in soil over years. PLoS One. 2007;2:e435. doi: 10.1371/journal.pone.0000435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown P, Rau EH, Johnson BK, Bacote AE, Gibbs CJ, Jr., Gajdusek DC. New studies on the heat resistance of hamster-adapted scrapie agent: threshold survival after ashing at 600 degrees C suggests an inorganic template of replication. Proc Natl Acad Sci U S A. 2000;97:3418–21. doi: 10.1073/pnas.050566797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wiggins RC. Prion stability and infectivity in the environment. Neurochem Res. 2009;34:158–68. doi: 10.1007/s11064-008-9741-6. [DOI] [PubMed] [Google Scholar]
- 15.Paspaltsis I, Kotta K, Lagoudaki R, Grigoriadis N, Poulios I, Sklaviadis T. Titanium dioxide photocatalytic inactivation of prions. J Gen Virol. 2006;87:3125–30. doi: 10.1099/vir.0.81746-0. [DOI] [PubMed] [Google Scholar]
- 16.Paspaltsis I, Berberidou C, Poulios I, Sklaviadis T. Photocatalytic degradation of prions using the photo-Fenton reagent. J Hosp Infect. 2009;71:149–56. doi: 10.1016/j.jhin.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 17.Fichet G, Antloga K, Comoy E, Deslys JP, McDonnell G. Prion inactivation using a new gaseous hydrogen peroxide sterilisation process. J Hosp Infect. 2007;67:278–86. doi: 10.1016/j.jhin.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 18.Rogez-Kreuz C, Yousfi R, Soufflet C, Quadrio I, Yan ZX, Huyot V, Aubenque C, Destrez P, Roth K, Roberts C, et al. Inactivation of animal and human prions by hydrogen peroxide gas plasma sterilization. Infect Control Hosp Epidemiol. 2009;30:769–77. doi: 10.1086/598342. [DOI] [PubMed] [Google Scholar]
- 19.Bautista P, Mohedano AF, Casas JA, Zazo JA, Rodriguez JJ. An overview of the application of Fenton oxidation to industrial wastewaters treatment. J Chem Technol Biotechnol. 2008;83:1323–38. doi: 10.1002/jctb.1988. [DOI] [Google Scholar]
- 20.Pignatello JJ, Oliveros E, MacKay A. Advanced oxidation processes for organic contaminant destruction based on the Fenton reaction and related chemistry. Crit Rev Environ Sci Technol. 2006;36:1–84. doi: 10.1080/10643380500326564. [DOI] [Google Scholar]
- 21.Malato S, Fernandez-Ibanez P, Maldonado MI, Blanco J, Gernjak W. Decontamination and disinfection of water by solar photocatalysis: Recent overview and trends. Catal Today. 2009;147:1–59. doi: 10.1016/j.cattod.2009.06.018. [DOI] [Google Scholar]
- 22.Rincon AG, Pulgarin C. Absence of E-coli regrowth after Fe3+ and TiO2 solar photoassisted disinfection of water in CPC solar photoreactor. Catal Today. 2007;124:204–14. doi: 10.1016/j.cattod.2007.03.039. [DOI] [Google Scholar]
- 23.Zobeley E, Flechsig E, Cozzio A, Enari M, Weissmann C. Infectivity of scrapie prions bound to a stainless steel surface. Mol Med. 1999;5:240–3. [PMC free article] [PubMed] [Google Scholar]
- 24.Stanley A. Brown KM, Terry O. Woods, Deanna N. Busick. Effects on Instruments of the World Health Organization–Recommended Protocols for Decontamination after Possible Exposure to Transmissible Spongiform Encephalopathy–Contaminated Tissue. Wiley InterScience, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Wassell DT, Embery G. Adsorption of bovine serum albumin on to titanium powder. Biomaterials. 1996;17:859–64. doi: 10.1016/0142-9612(96)83280-7. [DOI] [PubMed] [Google Scholar]
- 26.Fichet G, Comoy E, Duval C, Antloga K, Dehen C, Charbonnier A, McDonnell G, Brown P, Lasmézas CI, Deslys JP. Novel methods for disinfection of prion-contaminated medical devices. Lancet. 2004;364:521–6. doi: 10.1016/S0140-6736(04)16810-4. [DOI] [PubMed] [Google Scholar]
- 27.Baxter HC, Campbell GA, Whittaker AG, Jones AC, Aitken A, Simpson AH, Casey M, Bountiff L, Gibbard L, Baxter RL. Elimination of transmissible spongiform encephalopathy infectivity and decontamination of surgical instruments by using radio-frequency gas-plasma treatment. J Gen Virol. 2005;86:2393–9. doi: 10.1099/vir.0.81016-0. [DOI] [PubMed] [Google Scholar]
- 28.Flechsig E, Hegyi I, Enari M, Schwarz P, Collinge J, Weissmann C. Transmission of scrapie by steel-surface-bound prions. Mol Med. 2001;7:679–84. [PMC free article] [PubMed] [Google Scholar]
- 29.Fichet G, Comoy E, Dehen C, Challier L, Antloga K, Deslys JP, McDonnell G. Investigations of a prion infectivity assay to evaluate methods of decontamination. J Microbiol Methods. 2007;70:511–8. doi: 10.1016/j.mimet.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 30.Luhr KM, Löw P, Taraboulos A, Bergman T, Kristensson K. Prion adsorption to stainless steel is promoted by nickel and molybdenum. J Gen Virol. 2009;90:2821–8. doi: 10.1099/vir.0.012302-0. [DOI] [PubMed] [Google Scholar]
- 31.Hervé R, Secker TJ, Keevil CW. Current risk of iatrogenic Creutzfeld-Jakob disease in the UK: efficacy of available cleaning chemistries and reusability of neurosurgical instruments. J Hosp Infect. 2010;75:309–13. doi: 10.1016/j.jhin.2010.01.024. [DOI] [PubMed] [Google Scholar]
- 32.Polymenidou M, Verghese-Nikolakaki S, Groschup M, Chaplin MJ, Stack MJ, Plaitakis A, Sklaviadis T. A short purification process for quantitative isolation of PrPSc from naturally occurring and experimental transmissible spongiform encephalopathies. BMC Infect Dis. 2002;2:23. doi: 10.1186/1471-2334-2-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O’Farrell PH. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975;250:4007–21. [PMC free article] [PubMed] [Google Scholar]
- 34.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 35.Lipscomb IP, Sihota AK, Botham M, Harris KL, Keevil CW. Rapid method for the sensitive detection of protein contamination on surgical instruments. J Hosp Infect. 2006;62:141–8. doi: 10.1016/j.jhin.2005.07.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.